Abstract
In addition to imaging the lymphatics and detecting various types of lymphatic leakage, lymphangiography is a therapeutic option for patients with chylothorax, chylous ascites, and lymphatic fistula. Percutaneous thoracic duct embolization, transabdominal catheterization of the cisterna chyli or thoracic duct, and subsequent embolization of the thoracic duct is an alternative to surgical ligation of the thoracic duct. In this pictorial review, we present the detailed technique, clinical applications, and complications of lymphangiography and thoracic duct embolization.
Keywords: Lymphangiography, Chylothorax, Chylous ascites, Lymphatic fistula
INTRODUCTION
Lymphatic leakage is a rare but severe complication that can occur after various surgical procedures, such as pancreaticoduodenectomy, abdominothoracic esophagectomy, inguinal lymph node resection, and nephrouretectomy (1). It can also occur from non-surgical pathologies including lymphangioleiomyomatosis and post-radiation treatment. Lymphatic leaks can occur anywhere along the pathway of lymph that begins in all four extremities. The clinically more important pathway starts from the intestinal lymphatic ducts and continues through the cisterna chyli and into the thoracic duct (Fig. 1). Lymphatic leakage can induce local complications, such as infections or delayed wound healing, or cause severe malnutrition in patients with chylothorax or chylous ascites (1).
Fig. 1.
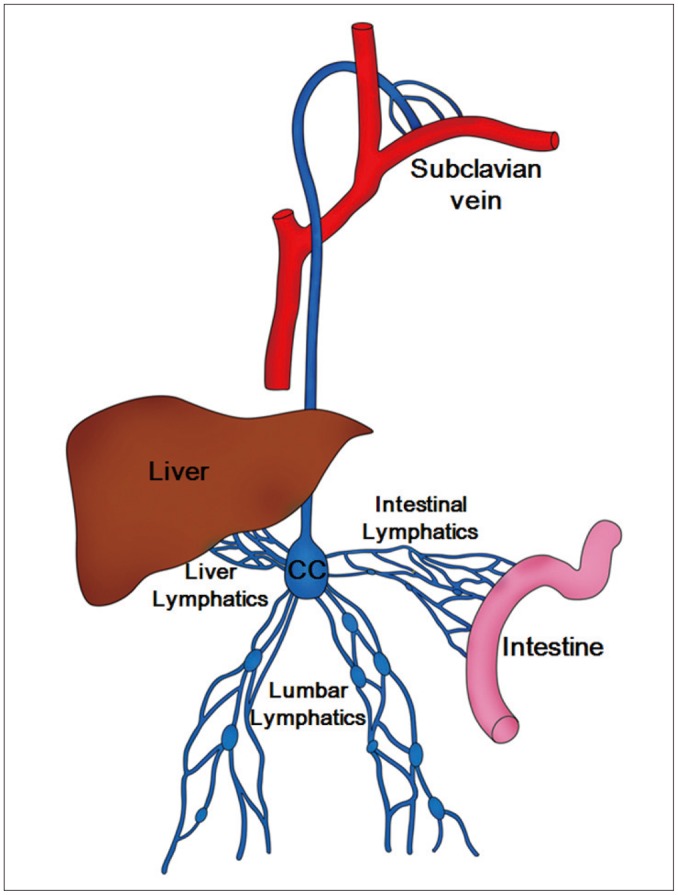
Schematic drawing of chyle pathway. Lumbar lymphatics drain not only lumbar region but also lower extremities. Lumbar, intestinal, and liver lymphatics join and form cisterna chyli (CC) which finally empties into left subclavian vein.
Lymphangiography (LG) is a valuable tool for the detection of various types of lymphatic leakage including chylothorax, chylous ascites, and lymphatic fistulae (2, 3, 4, 5, 6, 7, 8, 9). Previous studies have described a leakage detection rate of 64-78% (3, 4, 7).
Besides the diagnostic role of identifying the lymphatic leaks, recently published reports indicate LG plays a therapeutic role for lymphatic leakage (1, 3, 4, 5, 6, 7). The mechanism by which LG reduces lymphatic leaks has not yet been fully clarified, although some authors have suggested that Lipiodol, an ethiodized oil contrast agent, undergoes an inflammatory and granulomatous reaction during its extravasation (3, 10). The therapeutic effect is also attributed to the embolic properties of Lipiodol, as Lipiodol infused during LG accumulates at the point of leakage outside as well as within the lymphatics (3).
