Abstract
In directed C–H activation reactions, nitrogen and sulfur atoms present in heterocyclic substrates coordinate strongly with metal catalysts. This coordination, which can lead to catalyst poisoning or C–H functionalization at an undesired position, limits the application of C–H activation reactions in heterocycle-based drug discovery.1–5 Herein, we report a robust and synthetically useful reaction that overcomes the complications associated with performing C–H functionalization reactions on heterocycles. Our approach employs a simple N-methoxy amide group, which serves as both a directing group and an anionic ligand to promote the in situ generation of the reactive PdX2 (X = ArCONOMe) species from a Pd(0) source using air as the sole oxidant. In this way, the PdX2 species is inherently anchored in close proximity with the target C–H bond adjacent to CONHOMe group, thus avoiding the interference from various heterocycles. Remarkably, this reaction overrides the conventional positional selectivity patterns observed with substrates containing strongly coordinating heteroatoms, including nitrogen, sulfur, and phosphorus. Thus, this operationally simple aerobic reaction demonstrates the feasibility of bypassing a fundamental limitation that has long plagued applications of directed C–H activation in medicinal chemistry.
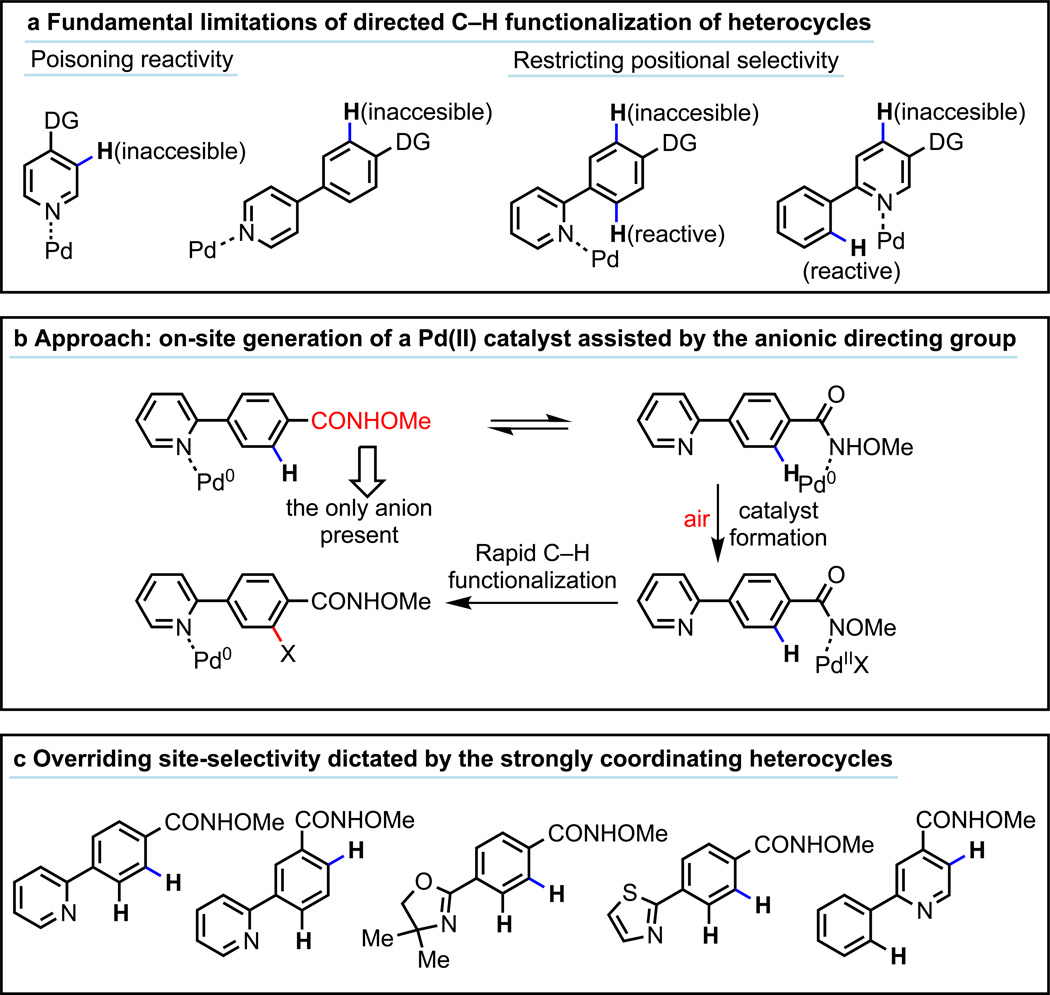
Heterocycles are commonly found in drug candidates owing to their ability to improve solubility and reduce the lipophilicity of a drug molecule.1–2 The potential application of C–H activation technologies in the rapid synthesis and diversification of novel heterocycles has attracted widespread attention from the pharmaceutical industry.3–5 One of the most significant challenges in the application of C–H functionalization reactions is achieving robust control of positional selectivity. Directed C–H metalation has recently emerged as a reliable approach for achieving a diverse collection of selective C–H functionalization reactions, and activation of both proximate6–13 and remote14 C–H bonds have proven feasible. The use of a weakly coordinating functional group to achieve high effective molarity of the catalyst around the C–H bond of interest has greatly expanded the substrate scope of these processes.12 Unfortunately, these C–H functionalization processes are generally incompatible with the majority of medicinally important heterocyclic substrates because the heteroatoms can interfere with the catalyst.15–18 For example, the Novartis team recently developed two strategies to protect the pyridyls with Lewis acid or N-oxide formation