Abstract
Objective
Tiny cerebral aneurysms are difficult to embolize because the aneurysm's sac is too small for a single small coil, and coils within the aneurysm may escape from the confinement of a stent. This study was performed to introduce the stent-assisted coil-jailing technique and to investigate its effect on the coil embolization of tiny intracranial aneurysms.
Materials and Methods
Sixteen patients with tiny intracranial aneurysms treated with the stent-assisted coil-jailing technique between January 2011 and December 2013 were retrospectively reviewed and followed-up.
Results
All aneurysms were successfully treated with the coil-jailing technique, and at the end of embolization, complete occlusion of the aneurysm was achieved in 9 cases (56.3%), incomplete occlusion in 6 (37.5%), and partial occlusion in 1 (6.3%). Intraprocedural complications included acute thrombosis in one case (6.3%) and re-rupture in another (6.3%). Both complications were managed appropriately with no sequela. Follow-up was performed in all patients for 3-24 months (mean, 7.7 months) after embolization. Complete occlusion was sustained in the 9 aneurysms with initial complete occlusion, progressive thrombosis to complete occlusion occurred in the 6 aneurysms with initial near-complete occlusion, and one aneurysm resulted in progressive thrombosis to complete occlusion after initial partial occlusion. No migration of stents or coils occurred at follow-up as compared with their positions immediately after embolization. At follow-up, all patients had recovered with no sequela.
Conclusion
The stent-assisted coil-jailing technique can be an efficient approach for tiny intracranial aneurysms, even though no definite conclusion regarding its safety can be drawn from the current data.
Keywords: Tiny intracranial aneurysm, Stent-assisted coiling, Redundant coil tails, Coil migration
INTRODUCTION
Endovascular coil embolization of ruptured and unruptured cerebral aneurysms has become an efficient alternative technique to surgical clipping (1). However, endovascular coiling of very small aneurysms remain controversial (2, 3, 4, 5) because of technical difficulties and the high risk of procedure-related rupture in spite of the improvement of endovascular devices and techniques (2). In these cases, surgical clipping is also prone to technical difficulty, because very small aneurysms are often thin-walled and too small to accept a clip without narrowing or tearing the parent artery (6). Although the likelihood of rupture of small, unruptured intracranial aneurysms (< 7 mm) is very low in both patients with or without a history of subarachnoid hemorrhage (7), there is no evidence confirming the benign natural course of very small unruptured aneurysms or the advantage of conservative treatment over endovascular management. Recent studies by van Rooij et al. (4) and Gupta et al. (8) revealed that very small aneurysms constituted 15% and 7% of overall ruptured aneurysms, respectively. Moreover, a Korean single center study regarding the size and location of ruptured aneurysms demonstrated that very small aneurysms (less than 3 mm in diameter) constituted 7.9% of overall ruptured aneurysms and that small aneurysms (≤ 7 mm) constituted 66% of all ruptured aneurysms (9). This indicates that very small ruptured aneurysms are not an infrequent clinical occurrence, and that the incidence of rupture of these aneurysms is higher than expected. Thus, the treatment of very small aneurysms, especially those that rupture, is necessary to prevent the rupture or re-rupture of these aneurysms. The stent-assisted coiling technique is essential for the embolization of small aneurysms due to its wide neck and small size. However, coil migration from the small stent-encaged aneurysm cavity through the stent mesh is also possible because the stent mesh is usually greater than 2 mm in diameter, leading to distal embolization of arteries (10, 11). To make things worse, it is very difficult to choose a coil of appropriate size to completely pack the small aneurysm cavity, especially for tiny aneurysms less than 2 mm in diameter. In China, the available smallest coil (1.5 mm × 1 cm) is only able to partially pack a tiny aneurysm sac, and second coil would be too large to completely insert into the sac, potentially resulting in a rupture of the aneurysm if inserted forcefully. Forceful insertion of the second coil may also squeeze the first coil through the stent mesh, leading to severe consequences. If a coil is too long for the tiny aneurysm sac, it has to be replaced by a shorter one, thus making the procedure longer and possibly adding complications. To solve the problems associated with embolizing small or tiny aneurysms, we designed the stent-assisted coil-jailing technique to intentionally jail redundant coil segments for a simple and complete embolization of the tiny cerebral aneurysm. In this technique, if the coil is too long for a tiny aneurysm, the redundant coil segment (tail) will be deliberately left and jailed between the stent and the parent artery wall at the end of the embolization procedure to simplify the procedure and prevent possible complications caused by the replacement of coils. This study was performed to introduce the stent-assisted coil-jailing technique for intentional redundant coil jailing and to investigate its effect on the coil embolization of tiny intracranial aneurysms.
