Abstract
SUMMARY
Basidiomycete fungi subsist on various types of plant material in diverse environments, from living and dead trees and forest litter to crops and grasses and to decaying plant matter in soils. Due to the variation in their natural carbon sources, basidiomycetes have highly varied plant-polysaccharide-degrading capabilities. This topic is not as well studied for basidiomycetes as for ascomycete fungi, which are the main sources of knowledge on fungal plant polysaccharide degradation. Research on plant-biomass-decaying fungi has focused on isolating enzymes for current and future applications, such as for the production of fuels, the food industry, and waste treatment. More recently, genomic studies of basidiomycete fungi have provided a profound view of the plant-biomass-degrading potential of wood-rotting, litter-decomposing, plant-pathogenic, and ectomycorrhizal (ECM) basidiomycetes. This review summarizes the current knowledge on plant polysaccharide depolymerization by basidiomycete species from diverse habitats. In addition, these data are compared to those for the most broadly studied ascomycete genus, Aspergillus, to provide insight into specific features of basidiomycetes with respect to plant polysaccharide degradation.
INTRODUCTION
Plant biomass is the most abundant renewable carbon source on Earth. Many microbes have central roles in the degradation of this biomass to ensure a global carbon cycle. Fungi are specialized to use plant biomass as a carbon source by producing enzymes that degrade plant cell wall polysaccharides into metabolizable sugars. Plant-polysaccharide-depolymerizing enzymes are of great interest to biotechnology, as the products of their catalysis can be used as precursors in the processes that generate bio-based products, e.g., fuels, paper, food, animal feed, and chemicals (1). The enzymes degrading or modifying plant polysaccharides are classified as carbohydrate-active enzymes (CAZymes) and are divided into families according to their amino acid sequence and structural similarity (2). The CAZy database (http://www.cazy.org/) is organized into families of glycoside hydrolases (GHs), carbohydrate esterases (CEs), polysaccharide lyases (PLs), glycosyltransferases (GTs), and auxiliary activities (AA) (2).
Basidiomycetes colonize or inhabit a diversity of plant material in forests, meadows, farmlands, and compost. Different species have various CAZyme sets to meet the needs of their ecological roles as saprobes (wood-rotting and litter-decomposing fungi), symbionts and endophytes (mycorrhizas and lichens), parasites, and plant and animal pathogens (3, 4). Basidiomycetes are the most efficient degraders of woody biomass (5) and therefore are essential for the global carbon cycle. The understanding of the mechanisms that basidiomycetes use for plant polysaccharide degradation is in its infancy compared to ascomycete studies, due largely to the traditional and well-established industrial relevance of several ascomycetes. Since the enzyme sets of basidiomycetes are likely to reflect adaptation to their unique natural niches, basidiomycetes contain a huge potential for applications in various industries, which has so far remained largely unexplored.
As mentioned above, our knowledge of basidiomycetes regarding their ability to decompose plant polysaccharides is limited compared to the wealth of information on ascomycetes. Before the genomics era, functional analyses of purified enzymes and expression studies of the corresponding genes were the main approaches for characterization of the fungal CAZyme machinery. However, these methods are laborious and cannot provide a full overview of a fungal CAZyme arsenal. More detailed insights into the entire polysaccharide-degrading capability of fungi with interesting ecologies have been obtained through genome sequencing (6–15) together with transcriptome and proteome analyses (16–18). However, only by combining these omics data with biochemical characteristics of the enzymes can we complete our understanding of the plant cell wall polysaccharide degradation ability of basidiomycete fungi.
This review explores the enzymatic potential of basidiomycetes from different biotopes and focuses on their ability to depolymerize cellulose, hemicelluloses, and pectin. The basidiomycetes are compared to species belonging to Aspergillus, which is one of the most extensively studied ascomycete genera, to dissect differences in their strategies for plant polysaccharide degradation. While there is also a large diversity among the ascomycete fungi, the aspergilli are among the few ascomycetes that have been studied with respect to the degradation of all plant polysaccharides (19). First, a comparison of the putative CAZyme-encoding genes found in the genomes of wood- and litter-decomposing basidiomycetes, plant pathogens, and ectomycorrhizal (ECM) fungi gives insight into their plant cell wall polysaccharide-degrading enzyme potential. Second, previously characterized CAZymes isolated from basidiomycetes are compared to those from genomic studies. Finally, the so far poorly addressed regulatory mechanisms of basidiomycetes in plant cell wall degradation are reviewed.
PLANT CELL WALL POLYSACCHARIDES
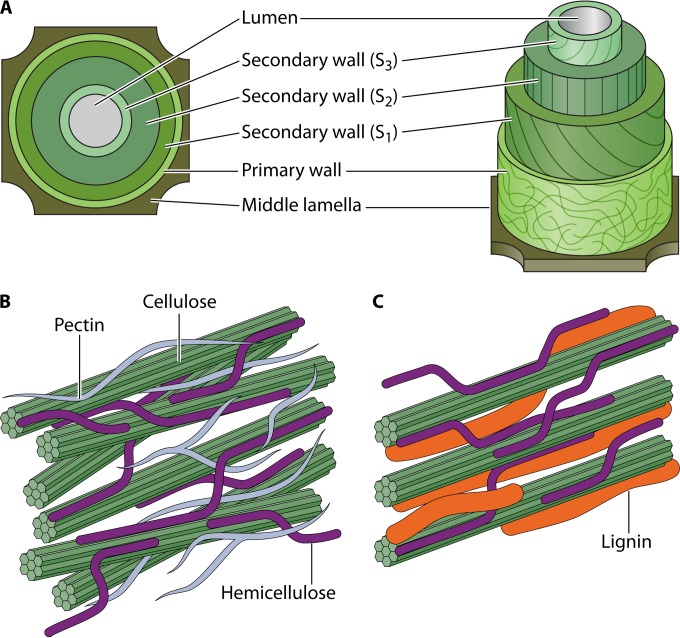
The three most important polysaccharide building blocks of plant cell walls are cellulose, hemicellulose, and pectin. Together with lignin, an aromatic heteropolymer, they form a degradation-resistant and functional complex that provides rigidity and structure to the plant and protects the cells from microbial attack. The plant cell wall consists of three main layers: the middle lamella and the primary and secondary walls (Fig. 1A) (20, 21). Each of these layers has a unique structure and chemical composition that also differ strongly between plant species, tissues, and the growth phase of the plant (Fig. 1B and C).
FIG 1.
Simplified model of plant cell wall structure. (A) The structure consists of three main layers: the middle lamella and the primary and secondary walls. (A and B) The main polysaccharides and lignin which form the surrounding structure for the plasma membrane are presented in the primary (B) and secondary wall (C). The lignin content in the primary cell wall (not illustrated) varies considerably depending on the plant species (Table 1). The illustrations are not to scale.
The major differences in the chemical compositions of softwood (e.g., pine and spruce) and hardwood (e.g., birch, aspen, and oak) are in the structure and content of hemicelluloses (Table 1). Hemicelluloses in softwood consist mainly of galactoglucomannans, whereas the majority of hardwood hemicelluloses are glucuronoxylans (Table 1) (20). On average, softwood has higher lignin content than hardwood, while the amount of cellulose in softwood is smaller than that in hardwood (Table 1) (20).
TABLE 1.
Approximate chemical compositions of softwood, hardwood, monocot, and dicot plant cell wallsa
| Plant material | Chemical composition (% dry wt)b |
||||||
|---|---|---|---|---|---|---|---|
| Cellulose | Hemicelluloses |
Pectin | Lignin | ||||
| Mannan | Xylan | β-Glucan | Xyloglucan | ||||
| Softwood | 33–42 | 10–15 | 5–11 | — | — | — | 27–32 |
| Hardwood | 38–47 | 2–5 | 15–30 | — | — | — | 21–31 |
| Monocots | |||||||
| Primary | 20–30 | Minor | 20–40 | 10–30 | 1–5 | 5 | Minor |
| Secondary | 35–45 | Minor | 40–50 | Minor | Minor | Minor | 20 |
| Dicots | |||||||
| Primary | 15–30 | 5–10 | 5 | ND | 20–25 | 20–30 | Minor |
| Secondary | 45–50 | 3–5 | 20–30 | ND | Minor | Minor | 7–10 |
The chemical compositions of cell walls in flowering plants also vary (Table 1). Monocots, i.e., grasses, are considered the most important renewable-energy crops, and their primary cell wall consists mainly of cellulose and hemicelluloses, whereas their secondary walls contain larger amounts of cellulose, a different composition of hemicelluloses, and significant amounts of lignin (Table 1) (22). The primary cell walls of dicots differ from those of grasses by their low xylan and high xyloglucan and mannan contents (Table 1) (22). In addition, the amount of pectin is notably larger in dicots than in grasses (Table 1). The secondary wall of dicots is composed of cellulose, hemicelluloses, and lignin (Table 1) (22).
Cellulose
Cellulose, found in both the primary and secondary cell walls, is the most abundant polysaccharide in plant matter (40 to 45% dry weight) and gives the plant cell wall its rigid structure (20). Repeating units of β-1,4-linked d-glucose form linear cellulose chains, which are held together by intermolecular hydrogen bonds and create linear crystalline structures (microfibrils) (23) and less crystalline, amorphous regions. The ratio of crystalline to amorphous regions varies between the layers of primary and secondary cell walls as well as between plant species. Cellulose microfibrils are more irregularly ordered in the outer layer than in the inner layer of the primary cell wall, where they are perpendicularly oriented (Fig. 1). Furthermore, the angles and directions of the cellulose microfibrils vary among the three sublayers (sublayer 1 [S1] to S3) of the secondary plant cell wall (20, 21).
Hemicellulose
Hemicelluloses (20 to 30% plant dry weight) support the structure of the cellulose microfibrils in the primary and secondary walls of plant cells (20). There are four types of amorphous hemicellulose structures with different main monosaccharide units in their hemicellulose backbone. Xylan is the most common hemicellulose polymer with a β-1,4-linked d-xylose backbone. Other hemicelluloses are xyloglucan (β-1,4-linked d-glucose), found mainly in the primary walls; β-glucan (β-1,3;1,4-linked d-glucose); and mannan (β-1,4-linked d-mannose) (21). Xylan, xyloglucan, and mannan backbones are decorated with branched monomers and short oligomers consisting of d-galactose, d-xylose, l-arabinose, l-fucose, d-glucuronic acid, acetate, ferulic acid, and p-coumaric acid that are cleaved by debranching enzymes (24).
Pectin
Pectin is a noncellulosic polysaccharide containing galacturonic acid that provides additional cross-links between the cellulose and hemicellulose polymers. It is found mainly in plant primary cell walls and middle lamella (25). The pectin concentration in the middle lamella is high at an early stage of plant growth, but the concentration decreases during lignification (20). The simplest pectin structure is homogalacturonan (HG), which is a linear polymer of α-1,4-linked d-galacturonic acid residues that can be methylated at the C-6 carboxyl group and acetylated at the O-2 or O-3 position. Xylogalacturonan (XGA) is a substituted galacturonan that has β-1,3-linked d-xylose residues attached to the galacturonic acid backbone. The second substituted galacturonan is rhamnogalacturonan II (RG-II). The structure of RG-II is more complex than the structure of XGA. Altogether, 12 different glycosyl residues, e.g., 2-O-methyl xylose, 2-O-methyl fucose, aceric acid, 2-keto-3-deoxy-d-lyxo heptulosaric acid, and 2-keto-3-deoxy-d-manno-octulosonic acid, can be attached to the galacturonic acid backbone (25). The most complex pectin structure, rhamnogalacturonan I (RG-I), has a backbone of alternating d-galacturonic acid and l-rhamnose residues, with branching structures consisting of d-galactose and l-arabinose chains attached to the l-rhamnose residues.
ENZYMES MODIFYING PLANT POLYSACCHARIDES
An overview of the known fungal plant-polysaccharide-degrading or -modifying enzymes is presented in Table 2. The enzymes are divided according to their substrates, and their EC numbers, abbreviations, and corresponding CAZyme families (2) are also shown.
TABLE 2.
Plant-polysaccharide-degrading enzymes
| Substrate | Enzyme activity | EC no.a | Abbreviation | CAZyme family(ies) |
|---|---|---|---|---|
| Cellulose | β-1,4-Endoglucanase | 3.2.1.4 | EG | GH3, -5, -6, -7, -9, -12, -45 |
| Cellobiohydrolase (reducing end) | 3.2.1.176 | CBHI | GH7 | |
| Cellobiohydrolase (nonreducing end) | 3.2.1.91 | CBHII | GH6 | |
| β-1,4-Glucosidase | 3.2.1.21 | BGL | GH1, -3 | |
| Cellobiose dehydrogenase | 1.1.99.18 | CDH | AA3_1, AA8 | |
| Lytic polysaccharide monooxygenase | NA | LPMO | AA9 | |
| Xylan | β-1,4-Endoxylanase | 3.2.1.8 | XLN | GH10, -11 |
| Xylobiohydrolase | 3.2.1.– | XBH | ||
| β-1,4-Xylosidase | 3.2.1.37 | BXL | GH3, -43 | |
| Galactomannan | β-1,4-Endomannanase | 3.2.1.78 | MAN | GH5, -26 |
| β-1,4-Mannosidase | 3.2.1.25 | MND | GH2 | |
| β-1,4-Galactosidase | 3.2.1.23 | LAC | GH2, -35 | |
| α-1,4-Galactosidase | 3.2.1.22 | AGL | GH27, -36 | |
| α-Arabinofuranosidase | 3.2.1.55 | ABF | GH51, -54 | |
| Galactomannan acetyl esterase | 3.1.1.– | GMAE | ||
| Xyloglucan | Xyloglucan β-1,4-endoglucanase | 3.2.1.151 | XEG | GH12, -74 |
| α-Arabinofuranosidase | 3.2.1.55 | ABF | GH51, -54 | |
| α-Xylosidase | 3.2.1.177 | AXL | GH31 | |
| α-Fucosidase | 3.2.1.51 | AFC | GH29, -95 | |
| α-1,4-Galactosidase | 3.2.1.22 | AGL | GH27, -36 | |
| β-1,4-Galactosidase | 3.2.1.23 | LAC | GH2, -35 | |
| Arabinoxylan | Arabinoxylan arabinofuranohydrolase/arabinofuranosidase | 3.2.1.55 | AXH | GH62 |
| α-Glucuronidase | 3.2.1.139 | AGU | GH67, -115 | |
| α-1,4-Galactosidase | 3.2.1.22 | AGL | GH27, -36 | |
| β-1,4-Galactosidase | 3.2.1.23 | LAC | GH2, -35 | |
| Acetyl xylan esterase | 3.1.1.72 | AXE | CE1, -5 | |
| Feruloyl esterase | 3.1.1.73 | FAE | CE1 | |
| Pectin | Endopolygalacturonases | 3.2.1.15 | PGA | GH28 |
| Exopolygalacturonases | 3.2.1.67 | PGX | GH28 | |
| Xylogalacturonan hydrolase | 3.2.1.– | XGH | ||
| Endorhamnogalacturonase | 3.2.1.171 | RHG | GH28 | |
| Exorhamnogalacturonase | 3.2.1.– | RHX | GH28 | |
| Rhamnogalacturonan rhamnohydrolase | 3.2.1.174 | RGXB | GH28 | |
| α-Rhamnosidase | 3.2.1.40 | RHA | GH78 | |
| α-Arabinofuranosidase | 3.2.1.55 | ABF | GH51, -54, -62 | |
| Endoarabinanase | 3.2.1.99 | ABN | GH43 | |
| Exoarabinanase | 3.2.1.– | ABX | GH93 | |
| β-1,4-Endogalactanase | 3.2.1.89 | GAL | GH53 | |
| Unsaturated glucuronyl hydrolase | 3.2.1.– | UGH | GH88 | |
| Unsaturated rhamnogalacturonan hydrolase | 3.2.1.172 | URH | GH105 | |
| β-1,4-Xylosidase | 3.2.1.37 | BXL | GH3, -43 | |
| β-1,4-Galactosidase | 3.2.1.23 | LAC | GH2, -35 | |
| Pectin lyase | 4.2.2.10 | PEL | PL1 | |
| Pectate lyase | 4.2.2.2 | PLY | PL1, -3, -9 | |
| Rhamnogalacturonan lyase | 4.2.2.23 | RGL | PL4, -11 | |
| Pectin methyl esterase | 3.1.1.11 | PME | CE8 | |
| Pectin acetyl esterase | 3.1.1.– | PAE | ||
| Rhamnogalacturonan acetyl esterase | 3.1.1.– | RGAE | CE12 | |
| Feruloyl esterase | 3.1.1.73 | FAE | CE1 |
NA, not categorized by the International Union of Biochemistry and Molecular Biology (IUBMB).
Cellulose Degradation
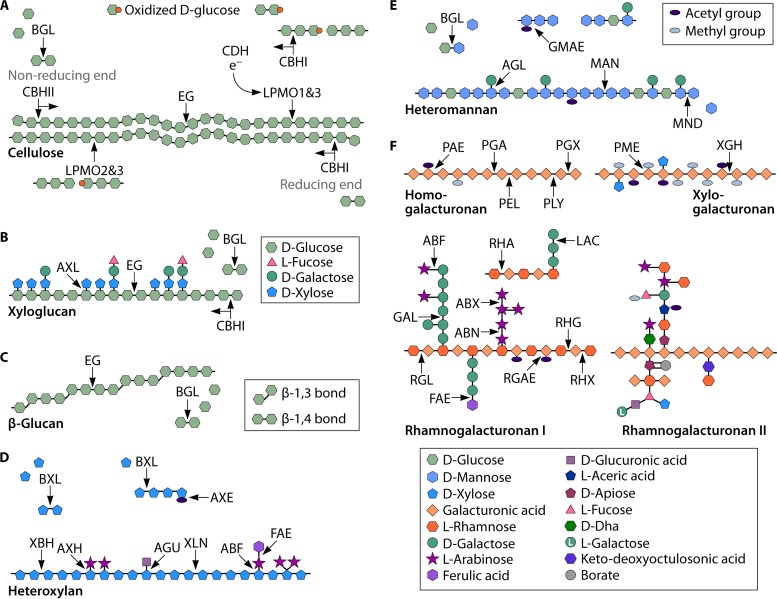
The main enzymes that hydrolyze cellulose, so-called classical cellulases, are endoglucanases, exoglucanases, and β-glucosidases (BGLs). β-1,4-Endoglucanase (EG) (EC 3.2.1.4) cleaves within the cellulose chains to release glucooligosaccharides (Fig. 2A). Exoglucanases or cellobiohydrolases (CBHs) release cellobiose from the end of the cellulose chains. The two types of cellobiohydrolases, CBHI and CBHII (EC 3.2.1.176 and EC 3.2.1.91, respectively), degrade cellulose from either the reducing or the nonreducing end, respectively, with different processivities, i.e., the efficiency of the sequential hydrolysis of the β-1,4-glycosidic bonds by the cellulase before the dissociation of the enzyme from the substrate (26). BGL (EC 3.2.1.21) releases the smallest unit, glucose, from shorter oligosaccharides.
FIG 2.
Schematic representation of plant cell wall polysaccharides and selected corresponding polysaccharide-degrading enzymes. (A) Cellulose; (B) xyloglucan; (C) β-glucan; (D) heteroxylan; (E) heteromannan; (F) pectin. Enzyme abbreviations are presented in Table 2. Polysaccharide structures were drawn by using data reported previously by Mohnen (203) and Doblin et al. (204).
Recently, oxidoreductive cleavage of the cellulose chain has been reported. Cellobiose dehydrogenase (CDH) (EC 1.1.99.18) and lytic polysaccharide monooxygenases (LPMOs) participate in cellulose degradation in combination with cellulases (Fig. 2A) (27, 28). CDH is the only known extracellular flavocytochrome that oxidizes cellobiose and cellooligosaccharides to the corresponding lactones (29, 30). The exact role of CDH in lignocellulose degradation is still unclear, although there is evidence of its relevance in both the cellulolytic and lignin-modifying machinery of fungi (29, 30). The ability of CDH to produce hydroxyl radicals through Fenton chemistry supports its role in lignin modification, while oxidation of cellobiose together with the production of electrons for LPMO-catalyzed cellulose depolymerization demonstrate the participation of CDH in the degradation of cellulose (29, 31, 32).
LPMOs are copper monooxygenases that catalyze the direct oxidation of the cellulose chain leading to cleavage of the glycosidic bond (28, 31, 32). Moreover, fungal LPMOs can be divided into at least three classes according to their sequence similarity and specific activities toward cellulose (33). Type 1 LPMOs catalyze oxidation of the glucose unit at the C-1 position, resulting in the formation of aldonic acids at the reducing end of the cellulose chain (28, 32). Type 2 LPMOs generate ketosugars at the nonreducing end of the cellulose chain by oxidizing at the C-4 position (34). LPMOs of type 3 are not as specific as type 1 or 2 enzymes, and they are able to oxidize both positions (32). Oxidation at C-6 has also been proposed (28). The reaction catalyzed by LPMOs requires an electron donor to reduce copper II to copper I in the active site of the enzyme and molecular oxygen to form the copper-oxygen complex, which is capable of oxidizing the glycosidic bond (35). In addition to the above-mentioned CDH, other naturally occurring electron donors for LPMOs have been proposed, e.g., gallic acid or lignin (28, 36). Also, several compounds, e.g., ascorbic acid, have been shown to act as reductants in LPMO catalysis in vitro (28, 34).
Hemicellulose Degradation
Due to variable structures, a specific set of CAZymes is needed to degrade the backbone and branching structures of each hemicellulose (Fig. 2B to E) (37). The xylan backbone is cleaved by β-1,4-endoxylanase (XLN) (EC 3.2.1.8) into shorter oligomers (Fig. 2D). A xylobiohydrolase that hydrolyzes xylan into xylobiose has also been described (38). β-1,4-Xylosidase (BXL) (EC 3.2.1.37) hydrolyzes xylobiose into its monomeric units and also releases d-xylose from larger xylooligosaccharides from the nonreducing terminus (24, 39). The xyloglucan backbone, the structure of which is similar to that of cellulose, is hydrolyzed by EGs, CBHs, and BGLs (Fig. 2B) (24). β-Glucan can be degraded by EGs into oligosaccharides (Fig. 2C). The β-1,4-linked d-mannose backbone of mannan is cleaved by β-1,4-endomannanase (MAN) (EC 3.2.1.78) to mannooligosaccharides (Fig. 2E). β-1,4-Mannosidase (MND) (EC 3.2.1.25) releases d-mannose from the terminal ends of mannan (24). In addition, BGL acts on the galactoglucomannan backbone.
The enzymatic oxidative cleavage of hemicelluloses was recently confirmed (40). First, the ability of CDH to accept electrons from xylooligosaccharides and interact with various LPMOs was detected, suggesting that these enzymes are able to act on hemicelluloses (41). Recently, LPMO9C of the ascomycete fungus Neurospora crassa was shown to cleave xyloglucan, β-glucan, and, to a lesser extent, glucomannan with ascorbic acid as a reductant (40).
Pectin Degradation
Endopolygalacturonases (PGAs) (EC 3.2.1.15) and exopolygalacturonases (PGXs) (EC 3.2.1.67) act within and at the terminal end of the α-1,4-linked d-galacturonic acid polymer, respectively, releasing d-galacturonic acid from the homogalacturonan backbone (Fig. 2F). Xylogalacturonan is cleaved specifically by xylogalacturonan hydrolases (XGHs) (EC 3.2.1.–). The backbone of rhamnogalacturonan I is hydrolyzed by exorhamnogalacturonase (RHX) (EC 3.2.1.–), endorhamnogalacturonase (RHG) (EC 3.2.1.171), rhamnogalacturonan rhamnohydrolase (RGXB) (EC 3.2.1.174), and α-rhamnosidase (RHA) (EC 3.2.1.40) (19, 24).
Pectin lyase (PEL) (EC 4.2.2.10), pectate lyase (PLY) (EC 4.2.2.2), and rhamnogalacturonan lyase (RGL) (EC 4.2.2.23) also cleave the pectin backbone, using a β-elimination mechanism. Lyases have different sensitivities to the acetylations (O-2 or O-3) or methyl esterifications (O-6) of the d-galacturonic acid backbone. In contrast to pectate lyases, pectin lyases prefer substrates with a high degree of methyl esterification. Rhamnogalacturonan lyases favor nonacetylated substrates (19, 24).
Debranching Enzymes
The enzymes described above cleave the main chains of cellulose and the backbone and branches of hemicellulose and pectin. However, smaller side branches extending from hemicellulose and pectin require a different set of CAZymes. The debranching enzymes (also known as accessory enzymes) α-d-xylosidase (AXL) (EC 3.2.1.177), α-l-arabinofuranosidase (ABF) (EC 3.2.1.55), arabinoxylan arabinofuranohydrolase (AXH), endoarabinase (ABN), exoarabinase (ABX), α-d-galactosidase (AGL) (EC 3.2.1.22), β-d-galactosidase (LAC) (EC 3.2.1.23), endogalactanase (GAL) (EC 3.2.1.89), exogalactanase (EC 3.2.1.–), α-glucuronidase (AGU) (EC 3.2.1.139), feruloyl esterase (FAE) (EC 3.1.1.73), p-coumaroyl esterase (pCAE) (EC 3.1.1.–), acetyl xylan esterase (AXE) (EC 3.1.1.72), galactomannan acetyl esterase (GMAE) (EC 3.1.1.–), rhamnogalacturonan acetyl esterase (RGAE) (EC 3.1.1.–), pectin acetyl esterase (PAE) (EC 3.1.1.–), and pectin methyl esterase (PME) (EC 3.1.1.11) work synergistically with the main-chain-depolymerizing enzymes to degrade plant polysaccharides (19).
BASIDIOMYCETE GENOMES AND PLANT POLYSACCHARIDE DEGRADATION
To date, an increasing number of basidiomycete genomes have been sequenced and annotated to understand fungal physiology and, in several cases, to search for enzymes of interest that could be of use in industrial applications (Table 3) (42). These fungi inhabit a wide range of ecological niches and colonize various growth substrates, such as conifers, deciduous trees, forest litter, crops, grassland soils, and roots of plants. Differences in the CAZyme sets can often be linked to fungal habitat. For example, the wood-decaying white rot fungus Phanerochaete chrysosporium has a larger repertoire of plant cell wall polysaccharide-degrading enzymes than the biotrophic phytopathogen Ustilago maydis, which possesses a minimal set of CAZyme-encoding genes in order to prevent host plant defense responses, as suggested in previous studies (6, 8). While it cannot be automatically concluded that an increase in the number of genes related to a particular polysaccharide also means an improved degradation of this polysaccharide, many studies have revealed such correlations (43–50). However, there are also clear exceptions to this. The most noteworthy exception is the ascomycete Hypocrea jecorina (anamorph Trichoderma reesei), which is a very efficient cellulose degrader but contains a relatively small number of cellulase-encoding genes in each genome. Its strategy appears to have focused on high production levels of a limited set of enzymes rather than expanding its enzyme repertoire (51). This approach appears to be used by only a minority of fungi, based on an extensive correlation analysis between genome content and growth on plant biomass substrates of >150 fungal species (R. P. de Vries, A. Wiebenga, M. Zhou, P. M. Coutinho, and B. Henrissat, unpublished data).
TABLE 3.
List of basidiomycete species with published genomes and CAZyme annotations
Wood-Rotting Fungi
Wood-rotting fungi are traditionally divided into white rot and brown rot fungi according to the modification that they cause to wood residue during decay. White rot fungi degrade both lignin and wood polysaccharides (cellulose and hemicelluloses) so that the residual wood is white or yellowish, moist, soft, and often fiber-like. More than 90% of all known wood-rotting basidiomycetes are of the white rot type (52), and they are found more commonly on angiosperm than on gymnosperm wood species in nature. Brown rot fungi degrade wood to yield brown, typically cubical cracks that are easily broken down. Less than 10% of all known wood-decaying basidiomycete species are classified into this group, which occurs most often on gymnosperm wood (53). Interestingly, the analyzed genome sequence data show that many cellulases of wood-rotting basidiomycetes lack the cellulose binding modules (CBMs) generally considered essential for efficient cellulose hydrolysis (54). More sequence data are needed to clarify possible ecological and evolutionary advantages for the occurrence of CBM-less cellulases and other polysaccharide-degrading enzymes in nature.
Genome information indicates that brown rot fungi evolved several times from ancestor white rot species (11). Thus, individual brown rot species may have different sets of characteristics left, which makes this group rather heterogeneous, and some of them resemble white rot fungi. Genome studies of wood-inhabiting basidiomycetes show that there is a need for a more detailed classification of the rot types, since some fungi, e.g., Botryobasidium botryosum and Jaapia argillacea, do not fulfill the traditional criteria for dichotomous grouping (55). However, it has been suggested that the definition “white rot” should be reserved for those fungi that degrade all cell wall polymers through the action of the lignin-modifying peroxidases and have enzymes capable of attacking crystalline cellulose (55).
White rot fungi.
White rot fungi are efficient degraders of the aromatic polymer lignin and cause a characteristic white appearance on degraded wood (56). White rot fungi also have the most extensive arsenal of putative CAZymes among the basidiomycetes (Table 4), allowing them to colonize a wide range of plants, from pine trees to poplars and grapevines (11). White rot fungi make up the majority of wood-rotting basidiomycetes, and the most intensively studied species are commonly isolated from hardwoods (56), which have slightly higher cellulose and hemicellulose (glucomannan and glucuronoxylan) contents than do softwoods (Table 1) (20).
TABLE 4.
