Abstract
Immune responses in the brain are thought to play a role in disorders of the central nervous system, but an understanding of the process underlying how immune cells get into the brain and their fate there remains unclear. In this study, we used a 2-photon microscopy to reveal that neutrophils infiltrate brain and migrate toward amyloid plaques in a mouse model of Alzheimer’s disease. These findings suggest a new molecular process underlying the pathophysiology of Alzheimer’s disease.
Keywords: Alzheimer’s disease, Neutrophils, Amyloid plaques, Immune cells
1. Introduction
Although the central nervous system has classically been considered a site of immune privilege, recent studies have shown that immune cells can infiltrate the brain under certain pathologic conditions (Friese et al., 2004; Lucin and Wyss-Coray, 2009). The major causative factor of Alzheimer’s disease (AD), beta-amyloid (Aβ), has been shown to be associated with brain inflammatory responses and to activate microglia and monocytes (Fiala et al., 2007; Hickman and El Khoury, 2013; Krstic and Knuesel, 2013), raising the possibility that immune responses might play a role in the pathophysiology of AD. However, it is unknown whether and how innate immune cells are involved in the progress of AD (Fortin et al., 2008). Here, we show that neutrophils, which are the most abundant type of white blood cell, infiltrate into the brain and migrate to amyloid plaques in a mouse model of AD (the 5XFAD mouse). We labeled neutrophils with a Ly6C/G (Gr-1) Alexa Fluor 488-conjugated antibody and followed their movements in the 5XFAD mouse with a 2-photon in vivo imaging technique. Some neutrophils in the blood vessels infiltrated into the brain, migrated toward amyloid plaques, and accumulated at the same locations where amyloid plaques were found. One pathologic hallmark of AD is the accumulation of senile plaques in the brain, and Aβ is their major constituent. Our results showed that chronic Aβ deposition attracted neutrophils from the blood vessels in AD animal model mice, suggesting that immune cell responses might be involved in the progress of AD. Although the role of immune cells that are colocalized with Aβ plaques is unknown, they might be involved in the pathology of senile plaques. Understanding this process might provide insights into the pathophysiology and potential treatments of AD.
2. Methods
2.1. Animals and surgery
The 5XFAD mice (Tg6799; B6SJL-Tg [APPSwFlLon, PSEN* M146L*L286V] 6799Vas/J, stock no. 006554) that were used in this experiment were 9–13 months old and were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). These mice express 3 mutations (Swedish, Florida, and London) of human amyloid precursor protein 695 and 2 mutations (M146L and L286V) of human presenilin-1 (Oakley et al., 2006). A craniotomy surgery was performed as described previously (Mostany and Portera-Cailliau, 2008). Briefly, the mice were initially anesthetized with isoflurane, and the hair on the skin of the imaging area was removed. Dexamethasone (0.2 mg/kg) and carprofen (5 mg/kg) were intramuscularly injected before the surgery to avoid inflammatory responses or swelling of the brain. After drilling, a lidocaine and epinephrine mixture was also applied to the periosteumto prevent pain or excessive bleeding. A 5-mm round coverslip was placed on top of the dura mater with cyanoacrylate-based glue and dental acrylic. The body temperature of the mice was maintained with a heating plate (Live Cell Instrument, Seoul, Korea) that was set to 37 °C. The animal treatment and maintenance protocols were approved by the Ethics Review Committee for Animal Experimentation in Seoul National University.
2.2. Multiphoton imaging
To image the neutrophils, 0.12 mg/kg of the Ly6C/G (Gr-1) Alexa Fluor 488-conjugated antibody was intravenously injected through the femoral vein immediately before the imaging. Texas red-dextran was also intravenously injected to label the vessels before imaging. To visualize amyloid plaques, methoxy-XO4 (5 mg/mL in 10% dimethyl sulfoxide, 45% propylene glycol, and 45% saline) was intraperitoneally injected 24 hours before the imaging. The multiphoton imaging was performed with a LSM 7 MP 2-photon laser scanning microscope system (Carl Zeiss Microscopy GmbH, Oberkochen, Germany). The Volocity image analysis software program (PerkinElmer Inc, Waltham, MA, USA) was used to process the 3D imaging and track the movements of the neutrophils.
