Abstract
Objective
Rheumatoid arthritis is thought to be a T cell mediated disease, based on its strong association with HLA class II alleles, clinical responsiveness to T cell directed therapies and the presence of CD4 T cells in rheumatoid joints. The presence of ACPA in RA serum and the association of these antibodies with HLA-DR4 alleles implicates citrullinated specific autoreactive T cells in the development and progression of RA. The goal of this study was to determine the character and specificity of auto-reactive T cell responses in RA.
Methods
We developed a panel of HLA-DRB1*04:01 tetramers, selecting citrullinated peptides from synovial antigens and verifying their immunogenicity in DRB1*04:01 transgenic mice. Seven tetramers were used to examine the ex vivo frequency and surface phenotype of cit-specific T cells in RA and healthy subjects with DRB1*04:01 haplotypes using a magnetic enrichment procedure.
Results
Cit-specific T cells were detectable in peripheral blood samples from both healthy subjects and RA patients. In comparison to healthy subjects, RA patients had significantly higher frequencies of cit-specific T cells and a greater proportion of these cells displayed a Th1 memory phenotype. Among RA subjects the frequency of cit-specific T cells was highest within the first 5 years after diagnosis of RA and was decreased in patients taking biologic therapies irrespective of disease duration.
Conclusion
These findings link the presence of ACPA in RA with Th1 cells specific to citrullinated epitopes and provide tools for disease-specific immunomonitoring of autoreactive T cells.
Rheumatoid arthritis (RA) is a chronic disease characterized by inflammation and destruction of joints and the surrounding tissue (1;2). This destruction is mediated through autoimmune processes as evidenced by the appearance of specific serologic markers, including rheumatoid factor and anti-citrullinated protein antibodies (ACPA) (3–6). ACPA develop years before disease onset, but are remarkably disease specific, suggesting an important etiologic role for immune recognition of self-proteins modified by citrullination (7;8). RA susceptibility in general and the appearance of ACPA in particular are linked to a limited subset of HLA-DR haplotypes, including DRB1*04:01 (DR0401), implying that recognition of self-peptides by CD4+ T cells is important in driving antibody responses in RA and also suggesting that some T cell epitopes could be citrullinated (1;9). CD4+ T cells that respond to citrullinated peptides (cit-specific T cells) have been implicated in the progression of arthritis in several murine models - both indirectly through T cell help and by direct infiltration into the joint (10;11). T cells from RA patients have been shown to expand and secrete cytokines in response to stimulation in vitro with citrullinated peptides (12–14). However these studies did not characterize cit-specific T cells directly ex vivo, leaving unanswered questions about the magnitude and phenotype of T cell responses to citrullinated antigens in human subjects.
Previous studies of T cells in autoimmunity have predominantly relied on assays that include in vitro manipulation, which therefore have a limited ability to examine T cell frequency and potentially skew T cell phenotype as a result of that manipulation. Direct ex vivo analysis, while preferable, has been a technical challenge due to the inherently low frequencies of epitope specific CD4+ T cells (15). In this paper we sought to visualize and characterize CD4+ T cell responses to citrullinated antigens directly ex vivo utilizing a panel of DR0401 tetramers. We developed this tetramer panel by first identifying a set of citrullinated peptides derived from synovial antigens with the capacity to bind HLA-DR0401, and then confirmed in vivo immune responsiveness using HLA-DR0401 transgenic mice. The corresponding DR0401 tetramers were able to detect cit-specific T cells in the peripheral blood of RA patients and healthy controls. However, in patients as compared to controls, the frequency of cit-specific T cells was increased, as was the percentage of these cells displaying a Th1 memory phenotype. Frequency of cit-specific T cells varied significantly with disease duration and with treatment using biologic therapies. Thus, we have directly identified cit-specific T cells from RA patients that are likely active in the pathogenesis of RA.
Materials and Methods
Human Subjects
RA and control subjects were recruited through the Benaroya Research Institute (BRI) immune-mediated disease registry. All RA patients met the 1987 American College of Rheumatology criteria for RA (16), ranged in age from 25–81 (mean, 52±13.5) and 18 of 19 were ACPA positive based on clinical CCP testing. Control subjects were selected based on a lack of autoimmune disease, or family history of RA, they ranged in age from 25–82 (mean, 44±17.5) and were ACPA-. All subjects studied had at least one copy of the HLA-DR0401 allele. PBMC were prepared from heparinized blood by centrifugation over Ficoll-Hypaque gradients. Frozen PBMCs were cryopreserved in liquid nitrogen in 10% DMSO and 90% heat-inactivated FBS. The research protocols were approved by the IRB at Benaroya Research Institute.
Peptide Selection and Binding Assays
Peptides were synthesized and purified by the manufacturer (Sigma). Putative citrullinated epitopes were predicted based on published binding data for anchor residues at pockets 1, 4, 6, 7, and 9 and our data about accommodation of citrulline versus arginine (17). As summarized in Supplemental Table 1, DR0401 motifs were defined by calculating the product of the coefficients for each possible combination of residues that included a citrulline and scores of 0.1 or higher were selected for synthesis.
For peptide binding assays, increasing concentrations of each non-biotinylated test peptide were incubated in competition with 0.01 µM biotinylated HA306–318 peptide in wells coated with HLA-DR0401 protein as previously described (18). Europium-conjugated streptavidin (PerkinElmer) was used to label biotinylated peptide bound to the HLA-DR protein and was quantified using a Victor2 multilabel time resolved-fluorometer (PerkinElmer). Binding curves were fitted by non-linear regression with a sigmoidal dose response curve model using Prism software (version5.0, GraphPad Software Inc.). Peptides selected for further study based on positive binding results were re-synthesized at a higher purity by another manufacturer (Genscript).
