Abstract
Objective
To compare the ablation characteristics of the moving-shot technique (MST) and the fixed electrode technique (FET) for radiofrequency (RF) ablation in an ex-vivo bovine liver tissue model.
Materials and Methods
We performed RF ablation using FET in 110 bovine liver blocks using 11 different ablation times ranging from 5 seconds to 5 minutes (10 blocks per each time duration). Ten bovine liver blocks at each ablation time of 1- or 2-minute, were ablated with MST, which treated conceptual ablation units by moving the electrode tip. We evaluated the ablation volume obtained with FET across ablation time lengths. The results of FET and MST performed with the same ablation time lengths, i.e., 1- and 2-minute ablation time were also compared.
Results
The ablation volume achieved with FET gradually increased with increasing ablation time; however, the pair-wise statistical comparison between 2 neighboring ablation time lengths was not significant after 30 seconds. MST with either 1- or 2-minute ablation time achieved larger ablation volumes (1.1 ± 0.2 mL vs. 2.7 ± 0.3 mL, p < 0.001; and 1.4 ± 0.2 mL vs. 5.6 ± 0.4 mL, p < 0.001, respectively), longer true RF times (46.7 ± 4.6 seconds vs. 60 seconds, p < 0.001; and 64.8 ± 4.6 seconds vs. 120 seconds, p < 0.001, respectively), fewer numbers of RF cut-offs (1.6 ± 0.5 vs. 0, p < 0.001; and 5.5 ± 0.5 vs. 0, p < 0.001, respectively), and greater energy deposition (2050.16 ± 209.2 J vs. 2677.76 ± 83.68 J, p < 0.001; and 2970.64 ± 376.56 J vs. 5564.72 ± 5439.2 J, p < 0.001, respectively), than FET.
Conclusion
The MST can achieve a larger ablation volume by preventing RF cut-off, compared with the FET in an ex-vivo bovine liver model.
Keywords: Radiofrequency ablation, Moving shot technique, Fixed electrode technique, Thyroid nodule, Intervention
INTRODUCTION
Radiofrequency (RF) ablation has been successfully used 843to treat various malignant tumors, especially liver tumors (1, 2, 3, 4). The main strategy for RF ablation of liver tumors is to create a large ablation zone that includes both tumor and surrounding normal liver parenchyma, in order to eliminate microscopic tumor foci (5, 6, 7). An electrode is inserted into the central portion of the liver tumor and remains fixed during the entire ablation time.
Recently, RF ablation has been successfully used for both benign thyroid nodules and recurrent thyroid cancers (8, 9, 10, 11, 12, 13), using the moving shot technique (MST) which differs from the fixed electrode technique (FET) used in liver tumors. Baek et al. (14) suggested that the FET was not suitable for the thyroid gland, since unlike the liver, the thyroid gland is a small organ, whereas thyroid nodules are relatively large and ellipsoid in shape. Therefore the treatment strategy of FET, i.e., making a round-shaped ablation zone, is dangerous to the surrounding critical structures in the thyroid gland (14). To overcome this problem, several studies have proposed MST for RF ablation of thyroid nodules and have achieved successful clinical outcomes (8, 9, 10, 11, 12, 13, 14, 15). Although the efficacy of MST was suggested by clinical studies (8, 16, 17, 18), comparison of the MST and the FET has not yet been demonstrated.
The purpose of our study was to evaluate the ablation volume according to the serial ablation time by FET and to compare the ablation characteristics of MST and FET in normal bovine liver blocks.
MATERIALS AND METHODS
This ex vivo study was approved by the Institutional Review Board of Asan Medical Center. This study was supported by the STARmed Co. (Goyang, Korea) who provided the required equipment.
Equipment and RF Ablation Procedure
We used an 18-gauge, monopolar, modified, internally cooled electrode (VIVA, STARmed, Goyang, Korea) with a 1-cm active tip and a 7-cm shaft length, specifically developed for thyroid lesions (10). An RF generator (VIVA RF generator, STARmed, Goyang, Korea) and a peristaltic pump (VIVA pump, STARmed, Goyang, Korea) were also used. The pump continuously infused the cold saline solution (0℃) into the lumen of the electrodes so as to maintain the temperature of the electrode at < 20℃.
