Abstract
The cyclic AMP (cAMP)-protein kinase A (PKA) signaling activates virulence expression during hyphal development in the fungal human pathogen Candida albicans. The hyphal growth is characterized by Golgi polarization toward the hyphal tips, which is thought to enhance directional vesicle transport. However, how the hypha-induction signal regulates Golgi polarization is unknown. Gyp1, a Golgi-associated protein and the first GTPase-activating protein (GAP) in the Rab GAP cascade, critically regulates membrane trafficking from the endoplasmic reticulum to the plasma membrane. Here, we report a novel pathway by which the cAMP-PKA signaling triggers Golgi polarization during hyphal growth. We demonstrate that Gyp1 plays a crucial role in actin-dependent Golgi polarization. Hyphal induction activates PKA, which in turn phosphorylates Gyp1. Phosphomimetic mutation of four PKA sites identified by mass spectrometry (Gyp14E) caused strong Gyp1 polarization to hyphal tips, whereas nonphosphorylatable mutations (Gyp14A) abolished it. Gyp14E exhibited enhanced association with the actin motor Myo2, while Gyp14A showed the opposite effect, providing a possible mechanism for Golgi polarization. A GAP-dead Gyp1 (Gyp1R292K) showed strong polarization similar to that seen with Gyp14E, indicating a role for the GAP activity. Mutating the PKA sites on Gyp1 also impaired the recruitment of a late Golgi marker, Sec7. Furthermore, proper PKA phosphorylation and GAP activity of Gyp1 are required for virulence in mice. We propose that the cAMP-PKA signaling directly targets Gyp1 to promote Golgi polarization in the yeast-to-hypha transition, an event crucial for C. albicans infection.
INTRODUCTION
Polarized cell growth is essential for cell division and morphogenesis in all organisms and is largely achieved through directional transport of secretory vesicles from the Golgi compartment to the growth sites in eukaryotes. In the budding yeast Saccharomyces cerevisiae, the transport from the endoplasmic reticulum (ER) to the plasma membrane (PM) involves a cascade of three Rab GTPases, Ypt1, Ypt31/32, and Sec4 (1, 2). Ypt1 controls ER-Golgi and intra-Golgi transport, Ypt31/32 regulates the transport from late-Golgi to secretory vesicles, and Sec4 drives the transport further to the PM. When Ypt32 is activated, it recruits the guanine nucleotide exchange factor (GEF) Sec2 to activate the downstream Rab, Sec4 (3). Meanwhile, Ypt32 also recruits the GTPase-activating protein (GAP), Gyp1, to deactivate its upstream Rab, Ypt1, by stimulating the hydrolysis of its bound GTP to GDP (4–6). Gyp1 plays a central role in this cascade mechanism to maintain the boundary between the Ypt1 and Ypt32 domains on Golgi compartments (4).
Golgi-derived secretory compartments migrate to the growth sites largely by riding on polarized cytoskeletal tracks (7). The unconventional myosin V motor proteins play a crucial role in moving these secretory vesicles along actin cables (8). In yeasts, Rab GTPases associated with different stages of the secretory vesicles interact with Myo2, a myosin V, which is thought to facilitate the loading and efficient transport of the respective compartments along actin cables during polarized growth (9–12).
The hyphal development of the fungal human pathogen Candida albicans provides a striking example of highly polarized morphogenesis. In response to environmental cues, C. albicans switches from a budding yeast growth form to a highly elongated hyphal growth form (13). This ability to switch is a key virulent trait of this pathogen (14). Upon switching to hyphal development, cell growth is focused in a narrow space of the hyphal tip, leading to rapid hyphal extension. To achieve this remarkable polarized growth, C. albicans has evolved hypha-specific means to facilitate rapid, long-distance vesicular transport to the hyphal tip. For example, a vesicular structure called the Spitzenkörper is formed at the subapical region immediately behind the hyphal apex, acting as a center for supply of secretory vesicles to the growth site (15, 16). Another example is the polarization of Golgi compartments toward the hyphal tip in a manner dependent on polarized actin cables (17).
A range of environmental signals activate C. albicans hyphal growth mainly through two signal transduction pathways, a mitogen-activated protein kinase pathway and the cyclic AMP (cAMP)-protein kinase A (PKA) pathway, although the latter plays a more prominent role (18). A central component of the cAMP-PKA pathway is Cyr1, the sole adenylyl cyclase in this fungus. In response to hyphal induction, Cyr1 increases the synthesis of cAMP, which in turn activates PKA (19). Once activated, PKA phosphorylates the Efg1 transcription factor to switch on the expression of hypha-specific genes (20, 21).
In the past 2 decades, tremendous progress has been made in elucidating the molecular mechanisms by which the hypha-induction signaling pathways regulate the cell's polarity machinery to achieve polarized growth during C. albicans hyphal development. The discovery of the hypha-specific Hgc1-Cdc28 kinase has led to findings of direct molecular links to several key regulators governing different aspects of polarized growth (22, 23). For example, Hgc1-Cdc28 activates the Cdc42 GTPase polarity promoter at the hyphal tip by phosphorylating and inhibiting Rga2, the negative regulator of Cdc42 (24). Hgc1-Cdc28 also phosphorylates Sec2 and the Exo84 exocyst component to facilitate the transport of secretory vesicles to the hyphal tip (25, 26). Efg1 is also a substrate of Hgc1-Cdc28, and this phosphorylation converts Efg1 to a transcription repressor to switch off genes normally required for cell separation during yeast growth, which ensures that hyphal cells remain attached after cytokinesis (27). In addition to these Hgc1-dependent posttranscriptional regulations, modifications of some polarity-related proteins have been found to occur immediately after hyphal induction in manners not requiring Hgc1, such as phosphorylation of the septin Cdc11 (28) and the dephosphorylation of actin patch protein SlaI (29). Indeed, hgc1Δ mutants show an immediate response to hyphal induction, although they are able to form only a wide surface protrusion that is unlike the thin germ tube typically seen in wild-type (WT) cells (22). These observations indicate that the hypha-induction signaling pathways may directly activate some early events of hyphal growth by as-yet-unknown mechanisms.
By screening a C. albicans haploid mutant library deleted of genes encoding GTPases and their regulators, we discovered that GYP1 is essential for C. albicans hyphal growth. Further characterizations revealed that hyphal induction activates rapid Gyp1 phosphorylation by PKA through the cAMP signaling pathway and that this phosphorylation enhances Gyp1's affinity to the Myo2 actin motor protein and determines the polarization of Golgi compartments. Deleting GYP1 or mutating the PKA phosphorylation sites on Gyp1 attenuated C. albicans virulence in mice. Our results establish a novel early event linking the cAMP-PKA pathway to direct regulation of membrane trafficking in C. albicans hyphal development.
MATERIALS AND METHODS
Plasmids, strains, and culture conditions.
Plasmids and C. albicans strains used in this study are listed in Table S1 and Table S2 in the supplemental material. Yeast cells were routinely grown at 30°C in yeast extract-peptone-dextrose (YPD) medium containing 2% yeast extract, 1% peptone, and 2% glucose or in glucose minimal medium (GMM) containing 6.79 g/liter yeast nitrogen base without amino acids and 2% glucose with appropriate supplementation (uridine at 80 μg/ml, arginine at 40 μg/ml, or histidine at 40 μg/ml) for auxotrophic mutants. For hyphal induction, yeast cells were inoculated into YPD medium or GMM supplemented with 20% fetal bovine serum (HyClone Laboratories) and incubated at 37°C.
Hyphal growth on solid medium.
