Abstract
Objective
The goal of the study was to compare conventional mammography (MG) and contrast-enhanced spectral mammography (CESM) in preoperative women.
Materials and Methods
The study was approved by the local Ethics Committee and all participants provided informed consent. The study included 152 consecutive patients with 173 breast lesions diagnosed on MG or CESM. All MG examinations and consults were conducted in one oncology centre. Non-ionic contrast agent, at a total dose of 1.5 mL/kg body weight, was injected intravenous. Subsequently, CESM exams were performed with a mammography device, allowing dual-energy acquisitions. The entire procedure was done within the oncology centre. Images from low and high energy exposures were processed together and the combination provided an "iodine" image which outlined contrast up-take in the breast.
Results
MG detected 157 lesions in 150 patients, including 92 infiltrating cancers, 12 non-infiltrating cancers, and 53 benign lesions. CESM detected 149 lesions in 128 patients, including 101 infiltrating cancers, 13 non-infiltrating cancers, and 35 benign lesions. CESM sensitivity was 100% (vs. 91% for MG), specificity was 41% (vs. 15% for MG), area under the receiver operating characteristic curve was 0.86 (vs. 0.67 for MG), and accuracy was 80% (vs. 65% for MG) for the diagnosis of breast cancer. Both MG and CESM overestimated lesion sizes compared to histopathology (p < 0.001).
Conclusion
CESM may provide higher sensitivity for breast cancer detection and greater diagnostic accuracy than conventional mammography.
Keywords: Breast cancer, Contrast enhanced spectral mammography, Mammography
INTRODUCTION
Mammography is the only breast imaging examination shown to reduce breast cancer mortality, with a population-based sensitivity of 75% to 80% (1). The sensitivity of mammography detection of breast cancer ranges from 63% to 98% (2, 3) and has been reported to be as low as 30-48% in the dense breasts (4, 5).
Full-field digital mammography (FFDM) has almost entirely replaced analog (screen-film) mammography (6). FFDM enables high-quality breast imaging with higher contrast resolution, improved dynamic range, and rapid processing of data and images compared to screen-film mammography; however, overall sensitivity remains the same as analog, and more than half of the cases go undetected (1, 7, 8). Applications like stereo mammography, breast tomosynthesis and contrast-enhanced digital mammography are under investigation. Such advances in technology may provide improved diagnostic information and reduce the effect of overlapping structures (9). Contrast-enhanced digital mammography with injection of an iodinated contrast agent is one such diagnostic tool.
Contrast-enhanced breast imaging techniques like CT and MRI (10, 11) are used for the detection of angiogenesis by tracking contrast agent uptake and washout in suspicious tissues. CT has the drawback of high radiation doses, despite its reported use in the detection of breast carcinoma (12, 13). Contrast-enhanced MRI is currently the most sensitive breast cancer detection technique, but may have high false positive rates, higher costs, and lower availability (14, 15, 16). Patients with pacemakers, certain aneurysm clips or other metal implants, or severe claustrophobia are unable to undergo MRI.
Contrast-enhanced digital mammography offers the potential to detect angiogenesis in the mammography suite. Two types of contrast-enhanced digital mammography have emerged: 1) digital subtraction mammography (DSM), which acquires images in a single breast with a single view projection before and after contrast agent injection, and 2) contrast-enhanced spectral mammography (CESM), also known as dual-energy mammography. CESM acquires both low- and high-energy images during a single, brief compression after injection of contrast agent, to highlight the conspicuity of iodine in the breast. Unlike DSM, which would require injection of contrast for each view, CESM can be performed in both breasts and in multiple views after a single injection, with a very short interval of about 1.5 second between low- and high-energy image acquisitions. This reduces misregistration between the 2 images. A weighted subtraction of low-energy (the X-ray spectrum entirely below the k-edge of iodine) and high-energy (mostly above the k-edge of iodine) images is performed to maximize the conspicuity of low concentrations of iodine in enhancing breast lesions (17). The aim of our study was to compare the diagnostic estimates of CESM and conventional mammography (MG) for breast cancer in preoperative women.
MATERIALS AND METHODS
The study protocol was approved by the local Ethics Committee at the Regional Board of Physicians. All study procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The prospective study included 152 patients diagnosed and treated between August 2011 and September 2012, from whom written informed consent was obtained. Their mean age was 56 years (range 26-82). All patients had previously undergone MG and were referred for further diagnostic examinations due to suspected breast cancer. Exclusion criteria were pregnancy or possible pregnancy, history of allergic reaction to iodinated contrast agent, or renal insufficiency.
Imaging Examinations
Conventional Mammography
Conventional mammography (Mammomat 3000, Siemens, Erlangen, Germany; Senographe Essential, GE Healthcare, Buc, France) was performed in all patients, either within the institution or in other imaging centres. Imaging performed in other centres was reviewed in the oncology centre. MG included craniocaudal and mediolateral oblique (MLO) views, as well as other projections (such as lateral, or spot views) when indicated by the standard of care. Images were available in hard or soft copy.
