Abstract
Poly(A)-binding protein-interacting protein 1 (Paip1) stimulates translational initiation by inducing the circularization of mRNA. However, the mechanisms underlying Paip1 regulation, particularly its protein stability, are still unclear. Here, we show that the E6AP carboxyl terminus (HECT)-type ubiquitin ligase WW domain-containing protein 2 (WWP2), a homolog of the HECT-type ubiquitin ligase WWP1, interacts with and targets Paip1 for ubiquitination and proteasomal degradation. Mapping of the region including the WW domain of WWP2 revealed the interaction between WWP2 and the PABP-binding motif 2 (PAM2) of Paip1. The two consecutive PXXY motifs in PAM2 are required for WWP2-mediated ubiquitination and degradation. Furthermore, ectopic expression of WWP2 decreases translational stimulatory activity with the degradation of Paip1. We therefore provide evidence that the stability of Paip1 can be regulated by ubiquitin-mediated degradation, thus highlighting the importance of WWP2 as a suppressor of translation.
INTRODUCTION
Regulation of gene expression occurs in eukaryotes during mRNA translation, specifically at the initiation of translation. Deregulation at this step of the translation process leads to abnormal gene expression, which in turn alters cell growth and possibly leads to cancer development (1–3). Translational initiation comprises a series of discrete steps and starts with the dissociation of 80S ribosomes into subunits. The eukaryotic translational initiation factors 1 (eIF1), 1A, and 5 and the eIF3 complex promote the binding of the eIF2-GTP-Met-initiator tRNA (tRNAi) ternary complex to the 40S subunit, thereby forming a 43S preinitiation complex (PIC) (4–7). The 43S PIC is loaded onto the mRNA near the 5′-7-methylguanosine cap by numerous factors, including eIF3, poly(A)-binding protein (PABP), eIF4B, and the eIF4F complex. The eIF4F complex comprises three subunits, namely, a cap-binding protein (eIF4E), an RNA helicase (eIF4A), and a scaffold protein (eIF4G) (8, 9). eIF4G harbors the binding domains for PABP, eIF4E, eIF4A, and eIF3 in mammals. The binding domains for eIF4E and PABP in eIF4G enable the assembly of a stable, circular messenger ribonucleoprotein (mRNP) by eIF4G, and eIF4G-eIF3 interaction generates a protein bridge between the mRNPs (10–13).
PABP-interacting protein 1 (Paip1) is a PABP-binding protein that contains two distinct PABP-binding motifs (PAMs). PAM1 binds to RNA recognition motif 2 in the N terminus of PABP; PAM2, which is a conserved region comprising approximately 15 amino acids, binds to the PABC domain of PABP (14, 15). Paip1 shows 39% similarity to eIF4G and the eIF4G-related protein p97/DAP5/NAT1 (16–18). A specific portion that is present in both Paip1 and eIF4G has one of two known eIF4A binding regions and an eIF3 binding site (19, 20). The above-mentioned findings indicate that Paip1 coimmunoprecipitates with eIF4A and eIF3 (21, 22). The presence of Paip1 in animal cells may indicate the involvement of a mechanism that links PABP to eIF4A, thereby causing the circularization of mRNA (21). Data from previous studies suggest that the interaction of Paip1 with eIF3 stabilizes the circular mRNP conformation, which is formed by eIF4G-PABP interaction (22). eIF3 is reportedly phosphorylated by S6K1/2, which stimulates the Paip1-eIF3 interaction and the initiation of translation (23). Paip1 is an important positive effector of translational initiation, but information on the mechanism underlying Paip1 regulation is lacking.
In the present study, we demonstrate the critical function of an E6AP carboxyl terminus (HECT) domain comprising an E3 ubiquitin ligase WW domain-containing protein 2 (WWP2), which is also known as atrophin 1-interacting protein 2 (AIP2), in the regulation of Paip1 protein stability. WWP2 is homologous to the HECT domain-type ubiquitin-protein ligase and participates in the regulation of craniofacial development and chondrogenesis (24, 25). WWP2 also participates in the maintenance of key oncogenic signaling pathways that are linked to cancer cell growth and survival and tumor spread (26, 27). Here, we show that WWP2 interacts with the PAM2 motif of Paip1 via the WW domain. Further investigation revealed that WWP2 targeted Paip1 for ubiquitination and degradation via the PEFYPSGY sequence in the PAM2 motif. Importantly, WWP2 was found to participate in translational initiation by regulating the Paip1 protein level.
MATERIALS AND METHODS
Cell culture and transfection.
HEK293T and HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 5 U/ml penicillin-streptomycin (Gibco, USA) in 5% CO2. The cells were transfected with Lipofectamine 2000 following the manufacturer's protocol (Invitrogen, USA).
Antibodies and reagents.
The proteasomal inhibitors MG132 and lactacystin were purchased from Sigma-Aldrich, USA. The WWP2 and Paip1 antibodies were purchased from Abcam, United Kingdom. Anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) and secondary antibodies were purchased from Santa Cruz Biotechnology, Inc., USA. Anti-hemagglutinin (anti-HA) was obtained from Roche Applied Science, Germany, and anti-Myc and anti-Flag antibodies were from MBL.
Immunoprecipitation and immunoblotting.
For general cell lysis, transfected cells were harvested and lysed in HEPES lysis buffer (containing 20 mM HEPES, pH 7.2, 50 mM NaCl, 0.5% Triton X-100, 1 mM NaF, and 1 mM dithiothreitol) and boiled with 2× SDS-PAGE loading buffer. For immunoprecipitation, cell lysates were prepared in 500 ml HEPES buffer supplemented with a protease inhibitor mixture (Roche Applied Science). Immunoprecipitation was performed by primary antibody incubation for 3 h, followed by overnight incubation with protein A/G-Sepharose beads (Santa Cruz). The beads were washed with HEPES buffer thrice and examined by immunoblotting.
Immunofluorescence.
For subcellular localization analyses, cells were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 (phosphate-buffered saline). Proteins were stained using the indicated antibodies and detected with a tetramethyl rhodamine isocyanate (TRITC)- or fluorescein isothiocyanate (FITC)-conjugated secondary antibody. The nuclei were stained with 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI) (Sigma) and visualized with a Zeiss LSM 510 Meta inverted confocal microscope.
RNA interference.
The WWP2 small interfering RNA 1 (siRNA-1) (5′-CACCTACTTTCGCTTTATA-3′) and siRNA-2 (5′-GGAGTACGTGCGCAACTAT-3′) and nontargeting siRNAs (5′-UUCUCCGAACGUGUCACGU-3′) were synthesized by Shanghai GenePharm. All siRNAs were transfected into the cells according to the manufacturer's protocol.
Determination of turnover of Paip1.
