Abstract
Zygosaccharomyces rouxii is a fructophilic yeast that consumes fructose preferably to glucose. This behavior seems to be related to sugar uptake. In this study, we constructed Z. rouxii single-, double-, and triple-deletion mutants in the UL4 strain background (a ura3 strain derived from CBS 732T) by deleting the genes encoding the specific fructose facilitator Z. rouxii Ffz1 (ZrFfz1), the fructose/glucose facilitator ZrFfz2, and/or the fructose symporter ZrFsy1. We analyzed the effects on the growth phenotype, on kinetic parameters of fructose and glucose uptake, and on sugar consumption profiles. No growth phenotype was observed on fructose or glucose upon deletion of FFZ genes. Deletion of ZrFFZ1 drastically reduced fructose transport capacity, increased glucose transport capacity, and eliminated the fructophilic character, while deletion of ZrFFZ2 had almost no effect. The strain in which both FFZ genes were deleted presented even higher consumption of glucose than strain Zrffz1Δ, probably due to a reduced repressing effect of fructose. This study confirms the molecular basis of the Z. rouxii fructophilic character, demonstrating that ZrFfz1 is essential for Z. rouxii fructophilic behavior. The gene is a good candidate to improve the fructose fermentation performance of industrial Saccharomyces cerevisiae strains.
INTRODUCTION
The most problematic spoilage yeasts in the food and beverage industry belong to the genus Zygosaccharomyces. Zygosaccharomyces bailii and Zygosaccharomyces rouxii are the main spoilage yeasts of sugar syrups, fruit juices, honey, and sweet sauces, due to their unique properties, such as high resistance to weak-acid preservatives, extreme osmotolerance (some Z. rouxii strains are able to grow on 90% [wt/vol] glucose), ability to vigorously ferment hexoses (which can cause swelling or bursting of packaging, due to gas accumulation), ability to adapt to high temperatures, and growth at low pH (1, 2). Z. rouxii is also industrially used in the fermentation of some salted condiments, such as soy sauce and miso paste, and in the production of balsamic vinegar (3, 4).
Z. rouxii and Z. bailii are fructophilic yeasts, meaning that they consume fructose faster than glucose, a behavior opposite to that of the glucophilic Saccharomyces cerevisiae, which prefers glucose over fructose (5). In 1956, Sols (6) showed that phosphorylation of sugars was not involved in fructophily of a “sauternes yeast” (probably Z. bailii [7]), suggesting that another step preceding phosphorylation was the main regulator of the fructophilic behavior. This was further supported by later studies that indicated that hexokinase activities were similar in Z. bailii and S. cerevisiae and that sugar transport should be involved in Z. bailii fructophily (5).
The transport across the plasma membrane is the first step at which the utilization of nutrients by cells can be controlled and regulated in response to extracellular conditions and intracellular requirements. The majority of sugar transporters belong to the sugar porter (SP) family, the largest member of the major facilitator superfamily (MFS) (8). Most yeasts use sugar facilitators and sugar-proton symporters, the latter being energy-consuming mechanisms that operate only when relatively low concentrations of sugars are available in the extracellular environment (9).
Fructose uptake in Z. bailii was shown to be mediated by a specific facilitator system with high capacity and low affinity and by a low-capacity and high-affinity facilitator system that also transports glucose (10). Fructose transport is thus privileged, mainly at high sugar concentrations. Three mechanisms seem to be responsible for this behavior: (i) fructose is transported by a specific transport system with high capacity, (ii) fructose competes with glucose for the hexose transport system, and (iii) high fructose concentrations inactivate the glucose facilitator system (10). Similar results were also found for Z. rouxii (11).
A peculiar type of sugar transporter was identified and characterized in Z. bailii ISA 1307. The Z. bailii Ffz1 (ZbFfz1) protein, when expressed in a hxt-null mutant of S. cerevisiae, behaved as a high-capacity and low-affinity (Km, ∼80 mM; Vmax, ∼3 mmol h−1 g−1) specific fructose facilitator with low protein sequence identity to the sugar porter family (12). Two similar proteins, Z. rouxii Ffz1 (ZrFfz1) (a fructose-specific facilitator; Km, ∼400 mM; Vmax, ∼13 mmol h−1 g−1) and ZrFfz2 (a fructose/glucose facilitator; Km glucose; fructose, ∼200 mM; Vmax glucose; fructose, ∼4 mmol h−1 g−1), were subsequently characterized in Z. rouxii CBS 732T, revealing the existence of a new family of sugar transporters (13). The Ffz proteins have highly conserved sequence motifs distinct from those of the sugar porter family (9) and are phylogenetically closer to the drug/H+ antiporter 1 (DHA1) family. This new family also includes several putative proteins from Aspergillus species and from several fungal plant pathogens (13). Another Ffz protein, CmFfz1, was recently characterized as a specific fructose transporter (Km, ∼100 mM; Vmax, ∼9 mmol h−1 g−1) in the osmotolerant fructophilic yeast Candida magnoliae (14). Besides the ZbFfz1 gene, three other putative FFZ-like genes were annotated in the recently sequenced genome of Z. bailii ISA 1307 (15). In the genome of the type strain Z. bailii CLIB13 (CBS 680) (16), we also detected two putative FFZ-like genes.
A high-affinity fructose/H+ symporter (Km, ∼0.4 mM; Vmax, ∼0.6 mmol h−1 g−1), Fsy1, was also recently described in Z. rouxii CBS 732T. The symporter was tightly regulated, being expressed in Z. rouxii only when the cells were grown in media with extremely low fructose concentrations (<0.2% [wt/vol]) or with nonfermentable carbon sources, such as mannitol and xylitol (17).
The fructophilic behavior of Z. rouxii has not yet been unequivocally associated with the expression of a specific gene(s). The detection of the unusual Ffz transporters in at least three fructophilic yeasts points to a possible correlation between their presence and the fructophilic behavior of these yeasts.