Overnight cultures were diluted to an optical density at 600 nm (OD600) of 0.1, and 2 μl of diluted cultures was spotted onto the Spider medium plates (30) or the Lee's medium plates (31). The plates were incubated at 30°C for 3 and 6 days, respectively.
Protein extraction, immunoprecipitation, and Western blotting.
Experiments were performed as described previously (32), except that an anti-PKA substrate antibody (Cell Signaling; product no. 9621) was used to detect PKA-phosphorylated proteins. Immunoprecipitated Gyp1-green fluorescent protein (Gyp1-GFP) was subjected to liquid chromatography-tandem mass spectrometry (LC/MS/MS) phosphorylation site identification, a service provided by the Proteomics Core Facility of University at Albany, Rensselaer, NY, USA.
In vitro kinase assay.
Immunoprecipitated WT or mutant Gyp1 proteins were incubated in the reaction buffer containing 40 mM Tris-HCl (pH 7.4), 20 mM MgCl2, and 500 μM ATP with or without 30 U of bovine PKA catalytic subunit (Promega) at 30°C for 5 min. The level of phosphorylation was determined by Western blot analysis using the anti-PKA substrate antibody.
Microscopy.
Colony morphologies on solid plates were examined using a Leitz DMRB inverted microscope (Leica) with a 5× objective lens. Cell morphology in liquid media and the subcellular localization of GFP-tagged proteins were examined using a Leica digital module R (DMR) fluorescence microscope with a 100× objective lens.
Virulence assays.
Mouse survival assays were performed as described previously (32). Periodic acid-Schiff staining of formaldehyde-fixed kidneys from infected mice and the slide scanning procedures were carried out by the Advanced Molecular Pathology Laboratory of the Institute of Molecular and Cell Biology, Singapore.
RESULTS
Gyp1 is required for the hyphal growth in C. albicans.
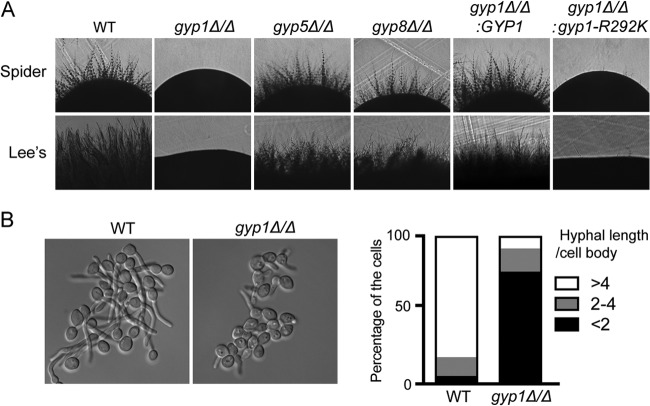
In an effort to generate and screen a mutant library of GTPase-related genes using C. albicans haploids (33), we found that among three reported Ypt1 GAPs, namely, Gyp1, Gyp5 and Gyp8 (5, 34), only Gyp1 was required for the hyphal growth (unpublished data). In this study, we deleted GYP1 (orf19.3811), GYP5 (orf19.5340), or GYP8 (orf19.4315) in diploid C. albicans and tested the mutants for hyphal growth. As in the case of haploid C. albicans, we again found Gyp1 to be the only Ypt1-GAP essential for hyphal growth on solid Spider medium (30) and Lee's medium (31), whereas gyp5Δ/Δ and gyp8Δ/Δ cells exhibited normal hyphal growth (Fig. 1A). Consistently, under conditions of induction with 20% serum at 37°C in liquid media, although gyp1Δ/Δ cells could form germ tubes, the hyphal growth was much slower than that seen in wild-type (WT) cells (Fig. 1B). Reintegration of a copy of WT GYP1 restored normal hyphal growth in gyp1Δ/Δ cells (Fig. 1A). Du and Novick (5) have reported that mutating arginine 343 (R343) of Saccharomyces cerevisiae Gyp1 to lysine (K) abolishes its GAP activity without disrupting the protein folding. To determine whether the GAP activity is required for Gyp1's function in C. albicans hyphal growth, we mutated the corresponding arginine residue (R292) to K and found that the GAP-dead gyp1R292K failed to restore the hyphal growth in gyp1Δ/Δ cells (Fig. 1A). Thus, Gyp1's GAP activity is required for hyphal growth.
FIG 1.
Gyp1 functions in C. albicans hyphal growth. (A) gyp1Δ/Δ and gyp1R292K strains have defects in hyphal development. Yeast cells of BWP17 (WT), HZX201 (gyp1Δ/Δ; for details on strain genotypes, please see Table S2 in the supplemental material), HZX250 (gyp5Δ/Δ), HZX251 (gyp8Δ/Δ), HZX202 (gyp1Δ/Δ:GYP1), and HZX203 (gyp1Δ/Δ:gyp1R292K) were grown overnight in YPD medium at 30°C, adjusted to an OD600 of 0.1, and spotted onto Spider medium plates or Lee's medium plates. The plates were incubated for 3 days or 6 days, respectively. (B) The gyp1Δ/Δ mutant shows markedly slower hyphal extension in liquid media. As shown in the left panel, cells of BWP17 (WT) and HZX201 (gyp1Δ/Δ) were induced for hyphal development in GMM–20% fetal bovine serum (FBS) at 37°C for 2 h before imaging. The right panel shows the distributions of hyphae with different ratios of hyphal length to the diameter of the mother cell body at 2 h of hyphal induction (n = 120).
Gyp1 localizes to and regulates hypha-specific translocation of the Golgi apparatus in C. albicans.
Unlike their polarized localization toward the tip of a growing bud in S. cerevisiae (35), the Golgi compartments appear as random puncta in C. albicans yeast cells. However, the Golgi compartment is polarized toward the tip of growing hyphae, which is thought to facilitate the long-distance directional vesicle transport required for rapid hyphal extension (17). To reveal the subcellular localization of Gyp1 in C. albicans, we expressed Gyp1-GFP from its endogenous promoter for examination by fluorescence microscopy. We observed that, consistent with its Golgi association (5), Gyp1 localized as random puncta in the cytoplasm in yeast cells and showed polarization toward the growing tip in hyphal cells (Fig. 2A). Golgi polarization is dependent on the formin Bni1 and actin cables in both S. cerevisiae (35) and C. albicans (17). Consistently, the polarized Gyp1 localization in hyphae was completely abolished when BNI1 was deleted (Fig. 2A). Interestingly, we observed that the polarized localization of the Vrg4-labeled early Golgi compartments (17, 36) was largely lost in gyp1Δ/Δ hyphal cells (Fig. 2B). Thus, the data demonstrate that C. albicans Gyp1 is a Golgi-associated protein required for the polarization of early Golgi compartments during hyphal development.
FIG 2.