For the treatment of chylothorax, percutaneous thoracic duct embolization (TDE) is an established alternative to surgical ligation of the thoracic duct (11, 12, 13). TDE is a minimally invasive procedure in which opacification of the lymphatic system by LG is followed by transabdominal catheterization of the cisterna chyli or thoracic duct and subsequent occlusion of the thoracic duct with embolization to prevent further leak.
The purpose of this pictorial essay is to acquaint radiologists and interventional radiologists to the details of technique and clinical applications of LG and TDE in the diagnosis and treatment of postoperative lymphatic leakage.
Technique
There are two methods of LG: pedal LG and intranodal LG. Conventional pedal LG, which involves the isolation and cannulation of pedal lymphatic vessels and injection of Lipiodol, is time-consuming and requires an incision to expose the pedal lymphatics (3, 4, 5, 6, 7).
Intranodal LG was developed to evaluate the tumoral involvement of inguinal, pelvic, and lumbar lymph nodes (14). The technique relies on ultrasound-guidance to access inguinal lymph nodes for direct lymph node access and injection of Lipiodol. Accessing the inguinal lymph nodes requires no incisions and bypasses the lower extremities, thus decreasing the procedure time for LG and TDE, radiation dose, and volume of the contrast medium (8, 9, 15). This technique seems appealing in children in view of the small size of the lymphatic channels and commonly enlarged inguinal lymph nodes (9).
Pedal LG
In patients with abdominal or thoracic lymphatic leakage, either the right or left foot is used for pedal LG. However, in patients with inguinal or pelvic lymphatic leakage, the ipsilateral foot to the site of the leakage is used (1). Five to 10 mL of indigo carmine (Korea United Pharm., Seoul, Korea) is injected into the cutaneous and subcutaneous web spaces of the first to third toe spaces after administering lidocaine as a local anesthetic. After waiting 20-30 minutes, the course of the lymphatic vessels is identified at the dorsum of the foot. A longitudinal or transverse cutaneous incision at the base of the first metatarsal bone is made to expose a lymphatic vessel with blue staining after dissection of the surrounding tissue. The isolated lymphatic vessel is then cannulated using a 30-G LG needle (Cook, Bloomington, IN, USA) (Fig. 2). Often, a surgical loop or magnifying loop is utilized to better visualize the lymphatics. The needle and the lymphatic vessel are firmly tied together using 3-0 silk thread and then secured with adhesive strips (Fig. 2).
Fig. 2.
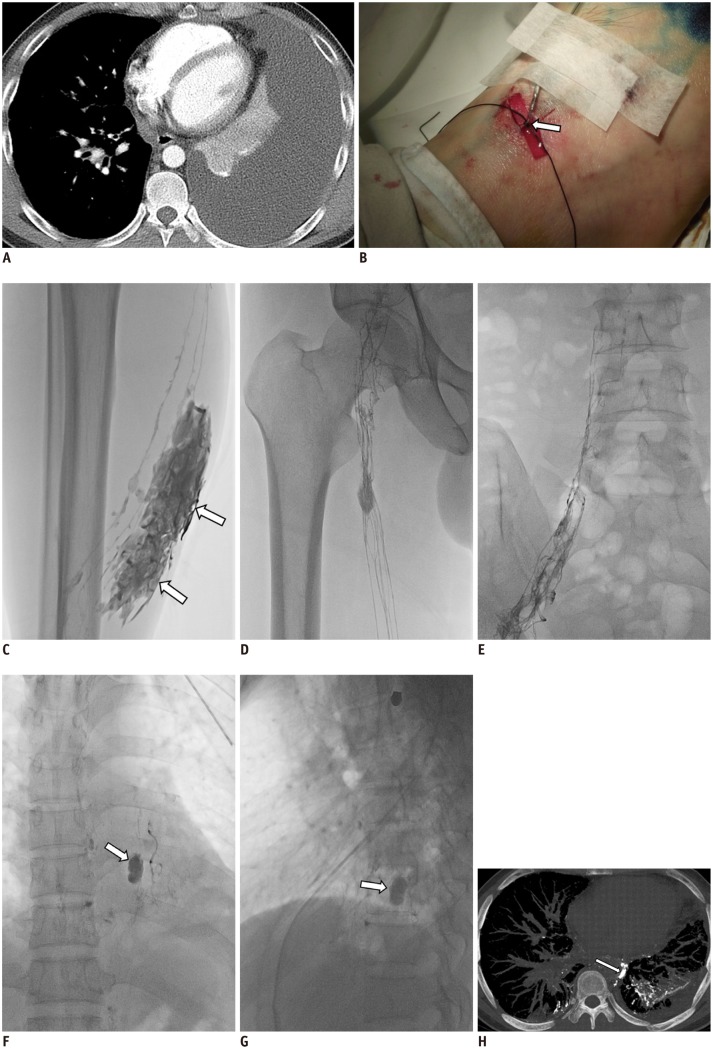
35-year-old man with left chylothorax after lung biopsy using video-assisted thoracoscopy.