In this paper, we analyze the effect of deletion of the genes encoding the atypical Ffz fructose transporters (ZrFFZ1 and ZrFFZ2) on the fructophilic character of Z. rouxii CBS 732T and demonstrate the importance of the expression of the specific fructose facilitator ZrFfz1 in this behavior.
MATERIALS AND METHODS
Strains and growth media.
The strains used or constructed in this study are listed in Table 1. S. cerevisiae strain BW31a was used for the construction of plasmids by homologous recombination. Yeast strains were grown in minimal YNB (yeast nitrogen base without amino acids containing the indicated carbon source and the required supplements) or rich YPD (1% yeast extract, 2% peptone, 2% dextrose) medium. Cultures were incubated at 30°C with orbital shaking. Sugar concentrations are given as percentages (weight/volume). Escherichia coli XL1-Blue (Stratagene) was used as the host for plasmid amplification. E. coli transformants were grown in standard Luria-Bertani medium supplemented with ampicillin (100 μg ml−1).
TABLE 1.
Strains used or constructed in this study
| Strain | Genotype | Reference |
|---|---|---|
| S. cerevisiae BW31a | MATa leu2-3/122 ura3-1 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2 mal10 ena1-4Δ::HIS3 nha1::LEU2 | 26 |
| Z. rouxii | ||
| CBS 732T | Wild type | CBS strain database |
| UL4 | ura3 | 19 |
| F1 | ura3 Zrffz1Δ::loxP | This study |
| F2 | ura3 Zrffz2Δ::loxP | This study |
| F1F2 | ura3 Zrffz1Δ::loxP Zrffz2Δ::loxP | This study |
| S1 | ura3 Zrfsy1Δ::loxP | 17 |
| F1F2S1 | ura3 Zrffz1Δ::loxP Zrffz2Δ::loxP Zrfsy1Δ::loxP | This study |
| F1F2x | ura3 Zrffz1Δ::loxP Zrffz2Δ::loxP | This study |
| F1F2(pZEU) | F1F2 + pZEU; Zrffz1Δ::loxP Zrffz2Δ::loxP | This study |
| F1F2(pZEU_ZrFFZ1) | F1F2 + pZEU_ZrFFZ1; Zrffz2Δ::loxP | This study |
| F1F2(pZCA_ZrFFZ1) | F1F2 + pZCA_ZrFFZ1; Zrffz2Δ::loxP | This study |
| F1F2(pZEU_ZrFFZ2) | F1F2 + pZEU_ZrFFZ2; Zrffz1Δ::loxP | This study |
| F1F2(pZGFP_ZrFFZ2) | F1F2 + pZGFP_ZrFFZ2; Zrffz1Δ::loxP | This study |
| F1F2(pZCA_ZrFFZ2) | F1F2 + pZCA_ZrFFZ2; Zrffz1Δ::loxP | This study |
Yeast transformations and construction of Z. rouxii deletion strains.
Z. rouxii and S. cerevisiae cells were transformed by electroporation, as described in reference 18. The deletion of the Z. rouxii ZrFFZ1 (ZYRO0E10054g; GenBank accession no. XM_002499318, GeneID 8204919) and ZrFFZ2 (ZYRO0F02090g; GenBank accession no. XM_002497242, GeneID 8205043) genes in strain UL4 (a ura3 auxotrophic strain derived from CBS 732T) (19) was performed with PCR-amplified loxP-kanMX-loxP deletion cassettes (20). The primers (obtained from Sigma-Aldrich) used for the cassette amplifications are listed in Table S1 in the supplemental material (ZrFFZ1-Kan-F and ZrFFZ1-Kan-R for ZrFFZ1; ZrFFZ2-Kan-F2 and ZrFFZ2-Kan-R2 for ZrFFZ2), and the pUG6 plasmid (21) was used as a template. The pZCRE plasmid expressing the Cre recombinase was used to remove the integrated kanMX marker (20).
The replacement of the original genes with the loxP sequence was confirmed by diagnostic PCR with the following combinations of primers (see Table S1 in the supplemental material): (i) for deletion of ZrFFZ1, ZrFFZ1-361up-F/KANX-R1, KANX-F1/ZrFFZ1-456d-R2, and ZrFFZ1-361up-F/ZrFFZ1-456d-R2; (ii) for deletion of ZrFFZ2, ZrFFZ2-521up-F2/KANX-R1, KANX-F1/ZrFFZ2-492d-R2, and ZrFFZ2-521up-F2/ZrFFZ2-492d-R2. The generated Z. rouxii deletion strains are listed in Table 1. Strain F1F2 was obtained by deletion of the ZrFFZ1 gene in strain F2 (Zrffz2Δ). Strain F1F2x was obtained by deletion of the ZrFFZ2 gene in strain F1 (Zrffz1Δ). Deletion of the ZrFSY1 gene, as described in reference 17, was performed in strain F1F2, producing the triple-deletion mutant F1F2S1.
Growth assays.
Yeast cells were grown at 30°C, and growth was monitored either in drop tests in solid media or in liquid media using an ELx808 Absorbance Microplate Reader (BioTek Instruments, Winooski, VT, USA) as described in reference 22 or by measuring optical densities at 640 nm (OD640) in an Ultrospec 2100 pro (Amersham Biosciences) spectrophotometer.
DNA manipulations.
DNA manipulations were performed according to standard protocols (23). Genomic DNA and plasmid DNA from yeast cells were isolated as described in reference 24. The high-fidelity DNA polymerase Phusion F-530 (Finnzymes) was used to avoid mismatch base pairing during the synthesis of PCR products. Plasmid DNA from E. coli was isolated using the GenElute Plasmid Miniprep Kit (Sigma-Aldrich). Restriction enzymes were purchased from Roche. Sequencing was performed at Stab Vida (Caparica, Portugal).
Plasmid and Z. rouxii strain construction.
The plasmids used for cloning were pZEU, pZGFP (20), and pZCA (18).