HEK293T cells were transfected with Myc-Paip1 with Flag-WWP2, Flag-WWP2-C838A, or empty vector (or interfering RNA). The cells were treated with cycloheximide (CHX) (15 mg/ml; Sigma) at 36 h after transfection and harvested at the indicated times. The protein level was measured by Western blotting using the indicated antibodies.
Ubiquitination assays.
Cells were transfected with Myc-Paip1 and Flag-WWP2 or its mutants to investigate Paip1 ubiquitination. Subsequently, the cells were treated with MG132 (20 μM) for 8 h before harvest. Cell lysis solution was prepared in modified RIPA lysis buffer containing 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.025% sodium dodecyl sulfate, and protease inhibitors. Immunoprecipitation was conducted using the indicated antibody. Subsequently, immunoblot analysis was performed. For in vitro ubiquitination assays, His-WWP2 and glutathione S-transferase (GST)–Paip1 were expressed in Escherichia coli and purified with Ni-nitrilotriacetate-agarose (Qiagen, Netherlands) and glutathione-Sepharose 4B beads (Amersham, United Kingdom), respectively. The assays were conducted in 30 μl ubiquitination assay buffer (containing 50 mM Tris, pH 8.0, 50 mM NaCl, 1 mM dithiothreitol, 5 mM MgCl2, and 3 mM ATP) with 0.7 μg of E1, 1 μg of UbcH5c (E2), 15 μg of HA-ubiquitin (Boston Biochem, MA, USA), 0.7 μg of His-WWP2 (wild type [WT] or C838A mutant [CA]), and 1.5 μg GST-Paip1. Samples were incubated at 30°C for 2 h. Reactions were terminated using the sample buffer.
Real-time reverse transcription-PCR (RT-PCR).
Total RNA was isolated from the cells using TRIzol (Invitrogen) and reverse transcribed using 1 μg of total RNA with an oligo(dT) primer. The following primers were used for real-time PCR: human GAPDH forward, 5′-GGGAAGGTGAAGGTCGGAGT-3′, and GAPDH reverse, 5′-TTGAGGTCAATGAAGGGGTCA-3′; human WWP2 forward, 5′-CGCAACTATGAGCAGTGGCA-3′, and human WWP2 reverse, 5′-GGTCGTGCGAGTGTTATGGT-3′; human Paip1 forward, 5′-GGAGAACTGGAAAGCCGAGGGTA-3′, and human Paip1 reverse, 5′-GTGTAACTGGAAGAATAACCTGAAGGG-3′; Renilla forward, 5′-CAGTGGTGGGCCAGATGTAAACAA-3′, and Renilla reverse 5′-TAATACACCGCGCTACTGGCTCAA-3′.
Translation assays.
HeLa cells were grown in 60-mm dishes and cotransfected with Myc-Paip1, pTet-HA-WWP2 (WT or CA), or the control vector pUHD-15-1 expressing the Tet-controlled transactivator (tTA) (28), and pRL-CMV (Promega, USA). pRL-CMV is a Renilla luciferase reporter construct. The medium was replaced with Tet at a final concentration of 0 or 300 ng/ml after 4 h. Cells were harvested 48 h after transfection, and extracts were used to quantify Renilla luciferase by a dual-luciferase reporter assay system (Promega). The protein concentration was determined using Bio-Rad protein assay reagent, and Renilla luciferase activity was corrected according to the protein concentration. The relative induction for each construct was determined by calculating the ratio of Renilla luciferase activity between the induced condition (no Tet) and the repressed condition (with 300 ng/ml Tet). Extracts were subjected to SDS-PAGE and Western blot analysis.
RESULTS
WWP2 negatively regulates the protein level of Paip1 in a proteasome-dependent manner.
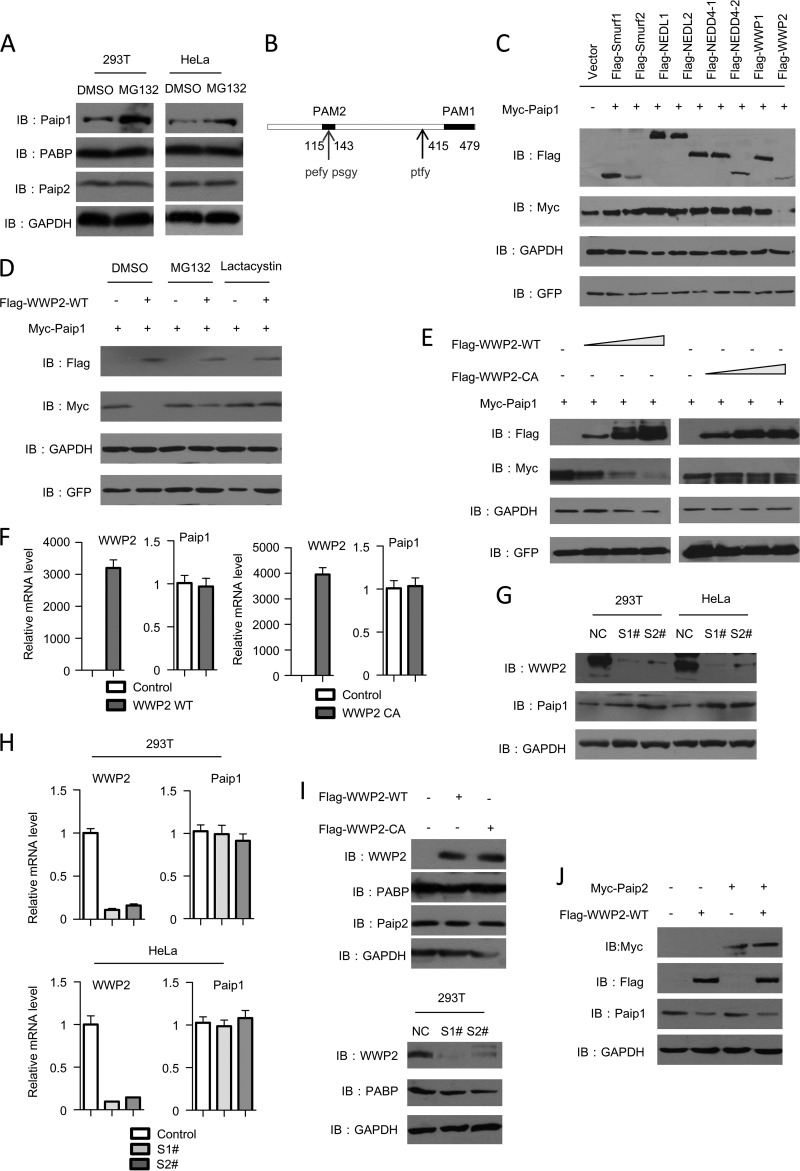
To explore the mechanism underlying Paip1 regulation, we first investigated whether the Paip1 protein was regulated through the proteasome pathway, which is the most critical pathway in the regulation of cellular protein stability and quality in eukaryotes. The level of endogenous Paip1 protein increased significantly after MG132 treatment in both HEK293T and HeLa cells (Fig. 1A), suggesting that Paip1 protein regulation depended on the proteasome system. We also examined the effect of MG132 treatment on the levels of other related translation factors, such as PABP and Paip2. The proteasome inhibitors failed to affect the PABP and Paip2 protein levels (Fig. 1A). The ubiquitin ligases controlled ubiquitination and determined the specificity of substrate recognition during ubiquitin-mediated proteasomal degradation, so we determined which ubiquitin ligase downregulated Paip1 protein stability. Sequence analysis showed that Paip1 comprised three putative PXXY-type PY motifs (Fig. 1B). These motifs are recognized by the Nedd4 family of ubiquitin ligases. Among the nine members of the Nedd4 ligases, only WWP2 specifically downregulated Paip1 protein levels (Fig. 1C). The downregulation of Paip1 by WWP2 was blocked by the proteasome inhibitors MG132 and lactacystin (Fig. 1D). Thus, we hypothesized that WWP2 functions as a ubiquitin ligase in Paip1 degradation.
