Abstract
Antimicrobial peptides (AMP) are ubiquitous innate immune elements in epithelial tissues. We recently discovered that a signaling lipid, the ceramide metabolite sphingosine-1-phosphate (S1P), regulates production of a major AMP, cathelicidin antimicrobial peptide (CAMP), in response to a subtoxic level of endoplasmic reticulum (ER) stress that can be induced by external perturbants in keratinocytes. We hypothesized that an ER stress-initiated signal could also regulate production of another major class of AMPs: i.e., the human beta-defensins 2 (hBD2) and 3 (hBD3). Keratinocytes stimulated with a pharmacological ER stressor, thapsigargin (Tg), increased hBD2/hBD3 as well as CAMP mRNA expression. While inhibition of sphingosine-1-phosphate production did not alter hBD expression following ER stress, blockade of ceramide-1-phosphate (C1P) suppressed Tg-induced hBD2/hBD3 but not CAMP expression. Exogenous C1P also increased hBD2/hBD3 production, indicating that C1P stimulates hBD expression. We showed further that C1P-induced hBD2/hBD3 expression is regulated by a novel pathway in which C1P stimulates downstream hBD via a cPLA2a→15d-PGJ2→PPARα/PPARβ/δ→Src kinase→STAT1/STAT3 transcriptional mechanism. Finally, conditioned medium from C1P-stimulated keratinocytes showed antimicrobial activity against Staphylococcus aureus. In summary, our present and recent studies discovered two new regulatory mechanisms of key epidermal AMP, hBD2/hBD3 and CAMP. The C1P and S1P pathways both signal to enhance innate immunity in response to ER stress.
INTRODUCTION
Antimicrobial peptides (AMP) are innate immune elements that are widely expressed in all living systems, including mammalian epithelia. While AMP production often is low under basal (nonstressed) conditions, production increases following external perturbations, including exposure to microbial pathogens (1). Epithelial tissues, including epidermis, are continuously threatened by exposure to external perturbants, such as oxidative stress, UV irradiation, and wounding, which attenuate barrier functions to increase the risk of microbial pathogen colonization and invasion. To protect the internal milieu from exogenous pathogens, these epithelia upregulate multiple innate immune elements, including several AMP that together provide an effective antimicrobial barrier against these external threats. We and others have demonstrated that AMP production increases following external perturbations, such as UV irradiation, oxidative stress, inflammation, and wounding (2–6). We also showed that most of these external perturbations induce endoplasmic reticulum (ER) stress, which in turn stimulates production of a major AMP, cathelicidin antimicrobial peptide (CAMP), in epithelial cells/tissues (7). We then identified and characterized a novel signaling mechanism that upregulates CAMP production through an ER stress→nuclear factor κB (NF-κB)→C/EBPα transcriptional mechanism that operates independently of the well-known vitamin D receptor (VDR)-mediated transcriptional pathway. Instead, VDR-induced transactivity is suppressed under the same ER-stressed conditions (7). We then identified the ER stress-induced signal: i.e., increased production of ceramide (Cer) and its metabolite, sphingosine-1-phosphate (S1P), which serves as an upstream activator of NF-κB in epithelial cells (8). Thus, VDR- and S1P-dependent mechanisms likely regulate CAMP production under basal and stressed conditions, respectively.
Expression of human beta-defensins 2 (hBD2) and 3 (hBD3), two other major AMP, increases following activation of the signal transducer and transcriptional activator 1 (STAT1), STAT3, and NF-κB, whose consensus-binding sequences are localized in the promoter sequences of both hBD2 and hBD3 (9). Moreover, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) increases after Toll-like receptor (TLR) activation, stimulating hBD2 and hBD3 expression through NF-κB activation (10). Although 15d-PGJ2 is a promiscuous activator of the peroxisome proliferator-activated receptors (PPARs), PPARα, -β/δ, and -γ (11, 12), PPARγ activation does not stimulate hBD2 or hBD3 expression (10).
Cellular Cer and its proximal metabolite, S1P, increase in response to external perturbations (13, 14), serving as signaling molecules that can modulate multiple important cellular functions, including cellular proliferation, differentiation, and apoptosis (13, 15, 16). In contrast to Cer itself, which generates antimitogenic and proapoptotic signals, both S1P and another Cer metabolite, ceramide-1-phosphate (C1P), display promitogenic and antiapoptotic activities, while also stimulating cell motility, wound healing, and cytokine production (17–26). S1P alters cellular function through either a G protein-coupled family of S1P membrane receptors, or S1P-receptor-independent mechanisms (15, 27). While the putative C1P receptor has not yet been fully identified, C1P binds and activates cytosolic phospholipase A2a (cPLA2a) (28, 29), which increases arachidonic acid production, thereby potentially altering multiple cellular functions.