Distribution of CAZyme-encoding genes in basidiomycetes and Aspergillus speciesd
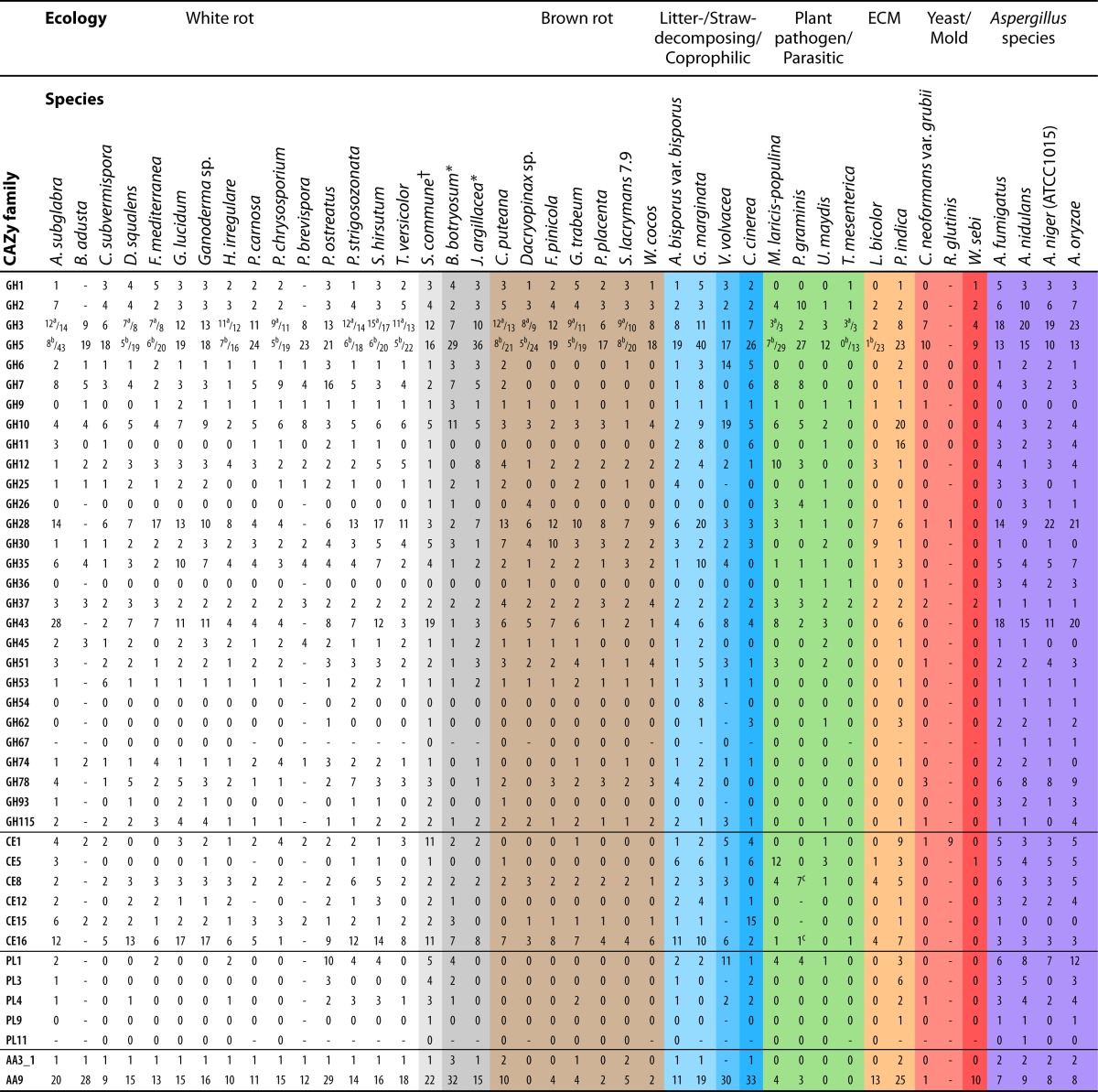
No β-N-acetylhexosaminidase was included.
β-1,4-Endoglucanase and β-1,4-endomannanase are included.
Can also include models associated with more than one category.
Gene numbers are based on previously reported data for the following organisms, and basidiomycete data are updated according to Riley et al. (55): Agaricus bisporus var. bisporus (9), Aspergillus fumigatus (208), Aspergillus nidulans (49, 209), Aspergillus niger (ATCC 1015) (84, 210), Aspergillus oryzae (211), Auricularia subglabra (11), Bjerkandera adusta (205), Botryobasidium botryosum (55), Ceriporiopsis subvermispora (12), Coniophora puteana (11), Coprinopsis cinerea (206), Cryptococcus neoformans var. grubii (81), Dacryopinax sp. (11), Dichomitus squalens (11), Fomitiporia mediterranea (11), Fomitopsis pinicola (11), Galerina marginata (55), Ganoderma lucidum (14), Ganoderma sp. (205), Gloeophyllum trabeum (11), Heterobasidion irregulare (66), Jaapia argillacea (55), Laccaria bicolor (7), Melampsora laricis-populina (10), Phanerochaete carnosa (45), Phanerochaete chrysosporium (6), Phlebia brevispora (205), Piriformospora indica (77), Pleurotus ostreatus (55), Postia placenta (18), Puccinia graminis (10), Punctularia strigosozonata (11), Rhodotorula glutinis (82), Schizophyllum commune (15), Serpula lacrymans 7.9 (71), Stereum hirsutum (11), Trametes versicolor (11), Tremella mesenterica (11), Ustilago maydis (8), Wallemia sebi (207), Wolfiporia cocos (11), and Volvariella volvacea (13). †, white rot-like; *, ecological classification uncertain; —, not in published papers.
Based on the sequenced genomes (Table 3), the white rot basidiomycetes harbor an extensive set of genes encoding putative cellulolytic enzymes. Genes encoding GH family 6 (GH6) and GH7 enzymes, which include mainly cellulose-hydrolyzing CBHs, are typically present with 1 to 7 copies in all white rot fungal species sequenced so far (Table 4). As an exception, Pleurotus ostreatus harbors 16 putative GH7-encoding genes (Table 4). Several genes from GH3 and GH5 (6 to 17 and 16 to 43 genes, respectively) (Table 4), which encode other putative cellulolytic enzymes, such as BGLs and EGs, occur in all white rot fungi. White rot fungi also possess a large set of genes encoding putative hemicellulose- and pectin-active enzymes from various CAZyme families. On average, they have more copies of genes from GH families 10 and 11 (xylan related), 28 (pectin related), 43 (xylan and pectin related), and 74 (xyloglucan related) and carbohydrate esterase (CE) families 1 (xylan related) and 12 (pectin related) than other wood-rotting and litter-decomposing basidiomycetes (Table 4). Genes belonging to polysaccharide lyase (PL) families PL3, -9, and -11 are almost absent, while some species have few representatives in PL1 and -4. Notably, high numbers of gene copies in PL1 were annotated for P. ostreatus (Table 4). For the oxidoreductases involved in plant polysaccharide degradation, white rot fungi possess typically 1 copy of a CDH (families AA3_1 and AA8)-encoding gene and up to 29 copies of LPMO (AA9)-encoding genes. In this respect, J. argillacea resembles white rot fungi, as it harbors similar numbers of genes encoding CDH and LPMOs (Table 4). Interestingly, B. botryosum has more genes encoding CDHs and LPMOs than any white rot fungus sequenced so far (55).
The first basidiomycete genome sequenced is the model white rot fungus P. chrysosporium (6, 57). Its CAZyme content shows many similarities to the genomes of other white rot basidiomycetes by carrying, for instance, several genes that encode putative cellulose-hydrolyzing enzymes (EGs, CBHs, and BGLs) (Table 4), which enables it to completely degrade cellulose (6). P. chrysosporium secretes CBHI, CBHII, EGs, and BGL when grown on microcrystalline cellulose (Avicel) (58). As these cellulases were not found in P. chrysosporium under ligninolytic culture conditions, they do not seem to be constitutively produced (57). In Avicel cultures of P. chrysosporium, the expression of oxidatively polysaccharide-degrading CDH- and putative LPMO-encoding genes was detected together with the expression of genes encoding classical cellulases (17). P. chrysosporium is also able to degrade hardwood hemicelluloses into their building blocks (6). Genes encoding hemicellulolytic and pectinolytic enzymes (e.g., GH10 xylanase, a putative GH28 exopolygalacturonase, and a putative CE1 acetyl xylan esterase) were expressed, and the corresponding proteins were secreted in both Avicel and carbon-limited liquid cultures, suggesting constitutive expression of the corresponding genes (17, 57, 58).
Only a limited number of pectinolytic genes are present in the genome of P. chrysosporium. For example, pectin/pectate lyase-, exoarabinanase-, or rhamnogalacturonan hydrolase-encoding genes were not detected (6). Despite this low pectinolytic potential, P. chrysosporium is able to grow on solid cultures of pectin substrates with a high degree of methyl esterification, such as soy, apple, and lemon pectins, possibly producing endopolygalacturonase together with galactan- and arabinan-hydrolyzing 1,4-β-endogalactanase (GH53), β-galactosidase (GH35), and α-arabinofuranosidase (GH51) (44). However, poor growth on rhamnogalacturonan and polygalacturonic acid was observed (44).
Several studies comparing the plant-polysaccharide-degrading ability of P. chrysosporium to those of other basidiomycetes have been conducted. The selective white rot fungus Ceriporiopsis (Gelatoporia) subvermispora, which depolymerizes mainly lignin and hemicelluloses and leaves cellulose almost intact, has a GH family distribution similar to that of P. chrysosporium. However, some key differences between these fungi can be pointed out. C. subvermispora possesses fewer GH3 (including BGL)-encoding genes, with only six copies in the genome (12), while P. chrysosporium and the other sequenced white rot species harbor at least 8 genes (Table 4). Also, modest transcript levels for the genes from GH5, -6, -7, and -12 were observed during the growth of C. subvermispora on semisolid aspen wood cultures compared to those observed during the growth of P. chrysosporium, suggesting a significant reduction in the expression levels of putative cellulase-encoding genes by the selective white rot fungus. This shortage and low-level expression of cellulase genes are compensated by a greater dependence on oxidoreductases, which is in line with the growth pattern of C. subvermispora showing preference for lignin depolymerization (12). C. subvermispora grows better on pectin and guar gum (galactomannan) than on cellulose (12). In fact, C. subvermispora has more endopolygalacturonase (GH28)-encoding genes (six) than P. chrysosporium (four), but significant differences in the amounts of other pectinolytic genes between these two white rot species were not detected.
Phanerochaete carnosa, a member of the same genus as P. chrysosporium, is found on softwoods, while most other studied white rot fungi are typically isolated from hardwood (45). The chemical compositions of the cell walls of softwoods and hardwoods differ particularly in their hemicelluloses structures (mainly galactoglucomannans are present in softwood, while glucuronoxylan is the most abundant hemicellulose in hardwood) and in the slightly higher lignin contents of softwoods (20). The genome of P. carnosa contains 193 GH gene models, which is higher than the number of gene models in the genome of P. chrysosporium (182 gene models) (45). When the secretome of P. carnosa grown in cellulose and spruce wood cultures was analyzed, the fungus produced a pattern of classical cellulases (GH3 EGs and BGLs and GH6 and -7 CBHs), xylanases (GH10 and -11), debranching hydrolases (GH43), and glucuronoyl esterases (CE1) together with putative LPMOs (AA9) that was similar to the pattern produced by P. chrysosporium (59). Interestingly, a GH2 β-mannosidase, which was not detected by proteomic analyses in cellulose or wood cultures of P. chrysosporium (17, 57), was present in cellulose-containing cultures of P. carnosa (59). Also, peptides corresponding to a GH5 mannanase were identified in cellulose cultures of P. carnosa. In addition, P. carnosa grows better (based on radial growth and mycelium density) on guar gum (galactomannan) than on xylan- and pectin-containing substrates (45), thus supporting its preference for softwood bioconversion. Biochemical characterization of P. carnosa hemicellulases is still needed to confirm a correlation between growth profiles and enzyme substrate specificities.
Another white rot fungus isolated mainly from softwood, e.g., western yellow pine (Pinus ponderosa) and old coniferous trunks (60), Dichomitus squalens, has a CAZyme repertoire typical of white rot species (11). It is able to grow on cellulose-, pectin-, and lignin-containing minimal media, and it shows better growth on galactomannan than on xylan. Together with Fomitiporia mediterranea, it lacks the CE1 genes encoding putative xylan- and pectin-debranching enzymes. D. squalens also shows a decreased ability to grow on pectin than on d-glucose, which is in contrast to the majority of the species studied so far (11). A recent study shows that the genes encoding CBHs, LPMOs, and CDH are coexpressed when D. squalens grows on spruce wood and in microcrystalline cellulose (Avicel)-containing cultures. Moreover, the simultaneous expression of the cdh and lpmo genes emphasizes the role of oxidative degradation of cellulose together with hydrolytic cellulases in white rot fungi (61).
Ganoderma lucidum is a wood-decaying white rot species and a model medicinal fungus traditionally used in Asia. It produces a large variety of bioactive compounds, thus harboring potential for medical applications (14). G. lucidum possesses a relatively large number of genes encoding putative CAZymes, including 288 GHs, compared to other white rot basidiomycetes with all the major cellulose-, hemicellulose-, and pectin-degrading genes (14, 62). Similar to most white rot fungi, its genome lacks the genes for putative pectin lyase, pectate lyase, and rhamnogalacturonan lyase (PL1, -3, -9, and -11) (14).
Based on morphological features, Auricularia subglabra belongs to a group of so-called jelly fungi. A. subglabra is found on dead and decaying wood, where it causes white rot (11). Compared to the genomes of other white rot species, the genome of A. subglabra (formerly deposited as Auricularia delicata in the JGI database) harbors a large number of GH43 and CE16 genes, which include putative β-1,4-xylosidase-, endoarabinanase-, α-l-arabinofuranosidase-, and acetylesterase-encoding activities. Cross sections of colonized wood demonstrate the ability of A. subglabra to extensively degrade all the main polymers of the wood cell wall (11). However, it lacks specific xylan side-chain-hydrolyzing enzymes, such as arabinoxylan arabinofuranohydrolases (11).
Schizophyllum commune is a model basidiomycete for mushroom development (15). It has been classified as a white rot fungus, although it has a limited lignin-degrading capacity and therefore does not correspond to the typical characteristics of white rot species. Instead, S. commune has one of the most extensive cellulose- and hemicellulose-degrading enzyme sets, and each fungal CAZyme family related to plant biomass degradation is represented in its genome (Table 4) (15). S. commune is found mainly on fallen hardwood, but it also colonizes softwood and grass silage. S. commune is rich in GH43 enzyme-encoding genes, which include β-1,4-xylosidase and endoarabinanase, and genes encoding xylan- and pectin-degrading enzymes. Another uncommon characteristic of S. commune is the wealth of putative pectin-degrading lyases (PL1, -2, and -4), which correlates with high-level pectinase production (15, 63). This is consistent with the strategy of S. commune to invade adjacent parenchymatic cells in plant xylem tissue through pectin-surrounded simple and bordered pits (15).
The dual life-style of the necrotrophic white rot fungus and economically important forest pathogen Heterobasidion irregulare (formerly known as H. annosum, intersterility group P [64]) involves pathogenic and saprobic life-styles, which are reflected in its genome and transcriptome (65). Similar to saprobes, it has all the enzymes for digesting cellulose/xyloglucan (GH5, -6, -7, -12, -27, -29, -45, and -74) and pectin (GH28, -43, -51, -53, -78, and -105; PL1 and -4; and CE8 and -12). However, the whole CAZyme arsenal is used only during the saprobic growth phase of H. irregulare, while fewer CAZyme-encoding genes are expressed during the pathogenic phase (66). This shows that H. irregulare has the ability to extensively degrade plant material, but the fungus uses its full CAZyme repertoire only when it becomes less dependent on its living host (66). Other plant-pathogenic basidiomycetes are discussed in “Plant-Pathogenic Fungi and Mycoparasites,” below.
Brown rot fungi.
Brown rot fungi represent ∼6 to 7% of the known wood-rotting basidiomycetes and occur mostly on conifers (gymnosperms), which are softwoods (53). While brown rot fungi are able to efficiently and rapidly break down wood cellulose and hemicelluloses, they only modify lignin, mainly by demethoxylation, resulting in a characteristic brown residue of decayed wood (56). In contrast to the enzymatic approach of white rot fungi (6, 67), brown rot fungi initiate cellulose breakdown with highly reactive oxidants, such as low-molecular-weight free radicals, including the hydroxyl radicals formed through the Fenton reaction (68, 69). The difference in cellulose-depolymerizing abilities between white and brown rot fungi is probably a result of multiple evolutionary steps that have led to these two different life-styles (11). This can be seen, for example, by the loss of lignin-modifying peroxidases, which has been proposed to have occurred several times, resulting in the divergence of brown rot fungi in the orders Polyporales (e.g., Fomitopsis pinicola, Postia placenta, and the plant-parasitic brown rot fungus Wolfiporia cocos) and Boletales (e.g., Coniophora puteana and Serpula lacrymans) and species Gloeophyllum trabeum and Dacryopinax sp. (11).
A comparison of the representatives of the different CAZyme families in each plant-biomass-modifying basidiomycete group indicates that the brown rot fungi studied up to now possess a significantly smaller set of plant-polysaccharide-depolymerizing enzymes than white rot and litter-decomposing fungi (Table 4). The most obvious reduction in the CAZymes of brown rot fungi can be seen in the small number of putative CBHs (GH6 and -7) (18, 70). Only the species of the order Boletales and closely related to ECM fungi, S. lacrymans and C. puteana, harbor one and four putative CBH-encoding genes, respectively. Also, the genome of Postia placenta lacks genes for CBHs and for carbohydrate binding modules from family 1 (CBM1) and contains only two putative β-1,4-endoglucanase-encoding genes (18). Although the genomes of brown rot fungi contain fewer genes encoding CDHs (AA3_1 and AA8) and LPMOs (AA9) than those of white rot fungi, it is possible that these putative oxidoreductases of brown rot fungi take part in enzymatic cellulose depolymerization (11, 18, 71). However, considering the overall lower number of LPMOs and greater variety in the absence and presence of CDH in brown rot fungi, this implies that their ability to utilize oxidized sugars is also more variable than in white rot fungi. When secretomes from semisolid aspen cultures of brown rot fungi were analyzed, only C. puteana and G. trabeum secreted a putative CDH and LPMO, respectively, while none of these proteins were detected in F. pinicola or W. cocos (11).
The substrate preference of brown rot basidiomycetes for softwoods can also be explained by the characteristics of their hemicellulose-degrading capacity. While hardwoods are known to have a higher proportion of xylan, softwoods have a higher mannan content. During the evolution of the brown rot fungal life-style, the number of genes encoding enzymes assigned to GH10 and -11 (endoxylanases) and CE15 was reduced (18, 70). Therefore, brown rot fungi have slightly lower numbers of xylanolytic enzymes than white rot fungi. In addition, the genomes of C. puteana, Dacryopinax sp., F. pinicola, P. placenta, and S. lacrymans lack genes encoding putative acetyl xylan or feruloyl esterases from CE1. Instead, brown rot fungi grow well on guar gum, which is a galactomannan similar in structure to softwood cell wall galactomannans (11). Several copies of genes encoding putative β-1,4-endomannanases involved in the degradation of mannan are present in the genomes of brown rot basidiomycetes, presumably helping them to colonize softwoods.
Litter- and Straw-Decomposing Fungi
Litter- and straw-decomposing basidiomycetes participate significantly in the Earth's carbon cycle, together with wood-decaying fungi. The genomes of the litter-decomposing fungus Agaricus bisporus and the straw-decomposing species Volvariella volvacea (Table 3) have been sequenced because of their importance as cultivated mushrooms and in recycling decaying plant matter. The genomes of two A. bisporus strains show similar gene contents with respect to plant polysaccharide degradation. The economically important white button mushroom A. bisporus var. bisporus originates from Europe, while A. bisporus var. burnettii grows on leaf litter in North America (9). Another edible fungus, V. volvacea, is widely cultivated in Asia, where it is grown on rice straw, cotton waste, and other agricultural by-products (13). In addition, the genome of another litter-decomposing species, Galerina marginata, was recently reported (55).
A. bisporus, G. marginata, and V. volvacea have a close evolutionary relationship with white rot basidiomycetes and ECM fungi (9, 55, 72), although their genome content resembles that of white rot rather than ECM genomes. All these fungi grow on partially decayed plant matter, have diverse sets of CAZymes, and are able to cause white rot (Table 4). Although litter- and straw-decomposing fungi and the ECM fungi are taxonomically closely related, their dissimilar ecological niches have resulted in different CAZyme repertoires (Table 4). Generally, saprobes are more capable of degrading plant polysaccharides than root symbionts. For example, the coprophilic fungus Coprinopsis cinerea and the litter decomposer G. marginata secrete a broader set of plant cell wall-degrading enzymes than the ECM fungus Amanita bisporigera (73).
Nonwoody plant tissues contain relatively large amounts of pectin (74). In accordance with this, some forest litter-decomposing basidiomycetes have been shown to produce pectinolytic enzymes (75). A. bisporus and G. marginata harbor two putative pectinolytic enzymes encoding genes from PL1, whereas V. volvacea possesses 11 PL1-encoding genes. Up to 5 CE1, 6 CE5, 3 CE8, 4 CE12, and 11 CE16 genes encoding putative carbohydrate esterases have been found in the genomes of these litter- and straw-decomposing fungi, while the CE1, -5, and -12 genes are missing from several white and brown rot fungal species (Table 4). While A. bisporus, G. marginata, and V. volvacea have a wide spectrum of CE genes, only one gene encoding a putative 4-O-methyl-glucuronoyl methyl esterase (CE15) has been detected in A. bisporus and G. marginata.
Ectomycorrhizal Fungi
Mycorrhizal fungi depend largely on their plant symbionts for their carbon source (76), and thus, they have a less extensive CAZyme arsenal than the wood-rotting and litter- and straw-decomposing fungi (7, 9, 72, 73). The limited plant-polysaccharide-degrading capability of ECM fungi is a result of evolutionary reduction in CAZyme families (7, 71) to suit their role as root symbionts. The few CAZymes of ECM fungi are most probably needed for the modification of cell walls of plant roots in order to establish contact with their host for nutrient exchange. This is supported by the tightly controlled expression of putative CAZyme-encoding genes of Piriformospora indica during the fungal colonization of living plant roots (77). Five LPMO-encoding genes are upregulated at the prepenetration stage, while GH10-, GH11-, GH18-, and GH62-encoding genes are induced during prepenetration, colonization, and postcolonization, thus suggesting a role of GHs in the local secretion of enzymes at the penetration site (77). The reduction in CAZymes is also observed for the genome of L. bicolor and Paxillus involutus (7, 78). L. bicolor possesses mostly enzymes that modify polysaccharide backbones, such as β-1,4-endoglucanase, polygalacturonases, and β-1,4-endomannanses for cellulose, pectin, and galactomannan degradation, respectively, but the number of putative genes encoding accessory enzymes is limited. The most abundant CAZyme family acting on the plant cell wall in the genome of L. bicolor is LPMO (7). P. involutus has a unique enzymatic system, similar to that of brown rot fungi, to decompose plant biomass (78, 79). Transcriptomic studies of P. involutus have revealed that only one β-1,4-endoglucanase (GH9) and two LPMO genes are expressed during growth on plant litter or cellulose (79). The oxidative depolymerization of cellulose in cooperation with CDH or low-molecular-weight reducing agents (28, 31) supports the role of LPMOs as important components of the radical-based cellulose-degrading mechanism of ECM fungi. We suggest that most CAZyme activities have been lost in ectomycorrhizal fungi as an adaptation to symbiotic growth on host photosynthate. The CAZyme arsenal of some ECM basidiomycetes, such as P. indica, reflects their ability to switch their life-styles from mutualist to saprobe. P. indica associates with living and dead barley roots and a variety of mono- and dicotyledonous plants. When exposed to dead plant matter instead of living plant roots, P. indica upregulates several of its pectin-related enzymes, thus indicating a switch from a mutualistic to a saprobic life-style (77).
Plant-Pathogenic Fungi and Mycoparasites
Ustilago maydis, Melampsora laricis-populina, and Puccinia graminis are obligate biotrophic pathogens that derive nutrients from living plant tissues and are not able to survive without their hosts. In contrast to the genomes of more aggressive ascomycete pathogens such as Magnaporthe grisea and Fusarium graminearum, these basidiomycete pathogens have few genes encoding CAZymes that are most likely employed for penetrating the cell surface of the host plant (8, 10, 11, 66). The limited CAZyme set also reflects the avoidance of extensive damage of the host cell walls, which can trigger the immune response of the plant (8). However, the GH5 (including β-1,4-endoglucanase and β-1,4-endomannanase activity)-encoding genes are present in several copies (up to 29) (Table 4), and they are suggested to modify the polysaccharide backbones of cellulose and hemicelluloses in order to loosen the plant cell wall structure and to further facilitate the entry of fungal hyphae into the host cell. In M. laricis-populina-infected cultures of wheat and barley, cellulose- and hemicellulose-depolymerizing CAZyme-encoding genes were highly upregulated (10). A similar upregulation was detected in poplar cultures infected with P. graminis (80). This suggests that invading hyphae of these rust fungi secrete polysaccharide-degrading enzymes to form haustoria on the plant surface (10). However, it is possible that these obligate biotrophic pathogens possess as-yet-unidentified strategies for virulence, such as the unexpected set of small genes with unknown function detected in the genome of the corn smut fungus U. maydis (8).
Tremella mesenterica is a wood-degrading fungus and mycoparasite of Peniophora species that is morphologically classified into the group of jelly fungi. The genome of T. mesenterica has a limited CAZyme repertoire similar to that of ECM fungi, containing only three genes encoding GH3 (no β-N-acetylhexosaminidase included) and no genes encoding GH families 6, 7, 10, 11, 12, 28, 43, and 74 (11). This may reflect the parasitic life-style of T. mesenterica. However, T. mesenterica and related species might have an alternative mechanism to degrade plant biomass, but these species have so far been only scarcely studied.
Basidiomycete Yeasts
So far, the genomes of only a few basidiomycete yeast species have been sequenced and analyzed for CAZymes. These unicellular basidiomycetes usually have a very limited pattern of polysaccharide-degrading enzymes, which has been shown for the genomes of Cryptococcus neoformans (81) and Rhodotorula glutinis (82). Similarly, Wallemia sebi, a xerophilic mold-like basidiomycete, has reduced CAZyme sets (55).
Comparison of the Genomes of Basidiomycetes and Aspergillus as a Representative of the Plant-Biomass-Degrading Ascomycetes
Aspergillus species are widely studied due to their relevance to human health and economic importance. These species include the industrial workhorses A. niger and A. oryzae as well as the opportunistic human pathogen A. fumigatus (83). Therefore, their genomes were also among the first sequenced fungal genomes. The genomes of aspergilli revealed that these species contain unexpectedly abundant sets of plant-biomass-degrading genes compared to the previously identified genes and enzymes (19, 84). This demonstrated that without genome sequence data, predominantly only the genes and enzymes that are highly expressed and produced under laboratory conditions have been characterized.
A study of six Aspergillus species (A. clavatus, A. flavus, A. fumigatus, A. niger, A. oryzae, and Neosartorya fischeri [teleomorph of A. fischerianus]) demonstrated that the genome content and organization of closely related species are very similar (85). However, differences in the contents of plant-polysaccharide-degrading genes of A. nidulans, A. niger, and A. oryzae have been detected. A. oryzae has a significantly higher number of xylan- and pectin-related genes than the other two species. Instead, A. nidulans harbors more galactomannan-related genes than A. niger and A. oryzae, whereas the number of inulin-related genes is highest in A. niger (49). While the aspergilli do not provide representative numbers of genes in CAZyme families for all ascomycete fungi, their generalistic life-style and ability to degrade every plant polysaccharide (49) make them suitable baselines to use for comparisons with other fungi.
Genes related to cellulose degradation.
The overall CAZyme contents of the genomes of basidiomycetes and Aspergillus species are similar. They both possess several genes encoding GH5 and -12 EGs. Aspergilli and basidiomycetes have similar numbers of genes encoding CBHs (GH6 and -7). However, aspergilli have notably more BGL genes in GH3 than do the basidiomycetes (Table 4). Interestingly, basidiomycetes harbor genes from GH9, while aspergilli lack the GH9-encoding genes (Table 4). LPMO-encoding genes are present in most of the basidiomycete and aspergillus genomes (86), but basidiomycetes have more LPMO gene models (up to 33) than do the Aspergillus species (7 to 9) (Table 4). Some ascomycetes have similarly high numbers of LPMOs, e.g., 33 in Podospora anserina (50).
Genes related to hemicellulose degradation.
Generally, aspergilli have more genes in the CAZyme families encoding putative GH11, GH62, and CE5 enzymes than do wood-decaying white rot and brown rot basidiomycetes (Table 4). GH11 xylanases are absent from the genomes of brown rot fungi (Table 4). GH11 endoxylanases require a different number of nonsubstituted xylose residues to be able to cleave xylan than GH10 xylanases (87), which are present in all basidiomycetes (Table 4). This indicates that the xylan oligosaccharide profile originating from the action of brown rot xylanases will be different from that originating from white rot fungi and Aspergillus, which will affect the overall process of xylan degradation by these fungi. Basidiomycete genomes almost universally lack the genes encoding GH62 enzymes (Table 4). There are representatives of GH67 and GH93 genes in the genomes of Aspergillus species, while they are almost missing from basidiomycete genomes. In contrast, genes encoding GH30 enzymes, e.g., β-1,4-exoxylanases, are widely present in basidiomycetes and absent from Aspergillus species. Basidiomycetes have more genes in CE15 and -16 than do aspergilli.
Genes related to pectin degradation.
While pectin is a minor component of wood, both basidiomycetes and Aspergillus species possess wide and variable sets of genes encoding pectin-degrading enzymes. Basidiomycetes and aspergilli have up to 20 and 22 genes, respectively, encoding putative GH28 polygalacturonases and rhamnogalacturonases (Table 4). All the brown rot fungi and the white rot species C. subvermispora, P. chrysosporium, and Trametes versicolor lack CE12 genes, which encode putative rhamnogalacturonan acetyl esterases.