Production of HLA-DR0401 monomers and Tetramers
Recombinant DR0401 protein was produced as previously described (19). Soluble DR0401 was purified from insect cell culture supernatants and biotinylated at a sequence-specific site using biotin ligase (Avidity) prior to dialysis into phosphate storage buffer. The biotinylated monomer was loaded with 0.2 mg/ml of peptide by incubating at 37°C for 72 hours in the presence of 2.5 mg/ml n-octyl-β-D-glucopyranoside and 1 mM Pefabloc SC (Sigma-Aldrich). Peptide loaded monomers were conjugated into tetramers using R-PE streptavidin (Invitrogen) at a molar ratio of 8 to 1.
Murine Assays
Class II deficient C57Bl/6 (I-Abo/o) mice transgenically expressing a chimeric class II containing the α1β1 domains of human DRA1*0101-B1*0401 on a mouse IE-d backbone (DR0401-IE mice) were obtained from Taconic and were housed under specific pathogen-free conditions. All animal work was approved by the BRI Animal Care and Use Committee (ACUC) and animals were housed in the BRI AAALAC-accredited animal facility.
Mice were immunized with 100 µg of peptide in 100 µl of 50% CFA/PBS, subcutaneously at the base of the tail. On day 14 spleens were harvested, brought to a single cell suspension and any remaining red blood cells were lysed with hemolytic buffer (0.15 M NH4Cl, 0.1 mM Na2EDTA & 10 mM KHCO3). For proliferation assays cells were plated at 0.5 × 106 cells/well in 96-well round bottom plates in complete DMEM (DMEM,10% FBS (ATLAS), 100 µg/ml Penicillin, 100 U/ml Streptomycin, 50 µM β-mercaptoethanol, 2mM glutamine and 1mM sodium pyruvate) either alone, with anti-CD3/antiCD28 (3.0/0.5 mg/ml) (BioLegend), or with peptide for 96 hrs. At 72 hrs 3H-thymidine (Moravek Biochemicals) was added at 1 µCi/well. Cells were harvested to glass fiber membranes and radioactivity was quantified with a MicroBeta TriLux 1450 LSC & luminescence counter (PerkinElmer).
In vitro detection of citrulline specific T cell by HLA class-II tetramers
Previously frozen PBMCs from RA subjects were cultured at 5 × 106 total cells/well within a 24 well plate in RPMI-1640 + 10% pooled human serum with 10 µg/ml of peptide. IL-2 (Novartis) was added at 325 IU/ml on day 6. After 14 days cells were stained for expression of CD25 FITC (BD), CD4 PerCP (BioLegend), Annexin V APC (BD), CD14 APC (BioLegend), CD19 APC (BioLegend), and tetramer before being run on a FACSCalibur. The data was analyzed by FlowJo software version 9.6.2 (Tree star).
Ex vivo detection of flu and citrulline specific T cell by HLA class-II tetramers
Freshly isolated PBMC were aliquotted for ex vivo analysis, with random assignment to HA control tests and citrulline peptide tests, where the total number of ex vivo tetramers tested was dependent on the number of PBMC available. All samples for ex vivo analysis were labeled, enriched (20) and subjected to phenotypic analysis as previously described (21). Briefly, 40 million PBMC in 200 µl of T cell culture medium were treated with dasatinib for 10 minutes at 37°C and then stained with 20 µg/ml PE-labeled tetramers at room temperature for 90 min. Cells were washed and incubated with anti-PE magnetic beads (Miltenyi Biotec) at 4°C for 20 minutes, washed again, and a 1/100th fraction saved for analysis. The other fraction was passed through a magnetic column (Miltenyi). Bound, PE-labeled cells were flushed and collected. Both enriched and non-enriched fractions were labeled with CD4 V500 (BD), CXCR3 Pacific Blue (BioLegend), CD45RO eFlour 650 NC (eBioscience), CD28 PE-Cy7 (BioLegend), CCR7 Alexa-700 (BD), CD25 APC (BD), CCR6 PerCP-Cy5.5 (BD), and CD14 (BioLegend), CD19 (BioLegend), & Annexin V (BD) conjugated in FITC. Samples were then run on a BD LSRII flow cytometer, and data was analyzed using FlowJo software version 9.6.2.
Ex vivo cloning of flu and citrulline specific T cells by HLA class-II tetramers
T cell clones specific for citrullinated epitopes or influenza HA306–318 were generated by staining PBMC samples directly ex vivo as described above, except no dasatinib was added. Tetramer-positive CD4+ cells were then sorted on a FACS Aria II at single-cell purity. Clones were expanded in 96-well plates in the presence of 1.0 × 105 irradiated PBMC and 2 µg/ml phytohemagglutinin (Remel Inc. Lenexa, KS), then screened by re-staining with tetramers. Antigen specific T cell proliferation was measured by stimulating 5.0 ×104 T cells in wells coated with peptide loaded DR0401 monomer in the presence of CD3/CD49d for 48 hours, pulsing with 3H thymidine for an additional 18 hours and measuring thymidine uptake by liquid scintillation counting (22).
Statistics
All statistical tests were performed using PRISM version 6 software (GraphPad Software Inc., La Jolla, CA, USA). Tests that were used (as appropriate) included unpaired T tests, unpaired T tests with Welch’s correction, Mann-Whitney tests, or ANOVA with Sidak's multiple comparisons test. P values < 0.05 were considered significant.
Results
CD4+ T cells recognize citrullinated epitopes derived from multiple synovial antigens
Previous studies have identified several peptides from synovial antigens that are preferentially bound and recognized in their citrullinated form (12–14;17;23). We measured the binding of substituted analogs of a known high affinity epitope with citrulline or arginine inserted within each of the pockets of DR0401 (fig. S1) and found citrulline to be preferred over arginine in pockets 4, 7, and 9.Using this information and published data, we used a scanning algorithm to identify arginine containing peptides from joint-associated antigens (vimentin, cartilage intermediate-layer protein (CILP), filaggrin, α-enolase, and fibrinogen α, β, and γ) with motifs that would be predicted to bind DR0401 in either the native form or after conversion of arginine to citrulline (table S1). Many of these peptides contained arginine in a position that would be predicted to enhance binding when converted to citrulline. Based on these predictions we synthesized 65 citrullinated peptides and the corresponding unmodified sequences and tested their ability to bind HLA-DR0401. In these studies 14 citrullinated peptides bound and among these 7 of the corresponding unmodified sequences also bound, as summarized in Table 1.