Contrast-Enhanced Spectral Mammography
Contrast-enhanced spectral mammography was performed about 3-4 weeks after MG. All CESM examinations were performed with a digital mammography device developed by GE Healthcare allowing dual-energy CESM acquisitions (SenoBright, OH, USA) (Fig. 1). It consisted of a current FFDM system (Senographe Essential, Buc, France) using a flat panel detector with a cesium iodide absorber, field size 24 × 31 cm, a 2394 × 3062 image matrix with a del pitch of 100 µm, and specific software and hardware for rapid acquisition and processing of dual-energy images.
Fig. 1.
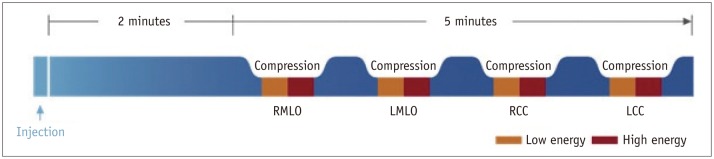
Contrast-enhanced spectral mammography examination scheme (example where left breast is most suspicious) (Courtesy of GE Healthcare).
LCC = left craniocaudal, LMLO = left mediolateral oblique, RCC = right craniocaudal, RMLO = right mediolateral oblique
The choice of anode and filter material was crucial to high image quality. Rhodium anode material was used for all acquisitions, with molybdenum and rhodium filters with kVp ranging from 26 to 32 used for low energy acquisitions similar to those in MG. The paired high-energy images were acquired at 45-49 kVp with rhodium filtration and an additional copper (Cu) filter in the X-ray beam to produce an X-ray spectrum above the K-edge of iodine (33.2 KeV), to increase the visibility of low concentrations of iodine (16).
The radiographer in charge ensured that the patient had no contraindications for iodine contrast injection and explained the steps of the procedure. A nurse prepared for the intravenous injection and placed a catheter into the antecubital vein of the arm contralateral to the affected breast. A one-shot intravenous injection of 1.5 mL/kg body weight of nonionic contrast agent (iopromide, Ultravist 370; Bayer Healthcare, Berlin, Germany) was then performed using a power injector (Optistar™ Elite Injector, Covidien, Cincinnati, OH, USA) at a rate of 3 mL/s with a bolus chaser of 30 mL saline.
After a countdown of 2 minutes, the radiographer positioned the patient as required for MG examinations, starting with a MLO view. The breast without a suspected lesion was imaged first, followed by the breast with a suspected lesion, to secure more contrast uptake for visualization. The device in the CESM mode automatically performed a pair of exposures (low- and high-energy) in each view. A combination of low-energy and high-energy images through specific image processing was performed to generate subtracted images with contrast agent uptake information in each view (18).
Patients were observed for 30 minutes after the examination, to ensure that they had no allergic reaction to the iodinated contrast agent. Compression time for each view was a maximum of 15 seconds. Depending on the patient and technologist, the entire imaging procedure was completed in as little as 4 minutes following the contrast media injection. The total duration of the examination was typically 10 minutes. Processed images were transferred directly to the workstation for review by the radiologist. The total X-ray dose delivered to the patient for a pair of low- and high-energy images ranged between 0.7 and 3.6 mGy, depending on breast thickness (30 to 80 mm) and tissue composition (0 to 100% glandularity) (17). This dose level corresponded to approximately 1.2 times the dose delivered for a standard digital mammogram in the "contrast" automatic exposure control (AEC) mode. The average glandular dose for the low energy image was equivalent to that of one conventional mammogram, while for high energy it was approximately 20% (1/5th) of the dose of a conventional mammogram in the AEC mode. All lesions visible either in MG or in CESM were verified histopathologically, after wire localization or core needle biopsy (Fig. 2).
Fig. 2.