in order to prevent the classic cyclopalladation and perform the desired allylic C–H acetoxylation.18 In directed C–H activation, strongly coordinating nitrogen, sulfur, and phosphorous heteroatoms often outcompete the directing groups for catalyst binding, thus preventing activation of the C–H bonds proximate to the directing groups (Fig. 1a). When coordinated to a heterocycle, the catalyst is either (a) unreactive due to the lack of a proximate C–H bond or (b) only capable of activating the C–H bonds adjacent to the coordinating heteroatom. This inherent drawback of directed C–H activation, especially with Pd(II) catalysts, is currently a major roadblock for widespread application of C–H functionalization in heterocycle-based medicinal chemistry. Similarly, C–H functionalization of heterocycles using non-directed approaches has found limited success in terms of substrate scope and efficiency.19–26
Figure 1. Development of a catalytic system to overcome fundamental limitations of heterocyclic C–H bond functionalizations.
a, Adverse effects of strong coordination between Pd(II) catalysts and heterocycles. b, Avoiding heterocycle poisoning via on-site generation of Pd(II) catalysts. c, Overriding the conventional positional selectivity directed by heterocycles.
Herein, we report an aerobic C–H functionalization reaction that effectively overcomes heterocycle poisoning and overrides the commonly observed positional selectivity dictated by heterocycles. The catalytic cycle begins with the on-site generation of a reactive Pd(II) species (Fig. 1b). To this end, a Pd(0) precursor coordinates with a simple, carboxylic acid–derived N-methoxy amide directing group (CONHOMe),27 which promotes subsequent oxidation of Pd(0) to Pd(II) by air present in the reaction mixture.28 The anionic directing group is the only X-type ligand in the reaction mixture that can be incorporated in to the resulting PdX2 species. Thus, any Pd(0) species in solution that are transiently coordinated to an L-type heterocycle must migrate to the CONHOMe directing group in order to form the reactive PdX2 species which then cleave adjacent C–H bonds thereby bypassing the adverse effects of heterocycles. Remarkably, the commonly observed positional selectivity patterns dictated by the well-known cyclopalladation in heterocycles, are overridden (Fig. 1c), even when C–H bonds are present ortho to strongly coordinating heteroatoms. Since C–H palladation is often the selectivity-determining step, we anticipate this switch of positional selectivity can be extended to other C–H activation transformations upon further development.