We used freshly excised bovine liver blocks. The liver blocks were exposed to room temperature for more than two hours. The temperature of tissue before ablation ranged from 22 to 26℃. The study procedure had 2 parts: 1) evaluation of the ablation volume according to the serial ablation time by FET; and 2) comparison of ablation characteristics of the MST and FET at 1- and 2-minutes of ablation time. Ablation was performed in both groups using 50 watts of RF power with a pulsing mode.
RF Ablation According to the Serial Ablation Time Using the FET
We performed RF ablation on 110 freshly excised bovine liver blocks. The livers were cut into 6 × 6 × 6-cm3 blocks (19) that were dipped into a 20 × 20 × 50-cm3 saline-filled bath at room temperature. A dispersive metallic pad (20 × 15 cm2) was attached to the lateral wall of the bath. The tip of the electrode was inserted into the liver to a depth of 2 cm, and the electrode was fixed during the ablation. We checked tissue impedance after insertion of the electrode. The ablation time ranged from 5 seconds to 5 minutes (5, 10, 20, 30, 40, 50, 60, 120, 180, 240, and 300 seconds).Ten liver blocks were obtained for each ablation time. RF ablation was feasible in all ex vivo studies. Ablation time > 5 minutes frequently causes the RF cut-off phenomenon, hence the ablation duration in this study was limited to 5 minutes.
RF Ablation Using the Moving-Shot Technique
We performed additional RF ablation using MST under ultrasound guidance on 20 freshly excised bovine liver blocks. The livers were cut into multiple, 10 × 10 × 10-cm3 blocks that were placed on a dispersive metallic pad (20 × 15 cm2). An operator with 10 years of clinical experience performing thyroid RF ablation, performed the RF ablation by MST (8, 10, 11, 14, 20). The electrode tip was initially positioned in the deepest (2.5-3.0 cm) portion of the liver tissue and ablation was then begun. The electrode tip moves slowly but continuously in order to prevent the RF cut-off phenomenon. When an echogenic area was detected at the tip of the electrode, we pulled the electrode tip along the long axis of the electrode, up to 3-4 cm in length. The electrode tip was then superficially re-positioned in the direction of DT2 (described in the measurement of ablation size and volume) and pulled along the long axis of the electrode again. After 3-4 repeated movements in the direction of DT2, we repositioned the electrode tip in the DT1 direction (described in the measurement of ablation size and volume) and we pulled the electrode tip along the long axis of the electrode again. Ten liver blocks were used for each ablation time of 1- and 2-minutes.
Measurement of Ablation Size and Volumes
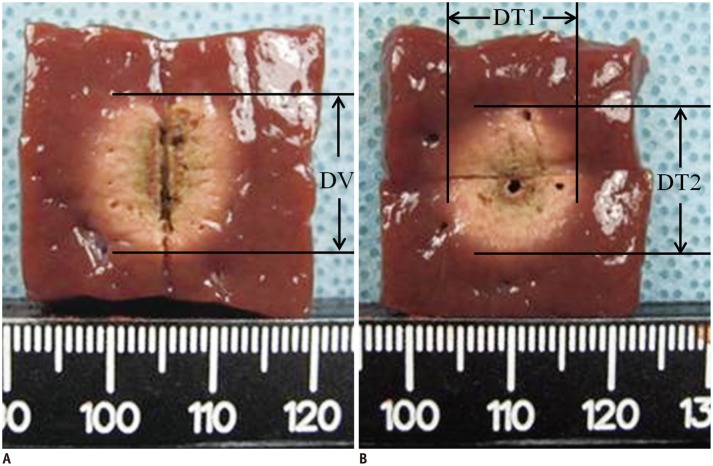
Following ablation the liver blocks were cut along the longitudinal plane passing through the longitudinal axis of the electrode (longitudinal plane, L-plane); the blocks were then cut transversely and perpendicular to the L-plane at the center of the ablation zone (transverse plane, T-plane). Two investigators measured the volume of the ablation zone. We measured the vertical diameter (DV) along the electrode, in the L-plane (Fig. 1A). In the T-plane, we measured the transverse diameter (DT1) perpendicular to the DV in the L-plane and another transverse diameter (DT2) in the T-plane (Fig. 1B). The ablated white area was defined as the ablation zone which reportedly corresponds to the zone of coagulation necrosis (21, 22, 23, 24, 25, 26). The volume of the ablation zone was approximated using the following formula (27): Volume = π·(DV × DT1 × DT2)/6 (28).