Plasmids containing the ZrFFZ1 and ZrFFZ2 genes with the respective promoters (828 and 869 bp long, respectively) were constructed by homologous recombination (25) in S. cerevisiae BW31a (26). ZrFFZ2 (amplified by PCR with primers ZrFFZ2-ZEU-F and ZrFFZ2-ZEU-R) was inserted into the plasmid pZEU (previously digested with XbaI), resulting in the plasmid pZEU_ZrFFZ2. The same gene was also inserted into the plasmids pZGFP (previously digested with XbaI) and pZCA (previously digested with XbaI) (see Table S2 in the supplemental material). The same procedure was used to clone ZrFFZ1 genes into plasmids pZEU and pZCA (see Table S2 in the supplemental material). The primers used are listed in Table S1 in the supplemental material. All the constructed plasmids and the control plasmid pZEU were used to transform the Z. rouxii deletion strain F1F2.
Fluorescence microscopy.
Cells grown to exponential phase were spotted onto microscope slides and observed with an Olympus AX70 fluorescence microscope. To visualize green fluorescent protein (GFP), a U-MWB fluorescence cube (Olympus) was used with a 450- to 480-nm excitation filter and a 515-nm barrier filter.
Sugar transport assays.
Initial [U-14C]fructose and [U-14C]glucose (GE Healthcare [formerly Amersham Biosciences]) uptake rates were performed as previously described (13). Kinetic parameters were estimated using GraphPad Prism version 5.00 (Graphpad Software) for Michaelis-Menten regression analysis. The existence of H+ movements associated with initial fructose uptake was assessed with a pH meter upon addition of fructose pulses to unbuffered cell suspensions, as described in reference 17. The dry weight was determined (in triplicate) by placing 100 μl of cell suspensions into preweighed aluminum foil cups and drying in a 70°C oven for 24 h.
Sugar consumption assays.
Strains were pregrown overnight in YNB plus 10 g liter−1 fructose plus 10 g liter−1 glucose (with supplements when necessary) or in YP (1% yeast extract, 2% peptone) plus 100 g liter−1 fructose plus 100 g liter−1 glucose, transferred to the same medium to an initial OD640 of 0.1 (in Erlenmeyer flasks with a medium/flask volume ratio of 1:5), and incubated at 25°C and 180 rpm. Growth was followed by measuring optical densities at 640 nm in an Ultrospec 2100 pro spectrophotometer (Amersham Biosciences). To monitor the evolution of sugar consumption, after removing the cells by filtration with a 0.2-μm cellulose acetate filter (Advantec), glucose and fructose concentrations in supernatants, collected at several time points, were measured by enzymatic assays (kit no. 10 139 106 035; Boehringer Mannheim/Roche/r-biopharm).
Reproducibility of results.
All experiments were performed at least three times. Mean values and corresponding standard deviations or results of typical experiments are presented, as indicated.
RESULTS
Construction and characterization of Z. rouxii FFZ deletion mutants.
We recently characterized the fructose-specific facilitator ZrFfz1, the fructose/glucose facilitator ZrFfz2 (13), and the fructose/H+ symporter ZrFsy1 (17) from Z. rouxii CBS 732T by heterologous expression in an S. cerevisiae strain lacking its own hexose transporters (hxt null). Z. rouxii strain UL4 is a ura3 auxotrophic strain derived from CBS 732T (19). Strain UL4 was used as background for deletion of the genes encoding these three Z. rouxii fructose transporters. The constructed deletion mutants, F1 (Zrffz1Δ), F2 (Zrffz2Δ), F1F2 (Zrffz1Δ Zrffz2Δ), F1F2x (Zrffz1Δ Zrffz2Δ), and F1F2S1 (Zrffz1Δ Zrffz2Δ Zrfsy1Δ), were confirmed by PCR. Strain F1F2 was obtained by deletion of the ZrFFZ1 gene in strain F2 (Zrffz2Δ). We also constructed strain F1F2x by deleting the ZrFFZ2 gene in strain F1 (Zrffz1Δ). An extremely low targeting efficiency (sometimes as low as 0.5%) was observed, as previously reported for Z. rouxii UL4 (20).
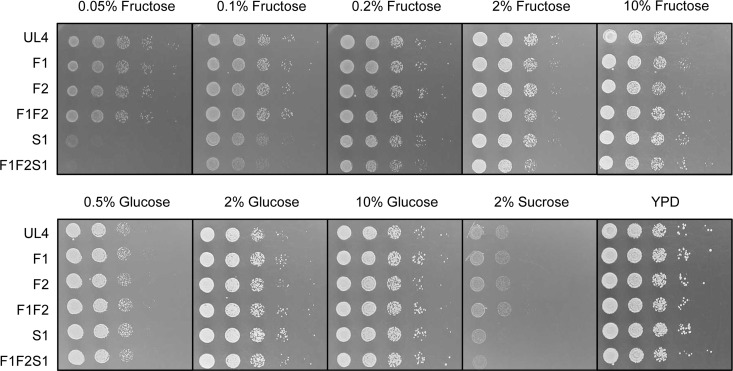
The growth phenotype of single-, double-, and triple-deletion mutants was tested on solid (Fig. 1) and in liquid (Fig. 2) YNB media supplemented with fructose (0.05% [wt/vol] to 20% [wt/vol]), glucose (0.1% [wt/vol] to 20% [wt/vol]), or sucrose (0.5% [wt/vol] and 2% [wt/vol]).
FIG 1.
Growth of Z. rouxii strains UL4, F1 (Zrffz1Δ), F2 (Zrffz2Δ), F1F2 (Zrffz1Δ Zrffz2Δ), S1 (Zrfsy1Δ), and F1F2S1 (Zrffz1Δ Zrffz2Δ Zrfsy1Δ) on YPD and YNB media with different carbon sources after 3 days (fructose and glucose media) or 8 days (sucrose medium) of incubation at 30°C. The results from one of two independent experiments are shown.