FIG 1.
WWP2 negatively regulates the Paip1 protein level. (A) MG132 treatment upregulates the Paip1 protein level. HEK293T cells were treated with MG132 (20 μM) for 8 h before harvest. Endogenous Paip1, PABP, and Paip2 levels were analyzed by immunoblotting (IB). GAPDH was used as an internal control. (B) Schematic of the Paip1 protein. The positions of PXXY motifs are labeled. (C) WWP2 specifically reduces the amount of Paip1 protein. The indicated Nedd4 family of E3 ligases was cotransfected with Paip1 into HEK293T cells, and cell lysates were analyzed by immunoblotting. (D) WWP2 downregulates Paip1 in a proteasome-dependent manner. Increasing concentrations of Flag-WWP2 plasmids were cotransfected into HEK293T cells with Myc-Paip1. The cells were treated with MG132 (20 μM) and lactacystin (30 μM) or dimethyl sulfoxide (DMSO) for 16 h, and the cell lysates were analyzed by immunoblotting. (E) WWP2 decreases the Paip1 protein level in a dose-dependent manner. Cells were transfected with increasing amounts of WWP2-WT or the catalytic mutant form WWP2-C838A. Subsequently, the Paip1 level was detected. (F) WWP2 cannot affect the mRNA level of Paip1. Paip1 mRNA prepared from the transfected HEK293T cells was analyzed by real-time PCR assay. The data are presented as means and standard deviations (SD) (n = 3). (G) WWP2 depletion increases the endogenous Paip1 protein level. HEK293T and HeLa cells were transfected with nontargeted control (NC) or with WWP2-specific siRNA (S1# and S2#). The endogenous WWP2 and Paip1 levels were analyzed by Western blotting. (H) WWP2 depletion cannot affect the Paip1 mRNA level. Paip1 mRNA prepared from the transfected HEK293T and HeLa cells was analyzed by real-time PCR assay. The data are presented as means and SD (n = 3). (I) WWP2 cannot affect the endogenous Paip2 and PABP protein levels. (Top) Cells were transfected with WWP2-WT or the catalytic mutant form WWP2-C838A, and Paip2 and PABP protein levels were detected by immunoblotting. (Bottom) HEK293T cells were transfected with nontargeted control or WWP2-specific siRNA, and the endogenous WWP2 and PABP protein levels were analyzed by immunoblotting. (J) Overexpression of Paip2 cannot affect WWP2-mediated degradation of Paip1. HEK293T cells were transfected with the vector or with Paip2. At 24 h after transfection, the cells were transfected with WWP2 and analyzed by immunoblotting.
To test our hypothesis, we transiently transfected HEK293T cells with a constant amount of Paip1 and an increasing amount of wild-type WWP2 (WWP2-WT) or ligase-inactive WWP2-C838A expression vectors. The Paip1 protein level progressively decreased with increasing WWP2-WT expression, but this phenomenon was not observed with increasing WWP2-C838A mutant expression (Fig. 1E and F). No change in the Paip1 mRNA level was observed. Thus, WWP2 could mediate Paip1 destruction, depending on its ubiquitin ligase activity.
To verify whether endogenous WWP2 regulates the Paip1 level, WWP2 was depleted using two independent siRNAs. The depletion of endogenous WWP2 in HEK293T and HeLa cells significantly increased the Paip1 protein level (Fig. 1G). However, the mRNA level was not affected by the above-mentioned changes (Fig. 1H). WWP2 can function as a ubiquitin ligase to maintain proper Paip1 protein levels in human cells.
Paip1 and Paip2 share similar domains and compete for PABP binding. According to previous studies, another HECT-type ubiquitin ligase, namely, EDD, targets Paip2 for degradation during PABP depletion. We examined the effect of WWP2 overexpression on Paip2 and found that WWP2 did not affect Paip2 protein levels (Fig. 1I). Furthermore, overexpression of Paip2 did not affect WWP2-mediated Paip1 degradation (Fig. 1J). Paip1 and Paip2 did not compete for WWP2 binding, and the degradation of the two proteins required different ubiquitin ligases. Moreover, the Paip1 binding protein, PABP, was not regulated upon WWP2 silencing and overexpression (Fig. 1I).
WWP2 promotes the degradation and ubiquitination of Paip1.