Fig. 1.
Measurement of ablation zone.
A. On longitudinal plane, we measured vertical diameter (DV) along electrode. B. On transverse plane, we measured transverse diameter (DT1) perpendicular to DV in L-plane and another transverse diameter (DT2) in T-plane.
Statistical Analysis
We performed statistical analyses using the commercially available software (SPSS-PC, version 12.0; SPSS Inc., Chicago, IL, USA). We used the Kruskal-Wallis test to evaluate the difference of impedance before and during the ablation, and compared the variables, i.e., DV, DT1, DT2, and the ablation volume, among 11 serial ablation groups (5-300 seconds) with FET. We performed the post hoc test with Bonferroni correction, if statistical significance was detected. The variables of FET and MST were compared by selecting only 1- and 2-minutes ablations among the 11 serial ablation groups of FET (5-300 seconds) and the 1- and 2-minutes ablations by MST. We used the Mann-Whitney test to compare the variables, i.e., true ablation time (total ablation time-cut-off time); number of RF cut-offs; applied energy; DV, DT1, DT2; and the ablation volume, of the moving shot and the fixed electrode group. A 2-sided p value < 0.05 was considered as statistical significance.
RESULTS
RF Ablation According to the Serial Ablation Time Using the Fixed Electrode Technique
The impedance of the liver blocks for each ablation time (5-300 seconds) was not significantly different before ablation (p = 0.891) (Table 1). The impedance of each group during the ablation showed a significant difference (p < 0.001), although the post hoc test showed no significant difference (p = 0.594) (Table 2). RF cut-off was not detected in the ablation groups in < 30 seconds.
Table 1.
Variables Before, During, and After Ablation According to Serial Ablation Time Using Fixed Electrode Technique
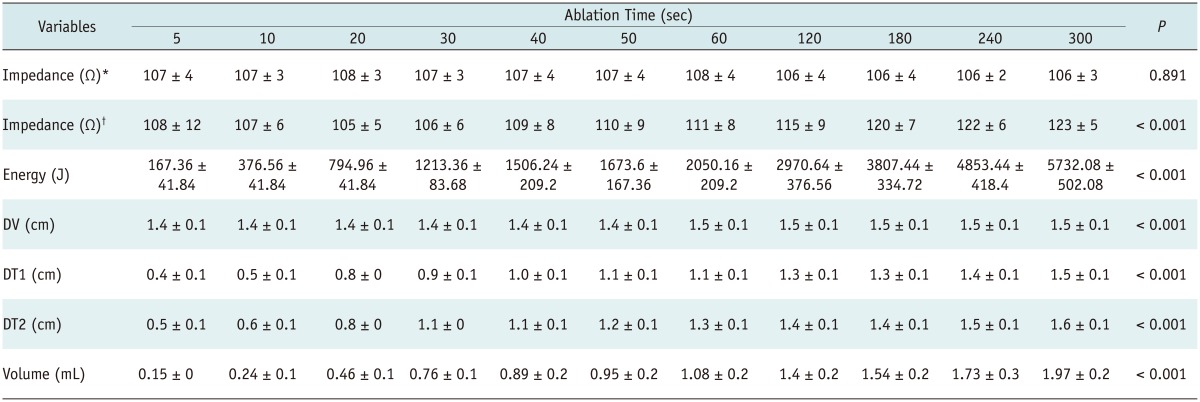
Note.- *Impedance before ablation, †Impedance during ablation (mean ± standard deviation). DT1 = transverse diameter, DT2 = another transverse diameter, DV = vertical diameter
Table 2.