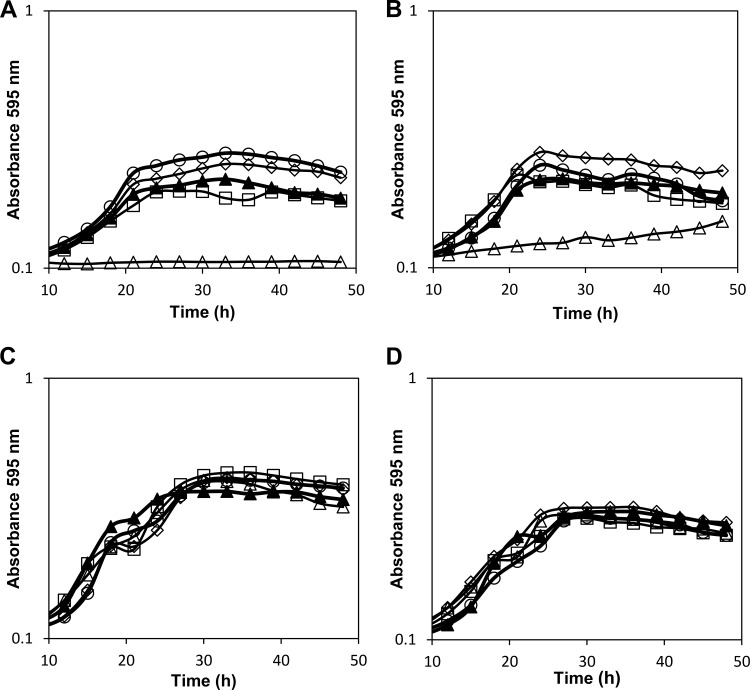
FIG 2.
Growth curves (in microplates) of Z. rouxii strains UL4 (▲), F1 (Zrffz1Δ) (◇), F2 (Zrffz2Δ) (□), F1F2 (Zrffz1Δ Zrffz2Δ) (○), and F1F2S1 (Zrffz1Δ Zrffz2Δ Zrfsy1Δ) (△) in YNB medium supplemented with 0.05% (A), 0.1% (B), and 2% (C) fructose or 2% glucose (D). The data are representative of at least two independent experiments.
Strains F1F2 (Fig. 1 and 2) and F1F2x (data not shown), both lacking the facilitators ZrFfz1 and ZrFfz2, displayed the same growth phenotype. For the single-, the double-, or even the triple-deletion mutants, there was not a visible difference in growth on solid (Fig. 1 and data not shown) or liquid (Fig. 2 and data not shown) medium for fructose concentrations above 0.2% or for any of the tested glucose concentrations. This means that under these growth conditions, other hexose transporters must be efficiently operating in Z. rouxii, compensating for the deletion of the three fructose transporters characterized so far. At fructose concentrations below 0.2%, the effect observed is probably due to the deletion of the fructose/H+ symporter ZrFsy1, which appears to be the main active fructose transporter at these extremely low concentrations, as previously observed for strain S1 (Zrfsy1Δ) (17).
Nevertheless, kinetic characterization of fructose and glucose transport in the deletion mutants presented some differences from strains CBS 732T and UL4 (Table 2). Z. rouxii CBS 732T presented a higher transport capacity for fructose than for glucose, in agreement with its fructophilic behavior. The ura3 auxotrophic strain UL4 (derived from CBS 732T) showed lower transport activities (a reduction of 36% for fructose and 48% for glucose Vmax values) than the Z. rouxii CBS 732T strain (Table 2; see Fig. S1 in the supplemental material). The differences between the two strains were also visible in the respective growth patterns on fructose or glucose, as Z. rouxii CBS 732T presented a shorter lag phase than UL4, slightly higher growth rates (0.114 ± 0.002 h−1 versus 0.096 ± 0.004 h−1 in 2% fructose and 0.093 ± 0.001 h−1 versus 0.085 ± 0.003 h−1 in 2% glucose media) and higher final biomass (see Fig. S2 in the supplemental material).
TABLE 2.
Kinetic parameters of fructose and glucose transport in Z. rouxii strains grown in 2% glucose
| Strain | Fructose transportb |
Glucose transportb |
||
|---|---|---|---|---|
| Km (mM) | Vmax (mmol g−1 h−1) | Km (mM) | Vmax (mmol g−1 h−1) | |
| CBS 732Ta | 284.5 ± 62.8 | 17.48 ± 2.45 | 14.28 ± 1.94 | 1.72 ± 0.04 |
| UL4 | 239.2 ± 97.0 | 11.11 ± 2.45 | 19.76 ± 1.28 | 0.89 ± 0.02 |
| F1 (Zrffz1Δ) | 138.5 ± 31.7 | 2.95 ± 0.30 | 25.88 ± 2.72 | 1.34 ± 0.06 |
| F2 (Zrffz2Δ) | 339.6 ± 101.9 | 10.79 ± 1.94 | 13.28 ± 1.90 | 0.96 ± 0.04 |
| F1F2 (Zrffz1Δ Zrffz2Δ) | 97.30 ± 34.71 | 2.71 ± 0.45 | 12.10 ± 6.54 | 1.27 ± 0.16 |
Reference 11.
The values result from GraphPad Prism Michaelis-Menten regression analysis of data from at least two independent experiments. Vmax values were calculated for biomass expressed as dry weight.
When ZrFFZ1 was deleted in Z. rouxii strain UL4, there was a strong reduction in fructose transport capacity (Vmax fructose was reduced 73%), whereas deletion of ZrFFZ2 had almost no effect (Vmax fructose was reduced only 3%). The double-deletion strain F1F2 exhibited fructose transport kinetic parameters very similar to those of strain F1 (Zrffz1Δ) (Table 2; see Fig. S1 in the supplemental material), which further supports the fact that ZrFfz1 seems to be the main transporter system responsible for the high fructose transport capacity of Z. rouxii, as previously suggested by the kinetic parameters of the individual transporters ZrFfz1 and ZrFfz2 when expressed in a hxt-null S. cerevisiae strain (Vmax fructose ZrFfz1, 12.7 ± 3.3 mmol h−1 g−1 versus Vmax fructose ZrFfz2, 4.51 ± 0.56 mmol h−1 g−1) (13).