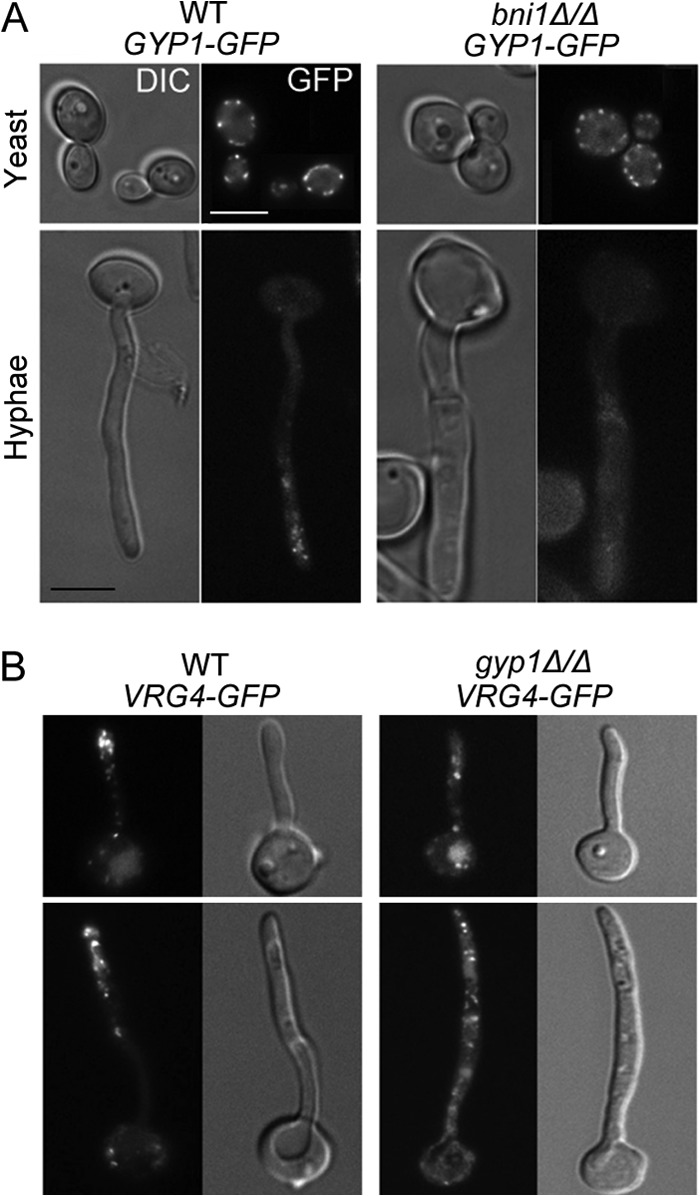
Gyp1 localizes to the Golgi compartment and is required for Golgi polarization in C. albicans. (A) Localization of Gyp1-GFP in WT (HZX202) and bni1Δ/Δ (HZX225) yeast and hyphal cells. Yeast cells were grown in GMM to the log phase at 30°C. Hyphal cells were grown in GMM–20% FBS at 37°C for 2 h. Size bar = 5 μm. Images of differential interference contrast (DIC) and GFP fluorescence microscopy results are shown. (B) Localization of Vrg4 in short and long hyphae in WT (HZX210) and gyp1Δ/Δ (HZX211) backgrounds. Hyphal cells were grown in GMM–20% FBS at 37°C for 2 to 3 h. Although gyp1Δ/Δ cells are defective in hyphal growth, a small percentage of long hyphae can be found. Size bar = 5 μm in all images.
Gyp1 is phosphorylated by PKA upon hyphal induction.
Global phosphoproteome analysis indicated that C. albicans Gyp1 is phosphorylated in vivo (37) at serine residues predicted to be PKA substrates (38). PKA activity is strongly activated by cAMP upon hyphal induction and plays a major role in transducing extracellular hypha-induction signals to the nucleus to activate hypha-specific gene expression (15). We hypothesized that Gyp1 could be a novel PKA substrate/effecter in cytoplasm that couples the hyphal signal to Golgi polarization. To test this hypothesis, we first demonstrated that Gyp1 was phosphorylated by PKA upon hyphal induction. We immunoprecipitated GFP-tagged Gyp1 at timed intervals after hyphal induction and then probed it with an antibody that specifically recognizes PKA-phosphorylated serine residues (PKA substrate antibody) in Western blot analysis. We detected a low level of PKA-phosphorylated Gyp1 in yeast cells. Strikingly, within 5 min of hyphal induction, a markedly higher level of PKA phosphorylation was detected which persisted for at least 2 h (Fig. 3A). Furthermore, immunoprecipitated Gyp1 can be phosphorylated by bovine PKA in vitro (Fig. 3B), strongly suggesting that Gyp1 is a direct substrate of PKA. PKA is activated by cAMP produced by adenylyl cyclase in response to hyphal induction (39). C. albicans has a single adenylyl cyclase, Cyr1 (39), and two isoforms of PKA catalytic subunits, Tpk1 and Tpk2 (40). As expected, PKA phosphorylation of Gyp1 is largely abolished when CYR1 is deleted (Fig. 3C). Furthermore, consistent with a previous report that the overall cellular PKA activity is lower in tpk2Δ/Δ cells than in WT and tpk1Δ/Δ cells (41), we detected markedly reduced PKA phosphorylation of Gyp1 in tpk2Δ/Δ cells in comparison with WT and tpk1Δ/Δ cells (Fig. 3D). Together, these results demonstrate that Gyp1 is phosphorylated by cAMP-activated PKA in response to hyphal induction.
FIG 3.
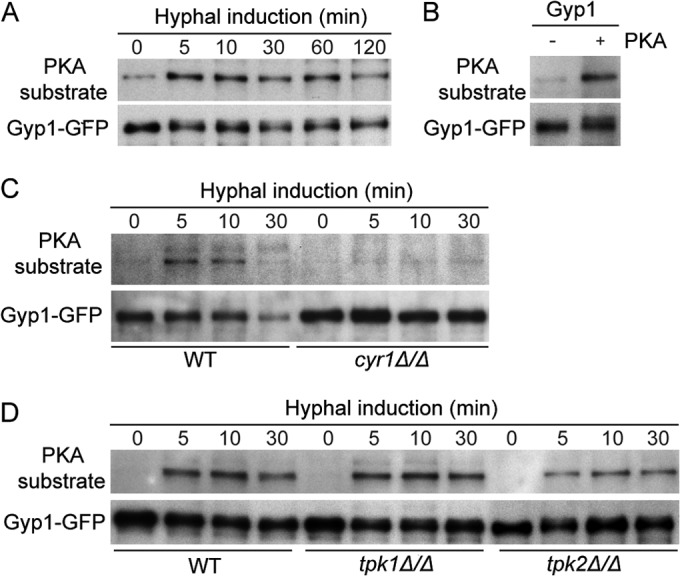
C. albicans Gyp1 is a PKA substrate. (A) Gyp1 is phosphorylated by PKA upon hyphal induction. Log-phase yeast cells expressing Gyp1-GFP (HZX202) were subjected to hyphal induction by switching from GMM at 30°C to prewarmed GMM–20% serum at 37°C. Aliquots were harvested at the indicated times after hyphal induction for preparation of cell lysates. PKA phosphorylation of Gyp1 was detected by Western blot analysis using the anti-PKA substrate antibody, and Gyp1 was probed with anti-GFP antibody. (B) Gyp1 can be phosphorylated by bovine PKA in vitro. Gyp1-GFP immunoprecipitated from yeast cells (HZX202) was subjected to in vitro kinase assay in the presence (+) or absence (−) of bovine PKA and then subjected to Western blot analysis using anti-PKA substrate antibody and anti-GFP antibody. (C and D) Gyp1 PKA phosphorylation levels are decreased in cyr1Δ/Δ and tpk2Δ/Δ cells upon hyphal induction. WT (HZX202), cyr1Δ/Δ (HZX232), tpk1Δ/Δ (HZX226), and tpk2Δ/Δ (HZX227) cells expressing Gyp1-GFP were processed and PKA phosphorylation of Gyp1 was analyzed as described for panel A.
PKA phosphorylation of Gyp1 dictates its polarized translocation.