Results of Post-Hoc Test for Variables during Ablation According to Serial Ablation Time
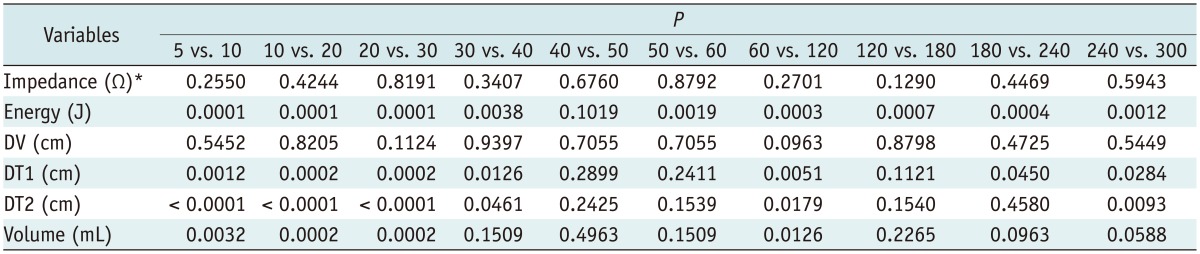
Note.- *Impedance during ablation, p < 0.005. DT1 = transverse diameter, DT2 = another transverse diameter, DV = vertical diameter
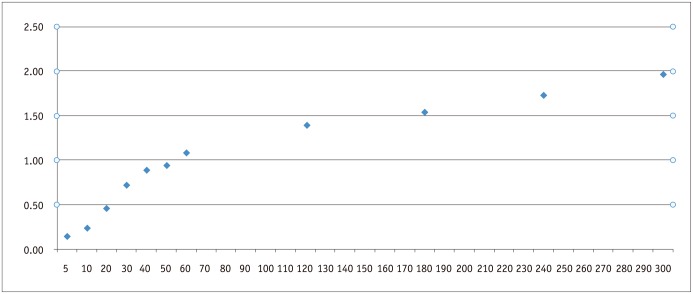
The size (DV, DT1, and DT2) and volume of the ablation zone increased significantly according to the ablation time (p < 0.001) (Table 1). In the post hoc test, DT1, DT2, and the volume of the ablation zone increased significantly up to 30 seconds, although there was no statistical significance after 30 seconds (Fig. 2). The DV did not increase significantly in the post hoc test in any of the study specimens (Table 2).
Fig. 2.
Relationship between ablation volume and time using fixed electrode technique. Ablation volume gradually increased with increasing ablation time; however, pair-wise statistical comparison between 2 neighboring ablation time lengths was not significant after 30 seconds.
Comparison of the Moving Shot and the Fixed Electrode Techniques
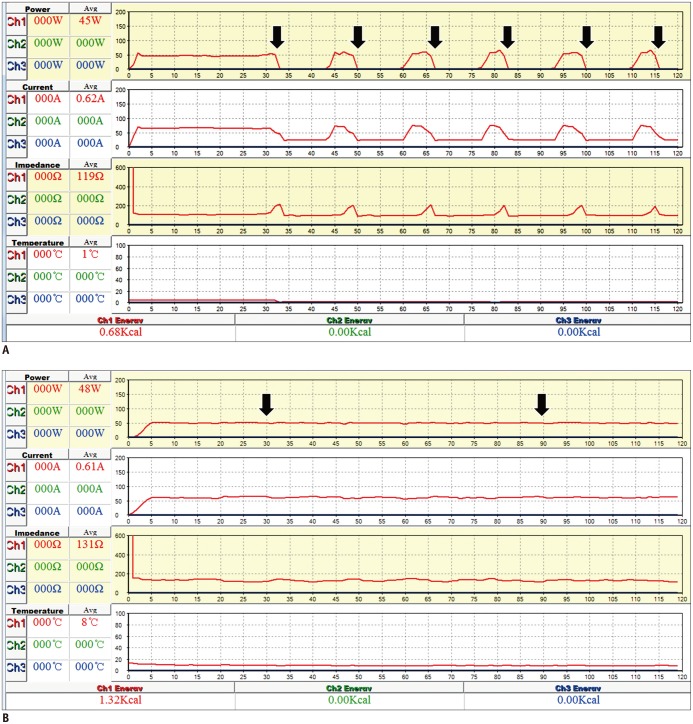
A comparison of the variables of the MST and the FET were summarized in Table 3. The first RF cut-off in the FET was detected in all specimens between 32-52 seconds (mean ± standard deviation [SD], 40.3 ± 6.5 seconds) and lasted for 8-11 seconds (mean ± SD, 9.8 ± 0.6 seconds) (Fig. 3A). Drops in RF current and sudden elevations of impedance were detected at the corresponding time. A second RF cut-off was detected in 6 specimens in the 1-minute group. However, the MST showed no RF cut-off in either the 1- or the 2-minute group (Fig. 3B).