Table 1.
Amino acid sequences and binding comparison of citrulline and arginine peptides chosen for further analysis.
| Peptide Name | Peptide Source | Sequence*^ | IC50 |
|---|---|---|---|
| Vim 1 | Vimentin 59–78# | GVYATRSSAVRLRSSVPGVR | >50 |
| Cit-Vim 1# | GVYATXSSAVXLXSSVPGVR | 1.3 | |
| Vim 2 | Vimentin 418–431 | SSLNLRETNLDSL | >50 |
| Cit-Vim 2 | SSLNLXETNLDSL | 14 | |
| Fib 1 | Fib B 69–81 | GYRARPAKAAAT | >50 |
| Cit Fib 1 | GYRAXPAXAAAT | 8.2 | |
| CILP 2 | CILP 297–311 | ATIKAEFVRAETPYM | >50 |
| Cit-CILP 2 | ATIKAEFVXAETPYM | 0.7 | |
| CILP 3 | CILP 982–996 | GKLYGIRDVRSTRDR | 17 |
| Cit-CILP 3 | GKLYGIXDVXSTRDR | 0.3 | |
| α-enolase | α enolase 11–25 | IFDSRGNPTVEVDLF | >50 |
| 3 Cit-α-enolase 3 | IFDSXGNPTVEVDLF | 2.2 | |
| α-enolase 4 | α enolase 326–340 | KRIAKAVNEKSCNCL | 1.7 |
| Cit-α-enolase 4 | KXIAKAVNEKSCNCL | 1.9 | |
| HA | Flu hemaglutanin | PKYVKQNTLKLAT | 0.03 |
Predicted binding register underlined with anchor residues in boldface.
X = citrulline
Previously published as a citrullinated DR401 epitope
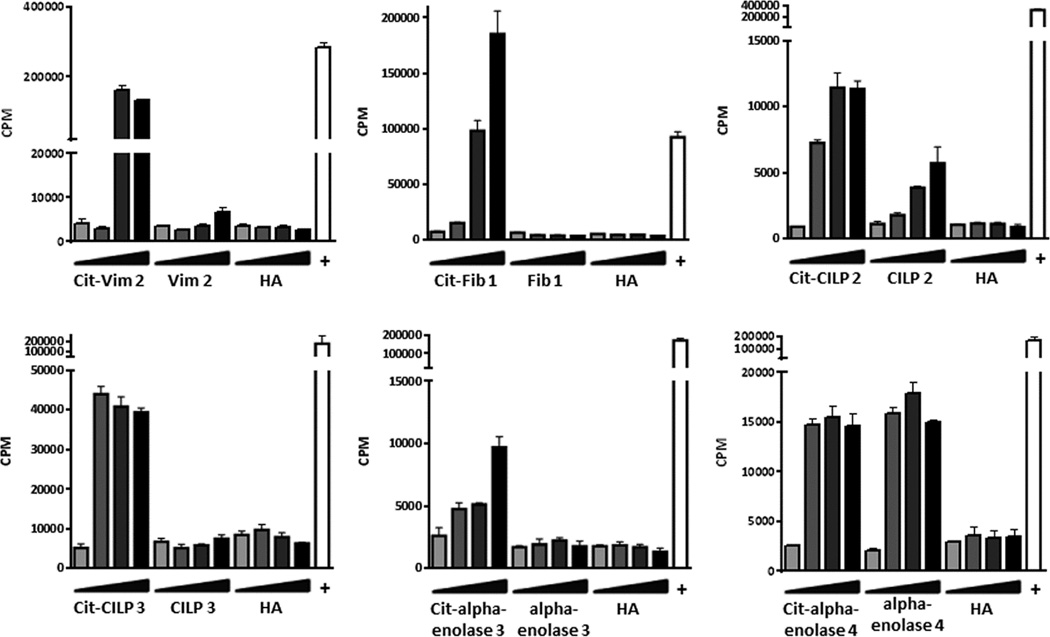
To assess the immunogenicity of the joint derived peptides that bound to DR0401(per Table 1), groups of DR0401-IE transgenic mice were immunized with one of the cit-peptides of interest and after 14 days recall responses were evaluated by assessing in vitro recall proliferation in response to the citrullinated peptide, unmodified peptide, or a control peptide. This approach verified that six of the newly identified citrullinated peptides were able to induce a recall response in DR0401-IE transgenic mice: Cit-Vim 2, Cit-Fib 1, Cit-CILP 2, Cit-CILP 3, Cit-a-enolase 3, and Cit-a-enolase 4 (fig.1). For three of these peptides, Cit-Vim 2, Cit-CILP 2, and Cit-a-enolase 4, the arginine version of the peptide also elicited a response.
Fig. 1.
Novel citrullinated peptides are antigenic in DR0401-IE mice. Representative 3H-thymidine incorporation from DR0401-IE mice immunized with Cit-Vim 2 (n=6), Cit-Fib 1 (n=6), Cit-CILP 2 (n=3), Cit-CILP 3 (n=3), Cit-alpha-enolase 3 (n=3), and Cit-alpha-enolase 4 (n=7). In vitro recall responses were measured with challenge from the immunizing citrullinated peptide, its arginine version, and HA using a dose titration of 0, 0.5, 5, and 50 µg/ml of peptide. Anti-CD3/anti-CD28 was used as a positive control in all assays (+).
We next utilized an in vitro assay to ascertain whether human T cells also recognized these citrullinated peptides. In these experiments, PBMC from RA subjects with DR0401 haplotypes were stimulated with citrullinated peptide or an influenza control peptide (HA306–318), cultured for two weeks, and stained with the corresponding DR0401 tetramers. In these cultures all six citrullinated peptides elicited a detectable population of tetramer positive CD4+ T cells that was absent in the HA306–318 stimulated control well (fig. S2). This panel of six peptides and the corresponding tetramers were selected to study T cell responses in RA subjects, along with our previously published vimentin epitope (14).