MATERIALS AND METHODS
This retrospective study was approved by the hospital ethics committee for scientific research, and all of the patients signed the informed consent for endovascular embolization of their tiny cerebral aneurysms with the stent-assisted coil-jailing technique. Tiny cerebral aneurysms are defined as those having a sac ≤ 2 mm in diameter as measured on a three-dimensional image. The inclusion criteria for this study were: tiny cerebral aneurysms less than 2 mm in diameter, with a wide neck, and treated with the coil-jailing technique. Tiny aneurysms treated without the coil-jailing technique were excluded. There were 16 consecutive patients, 7 males and 9 females (age range, 33-62 years; mean, 45), harboring tiny intracranial ruptured aneurysms treated with the coil-jailing technique between January 2011 and December 2013 (Table 1). The locations of the tiny intracranial aneurysms were the anterior communicating artery (4 patients), the posterior communicating artery (6 patients), the middle cerebral artery (4 patients), and the ophthalmic artery (2 patients). The greatest diameter of the aneurysms was ≤ 2 mm, and the dome-neck ratio was smaller than 1.5 in 14 aneurysms and larger than 1.5 in 2. All the aneurysms were seen ruptured at admission, with Hunt-Hess grades of I in 5 patients, II in 7, III in 3, and IV in 1. All patients received endovascular embolization of the aneurysms 1-15 (mean, 4.9) days after subarachnoid hemorrhage and were followed-up subsequently. The materials used for embolization included the Solitaire stent (eV3 Inc., Plymouth, MN, USA), the Enterprise stent (Codman, Raynham, MA, USA), and Microplex coils (MicroVention Inc., Tustin, CA, USA).
Table 1.
Characteristics of Patients and Tiny Aneurysms Treated with Stent-Assisted Coil-Jailing and Follow-Up Results
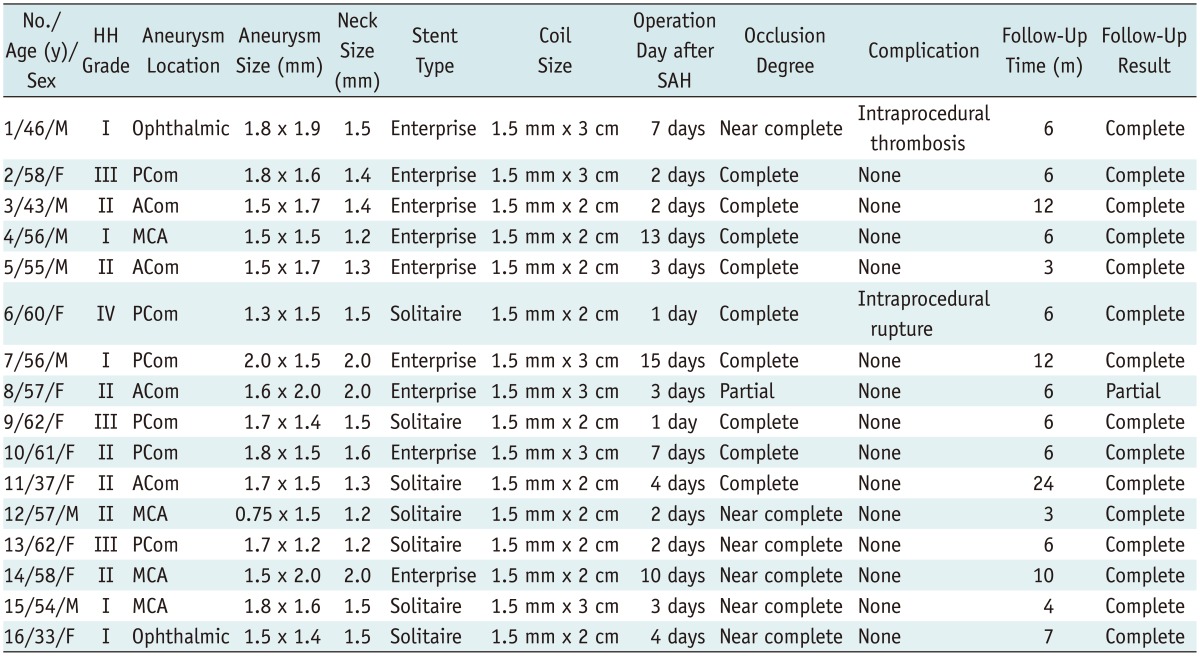
Note.- ACom = anterior communicating artery, DSA = digital subtraction angiography, HH grade = Hunt and Hess grade, MCA = middle cerebral artery, PCom = posterior communicating artery, SAH = subarachnoid hemorrhage
Embolization Procedure
All the procedures were performed under general anesthesia. All patients received antiplatelet medications before the procedure, with 300 mgs of each of clopidogrel and aspirin administered 30 minutes before the procedure. After the coil was inserted into the aneurysm cavity, a bolus of 3000 IU heparin was infused before the stent was deployed. Subsequently, 1000 IU heparin was infused per hour during the procedure. The Seldinger technique was employed to puncture the femoral artery, and digital subtraction angiography was performed. The best projection position was chosen to clearly show the relationship of the aneurysm neck and the parent artery and the