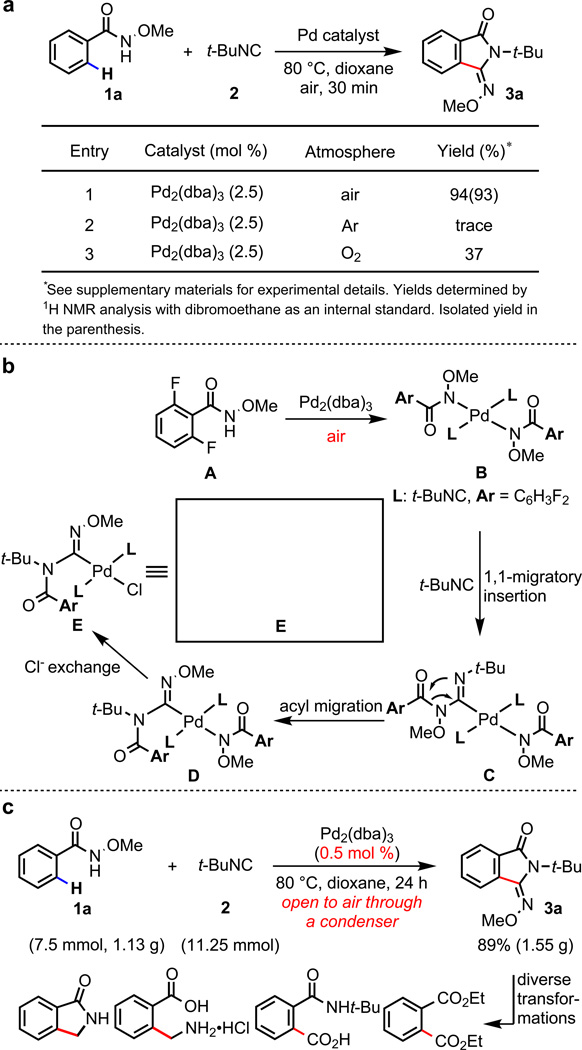
We began our investigations using a broadly used N-methoxy amide directing group (CONHOMe) developed in our laboratory in 2008.27 A lack of heterocyclic substrates among extensive reports on using this otherwise powerful directing group indicates widespread heterocycle poisoning in directed C–H activation. To verify this assessment, we performed an extensive survey by applying the previously reported reactions using the N-methoxy amide directing group to representative heterocyclic substrates shown in Fig.1a. Not a single protocol was compatible with these heterocyclic substrates (for details see supplementary information). We surmised that a novel approach would be needed to overcome the strong coordination of Pd(II) species with heterocycles. Pd(II)X2 catalysts are known to strongly coordinate with neutral σ-donors such as pyridines. On the other hand, Pd(0) species possess a comparatively weaker affinity for this type of ligand because they are more nucleophilic rather than electrophilic. We, therefore, focused on the design of a catalytic system that would begin with Pd(0) species, which could coordinate comparatively weakly with both pyridine and the directing group in a reversible manner. We hypothesized that a specifically designed anionic directing group, if coordinated to Pd(0) species, could accelerate the generation of the reactive PdX2(II) species if this directing group were the sole X-type ligand in the reaction mixture (Fig. 1b).28 Once generated on-site, the resulting PdX2 species could potentially cleave a C–H bond adjacent to the directing group prior to being scavenged by the pyridyl group. In essence, the pyridyl group would serve as a Pd(0) reservoir, rather than poisoning Pd(II). To establish the feasibility of this approach, we used a simple arene substrate 1a and employed CONHOMe to develop a highly efficient C–H functionalization reactions in the presence of catalytic amount of Pd2(dba)3. We anticipated that Pd(0) would be converted to Pd(II)(ArCONOMe)2 in the presence of an oxidant. Air was identified as an ideal oxidant in that it would avoid the introduction of other anions.28 Through extensive screening (see supplementary information), we found that arene 1a reacts with 1.5 equiv of isocyanide 2 in the presence of 2.5 mol% Pd2(dba)3 in 1,4-dioxane under 1 atm air at 80 °C for 30 min to give ortho-functionalized 3-(imino)isoindolinone 3a in 93% isolated yield (Fig. 2a).