2.3. Isolation of the mouse neutrophils and the flow cytometry analysis
The neutrophils were isolated from wild-type (WT) and 5XFAD mouse brains. In brief, we isolated the brains while the animals were under anesthesia at the age of 8 months and collected the frontal cortex. The brains were then homogenized with Hank’s Balanced Salt Solution (Welgene, Inc, Daegu, Korea) on ice. The cells were incubated at 37 °C with Collagenase D (2.5 mg/mL) for 1 hour. The neutrophils were then isolated with Percoll density gradient centrifugation. We rinsed these cells in fluorescence-activated cell sorting (FACS) buffer (1% fetal calf serum and 0.1% wt/vol sodium azide) and stained them with allophycocyanin-conjugated Ly6G (Gr-1) (1:1000) for 30 minutes on ice. After washing the cells in FACS buffer, we analyzed them with a FACSCalibur instrument (BD Biosciences, San Jose, CA, USA).
2.4. Fabrication of microfluidic platform
The microfluidic platform was fabricated as described (Cho et al., 2013). Briefly, inversed features were made on silicon wafers in multilayers: thicknesses of 5 µm for neutrophil migration channels, 10 µm for the entrance zone to the migration channels, 50 µm for all other compartments on the network wafer, and all features on the valve control wafer, using soft lithographic techniques. A mixture of polydimethylsiloxane (PDMS) and its curing agent (SYLGARD 184 A/ B, Dowcorning, Midland, MI, USA) at 10:1 was spun to a thickness of 150 µm on the network wafer and poured to the control wafer at a thickness of ~3 mm. The PDMS replica of network and control layers were peeled off from the wafers and then treated with oxygen plasma at 50 mW, 10 ccm, for 35 seconds (PX-250, March Plasma Systems, Petersburg, FL, USA) for bonding. The assembled PDMS layers were punched for reservoirs, treated with oxygen plasma, and bonded to a glass-bottomed UniWell plate (MGB001-1-2-LG, Matrical Bioscience, Spokane, WA, USA). The platforms were immediately treated for 30 minutes with human-fibronectin (Sigma-Aldrich, St Louis, MO) at 50-µg·mL−1 in distilled water (0.2 µm filter, AM9920, Life Technologies, Grand Island, NY, USA). Fibronectin-treated surfaces were then rinsed with sterile distilled water and the platform was filled with cell culture medium of Iscove’s Modified Dulbecco’s medium (30–2005, ATCC, Manassas, VA, USA) supplemented with 20% fetal bovine serum (Sigma-Aldrich, St Louis, MO) and 1% penicillin and/or streptomycin (Life Technologies).
2.5. Cell preparation
Human neutrophils were was prepared as described (Cho et al., 2013). Briefly, neutrophils were isolated from whole blood with HetaSep and an EasySepHuman Neutrophil Enrichment Kit (STEMCELL Technologies, Vancouver, Canada). The cell membrane was stained with red fluorescent dye (PKH26PCL, Sigma-Aldrich). The stained neutrophils were re-suspended in a culturing medium at a concentration of 20 × 106 cells·mL−1. Ten µL of cell suspension were injected into the cell compartment and incubated at 37 °C supplied with 5% CO2 for 30 minutes to recover their activities.
2.6. Chemokine preparation
We dissociated any higher molecular weight of Aβ by dissolving a synthesized clear human Aβ, 1–42 (4349-v, Peptide Institute, Inc, Japan) in HFIP and then fully evaporate hexafluoroisopropanol (HFIP). The dissociated Aβ was dissolved in dimethyl sulfoxide (DMSO) at 1 mg·mL−1 for a monomer form and further diluted in phosphate-buffered saline (PBS) at 0.1mg·mL−1. The prepared Aβ monomer was diluted in a neutrophil culturing medium at working concentrations and immediately used to minimize aggregation or degradation. N-formyl-methyl-leucyl-phenylalanine was diluted in sterile distilled water at 0.1 mg·mL−1 and further diluted in a culturing medium at a working concentration.