CHARACTERIZED PLANT CELL WALL POLYSACCHARIDE-DEGRADING ENZYMES IN BASIDIOMYCETES AND Aspergillus
Before the era of genome sequencing, various plant cell wall polysaccharide-degrading enzymes from basidiomycetes were isolated and characterized at the gene or protein level. Several basidiomycete CAZymes have unique biochemical properties, ranging from extreme temperature tolerance and pH to bifunctional catalytic activities. Most of the characterized CAZymes are from the white rot and litter- or straw-decomposing fungi (Tables 5 to 12). These fungi have more copies of putative CAZyme-encoding genes than any other group of basidiomycetes (Table 4). The extensive plant-polysaccharide-degrading ability of white rot fungi stems from their ecology as the dominant wood-degrading species (56).
TABLE 5.
Characterized basidiomycete β-1,4-endoglucanases and their biochemical properties
| Life-style | Species | CAZyme family | Gene | Enzymea | NCBI protein database accession no.b | Molecular mass (kDa) | pI | pHopt | Topt (°C) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| White rot | Cerrena unicolor | 44 | 4.0 | 212 | ||||||
| Dichomitus squalens | En I | 42 | 4.8 | 4.8 | 55 | 213 | ||||
| D. squalens | En II | 56 | 4.3 | 4.8 | 55 | 213 | ||||
| D. squalens | En III | 47 | 4.1 | 4.8 | 55 | 213 | ||||
| Ganoderma lucidum | 55 | 4.7 | 16 | |||||||
| G. lucidum | 43 | 4.7 | 16 | |||||||
| Irpex lacteus | 56 | 4.0 | 50 | 214 | ||||||
| I. lacteus | En-1 | 16 | 4.0 | 50 | 215 | |||||
| I. lacteus | E2-A | 216 | ||||||||
| I. lacteus | E2-B | 216 | ||||||||
| I. lacteus | GH5 | En-1* | 38 | 217 | ||||||
| Phanerochaete chrysosporium | GH5 | cel5A | EG36 | AAU12275 | 36 | 5.6–5.7 | 58, 90 | |||
| P. chrysosporium | GH5 | cel5A | EG38 | AAU12275 | 38 | 4.9 | 58, 90 | |||
| P. chrysosporium | GH5 | cel5B | EG44 | 44 | 4.3 | 58, 90 | ||||
| P. chrysosporium | GH12 | cel12A | Cel12A | AAU12276 | 28 | 5.2 | 58, 91 | |||
| P. chrysosporium | GH45 | PcCel45A | 18 | 92 | ||||||
| Polyporus arcularius | CMCase I | 39 | 4.4–4.6 | 68 | 218 | |||||
| P. arcularius | CMCase II | 36 | 4.4–4.6 | 68 | 218 | |||||
| P. arcularius | GH3 | cel3A | CMCase IIIa | BAD98315 | 24 | 4.9 | 52 | 218, 219 | ||
| Sporotrichum pulverulentumc | T1 | 32 | 5.3 | 220 | ||||||
| S. pulverulentumc | T2a | 37 | 4.7 | 220 | ||||||
| S. pulverulentumc | T2b | 28 | 4.4 | 220 | ||||||
| S. pulverulentumc | T3a | 38 | 4.7 | 220 | ||||||
| S. pulverulentumc | T3b | 37 | 4.2 | 220 | ||||||
| Trametes hirsuta | GH5 | ThEG | 44 | 221 | ||||||
| T. hirsuta | GH5 | rEG* | 50 | 5.0 | 50 | 221 | ||||
| Trametes versicolor | 30 | 222, 223 | ||||||||
| Brown rot | Coniophora cerebella | A | 42 | 4.7 | 224 | |||||
| C. cerebella | B | 39 | 4.2 | 224 | ||||||
| Fomitopsis palustris | 40 | 225 | ||||||||
| F. palustris | GH5 | EG47 | 47 | 105 | ||||||
| F. palustris | EG35 | 35 | 105 | |||||||
| F. palustris | GH12 | cel12 | 106 | |||||||
| F. palustris | GH12 | eg2 | EGII | BAF49602 | 24 | 3.5 | 55 | 226 | ||
| Gloeophyllum sepiarium (Lenzites sepiaria) | 85 | 227 | ||||||||
| G. sepiarium | EGS | 45 | 3.8 | 4.1 | 59 | 108 | ||||
| Gloeophyllum trabeum | EGT | 41 | 3.1 | 4.2 | 62 | 108 | ||||
| G. trabeum | GH5 | Cel5A | 42 | 4.9 | 103 | |||||
| G. trabeum | GH12 | Cel12A | 28 | 103 | ||||||
| G. trabeum (Lenzites trabea) | 29 | 4.4 | 70 | 145 | ||||||
| Serpula incrassata | Cel 25 | 25 | <3.6 | 50 | 228 | |||||
| S. incrassata | Cel 49 | 49 | <3.6 | 228 | ||||||
| S. incrassata | Cel 57 | 57 | <3.6 | 228 | ||||||
| Piptoporus betulinus | EG1 | 62 | 2.6–2.8 | 3.5 | 70 | 109 | ||||
| Postia placenta | 35–40 | 229 | ||||||||
| Straw decomposing | Volvariella volvacea | GH5 | eg1 | EG1 | AAG59832 | 42 | 7.7 | 7.5 | 55 | 89, 230 |
| Plant pathogen | Polyporus schweinitzii | 45 | 4.0 | 60 | 231, 232 | |||||
| Sclerotium rolfsii | Endo A | 52 | 4.6 | 4.0 | 74 | 233 | ||||
| S. rolfsii | Endo B | 27 | 4.2 | 2.3–3.0 | 50 | 233 | ||||
| S. rolfsii | Endo C | 78 | 4.5 | 4.0 | 50 | 233 | ||||
| Ustilago maydis | GH45 | egl1 | Egl1 | AAB36147 | 93 | |||||
| Yeast | Rhodotorula glutinis | 40 | 8.6 | 4.5 | 50 | 234 |
Asterisks indicate a heterologously produced enzyme.
Anamorph of P. chrysosporium.
TABLE 12.
Characterized basidiomycete-debranching enzymes and their biochemical properties
| Life-style | Species | CAZyme family | Gene | Enzymea | NCBI protein database accession no.b | Molecular mass (kDa) | pI | pHopt | Topt (°C) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| α-Arabinofuranosidase | ||||||||||
| White rot | Dichomitus squalens | 60 | 5.1 | 3.5 | 60 | 312 | ||||
| Coprophilic | Coprinopsis cinerea | GH62 | CcAbf62A | CcAbf62A* | BAK14423 | 48 | 7.0 | 45 | 313 | |
| α-Galactosidase | ||||||||||
| White rot | Ganoderma lucidum | 49 | 4.6 | 16 | ||||||
| G. lucidum | 249c | 6.0 | 70 | 166 | ||||||
| Lenzites elegans | GH36 | 158d | 4.0–4.2 | 4.5 | 60–80 | 167 | ||||
| Phanerochaete chrysosporium | GH27 | AAG24510, AAG24511 | 250c | 3.75 | 165, 314 | |||||
| Phlebia radiata | AgaS-b1 | 60 | 7.2 | 5.0 | 60 | 169 | ||||
| P. radiata | AgaS-b2 | 59 | 5.7 | 5.0 | 60 | 169 | ||||
| P. radiata | AgaS-b3 | 60 | 3.5 | 5.0 | 60 | 169 | ||||
| P. radiata | AgaS-m1 | 55 | 6.7 | 5.0 | 60 | 169 | ||||
| P. radiata | AgaS-m2 | 58 | 5.7 | 5.0 | 60 | 169 | ||||
| P. radiata | AgaS-m3 | 64 | 5.0 | 5.0 | 60 | 169 | ||||
| Pleurotus florida | 99 | 4.6–5.0 | 55 | 168 | ||||||
| Saprobic | Calvatia cyathiformis | 3.0–5.0 | 50 | 315 | ||||||
| β-Galactosidase | ||||||||||
| Plant pathogen | Sclerotium (Corticium) rolfsii | 2.0–2.5 | 316 | |||||||
| Yeast | Sporobolomyces (Bullera) singularis | 53 | 5.0 | 50 | 317 | |||||
| β-1,3-Endo/exogalactanase | ||||||||||
| White rot | Flammulina velutipes | GH16 | FvEn3GAL | FvEn3GAL* | BAK48741 | 30 | 5.5 | 50 | 318 | |
| Irpex lacteus | GH43 | Il1,3Gal | rIl1,3Gal* | BAH29957 | 45 | 4.5 | 40 | 319 | ||
| Galactan β-1,3-galactosidase | ||||||||||
| White rot | Phanerochaete chrysosporium | GH43 | 1,3Gal43A | 1,3Gal43A* | BAD98241 | 55 | 320 | |||
| α-Glucuronidase | ||||||||||
| White rot | Phanerochaete chrysosporium | 112 | 4.6 | 3.5 | 160 | |||||
| Phlebia radiata | 110 | 4.4 | 3.8 | 60 | 161 | |||||
| White rot-like | Schizophyllum commune | GH115 | Agu1 | Agu1* | ADV52250 | 163 | ||||
| S. commune | 125 | 3.6 | 4.5–5.5 | 162 | ||||||
| Litter decomposing | Agaricus bisporus | 3.3 | 52 | 321 | ||||||
| Acetyl xylan esterase | ||||||||||
| White rot | Phanerochaete chrysosporium | CE1 | axe1 | 58 | ||||||
| P. chrysosporium | CE1 | PcAxe2 | PcAxe2* | AEX99751 | 63 | 7.0 | 30–35 | 170 | ||
| White rot-like | Schizophyllum commune | 31 | 7.7 | 30–45 | 322 | |||||
| Straw decomposing | Volvariella volvacea | Vvaxe1 | VvAXE1* | ABI63599 | 45 | 8.0 | 60 | 323 | ||
| Coprophilic | Coprinopsis cinereae | CcEst1 | CcEst1* | BAJ10857 | 45 | 324 | ||||
| Feruloyl esterase | ||||||||||
| White rot | Auricularia auricula-judae | EstBC | 36 | 3.2 | 6.5 | 61–66 | 175 | |||
| Pleurotus eryngii | FaeA | 67 | 5.2 | 5.0 | 50 | 173 | ||||
| Pleurotus sapidus | Est1 | 55 | 6.0 | 50 | 174 | |||||
| Pectin methyl esterase | ||||||||||
| Plant pathogen | Sclerotium (Corticium) rolfsii | 37 | 2.5–4.5 | 45 | 159 |
Asterisks indicate a heterologously produced enzyme.
Tetramer.
Dimer.
Acetic acid- and ferulic acid-releasing activities.
Cellulose-Degrading Enzymes
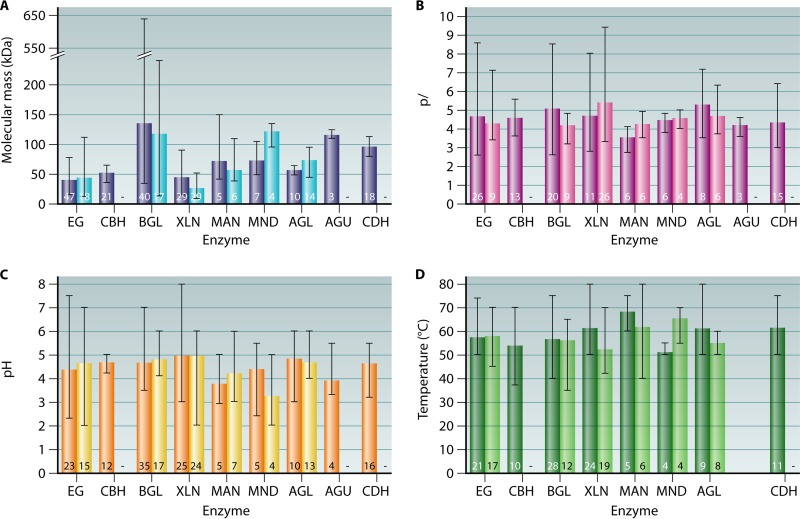
Cellulose, the most abundant plant polymer, is hydrolyzed by the extensive set of cellulolytic enzymes of basidiomycetes. EGs, CBHs, and BGLs have been isolated from species that represent various ecophysiological groups, but most of them belong to wood-degrading white rot fungi (Fig. 3 and Tables 5 to 7). On average, the molecular masses of basidiomycete EGs and CBHs are 41 kDa and 53 kDa, respectively (Fig. 3A and Tables 5 and 6). BGLs may be extracellular or cell wall associated, and their structure can be monomeric or multimeric (88). This is shown by the high level of variation in their molecular masses, ranging from 36 to 640 kDa (Fig. 3A and Table 7). In general, these cellulases have acidic pI values, with few exceptions, and acidic pH optima (Tables 5 and 6). The average optimum temperature of the characterized basidiomycete cellulases is between 54°C and 58°C (Fig. 3D and Tables 5 to 7).
FIG 3.
Average molecular masses (kDa for monomers) (A), isoelectric points (pI) (B), pH optima (C), and temperature optima (D) of selected CAZymes from basidiomycetous (first columns, in dark colors) and Aspergillus species (second columns, in light colors). EG, endoglucanase; CBH, cellobiohydrolase; BGL, β-glucosidase; XLN, endoxylanase; MAN, endomannanase; MND, β-mannosidase; AGL, α-galactosidase; AGU, α-glucuronidase; CDH, cellobiose dehydrogenase. The number of characterized enzymes used for calculation of mean values is marked at the root of each column. Error bars show the minimum and maximum values reported for each biochemical characteristic. −, no mean value was available.
TABLE 7.
Characterized basidiomycete β-glucosidases and their biochemical properties
| Life-style | Species | CAZyme family | Gene | Enzymea | NCBI protein database accession no.b | Molecular mass (kDa) | pI | pHopt | Topt (°C) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| White rot | Ceriporiopsis (Gelatoporia) subvermispora | 110 | 3.5 | 60 | 252 | |||||
| C. subvermispora | 53 | 3.5 | 60 | 252 | ||||||
| Fomes fomentarius | 58 | 6.7 | 4.5–5.0 | 60 | 100 | |||||
| Phanerochaete chrysosporium | 90 | 5.5 | 45 | 101 | ||||||
| P. chrysosporium | 410 | 7.0 | 45 | 101 | ||||||
| P. chrysosporium | 45 | 4.7 | 5.0 | 60 | 253 | |||||
| P. chrysosporium | 114 | 4.0–5.2 | 254 | |||||||
| P. chrysosporium | GH3 | cbgl-1 | AAC26490 | 255 | ||||||
| P. chrysosporium | GH3 | cbgl-2 | AAC26489 | 255 | ||||||
| P. chrysosporium | GH3 | wtBGL | BAB85988 | 116 | 256, 257 | |||||
| P. chrysosporium | GH3 | rBGL* | BAB85988 | 133 | 257 | |||||
| P. chrysosporium | GH1 | bgl1A | BG1A* | BAE87008 | 53 | 113 | ||||
| P. chrysosporium | GH1 | bgl1B | BG1B* | BAE87009 | 60 | 113 | ||||
| Pleurotus ostreatus | F1 | 35 | 7.5 | 4.0 | 40 | 99 | ||||
| P. ostreatus | F2 | 50 | 7.3 | 4.0 | 50 | 99 | ||||
| P. ostreatus | F3 | 66 | 8.5 | 5.0 | 50 | 99 | ||||
| Trametes gibbosa | 640 | 3.5 | 40–50 | 258 | ||||||
| Trametes versicolor | 300 | 4.3 | 45 | 185, 259 | ||||||
| Sporotrichum pulverulentumc | A1 | 165 | 4.8 | 4.0–4.5 | 260 | |||||
| S. pulverulentumc | A2 | 172 | 4.5 | 4.0–4.5 | 260 | |||||
| S. pulverulentumc | B1-3 | 165–182 | 4.6–5.2 | 4.0–4.5 | 260 | |||||
| Stereum hirsutum | BGL | 98 | 261 | |||||||
| White rot-like | Schizophyllum commune | 97 | 5.3 | 262 | ||||||
| S. commune | I | 102 | 3.4 | 5.8 | 263 | |||||
| S. commune | II | 96 | 3.3 | 5.1 | 263 | |||||
| Brown rot | Fomitopsis palustris | GH3 | Cel3A | Cel3A* | NR | 70 | 5.0 | 60 | 146 | |
| Gloeophyllum trabeum (Lenzites trabea) | 320 | 4.5 | 75 | 145, 264 | ||||||
| Piptoporus betulinus | BG1 | 36 | 2.6 | 4.0 | 60 | 109 | ||||
| Poria vaillantii | 4.2 | 50 | 265 | |||||||
| Litter decomposing | Agaricus bisporus | bg1 | CAC03462 | —d | ||||||
| Straw decomposing | Volvariella volvacea | BGL-I | 158 | 5.6 | 7.0 | 55–60 | 102 | |||
| V. volvacea | BGL-II | 256 | 5.0–5.2 | 6.2 | 55–60 | 102 | ||||
| V. volvacea | GH3 | bgl | AAG59831 | 95 | 266 | |||||
| Plant pathogen | Sclerotium rolfsii | BG-1 | 90 | 4.1 | 4.2 | 68 | 187 | |||
| S. rolfsii | BG-2 | 90 | 4.6 | 4.2 | 68 | 187 | ||||
| S. rolfsii | BG-3 | 107 | 5.1 | 4.2 | 68 | 187 | ||||
| S. rolfsii | BG-4 | 92 | 5.6 | 4.2 | 68 | 187 | ||||
| Ustilago esculenta | GH3 | UeBgl3A* | BAK61808 | 110 | 5.0 | 40 | 267 | |||
| Ectomycorrhiza | Tricholoma matsutake | 160 | 5.0 | 60 | 268 | |||||
| Pisolithus tinctorius | 450e | 3.8 | 4.0 | 65 | 269 | |||||
| Insect symbiont | Termitomyces clypeatus | 116 | 5.0 | 45 | 270 | |||||
| T. clypeatus | 110 | 4.5 | 5.0 | 65 | 271 | |||||
| Yeast | Rhodotorula minuta | 144 | 4.8 | 4.7–5.2 | 70 | 188 | ||||
| Sporobolomyces singularis | GH1 | bglA | BglA | BAD95570 | 74 | 3.5 | 45 | 112 |
Asterisks indicate a heterologously produced enzyme. wtBGL, wild-type BGL.
See http://www.ncbi.nlm.nih.gov/protein. NR, not reported.
Anamorph of P. chrysosporium.
Available in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank/).
Trimer.
TABLE 6.
Characterized basidiomycete cellobiohydrolases and their biochemical properties
| Life-style | Species | CAZyme family | Gene | Enzyme | NCBI protein database accession no.a | Molecular mass (kDa) | pI | pHopt | Topt (°C) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| White rot | Dichomitus squalens | Ex 1 | 39 | 4.6 | 5.0 | 60 | 235 | |||
| D. squalens | Ex 2 | 36 | 4.5 | 5.0 | 60 | 235 | ||||
| D. squalens | GH7 | cel7b | CDJ79665 | 61 | ||||||
| Flammulina velutipes | GH7 | cel7a | FvCel7A | BAJ07534 | 50 | 236 | ||||
| F. velutipes | GH7 | cel7b | FvCel7B | BAJ07535 | 60 | 236 | ||||
| Ganoderma lucidum | 56 | 5.2 | 16 | |||||||
| G. lucidum | 49 | 5.0 | 16 | |||||||
| G. lucidum | 50 | 5.6 | 16 | |||||||
| Irpex lacteus | GH7 | cel1 | Ex-1 | BAA76363 | 53 | 4.5 | 5.0 | 50 | 189, 237 | |
| I. lacteus | GH7 | cel2 | Ex-2 | BAA76364 | 56 | 4.8 | 5.0 | 50 | 189 | |
| I. lacteus | GH7 | cel3 | BAA76365 | 238 | ||||||
| I. lacteus | GH6 | cex3 | BAG48183 | 60 | 50 | 239 | ||||
| I. lacteus | 65 | 5.0 | 50 | 240 | ||||||
| Lentinula edodes | GH7 | cel7A | CEL7A | AAK95563 | 241 | |||||
| L. edodes | GH6 | cel6B | CEL6B | AAK95564 | 241 | |||||
| Phanerochaete chrysosporium | GH7 | cbh1-1 | Cel7A | CAA38274 | 95, 192, 242 | |||||
| P. chrysosporium | GH7 | cbh1-2 | Cel7B | CAA38275 | 95, 192, 242 | |||||
| P. chrysosporium | GH7 | cbh1 | Cel7C | AAB46373 | 62 | 4.9 | 94, 95, 192, 242, 243 | |||
| P. chrysosporium | GH7 | cbh1-4 | Cel7D | AAA19802 | 58 | 3.8 | 94, 95, 244 | |||
| P. chrysosporium | GH7 | cbh1-5 | Cel7E | 95 | ||||||
| P. chrysosporium | GH7 | cbh1-6/7 | Cel7F/G | 6, 95, 242 | ||||||
| P. chrysosporium | GH6 | CBH50 | 50 | 4.9 | 94 | |||||
| P. chrysosporium | GH6 | cbhII | CBHII | AAB32942 | 245 | |||||
| Polyporus arcularius | GH7 | cel1 | BAF80326 | 246 | ||||||
| P. arcularius | GH6 | cel2 | BAF80327 | 246 | ||||||
| Brown rot | Coniophora puteana | CBHI | 52 | 3.6 | 5.0 | 107 | ||||
| C. puteana | CBHII | 50 | 3.6 | 5.0 | 107 | |||||
| Fomitopsis palustris | 4.5 | 70 | 104 | |||||||
| Litter decomposing | Agaricus arvensis | GH7 | HM004552b | 130c | 4.0 | 65 | 247 | |||
| Agaricus bisporus | GH6 | cel3AC | CEL3 | AAA50607 | 52 | 186 | ||||
| A. bisporus | GH7 | cel2 | CAA90422 | 133 | ||||||
| Straw decomposing | Volvariella volvacea | GH7 | cbhI | CBHI | AAD41096 | 98 | ||||
| V. volvacea | GH6 | cbhII | CBHII | AAD41097 | 98 | |||||
| Coprophilic | Coprinopsis cinerea | GH6 | CcCel6A | CcCel6A | BAH08702 | 248, 249 | ||||
| C. cinerea | GH6 | CcCel6B | CcCel6B | BAH08703 | 248, 249 | |||||
| C. cinerea | GH6 | CcCel6C | CcCel6C | BAH08704 | 248, 249 | |||||
| C. cinerea | GH6 | CcCel6D | BAH08705 | 249 | ||||||
| C. cinerea | GH6 | CcCel6E | BAH08706 | 249 | ||||||
| White rot plant pathogen | Heterobasidion irregulare | GH7 | HirCel7A | 50 | 4.0 | 45 | 97 | |||
| Plant pathogen | Sclerotium rolfsii | 41.5–42.0 | 4.3 | 4.2 | 37 | 250 | ||||
| Insect symbiont | Termitomyces sp. | GH6 | Cellulase IF | 52 | 4.4 | 251 |
Generally, white rot fungi produce more isoenzymes for plant polysaccharide degradation than do other basidiomycetes. Isoenzymes of EGs have been isolated from several white rot species and characterized (Table 5). The molecular mass of the EGs from white rot fungi ranges from 18 to 78 kDa, and they have acidic pI values of 4.1 to 5.7. As an exception, EG of the straw-decomposing fungus V. volvacea has a neutral isoelectric point of 7.7 and also a neutral pH optimum (7.5) for the hydrolysis of carboxymethyl cellulose (CMC) (89). The most comprehensive view of characterized enzymes is from the model white rot fungus P. chrysosporium. Three GH5, one GH12, and one GH45 EG of P. chrysosporium have been biochemically characterized (58, 90–92). GH5 EGs of P. chrysosporium hydrolyze CMC more efficiently than Avicel (90). GH45 EGs have been characterized only for P. chrysosporium and the plant pathogen U. maydis (92, 93). P. chrysosporium GH45 EG hydrolyzes various glycan substrates, preferring substrates consisting mainly of β-1,3/1,4-glucan (92). These endoglucanases show the common synergistic action with CBHs from GH6 and -7 (90, 92).
P. chrysosporium has seven CBH-encoding genes, and three of them have been characterized at the protein level (Table 5). These isoenzymes work synergistically to cleave cellulose at the reducing and nonreducing ends (94). Multiple plant-polymer-degrading isoenzymes produced by one species are hypothesized to have different biochemical properties, such as the substrate specificity to enhance the degradation of plant biomass. The three-dimensional crystal structure of P. chrysosporium Cel7D (PDB accession number 1GPI) (95) shows that the catalytic domain is composed of a β-sandwich structure similar to that of ascomycete GH7 CBHI, first solved for the ascomycetous fungus H. jecorina Cel7A (PDB accession number 1CEL) (96). The crystal structures reveal that the cellulose binding tunnels of the CBHIs differ significantly, thus affecting the accessibility of the substrate to the active site. In P. chrysosporium Cel7D, the cellulose binding tunnel is more open than in H. irregulare Cel7A (HirCel7A) (PDB accession numbers 2YG1 and 2XSP) (97), while H. jecorina Cel7A has the most enclosed structure.
Differences in the three-dimensional structures of the six different P. chrysosporium CBH proteins were revealed by homology modeling, thus supporting the presence of multiple isoenzymes with different specificities and catalytic mechanisms (95). A function for the multiplicity of cellulolytic-enzyme-encoding genes is also supported by their expression at different phases of fungal growth and degradation of plant biomass. For example, V. volvacea has three GH7 CBHI-encoding genes that are expressed during different stages of mushroom development (98).
GH1 and GH3 β-glucosidases of white rot and straw-decomposing fungi have widely variable molecular masses (from 45 to 640 kDa) and isoelectric points (from 3.3 to 8.5) (Table 7). This diversity is due to the intra- and extracellular localizations of β-glucosidases. The exceptions of β-glucosidases with neutral pI values are those from Fomes fomentarius, P. ostreatus, P. chrysosporium, and V. volvacea (99–102).
The strategy used by brown rot basidiomycetes to degrade cellulose differs from that used by white rot fungi. Instead of using cellulolytic enzymes, the brown rot fungi rely on highly reactive oxidants for initial depolymerization of plant polysaccharides (18, 56, 71). Most brown rots are unable to degrade crystalline cellulose, with the majority preferring amorphous cellulose (88). However, some brown rot species, e.g., G. trabeum and Fomitopsis palustris, have been shown to degrade crystalline cellulose (103–106). The genomes of brown rot fungi harbor EG- but rarely any CBH-encoding genes, with the exception of the species belonging to the order Boletales, which can also degrade crystalline cellulose (Table 4). GH5 and GH12 EGs from G. trabeum and F. palustris have also been characterized (Table 5). In microcrystalline cellulose (Avicel) cultures, G. trabeum produces GH5 and GH12 EGs, of which GH5 EG was shown to hydrolyze Avicel into cellobiose (103). This processive EG has been suggested to compensate for the lack of CBHs in cellulose degradation of G. trabeum. Only C. puteana and S. lacrymans from the order Boletales possess either one or both GH6 and GH7 CBH gene models (Table 4). The GH6 and GH7 CBHs of C. puteana have also been isolated and characterized (Table 6) (107).
Both basidiomycetes and aspergilli have a complete set of hydrolytic cellulases, including EGs, CBHIs, CBHIIs, and BGLs. Only some of the brown rot, plant-pathogenic, and ECM fungi and basidiomycetous yeasts lack CBHs. Similar to the EGs and BGLs from Aspergillus species (19), several basidiomycete cellulases are able to degrade the backbone of hemicelluloses (88). EGs from P. chrysosporium, G. trabeum, Piptoporus betulinus, and Sclerotium rolfsii are able to hydrolyze xylan, galactoglucomannan, or mannan (91, 108–110). P. chrysosporium EG28 has activity toward xylan, mannan, and CMC (91), and EG of Aspergillus aculeatus does not hydrolyze cellulose and releases only xyloglucan oligosaccharides from plant cell walls (111). Basidiomycete BGLs from both GH1 and GH3 have been characterized (112, 113). Both basidiomycete and aspergillus BGLs show wide substrate specificity, and they are able to hydrolyze glucose, mannose, xylose, or galactose units from the corresponding oligosaccharides (19, 88).
CDHs are widely present in both basidiomycetes and ascomycetes. In contrast to the other plant cell wall polysaccharide-degrading enzymes, CDHs have been more commonly studied in basidiomycetes than in ascomycetes (114), probably because this enzyme was first found in the white rot fungus P. chrysosporium (115). So far, CDHs from 12 different white rot species, the brown rot fungus C. puteana, the coprophilic fungus C. cinerea, and the plant pathogen S. rolfsii have been characterized (Table 8). The average molecular mass of basidiomycete CDHs is 96 kDa (Table 8). The CDHs show acidic isoelectric points (from 3.0 to 6.4) and pH optima (pH 3.5 to 5). The optimum temperature for CDH-catalyzed reactions varies from 50°C to 75°C (Table 8).
TABLE 8.