Figure 2. Discovery of an efficient aerobic C–H activation reaction.
a, A catalytic C–H activation reaction using air as the sole oxidant. b, Characterization of a reactive C-amidinyl Pd(II) intermediate. c, Gram-scale reaction and diverse transformations.
The structure of 3a was unexpected based on earlier precedents in isocyanide insertion chemistry,29 indicating the involvement of a new isocyanide insertion pathway. To rationalize the formation of 3a, we reacted 2,6-difluoro-N-methoxybenzamide (1u) with 25 mol% Pd2(dba)3 under the reaction conditions, attempting to identify potential Pd(II) intermediates prior to the C–H activation event (Fig. 2b). We were able to characterize a new C-amidinyl Pd(II) species E by X-ray crystallography, which allows us to propose an intriguing reaction pathway. We speculate that the initially formed Pd(II) species B undergoes migratory insertion with t-BuNC to give C, which then rearranges to form C-amidinyl Pd(II) precursor D. The chloride in E is likely incorporated from the CHCl3 contained in commercial Pd2(dba)3 via anionic exchange with D. In hindsight, it is crucial that the unexpected C-amidinyl Pd(II) species D or E is able to cleave the C–H bonds in a highly efficient manner.
The use of air as an oxidant is essential for this transformation (Fig. 2a, entries 1, 2). Interestingly, a significantly lower yield is obtained when the reaction is conducted under O2 (1 atm). Presumably, in high concentration, O2 can intercept one of the intermediates in the catalytic cycle. The efficiency of this catalytic system was further demonstrated by running the reaction on gram-scale using 0.5 mol% Pd2(dba)3 to afford product in 89% isolated yield, albeit with a prolonged reaction time (24 h) (Fig. 2c). To demonstrate the synthetic utility of this C–H functionalization process, 3a was readily converted to a number of synthetically versatile building blocks including an ester, an amine and a lactam via one or two-step procedures (Fig. 2c, for details see supplementary information).
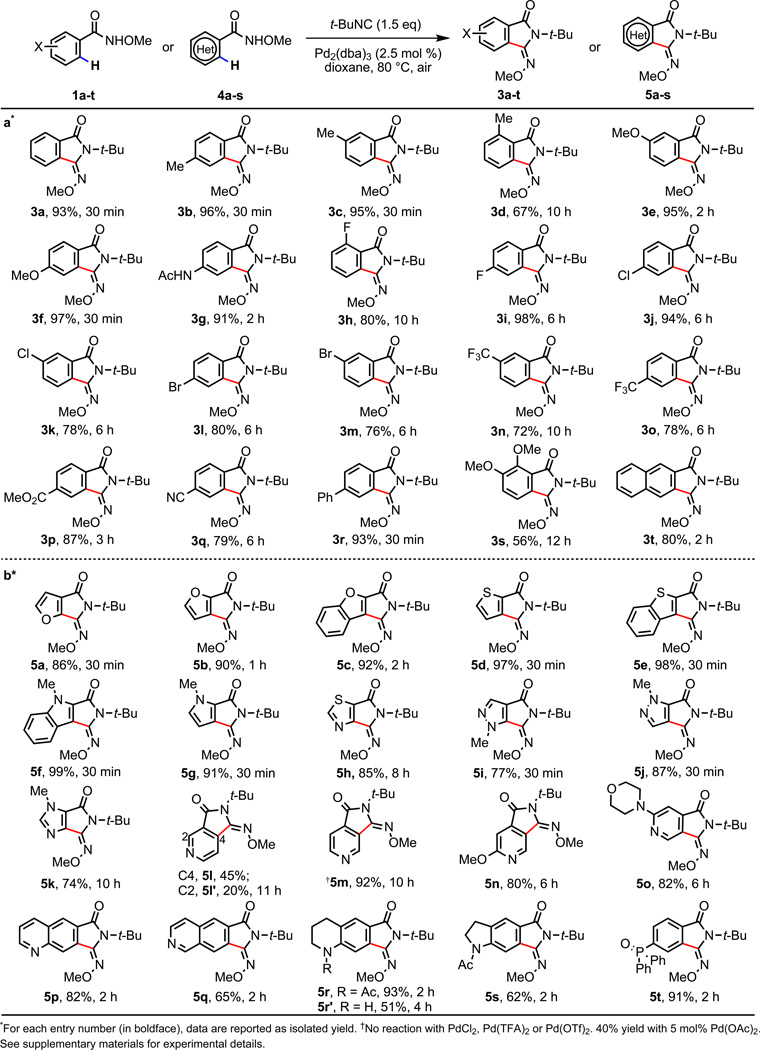