Direct detection of citrulline reactive-CD4 T cells in RA patients
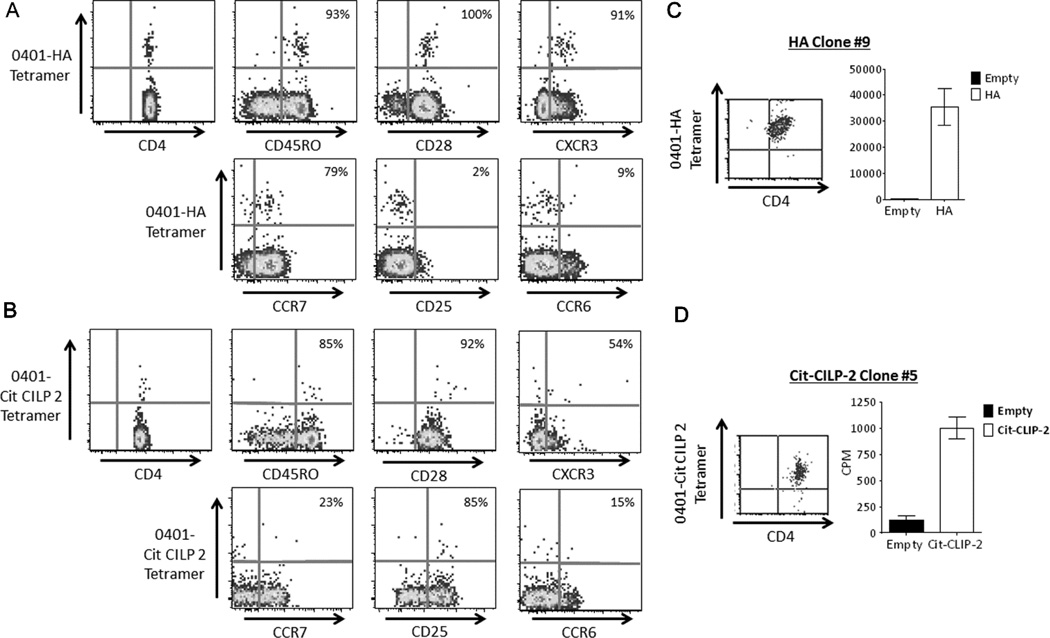
In our previous work, spiking and dilution experiments demonstrated that the limit of detection for our ex vivo tetramer staining assay is approximately 1 cell per 2 million CD4+ T cells (24). Therefore, in our study of T cells specific to our identified citrullinated epitopes responses above this cutoff were considered to be a detectable T cell population. Representative results of our ex vivo detection and phenotyping of influenza and citrulline specific T cells isolated from the periphery of RA subjects are seen in Figure 2 (A and B). By co-staining with antibodies against selected cell surface markers we were able to determine the phenotype as well as frequency of CD4 T cells specific for each citrullinated peptide and the influenza control. For this purpose we included CD45RO and CCR7 as memory T cell markers, CD25 as marker of Treg and activation, CXCR3 and CCR6 to distinguish Th1 and Th17 cells respectively, and CD28 to determine if the antigen specific CD4 T cells were CD28 negative, a population described to be increased in RA (25).
Fig. 2.
Flu and citrulline-specific CD4 T cells can reliably be detected ex vivo from the peripheral blood of RA patients. A) A representative example of ex vivo tetramer analysis for flu specific T cells from PBMC of an RA subject with a DR0401 haplotype after staining with DR0401-HA306–318 tetramer along with CD4, CXCR3, CD45RO, CD28, CCR7, CD25, and CCR6 antibodies. B) A representative example of ex vivo tetramer analysis for citrulline specific T cells from PBMC of an RA subject with a DR0401 haplotype after staining with DR0401-Cit-CILP 2 tetramer, CD4, CXCR3, CD45RO, CD28, CCR7, CD25, and CCR6 antibodies. C) DR0401- HA306–318 tetramer staining (left panel) and peptide specific proliferation (right panel) as seen by monomer stimulation (black bars=DR0401-empty monomer, white bars=DR0401-HA306–318 monomer) of a direct ex vivo sorted HA-T cell clone isolated from an RA patient. D) DR0401-Cit-CILP 2 tetramer staining (left panel) and peptide specific proliferation (right panel) as seen by monomer stimulation (black bars=DR0401-empty monomer, white bars=DR0401- Cit-CILP 2 monomer) of a direct ex vivo sorted Cit-CILP 2-T cell clone isolated from an RA patient.
Ex vivo tetramer staining is highly sensitive and specific (24). However to further verify that our ex vivo detection of antigen specific cells is reliable in RA subjects, we performed ex vivo tetramer staining and then directly isolated T cell clones by single cell sorting. As shown in figures 2C and 2D, we were able to clone T cells directly ex vivo from peripheral blood using both influenza and citrulline peptide specific tetramers. The expanded, clones remained tetramer positive and proliferated in an antigen specific manner toward the corresponding HLA-peptide combination.
In total we examined peripheral blood samples from 19 RA patients and 15 healthy control subjects using ex vivo tetramer analysis. Our allowable sample volume did not permit examination of every epitope in every subject, but each sample was divided and labeled with multiple tetramers to provide balanced data for all epitopes. For each of the seven citrullinated epitopes there was a detectable T cell population in at least 36% of the subjects tested – a minimum of 4 positive individuals for each epitope (table 2). There was no single “dominant” epitope that was positive in every RA patient tested, but every subject had a detectable population of T cells for at least one citrullinated peptide. An average of 3–4 cit-tetramers were tested per subject with a positive staining result for 2 tetramers on average. Although there was a higher percentage of responses to specific peptides among healthy controls in some instances and RA patients in the case of other epitopes, none of these differences were statistically significant. We also examined influenza specific T cells as a positive control. Using DR0401/HA306–318 tetramers we were able to detect a distinct population of influenza specific CD4+ T cells in peripheral blood of every RA patient tested (table 2). This is an important positive control as it allows us to determine if there are global alterations in CD4 T cells frequency and phenotype due to RA itself or the immunomodulatory therapy that the patients receive.