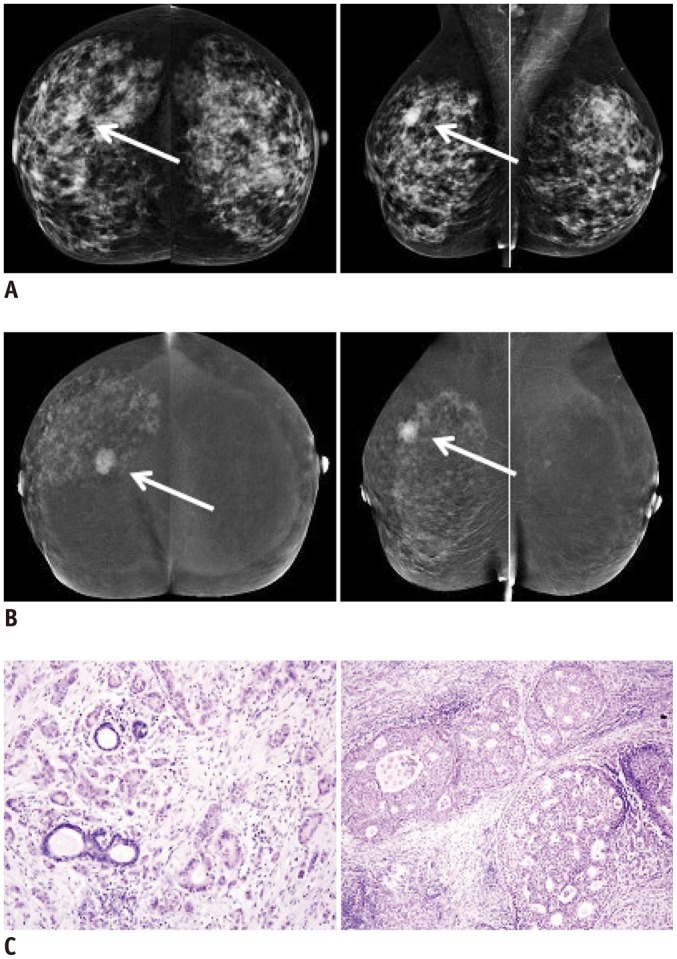
Infiltrating ductal carcinoma (arrows) of right breast in 63-year-old woman with dense fibroglandular breast tissue.
A. Digital conventional mammography (MG) with mediolateral oblique (MLO) view of right breast reveals oval, well demarcated shadow 15 mm in diameter. This mass is not visible in MG craniocaudal (CC) projection. B. Contrast-enhanced spectral mammography in same patient shows poorly demarcated focus of enhancement 15 mm in diameter visible in both MLO and CC projections. Additionally, in right breast, small enhancing foci are visible in upper and lower outer quadrants. C. In histopathological examination, infiltrating carcinoma (of no special type) grade 2 was found within main focus of enhancement. Ductal carcinoma in situ (DCIS) component was noticed in unaltered areas macroscopically. Hematoxylin and eosin staining. Objective magnification 10 × (invasive component, left) and 20 × (DCIS, right).
Image Analysis
A review workstation (GE Healthcare IDI Mammography Diagnostic Workstation, Buc, France) was used for image review. A radiologist with 20 years of breast imaging experience studied the MG and CESM images independently, with a time gap of 7-14 days between reviews of the 2 different modalities. MG images were reviewed using the Breast Imaging Reporting and Data System (BI-RADS) assessment scale: BI-RADS 1, negative; BI-RADS 2, benign finding; BI-RADS 3, probably benign finding; BI-RADS 4, likely malignant; and BI-RADS 5, malignant.
The evaluation forms for MG and CESM included the following data for each enhancing lesion found by the readers to be appropriate:
Localization (quadrant).
Degree of enhancement in the suspicious breast (none, slightly, medium, rapid).
-
Enhancement pattern:
- focal region with no focal findings (linear, ductal, segmental, regional);
- focal findings (form, margin, distribution with choices from BI-RADS-lexicon).
BI-RADS assessment class 1 to 5 (adapted for CESM) (19). All lesions detected in both examinations were verified with core biopsy. BI-RADS classification on MG and CESM was compared to histopathology.
In relation to the above, lesions visible on imaging (MG or CESM) were divided into the following groups:
True positive: BI-RADS ≥ 4 and proven cancer on histopathology;
False positive: BI-RADS ≥ 4 and benign lesion on histopathology;
False negative: BI-RADS ≤ 3 and proven cancer on histopathology;
True negative: BI-RADS ≤ 3 and benign lesion on histopathology.
The size of the lesions on both imaging methods, MG, and CESM, was measured and compared to the histopathological results.
Statistical Analysis
A receiver operating characteristic (ROC) per lesion analysis was performed to compare MG and CESM findings. ROC curves were drawn, and the areas under curves were compared using the Z test. Sensitivity, specificity, accuracy, as well as positive and negative predictive values were evaluated using BI-RADS scores ≥ 4 as positive assessments. The results were compared using McNamara's test corrected for continuity. Lesion size comparison analysis was performed using Student t tests for dependent variables. Alpha significance level was defined as 0.05. Calculations were performed using the STATISTICA 10.0 (StatSoft, Krakow, Poland) software.
RESULTS
The study included 152 patients with 173 breast lesions diagnosed on MG or CESM. Seventeen patients (11%) had 2 diagnosed lesions, and 2 patients (1%) had 3 diagnosed lesions. Histopathology revealed that 114 lesions (66%) were malignant, and 59 (34%) were benign. The detailed distribution of lesions was presented in Table 1.
Table 1.