Deletion of the specific fructose facilitator ZrFFZ1 also reduced Km values (Table 2), increasing the overall affinity for fructose, in agreement with the very low affinity of the transporter (Kmfructose ZrFfz1, 424.2 ± 163.1 mM, when expressed in a hxt-null S. cerevisiae strain).
Regarding glucose transport, when the specific fructose facilitator ZrFFZ1 was deleted, there was an increase (about 51%) in the glucose transport capacity (Table 2; see Fig. S1 in the supplemental material, strains F1 and F1F2), reaching a glucose transport capacity closer to that of the CBS 732T strain. Deletion of the high-capacity, low-affinity fructose/glucose transporter ZrFFZ2 did not significantly affect the glucose transport capacity (Vmax increased only 8%) and slightly increased the overall affinity for glucose.
Fructophilic behavior of constructed strains.
The Z. rouxii genes ZrFFZ1 and ZrFFZ2 were cloned into the Z. rouxii plasmids pZEU (multicopy), pZGFP (multicopy with C-terminal GFP tagging; only for ZrFFZ2), and pZCA (centromeric) by homologous recombination in S. cerevisiae BW31a and by plasmid rescue in E. coli XL-Blue. The constructed plasmids were confirmed by restriction analysis, PCR, and sequencing. Cloning of the constructed plasmids into the Z. rouxii deletion strain F1F2 produced the strains listed in Table 1.
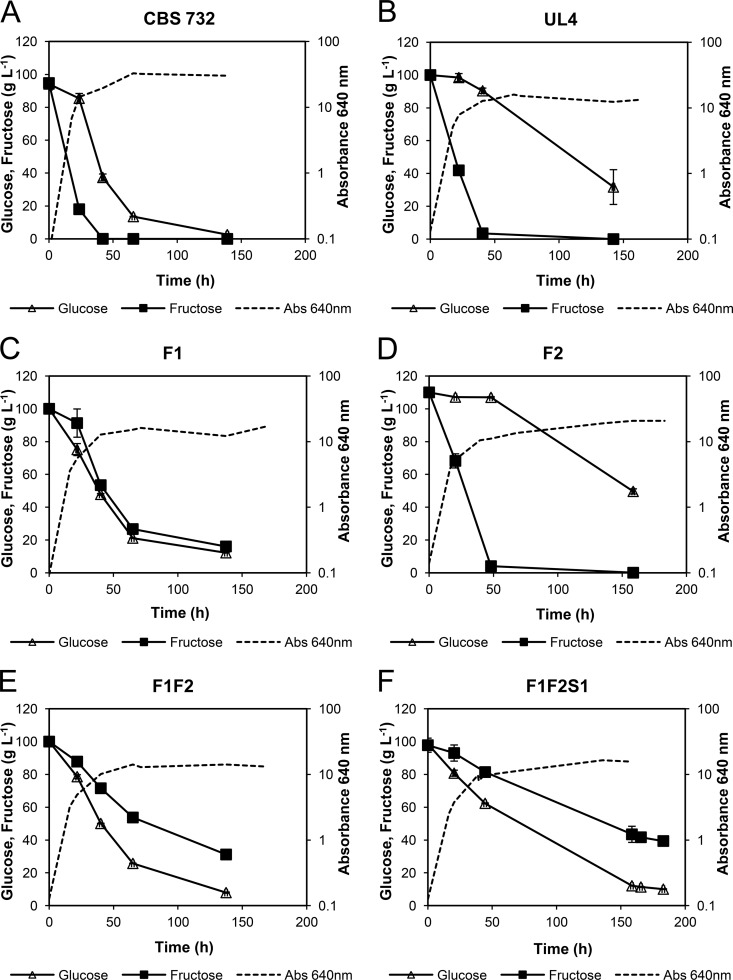
Z. rouxii fructophilic behavior is characterized by a preference for consumption of fructose over glucose, more pronounced at high sugar concentrations. To correlate this behavior with the presence of the peculiar Ffz transporters, we analyzed sugar consumption in minimal and in rich media containing equimolar concentrations of glucose and fructose. Initially, we used minimal synthetic medium in order to define and control its sugar content (10 g liter−1 fructose plus 10 g liter−1 glucose) and then repeated the experiments in YP medium with high sugar concentrations (100 g liter−1 fructose plus 100 g liter−1 glucose). For these high sugar concentrations, we used YP instead of YNB medium to prevent the lack of some other nutrient possibly affecting sugar utilization. The results obtained for strains CBS 732T and UL4 (ura3) (Fig. 3; see Fig. S3 in the supplemental material) show that in both media, fructose was consumed faster than glucose, with glucose consumption being much slower in strain UL4, especially in medium with a low sugar concentration. The phenotypic differences between strains CBS 732T and UL4 (high transport capacity and higher growth rates of the former) are further evidenced by sugar consumption profiles (Fig. 3; see Fig. S3 in the supplemental material), as UL4 left about 80% residual glucose, even after 140 h of incubation, in medium with 10 g liter−1 fructose plus 10 g liter−1 glucose and about 30% residual glucose in medium with 100 g liter−1 fructose plus 100 g liter−1 glucose, whereas strain CBS 732T was able to consume almost all the glucose available in both media (Fig. 3; see Fig. S3 in the supplemental material).
FIG 3.
Fructose and glucose consumption profiles of Z. rouxii strains CBS 732T (A), UL4 (B), F1 (Zrffz1Δ) (C), F2 (Zrffz2Δ) (D), F1F2 (Zrffz1Δ Zrffz2Δ) (E), and F1F2S1 (Zrffz1Δ Zrffz2Δ Zrfsy1Δ) (F) grown in YP plus 100 g liter−1 fructose plus 100 g liter−1 glucose. The data are representative of at least two independent experiments, and the error bars indicate standard deviations (SD).