Table 2.
Flu and citrullinated peptides are detectible directly ex vivo from the peripheral blood of healthy controls and RA patients
| Peptide Tested | Controls (15)*^ | RA Patients (19)*^ |
|---|---|---|
| HA | 100% (10/10) | 100% (17/17) |
| Cit-Vim 1 | 67% (4/6) | 36% (4/11) |
| Cit-Vim 2 | 60% (3/5) | 73% (8/11) |
| Cit-Fib 1 | 67% (4/6) | 88% (7/8) |
| Cit-CILP 2 | 100% (4/4) | 50% (5/10) |
| Cit-CILP 3 | 50% (4/8) | 44% (4/9) |
| Cit-α-enolase 3 | 38% (3/8) | 57% (4/7) |
| Cit-α-enolase 4 | 50% (4/8) | 83% (10/12) |
15 healthy controls and 19 RA patients were tested via ex vivo tetramer analysis
Percentages were calculated based on the number of individuals with a detectable ex vivo response out of the total number of individuals tested (indicated in parenthesis)
Citrulline reactive Th1 cells are more frequent in RA patients
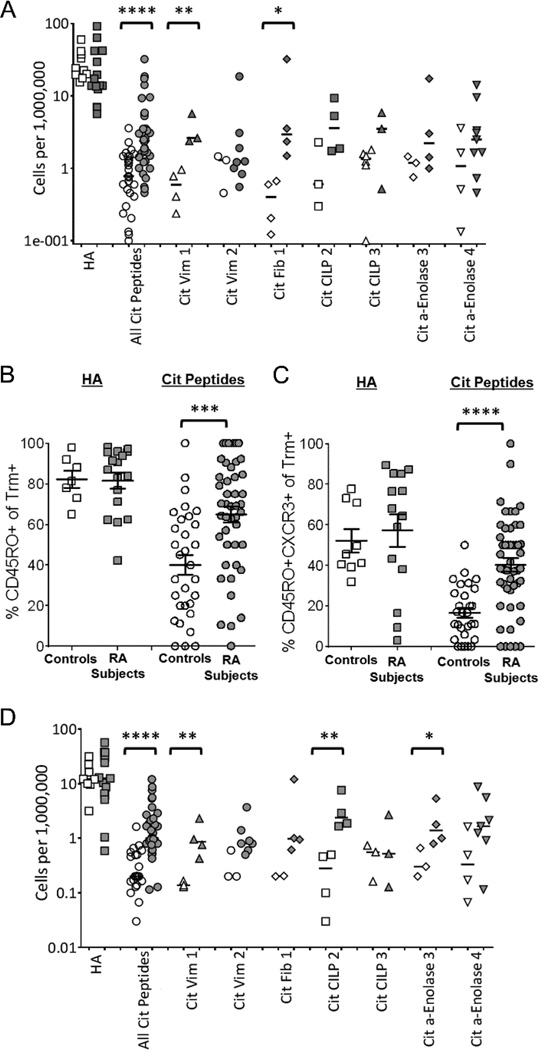
Previous studies have observed that while autoreactive T cells are present in the peripheral blood of healthy individuals, only individuals with autoimmunity have expanded populations of self-reactive memory T cells (26;27). Detectable populations of citrulline reactive T cells were present both in RA patients and healthy subjects, ranging from the limit of detection (1 epitope specific cell per two million CD4+ T cells) to as high as 40 epitope specific cells per one million CD4+ T cells. The overall frequency of citrulline reactive CD4+ T cells was significantly higher in RA patients than in controls (**p=0.0069; 5.4±8.6 versus 1.9±1.4 cit-specific cells/million) (fig. S3). While there was a significant overall increase in T cells reactive to citrullinated peptides in RA subjects as compared to controls, there was no dominant citrullinated epitope responsible for this increase. This finding is compatible with the varying responses to different citrullinated peptides in different individuals. In contrast to citrulline reactive T cells, there was no difference in the frequency of HA specific T cells between RA and healthy subjects (33.6±25.8 and 34.8±17.5 flu-specific cells/million respectively).
Given the observed differences in the overall frequency of citrulline reactive T cells in RA patients and controls, we next compared the frequency of CD45RO+ citrulline reactive T cells (fig. 3A). RA subjects and Healthy controls yielded similar frequencies of influenza specific memory CD4+ T cells (26.2±24.3 versus 29.2±14.0 RO+flu-specific cells/million). However, highly significant differences were seen in their respective frequencies of citrulline reactive memory CD4+ T cells (****p<0.0001; 5.1±7.4 versus 1.0±0.8 RO+ cit-specific cells/million). This difference between RA subjects and controls was also significant for two single epitopes: Cit Vim 1 (**p=0.0085) and Cit Fib 1 (*p=0.0160) (fig. 3B). The increase in the frequency of citrulline reactive memory T cells in RA patients compared to controls correlates to a significantly higher proportion of cit-tetramer+ T cells being cit-tetramer+CD45RO+ (mean 65% vs. 40%; ***p=0.0001) (fig. 3C). This difference was not simply reflecting global differences in the immune profile of RA patients, as similar proportions of HA specific T cells had a memory phenotype in both groups (RA = 81.7±16.5%; controls = 82.2±11.4%).
Fig. 3.
RA patients have an increased number of T cells recognizing citrullinated antigens, and these T cell are skewed towards a TH1 memory response. (A) T cell frequencies were determined directly ex vivo for DR0401-tetramer+CD45RO+ antigen specific T cells. All frequencies are expressed as number of antigen specific cells/million T cells. Comparisons are made between healthy controls (white symbols) and RA patients (gray symbols) for T cells specific for flu (HA306–318), all citrullinated antigens tested, and individual citrullinated peptides tested. Antigen specific T cells were further characterized for their memory phenotype by looking at the percentage of CD45RO+ (B) and CD45RO+CXCR3+ (C) among T cells that were tetramer+. Phenotypic comparisons were made between healthy controls and RA subjects for their flu specific T cells (controls=white boxes, RA=gray boxes) and their citrulline specific T cells (controls = white circles, RA=gray circles). (D) T cell frequencies were determined directly ex vivo for DR0401-tetramer+CD45RO+CXCR3+ antigen specific T cells. Symbols are identical to those used in panel A. Statistical significance was determined by an unpaired T test after normalization for logarithmic distribution (A&D) or by Mann-Whitney test (B&C). *=p<0.05, **=p<0.01, ***=p<0.001, and ****p=<0.0001.