Detailed Distribution of Malignant and Benign Lesions in Study Group
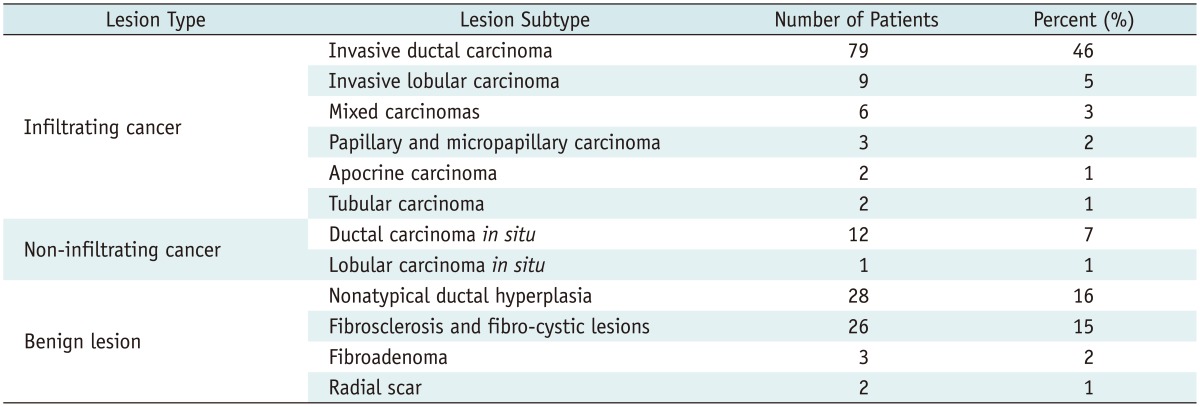
Conventional mammography detected 157 lesions (91% of the total number of lesions) in 150 patients (99% of all examined patients). The visible lesions included 92 cases of infiltrating cancer (59%), 12 (8%) of non-infiltrating cancer, and 53 (34%) with benign lesions. Two patients had no lesions detected on MG.
Contrast-enhanced spectral mammography detected 149 lesions (86% of the total number of lesions) in 128 patients (84% of all examined patients). The diagnosed lesions included 101 cases (68%) of infiltrating cancer, 13 (9%) of non-infiltrating cancer, and 35 (23%) benign cases. All lesions not diagnosed on CESM were benign.
Contrast-enhanced spectral mammography detected 16 lesions (11%) undetected on MG. Ten of the lesions diagnosed on CESM but not on MG were malignant, 9 of which were infiltrating carcinoma. The remaining 6 lesions were benign (proven fibroadenomas on histopathology). The distribution of lesions additionally diagnosed on CESM was presented in Table 2.
Table 2.
Distribution of Additional Lesions per Patient Diagnosed with CESM
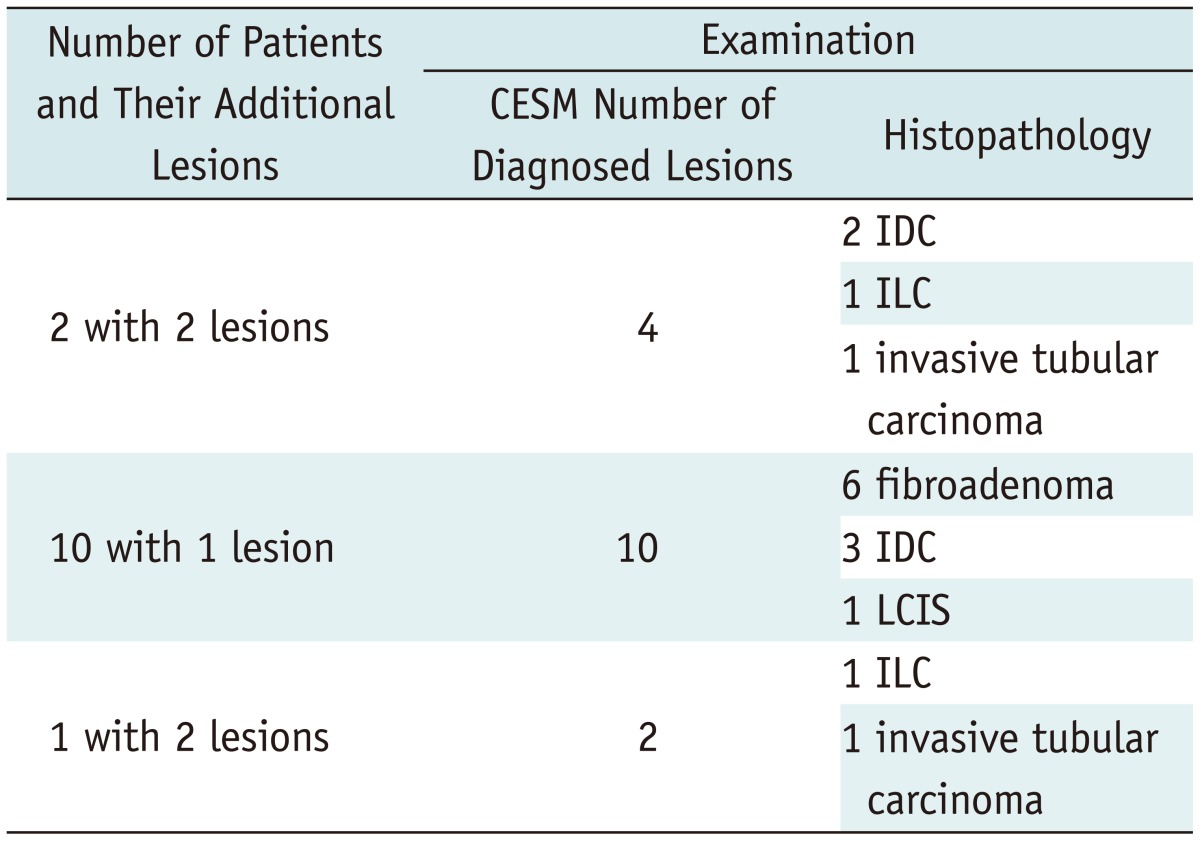
Note.- CESM = contrast-enhanced spectral mammography, IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma, LCIS = lobular carcinoma in situ, MG = conventional mammography
Contrast-enhanced spectral mammography did not detect 24 lesions (24%) that were visible on MG; all were proven benign on histopathology.