When the ZrFFZ1 gene (strain F1) was deleted, both sugars started to be consumed at the same rate, and the characteristic Z. rouxii fructophilic character was lost. Deletion of ZrFFZ2 (a fructose/glucose facilitator) did not affect the fructophilic behavior of Z. rouxii (Fig. 3; see Fig. S3 in the supplemental material). The double-deletion mutant F1F2 (as well as F1F2x [data not shown]) and the triple-deletion mutant F1F2S1 presented clear glucophilic behavior, consuming glucose faster than fructose (Fig. 3; see Fig. S3 in the supplemental material). These results reinforce the hypothesis that ZrFFZ1 is the main factor responsible for Z. rouxii fructophilic behavior.
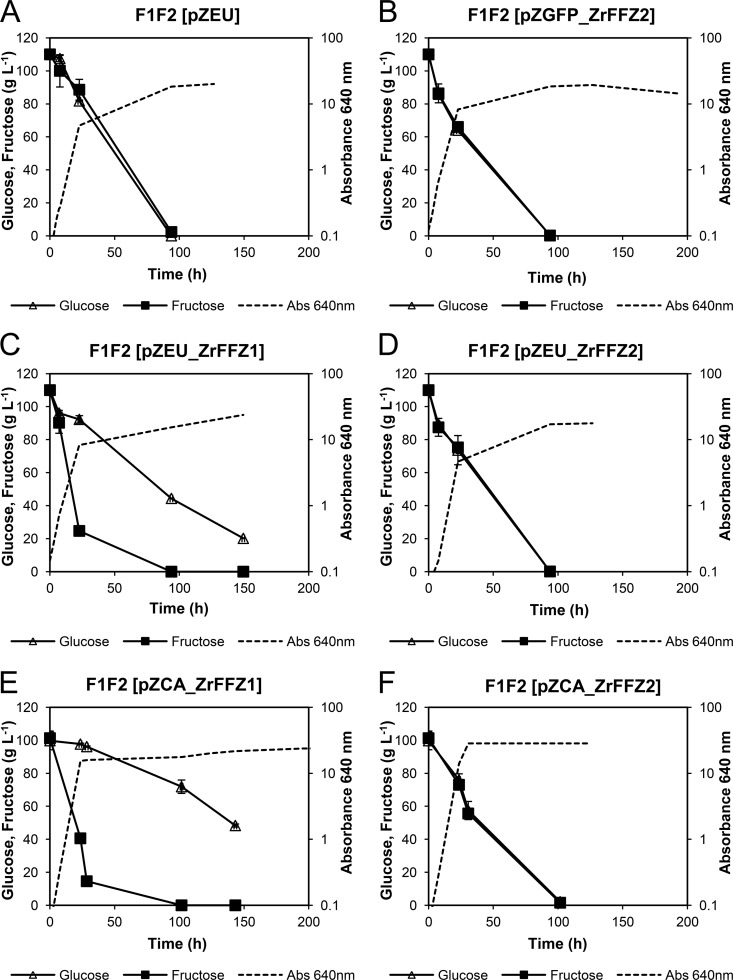
The ZrFFZ1 and ZrFFZ2 genes were individually cloned back into the double mutant F1F2 (Zrffz1Δ Zrffz2Δ). The resulting strains, expressing ZrFFZ1 from a multicopy (pZEU) or centromeric (pZCA) plasmid, are equivalent to strain F2 (Zrffz2Δ), lacking only the fructose/glucose facilitator ZrFfz2, and both strains recovered the original Z. rouxii fructophilic behavior at both high (Fig. 4) and low (see Fig. S4 in the supplemental material) sugar concentrations.
FIG 4.
Fructose and glucose consumption profiles of Z. rouxii strains F1F2(pZEU) (A), F1F2(pZGFP_ZrFFZ2) (B), F1F2(pZEU_ZrFFZ1) (C), F1F2(pZEU_ZrFFZ2) (D), F1F2(pZCA_ZrFFZ1) (E), and F1F2(pZCA_ZrFFZ2) (F) grown in YP plus 100 g liter−1 fructose plus 100 g liter−1 glucose. The data are representative of at least two independent experiments, and the error bars represent SD.
On the other hand, when the gene ZrFFZ2 expressed from a multicopy (with or without GFP) or centromeric plasmid was cloned back into the double mutant F1F2, generating strains equivalent to strain F1 (Zrffz1Δ), lacking only the high-capacity fructose facilitator ZrFfz1, both sugars were consumed simultaneously (Fig. 4; see Fig. S4 in the supplemental material) and faster than in the F1F2(pZEU) strain.
These results strengthen the direct involvement of ZrFFZ1 in Z. rouxii CBS 732T fructophilic behavior.
DISCUSSION
In wine fermentations, grape must usually contains similar amounts of glucose and fructose that are cofermented to ethanol and carbon dioxide. Due to climate changes, sugar levels in grapes have been increasing, which leads to wines with high alcohol content, lower acidity, and compromised flavor profiles (27). Due to the predilection of S. cerevisiae Hxt transporters for glucose, this sugar is consumed faster, producing a discrepancy between the amounts of glucose and fructose consumed during fermentation, and consequently, the concentration of residual fructose is higher than that of glucose at the end of fermentation. As fructose is almost twice as sweet as glucose, this has a strong effect on the final sweetness of wine. The discrepancy between the utilization of glucose and that of fructose depends on the yeast strain used and on the external conditions, such as nitrogen and ethanol levels (28). During fermentation of grape must, arrest of yeast growth generally occurs when the medium still contains high sugar concentrations, especially the nonpreferred sugar fructose, and may lead to sluggish or stuck (i.e., very delayed or incomplete) fermentations. The fermentation rate decreases due to loss of hexose transport capacity, and the factors related to stuck fermentations also regulate transporter expression, turnover, and function (29). The inhibition of fermentative metabolism that occurs under these conditions could be due to several mechanisms, such as sugar transport inefficiency, intracellular accumulation of ethanol, and/or toxicity of some yeast fermentation by-products. In nitrogen-limited stuck fermentations, glucose transport inactivation seems to play an important role (30).