Table 3.
Comparison of Variables of Moving Shot and Fixed Electrode Technique
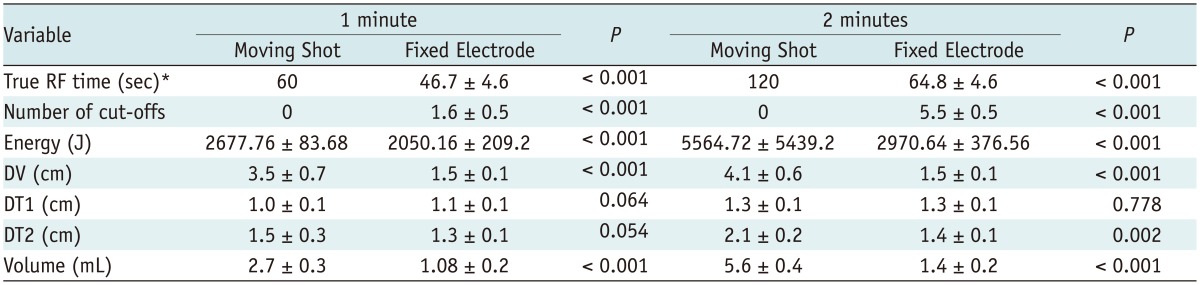
Note.- *Total ablation time-cut-off time. DT1 = transverse diameter, DT2 = another transverse diameter, DV = vertical diameter
Fig. 3.
Graphs of radiofrequency (RF) power, current, impedance, and temperature during 2-minute ablation using fixed electrode and moving-shot techniques.
A. With fixed electrode technique, power graphed over time demonstrates that first RF cut-off appears at 33 seconds (arrow) and 6 RF cut-offs times (arrows) were detected during 2-minute ablation procedure. Drops of RF current and sudden increases of impedance were detected in corresponding area. These RF cut-offs denote cooling period that serves to prevent tissue carbonization near electrode tip. Total energy deposition was 0.68 kcal. B. With moving shot technique, RF power is maintained at approximately 50 watts during entire ablation procedure (arrows). Moving electrode tip could prevent RF cut-off, and total energy deposition was 1.32 kcal.
The MST achieved longer mean true RF times as compared with the FET (46.7 ± 4.6 seconds vs. 60 seconds in 1-minute, p < 0.001; 64.8 ± 4.6 seconds vs. 120 seconds in 2-minute, p < 0.001, respectively), fewer mean numbers of RF cut-off (1.6 ± 0.5 vs. 0 in 1-minute, p < 0.001; 5.5 ± 0.5 vs. 0 in 2-minute, p < 0.001, respectively) and more mean energy deposition (2050.16 ± 209.2 J vs. 2677.76 ± 83.68 J, p < 0.001; 2970.64 ± 376.56 J vs. 5564.72 ± 5439.2 J, p < 0.001, respectively). Therefore, the MST achieved a significantly larger ablation volume zone than the FET at 1-minute (1.1 ± 0.2 mL vs. 2.7 ± 0.3 mL, p < 0.001) and 2-minute ablation time (1.4 ± 0.2 mL vs. 5.6 ± 0.4 mL, p < 0.001) (Table 3).
DISCUSSION
Our results demonstrated that the ablation volume achieved with FET gradually increased with increasing ablation time; however, the pair-wise statistical comparison between 2 neighboring ablation time lengths was not significant after 30 seconds. These results possibly indicated that an ablation longer than 30 seconds with the electrode fixed in the same position would not effectively increase the ablation volume. Rather, moving the electrode after 30 seconds could more effectively create a large ablation volume. The MST achieved a significantly larger ablation volume during both 1- and 2-minutes of ablation on comparison of the 2 techniques.