2.7. Time-lapse imaging
To simultaneously image the moving neutrophils in the arrayed 48 platforms, we used an automated microscope equipped with a motorized stage (EclipseTi, Nikon Inc, Melville, NY, USA) as described (Cho et al., 2013). To image live cells, we used a heated incubating stage (LiveCell 05-11-0032 Rev B, Pathology Devices Inc, Westminster, MD, USA), which was set at 37.7 °C, 5% CO2, and 85% humidity. The time-lapse imaging was taken every 3 minutes for 2 hours and in a large-area mode of 2 × 2mm2 using a 4× objective lens.
2.8. Analysis of the cellular motility
Acquired time-lapse images with the NIS Elements (Nikon Inc) were analyzed as described (Cho et al., 2013). Briefly, we identified and extracted positions of cells from each image automatically by using the open-sourced software, CellProfiler (Broad Institute, Boston, MA, USA). Extracted information was processed and analyzed to calculate migration speed by using MATLAB (Math-Works, Inc, Natick, MA, USA).
2.9. Antibodies and reagents
The anti-mouse Ly6C/G (Gr-1) Alexa Fluor 488 conjugate and Texas red-dextran (70,000 molecular weight) were purchased from Life Technologies Corporation (Grand Island, NY, USA). Antimouse Ly6G (Gr-1) allophycocyanin was purchased from eBioscience, Inc (San Diego, CA, USA). Methoxy XO-4 was synthesized with a protocol that has been described in a previous paper (Klunk et al., 2002).
2.10. Statistical analysis
All the data were analyzed by paired t tests with GraphPad Prism version 4.0 (GraphPad Software, Inc, La Jolla, CA, USA). p-values less than 0.05 were considered statistically significant.
3. Results
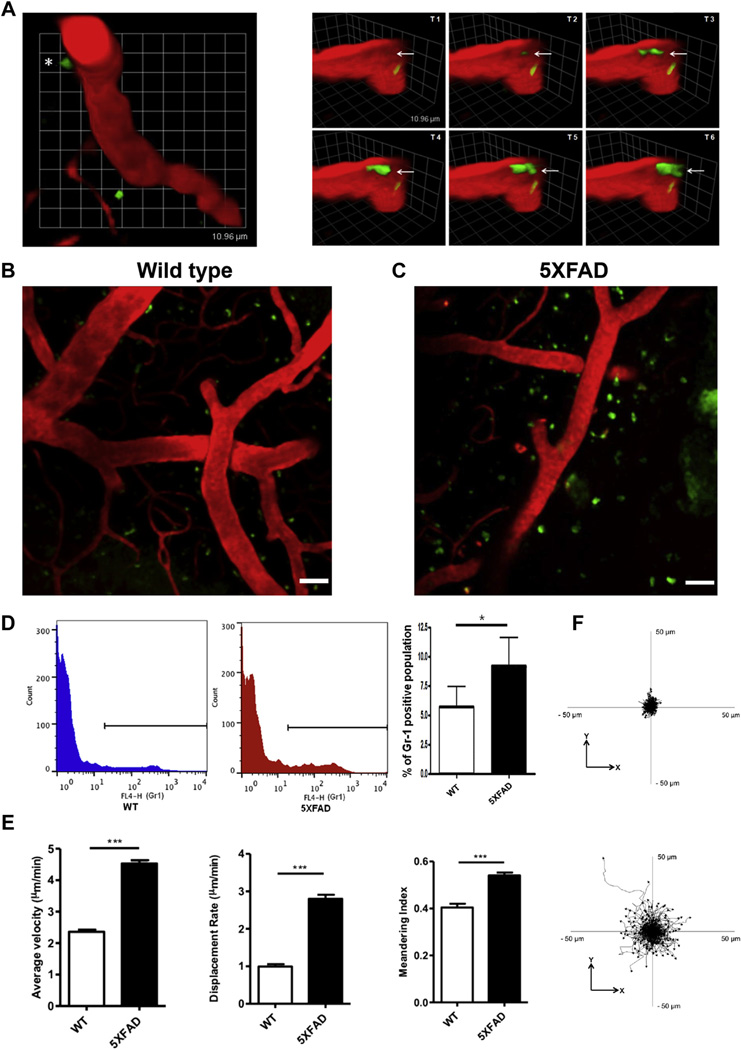
To examine the neutrophil movements in AD model animals, Ly6C/G (Gr-1) Alexa Fluor 488-conjugated antibody, which specifically binds to neutrophils, and Texas red-dextran were delivered to the blood circulating system with intravenous injections in craniotomized 5XFAD mice. Two-photon in vivo imaging showed that the neutrophils were rolling and crawling along the lumen of the blood vessels in WT and young 5XFAD mice. In contrast, dynamic extravasation of the neutrophils into the brain parenchyma was detected in the brains of 9- to 13-month-old 5XFAD mice, which showed pathologic signs of AD, such as massive Aβ plaque accumulation, and this was not seen in the brains of WT mice. In Fig. 1 and Video 1, the red represents the Texas red-conjugated 70-kDa dextran, which circulated in the blood vessel, and the green indicates the Ly6C/G (Gr-1) Alexa Fluor 488-conjugated antibody, which labels neutrophils. As shown in the figure, the neutrophils exited the blood vessels and moved into the brain parenchyma in 5XFAD mice. The time course for the transendothelial migration of the neutrophils showed that the extravasation of the neutrophils occurred within 2 minutes and 30 seconds (transendothelial migration of neutrophils, Fig. 1A, asterisk). Remarkably, these