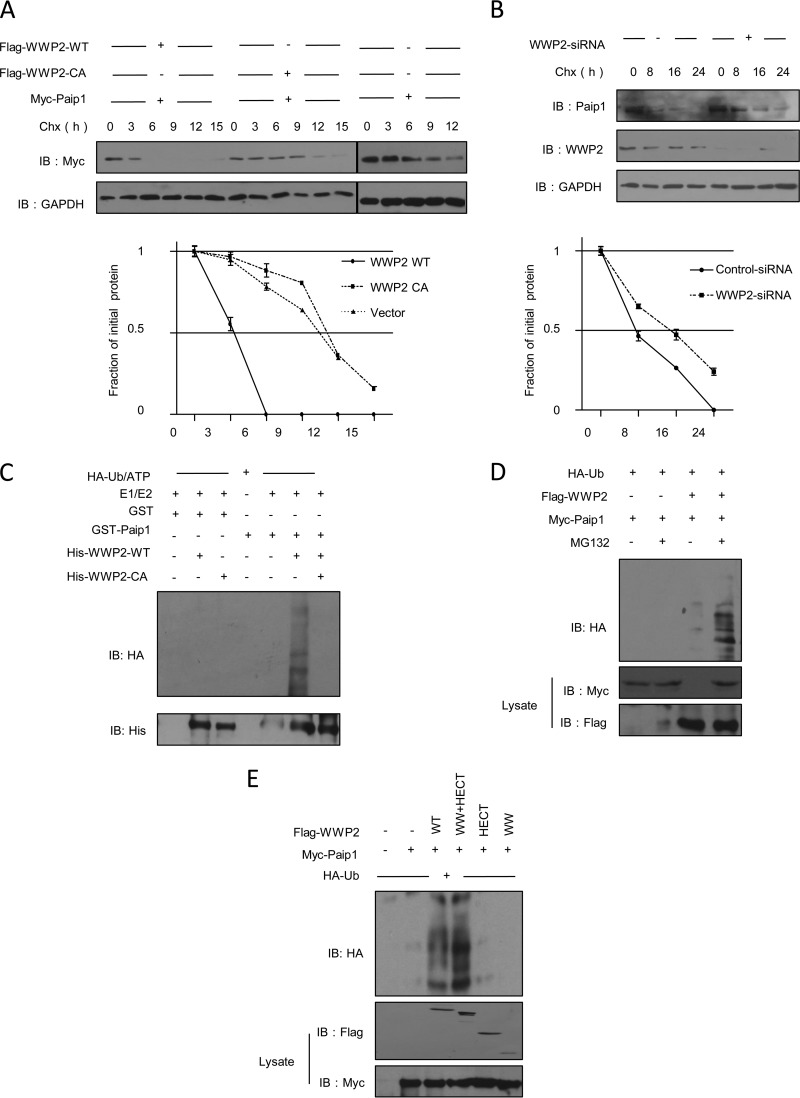
To assess whether the reduction in the protein level of Paip1, which was induced by WWP2 overexpression, was due to increasing Paip1 degradation, we analyzed the steady-state levels of Paip1 by applying CHX, an inhibitor of protein synthesis. The half-life of exogenously expressed Paip1 was greatly reduced by the expression of WWP2-WT but not by the expression of Paip1-C838A (Fig. 2A). However, the depletion of WWP2 prolonged the half-life of endogenous Paip1 (Fig. 2B). Thus, WWP2 promoted the degradation of the Paip1 protein.
FIG 2.
WWP2 promotes the ubiquitination and degradation of Paip1. (A) WWP2 reduces the half-life of endogenous Paip1 protein. HEK293T cells were transfected with control plasmid, Flag-WWP2-WT, or the catalytic mutant form Flag-WWP2-C838A, and the cells were treated with the protein synthesis inhibitor CHX (10 μg/ml) for the indicated durations before harvest. Paip1 protein expression was analyzed. Quantitative analysis was performed by measuring the integrated optical density using the program Gel-Pro Analyzer. The data are presented as means ± SD (n = 3). +, overexpression; −, vector control. Lines represents the same treatment at different time points. (B) Depletion of WWP2 prolongs the half-life of endogenous Paip1 protein. HEK293T cells were transfected with control siRNA or WWP2 siRNA and treated with CHX. Paip1 protein expression was subsequently analyzed. Quantitative analysis was performed by measuring the integrated optical density using the program Gel-Pro Analyzer. The data are presented as means ± SD (n = 3). (C) WWP2 catalyzes the ubiquitination of Paip1 in vitro. A mixture comprising purified HA-ubiquitin (HA-Ub), E1, E2 (UbcH5c), bacterium-expressed and purified His-WWP2-WT or -C838A, and GST-Paip1 or GST was used for in vitro ubiquitination assays and subsequent immunoblotting with anti-HA. (D) WWP2 enhances the ubiquitination of Paip1 in vivo. HEK293T cells were transfected with HA-Ub, Myc-Paip1, control vector, or Flag-WWP2 and treated with MG132 as indicated. Ubiquitinated Paip1 was immunoprecipitated by anti-Myc antibody and analyzed by immunoblotting. (E) The WW and HECT domains of WWP2 are involved in the ubiquitination of Paip1. HA-Ub, Myc-Paip1, and WWP2 constructs were cotransfected into HEK293T cells and treated with MG132. Ubiquitinated Paip1 was immunoprecipitated with anti-HA antibody and analyzed by immunoblotting.
We determined whether WWP2 functioned as a ubiquitin ligase and if WWP2 directly promoted the ubiquitination of Paip1. An in vitro ubiquitination assay was initially conducted using purified E1 and UbcH5c, bacterium-expressed His-WWP2-WT or His-WWP2-C838A, and GST-Paip1 or GST (control). Bacterium-expressed and purified WWP2-WT promoted the ubiquitination of Paip1, but a similar effect on Paip1 was not induced by WWP2-C838A (Fig. 2C). Overexpressed WWP2 enhanced the ubiquitination of Paip1 in the presence of MG132 in HEK293T cells (Fig. 2D). The required WW and HECT domains were sufficient for WWP2 to promote the degradation of Paip1 (Fig. 2E). These data suggested that WWP2 functioned as a biologically active ubiquitin ligase and induced the ubiquitination and degradation of Paip1.
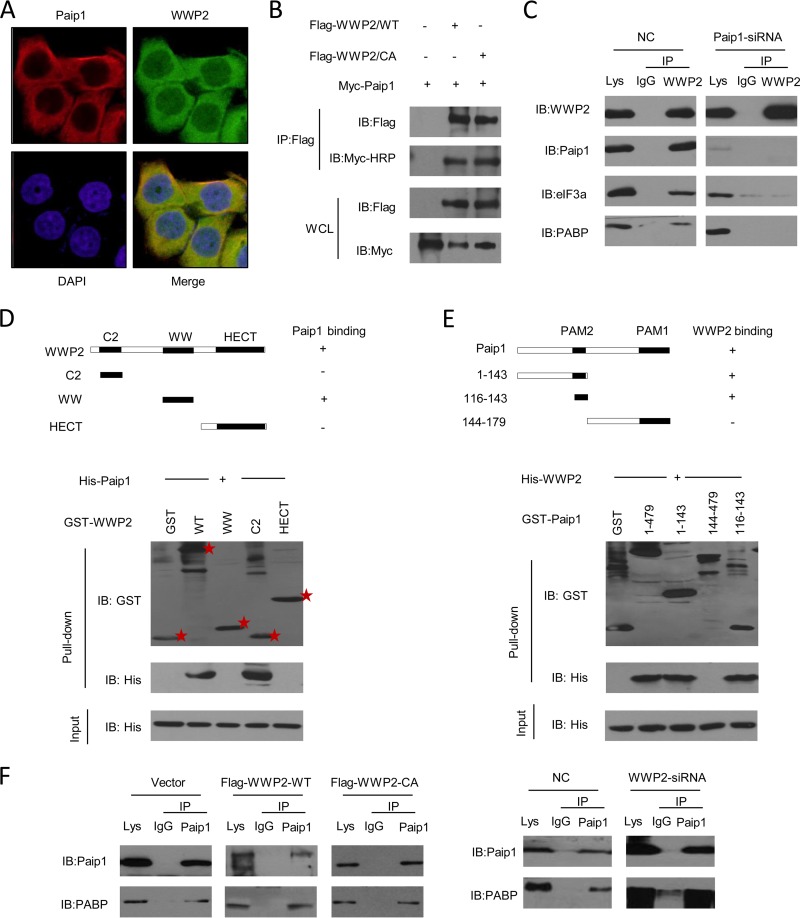
The WW domain of WWP2 is required for binding to the PAM2 motif of Paip1.