A. Axial CT scan obtained one week prior to LG shows large amount of left pleural effusion (chylothorax). B. Isolated lymphatic of dorsum of right foot was cannulated using 30-G LG needle and both needle and lymphatic were firmly tied (arrow). C. Radiographic image obtained 5 minutes following Lipiodol injection shows Lipiodol extravasation (arrows) at calf level. D, E. Radiographic images obtained 15 minutes following Lipiodol injection show good opacification of inguinal and pelvic lymph nodes as well as ascending lymphatics. LG = lymphangiography
F, G. Radiographic anteroposterior and lateral images obtained one hour following Lipiodol injection show pseudoaneurysm-like leakage (arrows) of Lipiodol at 9th thoracic spine level. H. CT reconstructed image obtained 5 hours following LG shows leakage site (arrow) adjacent to descending thoracic aorta and prominent Lipiodol leakage to left lung. Left chest tube draining 700 mL per day was eliminated three days after LG. LG = lymphangiography
After accessing the lymphatic vessel, 6-12 mL of Lipiodol (Guerbet, Paris, France) are injected at the rate of 0.2-0.4 mL/minute using the dedicated Cordis lymphangiogram pump (Johnson and Johnson, Miami Lakes, FL, USA) or, more commonly, an advanced anesthesia injection pump. Serial fluoroscopic spot images are obtained upwardly every 5-10 minutes over the course of the injected Lipiodol. If Lipiodol does not reach the area of interest, normal saline at the same rate can be injected to push Lipiodol further into the area of interest. The needles are then removed and the wounds are sutured after the injection is completed.
Pedal LG is considered to be technically successful if the lymphatic vessel is successfully selected and the lymphatic channels of interest including cisterna chyli are adequately visualized using Lipiodol.
Intranodal LG
The largest and most distal (away from the inguinal area) inguinal lymph node is directly accessed under ultrasound guidance using a 25-gauge spinal needle (Tae-Chang Industrial, Gongju, Korea) (Fig. 3). To minimize needle movement, the needle is assembled prior to nodal access. The stylet is removed and the needle is attached to a connecting tube (Mini-Volume Line; Insung Medical, Seoul, Korea) and is flushed with Lipiodol (Fig. 3). The needle tip is positioned in the transitional zone between the cortex and the hilum of the lymph node (Fig. 3).
Fig. 3.
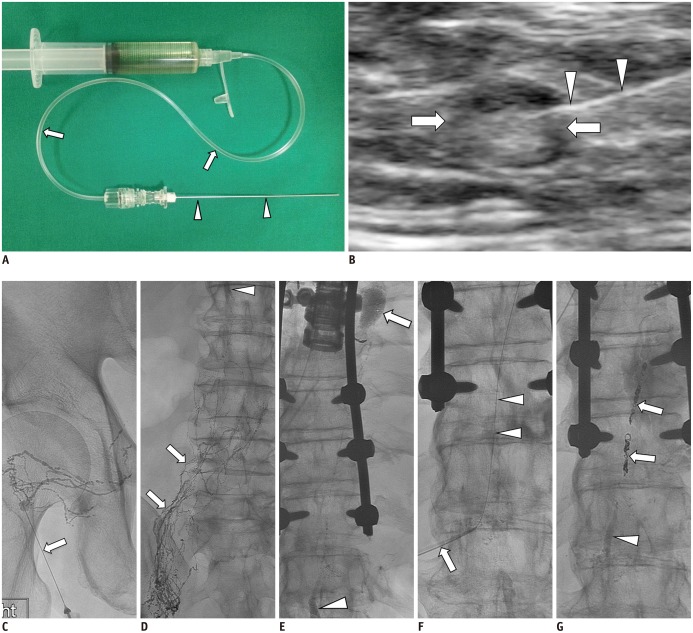
65-year-old man with history of esophageal cancer; status post total esophagectomy who presented with average of 2-3 L of left chylous effusion.