Characterized basidiomycete cellobiose dehydrogenases and lytic polysaccharide monooxygenases, their biochemical properties, and their corresponding genes
| Life-style | Species | CAZyme family(ies) | Gene | Enzymea | NCBI protein database accession no.b | Molecular mass (kDa) | pI | pHoptc | Toptc (°C) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cellobiose dehydrogenase | ||||||||||
| White rot | Ceriporiopsis subvermispora | AA3_1, AA8 | cdh | ACF60617 | 87–98 | 3.0 | 4.5 | 60 | 116 | |
| Irpex lacteus | CDHII | 97 | 4.0d | 50d | 118 | |||||
| Phanerochaete chrysosporium | cdh | AAC49277 | 89 | 4.2 | 5.0 | 272, 273 | ||||
| P. chrysosporium | cdh | CDH* | CAA61359 | 90 | 4.2 | 3.5–4d | 274–276 | |||
| Pycnoporus cinnabarinus | cdh | AAC32197 | 92 | 3.8 | 4.5 | 75 | 277, 278 | |||
| P. cinnabarinus | CDHI | 81 | 5.9 | 5.5 | 279 | |||||
| P. cinnabarinus | CDHII | 101 | 3.8 | 4.5 | 279 | |||||
| P. cinnabarinus | CDH* | ADX41688 | 110 | 4.5 | 70 | 280 | ||||
| Trametes hirsuta | 92 | 4.2 | 5.0e | 60–70 | 117 | |||||
| Trametes pubescens | TpCDH | 90 | 4.2 | 4.5–5.0e | 119 | |||||
| Trametes versicolor | AA3_1, AA8 | cdh | CDH 4.2* | AAC50004 | 97 | 4.2 | 4.5 | 55 | 281–283 | |
| T. versicolor | AA3_1 | CDH 6.4 | 6.4 | 55 | 281 | |||||
| Trametes villosa | TvCDH | 89 | 4.4 | 4.5–5.0e | 119 | |||||
| Grifola frondosa | cdh | GfrCDH | BAC20641 | 284 | ||||||
| Phlebia lindtneri | cdh | AGE45679 | 104 | 4.0 | 5.0e | 60e | 285 | |||
| Pycnoporus sanguineus | AA3_1, AA8 | 113 | 4.2 | 4.5 | 70 | 286 | ||||
| White rot-like | Schizophyllum commune | 102 | 287 | |||||||
| Brown rot | Coniophora puteana | 111 | 3.9 | 120, 121 | ||||||
| Coprophilic | Coprinopsis cinerea | AA3_1, AA8 | CDHcc* | ∼80 | 5.0 | 60 | 114 | |||
| Plant pathogen | Sclerotium rolfsii | AA3_1, AA8 | 101 | 4.2–5.0 | 3.2–4.8 | 55 | 288, 289 | |||
| Lytic polysaccharide monooxygenases | ||||||||||
| White rot | Heterobasidion irregulare | AA9 | HiGH61A | GH61A | AFO72232 | 290 | ||||
| H. irregulare | AA9 | HiGH61B | GH61B | AFO72233 | 290 | |||||
| H. irregulare | AA9 | HiGH61C | 290 | |||||||
| H. irregulare | AA9 | HiGH61D | GH61D | AFO72234 | 290 | |||||
| H. irregulare | AA9 | HiGH61E | 290 | |||||||
| H. irregulare | AA9 | HiGH61F | AFO72235 | 290 | ||||||
| H. irregulare | AA9 | HiGH61G | AFO72236 | 290 | ||||||
| H. irregulare | AA9 | HiGH61H | AFO72237 | 290 | ||||||
| H. irregulare | AA9 | HiGH61I | AFO72238 | 290 | ||||||
| H. irregulare | AA9 | HiGH61J | AFO72239 | 290 | ||||||
| Phanerochaete chrysosporium | AA9 | PcGH61D* | 25 | 4.8 | 125 |
Asterisks indicate a heterologously produced enzyme.
Activity measured with 2,6-dichlorophenolindophenol (DCIP) and cellobiose.
Activity measured with cytochrome c.
Activity measured with DCIP and lactose.
The substrate array of the characterized white rot fungal CDHs is variable. P. chrysosporium CDH is able to oxidize cellobiose and higher cellodextrins, lactose, mannobiose, and galactosylmannose (29). C. subvermispora and Trametes hirsuta CDHs are also able to oxidize maltose (116, 117), while CDH of Irpex lacteus oxidizes only cellobiose or higher cellodextrins efficiently (118). In addition, the CDHs of Trametes pubescens and Trametes villosa can oxidize xylobiose (119). The characterized basidiomycete CDHs have pH and temperature optima of 3.5 to 5.5 and 50°C to 75°C, respectively (Table 8). The only characterized brown rot fungal CDH is from C. puteana. It oxidizes both cellobiose and cellooligosaccharides but not glucose, which supports the typical catalytic properties of CDH (120, 121).
Basidiomycete and ascomycete CDHs are classified into two subgroups according to their primary amino acid sequences (122). Class I includes basidiomycete CDHs, which are shorter polypeptides than the more complex class II ascomycete CDHs, which have a C-terminal cellulose binding module. In addition, the linker regions in basidiomycete CDHs are more conserved than those in ascomycete CDHs (30). To our knowledge, CDHs from Aspergillus species have not been characterized at the protein level to date. The ascomycete CDHs isolated from N. crassa have a broader substrate spectrum and less glucose discrimination than basidiomycete CDHs (30). While basidiomycete CDHs catalyze the reactions at acidic pH, ascomycete CDHs are active at neutral or alkaline pH (41). Whether the differences in the biochemical characteristics of CDHs between basidiomycetes and ascomycetes are also valid for Aspergillus species remains to be clarified.
Biochemical studies of basidiomycete LPMOs are at the early stage, and most analyses have been conducted only at the gene level (Tables 4 and 8). In fact, enzymes from the white rot fungi P. chrysosporium and H. irregulare are the only isolated or characterized basidiomycete LPMOs. The structure of P. chrysosporium LPMO (GH61D) (PDB accession number 4B5Q) (123) together with five structures of ascomycete LPMOs, i.e., H. jecorina (Cel61B) (PDB accession number 2VTC), Thielavia terrestris (GH61E) (PDB accession numbers 3EII and 3EJA), Thermoascus aurantiacus (GH61A) (PDB accession number 2YET), and N. crassa (PMO-2 and PMO-3) (PDB accession numbers 4EIR and 4EIS) LPMOs (27, 28, 33, 124), have opened the path to describing the biochemical properties of various putative LPMOs harbored in fungal genomes.
Currently, cellulose-cleaving activities of LPMOs have been biochemically confirmed only for GH61D of the white rot fungus P. chrysosporium (PcGH61D) (125); the above-mentioned ascomycete LPMOs from H. jecorina, T. terrestris, T. aurantiacus, and N. crassa; and GH61A and GH61B of the ascomycete fungus P. anserina (27, 28, 31, 32, 126, 127). PcGH61D is not active on soluble cellooligosaccharides (125), but it is able to oxidize phosphoric acid-swollen cellulose in the presence of ascorbic acid and to release lactone, which is spontaneously converted to aldonic acid (125). The copper-bound active site that is common to LPMOs is present in PcGH61D (123). Nevertheless, it has significant differences in the loop structures near the binding face compared to the other characterized LPMO structures, which illustrates the diversity of the LPMOs.
Hemicellulose-Degrading Enzymes
Xylan degradation.
Xylanases break down the most common hemicellulose found in high quantities in hardwoods and cereals. Of the isolated and characterized basidiomycete xylan-degrading enzymes, 75% are from white rot fungi (Table 9). Moreover, basidiomycetes produce several xylanase isoenzymes, which is also reflected in the high copy number of the corresponding genes present in their genomes (Table 4). The average molecular mass of basidiomycete endoxylanases is 45 kDa (Fig. 3A and Table 9). Their isoelectric points vary from 2.8 to 8.0, and the pH optimum is between 3.0 and 8.0 (Fig. 3B and C and Table 9). Temperature optima for endoxylanase reactions range from 50°C to 80°C (Fig. 3D and Table 9).
TABLE 9.
Characterized basidiomycete β-1,4-endoxylanases and β-xylosidases and their biochemical properties
| Life-style | Species | CAZyme family | Gene | Enzymea | NCBI protein database accession no.b | Molecular mass (kDa) | pI | pHopt | Topt (°C) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Endoxylanase | ||||||||||
| White rot | Ceriporiopsis (Gelatoporia) subvermispora | Xylanase I | 79 | 8.0 | 50 | 128 | ||||
| C. subvermispora | 29 | 5.0 | 60 | 291 | ||||||
| Cerrena unicolor | 44 | 4.0 | 212 | |||||||
| Ganoderma lucidum | Xylanase B | 31 | 5.4 | 16 | ||||||
| Irpex lacteus | 38 | 7.6–8.0 | 4.6–5.2 | 60 | 292 | |||||
| I. lacteus | Xylanase I | 38 | 6.0 | 60 | 293 | |||||
| I. lacteus | Xylanase III | 62 | 6.0 | 70 | 293 | |||||
| Lentinula edodes | 41 | 3.6 | 4.5–5.0 | 60 | 294 | |||||
| L. edodes | GH11 | xyn11A | XYN11A* | AAL04152 | 4.5 | 50 | 295 | |||
| L. edodes | GH10 | 35 | 4.0 | 50 | 296 | |||||
| Phanerochaete chrysosporium | GH10 | xynA | XynA* | ABZ88797 | 52 | 4.5 | 70 | 297 | ||
| P. chrysosporium | GH11 | xynB | XynB* | ABZ88798 | 30 | 4.5 | 60 | 297 | ||
| P. chrysosporium | GH10 | xynC | XynC* | ABZ88799 | 50 | 4.5 | 70 | 297 | ||
| P. chrysosporium | GH10 | XynA | XynA* | AEK97220 | 50 | 5.0 | 70 | 298 | ||
| P. chrysosporium | GH10 | XynC | XynC* | AEK97221 | 55 | 5.0 | 70 | 299 | ||
| P. chrysosporium | GH10 | XynA | XYNA* | AAG44992 | 48 | 300 | ||||
| P. chrysosporium | GH11 | XynB | XYNB* | AAG44995 | 37 | 300 | ||||
| Phlebia radiata | XA-1 | 19 | 6.7 | 131 | ||||||
| P. radiata | XA-2 | 16 | 4.1 | 131 | ||||||
| Pycnoporus cinnabarinus | 50 | 4.0 | 5.0 | 60 | 277 | |||||
| White rot-like | Schizophyllum commune | GH11 | Xylanase A | 33 | 5.0 | 55 | 301 | |||
| S. commune | GH10 | XynB | 31 | 2.8 | 5.5 | 50 | 302 | |||
| S. commune | XynC | 30 | 3.6 | 5.5 | 50 | 302 | ||||
| Brown rot | Gloeophyllum trabeum | 39–42 | 5.0 | 4.0 | 80 | 303 | ||||
| G. trabeum | GH10 | Xyn10A | 39 | 4.8 | 103 | |||||
| G. trabeum | GH10 | Xyn10A* | AFR33046 | 3.4 | 50 | 129 | ||||
| G. trabeum | GH10 | Xyn10B* | AFR33047 | 4.5 | 70 | 129 | ||||
| G. trabeum | GH10 | Xyl10g | AEJ35165 | —c | ||||||
| Laetiporus sulfureus | 69 | 3.0 | 80 | 304 | ||||||
| Postia placenta | 43 | 3.8 | 305 | |||||||
| Litter decomposing | Agaricus bisporus | GH10 | xynA | XLNA* | CAB05665 | 34 | 190 | |||
| Coprophilic | Coprinopsis cinerea | GH11 | Xyn11C* | AFR33049 | 6.5 | 50–60 | 129 | |||
| Insect symbiont | Termitomyces clypeatus | 90 | 5.5 | 55 | 306 | |||||
| Termitomyces sp. | 87 | 5.6 | 65–70 | 307 | ||||||
| β-Xylosidase | ||||||||||
| White rot | Phanerochaete chrysosporium | GH43 | PcXyl | rPcXyl* | AFW16059 | 83 | 5.0 | 45 | 130 | |
| Phlebia radiata | XD-1 | 27 | 5.9 | 131 | ||||||
| Plant pathogen | Sclerotium rolfsii | 170 | 4.5 | 50 | 308 |
Asterisks indicate a heterologously produced enzyme.
Available in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank/).
GH10 and -11 endoxylanases from some white rot species, the litter-decomposing fungus A. bisporus, and the brown rot fungus G. trabeum have been characterized (Table 9). Optimum pH values of white rot fungal endoxylanases are most often between 4.0 and 6.0, but an alkaline optimum pH of 8.0 has been reported for C. subvermispora endoxylanase (128). G. trabeum endoxylanase (GtXyn10A) also exhibits activity for xyloglucan (129). β-Xylosidases have been isolated from only a few white rot fungal species, including C. subvermispora, G. lucidum, P. chrysosporium, and Phlebia radiata (Table 9). The GH43 β-xylosidase of P. chrysosporium has a molecular mass of 83 kDa (130), whereas the molecular mass of P. radiata β-xylosidase is only 27 kDa (131).
The isoelectric points of xylanases, endoxylanases, and β-xylosidases from aspergilli vary substantially (19). Variation in pI values is also seen in basidiomycetous xylanases, even though the number of characterized enzymes is lower than for ascomycetes (Table 5) (19). Ascomycete xylanases have different specificities toward the xylan polymer, some being strongly dependent on the substituents of the xylose residues neighboring the attacked residue and others cutting randomly between the unsubstituted xylose residues (19).
Mannan degradation.
Only a few basidiomycete mannanases have been characterized compared to cellulases and xylanases (Table 10). However, basidiomycetes provide an avenue for the isolation of unique mannan-degrading enzymes according to their genomes (Table 4). β-Endomannanases have been isolated only from the white rot fungi P. chrysosporium, T. versicolor, and C. subvermispora; the litter-decomposing fungus A. bisporus; and the plant pathogen Sclerotium rolfsii (128, 132–138). However, mannanase activities have also been measured in other white rot fungi, such as P. ostreatus (endomannanase [139]) and P. radiata (140). The molecular mass of β-endomannanases (42 to 65 kDa) is lower than that of β-mannosidases (49 to 105 kDa), with the exception of the C. subvermispora β-endomannanase, with an atypically high molecular mass (150 kDa) (128) (Fig. 3A and Table 10). The isoelectric points have been determined only for the β-endomannanases from S. rolfsii, and their pI values are very acidic, from 2.8 to 3.5 (135, 136, 138). Similarly, the pH optima of S. rolfsii β-endomannanases are also acidic (pH 2.9 to 3.5), while the optimum pH range for P. chrysosporium β-endomannanase is 4.0 to 6.0 (Table 10). β-Mannosidases from the white rot species G. lucidum and P. radiata, the brown rot fungus Laetiporus (Polyporus) sulfureus, and the plant pathogen S. rolfsii have been characterized (16, 135, 141, 142); their pI values are close to 4.5, and the optimum pH varies from 2.4 to 5.5 (Fig. 3B and C and Table 10). The average temperature optimum for the β-endomannanases (70°C) is higher than that for β-mannosidases (53°C) (Fig. 3D and Table 10).
TABLE 10.
Characterized basidiomycete β-1,4-endomannanases and mannosidases and their biochemical properties
| Life-style | Species | CAZyme family | Gene | Enzymea | NCBI protein database accession no.b | Molecular mass (kDa) | pI | pHopt | Topt (°C) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| β-Endomannanase | ||||||||||
| White rot | Ceriporiopsis (Gelatoporia) subvermispora | Mannanase I | 150 | 4.5 | 60 | 128 | ||||
| Heterobasidion irregulare (Fomes annosus) | 3.9–4.2 | 309 | ||||||||
| Phanerochaete chrysosporium | GH5 | man5D | Man5D* | ABG79370 | 65 | 4.0–6.0 | 60 | 132 | ||
| P. chrysosporium | GH5 | man5C | ABG79371 | —c | ||||||
| P. chrysosporium (Chrysosporium lignorum) | 4.11 | 309 | ||||||||
| Litter decomposing | Agaricus bisporus | GH5 | Cel4b | 133, 134 | ||||||
| Plant pathogen | Sclerotium rolfsii | 61 | 3.5 | 2.9 | 74 | 135, 136, 310, 311 | ||||
| S. rolfsii | 42 | 3.2 | 3.3 | 72 | 135, 310 | |||||
| S. rolfsii | 47 | 2.8 | 3.0–3.5 | 75 | 138 | |||||
| Stereum sanguinolentum | 3.58 | 309 | ||||||||
| β-Mannosidase | ||||||||||
| White rot | Ganoderma lucidum | 49 | 4.2 | 16 | ||||||
| G. lucidum | 49 | 4.8 | 16 | |||||||
| Phlebia radiata | GM-1 | 105 | 4.8 | 5.5 | 50 | 141 | ||||
| P. radiata | GM-2 | 90 | 3.8 | 5.5 | 50 | 141 | ||||
| P. radiata | OT-1 | 100 | 4.7 | 5.5 | 50 | 141 | ||||
| Brown rot | Laetiporus (Polyporus) sulfureus | 64 | 2.4–3.4 | 142 | ||||||
| Plant pathogen | Sclerotium rolfsii | 58 | 4.5 | 2.5 | 55 | 135 |
Asterisks indicate a heterologously produced enzyme.
Available in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank/).
Brown rot fungi are specialized in degrading conifers (143), which contain a higher percentage of mannan than hardwoods (20). The genomes of basidiomycetes possess several copies of genes encoding mannan-degrading enzymes assigned to GH2, -5, and -26 (Table 4). Correspondingly, the brown rot fungus Piptoporus betulinus has been observed to produce β-mannanase and β-mannosidase activities (109, 144). However, no mannanolytic enzymes from the brown rot basidiomycetes have been isolated or characterized (Table 5). Nevertheless, cellulolytic enzymes of brown rot fungi have been shown to hydrolyze substrates other than cellulose. Gloeophyllum sepiarium and G. trabeum produce β-1,4-endoglucanases that cleave galactoglucomannan and xylan, respectively (108, 145). F. palustris possesses a GH3 β-glucosidase that is active against p-nitrophenylxyloside, p-nitrophenylgalactoside, p-nitrophenylcellobioside, and p-nitrophenylmannoside (146). Similarly, P. betulinus β-glucosidase is able to release galactose, mannose, and xylose from xylan and galactomannan (109).
Aspergillus species produce both β-endomannanases and β-mannosidases, and characterized enzymes have been classified into GH5 and -2 (19). Altogether, at the level of characterized enzymes, both β-mannanases and β-mannosidases of basidiomycetes and aspergilli have gained less attention than several other CAZymes (Table 10).
Pectin-Degrading Enzymes
Basidiomycete genomes show a high level of variation with respect to pectin degradation (Table 4), but only a few pectinolytic enzymes from basidiomycetes have been characterized (Table 11). Instead, the main focus of studies of pectinases has been on A. niger and other Aspergillus species. Aspergilli produce variable hydrolases (endo- and exopolygalacturonases, endorhamnogalacturonan hydrolases, rhamnogalacturonan rhamnohydrolase, α-rhamnosidase, and rhamnogalacturonan galacturonohydrolases) and lyases (pectin, pectate, and rhamnogalacturonan lyases), with several isoenzymes that differ in their specific activity (19).
TABLE 11.
Characterized basidiomycete pectin-degrading enzymes and their biochemical properties
| Life-style | Species | CAZyme family | Gene | Enzymea | NCBI protein database accession no.b | Molecular mass (kDa) | pI(s) | pHopt | Topt (°C) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Endo/exopolygalacturonases and rhamnogalacturonases | ||||||||||
| White rot | Phanerochaete chrysosporium | 42 | 4.3, 4.6, 4.7 | 4.7 | 66 | 147 | ||||
| Plant pathogen | Chondrostereum purpureum | GH28 | epgA | AAF68401 | 150 | |||||
| C. purpureum | GH28 | epgB1 | AAF68402 | 150 | ||||||
| C. purpureum | GH28 | epgB2 | AAK29433 | 150 | ||||||
| C. purpureum | GH28 | epgC | AAF68403 | 150 | ||||||
| C. purpureum | GH28 | epgD | AAF68404 | 150 | ||||||
| C. purpureum | GH28 | cppg1 | PGA | BAA96351 | 149 | |||||
| C. purpureum | GH28 | Pg1 | PGA | BAA08102 | 39 | 8.8 | 148 | |||
| Sclerotium (Corticium) rolfsii | PGA | 2.5 | 151 | |||||||
| S. rolfsii | 46–48 | 5.2 | 4.0 | 152 | ||||||
| S. rolfsii | 28–31 | 5.2 | 4.0 | 152 | ||||||
| Yeast | Cystofilobasidium capitatum | PGA | 44 | 45 | 153, 154 | |||||
| Rhamnogalacturonan hydrolase | ||||||||||
| White rot | Irpex lacteus | RGH* | ACI26689 | 55 | 7.2 | 4.5–5.0 | 40–50 | 155, 156 |
The asterisk indicates a heterologously produced enzyme.
The pectinolytic enzymes of basidiomycetes isolated or characterized so far are endo/exorhamno- or polygalacturonases from the white rot fungus P. chrysosporium (147), the plant-pathogenic species Chondrostereum purpureum (148–150) and S. rolfsii (151, 152), and the basidiomycete yeast Cystofilobasidium capitatum (153, 154) as well as a rhamnogalacturonan lyase from the white rot fungus I. lacteus (155, 156) (Table 11). However, there is a great potential to find novel pectinases from basidiomycetes with unique properties because of the diverse ecological niches that basidiomycetes inhabit and the variety of putative pectinase-encoding genes in their genomes (Table 4). For example, several basidiomycetes, including the white rot fungi Lentinus sp., P. chrysosporium, Pycnoporus sanguineus, and S. commune, have been shown to produce higher polygalacturonase activities than A. niger on solid wheat bran cultures in a screening study of 75 basidiomycetes (63).
The plant pathogens S. rolfsii and C. purpureum produce an array of GH28 pectinolytic enzymes, which suggests that pectin degradation is important to their pathogenicity (148–150, 157, 158). C. purpureum, which causes a silver leaf disease in apple trees, produces five GH28 PGA isoenzymes corresponding to the five cloned PGA-encoding genes (150). A phylogenetic analysis has shown that the PGA-encoding genes of C. purpureum have undergone duplication after the divergence of ascomycetes and basidiomycetes, suggesting an adaptation to a pectin-rich environment (150). The pectinases of S. rolfsii act under extreme conditions. Pectin methyl esterase (CE8) of S. rolfsii has a very acidic pH optimum (pH 2.5) and also retains most of its activity at pH 1.1 and at 10°C (159). In addition, β-galactosidase of S. rolfsii tolerates acidic conditions, and its optimum pH is between 2 and 2.5 (151).
Very few pectin- and other plant cell wall polysaccharide-degrading enzymes have been isolated from basidiomycetous yeasts (Tables 5, 7, and 11). This corresponds to the limited number of CAZyme-encoding gene models found in the genome of R. glutinis (Table 4). However, the enzymes of basidiomycete yeasts may have interesting biochemical properties. For example, C. capitatum uses pectin as a sole carbon source and produces polygalacturonase (GH28), which is active even at 0°C and therefore has potential to be used in food processing applications (153, 154).
Hemicellulose- and Pectin-Debranching Enzymes
Debranching enzymes that cleave the side chains of hemicelluloses and pectin work synergistically with the enzymes that cleave the backbone and main branches of plant polysaccharides (19). Various debranching enzymes from ascomycetes have also been isolated from basidiomycetes, including α- and β-galactosidases, α-arabinofuranosidases, α-glucuronidases, acetyl xylan esterases, a pectin methyl esterase, and feruloyl esterases (Table 12). Despite the putative gene models present in basidiomycete genomes (Table 4), many debranching enzymes from basidiomycetes still remain uncharacterized, such as α-fucosidase, p-coumaroyl esterase, arabinoxylan arabinofuranohydrolase, endoarabinase, exoarabinase, and endogalactanase. Due to their importance for the complete degradation of plant biomass, future efforts should be directed at isolating debranching enzymes from basidiomycetes.
Only a few white rot fungal debranching enzymes that catalyze the cleavage of the smaller side branches of hemicellulose and pectin main chains have been characterized (Table 12). P. chrysosporium produced α-glucuronidase at high activity levels in a screening study of xylan-degrading fungi, where several ascomycetes, such as Aspergillus awamori and A. niger strains, and basidiomycetes, such as P. chrysosporium and S. commune, were included (160). The molecular mass of P. chrysosporium α-glucuronidase is 112 kDa, and its isoelectric point is 4.6. It has an acidic pH optimum and hydrolyzes short-chain xylooligosaccharides but shows low activity toward glucuronoxylan polysaccharides and xylans of birch, oat spelt, and wheat straw (160). α-Glucuronidase of the white rot fungus P. radiata has been shown to act together with an endoxylanase in the degradation of oat xylan (161). A. niger and A. tubingensis α-glucuronidases are also active mainly on small xylooligomers, and therefore, they are expected to be dependent on the action of endoxylanases (19). Interestingly, S. commune produced an α-glucuronidase that is active against polymeric glucuronoxylan (162) and for which the gene was recently cloned and demonstrated to be a member of GH115 (163). In contrast to this enzyme, a GH115 α-glucuronidase from the ascomycete Pichia stipitis was active only on oligomeric substrates (164).
α-Galactosidases have been isolated from some white rot species and have diverse properties. Both P. chrysosporium and G. lucidum glucomannan-debranching α-galactosidases are produced as tetramers, while the molecular masses of the monomers are 50 and 56 kDa, respectively (165, 166). P. chrysosporium α-galactosidase has an acidic pH optimum of 3.75, and the enzyme is stable from 0 to 80°C (165). The pH optimum of G. lucidum α-galactosidase is 6.0, and its optimum temperature is 70°C (165, 166) The white rot fungus Lenzites elegans secretes a homodimeric α-galactosidase with a molecular mass of 158 kDa (61 kDa for one subunit) (167). α-Galactosidase of L. elegans has an acidic pI value ranging from 4.0 to 4.2 and a pH optimum of 4.5. This enzyme shows activity against several α-galactosidases and is very thermostable, with an optimal temperature from 60°C to 80°C (167).
In contrast, α-galactosidase from the white rot fungus Pleurotus florida is a monomeric protein with a molecular mass of 99 kDa, and its temperature optimum is 55°C (168). The white rot fungus P. radiata produces several isoforms of α-galactosidase when grown in wheat bran- and locus bean gum-containing liquid media (169). Molecular masses of the α-galactosidase isoforms of P. radiata are between 55 and 64 kDa, and their isoelectric points vary from 3.5 to 7.15. P. radiata α-galactosidase isoforms have an optimum pH of 5.0 and show the highest activity at 60°C (169). Several different α-galactosidases have been purified from aspergilli. In addition, a few endo- and exogalactanases from aspergilli have been characterized (19), but these enzymes have not yet been characterized for basidiomycetes.
Acetyl xylan esterases (AXEs), which cleave ester linkages between acetic acid and xylan or mannan, and feruloyl esterases (FAEs), which cleave ester linkages between phenolic acid and the arabinose or galactose side chain of xylan or pectin, have been characterized for the white rot fungi P. chrysosporium, Pleurotus eryngii, Pleurotus sapidus, and S. commune; the jelly fungus Auricularia auricula-judae; and the coprophilic species C. cinerea (Table 12). The genome of P. chrysosporium harbors three AXEs, one of which, PcAxe2, has been biochemically characterized (170). PcAxe2 and AXEs from Aspergillus ficcum, A. awamori, and A. niger show a similar pH optimum (7.0). AXEs of A. ficcum, A. oryzae, and A. niger have slightly higher temperature optima (35°C to 50°C) than that of PcAxe2 (30°C to 35°C) (170–172). PcAxe2 displays low specific activity against birchwood xylan. However, synergistic action between PcAxe2 and the P. chrysosporium endoxylanase PcXynC in xylan degradation has been reported (170).
Only three basidomycete FAEs have been biochemically characterized (173–175). The molecular masses of FAEs of the white rot fungi P. eryngii and P. sapidus are 67 kDa and 55 kDa, respectively, which are higher than those of the FAEs of A. awamori (35 kDa) (176) and A. niger FaeA (36 kDa) (177, 178) but lower than that of A. niger FaeB (74 kDa) (178). P. eryngii FaeA (PeFaeA) and P. sapidus Est1 are 93% similar at the amino acid sequence level (173). However, they do not show significant homology to A. niger FaeA or FaeB, which is also reflected in their biochemical differences. P. sapidus Est1 hydrolyzes arabinosyl esters of ferulic acid more efficiently than methyl ferulate, which is the commonly used substrate for feruloyl esterases (174). PeFaeA had higher activity toward natural substrates, such as feruloylated mono-, di-, and trisaccharides (F-A, F-AX, and F-AXG, respectively), than toward the typical synthetic feruloyl ester substrates methyl ferulate, methyl coumarate, and methyl sinapate (173). In addition, it is not able to hydrolyze methyl caffeate (173). Both PeFaeA and P. sapidus Est1 prefer F-AX over F-A and F-AXG of the natural substrates, whereas A. niger FaeA prefers F-A over F-AX (173, 179). Recently, a novel type of FAE (EstBC), which hydrolyzes both benzoates and cinnamates, has been described for the jelly fungus A. auricula-judae (175).
Only two α-xylosidases from Aspergillus species (19) and, so far, none from basidiomycetes have been characterized. The α-xylosidases from aspergilli are specific for α-linked xylose residues but show differences in the type of glycosides that they are able to hydrolyze (19, 180, 181).
Several cellulases, xylanases, mannanases, and pectinases have been isolated from phytopathogenic basidiomycetes (Table 5). Most of the characterized enzymes are from S. rolfsii, which has a dual life-style as a soil saprotroph and a necrotrophic crop pathogen with a wide host range including >500 plant species (157, 182). S. rolfsii causes large economic losses by infecting several crop and ornamental plants, especially in tropical and subtropical regions. During infection, S. rolfsii produces oxalic acid as well as cellulolytic and pectinolytic enzymes (157, 182). Although the genome of another necrotrophic basidiomycete, the white rot fungus H. irregulare, is already available, the genome of S. rolfsii or a related facultative pathogenic basidiomycete has yet to be sequenced to reveal the full genetic potential of phytopathogens for carbohydrate degradation.
REGULATION OF PLANT POLYSACCHARIDE DEGRADATION IN BASIDIOMYCETES AND Aspergillus
Considering the highly varied composition of plant biomass, efficient degradation of these components by fungi depends on the production of the right combination of enzymes. Therefore, most genes encoding plant-biomass-degrading enzymes are under the control of transcriptional regulators. In aspergilli, several transcriptional regulators (all of the Zn2Cys6 type) that activate the expression of genes involved in plant biomass degradation have been identified, such as XlnR, AraR, GalR, GalX, and RhaR (183). None of these regulators have orthologs in basidiomycetes, but several are found across the phylum Ascomycota (184). This suggests that regulation of plant biomass degradation has developed after ascomycetes and basidiomycetes diverged during fungal evolution. No specific regulators involved in plant biomass degradation in basidiomycetes have been described, but indications for such systems can be derived from transcriptome studies with basidiomycetes, although these indications were not explicitly stated in those reports (17, 18, 70).