The volume increase after 30 seconds was not significant at any time interval in this study. This phenomenon could be explained by 2 factors. The first is the RF cut-off phenomenon. RF cut-off was first detected at 32-52 seconds after ablation and was more frequently encountered after 1-minute. Drops in the RF current and sudden elevations of impedance were detected at each corresponding time. These RF cut-offs denoted the cooling period that prevents tissue carbonization near the electrode tip. The second factor is the type of heat involved. The conventional ablation procedure, i.e., the FET, is achieved by both friction and conduction heats (14, 29). Friction heat creates immediate tissue necrosis by agitation of tissue ions; however, an ablation zone achieved by friction heat is only a few millimeters in size (29). Therefore, the majority of tumor tissue remote from the electrode is ablated more slowly by conduction heat, which increases the duration of the RF ablation to 10-30 minutes (5, 6, 29). Conduction heat is also affected by several factors such as perfusion-mediated tissue cooling and tissue characteristics (5, 7, 14, 29), thus leading to an unpredictable ablation zone. Friction heat is fast, powerful, and creates a more consistent ablation zone. Our study suggested that an ablation zone within 30 seconds of ablation time was achieved mainly by friction heat.
The FET group showed frequent RF cut-off after 30 seconds when compared the MST, which resulted in reduction of both the true ablation time and the ablation zone. We moved the electrode tip before the RF cut-off appeared with the MST, hence majority of tissue could thus be treated by friction heat without RF cut-off. Therefore, the MST achieved a significantly larger ablation zone than the FET during both, the 1- and 2-minutes of ablation. The optimal time for moving the electrode was within 30 seconds in this study.
Radiofrequency ablation of liver tumors is performed by fixing the electrode in position during the entire ablation time. Thus, a round ablation zone, including normal liver tissue, is obtained; however, thyroid nodules are usually ellipsoidal in shape and there is an insufficient safety margin as the thyroid gland is relatively small (14). Therefore, fixation of an electrode for a long time is dangerous to surrounding critical structures in the thyroid gland (14, 17). Many investigators have therefore proposed the MST for the treatment of benign thyroid nodules (9, 14, 15, 20, 30, 31, 32). This technique is regarded as safe for treating nodules with an ellipsoidal shape and has also revealed effective clinical results. RF ablation using the MST showed 44-81.9% of volume reduction in treated nodules at the 6-month follow-up and 93% at the 4-year follow-up. The complication rate was 3.3% in a multicenter study (14, 17, 20). However, the underlying mechanisms of the MST are as yet unclear. Our study suggested the plausible mechanisms and effectiveness of the MST compared to the FET. The collective findings from this experimental study together with previous clinical results, suggest that the MST is to be used in non-round-shaped tumors and tumors near critical structures.
Our study had several limitations. Firstly, application of the ex-vivo study data to in-vivo situations could result in different outcomes. Secondly, we performed this study in the bovine liver tissue, however, liver and other tissues such as thyroid could show different results. However, since it was not practical to obtain bovine thyroid tissue, we compared the ablation characteristics between the MST and FET in bovine liver blocks. Thirdly, we performed visual estimation of ablations aside from histological confirmation. Previous histological studies showed that it was difficult to clearly distinguish a distinct line between non-viable and viable cells. There is a "gray zone" between coagulative necrosis and viable cells, which indicates that many cells will undergo apoptosis and some cells will remain viable. Fourthly, we did not evaluate the shape of the ablation zone and the roundness index. However, the ablated zone was a smooth and oval shape in most cases. Interobserver variability is also required to be evaluated in the future.
In conclusion, our study demonstrated that the MST for the electrode tip during ablation could achieve a larger ablation volume by preventing RF cut-off, as compared with the FET.