Since levels of hBD2 and hBD3, like CAMP, increased in epidermis following external perturbations (2), we hypothesized that Cer and/or one of its metabolites could also regulate hBDs following external perturbations that induce ER stress. We describe here a novel ER stress→C1P→ cPLA2a→15d-PGJ2→PPARα and/or PPARβ/δ→Src kinase→STAT1 and/or STAT3 pathway that regulates hBD2 and hBD3 (but not hBD1) production in human keratinocytes (KC) and other epithelial cell types. Since activation of this pathway exhibits killing activity against Staphylococcus aureus, this innate immune mechanism likely is important in regulating epithelial antimicrobial defense.
MATERIALS AND METHODS
Reagents.
Thapsigargin (Tg) and acetylsalicylic acid were purchased from Sigma-Aldrich (St. Louis, MO). N-Acetyl-sphingosine (C2-ceramide [C2Cer]), N-acetyl-ceramide-1-phosphate (C2C1P), and N-palmitoyl-ceramide-1-phosphate (C16C1P) were from Avanti Polar Lipids (Alabaster, AL). NVP-231 was from Calbiochem (Billerica, MA). Deoxynojirimycin (DNM), sphingomyelinase, pyrrophenone (Pyr), N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS398), 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), prostaglandin E2 (PGE2), GW9578, GW0742, GW1929, and 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2) were from Cayman Chemical (Ann Arbor, MI). N-Oleoylethanolamine (NOE) was from Matreya LLC. (Pleasant Gap, PA). LL-37 was from InvivoGen (San Diego, CA). GW6471, GSK0660, and 1-[3-[1-([1,1′-biphenyl]-3-ylmethyl)-1H-1,2,3-triazol-5-yl]phenyl]-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d-]pyrimidin-4-amine (KB-SRC4) were from Tocris Bioscience (Minneapolis, MN). Fludarabine (Flu) and AG490 were from Selleckchem (Houston, TX). STA-21 and BAY 11-7085 were from Enzo Life Sciences (Farmingdale, NY).
Cell culture.
Normal human keratinocytes (KC), isolated from neonatal foreskins, were grown in serum-free KC growth medium containing 0.07 mM calcium chloride and growth supplements (Invitrogen, Carlsbad, CA), as described previously (7). Culture media were switched to a medium containing 0.07 mM calcium chloride, without growth supplements, prior to treatment with Tg, C2Cer, or C16C1P, as described previously (8).
C16C1P was dissolved in 98% ethanol–2% dodecane (final concentrations in cultures, 0.1% ethanol and 0.002% dodecane) (28, 29). C2C1P was prepared as either as a bovine serum albumin (BSA) conjugate or dimethyl sulfoxide (DMSO) solution (final concentration of DMSO, 0.1%) to be delivered into cells in some studies as indicated (C16C1P is dissolved in neither methanol, ethanol, nor DMSO). Cell toxicities were not evident following treatments with Tg, C2Cer, and C1P assessed by trypan blue exclusion assay.
qRT-PCR.
Quantitative real-time PCRs (qRT-PCR) were performed using cDNA prepared from mRNA fractions of cell lysates, as we described previously (7). Briefly, 30 ng of cDNA was prepared from the mRNA fraction of cell lysates, specific primer sets (final concentration, 250 nM), and SYBR green reagents (SensiMix SYBR Hi-ROX, Bioline, Taunton, MA). The following specific primer sets were used: hBD1 primer, 5′-TCGCCATGAGAACTTCCTACCT-3′ and 5′-CTCCACTGCTGACGCAATTGTA-3′; hBD2, 5′-GGTGTTTTTGGTGGTATAGGCG-3′ and 5′-GGGCAAAAGACTGGATGACA-3′; hBD3, 5′-TCAGCTGCCTTCCAAAGGA-3′ and 5′-TTCTTCGGCATTTTCG-3′; CAMP, 5′-CACAGCAGTCACCAGAGGATTG-3′ and 5′-GGCCTGGTTGAGGGTCACT-3′; CERK, 5′-ACCAACCAGCAGGACCAGTT-3′ and 5′-TCGCTGTCCTCATCCTCCAT-3′; human STAT1, 5′-TTCAGGAAGACCCAATCCAG-3′ and 5′-TGAATATTCCCCGACTGAGC-3′; human STAT3, 5′-GGCGTCACTTTCACTTGGGT-3′ and 5′-CCACGGACTGGATCTGGGT-3′; and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GGAGTCAACGGATTTGGTCGTA-3′ and 5′-GCAACAATATCCACTTTACCAGAGTTAA-3′. The thermal cycling conditions were 95°C for 10 min, followed by 95°C for 15 s, 60°C for 15 s, and 72°C for 15 s, repeated 40 times on ABI Prism 7700 (Applied Biosystems, Foster City, CA). mRNA expression was normalized to levels of GAPDH.