Mass spectrometry detected six phosphorylated serines in Gyp1, four of which, namely, S32, S96, S105, and S196, have one or two basic residues at the −3 position or at both the −3 and −2 positions (Fig. 4A), representing highly preferred PKA substrates (42, 43). Furthermore, the four serines are all located in the N-terminal regulatory domain (4), suggesting a regulatory function (Fig. 4A). To uncover the function of phosphorylation at these potential PKA sites, we mutated them to either the nonphosphorylatable alanine (gyp14A) or the phosphomimetic glutamic acid (gyp14E). We found that Gyp14A was significantly less phosphorylated by bovine PKA than the WT Gyp1 in vitro (Fig. 4B), indicating that these four sites are indeed phosphorylated by PKA. The observation that bovine PKA can still cause weak phosphorylation of Gyp14A suggests the existence of other unidentified PKA sites. Interestingly, Gyp14A was able to restore the hyphal growth in gyp1Δ/Δ cells on solid media, suggesting that a redundant pathway may exist that can compensate the function of Gyp1 phosphorylation in hyphal development. In contrast, Gyp14E was unable to restore the hyphal growth defect, indicating that constitutive Gyp1 phosphorylation by PKA blocks hyphal morphogenesis (Fig. 4C). This notion is supported by the report that mutants with constitutive activation of PKA are defective in hyphal growth (44, 45). However, neither gyp14A cells nor gyp14E cells exhibited significant hyphal defects under serum induction conditions.
FIG 4.
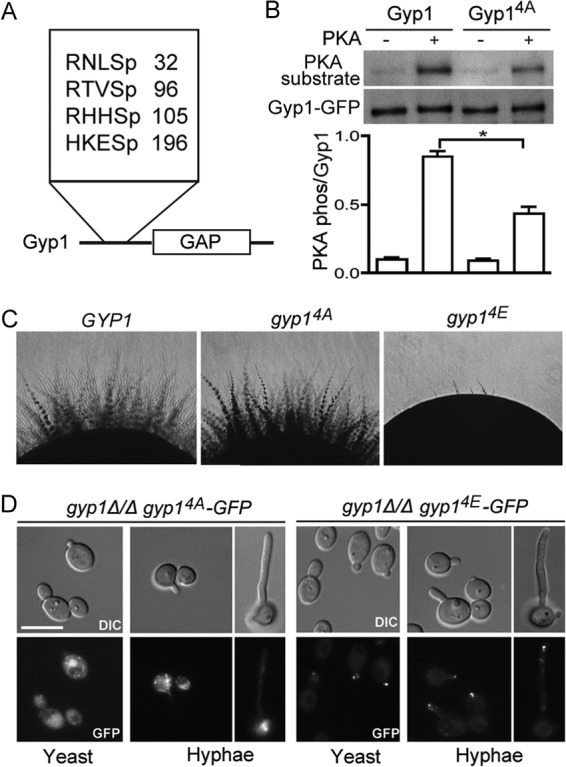
PKA phosphorylation of Gyp1 controls its polarized localization. (A) The diagram depicts the location of and amino acid sequences around four PKA-phosphorylated serines (Sp) identified by mass spectrometry in this study. (B) Gyp14A is less phosphorylated than WT Gyp1 by bovine PKA in vitro. A kinase assay was performed, and PKA phosphorylation of Gyp1 was analyzed as described for Fig. 2C. PKA phosphorylation of Gyp1 was quantified by dividing the density of the PKA substrate band (PKA phos) by that of the Gyp1 band (lower panel). The experiment was done three times, and the difference between PKA phosphorylation of Gyp1 and that of Gyp14A was statistically significant (*, P < 0.05 [t test]). Error bars represent standard errors of the means (SEM). (C) Hyphal development of GYP1 phosphorylation mutants (phosphomutants) on solid medium. A hyphal development assay was performed on Spider medium plates as described for Fig. 1A. The strains used were HZX202 (gyp1Δ/Δ GYP1), HZX204 (gyp1Δ/Δ gyp14A), and HZX205 (gyp1Δ/Δ gyp14E). (D) Cellular localization of Gyp14A-GFP (HZX204) and Gyp14E-GFP (HZX205) in yeast and hyphal cells.
To test our hypothesis that Gyp1 PKA phosphorylation controls Golgi polarization, GFP-tagged Gyp14A and Gyp14E were expressed in gyp1Δ/Δ cells and their localization in yeast and hyphal cells was examined. The two mutant versions of Gyp1 exhibited greatly distinct localization patterns. Gyp14A showed a random punctate pattern similar to that of WT Gyp1 in yeast cells. In hyphal cells, however, Gyp14A failed to polarize toward the hyphal tip, a result that was similar to the localization of WT Gyp1 in bni1Δ/Δ cells (Fig. 4D). In sharp contrast, Gyp14E was intensely polarized to the growing tips even during yeast budding, which resembles the behavior of WT Gyp1 in hyphal cells. During hyphal growth, Gyp14E was not only polarized but also highly concentrated at the hyphal tip (Fig. 4D). Taken together, our results demonstrate that PKA phosphorylation of Gyp1 governs its polarization to the growing site.
PKA phosphorylation of Gyp1 enhances its association with Myo2.
As mentioned above, Golgi polarization toward the hyphal tips depends on the presence of polarized actin cables in C. albicans (Fig. 2B). Actin cables, however, are oriented toward the growth sites during both yeast and hyphal growth in C. albicans. Thus, there must be a mechanism to ensure that the Golgi compartments ride only on the polarized actin cables during hyphal but not yeast growth. In S. cerevisiae, the myosin V motor protein, Myo2, is required for transporting Golgi compartments toward the growth sites by binding to proteins on Golgi compartments and Golgi-derived vesicles, such as Ypt11 (46), Ypt32 (11, 12), and Sec4 (10). In vertebrates, phosphorylation has been suggested to be a mechanism to regulate the affinity between myosin V and its cargoes (47). To test whether PKA phosphorylation of Gyp1 enhances its association with Myo2, we performed coimmunoprecipitation to compare the affinities of Myo2 with WT and mutant Gyp1 proteins. Consistent with the distinct localization patterns described above, Myo2 pulled down significantly more Gyp14E than WT Gyp1 and Gyp14A (Fig. 5A). The results lend strong support to our hypothesis that PKA phosphorylation of Gyp1 strengthens its association with Myo2, which facilitates Golgi transport toward the hyphal tips.
FIG 5.
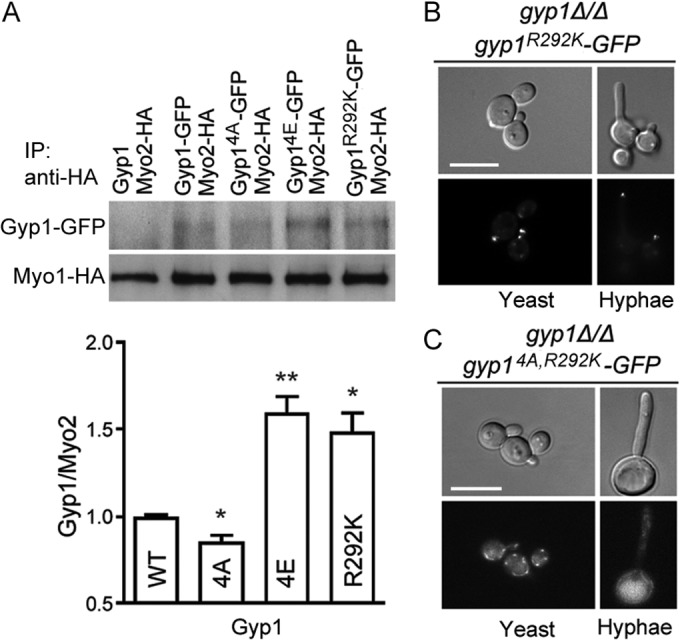
PKA phosphorylation and GAP activity inactivation of Gyp1 enhance its association with Myo2. (A) Log-phase yeast cells coexpressing Myo2-hemagglutinin (Myo2-HA) with Gyp1 (HZX232), Gyp1-GFP (HZX228), Gyp14A-GFP (HZX230), Gyp14E-GFP (HZX231), or Gyp1R292K-GFP (HZX229) were subjected to immunoprecipitation (IP) with anti-HA antibody to pull down Myo1, and the precipitation products were analyzed by Western blotting using anti-GFP and anti-HA antibody. The band intensity of the GFP-tagged Gyp1 protein was normalized to the band intensity of Myo2-HA in the same sample (lower panel). The experiment was done three times, and the differences between WT Gyp1 and mutant Gyp1 proteins were statistically significant (*, P < 0.05; **, P < 0.01 [t test]). Error bars represent SEM. (B and C) Localization of Gyp1R292K-GFP (HZX203) and Gyp14A,R292K-GFP (HZX233) in yeast and hyphal cells.