Size
Lesion size (mm) as evaluated on MG and CESM examinations were compared to histopathology. Mean lesion size in MG was 20.6 ± 0.9 mm, on CESM 19.5 ± 0.9 mm and on histopathology was 18.3 ± 0.8 mm. Both MG and CESM overestimated lesion sizes compared to histopathology (p < 0.001); the difference was smaller for CESM (Fig. 3).
Fig. 3.
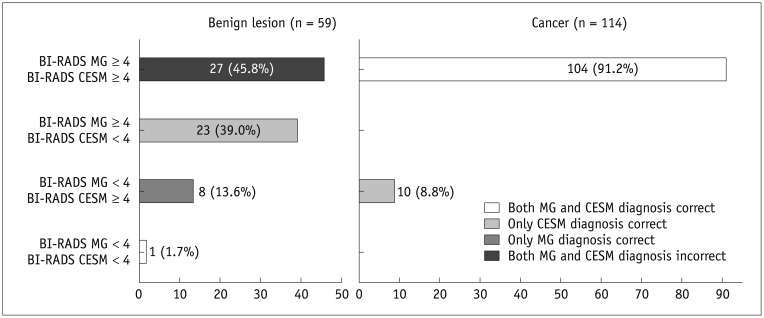
Comparison of correct and incorrect assessments on conventional mammography (MG) and contrast-enhanced spectral mammography (CESM) based on Breast Imaging Reporting and Data System (BI-RADS) scores (BI-RADS scores on MG and CESM were consistent and correct in 91% of proven malignant lesions).
Diagnosis was correct if lesion scored as BI-RADS < 4 was benign or scored ≥ 4 was malignant. Left picture presents agreement between MG and CESM diagnoses with pathological results in 59 benign lesions, right picture-in 114 malignant lesions.
Lesion Classifications on MG and CESM
Seventeen (10%) cases were classified as BI-RADS 1 on MG, 10 (59%) of which were proven cancers on histopathology. Two (1.1%) cases were classified as BI-RADS 2, both of which were proven benign. One-hundred and fourteen (66%) cases were classified as BI-RADS 4, and 65 (57%) were proven malignant. Of the 40 (25%) lesions classified as BI-RADS 5, 39 (98%) were proven malignant.
All lesions classified as BI-RADS Category 1 on CESM were proven benign on histopathology. Of the 41 (24%) lesions classified as BI-RADS 4 on CESM, 17 (41%) were malignant and 24 (59%) were benign. Among the 108 (62%) lesions classified as BI-RADS 5 on CESM, 97 (90%) were proven malignant and 11 (10%) were benign. One patient had 2 invasive malignant foci in the same breast that were detected by CESM, but not MG.
Diagnostic Accuracy
Breast Imaging Reporting and Data System scores on MG and CESM were consistent and accurate in 91% of the proven malignant lesions. The remaining 9% of malignant lesions were diagnosed correctly on CESM, but incorrectly on MG. For proven benign cases, only 2% of BI-RADS scores on MG and CESM were correct and in agreement. The diagnosis for benign findings was incorrect for both modalities in 46% of cases, while MG only was wrong in an additional 39% of cases (which were correctly assessed by CESM). As such, of benign findings were wrongly scored as malignant in 85% of cases on MG vs. 59% on CESM. These results were summarized in Figure 4.
Fig. 4.