angle formed between the aneurysm's parent artery and the major artery. After a microcatheter containing the stent and another microcatheter containing the coil both reached the aneurysm's location, the coil was partially inserted into the aneurysm cavity and the stent was deployed from the microcatheter to cover the aneurysm orifice. In this way, the microcatheter containing the coil was constrained between the parent artery wall and the deployed stent. Deployment of the coil continued until the aneurysm sac was completely or almost completely packed by the coil, at which point, further packing of the coil was difficult. At this time, the coil pusher was secured and the microcatheter containing the coil was slightly withdrawn so that the distal marker of the microcatheter overlapped with the detachment marker of the coil. Then, the coil was detached and the redundant coil tail remained outside the aneurysm's sac, thus being encaged between the stent and the parent artery wall to prevent possible coil migration out of the aneurysm's sac through the stent mesh.
Angiographic Results and Follow-Up
Aneurysm occlusion at the end of embolization procedure and at follow-up was considered complete when the aneurysm cavity and neck were fully packed with no filling of the aneurysm's sac by contrast material; near complete when the sac was occluded, but a neck remnant was present; and partially complete when there was persistent opacification of a sac remnant. Aneurysm recurrence was considered present when a previously completely occluded aneurysm exhibited partial or small neck recanalization during follow-up or when the size of the neck remnant of a previously near-completely occluded aneurysm increased in size during follow-up angiography. Angiographic follow-up was performed at 3 and 6 months and yearly thereafter to determine the occlusion status of the aneurysms. Clinical outcomes were evaluated with the Glasgow Outcome Scale.
RESULTS
The embolization procedure was performed 0-15 days after subarachnoid hemorrhage, and no re-rupture was observed during this period. All 16 tiny ruptured aneurysms were embolized successfully with only one coil by the stent-assisted coil-jailing technique, and the redundant coil tail was intentionally jailed between the stent and the parent artery wall in all cases (Table 1, Figs. 1, 2, 3). At the end of embolization, complete occlusion was achieved in 9 aneurysms (56.3%), near complete occlusion in 6 (37.5%), and partial occlusion in 1 (6.3%). Intraprocedural complications included acute thrombosis in one case (6.3%) (Fig. 2) and re-rupture in another (6.3%), caused by a microguidewire puncture in a case with an acute angle. Both complications were managed appropriately with no permanent sequela.