We observed that, similarly to Gyp14E, the GAP-dead Gyp1R292K also showed enhanced association with Myo2 (Fig. 5A). Consistently, the localization of Gyp1R292K was highly polarized to the growing sites during both yeast and hyphal growth, closely resembling that of Gyp14E (Fig. 5B). If PKA phosphorylation controlled Gyp1's localization only by inactivating its GAP activity, the nonphosphorylatable and GAP-dead Gyp1 (Gyp14A,R292K) would exhibit a localization pattern similar to those of Gyp1R292K and Gyp14E. However, the localization of Gyp14A,R292K was found to resemble that of Gyp14A (Fig. 5C), suggesting that PKA phosphorylation and the GAP activity of Gyp1 do not function in a simple linear pathway. Future research is needed to elucidate the interplay between these two important pathways.
PKA phosphorylation of Gyp1 is required for the recruitment of Sec7 to late Golgi compartments.
To investigate whether PKA phosphorylation of Gyp1 affects downstream Golgi-derived secretory compartments, we examined the localization of a marker protein, Sec7 (35, 48). As expected, GFP-tagged Sec7 markers appeared as random puncta in WT cells (Fig. 6A). Unlike the early Golgi marker Vrg4, the localization of Sec7 was apparently not affected by the deletion of GYP1 (Fig. 6B). Sec7 localization also appeared to be normal in the gyp14E mutant (Fig. 6D). However, in the gyp14A mutant, localizations of Sec7 became diffuse throughout the cytoplasm, with only weak enrichment at the site of growth (Fig. 6C). This localization pattern suggested a failure in Sec7 recruitment to the Golgi compartment. It has been recently reported that stable Sec7 membrane recruitment requires the initial GTP-bound Arf1 (Arf1-GTP) that is activated on early Golgi compartments to kick off a positive-feedback mechanism (49). We speculate that this initial Arf1-GTP activation may require signals from PKA-phosphorylated Gyp1. Future experiments, however, are required to elucidate the exact mechanisms. Nevertheless, the data suggest that PKA phosphorylation of Gyp1 is important for some downstream events in the Golgi trafficking pathway.
FIG 6.
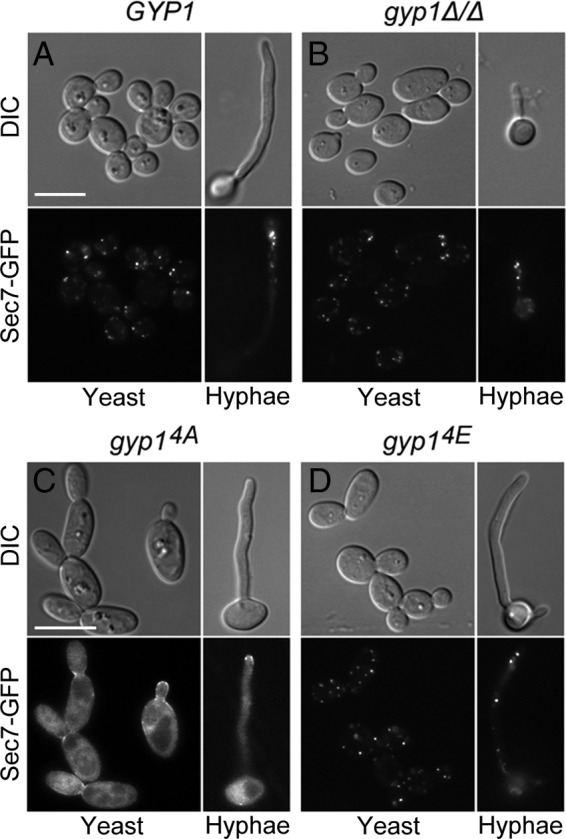
Recruitment of Sec7 to late Golgi compartments requires PKA phosphorylation of Gyp1. Actively growing yeast and hyphal cells of HZX215 (GYP1 SEC7-GFP) (A), HZX216 (gyp1Δ/Δ SEC7-GFP) (B), HZX218 (gyp14A SEC7-GFP) (C), and HZX219 (gyp14E SEC7-GFP) (D) were examined for Sec7-GFP localization by fluorescence microscopy. Yeast cells were grown in GMM at 30°C, and hyphae were induced in GMM–20% FBS at 37°C for 2 h.
PKA phosphorylation of Gyp1 plays an important role in the virulence of C. albicans.
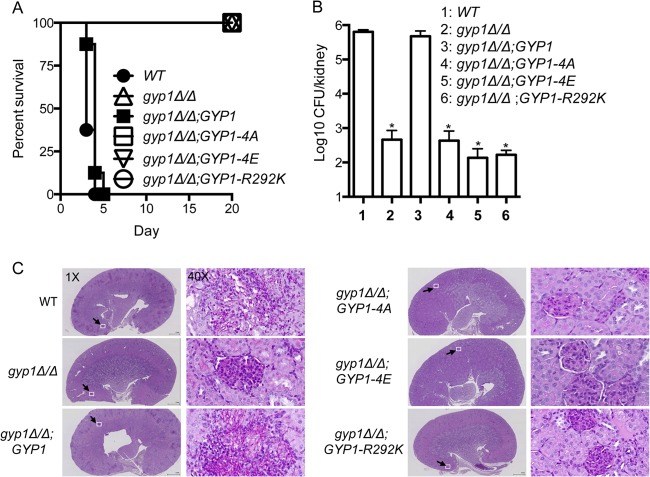
The cAMP-PKA signaling activates the expression of a range of virulence factors in C. albicans, including hyphal morphogenesis and host cell adhesion (50). Gyp1's role in coupling cAMP-PKA signals to the Golgi-mediated secretory pathway suggests that it could be explored as a potential therapeutic target. To test the impact of altering the PKA phosphorylation of Gyp1 on C. albicans virulence, we employed a mouse model of systemic infection in which C. albicans yeast cells were injected via the tail vein (32). All mice infected with the WT or the GYP1-reintegrant strain died within 5 days after inoculation (Fig. 7A). Similar results were observed in mice infected with the gyp5Δ/Δ mutant or the gyp8Δ/Δ mutant, both of which showed normal hyphal growth (see Fig. S1 in the supplemental material). In contrast, mice infected with the gyp14A mutant, the gyp14E mutant, or the gyp1R292K mutant all survived to the end of the experiment (Fig. 7A). Consistently, there was no significant difference in kidney fungal burdens between mice infected with the WT and GYP1-reintegrant cells 2 days after inoculation, whereas mice infected with gyp14A, gyp14E, or gyp1R292K mutant cells showed greater than 1,000 times less fungal burden in the kidney (Fig. 7B). Histological examination revealed that WT and GYP1-reintegrant cells in the infected kidney and brain were largely in the hyphal form, whereas few hyphae was observed in the kidney and brain of the mice infected with the mutant cells (Fig. 7C; see also Fig. S2). Taken together, our results show that proper PKA phosphorylation of Gyp1 is essential to the virulence of C. albicans. Thus, Gyp1 provides an attractive therapeutic target for the treatment of C. albicans infection.