The scope of arene substrates was surveyed using 2.5 mol% Pd2(dba)3 (Fig. 3a). A variety of substituents on the aryl ring were well tolerated (3a–t). These results demonstrate that the on-site generation of Pd(II) precursor B using air as the oxidant and subsequent C–H functionalizations are feasible. The fast rate of this reaction encouraged us to examine whether heteroatom poisoning could be overcome using this new reaction pathway. We were pleased to find that the reaction of furans, benzofurans and benzothiophenes proceed smoothly to afford the desired products 5a–e in 86–98% yields (Fig. 3b). Indole, pyrrole, thiazole, pyrazole, and imidazole substrates are also converted to the corresponding functionalized products 5f–k in good yields (74–99%). The strongly coordinating nitrogen atoms in pyridines and quinolines are well-known to poison directed C–H activation under Pd(II) catalysis. Thus, the excellent yields obtained with various pyridine substrates (5l–q), including an aminated pyridine (5o), provide further evidence that this catalytic system can overcome severe heteroatom poisoning. Acetyl-protected tetrahydroquinoline- and indoline-containing substrates can also be functionalized, giving 5r and 5s. A free amino group is tolerated, albeit resulting lower yield (5r', 51%). A phosphoryl group is also compatible (5t).
Figure 3. Scope of the Reaction.
a, Directed C–H functionalization of arenes. b, Directed C–H functionalization of heterocycles.
The importance of using a Pd(0) source to enter the catalytic cycle is further supported by the lack of reactivity using commonly employed Pd(II) sources, including PdCl2, Pd(TFA)2 and Pd(OTf)2, in place of Pd2(dba)3. In particular, exposing 4m, a representative pyridine-containing substrate, to the reaction conditions using these catalysts led to full recovery of starting material in the presence or absence of dba ligand (Fig 3b). The desired product, 5m, was formed in 40% yield, however, when 5 mol% Pd(OAc)2 was used as the catalyst. This is most likely due to the known facile reduction of Pd(OAc)2 to Pd(0) by isocyanide.30 To seek experimental evidence in support of this reasoning, we stirred Pd(OAc)2, PdCl2, Pd(TFA)2 and Pd(OTf)2 separately with t-BuNC in dioxane at 80 °C. We found that Pd(OAc)2 was completely reduced to Pd(0) within 30 min while other Pd(II) catalysts remained intact (For details see supplementary information). To further demonstrate the importance of the on-site generation of PdX2 (X = ArCONOMe) from Pd(0) in the absence of external anions, we also carried out the standard reaction in the presence of different anions, Cl-, TFA-, OTf-. We found these anions consistently prevent the desired reaction (see supplementary information).