results showed that the blood neutrophils entered the brain parenchyma in the AD model mice. We then examined whether the neutrophils were mobile once they entered the brain. The in vivo imaging revealed that the neutrophils moved around substantially in the brain parenchyma of 5XFAD mice (Video 2). The neutrophils from 5XFAD mice showed a higher migration velocity, displacement, and meandering index in the brain parenchyma compared with those of the WT (Fig. 1E and F). In contrast, neutrophils were rarely found in the brain parenchyma of the WT animals (Fig. 1B–D, and Video 7). The FACS analysis showed that the number of Gr-1-positive neutrophils was significantly increased in the brains of 5XFAD mice compared with WT mice (Fig. 1D, * p < 0.05). These results indicated that the old 5XFAD mice brains contained a substantial level of neutrophils, which had vigorous motility in the brain.
Fig. 1.
The transendothelial migration of neutrophils from blood vessels into the brain parenchyma occurs in 5XFAD mice. The brain of a 5XFAD mouse, which exhibited massive Aβ plaque burden and neuronal cell death, was imaged with a 2-photon microscope after craniotomy surgery, and the dynamic extravasation of neutrophils into the brain parenchyma was detected. Intravenously (i.v.) injected Texas red-conjugated 70-kDa Dextran (red) labels brain blood vessels, and the Ly6C/G (Gr-1) Alexa Fluor 488-conjugated antibody (green) labels neutrophils. (A) Left: neutrophils in the blood vessels exited the brain parenchyma (transendothelial migration of the neutrophils, asterisk). Right: the different side-view images of the transendothelial migration of the same neutrophils (white arrow) from Fig. 1A, left, were taken at intervals of 30 seconds; 1 unit, 10.96 µm. The in vivo imaging of the brain of wild-type ([WT]; B) and 5XFAD (C) mice with Texas red-conjugated 70-kDa Dextran (red) and Gr-1 Alexa Fluor 488-conjugated antibody (green). The neutrophils that migrated into the brain parenchyma were found in 5XFAD mice but not in WT mice. Scale bar, 40 µm. (D) A fluorescence-activated cell sorting analysis also shows that Gr-1-positive neutrophils were more abundant in the brain of 5XFAD mice (middle) than in WT mice (left). N = 2 per group; the experiments were repeated 4 times; * p < 0.05. (E) The populations of tracked neutrophils from the 5XFAD mice (Video 2) and WT mice (Video 8) were quantified with respect to their average velocity, displacement rate, and meandering index; *** p < 0.0001. (F) In addition, the populations of tracked neutrophils from 5XFAD mice (Video 2) and WT mice (Video 8) were also examined with respect to their XY track plot, which was relative to their starting position. These quantitative data and plots suggest that neutrophils migrated into the brain parenchyma and that they have vigorous behavior and movement in 5XFAD mice compared with WT mice. Abbreviation: Aβ, beta-amyloid.