A. 25-G spinal needle (arrowheads) is assembled to connecting tube (arrows) and is flushed with Lipiodol contained in syringe tube. B. Ultrasound-guided inguinal lymph node (arrows) access for initiation of intranodal LG using 25-G spinal needle (arrowheads). C. Initial spot image of right pelvis/inguinal region demonstrating needle (arrow) access of small lymph node and opacification of proximal lymphatic vessels and lymph node. D. Follow-up spot image of lower abdomen demonstrating upward move/flow of lymphatics (arrows) and appearance of cisterna chyli (arrowhead). E. Subsequent follow-up spot image of thoracolumbar region demonstrating continued upward flow of lymphatics with filling of cisterna chyli (arrowhead) and evidence of lymphatic leak showing in upper thoracic region with pooling of Lipiodol contrast (arrow). F. Cisterna chyli is percutaneously accessed using 22-G Chiba needle (arrow) and once it is accessed, microwire (arrowheads) is used to secure access by advancing it to mid thoracic duct. G. In this patient, microwire and microcatheter were unable to advance beyond leak along thoracic duct. Decision was made to coil embolize thoracic duct proximal to leak using several detachable coils (arrows) and it was further embolized using NBCA (arrowhead) down to cisterna chyli. On post-TDE day 1, chylous effusion completely stopped and chest tube drainage decreased to less than 50 mL from 3 L. LG = lymphangiography, NBCA = N-butyl-2-cyanoacrylate, TDE = thoracic duct embolization
Lipiodol injection is observed under fluoroscopic guidance to identify the efferent lymphatic or lymph node so as to confirm the proper positioning of the needle. A total volume of 10-20 mL of Lipiodol is injected using the dedicated lymphangiogram pump or, more commonly, an advanced anesthesia injection pump is used at the injection rate of 0.2-0.4 mL/minute. Upward serial fluoroscopic spot images are obtained every 5-10 minutes over the course of Lipiodol injection.
Intranodal LG is considered to be technically successful if the target lymph node is successfully selected and the lymphatic channels of interest including cisterna chyli are adequately visualized using Lipiodol.
Follow-Up CT Scans after LG
For a diagnostic purposes, follow-up non-contrast computed tomography (CT) scans are obtained to delineate the lymphatic vessels, assess the site of leakage, and potentially identify an appropriate puncture site for sclerotherapy.
Those follow-up CT scans are obtained immediately after injecting the full volume of Lipiodol in patients with inguinal or pelvic leakage, or 4-5 hours after the injection in patients with abdominal or thoracic leakage (Fig. 2) (1, 4).
Thoracic Duct Embolization
Once the retroperitoneal lymphatics along the upper lumbar region and cisterna chyli are opacified with Lipiodol, percutaneous cannulation of cisterna chyli is performed using 22-G Chiba needle (15 or 20 cm) (Fig. 3). Under the fluoroscopic guidance, the needle is entered at slightly right paramedian location in the supra-umbilicus region. Transabdominally, the needle traverses the abdomen to access the cisterna chyli. The needle should be slightly angled cranially to provide a less acute angle entering the cisterna chyli with a wire. During this process, a lateral projection angiography is useful to more accurately identify and access the cisterna chyli.
Once the cisterna chyli or large lymphatic duct is accessed with a needle, a 0.018 or 0.014 inch microwire is used to thread the thoracic duct. Once the microwire is in a secure location in the thoracic duct, a microcatheter (Renegade STC 18; Boston Scientific, Cork, Ireland) is advanced over the microwire in the thoracic duct. A small amount of contrast is injected to confirm the proper entry to the thoracic duct and also to verify the extravasation and leak. Once the leak is identified, the microwire and microcatheter are advanced past the leak and microcoils are used to embolize the thoracic duct. Usually, 2-4 mm long detachable fibered coils, either the Interlock coil (Boston Scientific, Cork, Ireland) or Concerto coil (Covidien, Plymouth, MN, USA), are used to shorten the procedure time. A shorter pushable coil (Tornado or MicroNester coils; Cook, Bloomington, IN, USA) can also be used. The proximal and distal thoracic duct around the leak is embolized with coils and the proximal thoracic duct is usually further embolized with N-butyl-2-cyanoacrylate or Onyx glue (Covidien, Plymouth, MN, USA) to completely occlude the thoracic duct.
Due to the size of the thoracic duct or cisterna chyli or the anatomic variants of thoracic duct, thoracic duct cannulation may not be possible. In those cases, instead of TDE, thoracic duct obliteration or cisterna chyli obliteration can be performed (10). Instead of accessing the cisterna chyli with a microwire and microcatheter, the proximal thoracic duct or cisterna chyli within the abdomen can be perforated with needle. This prevents chylous lymphatics from travelling up to the distal thoracic duct which may have leaks. Lymphatics leaking at the site of obliteration may cause chylous ascites that could be re-absorbed by the abdominal visceral organs.