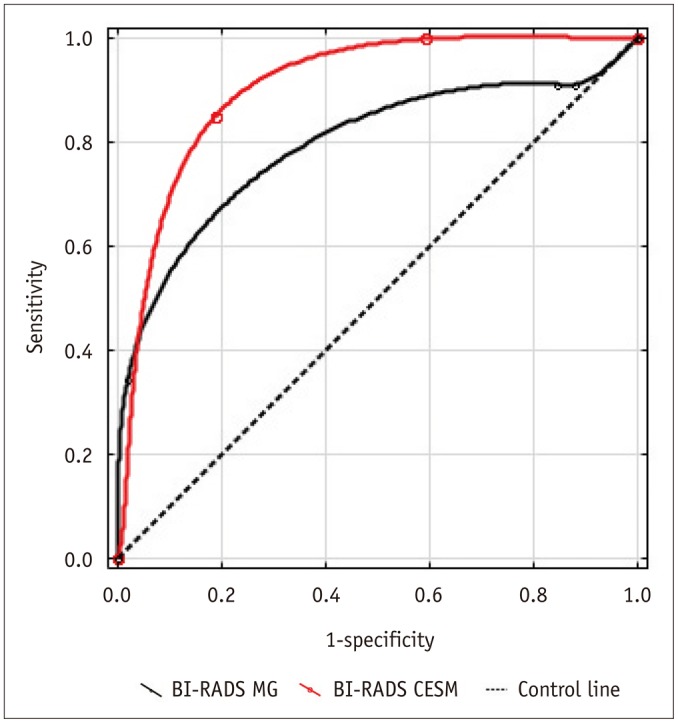
Receiver operating characteristic curves from Breast Imaging Reporting and Data System (BI-RADS) conventional mammography (MG) and contrast-enhanced spectral mammography (CESM) assessments.
There were 20% false positives and 0% false negatives on CESM, compared to 29% false positives and 6% false negatives on MG. The distribution of results based on diagnostic test evaluation and diagnostic test parameters were presented in Table 3.
Table 3.
Distribution of Results and Clinical Performance Based on Diagnostic Test Evaluation (per Lesion Analysis)
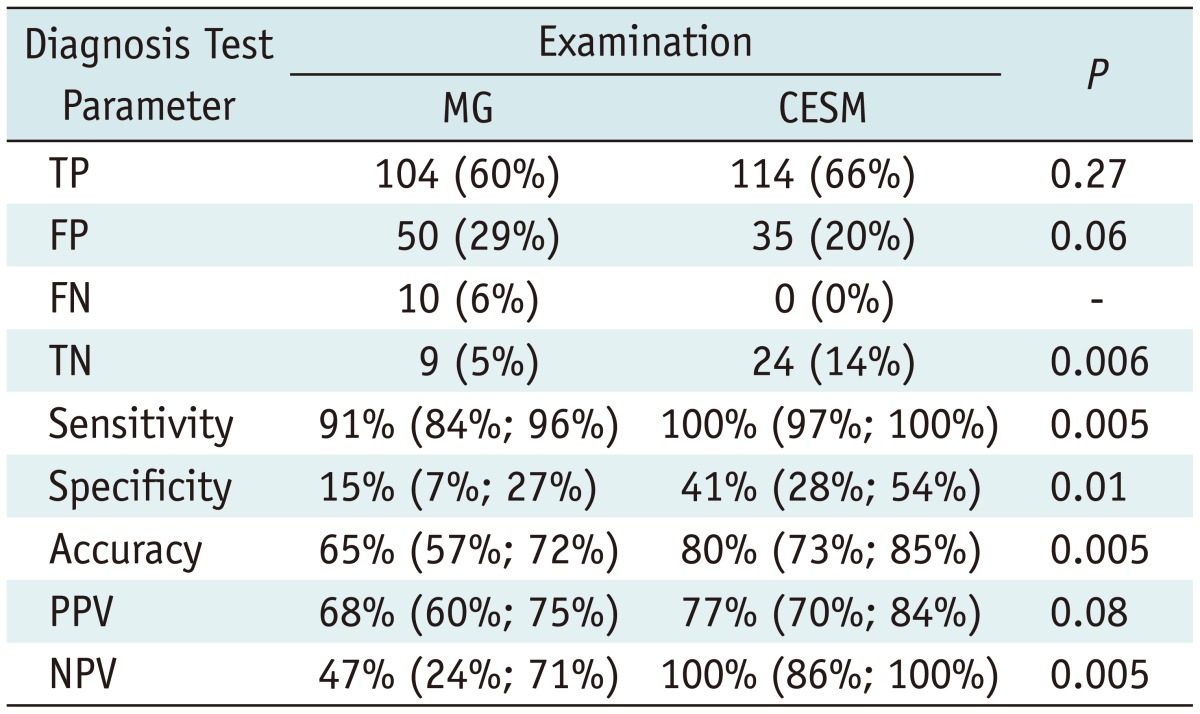
Note.- CESM = contrast-enhanced spectral mammography, FN = false negative, FP = false positive, MG = conventional mammography, NPV = negative predictive value, PPV = positive predictive value, TN = true negative, TP = true positive
The ROC curves based on BI-RADS assessments showed that CESM was superior to MG in clinical performance. The mean area under the ROC curve (AUC) was 0.86 (95% confidence interval [CI]: 0.80, 0.93) for CESM vs. 0.67 (95% CI: 0.59, 0.75) for MG. The AUC difference of 0.19 (95% CI: 0.12, 0.27) was statistically significant (p < 0.005).