In S. cerevisiae, ethanol seems to increase the glucophilic character, since addition of ethanol has a stronger inhibitory effect on fructose utilization than on glucose utilization, whereas addition of assimilable nitrogen has the opposite effect, stimulating fructose utilization more than glucose utilization (28). In the glucophilic yeast S. cerevisiae, glucose not only is an energy and carbon source, it also has an important role as a global regulator of metabolism and growth. In the fructophilic yeasts Z. bailii and Z. rouxii, this critical regulatory role seems to be played by fructose, as fructose regulates and inactivates the glucose transporter system, especially at high fructose concentrations, since glucose transport is completely abolished subsequent to addition of 10% fructose to 2% glucose-grown cells after 4 h of incubation in Z. bailii (10) and in Z. rouxii (11).
The growth phenotype in fructose (concentrations above 0.2% [wt/vol]) or in glucose medium of the constructed Z. rouxii triple-deletion mutant (lacking the facilitators ZrFfz1 and ZrFfz2 and the symporter ZrFsy1) was not affected, indicating that other hexose transporters were still efficiently working in this strain, enabling growth on those carbon sources. A BLASTP analysis of the Z. rouxii CBS 732T genome against known sugar transporters identified four putative hexose transporters similar to S. cerevisiae Hxt proteins and one putative transporter similar to the Kluyveromyces lactis glucose/fructose/galactose transporter Hgt1 (17, 31).
When the gene encoding the specific fructose facilitator ZrFfz1 was deleted in Z. rouxii, fructose transport capacity was drastically reduced, in agreement with the very high fructose transport capacity of the transporter, while an increase in glucose transport capacity was observed (Vmax glucose increased about 51% in strain F1 compared to strain UL4 [Table 2]). This increase may result from derepression of glucose transport, since fructose influx is drastically reduced when ZrFFZ1 is deleted. This effect is even more obvious in sugar consumption profiles of strain F1F2, lacking both Ffz facilitators, which has a lower fructose influx than strain F1 and, consequently, a reduced putative repressing effect by fructose, displaying higher glucose consumption (Fig. 3; see Fig. S3 in the supplemental material). These results are indicative of a regulatory role of fructose in Z. rouxii.
Since it was reported that the high capacity of fructose transport is directly linked to the fructophilic character of Z. bailii (10) and of Z. rouxii (11) and our results demonstrate that ZrFfz1 is responsible for the Z. rouxii fructophilic behavior, we advance the hypothesis that the presence of Ffz-like transporters can be an indicator of fructophily in other yeast species.
In C. magnoliae, also a fructophilic yeast, glucose is not consumed in the presence of fructose (32), and a comparative proteomic analysis of fructose-grown cells with glucose-grown cells revealed overexpression of proteins involved in stress response, in carbon metabolism, and in sugar uptake in fructose-grown cells, which further supports the crucial role of fructose uptake in the fructophilic behavior (33). This yeast also has a functional Ffz1 fructose transporter (CmFfz1) (14).
The fact that a wine strain with an altered Hxt3 hexose transporter has an enhanced fructose fermentation capacity, due to changes in fructose uptake (34), also supports the fundamental role of fructose uptake in fructophilic behavior.
To our knowledge, this is the first report that clearly demonstrates for a Zygosaccharomyces strain a striking change from an unambiguous fructophilic behavior to a glucophilic behavior due only to the deletion of one high-capacity specific fructose transporter gene, with complete reversal to the initial behavior when the gene is cloned back. This study confirms the molecular basis of the Z. rouxii fructophilic character, demonstrating the key role of ZrFfz1 in determining the fructose/glucose utilization preference during Z. rouxii fermentations.
Expression of this fructose transporter in industrial S. cerevisiae strains might improve the fructose utilization and fermentation performance of wine strains, accelerating the consumption of all sugars available and consequently reducing the risks of occurrence of stuck fermentations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Fundação para a Ciência e Tecnologia, Portugal (postdoctoral fellowships SFRH/BPD/41812/2007 to M.J.L. and SFRH/BPD/85143/2012 to C.P., FCT Ciência 2007, and Research Project “Improvement of fructose fermentation by industrial Saccharomyces cerevisiae strains” [PTDC/AGR-ALI/112802/2009]) and Czech GACR P503/10/0307 and RVO 6798582 grants.
Footnotes
Published ahead of print 29 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00137-14.
REFERENCES
- 1.Martorell P, Stratford M, Steels H, Fernández-Espinar MT, Querol A. 2007. Physiological characterization of spoilage strains of Zygosaccharomyces bailii and Zygosaccharomyces rouxii isolated from high sugar environments. Int. J. Food Microbiol. 114:234–242. 10.1016/j.ijfoodmicro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Dakal TC, Solieri L, Giudici P. 2014. Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 185:140–157. 10.1016/j.ijfoodmicro.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Deák T. 2007. Yeasts in specific types of foods, p 117–201 In Deák T. (ed), Handbook of food spoilage yeasts, 2nd ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 4.Solieri L, Giudici P. 2008. Yeasts associated to traditional balsamic vinegar: ecological and technological features. Int. J. Food Microbiol. 125:36–45. 10.1016/j.ijfoodmicro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Emmerich W, Radler F. 1983. The anaerobic metabolism of glucose and fructose by Saccharomyces bailii. Microbiology 129:3311–3318. 10.1099/00221287-129-11-3311. [DOI] [Google Scholar]
- 6.Sols A. 1956. Selective fermentation and phosphorylation of sugars by sauternes yeast. Biochim. Biophys. Acta 20:62–68. 10.1016/0006-3002(56)90263-3. [DOI] [PubMed] [Google Scholar]