Clinical Applications
Chylothorax and Chylous Ascites
Chylothorax is a rare but devastating complication associated with mediastinal lymph node or lymphatic injury within highly variable course of the thoracic duct (16). Similarly, any source of lymphatic obstruction or leakage can cause lymphatic accumulations in the peritoneal or retroperitoneal cavities, resulting in chylous ascites. Conservative management is recommended as the best initial treatment and includes drainage tube insertion, medium chain triglyceride diet and sometimes total parenteral nutrition. Chylothorax and chylous ascites with persistent high output may be treated with intravenous somatostatin analogues such as octreotide or pleurodesis (7).
If conservative management fails, surgical management, such as pleura- or peritoneovenous shunt implantation, lymphatic-venous anastomoses, and ligation or clipping of the lymphatics, is undertaken (4). General indications for surgery for postoperative chylothorax and chylous ascites include chylous leak greater than 1000 mL/day for more than 5 days, persistent leak for more than 2 weeks despite conservative management, or development of insurmountable nutritional or metabolic complications (4).
Lymphangiography has been used mainly to identify the site of the lymphatic leakage in patients with chylothorax and chylous ascites (Fig. 2). Based on three studies, the leakage sites were successfully identified between 64% to 86% of patients with chylothorax and chylous ascites and LG achieved successful therapeutic outcome in 56% to 86% of cases (3, 4, 7).
Percutaneous catheterization and embolization of the thoracic duct has been employed in patients with postoperative chylothoraces (Fig. 3). The procedure is technically challenging and catheterization of the thoracic duct is only successful in approximately 67% (12). When the thoracic duct is successfully catheterized and embolized, the success rate for resolution of chylothorax is 90% (12). Experience with this technique is limited and trials that compare this technique to thoracic duct ligation have not been performed.
Lymphatic Fistula
Lymphatic fistula occurs most commonly after lymphadenectomy (3, 7). Operations of aortic aneurysm or bypass can be the causes of lymphatic fistula (3, 7). Lymphatic fistula is connected with the surrounding organs including the urinary tract, gastrointestinal tract, or uterine cervix/vagina, or it can be opened to surrounding free tissue, causing lymphatic accumulation (Fig. 4) (7, 9, 17).
Fig. 4.
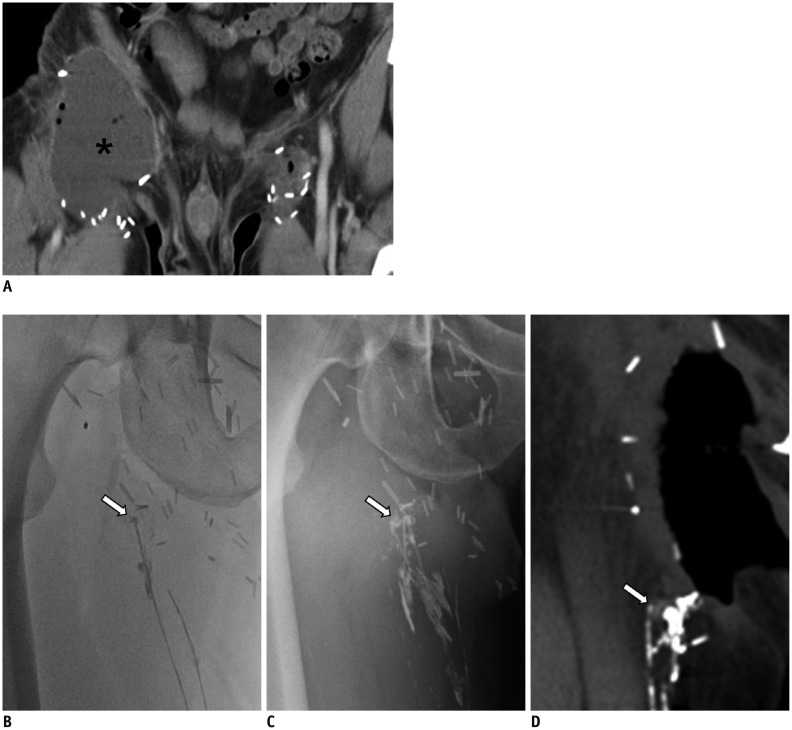
45-year-old man with right inguinal area swelling after deep inguinal lymph node dissection.