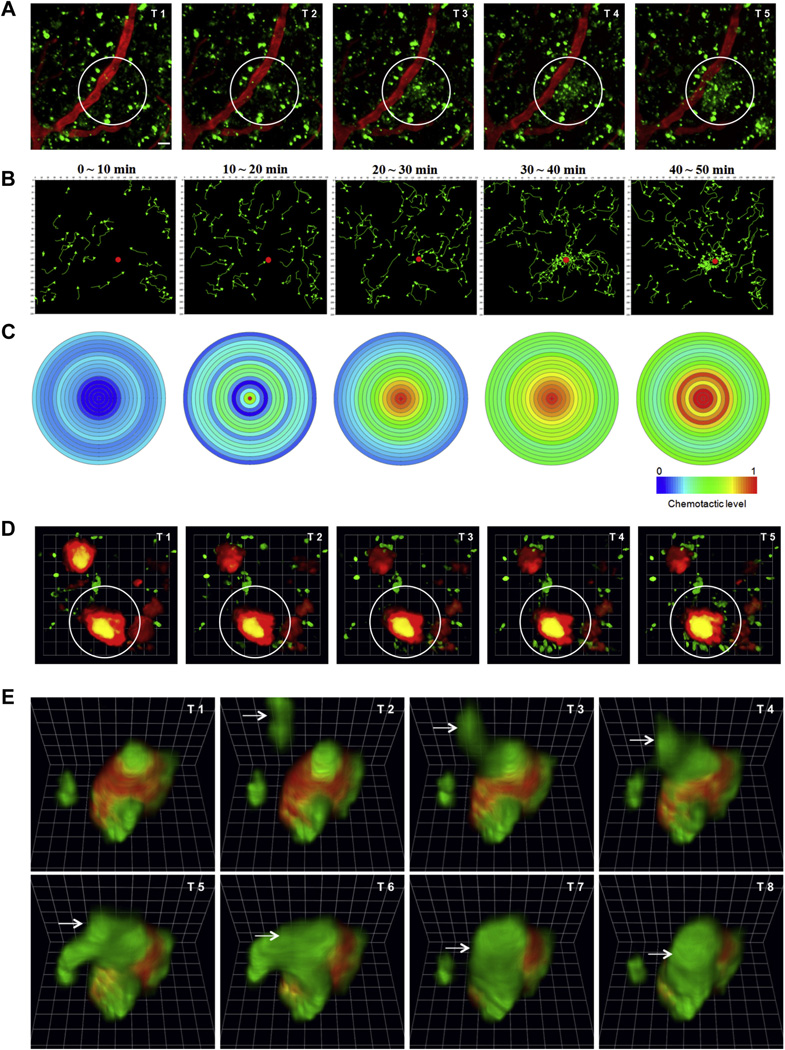
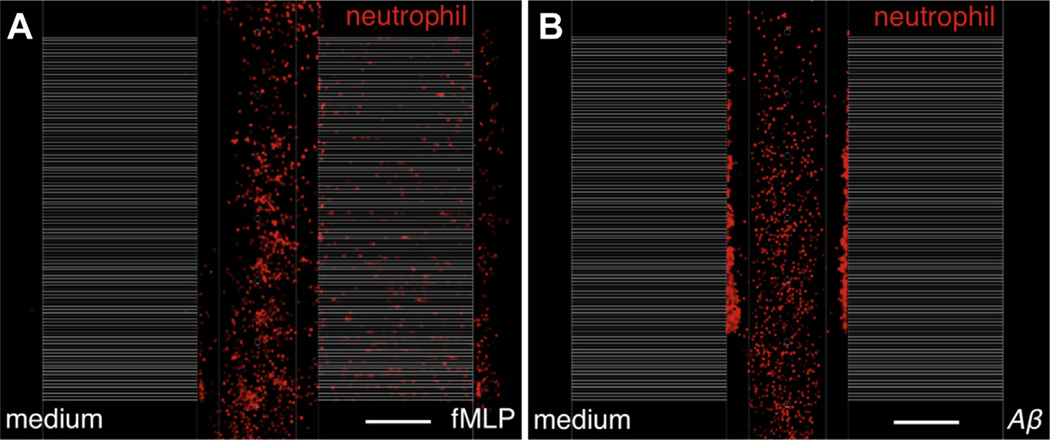
Our data also demonstrated that the blood-originated neutrophils did not make random movements but migrated towards certain locations within the brain. Twenty-four hours after the marker injections, the neutrophils rapidly migrated to and locally accumulated in specific areas of the brain. The neutrophils (green), which initially showed random migration outside of the blood vessels (red) following the marker injections, were suddenly and massively recruited to a specific spot (white circle) (Fig. 2A, Video 3). The neutrophils that were traced in Video 3 indicated the recruitment of neutrophils to a certain spot in the 5XFAD mice brains, and they showed chemotactic behavior (Fig. 2B and C). Such a clustering of neutrophils has never been observed in WT animals. This finding indicated that chronic AD pathology can recruit neutrophils into the brain parenchyma. To examine the destination of neutrophils that accumulated in certain regions, methoxy-XO4, which detects amyloid plaques in the brain (Klunk et al., 2002), was systemically injected into 5XFAD mice. Strikingly, we found that most of the neutrophils moved in a highly site-directed manner toward specific methoxy-XO4-stained amyloid plaques instead of taking the shortest path toward a nearby plaque (Fig. 2D and Videos 4–6). Thus, we found that blood neutrophils infiltrated into the brain and migrated to Aβ plaques in a mouse model of AD. Although the underlying mechanisms for this observation are currently unclear, Aβ itself might