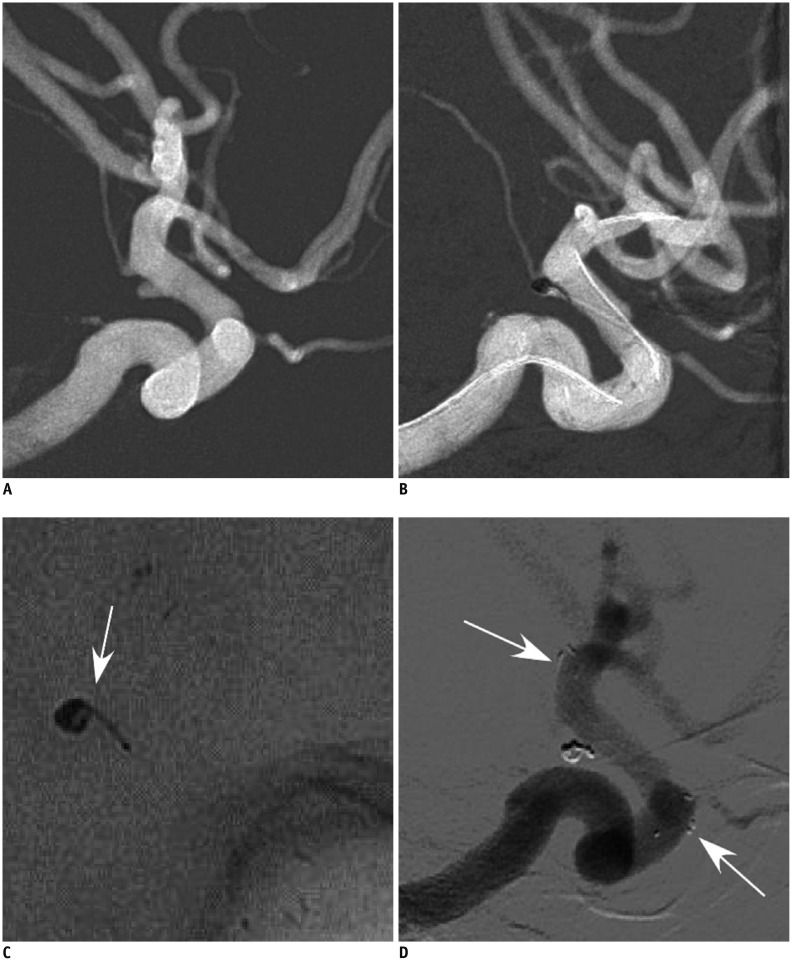
Fig. 1.
58-year-old woman (patient 2) had sudden headache, nausea and vomiting for 18 hours.
A. Tiny left posterior communicating artery aneurysm was shown. B. 1.5 mm × 3 cm coil was inserted into the aneurysm's sac and coil tail was constrained between stent and wall of internal carotid artery to increase coil stability. C. Deployed coil and its tail form shape of comma (arrow). D. Follow-up six months later revealed complete occlusion of aneurysm. Arrows indicate markers on distal and proximal ends of stent.
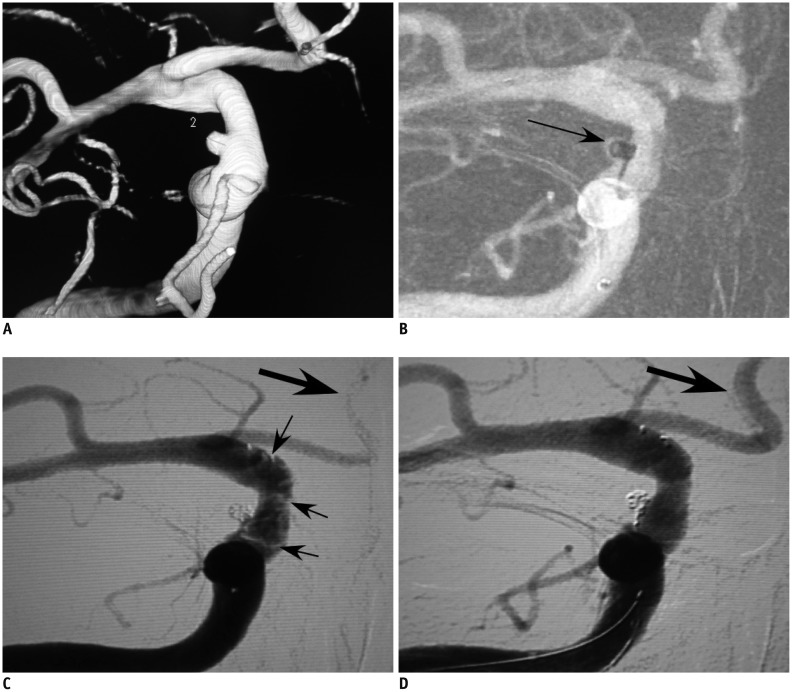
Fig. 2.
46-year-old man (patient 1) who had subarachnoid hemorrhage was referred to our hospital for embolization of ruptured tiny aneurysm.