A. Axial CT scan shows lymphocele (asterisk), which was subsequently dissected, in right inguinal area. Note minimal fluid collection at left superficial inguinal lymph node dissection site. B. Radiographic image obtained 30 minutes following Lipiodol injected into lymphatics of dorsum of foot, shows disruption of lymphatic with Lipiodol leakage (arrow) at entrance of open wound in right inguinal area. C. Radiographic image obtained 14 hours following Lipiodol injection, still shows leakage point (arrow). D. CT scan obtained 20 hours following Lipiodol injection shows disrupted lymphatic (arrow). Leakage was eliminated five days following LG. LG = lymphangiography
When a lymphatic fistula is located superficially, external compression may be applied (7). When conservative treatment or external compression fails, LG can offer diagnosis as well as therapy (Fig. 4). In lymphatic fistula, LG enables successful visualization of the leakage and therapeutic outcome in 75% of cases, which is comparable to the success rates in chylothorax and chylous ascites (7).
Lymphocele
Most postoperative lymphoceles are small and resolve without treatment. However, in 4-7% of patients with postoperative lymphoceles, lymphoceles persist resulting in pain, infection, or compression of vital structures, which require an intervention (18, 19). Lymphoceles seem to be difficult to treat with LG despite direct visualization of Lipiodol leakage during LG (Fig. 5) (7).
Fig. 5.
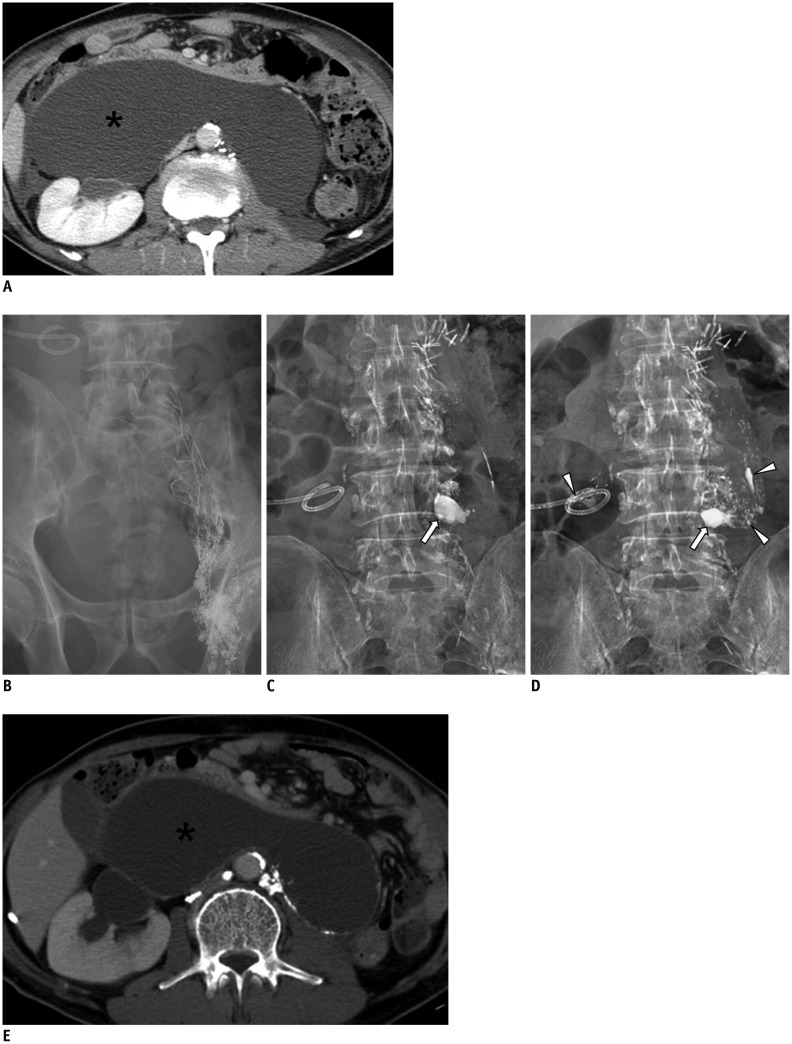
59-year-old man with history of nephroureterectomy and pelvic lymph node dissection.
A. Axial CT scan shows large retroperitoneal lymphocele (asterisk) displacing both bowel and right kidney. B. Radiographic image obtained 20 minutes following Lipiodol injection into left inguinal lymph node shows good opacification of lymphatics in left pelvic cavity. C, D. Radiographic images obtained four and 16 hours following Lipiodol injection shows Lipiodol leakage (arrows) at 4th lumbar spine level and scattered Lipiodols (arrowheads) within lymphocele cavity as well as drainage tube.