Proteins should at least partly colocalize to be functionally linked. Indirect immunofluorescence assays revealed that WWP2 and Paip1 colocalized predominantly in the cytoplasm (Fig. 3A). We further confirmed the interaction of WWP2 with Paip1 in mammalian cells. A coimmunoprecipitation assay revealed the association between Paip1 and WWP2-WT or the C838A mutant (Fig. 3B). Thus, ubiquitin ligase activity was not required for the interaction. In addition, endogenous Paip1 and its binding partners were coimmunoprecipitated with WWP2 but not with the control IgG from HeLa cells (Fig. 3C, left). Given the interactions between WWP2 and eIF3a, PABP disappeared in a Paip1 depletion cell extract (Fig. 3C, right), suggesting that eIF3a and PABP bonding to WWP2 depended on the presence of Paip1.
FIG 3.
Mapping of the interaction region between the truncated domains of WWP2 and Paip1. (A) WWP2 is colocalized with Paip1 within the cytoplasm. Myc-Paip1 and Flag-WWP2 were cotransfected into HEK293T cells, and the cells were stained 24 h later using mouse anti-Flag and rabbit anti-Myc antibodies for visualization by confocal microscopy. (B) Paip1 was coimmunoprecipitated with wild-type and catalytic mutant forms of WWP2. The wild-type and catalytic mutant forms of WWP2 and Myc-Paip1 were transfected into HEK293T cells. Cell lysates were immunoprecipitated (IP) with anti-Flag antibody, and the lysates and immunoprecipitates were analyzed by immunoblotting. HRP, horseradish peroxidase. (C) Immunoprecipitation analysis of WWP2 and Paip1 in vivo. HeLa cells were transfected with negative-control siRNA (NC) or siRNA-targeted Paip1, and cells were harvested after 36 h. The extracts were immunoprecipitated with anti-WWP2 antibody and subsequently analyzed by immunoblotting using anti-WWP2, anti-Paip1, anti-eIF3a, and anti-PABP antibodies. Preimmune IgG was used as the control. (D) Direct interaction between the WW domain of WWP2 and Paip1. GST pulldowns were conducted with GST or GST-WWP2 fragments, along with purified His-Paip1. Input and pulldown samples were subjected to immunoblotting with anti-GST and anti-His antibodies. The input represented 10% of the sample used for the pulldown. Stars denote protein bands. (E) The PAM2 motif of Paip1 showed direct binding to WWP2. GST pulldowns were performed with GST or GST-Paip1 fragments, along with purified His-WWP2. Input and pulldown samples were subjected to immunoblotting with anti-GST and anti-His antibodies. The input represented 10% of the sample used for pulldown. (F) WWP2 cannot affect the interaction between Paip1 and PABP. (Left) HEK293T cells were transfected with Flag-WWP2-WT or the catalytic mutant form Flag-WWP2-C838A. The cell lysates were immunoprecipitated with anti-Paip1 antibody. Both the lysate and immunoprecipitates were analyzed by immunoblotting. (Right) HEK293T cells were transfected with control siRNA or WWP2 siRNA. The cell lysates were immunoprecipitated with anti-Paip1 antibody. The lysates and immunoprecipitates were analyzed by immunoblotting.
The direct association between WWP2 and Paip1 was confirmed by a GST pulldown assay. WWP2 protein comprises three domains, namely, C2, WW (including four tandem WW domains), and HECT. The results of the GST pulldown assays indicated that the WW domain mediated the direct interaction between WWP2 and Paip1 (Fig. 3D). The C2 and HECT domains of WWP2 did not show the same regulatory action. The above-mentioned results contradicted a previous finding, which stated that the WW domains of Nedd4 family members preferred to recruit substrates (29). Paip1 contains two binding sites for PABP, namely, PAM1 and PAM2 (15). PAM2 was required for the binding of Paip1 to eIF3. Different parts of Paip1 were fused to GST to identify the WWP2-binding site in Paip1. His-WWP2 was detected in reactions involving the full-length protein, the fusion protein with a retained Paip1 N terminus (comprising amino acids 1 to 143), and the PAM2 motif (comprising amino acids 116 to 143). However, the large C-terminal region (comprising amino acids 144 to 479) was not required for the binding of Paip1 to WWP2 (Fig. 3E). The PAM2 motif was previously identified in numerous proteins with diverse functions (30), suggesting that PAM2 participated in protein-protein interactions in a wide range of cellular processes. Two of the three PXXY motifs were typical binding regions of the WW domains of the Nedd4 family members (31) and were located in the PAM2 motif of Paip1 (Fig. 1B). Paip1 interacted with PABP via the PAM2 motif. We measured the interaction of Paip1 and PABP upon WWP2 silencing and overexpression to determine whether WWP2 and PABP competed for Paip1 binding. WWP2 did not affect the interaction of Paip1 and PABP (Fig. 3F), suggesting that Paip1's PAM2 motif (comprising amino acids 116 to 143) sufficiently interacted with the WW domain of WWP2.
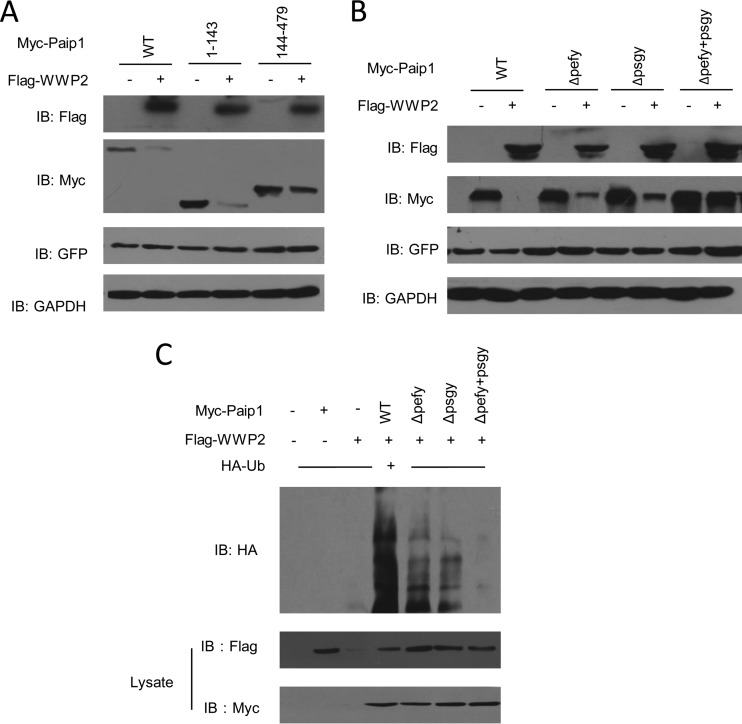
Degradation and ubiquitination of Paip1 by WWP2 depend on the presence of the PEFYPSGY sequence.