Repression of Gene Expression in Basidiomycetes
CreA-mediated repression is induced by monomeric products such as glucose, fructose, and xylose in ascomycetes (19), but the mechanism has not been studied for basidiomycetes. However, CreA homologs have been detected in all basidiomycete genomes sequenced so far (184). In line with the ascomycetes, various basidiomycetes, such as the white rot fungus Trametes (Coriolus) versicolor, the litter decomposers A. bisporus and V. volvacea, the phytopathogen S. rolfsii, and the basidiomycete yeast Rhodotorula minuta, have CAZyme-encoding genes that are repressed by glucose and other monosaccharides (89, 185–188). Some CAZyme-encoding genes are also repressed by monosaccharides that seem unrelated to the enzymes that these genes encode. For example, certain cellulolytic genes are inhibited by lactose, xylose, mannose, and fructose (89, 186, 189). Like ascomycetes, the xynA endoxylanase gene of A. bisporus is repressed by glucose (190).
Copies of a CreA-related binding site, SYGGRT (191), have been detected in the genomes of basidiomycetes. In the white rot fungus I. lacteus, a CreA binding site upstream of the cel2 gene (GH7 and CBHI) has been found between two CAAT boxes, similarly to what was observed for the cbhI gene of A. aculeatus (189). CreA binding elements were also identified in the glycosyl hydrolase promoters of the white rot fungi C. subvermispora and S. commune (12, 163) and the brown rot fungus P. placenta (18, 70). However, these elements were not present upstream of the bglA gene encoding the β-galactosidase of the basidiomycete yeast Sporobolomyces singularis (112). Information from the genomes of basidiomycetes and studies on their genes encoding CAZymes suggests that CreA homologs mediate the repression of some CAZyme-related genes, while other genes are possibly repressed by other mechanisms or constitutively expressed, such as the cbh1-1 and cbh1-2 genes from P. chrysosporium (192).
To relieve glucose repression at low sugar levels, the Snf1 protein kinase, found in Saccharomyces cerevisiae and other ascomycetes (193), targets Mig1, which is a functional homolog of CreA (194). An snf1 gene has also been identified in the phytopathogen U. maydis and was shown to mediate gene expression of at least one EG and one PGA (193). In mutants that did not have snf1, EG- and PGA-encoding genes were expressed at lower levels in high concentrations of glucose. However, xylanase gene expression levels were higher in mutants lacking snf1. This suggests that Snf1 negatively regulates xylanase expression and is required for the induction of EG- and PGA-encoding genes (193). An ortholog of Snf1, SnfA, has been identified in the brown rot fungus P. placenta (18), and it may also exist in other basidiomycetes. However, a direct interaction between Snf1 and CreA has yet to be studied in basidiomycetes.
All these data suggest that CreA homologs in basidiomycetes likely affect the expression of a range of CAZymes and respond to the presence of a variety of monosaccharides, similar to what has been described for Aspergillus species (19). The presence of CreA homologs across the fungal kingdom supports a central role for this regulator in fungal physiology in natural habitats (184).
Induction of Gene Expression in Basidiomycetes
CAZyme-encoding genes of basidiomycetes, from the brown rot fungi F. palustris and G. trabeum to the litter decomposer A. bisporus, are induced when exposed to long polymers of cellulose and hemicellulose (105, 186, 195). The CCAAT binding complex, which enhances the expression of genes located downstream of it, is found in the promoter regions of the genes involved in cellulose and hemicellulose degradation in many Aspergillus species (19). The white rot fungus I. lacteus possesses a CCAAT motif in the promoter region of cel2 (GH7 and CBHI) (189), which suggests that at least some basidiomycete CAZyme-encoding genes may be upregulated by mechanisms analogous to that of ascomycetes. In the plant pathogen C. purpureum, a CCAAT motif was found before the start codon in all five PGA-encoding genes (150). However, not all basidiomycetous CAZyme-encoding genes have a CCAAT binding complex. For example, β-galactosidase of the basidiomycete yeast S. singularis does not possess a CCAAT sequence (112).
The expression of multiple genes encoding plant-polysaccharide-degrading enzymes during growth on plant biomass, as evidenced for several basidiomycete species (9, 12, 17, 70, 196), indicates a regulatory system similar to that described for ascomycetes. The absence of homologs of the ascomycete regulators suggests that this may be due to parallel evolution, during which both fungal phyla developed different regulators that perform the same function. This poses an intriguing question regarding how the expression of plant-biomass-degrading enzymes was organized in the ancestral fungi before the divergence of basidiomycetes and ascomycetes.
CONCLUSIONS AND FUTURE PROSPECTS
The increasing number of genome sequences covering the wide range of diversity of biomass-decomposing fungi has widened our understanding of the enzymatic machinery that they possess for plant cell wall polysaccharide degradation. Since the first whole-genome sequencing of a basidiomycete, P. chrysosporium, next-generation sequencing has facilitated a growing number of genomes and transcriptomes of plant cell wall-decomposing basidiomycetes (197). These complementary “omics” studies have accelerated the process of discovering novel enzyme activities involved in plant cell wall decomposition. In line with the aspergilli, genome sequencing of basidiomycetes has revealed an unexpectedly large repertoire of GHs. For example, <20% of the putative GHs of P. chrysosporium were characterized before the genome sequence was published (59).
At the same time, the genomes of plant-biomass-degrading basidiomycete fungi have revealed putative novel protein-encoding genes, especially among those without known homology, providing enzymes related to plant polysaccharide degradation for further characterization. For instance, several new families, such as the second family of α-glucuronidases (GH115) (163) and a family of glucuronoyl esterases (CE15) (198, 199), have recently been added to the CAZy database. Furthermore, the novel concept of oxidoreductive polysaccharide degradation will provide challenges, as its significance in both the basidiomycete and ascomycete fungi is yet to be fully clarified. The abundance of the LPMO-encoding genes and the diversity of LPMO sequences and described activities for basidiomycetes and other plant-biomass-degrading microorganisms suggest that LPMO-catalyzed oxidation has a major role in plant cell wall polysaccharide conversion (40). The discovery of LPMO changed the concept of cellulose degradation (200, 201), and a recent study demonstrated the ability of LPMO to cleave not only cellulose but also hemicelluloses (40). In the near future, LPMO-catalyzed depolymerization of other plant cell wall polysaccharides besides cellulose and hemicelluloses will most likely be shown. In addition, a new family of fungal LPMOs from A. oryzae was characterized, expanding the substrate range of fungal LPMOs from plant cell wall polysaccharides to chitin (202).
Although genomic approaches have revealed an abundance of putative plant-polysaccharide-degrading enzymes, the number of biochemically characterized basidiomycete CAZymes is still relatively small compared to the number of Aspergillus enzymes. For example, representatives of basidiomycete β-galactosidases and endo- and exoarabinases remain to be studied. Future efforts should be directed toward revealing the catalytic potential of basidiomycete CAZymes in biomass utilization by studying enzymes from diverse species. Several complementary techniques have already been employed in secretome studies of basidiomycetes related to lignocellulose degradation (143). However, the number of basidiomycete secretomes examined is still small, and thus, only preliminary conclusions can be drawn. In the future, improved quality of genome assemblies will improve the detection of secreted proteins, thus also clarifying the overall picture of plant polysaccharide degradation (143). This will result in a better understanding of the relationship between fungi and their biotope and will aid in designing new and improved industrial applications.
Together with genomic comparisons between different species, transcriptome and proteome studies have opened up the way to untangle the complex regulatory mechanisms of basidiomycetes that underlie the plant polysaccharide conversion processes. However, more genome sequences are still needed to reveal the full potential and diversity of basidiomycetes for plant biomass degradation. In addition to functional genomics, metabolomics studies combined with knowledge on the regulation of basidiomycete CAZyme-encoding genes will increase our understanding of the complex processes of plant cell wall polysaccharide degradation. It will also facilitate a shift from a descriptive characterization of individual enzymes in plant biomass decay to detailed insight into complex decomposition mechanisms.
ACKNOWLEDGMENTS
J.R. acknowledges funding from the Doctoral Programme in Microbiology and Biotechnology. M.R.M. was supported by European Union grant (Optibiocat) EU FP7 201401.
Biographies

Johanna Rytioja obtained her B.Sc. in Biotechnology at the University of Helsinki, Finland, in 2008. She received her M.Sc. in Biotechnology from the University of Helsinki in 2010 under the supervision of Dr. T. Lundell and Dr. M. Mäkelä, studying the production and reactions of laccases of the white rot fungus Physisporinus rivulosus. She is currently finishing her Ph.D. on biotechnology in the Microbiology and Biotechnology Doctoral Programme at the University of Helsinki, under the supervision of Prof. A. Hatakka, Dr. M. Mäkelä, and Dr. K. Hildén. Her Ph.D. focuses on carbohydrate-active enzymes of basidiomycete white rot fungi, in particular cellobiohydrolases, cellobiose dehydrogenases, and the set of enzymes produced by the white rot fungus Dichomitus squalens, during growth on diverse plant biomass substrates.

Kristiina Hildén is principal investigator of the Fungal Genetics and Biotechnology group of the Department of Food and Environmental Sciences of the University of Helsinki, Finland. She received her M.Sc. in Genetics from the University of Helsinki in 1995 and a Ph.D. in Genetics from the same University under the supervision of Prof. O. Ritvos in 2003. She joined the Department of Food and Environmental Sciences of the University of Helsinki in 2000, and since then, her research has included various aspects of fungal molecular biology and enzymology. From 2009 to 2011, she interrupted her research at the University of Helsinki for a 2-year Marie Curie Intra-European Fellowship project at the University of Nottingham, United Kingdom. Upon returning to Helsinki, she developed her own research line at the group, focusing on the functional genomics of plant-biomass-degrading fungi from various biotopes. In addition, several more applied projects are ongoing in the group.

Jennifer Yuzon obtained her B.Sc. in Microbiology at the University of California, San Diego, in 2011. She received her M.Sc. in Environmental Biology from Utrecht University under the mentorship of Prof. R. de Vries. In the Fungal Physiology Laboratory of Prof. de Vries, she assisted with culturing of various strains of Agaricus bisporus and studied their extracellular carbohydrate-active enzymes. She also investigated the molecular phylogeny and taxonomy of the lichenized fungus Bagliettoa under the mentorship of Dr. C. Gueidan at the Natural History Museum, London, United Kingdom. She is currently in the doctoral program in Plant Pathology at the University of California, Davis, under the mentorship of Dr. T. Kasuga. Since she joined Dr. Kasuga's Phytophthora Genomics Laboratory in 2014, her research interests include the epigenetic mechanisms contributing to the invasive biology and pathogenicity of Phytophthora ramorum.

Annele Hatakka is a Professor in Environmental Biotechnology at the Division of Microbiology and Biotechnology, Department of Food and Environmental Sciences, University of Helsinki, Helsinki, Finland. Since the 1990s, she has been the leader of the research group on biotechnology of renewable natural resources. She obtained her Ph.D. in Microbiology at the University of Helsinki in 1986. The topic was degradation and conversion of lignin, lignin-related aromatic compounds. and lignocellulose by white rot fungi. She has worked for 1.5 years at the Swedish Forest Products Laboratory (STFI), Stockholm, Sweden, and for shorter periods at the INRA, France, and the United Kingdom. After working for 24 years in various research positions at the Academy of Finland, she became Professor in Environmental Biotechnology in 1997, which was converted to a permanent professorship in 2008. Her main research areas are lignocellulose degradation by white rot and other fungi, lignocellulose-degrading enzymes, applications in the pulp and paper industry, and bioremediation of polluted soils.

Ronald P. de Vries is head of the Fungal Physiology group of the CBS-KNAW Fungal Biodiversity Center in Utrecht, The Netherlands. He received his M.Sc. in Molecular Sciences in 1992 from Wageningen University, The Netherlands, and a Ph.D. in Fungal Molecular Biology from the same University under the supervision of Dr. J. Visser in 1999. Afterwards, he performed postdoc work at Wageningen University, the Institut Pasteur, and Utrecht University. In 2009, he was hired to build a group on Fungal Physiology at the CBS-KNAW Fungal Biodiversity Center, which mainly addresses plant biomass utilization by fungi. His current research focuses mainly on understanding fungal diversity with respect to plant biomass utilization, using a multidisciplinary approach combining (post)genomics with molecular biology, physiology, biochemistry, and microscopy. Special emphasis is placed on regulatory systems that control this aspect of fungal life. As of May 2014, he is also Professor in Fungal Molecular Physiology at Utrecht University, The Netherlands.

Miia R. Mäkelä is principal investigator of the Fungal Genetics and Biotechnology group of the Department of Food and Environmental Sciences of the University of Helsinki, Finland. She received her M.Sc. in Microbiology from the University of Helsinki in 2000 and a Ph.D. in Microbiology from the same University under the supervision of Prof. A. Hatakka and Dr. T. Lundell in 2009. Her Ph.D. work focused on the role of oxalate-converting enzymes related to lignin modification, which has remained a topic of interest in her research. She has performed postdoc projects related to various aspects of lignocellulose degradation by fungi and more recently also on the applications of various enzyme classes in industrial processes. She has recently expanded her work on oxalate metabolism to a more global look at carbon metabolism of wood-decaying fungi that also includes the conversion of plant-based monomeric carbon sources by basidiomycete fungi.
REFERENCES
- 1.Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. 2007. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 315:804–807. 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 2.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. 2014. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42:D490–D495. 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlile MJ, Watkinson SC, Gooday GW. 2001. The fungi, 2nd ed. Academic Press, London, United Kingdom. [Google Scholar]
- 4.Deacon JW. 2005. Fungal biology. Blackwell, Oxford, United Kingdom. [Google Scholar]
- 5.Eriksson K, Blanchette RA, Ander P. 1990. Microbial and enzymatic degradation of wood and wood components. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 6.Martinez D, Larrondo LF, Putnam N, Sollewijn Gelpke MD, Huang K, Chapman J, Helfenbein KG, Ramaiya P, Detter JC, Larimer F, Coutinho PM, Henrissat B, Berka R, Cullen D, Rokhsar D. 2004. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat. Biotechnol. 22:695–700. 10.1038/nbt967. [DOI] [PubMed] [Google Scholar]
- 7.Martin F, Aerts A, Ahrén D, Brun A, Danchin EGJ, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V, Salamov A, Shapiro HJ, Wuyts J, Blaudez D, Buée M, Brokstein P, Canbäck B, Cohen D, Courty PE, Coutinho PM, Delaruelle C, Detter JC, Deveau A, DiFazio S, Duplessis S, Fraissinet-Tachet L, Lucic E, Frey-Klett P, Fourrey C, Feussner I, Gay G, Grimwood J, Hoegger PJ, Jain P, Kilaru S, Labbé J, Lin YC, Legué V, Le Tacon F, Marmeisse R, Melayah D, Montanini B, Muratet M, Nehls U, Niculita-Hirzel H, Oudot-Le Secq MP, Peter M, Quesneville H, et al. 2008. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452:88–92. 10.1038/nature06556. [DOI] [PubMed] [Google Scholar]
- 8.Kämper J, Kahmann R, Bölker M, Ma L-J, Brefort T, Saville BJ, Banuett F, Kronstad JW, Gold SE, Müller O, Perlin MH, Wösten HAB, de Vries R, Ruiz-Herrera J, Reynaga-Peña CG, Snetselaar K, McCann M, Pérez-Martín J, Feldbrügge M, Basse CW, Steinberg G, Ibeas JI, Holloman W, Guzman P, Farman M, Stajich JE, Sentandreu R, González-Prieto JM, Kennell JC, Molina L, Schirawski J, Mendoza-Mendoza A, Greilinger D, Münch K, Rössel N, Scherer M, Vranes M, Ladendorf O, Vincon V, Fuchs U, Sandrock B, Meng S, Ho ECH, Cahill MJ, Boyce KJ, Klose J, Klosterman SJ, Deelstra HJ, Ortiz-Castellanos L, Li W, et al. 2006. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444:97–101. 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- 9.Morin E, Kohler A, Baker AR, Foulongne-Oriol M, Lombard V, Nagy LG, Ohm RA, Patyshakuliyeva A, Brun A, Aerts AL, Bailey AM, Billette C, Coutinho PM, Deakin G, Doddapaneni H, Floudas D, Grimwood J, Hildén K, Kües U, LaButti KM, Lapidus A, Lindquist EA, Lucas SM, Murat C, Riley RW, Salamov AA, Schmutz J, Subramanian V, Wösten HAB, Xu J, Eastwood DC, Foster GD, Sonnenberg ASM, Cullen D, de Vries RP, Lundell T, Hibbett DS, Henrissat B, Burton KS, Kerrigan RW, Challen MP, Grigoriev IV, Martin F. 2012. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. U. S. A. 109:17501–17506. 10.1073/pnas.1206847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duplessis S, Cuomo CA, Lin Y-C, Aerts A, Tisserant E, Veneault-Fourrey C, Joly DL, Hacquard S, Amselem J, Cantarel BL, Chiu R, Coutinho PM, Feaue N, Field M, Frey P, Gelhaye E, Goldberg J, Grabherr MG, Kodira CD, Kohler A, Kües U, Lindquist EA, Lucas SM, Mago R, Mauceli E, Morin E, Murat C, Pangilinan JL, Park R, Pearson M, Quesneville H, Rouhier N, Sakthikumar S, Salamov AA, Schmutz J, Selles B, Shapiro H, Tanguay P, Tuskan GA, Henrissat B, van de Peer Y, Rouzé P, Ellis JG, Dodds PN, Schein JE, Zhong S, Hamelin RC, Grigoriev IV, Szabo LJ, Martin F. 2011. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc. Natl. Acad. Sci. U. S. A. 108:9166–9171. 10.1073/pnas.1019315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TKA, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, et al. 2012. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719. 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Fueyo E, Ruiz-Dueñas FJ, Ferreira P, Floudas D, Hibbett DS, Canessa P, Larrondo LF, James TY, Seelenfreund D, Lobos S, Polanco R, Tello M, Honda Y, Watanabe T, Watanabe T, Ryu JS, Kubicek CP, Schmoll M, Gaskell J, Hammel KE, St John FJ, Vanden Wymelenberg A, Sabat G, Splinter BonDurant S, Syed K, Yadav JS, Doddapaneni H, Subramanian V, Lavín JL, Oguiza JA, Perez G, Pisabarro AG, Ramirez L, Santoyo F, Master E, Coutinho PM, Henrissat B, Lombard V, Magnuson JK, Kües U, Hori C, Igarashi K, Samejima M, Held BW, Barry KW, LaButti KM, Lapidus A, Lindquist EA, Lucas SM, Riley R, et al. 2012. Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis. Proc. Natl. Acad. Sci. U. S. A. 109:5458–5463. 10.1073/pnas.1119912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao D, Gong M, Zheng H, Chen M, Zhang L, Wang H, Jiang J, Wu L, Zhu Y, Zhu G, Zhou Y, Li C, Wang S, Zhao Y, Zhao G, Tan Q. 2013. Sequencing and comparative analysis of the straw mushroom (Volvariella volvacea) genome. PLoS One 8:e58294. 10.1371/journal.pone.0058294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, Li C, Wang L, Guo X, Sun Y, Luo H, Li Y, Song J, Henrissat B, Levasseur A, Qian J, Li J, Luo X, Shi L, He L, Xiang L, Xu X, Niu Y, Li Q, Han MV, Yan H, Zhang J, Chen H, Lv A, Wang Z, Liu M, Schwartz DC, Sun C. 2012. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 3:913. 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohm RA, De Jong JF, Lugones LG, Aerts A, Kothe E, Stajich JE, de Vries RP, Record E, Levasseur A, Baker SE, Bartholomew KA, Coutinho PM, Erdmann S, Fowler TJ, Gathman AC, Lombard V, Henrissat B, Knabe N, Kües U, Lilly WW, Lindquist E, Lucas S, Magnuson JK, Piumi F, Raudaskoski M, Salamov A, Schmutz J, Schwarze FWMR, van Kuyk PA, Horton JS, Grigoriev IV, Wösten HAB. 2010. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 28:957–963. 10.1038/nbt.1643. [DOI] [PubMed] [Google Scholar]
- 16.Manavalan T, Manavalan A, Thangavelu KP, Heese K. 2012. Secretome analysis of Ganoderma lucidum cultivated in sugarcane bagasse. J. Proteomics 77:298–309. 10.1016/j.jprot.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Vanden Wymelenberg A, Gaskell J, Mozuch M, Kersten P, Sabat G, Martinez D, Cullen D. 2009. Transcriptome and secretome analyses of Phanerochaete chrysosporium reveal complex patterns of gene expression. Appl. Environ. Microbiol. 75:4058–4068. 10.1128/AEM.00314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez D, Challacombe J, Morgenstern I, Hibbett D, Schmoll M, Kubicek CP, Ferreira P, Ruiz-Dueñas FJ, Martinez AT, Kersten P, Hammel KE, Vanden Wymelenberg A, Gaskell J, Lindquist E, Sabat G, BonDurant SS, Larrondo LF, Canessa P, Vicuña R, Yadav J, Doddapaneni H, Subramanian V, Pisabarro AG, Lavín JL, Oguiza JA, Master E, Henrissat B, Coutinho PM, Harris P, Magnuson JK, Baker SE, Bruno K, Kenealy W, Hoegger PJ, Kües U, Ramaiya P, Lucas S, Salamov A, Shapiro H, Tu H, Chee CL, Misra M, Xie G, Teter S, Yaver D, James T, Mokrejs M, Pospisek M, Grigoriev IV, Brettin T, Rokhsar D, Berka R, Cullen D. 2009. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc. Natl. Acad. Sci. U. S. A. 106:1954–1959. 10.1073/pnas.0809575106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vries RP, Visser J. 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 65:497–522. 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjöström E. 1993. Wood chemistry: fundamentals and applications. Academic Press, San Diego, CA. [Google Scholar]
- 21.Harris PJ, Stone BA. 3 March 2009. Chapter 4. Chemistry and molecular organization of plant cell walls, p 61–93 In Himmel ME. (ed), Biomass recalcitrance: deconstructing the plant cell wall for bioenergy. Blackwell Publishing Ltd, Oxford, United Kingdom. 10.1002/9781444305418.ch4. [DOI] [Google Scholar]
- 22.Vogel J. 2008. Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 11:301–307. 10.1016/j.pbi.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Kolpak FJ, Blackwell J. 1976. Determination of the structure of cellulose II. Macromolecules 9:273–278. 10.1021/ma60050a019. [DOI] [PubMed] [Google Scholar]
- 24.van den Brink J, de Vries RP. 2011. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 91:1477–1492. 10.1007/s00253-011-3473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caffall KH, Mohnen D. 2009. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344:1879–1900. 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 26.von Ossowski I, Ståhlberg J, Koivula A, Piens K, Becker D, Boer H, Harle R, Harris M, Divne C, Mahdi S, Zhao Y, Driguez H, Claeyssens M, Sinnott ML, Teeri TT. 2003. Engineering the exo-loop of Trichoderma reesei cellobiohydrolase, Cel7A. A comparison with Phanerochaete chrysosporium Cel7D. J. Mol. Biol. 333:817–829. 10.1016/S0022-2836(03)00881-7. [DOI] [PubMed] [Google Scholar]
- 27.Harris PV, Welner D, McFarland KC, Re E, Navarro Poulsen J-C, Brown K, Salbo R, Ding H, Vlasenko E, Merino S, Xu F, Cherry J, Larsen S, Lo Leggio L. 2010. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family. Biochemistry 49:3305–3316. 10.1021/bi100009p. [DOI] [PubMed] [Google Scholar]
- 28.Quinlan RJ, Sweeney MD, Lo Leggio L, Otten H, Navarro Poulsen J-C, Johansen KS, Krogh KBRM, Jørgensen CI, Tovborg M, Anthonsen A, Tryfona T, Walter CP, Dupree P, Xu F, Davies GJ, Walton PH. 2011. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. U. S. A. 108:15079–15084. 10.1073/pnas.1105776108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriksson G, Johansson G, Pettersson G. 2000. A critical review of cellobiose dehydrogenases. J. Biotechnol. 78:93–113. 10.1016/S0168-1656(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig R, Harreither W, Tasca F, Gorton L. 2010. Cellobiose dehydrogenase: a versatile catalyst for electrochemical applications. Chemphyschem 11:2674–2697. 10.1002/cphc.201000216. [DOI] [PubMed] [Google Scholar]
- 31.Langston JA, Shaghasi T, Abbate E, Xu F, Vlasenko E, Sweeney MD. 2011. Oxidoreductive cellulose depolymerization by the enzymes cellobiose dehydrogenase and glycoside hydrolase 61. Appl. Environ. Microbiol. 77:7007–7015. 10.1128/AEM.05815-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips CM, Beeson WT, Cate JH, Marletta MA. 2011. Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem. Biol. 6:1399–1406. 10.1021/cb200351y. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Beeson WT, IV, Phillips C, Marletta M, Cate JD. 2012. Structural basis for substrate targeting and catalysis by fungal polysaccharide monooxygenases. Structure 20:1051–1061. 10.1016/j.str.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isaksen T, Westereng B, Aachmann FL, Agger JW, Kracher D, Kittl R, Ludwig R, Haltrich D, Eijsink VGH, Horn SJ. 2014. A C4-oxidizing lytic polysaccharide monooxygenase cleaving both cellulose and cello-oligosaccharides. J. Biol. Chem. 289:2632–2642. 10.1074/jbc.M113.530196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemsworth GR, Davies GJ, Walton PH. 2013. Recent insights into copper-containing lytic polysaccharide mono-oxygenases. Curr. Opin. Struct. Biol. 23:660–668. 10.1016/j.sbi.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Dimarogona M, Topakas E, Olsson L, Christakopoulos P. 2012. Lignin boosts the cellulase performance of a GH-61 enzyme from Sporotrichum thermophile. Bioresour. Technol. 110:480–487. 10.1016/j.biortech.2012.01.116. [DOI] [PubMed] [Google Scholar]