play a role in chemotaxis (Joslin et al., 1992; Tiffany et al., 2001). To examine this possibility, we evaluated any chemotactic activity of neutrophils by soluble Aβ monomers in a microfluidic platform. The platform was composed of gradients of Aβ along migration channels from the central compartment filled with cells in buffer solution, a right compartment including Aβ solution or chemokines, and a left compartment with the same buffer solution as a negative control (Fig. 3). The gradients of soluble Aβ (molecular weight of 4.4 kDa) along the channels were estimated to provide the original gradients more than 80% for an hour (Cho et al., 2013) and neutrophil migration was monitored for 2 hours. In the experiments, neutrophils did not migrate toward the gradients of soluble Aβ monomers at the concentrations corresponding to either physiological (10 pM) or pathological levels (10 nM). In a positive control, neutrophils migrated persistently in a gradient of N-formyl-methyl-leucyl-phenylalanine at 100 nM at the speed of 14.0 × 0.6 µm·minute−1, which was consistent during the experimental period (Student t test. * p < 0.0001 with respect to no-chemokine condition), ncell > 100 for each condition. Data represent mean ± standard error of the mean. These experiments suggest that soluble Aβ monomer may not be a direct chemoattractant for neutrophils. Because there is a close association between neutrophils and Aβ plaques (Fig. 2E), however, Aβ plaques must be an important factor in recruiting neutrophils from the blood mediated with other cellular interactions.
Fig. 2.