Based on data from previous studies, we speculated that the two PXXY motifs (PEFYPSGY sequence) in PAM2 participated in WWP2's recognition and degradation of Paip1. We transfected different Paip1 deletion mutants into HEK293T cells with Flag-WWP2-WT to determine the importance of the two PXXY motifs. Degradation analysis showed that 144-to-479 mutants included one PXXY motif that was not degraded by overexpressed WWP2. In contrast, the N-terminal 1-to-143 mutants, which contained the first two PXXY motifs, were efficiently degraded by WWP2 (Fig. 4A). This phenomenon suggested that the critical degradation signal was present in the mutants. To further determine which PXXY motif was critical for WWP2-mediated degradation, we used the ΔPEFY, ΔPSGY, and ΔPEFYPSGY forms of Paip1 to analyze protein levels in the presence or absence of WWP2. Paip1-ΔPEFY and Paip1-ΔPSGY were both downregulated by WWP2. However, WWP2 did not downregulate Paip1-ΔPEFYPSGY (Fig. 4B). Thus, the two PXXY motifs and the PEFYPSGY sequence were necessary for WWP2 to recognize Paip1.
FIG 4.
The PEFYPSGY sequence is sufficient for the ubiquitination and degradation of Paip1. (A) HEK293T cells were transfected with plasmids expressing either full-length Paip1 or mutants of Paip1 with or without a plasmid encoding Flag-WWP2-WT. (B) The PEFYPSGY sequence maintains the WWP2-mediated degradation of Paip1. HEK293T cells were transfected with plasmids expressing Paip1-ΔPEFY, Paip1-ΔPSGY, or Paip1-ΔPEFYPSGY with or without Flag-WWP2. The cell lysates were analyzed by immunoblotting. (C) The PEFYPSGY sequence attenuated WWP2-mediated ubiquitination of Paip1. HEK293T cells were cotransfected with Myc-Paip1 deletions and HA-Ub with or without Flag-WWP2. Ubiquitinated Paip1 was immunoprecipitated with an anti-Myc antibody and protein A/G-agarose beads under denaturing conditions to eliminate any WWP2-associated protein by noncovalent bonding.
We performed a ubiquitination assay to test whether the resistance of the mutant Paip1-ΔPEFYPSGY to WWP2-mediated degradation was caused by abrogated ubiquitination. WWP2 did not ubiquitylate the truncated mutant (Fig. 4C). The PEFYPSGY sequence was critical for the catalytic activity of WWP2 during Paip1 ubiquitination and degradation.
WWP2 negatively regulates Paip1-mediated translation enhancement.
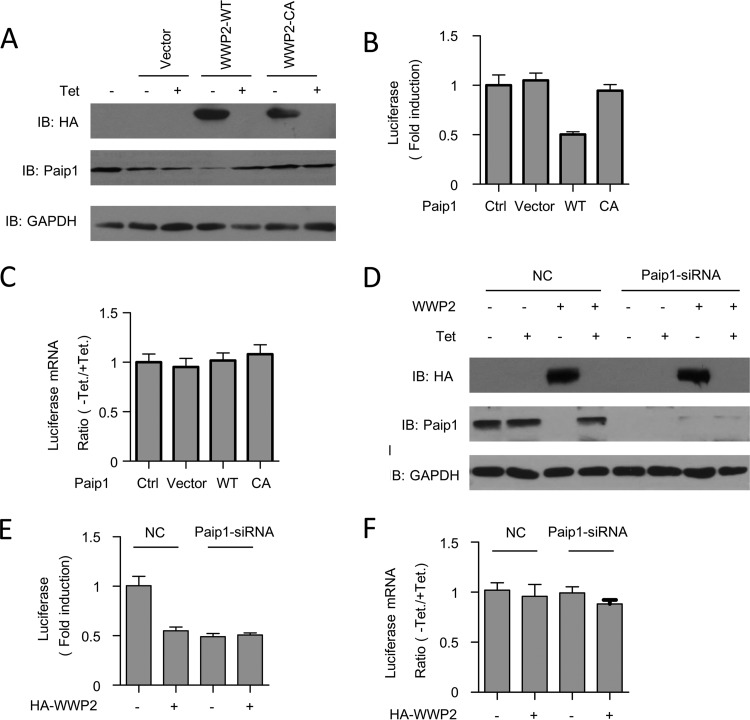
To gain insight into the functional relationship between WWP2 and Paip1, we initially determined the translational stimulatory activity via in vivo translation assays. Under the control of the Tet-off promoter expressing WWP2-WT or WWP2-C838A, DNA vectors were transfected into HeLa cells, along with constructs expressing Renilla luciferase and tTA. Each construct yielded comparable amounts of protein. Paip1 was absent in the extract expressing WWP2 (Fig. 5A). The relative induction of luciferase activity was determined by calculating the ratio of Renilla luciferase activity between induced (without Tet) and repressed (with Tet at 300 ng/ml) expression of WWP2. Luciferase activity was lower with the overexpression of the wild-type WWP2 plasmid than with the expression of the vector alone (Fig. 5B, lanes 1, 2, 3, 4, and 5). However, the overexpression of WWP2-C838A did not affect in vivo translation (Fig. 5B, lanes 6 and 7), suggesting that the ligase activity of WWP2 was required. WWP2 overexpression did not affect Renilla luciferase mRNA levels (Fig. 5C).
FIG 5.
Overexpression of WWP2 represses translation via the Paip1 protein. (A) Cells were transfected with the indicated pTet-HA-WWP2-WT or catalytic mutant form pTet-HA-WWP2-C838A plasmid, along with constructs expressing Renilla luciferase and tTA. The cells were cultured in medium containing 0 or 300 ng/ml of Tet. Cells were harvested and subjected to Western blot analysis with the indicated antibodies. (B) Renilla luciferase activity was quantified in HeLa cell extracts harvested as described for panel A and normalized on the total protein level. The relative induction of the luciferase reporter was determined by calculating the ratio of Renilla luciferase activity between induced (without Tet) and repressed (with 300 ng/ml Tet) expression of the indicated HA-WWP2. The error bars indicate the standard errors of the mean for the three independent experiments. (C) Quantitative real-time PCR of Renilla luciferase mRNA obtained from total RNA of duplicate HeLa cells harvested as described for panel A. The data are presented as means and SD (n = 3). (D) HeLa cells were transfected with the indicated siRNA sequences. At 24 h after siRNA transfection, the cells were transfected as described for panel A. The cells were subsequently placed in medium containing 0 or 300 ng/ml of Tet. Cells were harvested and subjected to Western blot analysis with the indicated antibodies. (E) Renilla luciferase activity was quantified in HeLa cell extracts harvested as described for panel D and normalized on the total protein level. The relative induction of the luciferase reporter was determined by calculating the ratio of Renilla luciferase activity between induced (without Tet) and repressed (with 300 ng/ml Tet) expression of the indicated HA-WWP2. The error bars indicate the standard errors of the mean for three independent experiments. (F) Quantitative RT-PCR of Renilla luciferase mRNA obtained from the total RNA of the duplicate HeLa cells harvested as described for panel D. The data are presented as means and SD (n = 3).