No differences were seen between RA patients and controls in the percentage of cit-tetramer+ T cells positive for CCR7, CCR6, CD28 or CD25 (fig. S4) and we found few citrulline specific T cells that were CD45RO+CCR6+ (not shown). However, a significantly higher percentage of citrulline reactive T cells were CD45RO+CXCR3+ in RA subjects as compared to controls (****p<0.0001; Figure 3C), suggesting a skewing toward a Th1 response of these autoantigen-specific T cells. This was further reflected in a significant increase in the frequency of citrulline reactive CD45RO+CXCR3+ T cells in RA subjects as compared to controls (p****p<0.0001; Figure 3D) and further demonstrated significant differences for Cit Vim 1 (**p=0.007), Cit CILP2 (**p=0.009) and Cit a-Enolase 3 (*p=0.02). There was no corresponding increase in CD45RO+CXCR3+ influenza specific T cells, indicating these differences are unique to the citrulline specific T cells of RA patients. These findings indicate that although citrulline reactive T cells are present both in healthy subjects and RA patients, a higher proportion of the citrulline specific T cells in RA subjects display signs of previous antigen experience and a skewing toward a proinflammatory Th1 phenotype.
Disease duration and treatment influence the frequency of citrulline reactive T cells
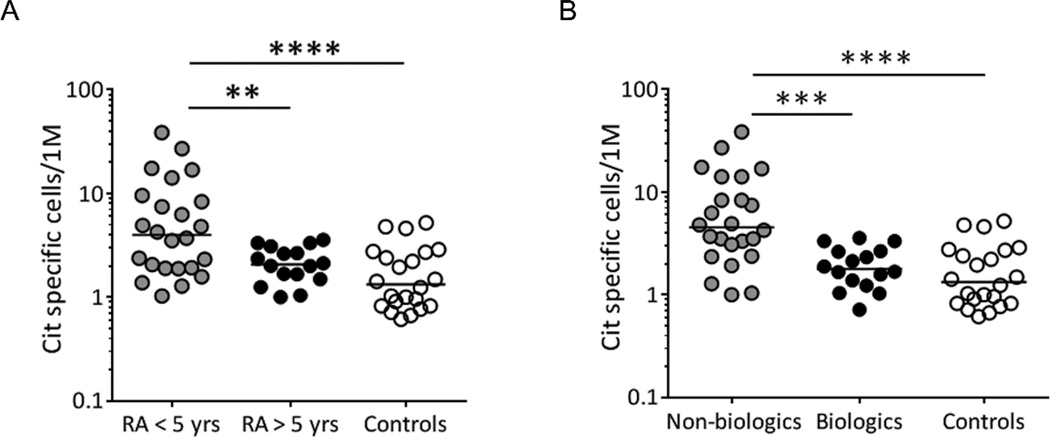
Given that the frequency and phenotype of citrulline reactive T cells differs between patients and healthy controls, we next sought to determine whether the frequency or phenotype of these cells is affected by disease duration. Stratifying our patients based on disease duration, we observed that the ex vivo frequency of citrulline reactive T cells was significantly higher in RA subjects within 5 years of diagnosis than in those with long standing disease (*p<0.01; RA <5 yrs=7.7 cit-specific cells/million±1.9; RA>5 yrs=2.2 cit-specific cells/ million±0.2) or controls (***p<0.0001; 1.9 cit specific cells/million±0.3) (Figure 4A). This finding was also significant when the analysis was performed on Th1 cells only (data not shown). This observed difference in the frequency of citrulline reactive T cells was independent of age, and no differences were seen in the frequency of influenza specific cells for these two groups of patients (fig. S5). In patients with long standing disease the frequencies of cit-specific T cells were comparable to those seen in controls, suggesting that T cell responses toward citrullinated epitopes may wane after prolonged disease.
Fig. 4.
Disease duration and treatment affect the frequency of cit-specific T cells found in RA patients. A) A comparison of ex vivo frequencies for cit-specific CD4+ T cells between 9 RA patients with disease <5 years (gray circles), 7 RA patients with disease >5 years (black circles), and 11 healthy controls (white circles) shows that RA patients with <5 year disease duration have significantly higher total frequencies of cit-specific CD4+T cells than RA patients with >5 year disease duration and healthy controls. B) A comparison of ex vivo frequencies of cit-specific CD4+ T cells in 8 RA patients on non-biologic therapies (gray circles), 8 RA patients on biologic therapies (black circles), and 11 healthy controls (white circles) shows that RA patients on non-biologic therapies have significantly more cit-specific CD4+T cells than RA patients on biologic therapies and healthy controls. Frequencies are shown as tetramer+ antigen specific T cells/million T cells. Statistical significance was determined by ANOVA using the Sidak multiple comparisons post-test after normalization for logarithmic distribution. **=p<0.01, ***=p<0.001, and ****p=<0.0001.
Because the majority of our patients had well-controlled disease, it was not possible to determine whether changes in the frequency or phenotype of cit-specific T cells correlate with disease activity or the effectiveness of therapy. However, our patient population could be stratified into groups whose treatment included biologics (including the anti-TNF therapies, abatacept and rituximab) or whose treatment only included non-biologics (including methotrexate, hydroxycholorquine, and leflunamide). Comparing these groups, we observed that RA patients on biologics had lower frequencies of cit-specific T cells compared to RA patients taking other pharmacological treatments (****p<0.0001; RA wo/biologics = 8.3±9.2 cit-specific cells/million; RA w/biologics = 2.0±0.8 cit-specific cells/million) (Figure 4B). This finding was also significant when the analysis was performed on Th1 cells only (fig. S6). The decrease in cit-specific T cells in RA patients taking biologics was seen irrespective of disease duration (fig. S7), indicating that the relationships between frequency and time from onset and therapy are independent effects.