- 7.Eddy AA, Barnett JA. 2007. A history of research on yeasts. 11. The study of solute transport: the first 90 years, simple and facilitated diffusion. Yeast 24:1023–1059. 10.1002/yea.1572. [DOI] [PubMed] [Google Scholar]
- 8.Saier MH., Jr 2000. Families of transmembrane sugar transport proteins. Mol. Microbiol. 35:699–710. 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 9.Leandro MJ, Fonseca C, Gonçalves P. 2009. Hexose and pentose transport in ascomycetous yeasts: an overview. FEMS Yeast Res. 9:511–525. 10.1111/j.1567-1364.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- 10.Sousa-Dias S, Gonçalves T, Leyva JS, Peinado JM, Loureiro-Dias MC. 1996. Kinetics and regulation of fructose and glucose transport systems are responsible for fructophily in Zygosaccharomyces bailii. Microbiology 142:1733–1738. 10.1099/13500872-142-7-1733. [DOI] [Google Scholar]
- 11.Sousa-Dias S. 2000. Ph.D. thesis Technical University of Lisbon, Lisbon, Portugal. [Google Scholar]
- 12.Pina C, Gonçalves P, Prista C, Loureiro-Dias MC. 2004. Ffz1, a new transporter specific for fructose from Zygosaccharomyces bailii. Microbiology 150:2429–2433. 10.1099/mic.0.26979-0. [DOI] [PubMed] [Google Scholar]
- 13.Leandro MJ, Sychrová H, Prista C, Loureiro-Dias MC. 2011. The osmotolerant fructophilic yeast Zygosaccharomyces rouxii employs two plasma-membrane fructose uptake systems belonging to a new family of yeast sugar transporters. Microbiology 157:601–608. 10.1099/mic.0.044446-0. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Kim SJ, Seo JH. 2014. Molecular cloning and characterization of two novel fructose-specific transporters from the osmotolerant and fructophilic yeast Candida magnoliae JH110. Appl. Microbiol. Biotechnol. 98:3569–3578. 10.1007/s00253-013-5225-y. [DOI] [PubMed] [Google Scholar]
- 15.Mira NP, Münsterkötter M, Dias-Valada F, Santos J, Palma M, Roque FC, Guerreiro JF, Rodrigues F, Sousa MJ, Leão C, Guldener U, Sá-Correia I. 2014. The genome sequence of the highly acetic acid-tolerant Zygosaccharomyces bailii-derived interspecies hybrid strain ISA1307, isolated from a sparkling wine plant. DNA Res. 21:299–313. 10.1093/dnares/dst058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galeote V, Bigey F, Devillers H, Neuveglise C, Dequin S. 2013. Genome sequence of the food spoilage yeast Zygosaccharomyces bailii CLIB 213T. Genome Announc. 1:e00606–13. 10.1128/genomeA.00606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leandro MJ, Sychrová H, Prista C, Loureiro-Dias MC. 2013. ZrFsy1, a high-affinity fructose/H+ symporter from fructophilic yeast Zygosaccharomyces rouxii. PLoS One 8:e68165. 10.1371/journal.pone.0068165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pribylová L, Straub ML, Sychrová H, de Montigny J. 2007. Characterisation of Zygosaccharomyces rouxii centromeres and construction of first Z. rouxii centromeric vectors. Chromosome Res. 15:439–445. 10.1007/s10577-007-1136-z. [DOI] [PubMed] [Google Scholar]
- 19.Pribylová L, Sychrová H. 2003. Efficient transformation of the osmotolerant yeast Zygosaccharomyces rouxii by electroporation. J. Microbiol. Methods 55:481–484. 10.1016/S0167-7012(03)00197-0. [DOI] [PubMed] [Google Scholar]
- 20.Pribylová L, de Montigny J, Sychrová H. 2007. Tools for the genetic manipulation of Zygosaccharomyces rouxii. FEMS Yeast Res. 7:1285–1294. 10.1111/j.1567-1364.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 21.Guldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519–2524. 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marešová L, Sychrová H. 2007. Applications of a microplate reader in yeast physiology research. Biotechniques 43:667–672. 10.2144/000112620. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 24.Hoffman CS, Winston F. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267–272. 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 25.Zaragoza O. 2003. Generation of disruption cassettes in vivo using a PCR product and Saccharomyces cerevisiae. J. Microbiol. Methods 52:141–145. 10.1016/S0167-7012(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 26.Kinclová-Zimmermannová O, Zavrel M, Sychrová H. 2005. Identification of conserved prolyl residue important for transport activity and the substrate specificity range of yeast plasma membrane Na+/H+ antiporters. J. Biol. Chem. 280:30638–30647. 10.1074/jbc.M506341200. [DOI] [PubMed] [Google Scholar]
- 27.Jones GV, White MA, Cooper OR, Storchmann K. 2005. Climate change and global wine quality. Climatic Change 73:319–343. 10.1007/s10584-005-4704-2. [DOI] [Google Scholar]
- 28.Berthels NJ, Cordero Otero RR, Bauer FF, Thevelein JM, Pretorius IS. 2004. Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 4:683–689. 10.1016/j.femsyr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Bisson LF. 1999. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 50:107–119. [Google Scholar]
- 30.Salmon JM. 1989. Effect of sugar transport inactivation in Saccharomyces cerevisiae on sluggish and stuck enological fermentations. Appl. Environ. Microbiol. 55:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palma M, Seret ML, Baret PV. 2009. Combined phylogenetic and neighbourhood analysis of the hexose transporters and glucose sensors in yeasts. FEMS Yeast Res. 9:526–534. 10.1111/j.1567-1364.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 32.Yu JH, Lee DH, Oh YJ, Han KC, Ryu YW, Seo JH. 2006. Selective utilization of fructose to glucose by Candida magnoliae, an erythritol producer. Appl. Biochem. Biotechnol. 131:870–879. 10.1385/ABAB:131:1:870. [DOI] [PubMed] [Google Scholar]
- 33.Yu JH, Lee DH, Park YC, Lee MG, Kim DO, Ryu YW, Seo JH. 2008. Proteomic analysis of fructophilic properties of osmotolerant Candida magnoliae. J. Microbiol. Biotechnol. 18:248–254. [PubMed] [Google Scholar]
- 34.Guillaume C, Delobel P, Sablayrolles JM, Blondin B. 2007. Molecular basis of fructose utilization by the wine yeast Saccharomyces cerevisiae: a mutated HXT3 allele enhances fructose fermentation. Appl. Environ. Microbiol. 73:2432–2439. 10.1128/AEM.02269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.