46-year-old man (patient 1) had tiny ruptured aneurysm at right ophthalmic artery segment of internal carotid artery (A). B. Aneurysm was treated with stent-assisted coiling technique. Small 1.5 mm × 2 cm coil was inserted into aneurysm's sac, and coil tail was compressed against wall of internal carotid artery by stent. Arrow indicates coil and constrained coil tail forming shape of comma. C. After stent was deployed, thrombi formed (small arrows) and 3000 IU heparin was infused together with 20 U urokinase. Bigger arrow indicates that anterior cerebral artery did not display very well because of thrombi. D. Fifteen minutes later, thrombi disappeared and patient was discharged with no sequela. Anterior cerebral artery was well displayed after thrombolysis (big arrow).
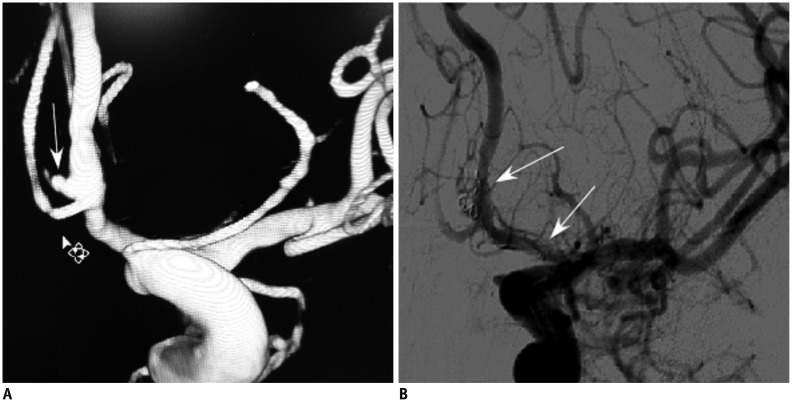
Fig. 3.
57-year-old woman had sudden headache and nausea (patient 8).
A. 57-year-old woman had tiny ruptured anterior communicating artery aneurysm (patient 8). B. Aneurysm was treated with stent-assisted coiling technique. Small 1.5 mm × 3 cm coil was inserted into aneurysm's sac and coil tail was compressed between stent (arrows) and vessel wall.
Follow-up was performed for 3-24 months (mean, 7.7 months) after embolization. All patients had angiographic follow-up post-embolization. Complete occlusion remained in 9 aneurysms with initial complete occlusion, progressive thrombosis to complete occlusion occurred in 6 aneurysms with initial near complete occlusion, and the same occlusion status was seen in one aneurysm with initial partial occlusion. No migration of stents or coils occurred at follow-up compared with immediately after embolization. At follow-up, all patients had recovered with no sequela.
DISCUSSION
This study introduced the stent-assisted coil-jailing technique and investigated its effect on the embolization of tiny intracranial aneurysms in 16 cases, with the redundant coil tail jailed between the stent and the parent artery wall for efficient and complete embolization, as well as for the prevention of possible coil migration through the stent mesh. After 3-24 months of follow-up, all the stents and coils remained in position with no delayed distal migration or recurrence.
Intraprocedural rupture of aneurysms is one of the most severe complications and may be caused by perforation from the microcatheter or micro-guidewire. It may also occur during coil placement. When embolizing tiny intracranial aneurysms, it is better not to use the micro-guidewire to select the aneurysm sac because the tip of the micro-guidewire could be inserted into the aneurysm sac. Instead, the microwire tip should be placed near the aneurysm's orifice and kept there during the guidance of the microcatheter. Moreover, the tip of the microcatheter should be appropriately shaped for good maneuverability. When inserting the coil into the aneurysm sac, partial coil loop deployment outside the aneurysm sac before entering the sac is safer and the tip of the coil in a small curved loop will not puncture the aneurysm wall, thus preventing possible re-rupture caused by coil puncture. If intraprocedural re-rupture of aneurysms is caused by the microcatheter or coils, continued coil packing inside the aneurysm sac usually stops the bleeding. If the re-rupture was caused by the micro-guidewire, the microcatheter should be advanced into the aneurysm sac for coil packing, however, if the microcatheter cannot be navigated into the aneurysm's sac for packing, further manipulation of both the microcatheter and the micro-guidewire should be stopped. This is because the rupture caused by the micro-guidewire is usually small and non-life-threatening especially for small aneurysms, and further maneuvering may cause greater damage to the aneurysm wall and subsequent excessive bleeding. Temporary occlusion of the parent artery may also be useful in addition to some generalized measures like induced hypotension and reversal of heparin.