- 37.Decker SR, Siika-Aho M, Viikari L. 3 March 2009. Chapter 10. Enzymatic depolymerization of plant cell wall hemicelluloses, p 352–373 In Himmel ME. (ed), Biomass recalcitrance: deconstructing the plant cell wall for bioenergy. Blackwell Publishing Ltd, Oxford, United Kingdom. 10.1002/9781444305418.ch10. [DOI] [Google Scholar]
- 38.Ghosh M, Nanda G. 1994. Purification and some properties of a xylanase from Aspergillus sydowii MG49. Appl. Environ. Microbiol. 60:4620–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS. 2005. Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67:577–591. 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- 40.Agger JW, Isaksen T, Várnai A, Vidal-Melgosa S, Willats WGT, Ludwig R, Horn SJ, Eijsink VGH, Westereng B. 2014. Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc. Natl. Acad. Sci. U. S. A. 111:6287–6292. 10.1073/pnas.1323629111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sygmund C, Kracher D, Scheiblbrandner S, Zahma K, Felice AKG, Harreither W, Kittl R, Ludwig R. 2012. Characterization of the two Neurospora crassa cellobiose dehydrogenases and their connection to oxidative cellulose degradation. Appl. Environ. Microbiol. 78:6161–6171. 10.1128/AEM.01503-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mäkelä MR, Hildén KS, de Vries RP. 2014. Degradation and modification of plant biomass by fungi, p 175–208 In Nowrousian M. (ed), The mycota, fungal genomics, 2nd ed, vol 13 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 43.de Wit PJGM, van der Burgt A, Ökmen B, Stergiopoulos I, Abd-Elsalam KA, Aerts AL, Bahkali AH, Beenen HG, Chettri P, Cox MP, Datema E, de Vries RP, Dhillon B, Ganley AR, Griffiths SA, Guo Y, Hamelin RC, Henrissat B, Kabir MS, Jashni MK, Kema G, Klaubauf S, Lapidus A, Levasseur A, Lindquist E, Mehrabi R, Ohm RA, Owen TJ, Salamov A, Schwelm A, Schijlen E, Sun H, van den Burg HA, van Ham RCHJ, Zhang S, Goodwin SB, Grigoriev IV, Collemare J, Bradshaw RE. 2012. The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 8:e1003088. 10.1371/journal.pgen.1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benoit I, Coutinho PM, Schols HA, Gerlach JP, Henrissat B, de Vries RP. 2012. Degradation of different pectins by fungi: correlations and contrasts between the pectinolytic enzyme sets identified in genomes and the growth on pectins of different origin. BMC Genomics 13:321. 10.1186/1471-2164-13-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki H, MacDonald J, Syed K, Salamov A, Hori C, Aerts A, Henrissat B, Wiebenga A, van Kuyk PA, Barry K, Lindquist E, LaButti K, Lapidus A, Lucas S, Coutinho P, Gong Y, Samejima M, Mahadevan R, Abou-Zaid M, de Vries RP, Igarashi K, Yadav JS, Grigoriev IV, Master ER. 2012. Comparative genomics of the white-rot fungi, Phanerochaete carnosa and P. chrysosporium, to elucidate the genetic basis of the distinct wood types they colonize. BMC Genomics 13:444. 10.1186/1471-2164-13-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Battaglia E, Benoit I, van den Brink J, Wiebenga A, Coutinho PM, Henrissat B, de Vries RP. 2011. Carbohydrate-active enzymes from the zygomycete fungus Rhizopus oryzae: a highly specialized approach to carbohydrate degradation depicted at genome level. BMC Genomics 12:38. 10.1186/1471-2164-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amselem J, Cuomo CA, van Kan JAL, Viaud M, Benito EP, Couloux A, Coutinho PM, de Vries RP, Dyer PS, Fillinger S, Fournier E, Gout L, Hahn M, Kohn L, Lapalu N, Plummer KM, Pradier J-M, Quévillon E, Sharon A, Simon A, Have A, Tudzynski B, Tudzynski P, Wincker P, Andrew M, Anthouard V, Beever RE, Beffa R, Benoit I, Bouzid O, Brault B, Chen Z, Choquer M, Collémare J, Cotton P, Danchin EG, Da Silva C, Gautier A, Giraud C, Giraud T, Gonzalez C, Grossetete S, Güldener U, Henrissat B, Howlett BJ, Kodira C, Kretschmer M, Lappartient A, Leroch M, Levis C, et al. 2011. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 7:e1002230. 10.1371/journal.pgen.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berka RM, Grigoriev IV, Otillar R, Salamov A, Grimwood J, Reid I, Ishmael N, John T, Darmond C, Moisan M-C, Henrissat B, Coutinho PM, Lombard V, Natvig DO, Lindquist E, Schmutz J, Lucas S, Harris P, Powlowski J, Bellemare A, Taylor D, Butler G, de Vries RP, Allijn IE, van den Brink J, Ushinsky S, Storms R, Powell AJ, Paulsen IT, Elbourne LDH, Baker SE, Magnuson J, Laboissiere S, Clutterbuck AJ, Martinez D, Wogulis M, De Leon AL, Rey MW, Tsang A. 2011. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 29:922–929. 10.1038/nbt.1976. [DOI] [PubMed] [Google Scholar]
- 49.Coutinho PM, Andersen MR, Kolenová K, van Kuyk PA, Benoit I, Gruben BS, Trejo-Aguilar B, Visser H, van Solingen P, Pakula T, Seiboth B, Battaglia E, Aguilar-Osorio G, de Jong JF, Ohm RA, Aguilar M, Henrissat B, Nielsen J, Stålbrand H, de Vries RP. 2009. Post-genomic insights into the plant polysaccharide degradation potential of Aspergillus nidulans and comparison to Aspergillus niger and Aspergillus oryzae. Fungal Genet. Biol. 46(Suppl 1):S161–S169. 10.1016/j.fgb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 50.Espagne E, Lespinet O, Malagnac F, Da Silva C, Jaillon O, Porcel BM, Couloux A, Aury JM, Ségurens B, Poulain J, Anthouard V, Grossetete S, Khalili H, Coppin E, Déquard-Chablat M, Picard M, Contamine V, Arnaise S, Bourdais A, Berteaux-Lecellier V, Gautheret D, de Vries RP, Battaglia E, Coutinho PM, Danchin EG, Henrissat B, Khoury RE, Sainsard-Chanet A, Boivin A, Pinan-Lucarré B, Sellem CH, Debuchy R, Wincker P, Weissenbach J, Silar P. 2008. The genome sequence of the model ascomycete fungus Podospora anserina. Genome Biol. 9:R77. 10.1186/gb-2008-9-5-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, Danchin EGJ, Grigoriev IV, Harris P, Jackson M, Kubicek CP, Han CS, Ho I, Larrondo LF, De Leon AL, Magnuson JK, Merino S, Misra M, Nelson B, Putnam N, Robbertse B, Salamov AA, Schmoll M, Terry A, Thayer N, Westerholm-Parvinen A, Schoch CL, Yao J, Barbote R, Nelson MA, Detter C, Bruce D, Kuske CR, Xie G, Richardson P, Rokhsar DS, Lucas SM, Rubin EM, Dunn-Coleman N, Ward M, Brettin TS. 2008. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat. Biotechnol. 26:553–560. 10.1038/nbt1403. [DOI] [PubMed] [Google Scholar]
- 52.Gilbertson RL. 1980. Wood-rotting fungi of North America. Mycologia 72:1–49. 10.2307/3759417. [DOI] [Google Scholar]
- 53.Ryvarden L. 1991. Genera of polypores, nomenclature and taxonomy. Syn. Fungorum 5:1–363. [Google Scholar]
- 54.Várnai A, Mäkelä MR, Djajadi DT, Rahikainen J, Hatakka A, Viikari L. 2014. Carbohydrate-binding modules of fungal cellulases. Occurrence in nature, function, and relevance in industrial biomass conversion. Adv. Appl. Microbiol. 88:103–165. 10.1016/B978-0-12-800260-5.00004-8. [DOI] [PubMed] [Google Scholar]
- 55.Riley R, Salamov AA, Brown DW, Nagy LG, Floudas D, Held BW, Levasseur A, Lombard V, Morin E, Otillar R, Lindquist EA, Sun H, LaButti KM, Schmutz J, Jabbour D, Luo H, Baker SE, Pisabarro AG, Walton JD, Blanchette RA, Henrissat B, Martin F, Cullen D, Hibbett DS, Grigoriev IV. 2014. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. U. S. A. 111:9923–9928. 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatakka A, Hammel K. 2011. Fungal biodegradation of lignocelluloses, p 319–340 In Hofrichter M. (ed), The mycota, industrial applications, 2nd ed, vol 10 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 57.Vanden Wymelenberg A, Minges P, Sabat G, Martinez D, Aerts A, Salamov A, Grigoriev I, Shapiro H, Putnam N, Belinky P, Dosoretz C, Gaskell J, Kersten P, Cullen D. 2006. Computational analysis of the Phanerochaete chrysosporium v2.0 genome database and mass spectrometry identification of peptides in ligninolytic cultures reveal complex mixtures of secreted proteins. Fungal Genet. Biol. 43:343–356. 10.1016/j.fgb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Vanden Wymelenberg A, Sabat G, Martinez D, Rajangam AS, Teeri TT, Gaskell J, Kersten PJ, Cullen D. 2005. The Phanerochaete chrysosporium secretome: database predictions and initial mass spectrometry peptide identifications in cellulose-grown medium. J. Biotechnol. 118:17–34. 10.1016/j.jbiotec.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Mahajan S, Master ER. 2010. Proteomic characterization of lignocellulose-degrading enzymes secreted by Phanerochaete carnosa grown on spruce and microcrystalline cellulose. Appl. Microbiol. Biotechnol. 86:1903–1914. 10.1007/s00253-010-2516-4. [DOI] [PubMed] [Google Scholar]
- 60.Niemelä T, Kinnunen J. 2005. Käävät, puiden sienet. Luonnontieteellinen Keskusmuseo, Kasvimuseo, Helsinki, Finland. [Google Scholar]
- 61.Rytioja J, Hildén K, Hatakka A, Mäkelä MR. 3 January 2014. Transcriptional analysis of selected cellulose-acting enzymes encoding genes of the white-rot fungus Dichomitus squalens on spruce wood and microcrystalline cellulose. Fungal Genet. Biol. 10.1016/j.fgb.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Liu D, Gong J, Dai W, Kang X, Huang Z, Zhang H-M, Liu W, Liu L, Ma J, Xia Z, Chen Y, Chen Y, Wang D, Ni P, Guo A-Y, Xiong X. 2012. The genome of Ganderma lucidum provide insights into triterpense biosynthesis and wood degradation. PLoS One 7:e36146. 10.1371/journal.pone.0036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xavier-Santos S, Carvalho CC, Bonfá M, Silva R, Capelari M, Gomes E. 2004. Screening for pectinolytic activity of wood-rotting basidiomycetes and characterization of the enzymes. Folia Microbiol. (Praha) 49:46–52. 10.1007/BF02931645. [DOI] [PubMed] [Google Scholar]
- 64.Otrosina WJ, Garbelotto M. 2010. Heterobasidion occidentale sp. nov. and Heterobasidion irregulare nom. nov.: a disposition of North American Heterobasidion biological species. Fungal Biol. 114:16–25. 10.1016/j.mycres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Lind M, Stenlid J, Olson T. 2014. Heterobasidion annosum s.l. genomics. Adv. Bot. Res. 70:371–396. 10.1016/B978-0-12-397940-7.00012-4. [DOI] [Google Scholar]
- 66.Olson Å, Aerts A, Asiegbu F, Belbahri L, Bouzid O, Broberg A, Canbäck B, Coutinho PM, Cullen D, Dalman K, Deflorio G, van Diepen LTA, Dunand C, Duplessis S, Durling M, Gonthier P, Grimwood J, Fossdal CG, Hansson D, Henrissat B, Hietala A, Himmelstrand K, Hoffmeister D, Högberg N, James TY, Karlsson M, Kohler A, Kües U, Lee Y-H, Lin Y-C, Lind M, Lindquist E, Lombard V, Lucas S, Lundén K, Morin E, Murat C, Park J, Raffaello T, Rouzé P, Salamov A, Schmutz J, Solheim H, Ståhlberg J, Vélëz H, de Vries RP, Wiebenga A, Woodward S, Yakovlev I, Garbelotto M, Martin F, Grigoriev IV, Stenlid J. 2012. Insight into trade-off between wood decay and parasitism from the genome of a fungal forest pathogen. New Phytol. 194:1001–1013. 10.1111/j.1469-8137.2012.04128.x. [DOI] [PubMed] [Google Scholar]
- 67.Hori C, Gaskell J, Igarashi K, Samejima M, Hibbett D, Henrissat B, Cullen D. 2013. Genomewide analysis of polysaccharides degrading enzymes in 11 white- and brown-rot Polyporales provides insight into mechanisms of wood decay. Mycologia 105:1412–1427. 10.3852/13-072. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE. 2006. Fungal hydroquinones contribute to brown rot of wood. Environ. Microbiol. 8:2214–2223. 10.1111/j.1462-2920.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- 69.Xu G, Goodell B. 2001. Mechanisms of wood degradation by brown-rot fungi: chelator-mediated cellulose degradation and binding of iron by cellulose. J. Biotechnol. 87:43–57. 10.1016/S0168-1656(00)00430-2. [DOI] [PubMed] [Google Scholar]
- 70.Vanden Wymelenberg A, Gaskell J, Mozuch M, Sabat G, Ralph J, Skyba O, Mansfield SD, Blanchette RA, Martinez D, Grigoriev I, Kersten PJ, Cullen D. 2010. Comparative transcriptome and secretome analysis of wood decay fungi Postia placenta and Phanerochaete chrysosporium. Appl. Environ. Microbiol. 76:3599–3610. 10.1128/AEM.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eastwood DC, Floudas D, Binder M, Majcherczyk A, Schneider P, Aerts A, Asiegbu FO, Baker SE, Barry K, Bendiksby M, Blumentritt M, Coutinho PM, Cullen D, de Vries RP, Gathman A, Goodell B, Henrissat B, Ihrmark K, Kauserud H, Kohler A, LaButti K, Lapidus A, Lavin JL, Lee Y-H, Lindquist E, Lilly W, Lucas S, Morin E, Murat C, Oguiza JA, Park J, Pisabarro AG, Riley R, Rosling A, Salamov A, Schmidt O, Schmutz J, Skrede I, Stenlid J, Wiebenga A, Xie X, Kües U, Hibbett DS, Hoffmeister D, Högberg N, Martin F, Grigoriev IV, Watkinson SC. 2011. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 333:762–765. 10.1126/science.1205411. [DOI] [PubMed] [Google Scholar]
- 72.Chen B, Gui F, Xie B, Deng Y, Sun X, Lin M, Tao Y, Li S. 2013. Composition and expression of genes encoding carbohydrate-active enzymes in the straw-degrading mushroom Volvariella volvacea. PLoS One 8:e58780. 10.1371/journal.pone.0058780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagendran S, Hallen-Adams HE, Paper JM, Aslam N, Walton JD. 2009. Reduced genomic potential for secreted plant cell-wall-degrading enzymes in the ectomycorrhizal fungus Amanita bisporigera, based on the secretome of Trichoderma reesei. Fungal Genet. Biol. 46:427–435. 10.1016/j.fgb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 74.Sakai T, Sakamoto T, Hallaert J, Vandamme EJ. 1993. Pectin, pectinase, and protopectinase: production, properties, and applications. Adv. Appl. Microbiol. 39:213–294. 10.1016/S0065-2164(08)70597-5. [DOI] [PubMed] [Google Scholar]
- 75.Lindeberg G, Lindeberg M. 1977. Pectinolytic ability of some mycorrhizal and saprophytic hymenomycetes. Arch. Microbiol. 115:9–12. 10.1007/BF00427838. [DOI] [PubMed] [Google Scholar]
- 76.Divon HH, Fluhr R. 2007. Nutrition acquisition strategies during fungal infection of plants. FEMS Microbiol. Lett. 266:65–74. 10.1111/j.1574-6968.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 77.Zuccaro A, Lahrmann U, Güldener U, Langen G, Pfiffi S, Biedenkopf D, Wong P, Samans B, Grimm C, Basiewicz M, Murat C, Martin F, Kogel K-H. 2011. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 7:e1002290. 10.1371/journal.ppat.1002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rineau F, Roth D, Shah F, Smits M, Johansson T, Canbäck B, Olsen PB, Persson P, Grell MN, Lindquist E, Grigoriev IV, Lange L, Tunlid A. 2012. The ectomycorrhizal fungus Paxillus involutus converts organic matter in plant litter using a trimmed brown-rot mechanism involving Fenton chemistry. Environ. Microbiol. 14:1477–1487. 10.1111/j.1462-2920.2012.02736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rineau F, Shah F, Smits MM, Persson P, Johansson T, Carleer R, Troein C, Tunlid A. 2013. Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. ISME J. 7:2010–2022. 10.1038/ismej.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leonard KJ, Szabo LJ. 2005. Stem rust of small grains and grasses caused by Puccinia graminis. Mol. Plant Pathol. 6:99–111. 10.1111/j.1364-3703.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 81.Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, Vamathevan J, Miranda M, Anderson IJ, Fraser JA, Allen JE, Bosdet IE, Brent MR, Chiu R, Doering TL, Donlin MJ, D'Souza CA, Fox DS, Grinberg V, Fu J, Fukushima M, Haas BJ, Huang JC, Janbon G, Jones SJM, Koo HL, Krzywinski MI, Kwon-Chung JK, Lengeler KB, Maiti R, Marra MA, Marra RE, Mathewson CA, Mitchell TG, Pertea M, Riggs FR, Salzberg SL, Schein JE, Shvartsbeyn A, Shin H, Shumway M, Specht CA, Suh BB, Tenney A, Utterback TR, Wickes BL, Wortman JR, Wye NH, Kronstad JW, Lodge JK, Heitman J, Davis RW, Fraser CM, Hyman RW. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321–1324. 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paul D, Magbanua Z, Arick M, French T, Bridges SM, Burgess SC, Lawrence ML. 2014. Genome sequence of the oleaginous yeast Rhodotorula glutinis ATCC 204091. Genome Announc. 2(1):e00046-14. 10.1128/genomeA.00046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benoit I, Malavazi I, Goldman G, Baker S, de Vries R. 2013. Aspergillus: genomics of a cosmopolitan fungus, p 89–126 In Horwitz BA, Mukherjee PK, Mukherjee M, Kubicek CP. (ed), Genomics of soil- and plant-associated fungi, vol 36 Springer, Berlin, Germany. [Google Scholar]
- 84.Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K, Andersen MR, Bendtsen JD, Benen JAE, van den Berg M, Breestraat S, Caddick MX, Contreras R, Cornell M, Coutinho PM, Danchin EGJ, Debets AJM, Dekker P, van Dijck PWM, van Dijk A, Dijkhuizen L, Driessen AJM, D'Enfert C, Geysens S, Goosen C, Groot GSP, de Groot PWJ, Guillemette T, Henrissat B, Herweijer M, van den Hombergh JPTW, van den Hondel CAMJJ, van der Heijden RTJM, van der Kaaij RM, Klis FM, Kools HJ, Kubicek CP, van Kuyk PA, Lauber J, Lu X, van der Maarel MJEC, Meulenberg R, Menke H, Mortimer MA, Nielsen J, Oliver SG, et al. 2007. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 25:221–231. 10.1038/nbt1282. [DOI] [PubMed] [Google Scholar]
- 85.Rokas A, Payne G, Fedorova ND, Baker SE, Machida M, Yu J, Georgianna DR, Dean RA, Bhatnagar D, Cleveland TE, Wortman JR, Maiti R, Joardar V, Amedeo P, Denning DW, Nierman WC. 2007. What can comparative genomics tell us about species concepts in the genus Aspergillus? Stud. Mycol. 59:11–17. 10.3114/sim.2007.59.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Žifcáková L, Baldrian P. 2012. Fungal polysaccharide monooxygenases: new players in the decomposition of cellulose. Fungal Ecol. 5:481–489. 10.1016/j.funeco.2012.05.001. [DOI] [Google Scholar]
- 87.Biely P, Vršanská M, Tenkanen M, Kluepfel D. 1997. Endo-β-1,4-xylanase families: differences in catalytic properties. J. Biotechnol. 57:151–166. 10.1016/S0168-1656(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 88.Baldrian P, Valášková V. 2008. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 32:501–521. 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 89.Ding S-J, Ge W, Buswell JA. 2001. Endoglucanase I from the edible straw mushroom, Volvariella volvacea: purification, characterization, cloning and expression. Eur. J. Biochem. 268:5687–5695. 10.1046/j.0014-2956.2001.02503.x. [DOI] [PubMed] [Google Scholar]
- 90.Uzcategui E, Johansson G, Ek B, Pettersson G. 1991. The 1,4-β-d-glucan glucanohydrolases from Phanerochaete chrysosporium. Re-assessment of their significance in cellulose degradation mechanisms. J. Biotechnol. 21:143–159. [DOI] [PubMed] [Google Scholar]
- 91.Henriksson G, Nutt A, Henriksson H, Pettersson B, Ståhlberg J, Johansson G, Pettersson G. 1999. Endoglucanase 28 (Cel12A), a new Phanerochaete chrysosporium cellulase. Eur. J. Biochem. 259:88–95. 10.1046/j.1432-1327.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 92.Igarashi K, Ishida T, Hori C, Samejima M. 2008. Characterization of an endoglucanase belonging to a new subfamily of glycoside hydrolase family 45 of the basidiomycete Phanerochaete chrysosporium. Appl. Environ. Microbiol. 74:5628–5634. 10.1128/AEM.00812-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schauwecker F, Wanner G, Kahmann R. 1995. Filament-specific expression of a cellulase gene in the dimorphic fungus Ustilago maydis. Biol. Chem. Hoppe Seyler 376:617–625. 10.1515/bchm3.1995.376.10.617. [DOI] [PubMed] [Google Scholar]
- 94.Uzcategui E, Ruiz A, Montesino R, Johansson G, Pettersson G. 1991. The 1,4-β-d-glucan cellobiohydrolases from Phanerochaete chrysosporium. I. A system of synergistically acting enzymes homologous to Trichoderma reesei. J. Biotechnol. 19:271–285. 10.1016/0168-1656(91)90064-3. [DOI] [PubMed] [Google Scholar]
- 95.Muñoz IG, Ubhayasekera W, Henriksson H, Szabó I, Pettersson G, Johansson G, Mowbray SL, Ståhlberg J. 2001. Family 7 cellobiohydrolases from Phanerochaete chrysosporium: crystal structure of the catalytic module of Cel7D (CBH58) at 1.32 Å resolution and homology models of the isozymes. J. Mol. Biol. 314:1097–1111. 10.1006/jmbi.2000.5180. [DOI] [PubMed] [Google Scholar]
- 96.Divne C, Ståhlberg J, Reinikainen T, Ruohonen L, Pettersson G, Knowles JKC, Teeri TT, Jones TA. 1994. The three-dimensional crystal structure of the catalytic core of cellobiohydrolase I from Trichoderma reesei. Science 265:524–528. 10.1126/science.8036495. [DOI] [PubMed] [Google Scholar]
- 97.Momeni MH, Payne CM, Hansson H, Mikkelsen NE, Svedberg J, Engström A, Sandgren M, Beckham GT, Stahlberg J. 2013. Structural, biochemical, and computational characterization of the glycoside hydrolase family 7 cellobiohydrolase of the tree-killing fungus Heterobasidion irregulare. J. Biol. Chem. 288:5861–5872. 10.1074/jbc.M112.440891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jia J, Dyer PS, Buswell JA, Peberdy JF. 1999. Cloning of the cbhI and cbhII genes involved in cellulose utilisation by the straw mushroom Volvariella volvacea. Mol. Gen. Genet. 261:985–993. 10.1007/s004380051047. [DOI] [PubMed] [Google Scholar]
- 99.Morais H, Ramos C, Matos N, Forgács E, Cserháti T, Almeida V, Oliveira J, Darwish Y, Illés Z. 2002. Liquid chromatographic and electrophoretic characterisation of extracellular β-glucosidase of Pleurotus ostreatus grown in organic waste. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 770:111–119. 10.1016/S0378-4347(01)00561-8. [DOI] [PubMed] [Google Scholar]
- 100.Větrovský T, Baldrian P, Gabriel J. 2013. Extracellular enzymes of the white-rot fungus Fomes fomentarius and purification of 1,4-β-glucosidase. Appl. Biochem. Biotechnol. 169:100–109. 10.1007/s12010-012-9952-9. [DOI] [PubMed] [Google Scholar]
- 101.Smith MH, Gold MH. 1979. Phanerochaete chrysosporium β-glucosidases: induction, cellular localization, and physical characterization. Appl. Environ. Microbiol. 37:938–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cai YJ, Buswell JA, Chang ST. 1998. β-Glucosidase components of the cellulolytic system of the edible straw mushroom, Volvariella volvacea. Enzyme Microb. Technol. 22:122–129. 10.1016/S0141-0229(97)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen R, Suzuki MR, Hammel KE. 2005. Processive endoglucanase active in crystalline cellulose hydrolysis by the brown rot basidiomycete Gloeophyllum trabeum. Appl. Environ. Microbiol. 71:2412–2417. 10.1128/AEM.71.5.2412-2417.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoon J-J, Kim Y-K. 2005. Degradation of crystalline cellulose by the brown-rot basidiomycete Fomitopsis palustris. J. Microbiol. 43:487–492. [PubMed] [Google Scholar]
- 105.Yoon J-J, Cha C-J, Kim Y-S, Son D-W, Kim Y-K. 2007. The brown-rot basidiomycete Fomitopsis palustris has the endo-glucanases capable of degrading microcrystalline cellulose. J. Microbiol. Biotechnol. 17:800–805. [PubMed] [Google Scholar]
- 106.Song B-C, Kim K-Y, Yoon J-J, Sim S-H, Lee K, Kim Y-S, Kim Y-K, Cha C-J. 2008. Functional analysis of a gene encoding endoglucanase that belongs to glycosyl hydrolase family 12 from the brown-rot basidiomycete Fomitopsis palustris. J. Microbiol. Biotechnol. 18:404–409. [PubMed] [Google Scholar]
- 107.Schmidhalter DR, Canevascini G. 1993. Purification and characterization of two exo-cellobiohydrolases from the brown-rot fungus Coniophora puteana (Schum ex Fr) Karst. Arch. Biochem. Biophys. 300:551–558. 10.1006/abbi.1993.1076. [DOI] [PubMed] [Google Scholar]
- 108.Mansfield SD, Saddler JN, Gübitz GM. 1998. Characterization of endoglucanases from the brown rot fungi Gloeophyllum sepiarium and Gloeophyllum trabeum. Enzyme Microb. Technol. 23:133–140. 10.1016/S0141-0229(98)00033-7. [DOI] [Google Scholar]
- 109.Valášková V, Baldrian P. 2006. Degradation of cellulose and hemicelluloses by the brown rot fungus Piptoporus betulinus—production of extracellular enzymes and characterization of the major cellulases. Microbiology 152:3613–3622. 10.1099/mic.0.29149-0. [DOI] [PubMed] [Google Scholar]
- 110.Lachke AH, Deshpande MV. 1988. Sclerotium rolfsii: status in cellulase research. FEMS Microbiol. Lett. 54:177–193. 10.1111/j.1574-6968.1988.tb02742.x. [DOI] [Google Scholar]
- 111.Pauly M, Andersen LN, Kauppinen S, Kofod LV, York WS, Albersheim P, Darvill A. 1999. A xyloglucan-specific endo-β-1,4-glucanase from Aspergillus aculeatus: expression cloning in yeast, purification and characterization of the recombinant enzyme. Glycobiology 9:93–100. 10.1093/glycob/9.1.93. [DOI] [PubMed] [Google Scholar]
- 112.Ishikawa E, Sakai T, Ikemura H, Matsumoto K, Abe H. 2005. Identification, cloning, and characterization of a Sporobolomyces singularis β-galactosidase-like enzyme involved in galacto-oligosaccharide production. J. Biosci. Bioeng. 99:331–339. 10.1263/jbb.99.331. [DOI] [PubMed] [Google Scholar]
- 113.Tsukada T, Igarashi K, Yoshida M, Samejima M. 2006. Molecular cloning and characterization of two intracellular β-glucosidases belonging to glycoside hydrolase family 1 from the basidiomycete Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 73:807–814. 10.1007/s00253-006-0526-z. [DOI] [PubMed] [Google Scholar]
- 114.Turbe-Doan A, Arfi Y, Record E, Estrada-Alvarado I, Levasseur A. 2013. Heterologous production of cellobiose dehydrogenases from the basidiomycete Coprinopsis cinerea and the ascomycete Podospora anserina and their effect on saccharification of wheat straw. Appl. Microbiol. Biotechnol. 97:4873–4885. 10.1007/s00253-012-4355-y. [DOI] [PubMed] [Google Scholar]
- 115.Westermark U, Eriksson KE. 1975. Purification and properties of cellobiose: quinone oxidoreductase from Sporotrichum pulverulentum. Acta Chem. Scand. B 29:419–424. [PubMed] [Google Scholar]
- 116.Harreither W, Sygmund C, Dünhofen E, Vicuña R, Haltrich D, Ludwig R. 2009. Cellobiose dehydrogenase from the ligninolytic basidiomycete Ceriporiopsis subvermispora. Appl. Environ. Microbiol. 75:2750–2757. 10.1128/AEM.02320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakagame S, Furujyo A, Sugiura J. 2006. Purification and characterization of cellobiose dehydrogenase from white-rot basidiomycete Trametes hirsuta. Biosci. Biotechnol. Biochem. 70:1629–1635. 10.1271/bbb.50692. [DOI] [PubMed] [Google Scholar]
- 118.Hai PQ, Nozaki K, Amano Y, Kanda T. 2000. Purification and characterization of cellobiose dehydrogenase from Irpex lacteus and its adsorption on cellulose. J. Appl. Glycosci. 47:311–318. 10.5458/jag.47.311. [DOI] [Google Scholar]
- 119.Ludwig R, Salamon A, Varga J, Zámocky M, Peterbauer CK, Kulbe KD, Haltrich D. 2004. Characterisation of cellobiose dehydrogenases from the white-rot fungi Trametes pubescens and Trametes villosa. Appl. Microbiol. Biotechnol. 64:213–222. 10.1007/s00253-003-1501-6. [DOI] [PubMed] [Google Scholar]
- 120.Schmidhalter DR, Canevascini G. 1993. Isolation and characterization of the cellobiose dehydrogenase from the brown-rot fungus Coniophora puteana (Schum ex Fr.) Karst. Arch. Biochem. Biophys. 300:559–563. 10.1006/abbi.1993.1077. [DOI] [PubMed] [Google Scholar]