E. Axial CT scan obtained 20 days following initial LG shows decreased size of lymphocele (asterisk) and with decrease in amount of drainage. LG = lymphangiography
In patients with lymphocele, the therapeutic success of LG seems discouraging. Kos et al. (7) reported that there was no therapeutic success with pedal LG in three patients with lymphocele. As a lymphocele is a lymph-filled space without a distinct epithelial lining, Lipiodol seems to be passed into the open space without inducing inflammatory or granulomatous reaction at the leakage site. In cases of superficial lymphocele, external compression may be applied (7). Local sclerotherapy has shown a high success rate of 77-94% in patients with postoperative lymphocele (18, 20, 21) and so is preferable to LG in patients with lymphocele.
Complications
Since pedal and intranodal LG procedures are invasive procedures including incision or puncture, there are inherent risks such as infection, pain, and Lipiodol extravasation during injection (Fig. 2) (9, 12).
Serious complications have also been reported including intra-alveolar hemorrhage, Lipiodol emboli in the pulmonary vasculature, allergic reactions to Lipiodol, and extravasation of Lipiodol into the soft tissue (2, 22, 23, 24). Contraindications for LG include pulmonary insufficiency or right-to-left cardiac shunt; the former increases the potential risk of exacerbation by pulmonary embolism through Lipiodol, and the latter creates a risk of cerebral embolism (25).
As complications are frequently related to the volume of Lipiodol injected, the overall complications associated with LG are low when the volume injected is less than 10 mL for the extremity (2).
As for TDE, due to its current approach of percutaneous transabdominal access of cisterna chyli/thoracic duct, inevitable punctures of different organs including bowels, liver, pancreas, inferior vena cava, and aorta may occur. However, only a 22-G Chiba needle is being used and no clinically significant complications have been experienced by the authors or been reported (12, 13). Two types of major complications can occur Glue pulmonary embolism results when excess glue escapes through the thoracic duct into the subclavian vein and causes symptomatic pulmonary embolism. Peripheral lower extremity edema can result due to disruption of peripheral lymphatics with pedal LG; this is no longer an issue with nodal LG.
Summary
Lymphangiography in conjunction with thoracic duct embolization is a relatively safe, powerful, and reliable interventional method. It enables identification of lymphatic leakage and provides complete occlusion, which results in complete resolution of one of the most devastating complications of thoraco-abdominal surgery.
Due to its minimally invasive nature and its proven effectiveness, the need for reoperation and exposure to perioperative morbidity may be avoided.
References
- 1.Kortes N, Radeleff B, Sommer CM, Bellemann N, Ott K, Richter GM, et al. Therapeutic lymphangiography and CT-guided sclerotherapy for the treatment of refractory lymphatic leakage. J Vasc Interv Radiol. 2014;25:127–132. doi: 10.1016/j.jvir.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Guermazi A, Brice P, Hennequin C, Sarfati E. Lymphography: an old technique retains its usefulness. Radiographics. 2003;23:1541–1558. doi: 10.1148/rg.236035704. discussion 1559-1560. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto T, Yamagami T, Kato T, Hirota T, Yoshimatsu R, Masunami T, et al. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. 2009;82:286–290. doi: 10.1259/bjr/64849421. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki R, Sugimoto K, Fujii M, Miyamoto N, Okada T, Yamaguchi M, et al. Therapeutic effectiveness of diagnostic lymphangiography for refractory postoperative chylothorax and chylous ascites: correlation with radiologic findings and preceding medical treatment. AJR Am J Roentgenol. 2013;201:659–666. doi: 10.2214/AJR.12.10008. [DOI] [PubMed] [Google Scholar]
- 5.Ngan H, Fok M, Wong J. The role of lymphography in chylothorax following thoracic surgery. Br J Radiol. 1988;61:1032–1036. doi: 10.1259/0007-1285-61-731-1032. [DOI] [PubMed] [Google Scholar]
- 6.Sachs PB, Zelch MG, Rice TW, Geisinger MA, Risius B, Lammert GK. Diagnosis and localization of laceration of the thoracic duct: usefulness of lymphangiography and CT. AJR Am J Roentgenol. 1991;157:703–705. doi: 10.2214/ajr.157.4.1892021. [DOI] [PubMed] [Google Scholar]