Neutrophils migrate toward β-amyloid plaques in the 5XFAD mouse brain. (A) The 2-photon in vivo time series imaging of the 5XFAD mouse brain reveals the interaction between transmigrated neutrophils, which seem to be activated, and some spots in the brain parenchyma. Neutrophils (green), which moved randomly outside the vessels (red), were suddenly and massively recruited to the specific spot (white circle). T1: 10 minutes, 0 seconds; T2: 28 minutes, 0 seconds; T3: 32 minutes, 30 seconds; T4: 39 minutes, 0 seconds; T5: 46 minutes, 0 seconds; and T6: 48 minutes, 30 seconds from Video 3. Scale bar, 20 µm. (B) The traced neutrophils from Video 3 were shown as green color at each time point. This tracking data also indicated recruitment of neutrophils in the 5XFAD mouse brain. The Volocity software program (PerkinElmer Inc, Waltham, MA, USA) was used. (C) Chemotactic effects are expressed by the color index. The blue area indicates less chemotactic, while more chemotactic movement is indicated by the red color. The center of the circle is represented by a red dot in Fig. 3B at each time point, and the chemotactic neutrophils were counted at intervals of 5 µm in the vector states. The Volocity software program was used. Neutrophils were recruited to β-amyloid plaques in the cortex of 5XFAD mice. To visualize the plaques, methoxy-X04 dye, which can cross the blood-brain barrier well, was intraperitoneally (i.p.) injected 24 hours before the imaging. (D) The in vivo time series imaging in the brain of a 5XFAD mouse. Gr-1-positive neutrophils are recruited near βamyloid plaques (white circle, red), and they stick. T1: 0 minutes, T2: 10 minutes, T3: 20 minutes, T4: 30 minutes, T5: 40 minutes, and T6: 50 minutes from Video 4; 1 unit, 13.87 µm. (E) The direct binding of neutrophils to β-amyloid plaques. A neutrophil (arrow) is directly bound to the small plaque (red) that is already covered with a few neutrophils. T1: 45 minutes, 30 seconds; T2: 46 minutes, 0 seconds; T3: 46 minutes, 30 seconds; T4: 47 minutes, 0 seconds; T5: 47 minutes, 30 seconds; T6: 48 minutes, 0 seconds; T7: 48 minutes, 30 seconds; and T8: 49 minutes, 0 seconds from Video 5; 1 unit, 4.3 µm.
Fig. 3.
Quantification of neutrophil chemotaxis by using a microfluidic platform. The platform is composed of a central compartment for cells, both side compartments for 2 different conditions, and migration channels connecting the compartments. The cell compartment is separated from migration channels by a central valve (default-open) and the migration channels are separated from chemokine compartment by 2 side valves (default-closed). Neutrophils were stained in a red fluorescence for easy tracking and white migration channels are overlaid for visualization after imaging. (A) The photo shows the strong chemotactic activity of neutrophils by fMLP 100 nM, which is taken at 28 minutes after starting the experiment. (B) On the other hand, Aβ at 10 nM does not induce any chemotatic activity on the neutrophils from the observation for 2 hours. Scale bar, 200 µm. Abbreviations: Aβ, beta-amyloid; fMLP, N-formyl-methyl-leucyl-phenylalanine.