Discussion
The strong associations between RA and susceptible HLA-DR haplotypes, suggest a critical role for CD4+ T cell responses in initiating or propagating joint inflammation in RA. Previous studies have demonstrated enhanced binding and presentation of citrullinated peptides by ‘shared epitope’ HLA-DR and enhanced in vitro T cell responses directed against individual citrullinated epitopes (12;14) (17;28). However, in order to understand the contribution of T cells to disease it is vital to unambiguously interrogate autoreactive T cells that recognize relevant antigens. Here we utilize a recently developed methodology to detect potentially pathogenic autoreactive T cells directly ex vivo and to examine multiple cell surface markers on a per cell basis without the hazard of bias due to in vitro manipulation. Autoreactive T cells are rare in peripheral blood, and their specificities are multiple and appear to differ between patients. By using a panel of peptides, we have overcome the limitations of studies using only one antigen or peptide. Using this approach we have observed that citrulline specific T cells are present in RA patients and healthy controls. This observation is consistent with studies of other self-antigens (29) and suggests that possessing a susceptible HLA is sufficient to generate a self-reactive CD4+ T cell repertoire. However, unlike controls, cit-specific T cells in patients are characterized by markers of Th1 memory. Based on previous work in animal models of arthritis, it might have been expected that cit-specific T cells in RA patients would have a Th17 phenotype (10;30). Indeed, several groups have described a global increase in the number of Th17 CD4 T cells in the peripheral blood and synovial fluid (31;32) of RA subjects which is modulated in response to therapy (33–35). Yet the observed predominance of cit-specific Th1 T cells in our patients with RA is consistent with the findings of other groups who have examined T cells from the peripheral blood and joints of RA patients (36;37) and several reports of Th1 predominant cit-specific responses of RA subjects from in vitro studies(12;14). Our findings confirm that a loss of T as well as B cell tolerance to citrullinated self-antigens is present in RA and further reinforce the notion that Th1 cells may play an important role in RA.
Our study does have limitations. Our tetramer panel is composed of peptides derived from 5 joint associated antigens. It is likely that additional epitopes are recognized by pathogenic T cells in RA, the attributes of which may differ from those studied here. Further we have examined subjects with established RA, the majority of whom are on either a DMARD and/or a biologic therapy the majority of whom had well controlled disease. Although we were able to demonstrate differences in the frequency of cit-specific CD4 T cells in RA patients based on time of diagnosis and the use of biologic therapy, we were unable to determine whether the profile or frequency of cit-specific T cells is directly altered by disease activity. In addition our selection of established RA patients for our study did not allow us to examine the CD4 T cell profile at the time of, or prior to, the development of clinical disease. This is an important question, as future studies may demonstrate that the diversity of the antigens recognized, the frequency of autoreactive T cells in the periphery, or the cytokine profile of those cells may differ prior to or at onset of disease from those of subjects with established disease. Our ability to address the question of how ACPA level and specificity may correlate with cit-specific T cell frequencies was also limited in this study as 18 of the 19 RA subjects were ACPA positive and we observed no significant correlation between ACPA level and T cell frequency. However our approach of using ex vivo Tmr combined with studies of discrete antibody epitopes should allow this to be tested in future studies. Our study utilized peripheral blood. As RA is a disease of the joints, a vital question is whether the T cell profile in peripheral blood is an accurate reflection of the inflammatory process in the synovium. This again is an important question that we believe can be addressed in future studies using the tools developed in this paper.
Although previous studies have documented T cell responses to citrullinated epitopes (26;27;38;39), our study represents the first to define the frequency and ex vivo phenotype of these cells, thereby providing direct evidence that both frequency and phenotype vary with respect to disease parameters. Together these results strongly suggest that citrulline specific CD4+ T cell responses may have a role in the etiology of RA. ACPA antibodies are highly correlated with disease and disease risk (6;7). However, following disease onset, the clinical significance of ACPA levels is less clear (40;41). Our current observations suggest that the number and frequency of these T cells may vary in meaningful ways based on clinical status. The approach described in this paper can be applied to assist in addressing this issue and other important questions about the diversity, frequency, phenotype, and role of autoreactive T cells in various stages of disease.
Supplementary Material
Acknowledgements
We wish to thank the BRI Translational Research Program including Christine Chan and Kevin Criste, the BRI clinical core and Tuan Nguyen for sample processing and handling and Drs. Stanford Peng, Jeffery Carlin, Pedro Truillo and Viviane Stone for subject recruitment. We would like to acknowledge Anton Preisinger for his assistance with murine handling, and Michael Mason for his assistance with statistical analysis.
Funding
Work performed at BRI was supported by grants from the NIH including NIAID 5U19 AI050864, NIMS 5 R01 AR037296, and NIAID UO1 AI101981 while the studies performed at Karolinska Institutet were supported by grants from the Margaretha af Ugglas Foundation, the Swedish Association against Rheumatism, the Swedish Medical Association, the King Gustaf V 80 year Foundation, the Swedish Research Council, the EU FP7 project Masterswitch (HEALTH-F2-2008-223404) and the IMI JU funded project BTCure 115142-2 and Boehringer Ingelheim Fonds and the European Research Council (ERC)
Footnotes
Conflict of Interest
No conflicts of interest
Author Contributions:
EJ and MR designed and performed the research and analysis and contributed to the writing of the paper, J.P., J.A.G, B.B.Y., M.T., C.S. contributed to the performance of the research, M.P. was involved with patient recruitment and clinical data analysis, L.K. and V.M. contribute to the conception and design of the study, JHB was the principal investigator and wrote the paper.