FIG 7.
Gyp1 function is required for virulence of C. albicans. (A) Survival curves of mice inoculated with 1 × 106 yeast cells with the indicated genotype. Eight mice were used for each C. albicans strain (SC5314, HZX201, HZX202, HZX204, HZX205, and HZX203). (B) Kidney fungal burden of the infected mice. Two mice for each C. albicans strain were sacrificed 2 days after injection to determine the log10CFU/kidney (*, P < 0.01 [t test]). (C) Histological examination of the kidneys of infected mice. The kidneys of mice infected with C. albicans with the indicated genotypes were removed 2 days after infection. Kidney sections were stained using the periodic acid-Schiff staining method, which stains C. albicans cells dark magenta. The square in the 1× image marks that region shown in the 40× image.
DISCUSSION
In this study, we have discovered a novel role for Gyp1 in transducing hypha-induction signals to the Golgi-derived vesicular transport during the yeast-to-hypha transition in C. albicans. Gyp1 is the only Rab1/Ypt1 GAP that is essential for the hyphal growth and virulence in this pathogen. Gyp1 receives the hypha-induction signal via direct phosphorylation by cAMP-activated PKA. This phosphorylation triggers a switch of the transport machinery from the yeast mode to the hyphal mode by, at least in part, enhancing the interaction between the proteins on Golgi compartments to the actin motor Myo2 on polarized actin cables. Altering PKA phosphorylation of Gyp1 has dramatic effects on Golgi polarization, impairs the hyphal growth, and attenuates the virulence of this pathogen. The idea of the importance of Gyp1 in the hyphal growth is supported by previous reports that the transcript of GYP1 is coregulated by three hyphal gene repressors, Tup1, Nrg1, and Mig1 (51), and several prevacuolar Rab GTPases have recently been reported to impact the hyphal growth (52, 53). Thus, our results provide the first direct link between the hypha-induction signal and the vesicular transport machinery, which is essential for the morphogenetic transition and virulence in C. albicans.
The cAMP-PKA pathway is the major signaling pathway that transduces hyphal inducing signals to its downstream effectors to turn on the hyphal program (18). Studies of the role of PKA in the yeast-to-hypha transition have been focused on its downstream transcription factors, including Efg1 and Flo8 (54–57). Our results identify Gyp1 as the first PKA effector in the vesicular transport pathway. Our findings also suggest that the role of the cAMP-PKA signaling pathway is broader than just regulating hyphal gene expression. As Gyp1 functions in the early steps of the vesicular transport pathway from the ER to the PM, it could act as a receiver of hypha-induction signals, which are then passed on along the pathway via established signaling cascades. Our finding that localization of the late Golgi marker Sec7 requires PKA phosphorylation of Gyp1 (Fig. 6) supports this model. Another possible model could be that switching to the hyphal mode would require PKA to simultaneously phosphorylate its effectors at multiple steps of the vesicular transport pathway to alter their functions, such as increasing vesicle binding to Myo2 to meet the need for rapid hyphal growth. The recent discovery that S. cerevisiae Myo2 is a potential PKA substrate and that its phosphorylation regulates its polarized localization to the growing bud seems to support this model (58). A third possibility is the combination of these two models, i.e., that PKA phosphorylates Gyp1 to activate the signaling cascade along the transport pathway and at the same time PKA also phosphorylates other effectors along the pathway to reinforce the signal initiated from Gyp1. Further efforts are justified to identify more PKA effectors and elucidate how the hypha-induction signals are integrated into the Rab GTPase cascade in vesicular transport.
Besides PKA, other kinases, such as the cyclin-dependent kinases (CDKs), also play critical roles in C. albicans hyphal growth. In the past decade, a series of studies have shown that CDKs control C. albicans hyphal growth by phosphorylating components in key cellular machines that execute polarized growth (23), including the Cdc42 GTPase module (24), actomyosin ring components (59), septins (28, 60), endocytic machinery (29), and the Cbk1/Mob2 complex in the regulation of Ace2 and morphogenesis network (61). Recently, Sudbery and his colleagues have reported that the hypha-specific Hgc-Cdc28 phosphorylates and regulates Sec2 and Exo84, two downstream components of the vesicular transport pathway during C. albicans hyphal growth (25, 26). Together with the results in this study, we propose that PKA and CDK work in a coordinated manner to control early and late events in the vesicular transport pathway during hyphal growth. As PKA is activated first by hyphal induction, it phosphorylates some upstream components on Golgi compartments, such as Gyp1, to initiate changes in the transport machinery, and at the same time PKA phosphorylates a transcription factor(s) to turn on the expression of the hypha-specific G1 cyclin Hgc1 (22). After Hgc1 has been synthesized, the Hgc1-CDK complex starts to phosphorylate some downstream components on Golgi-derived secretory vesicles, such as Sec2 and Exo84 (25, 26), to complete the transition of the entire vesicular transport machinery from the yeast mode to the hyphal mode. This PKA-CDK relay would ensure rapid establishment of the hyphal growth in response to environmental cues.
There have been a number of reports on a strategy used in the evolution of several key traits in C. albicans to specifically meet the needs of its commensal/pathogen life style in the human host: it has modified and converted some existing developmental pathways for specific purposes. One example is the evolution of Hgc1 from a cell cycle regulator to a specific promoter of hyphal growth (22). In spite of its homology to G1 cyclins, Hgc1 has nearly completely lost its function in cell cycle control but has specifically retained and possibly enhanced the ability of the yeast G1 cyclins such as Cln1 and Cln2 to promote apical growth (62, 63). Furthermore, the expression of HGC1 has also been uncoupled from cell cycle control and placed under the control of the hypha-induction signals to ensure the continuous polarized growth in hyphal development (22). Furthermore, Hgc1-Cdc28 phosphorylation of Rga2 (24), Efg1 (27), Sec2 (25), Exo84 (26), and Cdc11 (60) leads to modification of highly conserved cellular processes to suit the requirement by different aspects of hyphal development. The evolution of C. albicans has also involved extensive rewiring of existing transcription regulation circuits (64–67). The phosphoregulation of Gyp1 by PKA found in this study may provide another example of this evolutionary strategy. In S. cerevisiae, the Golgi compartments are polarized toward the growing bud (35), whereas in C. albicans budding yeast growth, the Golgi compartments exhibit no such polarity. Interestingly, Gyp1 in S. cerevisiae has been reported to be among the proteins that are most highly phosphorylated by CDK1 in the secretory pathways (37, 68). In contract, C. albicans Gyp1 does not have a CDK consensus site; instead, it has multiple PKA sites. Budding is tightly coupled with the cell cycle, while the hyphal extension is independent of it (69) but dependent on PKA activation by cAMP (50, 70). We speculate that the mechanism by which Gyp1 phosphorylation regulates Golgi polarization may be evolutionarily conserved. However, Gyp1 has evolved different regulatory targets for different kinases, thus linking Golgi polarization to different upstream signaling cascades to adapt to various developmental programs in different species.