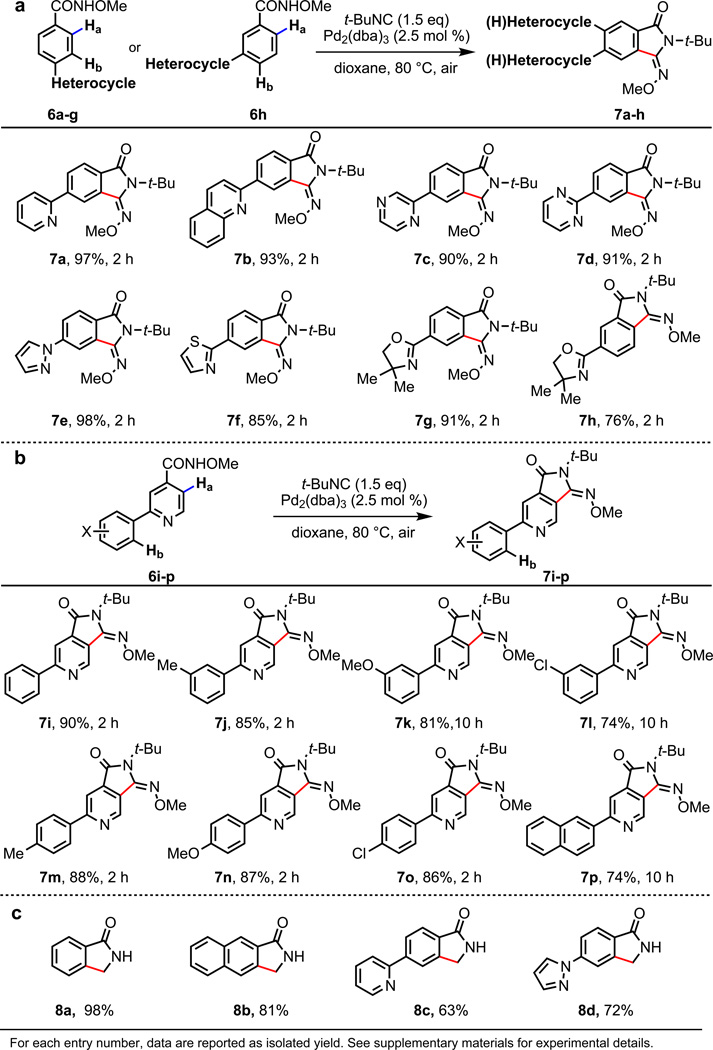
It is well established that substrates containing C–H bonds ortho to strongly coordinating heterocyles will undergo facile heterocycle-directed ortho-cyclopalladation. This reactivity can inhibit the activation of a target C–H bond that is proximate to a weaker directing group (here, the CONHOMe functional group),10–12 which may prevent the employment of directed C–H functionalization reactions in substrates containing heterocyles. Not surprisingly, reaction of para-(2-pyridyl)benzamide (6a) with Pd(OAc)2 or Pd(TFA)2 in the absence of t-BuNC gave exclusively cyclopalladation product directed by the pyridine, suggesting pyridine is a stronger coordinating group than CONHOMe group (for X-ray characterization of the cyclopalladation intermediate formed from 6a see supplementary information). The unprecedented compatibility of our catalytic system with heterocycles substrates, however, prompted us to examine whether our new catalytic system can override the conventional heterocycle-directed cyclopalladation.
We chose as a test substrate para-(2-pyridyl)benzamide (6a) (Fig. 4a), which has a 2-pyridyl group para to the N-methoxy amide directing group. Remarkably, with our new catalytic system, C–H functionalization proceeds exclusively at the position ortho to the CONHOMe group to provide the desired product 7a in 97% isolated yield. To investigate the origin of the observed switch of positional selectivity, we reacted 6a with various Pd(II) catalysts under the classic cyclopalladation conditions. As expected, palladation at the position ortho to the pyridyl group occurs to give the cyclopalladate intermediate in quantitative yield (see supplementary information). In contrast, not traces of this intermediate can be detected throughout our standard reaction when Pd2dba3 is used as the catalyst. These experiments suggest that the use of Pd2(dba)3 catalyst under our aerobic conditions effectively avoids the conventional pyridyl-directed ortho-palladation pathway. We subsequently replaced the pyridine with other medicinally important heterocycles including a quinoline, pyrazine, pyrimidine, pyrazole and thiazole. Uniformly excellent yields of the desired C–H functionalization products are obtained (7b–f, 76–98% yield) for these substrates. In light of the well-known directing power of oxazoline in ortho-palladation,12 para- and meta-oxazolinyl substituted substrates 6g and 6h were also subjected our standard reaction conditions. In both cases, only the desired C–H functionalization products are formed (7g and 7h, 91 and 76% yield, respectively).
Figure 4. Overriding the conventional positional selectivity dictated by heterocycles.
a, b, Reactions of substrates prone to heterocycle-directed cyclometalation. c, Lactam products formed via hydrogenation and removal of the t-butyl groups (yields over two steps are shown).