- 121.Kajisa T, Yoshida M, Igarashi K, Katayama A, Nishino T, Samejima M. 2004. Characterization and molecular cloning of cellobiose dehydrogenase from the brown-rot fungus Coniophora puteana. J. Biosci. Bioeng. 98:57–63. 10.1016/S1389-1723(04)70242-X. [DOI] [PubMed] [Google Scholar]
- 122.Zámocký M, Hallberg M, Ludwig R, Divne C, Haltrich D. 2004. Ancestral gene fusion in cellobiose dehydrogenases reflects a specific evolution of GMC oxidoreductases in fungi. Gene 338:1–14. 10.1016/j.gene.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 123.Wu M, Beckham GT, Larsson AM, Ishida T, Kim S, Payne CM, Himmel ME, Crowley MF, Horn SJ, Westereng B, Igarashi K, Samejima M, Ståhlberg J, Eijsink VGH, Sandgren M. 2013. Crystal structure and computational characterization of the lytic polysaccharide monooxygenase GH61D from the basidiomycota fungus Phanerochaete chrysosporium. J. Biol. Chem. 288:12828–12839. 10.1074/jbc.M113.459396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karkehabadi S, Hansson H, Kim S, Piens K, Mitchinson C, Sandgren M. 2008. The first structure of a glycoside hydrolase family 61 member, Cel61B from Hypocrea jecorina, at 1.6 Å resolution. J. Mol. Biol. 383:144–154. 10.1016/j.jmb.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 125.Westereng B, Ishida T, Vaaje-Kolstad G, Wu M, Eijsink VGH, Igarashi K, Samejima M, Ståhlberg J, Horn SJ, Sandgren M. 2011. The putative endoglucanase PcGH61D from Phanerochaete chrysosporium is a metal-dependent oxidative enzyme that cleaves cellulose. PLoS One 6:e27807. 10.1371/journal.pone.0027807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beeson WT, Phillips CM, Cate JHD, Marletta MA. 2012. Oxidative cleavage of cellulose by fungal copper-dependent polysaccharide monooxygenases. J. Am. Chem. Soc. 134:890–892. 10.1021/ja210657t. [DOI] [PubMed] [Google Scholar]
- 127.Bey M, Zhou S, Poidevin L, Henrissat B, Coutinho PM, Berrin J, Sigoillot J. 2013. Cello-oligosaccharide oxidation reveals differences between two lytic polysaccharide monooxygenases (family GH61) from Podospora anserina. Appl. Environ. Microbiol. 79:488–496. 10.1128/AEM.02942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Magalhães PO, Milagres AMF. 2009. Biochemical properties of a β-mannanase and a β-xylanase produced by Ceriporiopsis subvermispora during biopulping conditions. Int. Biodeterior. Biodegradation 63:191–195. 10.1016/j.ibiod.2008.08.008. [DOI] [Google Scholar]
- 129.Sydenham R, Zheng Y, Riemens A, Tsang A, Powlowski J, Storms R. 2014. Cloning and enzymatic characterization of four thermostable fungal endo-1,4-β-xylanases. Appl. Microbiol. Biotechnol. 98:3613–3628. 10.1007/s00253-013-5244-8. [DOI] [PubMed] [Google Scholar]
- 130.Huy ND, Thayumanavan P, Kwon T, Park S. 2013. Characterization of a recombinant bifunctional xylosidase/arabinofuranosidase from Phanerochaete chrysosporium. J. Biosci. Bioeng. 116:152–159. 10.1016/j.jbiosc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 131.Rogalski J, Oleszek M, Tokarzewska-Zadora J. 2001. Purification and characterization of two endo-1,4-β-xylanases and a β-xylosidase from Phlebia radiata. Acta Microbiol. Pol. 50:117–128. [PubMed] [Google Scholar]
- 132.Benech R-O, Li X, Patton D, Powlowski J, Storms R, Bourbonnais R, Paice M, Tsang A. 2007. Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1,4-mannanase. Enzyme Microb. Technol. 41:740–747. 10.1016/j.enzmictec.2007.06.012. [DOI] [Google Scholar]
- 133.Yagüe E, Mehak-Zunic M, Morgan L, Wood DA, Thurston CF. 1997. Expression of CEL2 and CEL4, two proteins from Agaricus bisporus with similarity to fungal cellobiohydrolase I and β-mannanase, respectively, is regulated by the carbon source. Microbiology 143:239–244. 10.1099/00221287-143-1-239. [DOI] [PubMed] [Google Scholar]
- 134.Tang CM, Waterman LD, Smith MH, Thurston CF. 2001. The cel4 gene of Agaricus bisporus encodes a β-mannanase. Appl. Environ. Microbiol. 67:2298–2303. 10.1128/AEM.67.5.2298-2303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gübitz GM, Hayn M, Sommerauer M, Steiner W. 1996. Mannan-degrading enzymes from Sclerotium rolfsii: characterisation and synergism of two endo β-mannanases and a β-mannosidase. Bioresour. Technol. 58:127–135. 10.1016/S0960-8524(96)00093-4. [DOI] [Google Scholar]
- 136.Gübitz GM, Hayn M, Urbanz G, Steiner W. 1996. Purification and properties of an acidic β-mannanase from Sclerotium rolfsii. J. Biotechnol. 45:165–172. 10.1016/0168-1656(95)00158-1. [DOI] [Google Scholar]
- 137.Johnson KG, Ross NW. 1990. Enzymic properties of β-mannanase from Polyporus versicolor. Enzyme Microb. Technol. 12:960–964. 10.1016/0141-0229(90)90117-9. [DOI] [Google Scholar]
- 138.Sachslehner A, Haltrich D. 1999. Purification and some properties of a thermostable acidic endo-β-1,4-D-mannanase from Sclerotium (Athelia) rolfsii. FEMS Microbiol. Lett. 177:47–55. 10.1111/j.1574-6968.1999.tb13712.x. [DOI] [Google Scholar]
- 139.Baldrian P, Valášková V, Merhautová V, Gabriel J. 2005. Degradation of lignocellulose by Pleurotus ostreatus in the presence of copper, manganese, lead and zinc. Res. Microbiol. 156:670–676. 10.1016/j.resmic.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 140.Rogalski J, Hatakka A, Longa A, Wojtas-Wasilewska M. 1993. Hemicellulolytic enzymes of the ligninolytic white-rot fungus Phlebia radiata. Influence of phenolic compounds on the synthesis of hemicellulolytic enzymes. Acta Biotechnol. 13:53–57. [Google Scholar]
- 141.Prendecka M, Buczyńska A, Rogalski J. 2007. Purification and characterization of β-mannosidases from white rot fungus Phlebia radiata. Pol. J. Microbiol. 56:139–147. [PubMed] [Google Scholar]
- 142.Wan CC, Muldrey JE, Li SC, Li YT. 1976. β-Mannosidase from the mushroom Polyporus sulfureus. J. Biol. Chem. 251:4384–4388. [PubMed] [Google Scholar]
- 143.Alfaro M, Oguiza JA, Ramírez L, Pisabarro AG. 2014. Comparative analysis of secretomes in basidiomycete fungi. J. Proteomics 102:28–43. 10.1016/j.jprot.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 144.Valášková V, Baldrian P. 2006. Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Res. Microbiol. 157:119–124. 10.1016/j.resmic.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 145.Herr D, Baumer F, Dellweg H. 1978. Purification and properties of an extracellular endo-1,4-β-glucanase from Lenzites trabea. Arch. Microbiol. 117:287–292. 10.1007/BF00738548. [DOI] [Google Scholar]
- 146.Ji H-W, Cha C-J. 2010. Identification and functional analysis of a gene encoding β-glucosidase from the brown-rot basidiomycete Fomitopsis palustris. J. Microbiol. 48:808–813. 10.1007/s12275-010-0482-2. [DOI] [PubMed] [Google Scholar]
- 147.Shanley NA, Van Den Broek LAM, Voragen AGJ, Coughlan MP. 1993. Isolation and characterization of an endopolygalacturonase from Phanerochaete chrysosporium. J. Biotechnol. 28:179–197. 10.1016/0168-1656(93)90169-N. [DOI] [Google Scholar]
- 148.Miyairi K, Senda M, Watanabe M, Hasui Y, Okuno T. 1997. Cloning and sequence analysis of cDNA encoding endopolygalacturonase I from Stereum purpureum. Biosci. Biotechnol. Biochem. 61:655–659. 10.1271/bbb.61.655. [DOI] [PubMed] [Google Scholar]
- 149.Senda M, Narita T, Akada S, Okuno T, Miyairi K. 2001. Characterization of an endopolygalacturonase gene cppg1 from phytopathogenic fungus Chondrostereum purpureum. J. Gen. Plant Pathol. 67:41–44. 10.1007/PL00012985. [DOI] [Google Scholar]
- 150.Williams HL, Tang Y, Hintz WE. 2002. Endopolygalacturonase is encoded by a multigene family in the basidiomycete Chondrostereum purpureum. Fungal Genet. Biol. 36:71–83. 10.1016/S1087-1845(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 151.Kaji A, Okada T. 1969. Purification and properties of an unusually acid-stable endo-polygalacturonase produced by Corticium rolfsii. Arch. Biochem. Biophys. 131:203–209. 10.1016/0003-9861(69)90122-2. [DOI] [PubMed] [Google Scholar]
- 152.Bateman DF. 1972. The polygalacturonase complex produced by Sclerotium rolfsii. Physiol. Plant Pathol. 2:175–184. 10.1016/0048-4059(72)90025-2. [DOI] [Google Scholar]
- 153.Nakagawa T, Yamada K, Miyaji T, Tomizuka N. 2002. Cold-active pectinolytic activity of psychrophilic-basidiomycetous yeast Cystofilobasidium capitatum strain PPY-1. J. Biosci. Bioeng. 94:175–177. 10.1016/S1389-1723(02)80140-2. [DOI] [PubMed] [Google Scholar]
- 154.Nakagawa T, Nagaoka T, Miyaji T, Tomizuka N. 2005. Cold-active polygalacturonase from psychrophilic-basidiomycetous yeast Cystofilobasidium capitatum strain PPY-1. Biosci. Biotechnol. Biochem. 69:419–421. 10.1271/bbb.69.419. [DOI] [PubMed] [Google Scholar]
- 155.Normand J, Ralet M-C, Thibault J-F, Rogniaux H, Delavault P, Bonnin E. 2010. Purification, characterization, and mode of action of a rhamnogalacturonan hydrolase from Irpex lacteus, tolerant to an acetylated substrate. Appl. Microbiol. Biotechnol. 86:577–588. 10.1007/s00253-009-2310-3. [DOI] [PubMed] [Google Scholar]
- 156.Normand J, Bonnin E, Delavault P. 2012. Cloning and expression in Pichia pastoris of an Irpex lacteus rhamnogalacturonan hydrolase tolerant to acetylated rhamnogalacturonan. Appl. Microbiol. Biotechnol. 94:1543–1552. 10.1007/s00253-011-3705-5. [DOI] [PubMed] [Google Scholar]
- 157.Leoni C, ter Braak CJF, Gilsanz JC, Dogliotti S, Rossing WAH, van Bruggen AHC. 2014. Sclerotium rolfsii dynamics in soil as affected by crop sequences. Appl. Soil Ecol. 75:95–105. 10.1016/j.apsoil.2013.11.002. [DOI] [Google Scholar]
- 158.Xu Z, Gleason ML, Mueller DS, Esker PD, Bradley CA, Buck JW, Benson DM, Dixon PM, Monteiro JEBA. 2008. Overwintering of Sclerotium rolfsii and S. rolfsii var. delphinii in different latitudes of the United States. Plant Dis. 92:719–724. 10.1094/PDIS-92-5-0719. [DOI] [PubMed] [Google Scholar]
- 159.Yoshihara O, Matsuo T, Kaji A. 1977. Purification and properties of acid pectinesterase from Corticium rolfsii. Agric. Biol. Chem. 41:2335–2341. 10.1271/bbb1961.41.2335. [DOI] [Google Scholar]
- 160.Castanares A, Hay AJ, Gordon AH, McCrae SI, Wood TM. 1995. D-Xylan-degrading enzyme system from the fungus Phanerochaete chrysosporium: isolation and partial characterisation of an α-(4-O-methyl)-D-glucuronidase. J. Biotechnol. 43:183–194. 10.1016/0168-1656(95)00128-X. [DOI] [PubMed] [Google Scholar]
- 161.Mierzwa M, Tokarzewska-Zadora J, Deptula T, Rogalski J, Szczodrak J. 2005. Purification and characterization of an extracellular α-D-glucuronidase from Phlebia radiata. Prep. Biochem. Biotechnol. 35:243–256. 10.1081/PB-200065648. [DOI] [PubMed] [Google Scholar]
- 162.Tenkanen M, Siika-Aho M. 2000. An α-glucuronidase of Schizophyllum commune acting on polymeric xylan. J. Biotechnol. 78:149–161. 10.1016/S0168-1656(99)00240-0. [DOI] [PubMed] [Google Scholar]
- 163.Chong S-L, Battaglia E, Coutinho PM, Henrissat B, Tenkanen M, de Vries RP. 2011. The α-glucuronidase Agu1 from Schizophyllum commune is a member of a novel glycoside hydrolase family (GH115). Appl. Microbiol. Biotechnol. 90:1323–1332. 10.1007/s00253-011-3157-y. [DOI] [PubMed] [Google Scholar]
- 164.Ryabova O, Vršanská M, Kaneko S, van Zyl WH, Biely P. 2009. A novel family of hemicellulolytic α-glucuronidase. FEBS Lett. 583:1457–1462. 10.1016/j.febslet.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 165.Brumer H, III, Sims PFG, Sinnott ML. 1999. Lignocellulose degradation by Phanerochaete chrysosporium: purification and characterization of the main α-galactosidase. Biochem. J. 339:43–53. 10.1042/0264-6021:3390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sripuan T, Aoki K, Yamamoto K, Tongkao D, Kumagai H. 2003. Purification and characterization of thermostable α-galactosidase from Ganoderma lucidum. Biosci. Biotechnol. Biochem. 67:1485–1491. 10.1271/bbb.67.1485. [DOI] [PubMed] [Google Scholar]
- 167.Sampietro D, Quiroga E, Sgariglia M, Soberón J, Vattuone MA. 2012. A thermostable α-galactosidase from Lenzites elegans (spreng.) ex pat. mb445947: purification and properties. Antonie Van Leeuwenhoek 102:257–267. 10.1007/s10482-012-9734-y. [DOI] [PubMed] [Google Scholar]
- 168.Ramalingam Saraswathy N, Sadasivam S, Subha K, Poorani N. 2007. Purification and properties of α-galactosidase from white-rot fungus Pleurotus florida. Indian J. Biochem. Biophys. 44:76–81. [PubMed] [Google Scholar]
- 169.Prendecka M, Szyjka K, Rogalski J. 2003. Purification and properties of α-galactosidase isozymes from Phlebia radiata. Acta Microbiol. Pol. 52:25–33. [PubMed] [Google Scholar]
- 170.Huy ND, Thiyagarajan S, Kim D-H, Park S-M. 2013. Cloning and characterization of a novel bifunctional acetyl xylan esterase with carbohydrate binding module from Phanerochaete chrysosporium. J. Biosci. Bioeng. 115:507–513. 10.1016/j.jbiosc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 171.Koseki T, Miwa Y, Akao T, Akita O, Hashizume K. 2006. An Aspergillus oryzae acetyl xylan esterase: molecular cloning and characteristics of recombinant enzyme expressed in Pichia pastoris. J. Biotechnol. 121:381–389. 10.1016/j.jbiotec.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 172.Linden J, Samara M, Decker S, Johnson E, Boyer M, Pecs M, Adney W, Himmel M. 1994. Purification and characterization of an acetyl esterase from Aspergillus niger. Appl. Biochem. Biotechnol. 45–46:383–393. [DOI] [PubMed] [Google Scholar]
- 173.Nieter A, Haase-Aschoff P, Linke D, Nimtz M, Berger RG. 2014. A halotolerant type A feruloyl esterase from Pleurotus eryngii. Fungal Biol. 118:348–357. 10.1016/j.funbio.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 174.Linke D, Matthes R, Nimtz M, Zorn H, Bunzel M, Berger RG. 2013. An esterase from the basidiomycete Pleurotus sapidus hydrolyzes feruloylated saccharides. Appl. Microbiol. Biotechnol. 97:7241–7251. 10.1007/s00253-012-4598-7. [DOI] [PubMed] [Google Scholar]
- 175.Haase-Aschoff P, Linke D, Nimtz M, Popper L, Berger RG. 2013. An enzyme from Auricularia auricula-judae combining both benzoyl and cinnamoyl esterase activity. Process Biochem. 48:1872–1878. 10.1016/j.procbio.2013.09.016. [DOI] [Google Scholar]
- 176.Koseki T, Furuse S, Iwano K, Matsuzawa H. 1998. Purification and characterization of a feruloylesterase from Aspergillus awamori. Biosci. Biotechnol. Biochem. 62:2032–2034. 10.1271/bbb.62.2032. [DOI] [PubMed] [Google Scholar]
- 177.de Vries RP, Michelsen B, Poulsen CH, Kroon PA, van den Heuvel RHH, Faulds CB, Williamson G, van den Hombergh JPTW, Visser J. 1997. The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Appl. Environ. Microbiol. 63:4638–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.de Vries RP, van Kuyk PA, Kester HCM, Visser J. 2002. The Aspergillus niger faeB gene encodes a second feruloyl esterase involved in pectin and xylan degradation and is specifically induced in the presence of aromatic compounds. Biochem. J. 363:377–386. 10.1042/0264-6021:3630377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Benoit I, Navarro D, Marnet N, Rakotomanomana N, Lesage-Meessen L, Sigoillot J, Asther M, Asther M. 2006. Feruloyl esterases as a tool for the release of phenolic compounds from agro-industrial by-products. Carbohydr. Res. 341:1820–1827. 10.1016/j.carres.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 180.Yoshikawa K, Yamamoto K, Okada S. 1993. Isolation of Aspergillus flavus MO-5 producing two types of intracellular α-D-xylosidases: purification and characterization of α-D-xylosidase I. Biosci. Biotechnol. Biochem. 57:1275–1280. 10.1271/bbb.57.1275. [DOI] [PubMed] [Google Scholar]
- 181.Yoshikawa K, Yamamoto K, Okada S. 1993. Purification and characterization of an intracellular α-D-xylosidase II from Aspergillus flavus MO-5. Biosci. Biotechnol. Biochem. 57:1281–1285. 10.1271/bbb.57.1281. [DOI] [PubMed] [Google Scholar]
- 182.Punja ZK. 1985. The biology, ecology, and control of Sclerotium rolfsii. Annu. Rev. Phytopathol. 23:97–127. 10.1146/annurev.py.23.090185.000525. [DOI] [Google Scholar]
- 183.Kowalczyk JE, Benoit I, de Vries RP. 2014. Regulation of plant biomass utilization in Aspergillus. Adv. Appl. Microbiol. 88:31–56. 10.1016/B978-0-12-800260-5.00002-4. [DOI] [PubMed] [Google Scholar]
- 184.Todd RB, Zhou M, Ohm RA, Leeggangers HACF, Visser L, de Vries RP. 2014. Prevalence of transcription factors in ascomycete and basidiomycete fungi. BMC Genomics 15:214. 10.1186/1471-2164-15-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Evans CS. 1985. Properties of the β-D-glucosidase (cellobiase) from the wood-rotting fungus, Coriolus versicolor. Appl. Microbiol. Biotechnol. 22:128–131. 10.1007/BF00250032. [DOI] [Google Scholar]
- 186.Chow C-M, Yagüe E, Raguz S, Wood DA, Thurston CF. 1994. The cel3 gene of Agaricus bisporus codes for a modular cellulase and is transcriptionally regulated by the carbon source. Appl. Environ. Microbiol. 60:2779–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shewale JG, Sadana J. 1981. Purification, characterization, and properties of β-glucosidase enzymes from Sclerotium rolfsii. Arch. Biochem. Biophys. 207:185–196. 10.1016/0003-9861(81)90024-2. [DOI] [PubMed] [Google Scholar]
- 188.Onishi N, Tanaka T. 1996. Purification and properties of a galacto- and gluco-oligosaccharide-producing β-glycosidase from Rhodotorula minuta IFO879. J. Ferment. Bioeng. 82:439–443. 10.1016/S0922-338X(97)86979-6. [DOI] [Google Scholar]
- 189.Hamada N, Ishikawa K, Fuse N, Kodaira R, Shimosaka M, Amano Y, Kanda T, Okazaki M. 1999. Purification, characterization and gene analysis of exo-cellulase II (Ex-2) from the white rot basidiomycete Irpex lacteus. J. Biosci. Bioeng. 87:442–451. 10.1016/S1389-1723(99)80092-9. [DOI] [PubMed] [Google Scholar]
- 190.De Groot PWJ, Basten DEJW, Sonnenberg ASM, Van Griensven LJLD, Visser J, Schaap PJ. 1998. An endo-1,4-β-xylanase-encoding gene from Agaricus bisporus is regulated by compost-specific factors. J. Mol. Biol. 277:273–284. 10.1006/jmbi.1997.1605. [DOI] [PubMed] [Google Scholar]
- 191.Kulmburg P, Mathieu M, Dowzer C, Kelly J, Felenbok B. 1993. Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon catabolite repression in Aspergillus nidulans. Mol. Microbiol. 7:847–857. 10.1111/j.1365-2958.1993.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 192.Covert SF, Bolduc J, Cullen D. 1992. Genomic organization of a cellulase gene family in Phanerochaete chrysosporium. Curr. Genet. 22:407–413. 10.1007/BF00352442. [DOI] [PubMed] [Google Scholar]
- 193.Nadal M, Garcia-Pedrajas MD, Gold SE. 2010. The snf1 gene of Ustilago maydis acts as a dual regulator of cell wall degrading enzymes. Phytopathology 100:1364–1372. 10.1094/PHYTO-01-10-0011. [DOI] [PubMed] [Google Scholar]
- 194.Östling J, Carlberg M, Ronne H. 1996. Functional domains in the Mig1 repressor. Mol. Cell. Biol. 16:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cotoras M, Agosin E. 1992. Regulatory aspects of endoglucanase production by the brown-rot fungus Gloeophyllum trabeum. Exp. Mycol. 16:253–260. 10.1016/0147-5975(92)90001-8. [DOI] [Google Scholar]
- 196.MacDonald J, Doering M, Canam T, Gong Y, Guttman DS, Campbell MM, Master ER. 2011. Transcriptomic responses of the softwood-degrading white-rot fungus Phanerochaete carnosa during growth on coniferous and deciduous wood. Appl. Environ. Microbiol. 77:3211–3218. 10.1128/AEM.02490-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ohm RA, Riley R, Salamov A, Min B, Choi I-G, Grigoriev IV. 20 May 2014. Genomics of wood-degrading fungi. Fungal Genet. Biol 10.1016/j.fgb.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 198.Li X-L, Špániková S, de Vries RP, Biely P. 2007. Identification of genes encoding microbial glucuronoyl esterases. FEBS Lett. 581:4029–4035. 10.1016/j.febslet.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 199.Duranová M, Špániková S, Wösten HAB, Biely P, de Vries RP. 2009. Two glucuronoyl esterases of Phanerochaete chrysosporium. Arch. Microbiol. 191:133–140. 10.1007/s00203-008-0434-y. [DOI] [PubMed] [Google Scholar]
- 200.Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VGH. 2012. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 5:45. 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, Sørlie M, Eijsink VGH. 2010. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330:219–222. 10.1126/science.1192231. [DOI] [PubMed] [Google Scholar]
- 202.Hemsworth GR, Henrissat B, Davies GJ, Walton PH. 2014. Discovery and characterization of a new family of lytic polysaccharide monooxygenases. Nat. Chem. Biol. 10:122–126. 10.1038/nchembio.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mohnen D. 2008. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11:266–277. 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 204.Doblin MS, Pettolino F, Bacic A. 2010. Plant cell walls: the skeleton of the plant world. Funct. Plant Biol. 37:357–381. 10.1071/FP09279. [DOI] [Google Scholar]
- 205.Binder M, Justo A, Riley R, Salamov A, Lopez-Giraldez F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH, Townsend J, Grigoriev IV, Hibbett DS. 2013. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105:1350–1373. 10.3852/13-003. [DOI] [PubMed] [Google Scholar]
- 206.Stajich JE, Wilke SK, Ahrén D, Au CH, Birren BW, Borodovsky M, Burns C, Canbäck B, Casselton LA, Cheng CK, Deng J, Dietrich FS, Fargo DC, Farman ML, Gathman AC, Goldberg J, Guigó R, Hoegger PJ, Hooker JB, Huggins A, James TY, Kamada T, Kilaru S, Kodira C, Kües U, Kupfer D, Kwan HS, Lomsadze A, Li W, Lilly WW, Ma L-J, Mackey AJ, Manning G, Martin F, Muraguchi H, Natvig DO, Palmerini H, Ramesh MA, Rehmeyer CJ, Roe BA, Shenoy N, Stanke M, Ter-Hovhannisyan V, Tunlid A, Velagapudi R, Vision TJ, Zeng Q, Zolan ME, Pukkila PJ. 2010. Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proc. Natl. Acad. Sci. U. S. A. 107:11889–11894. 10.1073/pnas.1003391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Padamsee M, Kumar TKA, Riley R, Binder M, Boyd A, Calvo AM, Furukawa K, Hesse C, Hohmann S, James TY, LaButti K, Lapidus A, Lindquist E, Lucas S, Miller K, Shantappa S, Grigoriev IV, Hibbett DS, McLaughlin DJ, Spatafora JW, Aime MC. 2012. The genome of the xerotolerant mold Wallemia sebi reveals adaptations to osmotic stress and suggests cryptic sexual reproduction. Fungal Genet. Biol. 49:217–226. 10.1016/j.fgb.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 208.Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, Bennett J, Bowyer P, Chen D, Collins M, Coulsen R, Davies R, Dyer PS, Farman M, Fedorova N, Fedorova N, Feldblyum TV, Fischer R, Fosker N, Fraser A, García JL, García MJ, Goble A, Goldman GH, Gomi K, Griffith-Jones S, Gwilliam R, Haas B, Haas H, Harris D, Horiuchi H, Huang J, Humphray S, Jiménez J, Keller N, Khouri H, Kitamoto K, Kobayashi T, Konzack S, Kulkarni R, Kumagai T, Lafton A, Latgé J-P, Li W, Lord A, Lu C, et al. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151–1156. 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 209.Galagan JE, Calvo SE, Cuomo C, Ma L-J, Wortman JR, Batzoglou S, Lee S-I, Bastürkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D'Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M, Selker EU, Archer DB, Peñalva MÁ, Oakley BR, Momany M, Tanaka T, Kumagai T, Asai K, Machida M, Nierman WC, Denning DW, Caddick M, Hynes M, Paoletti M, Fischer R, Miller B, Dyer P, Sachs MS, Osmani SA, Birren BW. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105–1115. 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- 210.Andersen MR, Salazar MP, Schaap PJ, van de Vondervoort PJI, Culley D, Thykaer J, Frisvad JC, Nielsen KF, Albang R, Albermann K, Berka RM, Braus GH, Braus-Stromeyer SA, Corrochano LM, Dai Z, van Dijck PWM, van de Vondervoort PJI, Lasure LL, Magnuson JK, Menke H, Meijer M, Meijer SL, Nielsen JB, Nielsen ML, van Ooyen AJJ, Pel HJ, Poulsen L, Samson RA, Stam H, Tsang A, van den Brink JM, Atkins A, Aerts A, Shapiro H, Pangilinan J, Salamov A, Lou Y, Lindquist E, Lucas S, Grimwood J, Grigoriev IV, Kubicek CP, Martinez D, Van Peij NNME, Roubos JA, Nielsen J, Baker SE. 2011. Comparative genomics of citric-acid-producing Aspergillus niger ATCC 1015 versus enzyme-producing CBS 513.88. Genome Res. 21:885–897. 10.1101/gr.112169.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto K-I, Arima T, Akita O, Kashiwagi Y, Abe K, Gomi K, Horiuchi H, Kitamoto K, Kobayashi T, Takeuchi M, Denning DW, Galagan JE, Nierman WC, Yu J, Archer DB, Bennett JW, Bhatnagar D, Cleveland TE, Fedorova ND, Gotoh O, Horikawa H, Hosoyama A, Ichinomiya M, Igarashi R, Iwashita K, Juvvadi PR, Kato M, Kato Y, Kin T, Kokubun A, Maeda H, Maeyama N, Maruyama J-I, Nagasaki H, Nakajima T, Oda K, Okada K, Paulsen I, Sakamoto K, Sawano T, Takahashi M, Takase K, Terabayashi Y, Wortman JR, et al. 2005. Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157–1161. 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 212.Belova OV, Lisov AV, Vinokurova NG, Kostenevich AA, Sapunova LI, Lobanok AG, Leontievsky AA. 2014. Xylanase and cellulase of fungus Cerrena unicolor VKM F-3196: production, properties, and applications for the saccharification of plant material. Appl. Biochem. Microbiol. 50:148–153. 10.1134/S0003683814020057. [DOI] [PubMed] [Google Scholar]
- 213.Rouau X, Foglietti M-J. 1985. Purification and partial characterisation of three endo-glucanases from Dichomitus squalens. Carbohydr. Res. 142:299–314. 10.1016/0008-6215(85)85031-X. [DOI] [Google Scholar]
- 214.Kanda T, Wakabayashi K, Nisizawa K. 1976. Purification and properties of an endo cellulase of Avicelase type from Irpex lacteus (Polyporus tulipiferae). J. Biochem. 79:977–988. [DOI] [PubMed] [Google Scholar]
- 215.Kanda T, Wakabayashi K, Nisizawa K. 1980. Purification and properties of a lower-molecular-weight endo-cellulase from Irpex lacteus (Polyporus tulipiferae). J. Biochem. 87:1625–1634. [DOI] [PubMed] [Google Scholar]
- 216.Kubo K, Nisizawa K. 1983. Purification and properties of 2 endo-type cellulases from Irpex lacteus (Polyporus tulipiferae). J. Ferment. Biotechnol. 61:383–389. [Google Scholar]
- 217.Toda H, Takada S, Oda M, Amano Y, Kanda T, Okazaki M, Shimosaka M. 2005. Gene cloning of an endoglucanase from the basidiomycete Irpex lacteus and its cDNA expression in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 69:1262–1269. 10.1271/bbb.69.1262. [DOI] [PubMed] [Google Scholar]
- 218.Ishihara H, Imamura K, Kita M, Aimi T, Kitamoto Y. 2005. Enhancement of the viscometric endocellulase activity of Polyporus arcularius CMCase IIIa by cellobiose and cellooligosaccharides. Mycoscience 46:148–153. 10.1007/s10267-005-0226-z. [DOI] [Google Scholar]
- 219.Ishihara H, Aimi T, Takahashi K, Kitamoto Y. 2005. Heterologous expression and characterization of the endocellulase encoding gene cel3A from the basidiomycete Polyporus arcularius. Mycoscience 46:154–161. 10.1007/s10267-005-0225-0. [DOI] [Google Scholar]