Several proteins associated with post-Golgi secretory vesicles have been reported to play important roles in C. albicans hyphal growth and virulence (15, 25, 26, 71), which underscores the importance of the vesicular transport pathway in these processes. The formation of the hypha-specific Spitzenkörper and the polarization of the Golgi compartment toward hyphal tips are clear indications that hypha-induction signals regulate the vesicular transport machinery (16). Our discovery of Gyp1 as a direct substrate of PKA reveals an important signaling node for this control. Interestingly, it has been reported that the bacterial pathogen Legionella pneumophila secrets proteins that mimic the activity of eukaryotic Rab1 GAP to manipulate the host secretory pathway (72, 73). These findings suggest that Rab1/Ypt1 GAPs could be targets for effective regulation and manipulation of the eukaryotic secretory process throughout evolution. Moreover, as the activity of Gyp1 is crucial for the virulence of C. albicans, it may represent an attractive target for developing new antifungal strategies.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Neta Dean and Joachim Ernst for providing constructs and strains. We acknowledge the technical expertise provided by the Advanced Molecular Pathology Laboratory at IMCB. We thank members of laboratory of Y.W. for comments on the manuscript.
This study was supported by the Agency for Science, Technology and Research, Singapore.
Footnotes
Published ahead of print 17 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00231-14.
REFERENCES
- 1.Hutagalung AH, Novick PJ. 2011. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91:119–149. 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr F, Lambright DG. 2010. Rab GEFs and GAPs. Curr. Opin. Cell Biol. 22:461–470. 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortiz D, Medkova M, Walch-Solimena C, Novick P. 2002. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157:1005–1015. 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera-Molina FE, Novick PJ. 2009. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc. Natl. Acad. Sci. U. S. A. 106:14408–14413. 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du LL, Novick P. 2001. Yeast rab GTPase-activating protein Gyp1p localizes to the Golgi apparatus and is a negative regulator of Ypt1p. Mol. Biol. Cell 12:1215–1226. 10.1091/mbc.12.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan X, Eathiraj S, Munson M, Lambright DG. 2006. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 442:303–306. 10.1038/nature04847. [DOI] [PubMed] [Google Scholar]
- 7.Pruyne DW, Schott DH, Bretscher A. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931–1945. 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- 8.Johnston GC, Prendergast JA, Singer RA. 1991. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 113:539–551. 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. 2011. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev. Cell 20:47–59. 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y, Sultana A, Gandhi P, Franklin E, Hamamoto S, Khan AR, Munson M, Schekman R, Weisman LS. 2011. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev. Cell 21:1156–1170. 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casavola EC, Catucci A, Bielli P, Di Pentima A, Porcu G, Pennestri M, Cicero DO, Ragnini-Wilson A. 2008. Ypt32p and Mlc1p bind within the vesicle binding region of the class V myosin Myo2p globular tail domain. Mol. Microbiol. 67:1051–1066. 10.1111/j.1365-2958.2008.06106.x. [DOI] [PubMed] [Google Scholar]
- 12.Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS, Segev N. 2008. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol. Biol. Cell 19:4177–4187. 10.1091/mbc.E08-02-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berman J, Sudbery PE. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918–930. 10.1038/nrg948. [DOI] [PubMed] [Google Scholar]
- 14.Sudbery P, Gow N, Berman J. 2004. The distinct morphogenic states of Candida albicans. Trends Microbiol. 12:317–324. 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 9:737–748. 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 16.Crampin H, Finley K, Gerami-Nejad M, Court H, Gale C, Berman J, Sudbery P. 2005. Candida albicans hyphae have a Spitzenkorper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 118:2935–2947. 10.1242/jcs.02414. [DOI] [PubMed] [Google Scholar]
- 17.Rida PC, Nishikawa A, Won GY, Dean N. 2006. Yeast-to-hyphal transition triggers formin-dependent Golgi localization to the growing tip in Candida albicans. Mol. Biol. Cell 17:4364–4378. 10.1091/mbc.E06-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst JF. 2000. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology 146(Pt 8):1763–1774. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y. 2013. Fungal adenylyl cyclase acts as a signal sensor and integrator and plays a central role in interaction with bacteria. PLoS Pathog. 9:e1003612. 10.1371/journal.ppat.1003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane S, Birse C, Zhou S, Matson R, Liu H. 2001. DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans. J. Biol. Chem. 276:48988–48996. 10.1074/jbc.M104484200. [DOI] [PubMed] [Google Scholar]
- 21.Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982–1991. 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Wang Y, Wang Y. 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 23:1845–1856. 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y. 2009. CDKs and the yeast-hyphal decision. Curr. Opin. Microbiol. 12:644–649. 10.1016/j.mib.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Zheng XD, Lee RT, Wang YM, Lin QS, Wang Y. 2007. Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J. 26:3760–3769. 10.1038/sj.emboj.7601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop A, Lane R, Beniston R, Chapa-y-Lazo B, Smythe C, Sudbery P. 2010. Hyphal growth in Candida albicans requires the phosphorylation of Sec2 by the Cdc28-Ccn1/Hgc1 kinase. EMBO J. 29:2930–2942. 10.1038/emboj.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caballero-Lima D, Sudbery PE. 5 February 2014, posting date In Candida albicans, phosphorylation of Exo84 by Cdk1-Hgc1 is necessary for efficient hyphal extension. Mol. Biol. Cell 10.1091/mbc.E13-11-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang A, Raniga PP, Lane S, Lu Y, Liu H. 2009. Hyphal chain formation in Candida albicans: Cdc28-Hgc1 phosphorylation of Efg1 represses cell separation genes. Mol. Cell. Biol. 29:4406–4416. 10.1128/MCB.01502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha I, Wang YM, Philp R, Li CR, Yap WH, Wang Y. 2007. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev. Cell 13:421–432. 10.1016/j.devcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Zeng G, Wang YM, Wang Y. 2012. Cdc28-Cln3 phosphorylation of SlaI regulates actin patch dynamics in different modes of fungal growth. Mol. Biol. Cell 23:3485–3497. 10.1091/mbc.E12-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Kohler J, Fink GR. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723–1726. 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 31.Lee KL, Buckley HR, Campbell CC. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148–153. 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 32.Huang ZX, Zhao P, Zeng GS, Wang YM, Sudbery I, Wang Y. 2014. Phosphoregulation of Nap1 plays a role in septin ring dynamics and morphogenesis in Candida albicans. mBio 5:e00915-13. 10.1128/mBio.00915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickman MA, Zeng G, Forche A, Hirakawa MP, Abbey D, Harrison BD, Wang YM, Su CH, Bennett RJ, Wang Y, Berman J. 2013. The ‘obligate diploid' Candida albicans forms mating-competent haploids. Nature 494:55–59. 10.1038/nature11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Antoni A, Schmitzova J, Trepte HH, Gallwitz D, Albert S. 2002. Significance of GTP hydrolysis in Ypt1p-regulated endoplasmic reticulum to Golgi transport revealed by the analysis of two novel Ypt1-GAPs. J. Biol. Chem. 277:41023–41031. 10.1074/jbc.M205783200. [DOI] [PubMed] [Google Scholar]