We further explored the utility of this catalytic system for 2-phenylpyridine substrates containing the CONHOMe group on the pyridine ring (6i–p) (Fig. 4b). We anticipated that achieving reactivity and positional selectivity with these substrates could be particularly challenging due to the electron-deficiency of the pyridine ring, which deactivates the C–H bonds ortho to the CONHOMe group. We were pleased to find that C–H functionalization of these 2-phenylpyridine substrates occurs exclusively ortho to the N-methoxy amide group, affording the desired products in good to excellent yields (7i–p, 74–90% yield).
Finally, representative C–H functionalization products from this reaction were converted to synthetically useful lactams by hydrogenolysis with Pd/C under H2 followed by treatment with trifluoroacetic acid. This new catalytic system provides an operationally simple and versatile route to access medicinally important lactams (8a–d).1–2
In summary, we have developed a catalytic system that overcomes the poisoning effect of heterocycles and overrides the inherent positional selectivity dictated by strongly coordinating heteroatoms. The switch of the positional selectivity in the cyclopalladation step, often as the selectivity-determining step, will be exploited in other catalytic C–H activation transformations.
Supplementary Material
Acknowledgements
We gratefully acknowledge the Shanghai Institute of Organic Chemistry, the Chinese Academy of Sciences, the CAS/SAFEA International Partnership Program for Creative Research Teams, NSFC-21121062, and The Recruitment Program of Global Experts for financial support. We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 1R01 GM102265) for their financial support.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions Y.-J.L. and and H.X. performed the reaction discovery experiments and contributed equally. W.-J.K., H.X. and M.S. performed the reactions with the heterocyclic substrates. H.-X.D. and J.-Q.Y. conceived the concept and directed the project and prepared this manuscript.
The authors declare no competing financial interests: details accompany the full-text HTML version of the paper at www.nature.com/nature.
References
- 1.Meanwell NA. Improving drug candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 2011;24:1420–1456. doi: 10.1021/tx200211v. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie TJ, Macdonald SJF, Young RJ, Pickett SD. The impact of aromatic ring count on compound developability: further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discovery Today. 2011;16:164–171. doi: 10.1016/j.drudis.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Schöenherr H, Cernak T. Profound methyl effects in drug discovery and a call for new C–H methylation reactions. Angew. Chem., Int. Ed. 2013;52:12256–12267. doi: 10.1002/anie.201303207. [DOI] [PubMed] [Google Scholar]
- 4.Bryan MC, et al. Sustainable practices in medicinal chemistry: current state and future directions. J. Med. Chem. 2013;56:6007–6021. doi: 10.1021/jm400250p. [DOI] [PubMed] [Google Scholar]
- 5.Davies IW, Welch CJ. Looking forward in pharmaceutical process chemistry. Science. 2009;325:701–704. doi: 10.1126/science.1174501. [DOI] [PubMed] [Google Scholar]
- 6.Snieckus V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 1990;90:879–933. [Google Scholar]
- 7.Kakiuchi F, et al. Catalytic addition of aromatic carbon–hydrogen bonds to olefins with the aid of ruthenium complexes. Bull. Chem. Soc. Jpn. 1995;68:62–83. [Google Scholar]
- 8.Jun C-H, Hong J-B, Lee D-Y. Chelation-assisted hydroacylation. Synlett. 1999:1–12. [Google Scholar]
- 9.Colby DA, Bergman RG, Ellman JA. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugulis O, Do H-Q, Shabashov D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engle KM, Mei T-S, Wasa M, Yu J-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung CS, Dong VM. Catalytic dehydrogenative cross-coupling: forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]