Contrast-enhanced spectral mammography had 100% sensitivity, 65% accuracy, and 100% negative predictive value (vs. 47% for MG); all differences with MG were statistically significant (p < 0.05). Positive predictive value was 77% for CESM and 68% for MG, which was not statistically significant.
DISCUSSION
Conventional mammography (20), ultrasonography and physical examination are currently the most widely employed non-invasive screening methods for the detection of breast cancer and are an integral part of routine examinations. However, these techniques have limited sensitivity and specificity for the detection and characterization of breast lesions. The sensitivity of mammography for the index cancer varies from 63% to 98% (21) and was reportedly as low as 30% to 48% in dense breasts which are more frequently associated with increased risk of breast cancer (4, 5, 22).
Contrast-enhanced spectral mammography is a new technique that may improve the clinical performance of mammography. The aim of our study was to compare MG and CESM in a diagnostic setting. CESM detected 16 lesions that were undetected on MG: 9 were invasive carcinomas and 1 was a lobular carcinoma in situ; 6 of these lesions were benign (fibroadenomas). 24 lesions that were identified as suspicious on MG did not enhance on CESM and were all proven benign at histopathology.
Our results showed that CESM was significantly better than MG. CESM sensitivity was 100% (vs. 91% for MG), AUC was 0.86 (vs. 0.67 for MG) and accuracy was 80% (vs. 65% for MG). These results showed that CESM was more effective than MG in the detection and characterization of breast lesions, with 20% false positives and 0% false negatives compared to the 29% false positives and 6% false negatives, respectively, for MG.
There are four previous reports on contrast-enhanced mammography, in which different examination techniques were described. Lewin and Niklason (23) used a temporal subtraction technique with only the MLO projection performed before and after contrast administration. The same technique was described by Dromain et al. (16) and Diekmann et al. (19).
Dromain et al. (17) assessed the diagnostic accuracy of CESM as an adjunct to MG vs. MG alone and vs. MG plus ultrasonography. They reported results for 120 patients with 80 malignant, 50 benign and 3 pre-cancerous lesions (1 case of atypical hyperplasia and 2 cases of lobular carcinoma in situ). Dromain's study confirmed the superior diagnostic accuracy of CESM for the detection of breast carcinoma than mammography alone, and mammography interpreted in conjunction with ultrasonography. They reported that the sensitivity for CESM was 93% compared with 78% for mammography alone (p = 0.001), which was comparable to our results on a larger patient cohort.
Jochelson et al. (14) reported a similar technique to the one used in our study, but on a smaller group of patients. Both breasts were examined in 2 projections after contrast administration using low and high energy acquisitions. Their analysis included 52 patients with 47 invasive ductal carcinomas, 3 infiltrating lobular carcinomas, and 2 cases of ductal carcinoma in situ, 1 with an invasive component. MG detected 42 (81%) of the 52 index cancers and CESM visualized 14 of 25 (56%) additional ipsilateral malignant foci.
The previously reported analyses described above showed that CESM detected suspicious lesions at a higher frequency than MG, which corroborated the results from our current study.
Our data showed that lesion sizes in CESM and MG were comparable to each other, but were overestimated by an average of 3.0 mm on CESM and 3.3 mm on MG, as compared to histopathology; this was in line with the results presented by Jochelson et al., where the sizes of index lesions (both infiltrative and in situ components) were believed to be accurate if the difference from the lesion size at lumpectomy or mastectomy were ≤ 0.5 cm. CESM and MG are performed under breast compression, which may lead to an apparently increased size of the lesion vs. histopathology. It also may also be due to the fact that iodine-based and gadolinium-based contrast agents suitable for CESM, breast CT, and breast MRI lesion enhancement are extravascular agents, leaking through newly-formed vessels into areas adjacent to the lesion itself. Increased lesion conspicuity thus occurs at the cost of overestimating the lesion size. Other publications described similar overestimations of lesion size in CESM compared to histopathology (14, 16, 17).
Previously reported studies, as well as the current data, collectively indicated that CESM may provide fast and accurate breast lesion detection and characterization. CESM may reveal multiple pathological foci within the breast and may help patient management decisions, particularly for surgical procedures.
Clinical studies proved the feasibility of CESM and simultaneously revealed its several major limitations. The breast is compressed for several minutes which may prevent contrast uptake in the suspicious lesions; additionally, during contrast agent injection decreasing patient comfort may cause patient motion and resultant image artefacts. CESM is not able to provide temporal information on contrast uptake and washout; hence it may be more difficult to distinguish some benign lesions from cancer.
Our study had several limitations. Firstly the images were reviewed by only one radiologist with relatively less experience (2 years) in CESM assessment. Secondly, there was a relatively high rate-20%-of false-positive results, and no false-negative findings with CESM, which was greater than Jochelson's findings on a smaller patient cohort (14). Benign lesions, such as fibroadenomas (26 cases), fibrosclerosis (1 case), hamartoma (1 case) or intraductal papillomas (4 cases) showed contrast enhancement. The third limitation was the discrepancy in lesion size between MG, CESM and histopathology. This could be partially explained by breast compression in imaging, but may also be due to different measurement methods in imaging and histopathology.