Reference List
- 1.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Aho K, Heliovaara M, Maatela J, Tuomi T, Palosuo T. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol. 1991;18:1282–1284. [PubMed] [Google Scholar]
- 4.Aho K, von Essen R, Kurki P, Palosuo T, Heliovaara M. Antikeratin antibody and antiperinuclear factor as markers for subclinical rheumatoid disease process. J Rheumatol. 1993;20:1278–1281. [PubMed] [Google Scholar]
- 5.Schellekens GA, de Jong BA, van den Hoogen FH, Van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurments in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 8.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44:291–296. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordova KN, Willis VC, Haskins K, Holers VM. A citrullinated fibrinogen-specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J Immunol. 2013;190:1457–1465. doi: 10.4049/jimmunol.1201517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008;205:967–979. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Delwig A, Locke J, Robinson J, Ng W. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:143–149. doi: 10.1002/art.25064. [DOI] [PubMed] [Google Scholar]
- 14.Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G, et al. Identification and functional characterization of T cells reactive to citrullinated-vimentin in HLA-DRB1*0401 humanized mice and RA patients. Arthritis Rheum. 2011 doi: 10.1002/art.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas M, Day CL, Wyer JR, Cunliffe SL, Loughry A, McMichael AJ, et al. Ex vivo phenotype and frequency of influenza virus-specific CD4 memory T cells. J Virol. 2004;78:7284–7287. doi: 10.1128/JVI.78.13.7284-7287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.James EA, Moustakas AK, Bui J, Papadopoulos GK, Bondinas G, Buckner JH, et al. HLA-DR1001 presents "altered-self" peptides derived from joint-associated proteins by accepting citulline in three of its binding pockets. Arthritis Rheum. 2010;62:2909–2918. doi: 10.1002/art.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger RA, Papadopoulos GK, Moustakas AK, Nepom GT, Kwok WW. Allelic variation in key peptide-binding pockets discriminates between closely related diabetes-protective and diabetes-susceptibleHLA-DQB1*06 alleles. J Immunol. 2006 Feb 1;176:1988–1998. doi: 10.4049/jimmunol.176.3.1988. [DOI] [PubMed] [Google Scholar]
- 19.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007 Aug 27;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wambre E, DeLong JH, James EA, Lafond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergenspecific CD4(+) T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129:544–551. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebe JA, Novak EJ, Kwok WW, Farr AG, Nepom GT, Buckner JH. T cell selection and differential activation on structurally related HLA-DR4 ligands. J Immunol. 2001;167:3250–3256. doi: 10.4049/jimmunol.167.6.3250. [DOI] [PubMed] [Google Scholar]
- 23.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 24.Kwok WW, Tan V, Gillette L, Littell CT, Soltis MA, LaFond RB, et al. Frequency of epitope-specific naive CD4(+) T cells correlates with immunodominance in the human memory repertoire. J Immunol. 2012 Mar 15;188:2537–2534. doi: 10.4049/jimmunol.1102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasth AE, Snir O, Johansson A, Nordmark B, Rahbar A, Af KE, et al. Skewed distribution of proinflammatory CD4+CD28null T cells is rheumatoid arthritis. Arthritis Res Ther. 2007;9:R87. doi: 10.1186/ar2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J Autoimmun. 2005;25:303–311. doi: 10.1016/j.jaut.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Monti P, Scirpoli M, Rigamonti A, Mayr A, Jaeger A, Bonfanti R, et al. Evidence for in vivo primed and expanaded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol. 2007 Nov 1;179:5785–5792. doi: 10.4049/jimmunol.179.9.5785. [DOI] [PubMed] [Google Scholar]
- 28.Hill JA, Wang D, Jevnikar AM, Cairns E, Bell DA. The relationship between predicted peptide-MHC class II affinity and T-cell activation in a HLADRbeta1* 0401 transgenic mouse model. Arthritis Res Ther. 2003;5:R40–R48. doi: 10.1186/ar605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, James EA, Sanda S, Greenbaum C, Kwok WW. CD4+ T cells recognize diverse epitopes within GAD65: implications for repertoire development and diabetes monitoring. Immunology. 2013 Mar 1;138:269–279. doi: 10.1111/imm.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy C, Langrish C, Chen Y, Blumenschein W, McClanahan T, Kastelein R, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-21 in joint autoimmune inflammation. J Exp Med. 2003 Dec 15;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colin E, Asmawidjaja P, van Hamburg J, Mus A, van Driel M, Hazes J, et al. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–142. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 32.Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–R795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Wang C, Leng N, Zhu P. Combined Treatment of Etanercept and MTX Reverses TH1/Th2, Th17/Treg Imbalances in Patients with Rheumatoid Arthritis. J Clin Imm. 2011;31:596–605. doi: 10.1007/s10875-011-9542-6. [DOI] [PubMed] [Google Scholar]
- 34.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Jiang L, Zhang S, Yin L, Ma L, He D, et al. Methotrexate attenuates the Th17/IL-17 levels in peripheral blood mononuclear cells from healthy individuals and RA patients. Rheumatol Int. 2013;32:2415–2422. doi: 10.1007/s00296-011-1867-1. [DOI] [PubMed] [Google Scholar]
- 36.Janson P, Linton L, Bergman E, Marits P, Eberhardson M, Piehl F, et al. Profiling of CD4+ T cells with epigenetic immune lineage analysis. J Immunol. 2011 Jan 1;186:92–102. doi: 10.4049/jimmunol.1000960. [DOI] [PubMed] [Google Scholar]
- 37.Yamada H, Nakashima Y, Okazaki K, Mawatari T, Fukushi J, Kaibara N, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–1304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 38.Hertl M, Amagai M, Sundaram H, Stanley J, Ishii K, Katz SI. Recognition of desmoglein 3 by autoreactive T cells in pemphigus vulgaris patients and normals. J Invest Dermatol. 1998 Jan 1;110:62–66. doi: 10.1046/j.1523-1747.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- 39.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 40.Ronnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis. 2005;64:1744–1749. doi: 10.1136/ard.2004.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modi S, Soejima M, Levesque MC. The effect of targeted rheumatoid arthritis therapies on anti-citrullinated protein autoantibody levels and B cell responses. Clin Exp Immunol. 2013;173:8–17. doi: 10.1111/cei.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.