References
- 1.Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 2.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 3.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 4.Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:34–42. doi: 10.3348/kjr.2009.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129–147. doi: 10.1016/s0929-8266(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 7.Rhim H, Goldberg SN, Dodd GD, 3rd, Solbiati L, Lim HK, Tonolini M, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21 Spec No:S17–S35. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- 8.Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194:1137–1142. doi: 10.2214/AJR.09.3372. [DOI] [PubMed] [Google Scholar]
- 9.Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011;197:W331–W336. doi: 10.2214/AJR.10.5345. [DOI] [PubMed] [Google Scholar]
- 10.Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009;33:1971–1977. doi: 10.1007/s00268-009-0130-3. [DOI] [PubMed] [Google Scholar]
- 11.Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250. doi: 10.1007/s00330-008-0880-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA) World J Surg. 2010;34:1488–1493. doi: 10.1007/s00268-010-0565-6. [DOI] [PubMed] [Google Scholar]
- 13.Sung JY, Kim YS, Choi H, Lee JH, Baek JH. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol. 2011;196:W210–W214. doi: 10.2214/AJR.10.5172. [DOI] [PubMed] [Google Scholar]
- 14.Baek JH, Lee JH, Valcavi R, Pacella CM, Rhim H, Na DG. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540. doi: 10.3348/kjr.2011.12.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha EJ, Baek JH, Lee JH. The efficacy and complications of radiofrequency ablation of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2011;18:310–314. doi: 10.1097/MED.0b013e32834a9168. [DOI] [PubMed] [Google Scholar]
- 16.Baek JH, Jeong HJ, Kim YS, Kwak MS, Lee D. Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid. 2008;18:675–676. doi: 10.1089/thy.2007.0274. [DOI] [PubMed] [Google Scholar]
- 17.Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342. doi: 10.1148/radiol.11110416. [DOI] [PubMed] [Google Scholar]
- 18.Spiezia S, Garberoglio R, Milone F, Ramundo V, Caiazzo C, Assanti AP, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19:219–225. doi: 10.1089/thy.2008.0202. [DOI] [PubMed] [Google Scholar]
- 19.Ha EJ, Baek JH, Lee JH, Kim JK, Shong YK. Clinical significance of vagus nerve variation in radiofrequency ablation of thyroid nodules. Eur Radiol. 2011;21:2151–2157. doi: 10.1007/s00330-011-2167-6. [DOI] [PubMed] [Google Scholar]
- 20.Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125. doi: 10.3348/kjr.2012.13.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2:151–158. doi: 10.3348/kjr.2001.2.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002;35:1467–1475. doi: 10.1053/jhep.2002.33635. [DOI] [PubMed] [Google Scholar]
- 23.Clasen S, Schmidt D, Dietz K, Boss A, Kröber SM, Schraml C, et al. Bipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: prediction of coagulation volume from applied energy. Invest Radiol. 2007;42:29–36. doi: 10.1097/01.rli.0000248973.95949.eb. [DOI] [PubMed] [Google Scholar]
- 24.Crocetti L, Lencioni R, Debeni S, See TC, Pina CD, Bartolozzi C. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol. 2008;43:33–39. doi: 10.1097/RLI.0b013e31815597dc. [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007;42:163–171. doi: 10.1097/01.rli.0000252495.44818.b3. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 27.Lee JM, Han JK, Kim SH, Shin KS, Lee JY, Park HS, et al. Comparison of wet radiofrequency ablation with dry radiofrequency ablation and radiofrequency ablation using hypertonic saline preinjection: ex vivo bovine liver. Korean J Radiol. 2004;5:258–265. doi: 10.3348/kjr.2004.5.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Na DG, Lee JH, Kim SM, Lim HK, Baek JH. Unidirectional ablation electrode to minimize thermal injury during radiofrequency ablation: an experimental study in an ex vivo bovine liver model. J Vasc Interv Radiol. 2011;22:935–940. doi: 10.1016/j.jvir.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Haemmerich D, Laeseke PF. Thermal tumour ablation: devices, clinical applications and future directions. Int J Hyperthermia. 2005;21:755–760. doi: 10.1080/02656730500226423. [DOI] [PubMed] [Google Scholar]
- 30.Jang SW, Baek JH, Kim JK, Sung JY, Choi H, Lim HK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012;81:905–910. doi: 10.1016/j.ejrad.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 31.Huh JY, Baek JH, Choi H, Kim JK, Lee JH. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session--prospective randomized study. Radiology. 2012;263:909–916. doi: 10.1148/radiol.12111300. [DOI] [PubMed] [Google Scholar]
- 32.Gharib H, Hegedüs L, Pacella CM, Baek JH, Papini E. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98:3949–3957. doi: 10.1210/jc.2013-1806. [DOI] [PubMed] [Google Scholar]