The clinical consequences of intraprocedural rupture are variable, ranging from minimal to massive subarachnoid hemorrhage (3, 12). A small-sized aneurysm is considered a risk factor (13, 14), with the reported rate of procedural rupture of small aneurysms more than twice (7.7%) or five times (11.7%) as high as the rate of larger aneurysms (4, 12). Two factors that may play a role in the relatively higher rupture risk of very small aneurysms are the direct transmission of force via the microcatheter and coils toward the aneurysm, as well as the structural limitation of currently available microcatheters and coils (15). The structural limitation is caused by the difference of the distance between the distal end of the distal markers of the microcatheter and the detachment zone of the coil in currently available devices, which indicates the need for device refinement and the importance of selecting proper microcatheters and coils (15). However, delicate maneuvering of therapeutic devices like microcatheters and coils is more important to prevent intraoperative rupture, and tiny aneurysms require greater control due to the extremely limited space within the aneurysm's sac. If the coil is too long to stay completely within the tiny aneurysm sac, trying to insert the full coil into the sac may result in aneurysm re-rupture. However, jailing of the redundant coil tail between the stent and the arterial wall would be able to solve the problem of a longer coil unsuitable for the tiny aneurysm sac.
Another significant complication during endovascular treatment of cerebral aneurysms is thromembolism, and the related intraarterial or systemic application of glycoprotein IIb/IIIa inhibitor during embolization is an effective and safe method to treat the complication (16, 17, 18), even though there was a report about fatal re-bleeding from the ruptured aneurysm in patients treated with abciximab for a thromboembolic event during coil embolization (19). In our series, the case with intraprocedrual thrombosis was managed with an intravenous injection of tirofiban hydrochloride until the thrombus disappeared.
Recurrence in embolized aneurysms is possible in the chronic stage after endovascular embolization, especially for large/giant aneurysms (20). The reported incidence of recurrence for large aneurysms (larger than 10 mm in diameter) is as high as 50.6% while that of small aneurysms (less than 10 mm in diameter) is only 21.3% (21). No recurrence was found in our series during the follow-up, however, long-term follow-up may detect recurrences. Moreover, the long-term rate of recurrence for tiny aneurysms has not been reported yet. Even with a stent to confine the coil within the small aneurysm sac, delayed coil migration through the stent mesh has been reported several months after stent-assisted coil embolization in two small aneurysms (10, 11). Long-term follow-up is still necessary to confirm the occlusion status of the aneurysms and the stability of the coils within the aneurysm sac.
Although the stent-jailing technique has been available for years, our coil-jailing technique is a renovation because it permanently jails the coil tail between the stent and the arterial wall. This technique is useful for the dense packing of the aneurysm, preventing the delayed coil escaping from the stent confinement and avoiding the replacement of an inappropriate coil, which might cause complications, thus simplifying the procedure. If the selected coil is too long for the aneurysm, one does not have to replace the longer coil with a shorter one, instead the redundant coil tail is jailed between the stent and the arterial wall, saving time and preventing possible complications related to the replacement of the longer coil. This refinement to the technique has not been reported previously and this article serves to remind researchers of this renovation to simplify the embolization procedure, as well as to prevent delayed coil migration through the stent mesh.
This study has some limitations. First, it has a limited number of patients. A large number of patients should be included in future studies of tiny aneurysms treated by the use of the stent-assisted coil-jailing technique. Second, a long-term follow-up should be performed to confirm the results and possible recurrence rate. Third, a randomized controlled study should be performed strictly for confirmation of the effect of the coil-jailing technique in embolizing tiny intracranial aneurysms as compared to other techniques that do not use coil-tail-jailing, such as coiling alone or stenting alone.
In conclusion, the stent-assisted coil-jailing technique may be an efficient approach for the treatment of tiny intracranial aneurysms even though no definite conclusion regarding its safety can be drawn presently because of the small size of the patient cohort and short follow-up period in this study.