To determine whether the translational suppression induced by WWP2 depended on the presence of Paip1, we examined the effect of Paip1 silencing via siRNA on WWP2 activity. The expression of WWP2 and Paip1 was confirmed by Western blotting (Fig. 5D). No significant translational repression was observed when Paip1 was knocked down compared with the control (Fig. 5E). Renilla luciferase mRNA levels were not affected by siRNA treatments (Fig. 5F). Therefore, WWP2 repression of mRNA translation depended on the presence of Paip1. These data confirmed the importance of the protein level regulation of Paip1 by WWP2. We demonstrated that WWP2 is a novel regulator of translational initiation.
DISCUSSION
Translation is important in the regulation of gene expression and is implicated in the control of cell growth, proliferation, and differentiation (32–34). In eukaryotes, initiation is the rate-limiting step of translation under most circumstances, and initiation is a major target for regulation (33). Paip1 is a mammalian PABP that binds to eIF4A and eIF3 and stimulates translational initiation. In the present study, we showed that the Paip1 protein was degraded by the HECT ubiquitin ligase WWP2. The following findings from the present study directly support the use of Paip1 as a physiological substrate of WWP2. WWP2 directly interacted with Paip1, and the interaction depended on the integrity of the WW domain of WWP2. The loss of WWP2 impeded Paip1 turnover. WWP2 promoted Paip1 ubiquitination both in vivo and in a reconstituted in vitro system. The ubiquitin ligase activity of WWP2 and the PXXY motif of Paip1 were critical for ubiquitination and degradation. Previous studies have demonstrated the involvement of WWP2 in the regulation of transcription, embryonic stem cell development, cellular transport, T-cell activation processes, and tumorigenesis by targeting distinct substrates (35). In the present study, we revealed that Paip1 is a novel substrate of WWP2. We also emphasized the importance of WWP2 in the regulation of translation.
The involvement of deregulation of translational initiation in cancer development and progression became the focus of various studies only recently. WWP2 mediates the depletion of phosphatase and a tensin homolog and consequently elevates phosphoinositide-3-kinase–protein kinase B pathway activity (36). Such regulatory activities may enhance eIF4E function and activate cap-dependent translation initiation, leading to the selective increase of translation of key mRNAs; these mRNAs are involved in tumor growth, angiogenesis, and cell survival (37). Thus, we hypothesized that WWP2-mediated degradation of Paip1 selectively reduced the expression of numerous potent growth and survival factors. The role of WWP2 in tumorigenesis, which depends on WWP2's regulation of translation, requires further investigation.
Circularization of mRNA by bridging the mRNA 5′ and 3′ ends is an important step in the process of translation initiation. In Saccharomyces cerevisiae, eIF4G interacts with PABP to contribute to the circularization of the mRNA and mediation of the poly(A) tail-dependent translation (11). In plants, PABP binds to eIF-iso4G and eIF4B, thereby increasing the RNA-binding activity of PABP (13). However, mammalian cells possess dual systems, namely, PABP-Paip1 and PABP-eIF4G. These systems regulate the formation of mRNP. The presence of Paip1 in animal cells reflects evolutionary advancement and allows higher eukaryotes to link PABP to eIF4A function. Information on whether the degradation of Paip1 by WWP2 reduces the rate of circular mRNA formation and inhibits mRNA translation is lacking. Paip1's sequence and function are similar to those of the eIF4G protein. WWP2 may also regulate the protein level of eIF4G in human cells, but the relationship between WWP2 and eIF4G requires further study.
The stimulation of translation by Paip1 in vivo decreased upon deletion of the N-terminal sequence containing PABP and the eIF3 binding domain known as PAM2 (22). In the present study, PAM2 was critical for the binding of PABP to WWP2. Paip1-interacting proteins can potentially compete for Paip1 binding via the PAM2 domain, thereby regulating translation. Further studies are required to determine the presence of an overlap among the binding sites of WWP2, eIF3, and PABP in Paip1.
WWP2 was identified as a typical ubiquitin ligase for Paip1. We showed that the overexpression of WWP2 significantly decreased the Paip1 protein level, and the decrease suppressed translation. We also discovered that the WW domain of WWP2 interacted with Paip1 fragments, which contained two PXXY motifs. The degradation and ubiquitination of Paip1 by WWP2 was transiently abolished by the deletion of the two PXXY motifs. WWP2 is an important translational suppressor that participates in Paip1 degradation. The function of WWP2 in the regulation of translation was clarified for the first time. The mechanisms underlying WWP2's regulation of Paip1 should be studied further to reveal the details of WWP2's function.
ACKNOWLEDGMENTS
This work was supported by grants from the Nature Science of Shandong Province (Q2008C08).