- 7.Kos S, Haueisen H, Lachmund U, Roeren T. Lymphangiography: forgotten tool or rising star in the diagnosis and therapy of postoperative lymphatic vessel leakage. Cardiovasc Intervent Radiol. 2007;30:968–973. doi: 10.1007/s00270-007-9026-5. [DOI] [PubMed] [Google Scholar]
- 8.Nadolski GJ, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23:613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 9.Rajebi MR, Chaudry G, Padua HM, Dillon B, Yilmaz S, Arnold RW, et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol. 2011;22:1300–1305. doi: 10.1016/j.jvir.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Binkert CA, Yucel EK, Davison BD, Sugarbaker DJ, Baum RA. Percutaneous treatment of high-output chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol. 2005;16:1257–1262. doi: 10.1097/01.rvi.0000167869.36093.43. [DOI] [PubMed] [Google Scholar]
- 11.Cope C, Salem R, Kaiser LR. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10:1248–1254. doi: 10.1016/s1051-0443(99)70227-7. [DOI] [PubMed] [Google Scholar]
- 12.Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139:584–589. doi: 10.1016/j.jtcvs.2009.11.025. discussion 589-590. [DOI] [PubMed] [Google Scholar]
- 13.Nadolski GJ, Itkin M. Thoracic duct embolization for nontraumatic chylous effusion: experience in 34 patients. Chest. 2013;143:158–163. doi: 10.1378/chest.12-0526. [DOI] [PubMed] [Google Scholar]
- 14.Zheutlin N, Shanbrom E. Contrast visualization of lymph nodes. Radiology. 1958;71:702–708. doi: 10.1148/71.5.702. [DOI] [PubMed] [Google Scholar]
- 15.Parvinian A, Mohan GC, Gaba RC, Saldanha DF, Knuttinen MG, Bui JT, et al. Ultrasound-guided intranodal lymphangiography followed by thoracic duct embolization for treatment of postoperative bilateral chylothorax. Head Neck. 2014;36:E21–E24. doi: 10.1002/hed.23425. [DOI] [PubMed] [Google Scholar]
- 16.Cho HJ, Kim DK, Lee GD, Sim HJ, Choi SH, Kim HR, et al. Chylothorax complicating pulmonary resection for lung cancer: effective management and pleurodesis. Ann Thorac Surg. 2014;97:408–413. doi: 10.1016/j.athoracsur.2013.10.065. [DOI] [PubMed] [Google Scholar]
- 17.Deso S, Kabutey NK, Vilvendhan R, Kim D, Guermazi A. Lymphangiography in the diagnosis, localization, and treatment of a lymphaticopelvic fistula causing chyluria: a case report. Vasc Endovascular Surg. 2010;44:710–713. doi: 10.1177/1538574410377123. [DOI] [PubMed] [Google Scholar]
- 18.Mahrer A, Ramchandani P, Trerotola SO, Shlansky-Goldberg RD, Itkin M. Sclerotherapy in the management of postoperative lymphocele. J Vasc Interv Radiol. 2010;21:1050–1053. doi: 10.1016/j.jvir.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Sawhney R, D'Agostino HB, Zinck S, Rose SC, Kinney TB, Oglevie SB, et al. Treatment of postoperative lymphoceles with percutaneous drainage and alcohol sclerotherapy. J Vasc Interv Radiol. 1996;7:241–245. doi: 10.1016/s1051-0443(96)70769-8. [DOI] [PubMed] [Google Scholar]
- 20.Akhan O, Karcaaltincaba M, Ozmen MN, Akinci D, Karcaaltincaba D, Ayhan A. Percutaneous transcatheter ethanol sclerotherapy and catheter drainage of postoperative pelvic lymphoceles. Cardiovasc Intervent Radiol. 2007;30:237–240. doi: 10.1007/s00270-006-0180-y. [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman DA, Yeager TD. Percutaneous ethanol sclerotherapy of postoperative lymphoceles. AJR Am J Roentgenol. 1997;169:433–437. doi: 10.2214/ajr.169.2.9242748. [DOI] [PubMed] [Google Scholar]
- 22.Deso S, Ludwig B, Kabutey NK, Kim D, Guermazi A. Lymphangiography in the diagnosis and localization of various chyle leaks. Cardiovasc Intervent Radiol. 2012;35:117–126. doi: 10.1007/s00270-010-0066-x. [DOI] [PubMed] [Google Scholar]
- 23.Dupont H, Timsit JF, Souweine B, Gachot B, Bedos JP, Wolff M. Intra-alveolar hemorrhage following bipedal lymphography. Intensive Care Med. 1996;22:614–615. doi: 10.1007/BF01708114. [DOI] [PubMed] [Google Scholar]
- 24.Bron KM, Baum S, Abrams HL. Oil embolism in lymphangiography. Incidence, manifestations, and mechanism. Radiology. 1963;80:194–120. doi: 10.1148/80.2.194. [DOI] [PubMed] [Google Scholar]
- 25.Kusumoto S, Imamura A, Watanabe K. Case report: the incidental lipid embolization to the brain and kidney after lymphography in a patient with malignant lymphoma: CT findings. Clin Radiol. 1991;44:279–280. doi: 10.1016/s0009-9260(05)80199-0. [DOI] [PubMed] [Google Scholar]