References
- 1.Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF. Endovascular treatment of very small (3 mm or smaller) intracranial aneurysms: report of a consecutive series and a meta-analysis. Stroke. 2010;41:116–121. doi: 10.1161/STROKEAHA.109.566356. [DOI] [PubMed] [Google Scholar]
- 3.Doerfler A, Wanke I, Egelhof T, Dietrich U, Asgari S, Stolke D, et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. AJNR Am J Neuroradiol. 2001;22:1825–1832. [PMC free article] [PubMed] [Google Scholar]
- 4.van Rooij WJ, Keeren GJ, Peluso JP, Sluzewski M. Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am J Neuroradiol. 2009;30:835–883. doi: 10.3174/ajnr.A1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang MS, Wong HF, Yang TH, Chen YL, Chan SW, Lee HJ, et al. Alternative option in the treatment of very small ruptured intracranial aneurysms. Surg Neurol. 2009;72(Suppl 2):S41–S46. doi: 10.1016/j.wneu.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Nussbaum ES, Erickson DL. The fate of intracranial microaneurysms treated with bipolar electrocoagulation and parent vessel reinforcement. Neurosurgery. 1999;45:1172–1174. doi: 10.1097/00006123-199911000-00031. discussion 1174-1175. [DOI] [PubMed] [Google Scholar]
- 7.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Chugh M, Jha AN, Walia BS, Vaishya S. Coil embolization of very small (2 mm or smaller) berry aneurysms: feasibility and technical issues. AJNR Am J Neuroradiol. 2009;30:308–314. doi: 10.3174/ajnr.A1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong YG, Jung YT, Kim MS, Eun CK, Jang SH. Size and location of ruptured intracranial aneurysms. J Korean Neurosurg Soc. 2009;45:11–15. doi: 10.3340/jkns.2009.45.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho CS. Coil Migration under Stent-Assisted Embolization. A Case Report. Interv Neuroradiol. 2006;12:65–66. doi: 10.1177/159101990601200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao BL, Li MH, Wang YL, Fang C. Delayed coil migration from a small wide-necked aneurysm after stent-assisted embolization: case report and literature review. Neuroradiology. 2006;48:333–337. doi: 10.1007/s00234-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TN, Raymond J, Guilbert F, Roy D, Bérubé MD, Mahmoud M, et al. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg. 2008;108:1088–1092. doi: 10.3171/JNS/2008/108/6/1088. [DOI] [PubMed] [Google Scholar]
- 13.Proust F, Debono B, Hannequin D, Gerardin E, Clavier E, Langlois O, et al. Treatment of anterior communicating artery aneurysms: complementary aspects of microsurgical and endovascular procedures. J Neurosurg. 2003;99:3–14. doi: 10.3171/jns.2003.99.1.0003. [DOI] [PubMed] [Google Scholar]
- 14.Sluzewski M, Bosch JA, van Rooij WJ, Nijssen PC, Wijnalda D. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg. 2001;94:238–240. doi: 10.3171/jns.2001.94.2.0238. [DOI] [PubMed] [Google Scholar]
- 15.Lim YC, Kim BM, Shin YS, Kim SY, Chung J. Structural limitations of currently available microcatheters and coils for endovascular coiling of very small aneurysms. Neuroradiology. 2008;50:423–427. doi: 10.1007/s00234-008-0365-y. [DOI] [PubMed] [Google Scholar]
- 16.Bruening R, Mueller-Schunk S, Morhard D, Seelos KC, Brueckmann H, Schmid-Elsaesser R, et al. Intraprocedural thrombus formation during coil placement in ruptured intracranial aneurysms: treatment with systemic application of the glycoprotein IIb/IIIa antagonist tirofiban. AJNR Am J Neuroradiol. 2006;27:1326–1331. [PMC free article] [PubMed] [Google Scholar]
- 17.Gralla J, Rennie AT, Corkill RA, Lalloo ST, Molyneux A, Byrne JV, et al. Abciximab for thrombolysis during intracranial aneurysm coiling. Neuroradiology. 2008;50:1041–1047. doi: 10.1007/s00234-008-0457-8. [DOI] [PubMed] [Google Scholar]
- 18.Kang HS, Kwon BJ, Roh HG, Yoon SW, Chang HW, Kim JE, et al. Intra-arterial tirofiban infusion for thromboembolism during endovascular treatment of intracranial aneurysms. Neurosurgery. 2008;63:230–237. doi: 10.1227/01.NEU.0000320440.85178.CC. discussion 237-238. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Kim JE, Sheen SH, Jung CK, Kwon BJ, Kwon OK, et al. Intraarterial abciximab for treatment of thromboembolism during coil embolization of intracranial aneurysms: outcome and fatal hemorrhagic complications. J Neurosurg. 2008;108:450–457. doi: 10.3171/JNS/2008/108/3/0450. [DOI] [PubMed] [Google Scholar]
- 20.Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50:239–249. doi: 10.1097/00006123-200202000-00003. discussion 249-250. [DOI] [PubMed] [Google Scholar]
- 21.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]