- 14.Leow D, Li G, Mei T-S, Yu J-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasa M, Worrell BT, Yu J-Q. Pd(0)/PR3-catalyzed arylation of nicotinic acid and isonicotinic acid derivatives. Angew. Chem., Int. Ed. 2010;49:1275–1277. doi: 10.1002/anie.200906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann L, Lygin AV. Ruthenium-catalyzed direct C–H bond arylations of heteroarenes. Org. Lett. 2011;13:3332–3335. doi: 10.1021/ol2010648. [DOI] [PubMed] [Google Scholar]
- 17.Cho J-Y, Iverson CN, Smith MR., III Steric and chelate directing effects in aromatic borylation. J. Am. Chem. Soc. 2000;122:12868–12869. [Google Scholar]
- 18.Malik HA, Taylor BLH, Kerrigan JR, Grob JE, Houk KN, Du Bois J, Hamann LG, Patterson AW. Non-directed allylic C–H acetoxylation in the presence of Lewis basic heterocycles. Chem. Sci. 2014;5:2352–2361. doi: 10.1039/C3SC53414F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi J, Sato K, Hartwig JF, Ishiyama T, Miyaura N. Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates. Tetrahedron Lett. 2002;43:5649–5651. [Google Scholar]
- 20.Hurst TE, et al. Iridium-catalyzed C–H activation versus directed ortho metalation: complementary borylation of aromatics and heteroaromatics. Chem. Eur. J. 2010;16:8155–8161. doi: 10.1002/chem.201000401. [DOI] [PubMed] [Google Scholar]
- 21.Nakao Y, Yamada Y, Kashihara N, Hiyama T. Selective C-4 alkylation of pyridine by nickel/lewis acid catalysis. J. Am. Chem. Soc. 2010;132:13666–13668. doi: 10.1021/ja106514b. [DOI] [PubMed] [Google Scholar]
- 22.Tsai C-C, et al. Bimetallic nickel aluminun mediated para-selective alkenylation of pyridine: direct observation of η2, η1-pyridine Ni(0)−Al(III) intermediates prior to C−H bond activation. J. Am. Chem. Soc. 2010;132:11887–11889. doi: 10.1021/ja1061246. [DOI] [PubMed] [Google Scholar]
- 23.Kwak J, Kim M, Chang S. Rh(NHC)-catalyzed direct and selective arylation of quinolines at the 8-position. J. Am Chem. Soc. 2011;133:3780–3783. doi: 10.1021/ja111670s. [DOI] [PubMed] [Google Scholar]
- 24.Wencel-Delord J, Nimphius C, Wang H, Glorius F. Rhodium(III) and hexabromobenzene—a catalyst system for the cross-dehydrogenative coupling of simple arenes and heterocycles with arenes bearing directing groups. Angew. Chem. Int. Ed. 2012;51:13001–13005. doi: 10.1002/anie.201205734. [DOI] [PubMed] [Google Scholar]
- 25.Fu HY, Chen L, Doucet H. Phosphine-free Palladium-catalyzed direct arylation of imidazo[1,2-a]pyridines with aryl bromides at low catalyst loading. J. Org. Chem. 2012;77:4473–4478. doi: 10.1021/jo300528b. [DOI] [PubMed] [Google Scholar]
- 26.Kuznetsov A, Onishi Y, Inamoto Y, Gevorgyan V. Fused heteroaromatic dihydrosiloles: synthesis and double-fold modification. Org. Lett. 2013;15:2498–2501. doi: 10.1021/ol400977r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D-H, Wasa M, Giri R, Yu J-Q. Pd(II)-catalyzed cross-coupling of sp3 C–H bonds with sp2 and sp3 boronic acids using air as the oxidant. J. Am. Chem. Soc. 2008;130:7190–7191. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]
- 28.Campbell AN, Stahl SS. Overcoming the "oxidant problem": strategies to use O2 as the oxidant in organometallic C–H oxidation reactions catalyzed by Pd (and Cu) Acc. Chem. Res. 2012;45:851–863. doi: 10.1021/ar2002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang S. Unravelling the labyrinth of palladium-catalysed reactions involving isocyanides. Chem. Soc. Rev. 2013;42:4867–4880. doi: 10.1039/c3cs60022j. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Suginome M, Matsuura T, Murakami M. Palladium-catalyzed insertion of isocyanides into the silicon-silicon linkages of oligosilanes. J. Am. Chem. Soc. 1991;113:8899–8908. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.