4. Discussion
How neutrophils migrate and whether they are a protective or detrimental factor will be important questions to answer in the understanding of AD pathogenesis, especially in relation to brain inflammation. There have been several reports that infiltrated immune cells contribute to tissue repair by phagocytosing Aβ (Gate et al., 2010; Lee and Landreth, 2010; Rezai-Zadeh et al., 2011). Moreover, a recent study has shown that microglia is effective in invading neutrophil granulocytes (Neumann et al., 2008). Collectively, these studies raise the possibility that amyloid plaques might be engulfed by activated neutrophils and then eliminated by microglia. Alternatively, senile plaque-associated glial cells, such as microglia and astrocytes, might secrete chemokines to recruit neutrophils. It is obscure at the moment what factors trigger the recruitment of blood neutrophils toward Aβ plaques since Aβ itself cannot recruit neutrophils directly as shown in Fig. 3. However, controversial findings regarding the toxic properties of infiltrated immune cells in the central nervous system diseases have been reported (Halle et al., 2008; Heneka et al., 2013). Although the role of infiltrated immune cells in the brain is currently controversial, our results showed for the first time the dynamic extravasation and migration of neutrophils to brain amyloid plaques with live in vivo imaging. Our results suggested the exciting possibility that immune cells interact with senile plaques in the brain, thereby influencing the progress of AD. Understanding the interaction between neutrophils and senile plaques might provide new insights into the pathophysiology and potential treatments of AD.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (2012R1A2A1A01002881, MRC [2011–0030738]); the Korea National Institute of Health ROAD R&D Program Project (A092058), Korea Institute of Science and Technology Institutional Program (2E24242-13–135) for Inhee Mook-Jung, and the Cooperative Research Program for Agriculture Science & Technology Development (PJ009103) for Kyung Ho Kim.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging. 2014.01.003.
Footnotes
Disclosure statement
The authors declare that they have no conflicts of interest, financial or otherwise, that are related to the present work.
References
- Cho H, Hamza B, Wong EA, Irimia D. On-demand, competing gradient arrays for neutrophil chemotaxis. LOC. Accepted. 2013 doi: 10.1039/c3lc50959a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Cribbs DH, Rosenthal M, Bernard G. Phagocytosis of amyloid-beta and inflammation: two faces of innate immunity in Alzheimer’s disease. J. Alzheimer’s Dis. 2007;11:457–463. doi: 10.3233/jad-2007-11406. [DOI] [PubMed] [Google Scholar]
- Fortin CF, McDonald PP, Lesur O, Fulop T., Jr Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11:873–882. doi: 10.1089/rej.2008.0750. http://dx.doi.org/10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- Friese MA, Steinle A, Weller M. The innate immune response in the central nervous system and its role in glioma immune surveillance. Onkologie. 2004;27:487–491. doi: 10.1159/000080371. http://dx.doi.org/10.1159/000080371. [DOI] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer’s disease: the blood-borne identity. J. Neural Transm. 2010;117:961–970. doi: 10.1007/s00702-010-0422-7. http://dx.doi.org/10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. http://dx.doi.org/10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. http://dx.doi.org/10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, El Khoury J. The neuroimmune system in Alzheimer’s disease: the glass is half full. J. Alzheimer’s Dis. 2013;33:S295–S302. doi: 10.3233/JAD-2012-129027. http://dx.doi.org/10.3233/JAD-2012-129027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslin G, Griffin GL, August AM, Adams S, Fallon RJ, Senior RM, Perlmutter DH. The serpin-enzyme complex (SEC) receptor mediates the neutrophil chemotactic effect of alpha-1 antitrypsin-elastase complexes and amyloid-beta peptide. J. Clin. Invest. 1992;90:1150–1154. doi: 10.1172/JCI115934. http://dx.doi.org/10.1172/JCI115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT. Imaging abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J. Neuropathol. Exp. Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat. Rev. Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. http://dx.doi.org/10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. http://dx.doi.org/10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. http://dx.doi.org/10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostany R, Portera-Cailliau C. A craniotomy surgery procedure for chronic brain imaging. J. Vis. Exp. 2008 doi: 10.3791/680. http://dx.doi.org/10.3791/680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Sauerzweig S, Ronicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J. Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Gate D, Gowing G, Town T. How to get from here to there: macrophage recruitment in Alzheimer’s disease. Curr. Alzheimer Res. 2011;8:156–163. doi: 10.2174/156720511795256017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany HL, Lavigne MC, Cui YH, Wang JM, Leto TL, Gao JL, Murphy PM. Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J. Biol. Chem. 2001;276:23645–23652. doi: 10.1074/jbc.M101031200. http://dx.doi.org/10.1074/jbc.M101031200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.