- 35.Rossanese OW, Reinke CA, Bevis BJ, Hammond AT, Sears IB, O'Connor J, Glick BS. 2001. A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J. Cell Biol. 153:47–62. 10.1083/jcb.153.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishikawa A, Poster JB, Jigami Y, Dean N. 2002. Molecular and phenotypic analysis of CaVRG4, encoding an essential Golgi apparatus GDP-mannose transporter. J. Bacteriol. 184:29–42. 10.1128/JB.184.1.29-42.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beltrao P, Trinidad JC, Fiedler D, Roguev A, Lim WA, Shokat KM, Burlingame AL, Krogan NJ. 2009. Evolution of phosphoregulation: comparison of phosphorylation patterns across yeast species. PLoS Biol. 7:e1000134. 10.1371/journal.pbio.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X. 2008. GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol. Cell. Proteomics 7:1598–1608. 10.1074/mcp.M700574-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12:3631–3643. 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bockmühl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF. 2001. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 42:1243–1257. [DOI] [PubMed] [Google Scholar]
- 41.Giacometti R, Kronberg F, Biondi RM, Hernandez AI, Passeron S. 2012. Cross regulation between Candida albicans catalytic and regulatory subunits of protein kinase A. Fungal Genet. Biol. 49:74–85. 10.1016/j.fgb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Kemp BE, Bylund DB, Huang TS, Krebs EG. 1975. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc. Natl. Acad. Sci. U. S. A. 72:3448–3452. 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp BE, Graves DJ, Benjamini E, Krebs EG. 1977. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J. Biol. Chem. 252:4888–4894. [PubMed] [Google Scholar]
- 44.Cassola A, Parrot M, Silberstein S, Magee BB, Passeron S, Giasson L, Cantore ML. 2004. Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit. Eukaryot. Cell 3:190–199. 10.1128/EC.3.1.190-199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giacometti R, Souto G, Silberstein S, Giasson L, Cantore ML, Passeron S. 2006. Expression levels and subcellular localization of Bcy1p in Candida albicans mutant strains devoid of one BCY1 allele results in a defective morphogenetic behavior. Biochim. Biophys. Acta 1763:64–72. 10.1016/j.bbamcr.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Arai S, Noda Y, Kainuma S, Wada I, Yoda K. 2008. Ypt11 functions in bud-directed transport of the Golgi by linking Myo2 to the coatomer subunit Ret2. Curr. Biol. 18:987–991. 10.1016/j.cub.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 47.Karcher RL, Roland JT, Zappacosta F, Huddleston MJ, Annan RS, Carr SA, Gelfand VI. 2001. Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science 293:1317–1320. 10.1126/science.1061086. [DOI] [PubMed] [Google Scholar]
- 48.Franzusoff A, Redding K, Crosby J, Fuller RS, Schekman R. 1991. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J. Cell Biol. 112:27–37. 10.1083/jcb.112.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson BC, McDonold CM, Fromme JC. 2012. The Sec7 Arf-GEF is recruited to the trans-Golgi network by positive feedback. Dev. Cell 22:799–810. 10.1016/j.devcel.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogan DA, Sundstrom P. 2009. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 4:1263–1270. 10.2217/fmb.09.106. [DOI] [PubMed] [Google Scholar]
- 51.Murad AM, d'Enfert C, Gaillardin C, Tournu H, Tekaia F, Talibi D, Marechal D, Marchais V, Cottin J, Brown AJ. 2001. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors CaTup1, CaMig1 and CaNrg1. Mol. Microbiol. 42:981–993. 10.1046/j.1365-2958.2001.02713.x. [DOI] [PubMed] [Google Scholar]
- 52.Johnston DA, Eberle KE, Sturtevant JE, Palmer GE. 2009. Role for endosomal and vacuolar GTPases in Candida albicans pathogenesis. Infect. Immun. 77:2343–2355. 10.1128/IAI.01458-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston DA, Tapia AL, Eberle KE, Palmer GE. 2013. Three prevacuolar compartment Rab GTPases impact Candida albicans hyphal growth. Eukaryot. Cell 12:1039–1050. 10.1128/EC.00359-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao F, Lane S, Raniga PP, Lu Y, Zhou Z, Ramon K, Chen J, Liu H. 2006. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 17:295–307. 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bockmühl DP, Ernst JF. 2001. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics 157:1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tebarth B, Doedt T, Krishnamurthy S, Weide M, Monterola F, Dominguez A, Ernst JF. 2003. Adaptation of the Efg1p morphogenetic pathway in Candida albicans by negative autoregulation and PKA-dependent repression of the EFG1 gene. J. Mol. Biol. 329:949–962. 10.1016/S0022-2836(03)00505-9. [DOI] [PubMed] [Google Scholar]
- 57.Schaekel A, Desai PR, Ernst JF. 2013. Morphogenesis-regulated localization of protein kinase A to genomic sites in Candida albicans. BMC Genomics 14:842. 10.1186/1471-2164-14-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Legesse-Miller A, Zhang S, Santiago-Tirado FH, Van Pelt CK, Bretscher A. 2006. Regulated phosphorylation of budding yeast's essential myosin V heavy chain, Myo2p. Mol. Biol. Cell 17:1812–1821. 10.1091/mbc.E05-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li CR, Wang YM, Wang Y. 2008. The IQGAP Iqg1 is a regulatory target of CDK for cytokinesis in Candida albicans. EMBO J. 27:2998–3010. 10.1038/emboj.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li CR, Yong JY, Wang YM, Wang Y. 2012. CDK regulates septin organization through cell-cycle-dependent phosphorylation of the Nim1-related kinase Gin4. J. Cell Sci. 125:2533–2543. 10.1242/jcs.104497. [DOI] [PubMed] [Google Scholar]
- 61.Gutiérrez-Escribano P, González-Novo A, Suárez MB, Li CR, Wang Y, de Aldana CR, Correa-Bordes J. 2011. CDK-dependent phosphorylation of Mob2 is essential for hyphal development in Candida albicans. Mol. Biol. Cell 22:2458–2469. 10.1091/mbc.E11-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lew DJ, Reed SI. 1993. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 120:1305–1320. 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rua D, Tobe BT, Kron SJ. 2001. Cell cycle control of yeast filamentous growth. Curr. Opin. Microbiol. 4:720–727. 10.1016/S1369-5274(01)00274-0. [DOI] [PubMed] [Google Scholar]
- 64.Booth LN, Tuch BB, Johnson AD. 2010. Intercalation of a new tier of transcription regulation into an ancient circuit. Nature 468:959–963. 10.1038/nature09560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diezmann S, Michaut M, Shapiro RS, Bader GD, Cowen LE. 2012. Mapping the Hsp90 genetic interaction network in Candida albicans reveals environmental contingency and rewired circuitry. PLoS Genet. 8:e1002562. 10.1371/journal.pgen.1002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moran GP. 2012. Transcript profiling reveals rewiring of iron assimilation gene expression in Candida albicans and C. dubliniensis. FEMS Yeast Res. 12:918–923. 10.1111/j.1567-1364.2012.00841.x. [DOI] [PubMed] [Google Scholar]
- 67.Sandai D, Yin Z, Selway L, Stead D, Walker J, Leach MD, Bohovych I, Ene IV, Kastora S, Budge S, Munro CA, Odds FC, Gow NA, Brown AJ. 2012. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. mBio 3:e00495-12. 10.1128/mBio.00495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holt LJ, Tuch BB, Villen J, Johnson AD, Gygi SP, Morgan DO. 2009. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325:1682–1686. 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hazan I, Sepulveda-Becerra M, Liu H. 2002. Hyphal elongation is regulated independently of cell cycle in Candida albicans. Mol. Biol. Cell 13:134–145. 10.1091/mbc.01-03-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Zou H, Fang HM, Zhu Y. 2010. Linking cellular actin status with cAMP signaling in Candida albicans. Virulence 1:202–205. 10.4161/viru.1.3.11836. [DOI] [PubMed] [Google Scholar]
- 71.Franke K, Nguyen M, Hartl A, Dahse HM, Vogl G, Wurzner R, Zipfel PF, Kunkel W, Eck R. 2006. The vesicle transport protein Vac1p is required for virulence of Candida albicans. Microbiology 152:3111–3121. 10.1099/mic.0.29115-0. [DOI] [PubMed] [Google Scholar]
- 72.Machner MP, Isberg RR. 2006. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev. Cell 11:47–56. 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Yu Q, Hu L, Yao Q, Zhu Y, Dong N, Wang DC, Shao F. 2013. Structural analyses of Legionella LepB reveal a new GAP fold that catalytically mimics eukaryotic RasGAP. Cell Res. 23:775–787. 10.1038/cr.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.