- 220.Eriksson KE, Pettersson B. 1975. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. I. Separation, purification and physico chemical characterization of five endo-1,4-β-glucanases. Eur. J. Biochem. 51:193–206. [DOI] [PubMed] [Google Scholar]
- 221.Nozaki K, Seki T, Matsui K, Mizuno M, Kanda T, Amano Y. 2007. Structure and characteristics of an endo-β-1,4-glucanase, isolated from Trametes hirsuta, with high degradation to crystalline cellulose. Biosci. Biotechnol. Biochem. 71:2375–2382. 10.1271/bbb.60385. [DOI] [PubMed] [Google Scholar]
- 222.Petterson G, Porath J. 1963. Studies on cellulolytic enzymes. 2. Multiplicity of cellulolytic enzymes of Polyporus versicolor. Biochim. Biophys. Acta 67:9–15. [DOI] [PubMed] [Google Scholar]
- 223.Idogaki H, Kitamoto Y. 1992. Purification and some properties of a carboxymethyl cellulase from Coriolus versicolor. Biosci. Biotechnol. Biochem. 56:970–971. 10.1271/bbb.56.970. [DOI] [PubMed] [Google Scholar]
- 224.Goksoyr J, Eriksen J. 1980. Cellulases, p 283–330 In Rose AH. (ed), Microbial enzymes and bioconversions. Academic Press, London, United Kingdom. [Google Scholar]
- 225.Ishihara M, Shimizu K. 1984. Purification and properties of two extracellular endo-cellulases from the brown-rotting fungus Tyromyces palustris. Mokuzai Gakkaishi 30:79–87. [Google Scholar]
- 226.Shimokawa T, Shibuya H, Nojiri M, Yoshida S, Ishihara M. 2008. Purification, molecular cloning, and enzymatic properties of a family 12 endoglucanase (EG-II) from Fomitopsis palustris: role of EG-II in larch holocellulose hydrolysis. Appl. Environ. Microbiol. 74:5857–5861. 10.1128/AEM.00435-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bhattacharjee B, Roy A, Majumder AL. 1993. Carboxymethylcellulase from Lenzites saepiaria, a brown-rotter. Biochem. Mol. Biol. Int. 30:1143–1152. [PubMed] [Google Scholar]
- 228.Kleman-Leyer KM, Kirk TK. 1994. Three native cellulose-depolymerizing endoglucanases from solid-substrate cultures of the brown rot fungus Meruliporia (Serpula) incrassata. Appl. Environ. Microbiol. 60:2839–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Clausen CA. 1995. Dissociation of the multi-enzyme complex of the brown-rot fungus Postia placenta. FEMS Microbiol. Lett. 127:73–78. 10.1016/0378-1097(95)00040-C. [DOI] [Google Scholar]
- 230.Zheng F, Ding S. 2013. Processivity and enzymatic mode of a glycoside hydrolase family 5 endoglucanase from Volvariella volvacea. Appl. Environ. Microbiol. 79:989–996. 10.1128/AEM.02725-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bailey PJ, Liese W, Roesch R, Keilich G, Afting EG. 1969. Cellulase (β-1,4-glucan 4-glucanohydrolase) from the wood-degrading fungus Polyporus schweinitzii fr. I. Purification. Biochim. Biophys. Acta 185:381–391. 10.1016/0005-2744(69)90431-8. [DOI] [PubMed] [Google Scholar]
- 232.Keilich G, Bailey PJ, Afting EG, Liese W. 1969. Cellulase (β-1,4-glucan 4-glucanohydrolase) from the wood-degrading fungus Polyporus schweinitzii fr. II. Characterization. Biochim. Biophys. Acta 185:392–401. 10.1016/0005-2744(69)90432-X. [DOI] [PubMed] [Google Scholar]
- 233.Sadana JC, Lachke AH, Patil RV. 1984. Endo-1,4-β-d-glucanases from Sclerotium rolfsii. Purification, substrate specificity, and mode of action. Carbohydr. Res. 133:297–312. 10.1016/0008-6215(84)85206-4. [DOI] [Google Scholar]
- 234.Oikawa T, Tsukagawa Y, Soda K. 1998. Endo-β-glucanase secreted by a psychrotrophic yeast: purification and characterization. Biosci. Biotechnol. Biochem. 62:1751–1756. 10.1271/bbb.62.1751. [DOI] [PubMed] [Google Scholar]
- 235.Rouau X, Odier E. 1986. Purification and properties of two enzymes from Dichomitus squalens which exhibit both cellobiohydrolase and xylanase activity. Carbohydr. Res. 145:279–292. 10.1016/S0008-6215(00)90435-X. [DOI] [Google Scholar]
- 236.Ishiguro M, Hori T, Ishida T, Yoshida M, Takabatake K, Kaneko S, Igarashi K, Samejima M. 2010. Molecular cloning of cDNAs encoding two glycoside hydrolase family 7 cellobiohydrolases from the basidiomycete Flammulina velutipes. Plant Biotechnol. 27:273–281. 10.5511/plantbiotechnology.27.273. [DOI] [Google Scholar]
- 237.Kanda T, Nakakubo S, Wakabayashi K, Nisizawa K. 1978. Purification and properties of an exo-cellulase of Avicelase type from a wood-rotting fungus, Irpex lacteus (Polyporus tulipiferae). J. Biochem. 84:1217–1226. [DOI] [PubMed] [Google Scholar]
- 238.Hamada N, Fuse N, Shimosaka M, Kodaira R, Amano Y, Kanda T, Okazaki M. 1999. Cloning and characterization of a new exo-cellulase gene, cel3, in Irpex lacteus. FEMS Microbiol. Lett. 172:231–237. 10.1111/j.1574-6968.1999.tb13473.x. [DOI] [PubMed] [Google Scholar]
- 239.Toda H, Nagahata N, Amano Y, Nozaki K, Kanda T, Okazaki M, Shimosaka M. 2008. Gene cloning of cellobiohydrolase II from the white rot fungus Irpex lacteus MC-2 and its expression in Pichia pastoris. Biosci. Biotechnol. Biochem. 72:3142–3147. 10.1271/bbb.80316. [DOI] [PubMed] [Google Scholar]
- 240.Kanda T, Nisizawa K. 1988. Exocellulase of Irpex lacteus (Polyporus tulipiferae). Methods Enzymol. 160:403–408. 10.1016/0076-6879(88)60146-7. [DOI] [Google Scholar]
- 241.Lee CC, Wong DWS, Robertson GH. 2001. Cloning and characterization of two cellulase genes from Lentinula edodes. FEMS Microbiol. Lett. 205:355–360. 10.1111/j.1574-6968.2001.tb10972.x. [DOI] [PubMed] [Google Scholar]
- 242.Covert SF, Wymelenberg AV, Cullen D. 1992. Structure, organization, and transcription of a cellobiohydrolase gene cluster from Phanerochaete chrysosporium. Appl. Environ. Microbiol. 58:2168–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sims P, James C, Broda P. 1988. The identification, molecular cloning and characterisation of a gene from Phanerochaete chrysosporium that shows strong homology to the exo-cellobiohydrolase I gene from Trichoderma reesei. Gene 74:411–422. 10.1016/0378-1119(88)90174-6. [DOI] [PubMed] [Google Scholar]
- 244.Vanden Wymelenberg A, Covert S, Cullen D. 1993. Identification of the gene encoding the major cellobiohydrolase of the white rot fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 59:3492–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tempelaars CAM, Birch PRJ, Sims PFG, Broda P. 1994. Isolation, characterization, and analysis of the expression of the cbhII gene of Phanerochaete chrysosporium. Appl. Environ. Microbiol. 60:4387–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ohnishi Y, Nagase M, Ichiyanagi T, Kitamoto Y, Aimi T. 2007. Transcriptional regulation of two cellobiohydrolase encoding genes (cel1 and cel2) from the wood-degrading basidiomycete Polyporus arcularius. Appl. Microbiol. Biotechnol. 76:1069–1078. 10.1007/s00253-007-1090-x. [DOI] [PubMed] [Google Scholar]
- 247.Lee K-M, Moon H-J, Kalyani D, Kim H, Kim I-W, Jeya M, Lee J-K. 2011. Characterization of cellobiohydrolase from a newly isolated strain of Agaricus arvensis. J. Microbiol. Biotechnol. 21:711–718. 10.4014/jmb.1102.02001. [DOI] [PubMed] [Google Scholar]
- 248.Liu Y, Igarashi K, Kaneko S, Tonozuka T, Samejima M, Fukuda K, Yoshida M. 2009. Characterization of glycoside hydrolase family 6 enzymes from Coprinopsis cinerea. Biosci. Biotechnol. Biochem. 73:1432–1434. 10.1271/bbb.80888. [DOI] [PubMed] [Google Scholar]
- 249.Yoshida M, Sato K, Kaneko S, Fukuda K. 2009. Cloning and transcript analysis of multiple genes encoding the glycoside hydrolase family 6 enzyme from Coprinopsis cinerea. Biosci. Biotechnol. Biochem. 73:67–73. 10.1271/bbb.80477. [DOI] [PubMed] [Google Scholar]
- 250.Sadana JC, Patil RV. 1988. 1,4-β-d-Glucan cellobiohydrolase from Sclerotium rolfsii. Methods Enzymol. 160:307–314. 10.1016/0076-6879(88)60132-7. [DOI] [Google Scholar]
- 251.Rouland C, Civas A, Renoux J, Petek F. 1988. Purification and properties of cellulases from the termite Macrotermes mülleri (Termitidae, Macrotermitinae) and its symbiotic fungus Termitomyces sp. Comp. Biochem. Physiol. Biochem. Mol. Biol. 91:449–458. 10.1016/0305-0491(88)90004-1. [DOI] [PubMed] [Google Scholar]
- 252.Magalhães PO, Ferraz A, Milagres AFM. 2006. Enzymatic properties of two β-glucosidases from Ceriporiopsis subvermispora produced in biopulping conditions. J. Appl. Microbiol. 101:480–486. 10.1111/j.1365-2672.2006.02946.x. [DOI] [PubMed] [Google Scholar]
- 253.Copa-Patino JL, Broda P. 1994. A Phanerochaete chrysosporium β-D-glucosidase/β-D-xylosidase with specificity for (1→3)-β-D-glucan linkages. Carbohydr. Res. 253:265–275. 10.1016/0008-6215(94)80071-5. [DOI] [PubMed] [Google Scholar]
- 254.Lymar ES, Li B, Renganathan V. 1995. Purification and characterization of a cellulose-binding β-glucosidase from cellulose-degrading cultures of Phanerochaete chrysosporium. Appl. Environ. Microbiol. 61:2976–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li B, Renganathan V. 1998. Gene cloning and characterization of a novel cellulose-binding β-glucosidase from Phanerochaete chrysosporium. Appl. Environ. Microbiol. 64:2748–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Igarashi K, Tani T, Kawai R, Samejima M. 2003. Family 3 β-glucosidase from cellulose-degrading culture of the white-rot fungus Phanerochaete chrysosporium is a glucan 1,3-β-glucosidase. J. Biosci. Bioeng. 95:572–576. 10.1016/S1389-1723(03)80164-0. [DOI] [PubMed] [Google Scholar]
- 257.Kawai R, Yoshida M, Tani T, Igarashi K, Ohira T, Nagasawa H, Samejima M. 2003. Production and characterization of recombinant Phanerochaete chrysosporium β-glucosidase in the methylotrophic yeast Pichia pastoris. Biosci. Biotechnol. Biochem. 67:1–7. 10.1271/bbb.67.1. [DOI] [PubMed] [Google Scholar]
- 258.Bhattacharjee B, Roy A, Majumder AL. 1992. β-Glucosidase of a white-rot fungus Trametes gibbosa. Biochem. Int. 28:783–793. [PubMed] [Google Scholar]
- 259.Gallagher IM, Evans CS. 1990. Immunogold-cytochemical labelling of β-glucosidase in the white-rot fungus Coriolus versicolor. Appl. Microbiol. Biotechnol. 32:588–593. 10.1007/BF00173732. [DOI] [Google Scholar]
- 260.Deshpande V, Eriksson KE, Pettersson B. 1978. Production, purification and partial characterization of 1,4-β-glucosidase enzymes from Sporotrichum pulverulentum. Eur. J. Biochem. 90:191–198. 10.1111/j.1432-1033.1978.tb12590.x. [DOI] [PubMed] [Google Scholar]
- 261.Ramachandran P, Nguyen NP, Choi JH, Kang YC, Jeya M, Lee JK. 2013. Optimization of β-glucosidase production by a strain of Stereum hirsutum and its application in enzymatic saccharification. J. Microbiol. Biotechnol. 23:351–356. 10.4014/jmb.1210.10060. [DOI] [PubMed] [Google Scholar]
- 262.Desrochers M, Jurasek L, Paice MG. 1981. High production of β-glucosidase in Schizophyllum commune: isolation of the enzyme and effect of the culture filtrate on cellulose hydrolysis. Appl. Environ. Microbiol. 41:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lo AC, Barbier J-R, Willick GE. 1990. Kinetics and specificities of two closely related β-glucosidases secreted by Schizophyllum commune. Eur. J. Biochem. 192:175–181. 10.1111/j.1432-1033.1990.tb19211.x. [DOI] [PubMed] [Google Scholar]
- 264.Herr D, Baumer F, Dellweg H. 1978. Purification and properties of an extracellular β-glucosidase from Lenzites trabea. Eur. J. Appl. Microbiol. 5:29–36. 10.1007/BF00515684. [DOI] [Google Scholar]
- 265.Sison BC, Jr, Schubert WJ. 1958. On the mechanism of enzyme action. LXVIII. The cellobiase component of the cellulolytic enzyme system of Poria vaillantii. Arch. Biochem. Biophys. 78:563–572. 10.1016/0003-9861(58)90381-3. [DOI] [PubMed] [Google Scholar]
- 266.Ding S, Ge W, Buswell JA. 2007. Molecular cloning and transcriptional expression analysis of an intracellular β-glucosidase, a family 3 glycosyl hydrolase, from the edible straw mushroom, Volvariella volvacea. FEMS Microbiol. Lett. 267:221–229. 10.1111/j.1574-6968.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- 267.Nakajima M, Yamashita T, Takahashi M, Nakano Y, Takeda T. 2012. Identification, cloning, and characterization of β-glucosidase from Ustilago esculenta. Appl. Microbiol. Biotechnol. 93:1989–1998. 10.1007/s00253-011-3538-2. [DOI] [PubMed] [Google Scholar]
- 268.Kusuda M, Ueda M, Konishi Y, Araki Y, Yamanaka K, Nakazawa M, Miyatake K, Terashita T. 2006. Detection of β-glucosidase as saprotrophic ability from an ectomycorrhizal mushroom, Tricholoma matsutake. Mycoscience 47:184–189. 10.1007/s10267-005-0289-x. [DOI] [Google Scholar]
- 269.Cao W, Crawford DL. 1993. Purification and some properties of β-glucosidase from the ectomycorrhizal fungus Pisolithus tinctorius strain SMF. Can. J. Microbiol. 39:125–129. 10.1139/m93-018. [DOI] [Google Scholar]
- 270.Pal S, Banik SP, Ghorai S, Chowdhury S, Khowala S. 2010. Purification and characterization of a thermostable intra-cellular β-glucosidase with transglycosylation properties from filamentous fungus Termitomyces clypeatus. Bioresour. Technol. 101:2412–2420. 10.1016/j.biortech.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 271.Sengupta S, Ghosh AK, Sengupta S. 1991. Purification and characterisation of a β-glucosidase (cellobiase) from a mushroom Termitomyces clypeatus. Biochim. Biophys. Acta 1076:215–220. 10.1016/0167-4838(91)90269-6. [DOI] [PubMed] [Google Scholar]
- 272.Henriksson G, Ander P, Pettersson B, Pettersson G. 1995. Cellobiose dehydrogenase (cellobiose oxidase) from Phanerochaete chrysosporium as a wood-degrading enzyme. Studies on cellulose, xylan and synthetic lignin. Appl. Microbiol. Biotechnol. 42:790–796. 10.1007/s002530050332. [DOI] [Google Scholar]
- 273.Li B, Nagalla SR, Renganathan V. 1996. Cloning of a cDNA encoding cellobiose dehydrogenase, a hemoflavoenzyme from Phanerochaete chrysosporium. Appl. Environ. Microbiol. 62:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Raices M, Paifer E, Cremata J, Montesino R, Stahlberg J, Divne C, Szabo IJ, Henriksson G, Johansson G, Pettersson G. 1995. Cloning and characterization of a cDNA encoding a cellobiose dehydrogenase from the white rot fungus Phanerochaete chrysosporium. FEBS Lett. 369:233–238. 10.1016/0014-5793(95)00758-2. [DOI] [PubMed] [Google Scholar]
- 275.Igarashi K, Verhagen MFJM, Samejima M, Schülein M, Eriksson K-L, Nishino T. 1999. Cellobiose dehydrogenase from the fungi Phanerochaete chrysosporium and Humicola insolens. A flavohemoprotein from Humicola insolens contains 6-hydroxy-fad as the dominant active cofactor. J. Biol. Chem. 274:3338–3344. [DOI] [PubMed] [Google Scholar]
- 276.Yoshida M, Ohira T, Igarashi K, Nagasawa H, Aida K, Hallberg BM, Divne C, Nishino T, Samejima M. 2001. Production and characterization of recombinant Phanerochaete chrysosporium cellobiose dehydrogenase in the methylotrophic yeast Pichia pastoris. Biosci. Biotechnol. Biochem. 65:2050–2057. 10.1271/bbb.65.2050. [DOI] [PubMed] [Google Scholar]
- 277.Sigoillot C, Lomascolo A, Record E, Robert JL, Asther M, Sigoillot JC. 2002. Lignocellulolytic and hemicellulolytic system of Pycnoporus cinnabarinus: isolation and characterization of a cellobiose dehydrogenase and a new xylanase. Enzyme Microb. Technol. 31:876–883. 10.1016/S0141-0229(02)00208-9. [DOI] [Google Scholar]
- 278.Moukha SM, Dumonceaux TJ, Record E, Archibald FS. 1999. Cloning and analysis of Pycnoporus cinnabarinus cellobiose dehydrogenase. Gene 234:23–33. 10.1016/S0378-1119(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 279.Temp U, Eggert C. 1999. Novel interaction between laccase and cellobiose dehydrogenase during pigment synthesis in the white rot fungus Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 65:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bey M, Berrin J-G, Poidevin L, Sigoillot J-C. 2011. Heterologous expression of Pycnoporus cinnabarinus cellobiose dehydrogenase in Pichia pastoris and involvement in saccharification processes. Microb. Cell Fact. 10:113. 10.1186/1475-2859-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Roy BP, Dumonceaux T, Koukoulas AA, Archibald FS. 1996. Purification and characterization of cellobiose dehydrogenases from the white rot fungus Trametes versicolor. Appl. Environ. Microbiol. 62:4417–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Dumonceaux TJ, Bartholomew KA, Charles TC, Moukha SM, Archibald FS. 1998. Cloning and sequencing of a gene encoding cellobiose dehydrogenase from Trametes versicolor. Gene 210:211–219. 10.1016/S0378-1119(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 283.Stapleton PC, O'Brien MM, O'Callaghan J, Dobson ADW. 2004. Molecular cloning of the cellobiose dehydrogenase gene from Trametes versicolor and expression in Pichia pastoris. Enzyme Microb. Technol. 34:55–63. 10.1016/j.enzmictec.2003.08.006. [DOI] [Google Scholar]
- 284.Yoshida M, Ohira T, Igarashi K, Nagasawa H, Samejima M. 2002. Molecular cloning and characterization of a cDNA encoding cellobiose dehydrogenase from the wood-rotting fungus Grifola frondosa. FEMS Microbiol. Lett. 217:225–230. 10.1111/j.1574-6968.2002.tb11479.x. [DOI] [PubMed] [Google Scholar]
- 285.Sulej J, Janusz G, Mazur A, Zuber K, Zebracka A, Rogalski J. 2013. Cellobiose dehydrogenase from the ligninolytic basidiomycete Phlebia lindtneri. Process Biochem. 48:1715–1723. 10.1016/j.procbio.2013.08.003. [DOI] [Google Scholar]
- 286.Sulej J, Janusz G, Osinska-Jaroszuk M, Malek P, Mazur A, Komaniecka I, Choma A, Rogalski J. 2013. Characterization of cellobiose dehydrogenase and its FAD-domain from the ligninolytic basidiomycete Pycnoporus sanguineus. Enzyme Microb. Technol. 53:427–437. 10.1016/j.enzmictec.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 287.Fang J, Liu W, Gao PJ. 1998. Cellobiose dehydrogenase from Schizophyllum commune: purification and study of some catalytic, inactivation, and cellulose-binding properties. Arch. Biochem. Biophys. 353:37–46. 10.1006/abbi.1998.0602. [DOI] [PubMed] [Google Scholar]
- 288.Sadana JC, Patil RV. 1988. Cellobiose dehydrogenase from Sclerotium rolfsii. Methods Enzymol. 160:448–454. 10.1016/0076-6879(88)60153-4. [DOI] [Google Scholar]
- 289.Baminger U, Subramaniam SS, Renganathan V, Haltrich D. 2001. Purification and characterization of cellobiose dehydrogenase from the plant pathogen Sclerotium (Athelia) rolfsii. Appl. Environ. Microbiol. 67:1766–1774. 10.1128/AEM.67.4.1766-1774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yakovlev I, Vaaje-Kolstad G, Hietala AM, Stefanczyk E, Solheim H, Fossdal CG. 2012. Substrate-specific transcription of the enigmatic GH61 family of the pathogenic white-rot fungus Heterobasidion irregulare during growth on lignocellulose. Appl. Microbiol. Biotechnol. 95:979–990. 10.1007/s00253-012-4206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Milagres AMF, Magalhães PO, Ferraz A. 2005. Purification and properties of a xylanase from Ceriporiopsis subvermispora cultivated on Pinus taeda. FEMS Microbiol. Lett. 253:267–272. 10.1016/j.femsle.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 292.Hoebler C, Brillouet J-M. 1984. Purification and properties of an endo-(1→4)-β-d-xylanase from Irpex lacteus (Polyporus tulipiferae). Carbohydr. Res. 128:141–155. 10.1016/0008-6215(84)85092-2. [DOI] [Google Scholar]
- 293.Kanda T, Amano Y, Nisizawa K. 1985. Purification and properties of two endo-1,4-β-xylanases from Irpex lacteus (Polyporus tulipiferae). J. Biochem. 98:1545–1554. [DOI] [PubMed] [Google Scholar]
- 294.Mishra C, Forrester IT, Kelley BD, Burgess RR, Leatham GF. 1990. Characterization of a major xylanase purified from Lentinula edodes cultures grown on a commercial solid lignocellulosic substrate. Appl. Microbiol. Biotechnol. 33:226–232. 10.1007/BF00176530. [DOI] [PubMed] [Google Scholar]
- 295.Lee CC, Wong DWS, Robertson GH. 2005. Cloning and characterization of the Xyn11A gene from Lentinula edodes. Protein J. 24:21–26. 10.1007/s10930-004-0602-0. [DOI] [PubMed] [Google Scholar]
- 296.Lee J-W, Gwak K-S, Kim S-I, Kim M, Choi D-H, Choi I-G. 2007. Characterization of xylanase from Lentinus edodes M290 cultured on waste mushroom logs. J. Microbiol. Biotechnol. 17:1811–1817. [PubMed] [Google Scholar]
- 297.Decelle B, Tsang A, Storms RK. 2004. Cloning, functional expression and characterization of three Phanerochaete chrysosporium endo-1,4-β-xylanases. Curr. Genet. 46:166–175. 10.1007/s00294-004-0520-x. [DOI] [PubMed] [Google Scholar]
- 298.Huy ND, Thiyagarajan S, Son Y-L, Park S-M. 2011. Heterologous expression of endo-1,4-β-xylanaseA from Phanerochaete chrysosporium in Pichia pastoris. Mycobiology 39:121–124. 10.4489/MYCO.2011.39.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Huy ND, Kim S-W, Park S-M. 2011. Heterologous expression of endo-1,4-β-xylanaseC from Phanerochaete chrysosporium in Pichia pastoris. J. Biosci. Bioeng. 111:654–657. 10.1016/j.jbiosc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 300.Khan SN, Akter MZ, Sims PFG. 2002. Cloning and expression analysis of two endo-1,4-β-xylanase genes from Phanerochaete chrysosporium. Plant Tissue Cult. 12:57–68. [Google Scholar]
- 301.Paice MG, Jurasek L, Carpenter MR, Smillie LB. 1978. Production, characterization, and partial amino acid sequence of xylanase A from Schizophyllum commune. Appl. Environ. Microbiol. 36:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kolenová K, Vršanská M, Biely P. 2005. Purification and characterization of two minor endo-β-1,4-xylanases of Schizophyllum commune. Enzyme Microb. Technol. 36:903–910. 10.1016/j.enzmictec.2005.01.006. [DOI] [Google Scholar]
- 303.Ritschkoff A-C, Buchert J, Viikari L. 1994. Purification and characterization of a thermophilic xylanase from the brown-rot fungus Gloeophyllum trabeum. J. Biotechnol. 32:67–74. 10.1016/0168-1656(94)90121-X. [DOI] [Google Scholar]
- 304.Lee J-W, Park J-Y, Kwon M, Choi I-G. 2009. Purification and characterization of a thermostable xylanase from the brown-rot fungus Laetiporus sulphureus. J. Biosci. Bioeng. 107:33–37. 10.1016/j.jbiosc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 305.Green F, III, Clausen CA, Micales JA, Highley TL, Wolter KE. 1989. Carbohydrate-degrading complex of the brown-rot fungus Postia placenta: purification of β-1,4-xylanase. Holzforschung 43:25–31. 10.1515/hfsg.1989.43.1.25. [DOI] [Google Scholar]
- 306.Ghosh AK, Banerjee PC, Sengupta S. 1980. Purification and properties of xylan hydrolase from mushroom Termitomyces clypeatus. Biochim. Biophys. Acta 612:143–152. 10.1016/0005-2744(80)90287-9. [DOI] [PubMed] [Google Scholar]
- 307.Faulet BM, Niamké S, Gonnety JT, Kouamé LP. 2006. Purification and biochemical properties of a new thermostable xylanase from symbiotic fungus, Termitomyces sp. Afr. J. Biotechnol. 5:273–282. [Google Scholar]
- 308.Lachke AH, Deshpande MV, Srinivasan MC. 1985. Extracellular β-D-xylosidase of Sclerotium rolfsii. Enzyme Microb. Technol. 7:445–448. 10.1016/0141-0229(85)90045-6. [DOI] [Google Scholar]
- 309.Ahlgren E, Eriksson K. 1967. Characterization of celullases and related enzymes by isoelectric focusing, gel filtration, and zone electrophoresis. II. Studies on Stereum sanguinolentum, Fomes annosus, and Chrysosporium lignorum enzymes. Acta Chem. Scand. 21:1193–1200. 10.3891/acta.chem.scand.21-1193. [DOI] [Google Scholar]
- 310.Gübitz GM, Schnitzhoter W, Balakrishnan H, Steiner W. 1996. Two mannanases from Sclerotium rolfsii in total chlorine free bleaching of softwood kraft pulp. J. Biotechnol. 50:181–188. 10.1016/0168-1656(96)01563-5. [DOI] [Google Scholar]
- 311.Gübitz GM, Laussamauer B, Schubert-Zsilavccz M, Steiner W. 2000. Production of 61-α-D-galactosyl-β-D-mannotriose with endo-1,4-β-mannanases from Schizophyllum commune and Sclerotium rolfsii. Enzyme Microb. Technol. 26:15–21. 10.1016/S0141-0229(99)00122-2. [DOI] [Google Scholar]
- 312.Brillouet J-M, Moulin J-C, Agosin E. 1985. Production, purification, and properties of an α-l-arabinofuranosidase from Dichomitus squalens. Carbohydr. Res. 144:113–126. 10.1016/0008-6215(85)85012-6. [DOI] [Google Scholar]
- 313.Hashimoto K, Yoshida M, Hasumi K. 2011. Isolation and characterization of CcAbf62A, a GH62 α-L-arabinofuranosidase, from the basidiomycete Coprinopsis cinerea. Biosci. Biotechnol. Biochem. 75:342–345. 10.1271/bbb.100434. [DOI] [PubMed] [Google Scholar]
- 314.Hart DO, He S, Chany CJ, II, Withers SG, Sims PFG, Sinnott ML, Brumer H., III 2000. Identification of Asp-130 as the catalytic nucleophile in the main α-galactosidase from Phanerochaete chrysosporium, a family 27 glycosyl hydrolase. Biochemistry 39:9826–9836. 10.1021/bi0008074. [DOI] [PubMed] [Google Scholar]
- 315.Li Y-T, Shetlar MR. 1964. Occurrence of α-galactosidase in higher fungi: isolation of α-galactosidase from Calvatia cyathiformis. Arch. Biochem. Biophys. 108:523–530. 10.1016/0003-9861(64)90437-0. [DOI] [PubMed] [Google Scholar]
- 316.Kaji A, Sato M, Shinmyo N, Yasuda M. 1972. Purification and properties of acid β-D-galactosidase from Corticium rolfsii. Agric. Biol. Chem. 36:1729–1735. 10.1271/bbb1961.36.1729. [DOI] [Google Scholar]
- 317.Cho Y-J, Shin H-J, Bucke C. 2003. Purification and biochemical properties of a galactooligosaccharide producing β-galactosidase from Bullera singularis. Biotechnol. Lett. 25:2107–2111. 10.1023/B:BILE.0000007077.58019.bb. [DOI] [PubMed] [Google Scholar]
- 318.Kotake T, Hirata N, Degi Y, Ishiguro M, Kitazawa K, Takata R, Ichinose H, Kaneko S, Igarashi K, Samejima M, Tsumuraya Y. 2011. Endo-β-1,3-galactanase from winter mushroom Flammulina velutipes. J. Biol. Chem. 286:27848–27854. 10.1074/jbc.M111.251736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kotake T, Kitazawa K, Takata R, Okabe K, Ichingse H, Kaneko S, Tsumuraya Y. 2009. Molecular cloning and expression in Pichia pastoris of a Irpex lacteus exo-/β-(1→3)-galactanase gene. Biosci. Biotechnol. Biochem. 73:2303–2309. 10.1271/bbb.90433. [DOI] [PubMed] [Google Scholar]
- 320.Ichinose H, Yoshida M, Kotake T, Kuno A, Igarashi K, Tsumuraya Y, Samejima M, Hirabayashi J, Kobayashi H, Kaneko S. 2005. An exo-β-1,3-galactanase having a novel β-1,3-galactan-binding module from Phanerochaete chrysosporium. J. Biol. Chem. 280:25820–25829. 10.1074/jbc.M501024200. [DOI] [PubMed] [Google Scholar]
- 321.Puls J, Schmidt O, Granzow C. 1987. α-Glucuronidase in two microbial xylanolytic systems. Enzyme Microb. Technol. 9:83–88. 10.1016/0141-0229(87)90147-5. [DOI] [Google Scholar]
- 322.Halgašová N, Kutejová E, Timko J. 1994. Purification and some characteristics of the acetylxylan esterase from Schizophyllum commune. Biochem. J. 298:751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ding S, Cao J, Zhou R, Zheng F. 2007. Molecular cloning, and characterization of a modular acetyl xylan esterase from the edible straw mushroom Volvariella volvacea. FEMS Microbiol. Lett. 274:304–310. 10.1111/j.1574-6968.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 324.Hashimoto K, Kaneko S, Yoshida M. 2010. Extracellular carbohydrate esterase from the basidiomycete Coprinopsis cinerea released ferulic and acetic acids from xylan. Biosci. Biotechnol. Biochem. 74:1722–1724. 10.1271/bbb.100299. [DOI] [PubMed] [Google Scholar]