Conclusions
Our results indicated that CESM may have improved sensitivity and specificity of breast cancer detection.
References
- 1.Jochelson M. Advanced imaging techniques for the detection of breast cancer. Am Soc Clin Oncol Educ Book. 2012:65–69. doi: 10.14694/EdBook_AM.2012.32.223. [DOI] [PubMed] [Google Scholar]
- 2.Burhenne HJ, Burhenne LW, Goldberg F, Hislop TG, Worth AJ, Rebbeck PM, et al. Interval breast cancers in the Screening Mammography Program of British Columbia: analysis and classification. AJR Am J Roentgenol. 1994;162:1067–1071. doi: 10.2214/ajr.162.5.8165983. discussion 1072-1075. [DOI] [PubMed] [Google Scholar]
- 3.Robertson CL. A private breast imaging practice: medical audit of 25,788 screening and 1,077 diagnostic examinations. Radiology. 1993;187:75–79. doi: 10.1148/radiology.187.1.8451440. [DOI] [PubMed] [Google Scholar]
- 4.Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 5.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 6.Pisano ED, Hendrick RE, Yaffe MJ, Baum JK, Acharyya S, Cormack JB, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008;246:376–383. doi: 10.1148/radiol.2461070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 8.Fischer U, Baum F, Obenauer S, Luftner-Nagel S, von Heyden D, Vosshenrich R, et al. Comparative study in patients with microcalcifications: full-field digital mammography vs screen-film mammography. Eur Radiol. 2002;12:2679–2683. doi: 10.1007/s00330-002-1354-x. [DOI] [PubMed] [Google Scholar]
- 9.Smith A. Fundamentals of digital mammography: physics, technology and practical considerations. Radiol Manage. 2003;25:18–24. 26–31. quiz 32-34. [PubMed] [Google Scholar]
- 10.Knopp MV, Giesel FL, Marcos H, von Tengg-Kobligk H, Choyke P. Dynamic contrast-enhanced magnetic resonance imaging in oncology. Top Magn Reson Imaging. 2001;12:301–308. doi: 10.1097/00002142-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Padhani AR, Dzik-Jurasz A. Perfusion MR imaging of extracranial tumor angiogenesis. Top Magn Reson Imaging. 2004;15:41–57. doi: 10.1097/00002142-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Jeswani T, Padhani AR. Imaging tumour angiogenesis. Cancer Imaging. 2005;5:131–138. doi: 10.1102/1470-7330.2005.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer AO, Langer M. [MRI mammography screening in women with lobular carcinoma in situ] Strahlenther Onkol. 2012;188:716–717. doi: 10.1007/s00066-012-0138-8. [DOI] [PubMed] [Google Scholar]
- 14.Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. 2013;266:743–751. doi: 10.1148/radiol.12121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg WA. Rationale for a trial of screening breast ultrasound: American College of Radiology Imaging Network (ACRIN) 6666. AJR Am J Roentgenol. 2003;180:1225–1228. doi: 10.2214/ajr.180.5.1801225. [DOI] [PubMed] [Google Scholar]
- 16.Dromain C, Balleyguier C, Muller S, Mathieu MC, Rochard F, Opolon P, et al. Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. AJR Am J Roentgenol. 2006;187:W528–W537. doi: 10.2214/AJR.05.1944. [DOI] [PubMed] [Google Scholar]
- 17.Dromain C, Thibault F, Diekmann F, Fallenberg EM, Jong RA, Koomen M, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Res. 2012;14:R94. doi: 10.1186/bcr3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puong S, Bouchevreau X, Patoureaux F, Iordache R, Muller S. Dual-energy contrast enhanced digital mammography using a new approach for breast tissue canceling. Proc SPIE. 2007;6510:65102H. [Google Scholar]
- 19.Diekmann F, Freyer M, Diekmann S, Fallenberg EM, Fischer T, Bick U, et al. Evaluation of contrast-enhanced digital mammography. Eur J Radiol. 2011;78:112–121. doi: 10.1016/j.ejrad.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Boetes C, Mus RD, Holland R, Barentsz JO, Strijk SP, Wobbes T, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 1995;197:743–747. doi: 10.1148/radiology.197.3.7480749. [DOI] [PubMed] [Google Scholar]
- 21.Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220:13–30. doi: 10.1148/radiology.220.1.r01jl3113. [DOI] [PubMed] [Google Scholar]
- 22.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA. 1996;276:33–38. [PubMed] [Google Scholar]
- 23.Lewin JM, Niklason L. Advanced applications of digital mammography: tomosynthesis and contrast-enhanced digital mammography. Semin Roentgenol. 2007;42:243–252. doi: 10.1053/j.ro.2007.06.006. [DOI] [PubMed] [Google Scholar]