The funders had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 29 September 2014
REFERENCES
- 1.Parsyan A, Hernandez G, Meterissian S. 2012. Translation initiation in colorectal cancer. Cancer Metastasis Rev. 31:387–395. 10.1007/s10555-012-9349-9. [DOI] [PubMed] [Google Scholar]
- 2.Jia Y, Polunovsky V, Bitterman PB, Wagner CR. 2012. Cap-dependent translation initiation factor eIF4E: an emerging anticancer drug target. Med. Res. Rev. 32:786–814. 10.1002/med.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graff JR, Konicek BW, Carter JH, Marcusson EG. 2008. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 68:631–634. 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 4.Cheung YN, Maag D, Mitchell SF, Fekete CA, Algire MA, Takacs JE, Shirokikh N, Pestova T, Lorsch JR, Hinnebusch AG. 2007. Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev. 21:1217–1230. 10.1101/gad.1528307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu WL, Wagner S, Herrmannova A, Burela L, Zhang F, Saini AK, Valasek L, Hinnebusch AG. 2010. The C-terminal region of eukaryotic translation initiation factor 3a (eIF3a) promotes mRNA recruitment, scanning, and, together with eIF3j and the eIF3b RNA recognition motif, selection of AUG start codons. Mol. Cell. Biol. 30:4415–4434. 10.1128/MCB.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisarev AV, Unbehaun A, Hellen CU, Pestova TV. 2007. Assembly and analysis of eukaryotic translation initiation complexes. Methods Enzymol. 430:147–177. 10.1016/S0076-6879(07)30007-4. [DOI] [PubMed] [Google Scholar]
- 7.Lorsch JR, Dever TE. 2010. Molecular view of 43 S complex formation and start site selection in eukaryotic translation initiation. J. Biol. Chem. 285:21203–21207. 10.1074/jbc.R110.119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preiss T, Hentz WM. 2003. Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays 25:1201–1211. 10.1002/bies.10362. [DOI] [PubMed] [Google Scholar]
- 9.Prevot D, Darlix JL, Ohlmann T. 2003. Conducting the initiation of protein synthesis: the role of eIF4G. Biol. Cell 95:141–156. 10.1016/S0248-4900(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 10.Kessler SH, Sachs AB. 1998. RNA recognition motif 2 of yeast Pab1p is required for its functional interaction with eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 18:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarun SZ, Jr, Sachs AB. 1996. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 12.Tarun SZ, Jr, Wells SE, Deardorff JA, Sachs AB. 1997. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc. Natl. Acad. Sci. U. S. A. 94:9046–9051. 10.1073/pnas.94.17.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le H, Tanguay RL, Balasta ML, Wei CC, Browning KS, Metz AM, Goss DJ, Gallie DR. 1997. Translation initiation factors eIF-iso4G and eIF-4B interact with the poly(A)-binding protein and increase its RNA binding activity. J. Biol. Chem. 272:16247–16255. 10.1074/jbc.272.26.16247. [DOI] [PubMed] [Google Scholar]
- 14.Khaleghpour K, Kahvejian A, De Crescenzo G, Roy G, Svitkin YV, Imataka H, O'Connor-McCourt M, Sonenberg N. 2001. Dual interactions of the translational repressor Paip2 with poly(A) binding protein. Mol. Cell. Biol. 21:5200–5213. 10.1128/MCB.21.15.5200-5213.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy G, De Crescenzo G, Khaleghpour K, Kahvejian A, O'Connor-McCourt M, Sonenberg N. 2002. Paip1 interacts with poly(A) binding protein through two independent binding motifs. Mol. Cell. Biol. 22:3769–3782. 10.1128/MCB.22.11.3769-3782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imataka H, Olsen HS, Sonenberg N. 1997. A new translational regulator with homology to eukaryotic translation initiation factor 4G. EMBO J. 16:817–825. 10.1093/emboj/16.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy-Strumpf N, Deiss LP, Berissi H, Kimchi A. 1997. DAP-5, a novel homolog of eukaryotic translation initiation factor 4G isolated as a putative modulator of gamma interferon-induced programmed cell death. Mol. Cell. Biol. 17:1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanaka S, Poksay KS, Arnold KS, Innerarity TL. 1997. A novel translational repressor mRNA is edited extensively in livers containing tumors caused by the transgene expression of the apoB mRNA-editing enzyme. Genes Dev. 11:321–333. 10.1101/gad.11.3.321. [DOI] [PubMed] [Google Scholar]
- 19.Morino S, Imataka H, Svitkin YV, Pestova TV, Sonenberg N. 2000. Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of eIF4GI constitute the core domain for cap-dependent translation, and the C-terminal one-third functions as a modulatory region. Mol. Cell. Biol. 20:468–477. 10.1128/MCB.20.2.468-477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imataka H, Sonenberg N. 1997. Human eukaryotic translation initiation factor 4G (eIF4G) possesses two separate and independent binding sites for eIF4A. Mol. Cell. Biol. 17:6940–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig AW, Haghighat A, Yu AT, Sonenberg N. 1998. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature 392:520–523. 10.1038/33198. [DOI] [PubMed] [Google Scholar]
- 22.Martineau Y, Derry MC, Wang X, Yanagiya A, Berlanga JJ, Shyu AB, Imataka H, Gehring K, Sonenberg N. 2008. Poly(A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Mol. Cell. Biol. 28:6658–6667. 10.1128/MCB.00738-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martineau Y, Wang X, Alain T, Petroulakis E, Shahbazian D, Fabre B, Bousquet-Dubouch MP, Monsarrat B, Pyronnet S, Sonenberg N. 2014. Control of Paip1-eukayrotic translation initiation factor 3 interaction by amino acids through S6 kinase. Mol. Cell. Biol. 34:1046–1053. 10.1128/MCB.01079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou W, Chen X, Shim JH, Huang Z, Brady N, Hu D, Drapp R, Sigrist K, Glimcher LH, Jones D. 2011. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of Goosecoid. Nat. Cell Biol. 13:59–65. 10.1038/ncb2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura Y, Yamamoto K, He X, Otsuki B, Kim Y, Murao H, Soeda T, Tsumaki N, Deng JM, Zhang Z, Behringer RR, Crombrugghe B, Postlethwait JH, Warman ML, Nakamura T, Akiyama H. 2011. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat. Commun. 2:251. 10.1038/ncomms1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, Chen J. 2011. WWP2 is an E3 ubiquitin ligase for PTEN. Nat. Cell Biol. 13:728–733. 10.1038/ncb2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soond SM, Chantry A. 2011. Selective targeting of activating and inhibitory Smads by distinct WWP2 ubiquitin ligase isoforms differentially modulates TGFbeta signalling and EMT. Oncogene 30:2451–2462. 10.1038/onc.2010.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gossen M, Bujard H. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U. S. A. 89:5547–5551. 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernassola F, Karin M, Ciechanover A, Melino G. 2008. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 14:10–21. 10.1016/j.ccr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Albrecht M, Lengauer T. 2004. Survey on the PABC recognition motif PAM2. Biochem. Biophys. Res. Commun. 316:129–138. 10.1016/j.bbrc.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Ingham RJ, Gish G, Pawson T. 2004. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23:1972–1984. 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 32.Conlon I, Raff M. 1999. Size control in animal development. Cell 96:235–244. 10.1016/S0092-8674(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 33.Gingras AC, Raught B, Sonenberg N. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913–963. 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 34.Miron M, Verdu J, Lachance PE, Birnbaum MJ, Lasko PF, Sonenberg N. 2001. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat. Cell Biol. 3:596–601. 10.1038/35078571. [DOI] [PubMed] [Google Scholar]
- 35.Chen A, Gao B, Zhang J, McEwen T, Ye SQ, Zhang D, Fang D. 2009. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol. Cell. Biol. 29:5348–5356. 10.1128/MCB.00407-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chantry A. 2011. WWP2 ubiquitin ligase and its isoforms: new biological insight and promising disease targets. Cell Cycle 10:2437–2439. 10.4161/cc.10.15.16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graff JR, Konicek BW, Carter JH, Marcusson EG. 2008. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 68:631–634. 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]