Measurement of intercellular levels of C1P.
To assess the levels of cellular C1P, KC were incubated with Tg or C2Cer and washed with phosphate-buffered saline followed by extraction of total C1P, as we reported previously (30). C1P was derivatized with o-phthalaldehyde reagent and then quantitated using a high-performance liquid chromatography (HPLC) system equipped with a fluorometric detector system (JASCO, Tokyo, Japan), as described previously (30). C1P levels were expressed as pmol per mg protein.
siRNAs and transfections.
KC were transfected with 20 nM siRNA for CERK or STAT3 from Qiagen (Valencia, CA), STAT1 from Cell Signaling Technology, or nontargeted control siRNA from Dharmacon (Lafayette, CO) using siLentFect (Bio-Rad, Hercules, CA) for 24 h and then were treated with Tg, C2Cer, or C16C1P for a further 24 h. The efficiency of blockage was evaluated by qRT-PCR. siRNA significantly suppressed CERK mRNA, STAT1, or STAT 3 mRNA expression, with CERK at 30% of control siRNA-treated cells, STAT1 at 30%, or STAT3 at 15%.
Western blot analysis.
Western blot analyses were performed as described previously (7). The following antibodies were used: anti-CERK or anti-lamin B1 from Santa Cruz Biotechnology (Dallas, TX) or anti-phospho-STAT1 (Tyr-701) or anti-phospho-STAT3 (Tyr-705) antibody from Cell Signaling Technology (Boston, MA).
ELISA for 15d-PGJ2, BD2, or hBD3 secretion.
The contents of 15d-PGJ2 and hBD3 in conditioned medium from KC incubated with C16C1P were measured with enzyme-linked immunosorbent assay (ELISA) kits for either 15d-PGJ2 (Enzo Life Sciences) or hBD3 (GenWay Biotech, Inc., San Diego, CA), respectively.
Immunofluorescence analysis.
KC were pretreated with PPAR antagonists for 30 min prior to treatment with C16C1P for 15 min. Cellular localization of pSTAT1 or pSTAT3 was assessed using anti-phospho-STAT1 (Tyr-701) or anti-phospho-STAT3 (Tyr-705) (Cell Signaling Technology) and anti-rabbit IgG conjugated with fluorescein isothiocyanate (Invitrogen). Nuclei were stained with the marker 4′,6-diaminido-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA).
Antimicrobial assay.
Antimicrobial activity of conditioned medium collected from KC, previously treated with C16C1P, was tested against Staphylococcus aureus (RN6980 strain), as described previously (7). Briefly, S. aureus cells were grown to mid-log phase and adjusted to a concentration of 106 CFU/ml in phosphate-buffered saline (PBS), incubated with synthetic LL-37 (as a positive control) or with conditioned medium of KC cotreated C16C1P with or without fludarabine or STA-21 for 2 h. The number of bacteria that survived was determined by plating serial dilutions in PBS onto THY agar plates. After 24 h, the number of bacterial colonies was counted, and bacterial killing was calculated as recovered CFU/initial inoculum CFU × 100 (%).
Statistical analyses.
Statistical comparisons were performed using an unpaired Student's t test.
RESULTS
Identification of ceramide-1-phosphate as the sphingolipid metabolite that stimulates hBD2 and hBD3 production.
To investigate whether ER stress increases hBD production, we first treated cultured human keratinocytes (KC) with the established pharmacological ER stressor thapsigargin (Tg). qRT-PCR analysis showed that ER stress, induced by Tg, significantly increased mRNA expression not only of CAMP, as we showed recently (7), but also of hBD2 and hBD3 (but not hBD1) (Fig. 1A). In addition, exogenous cell-permeable, short-chain Cer (C2Cer) treatment also increased hBD2, hBD3, and CAMP (but not hBD1) mRNA production (Fig. 1A). Thus, ER stress, as well as Cer and/or one of its metabolites, increases production of CAMP, hBD2, and hBD3.
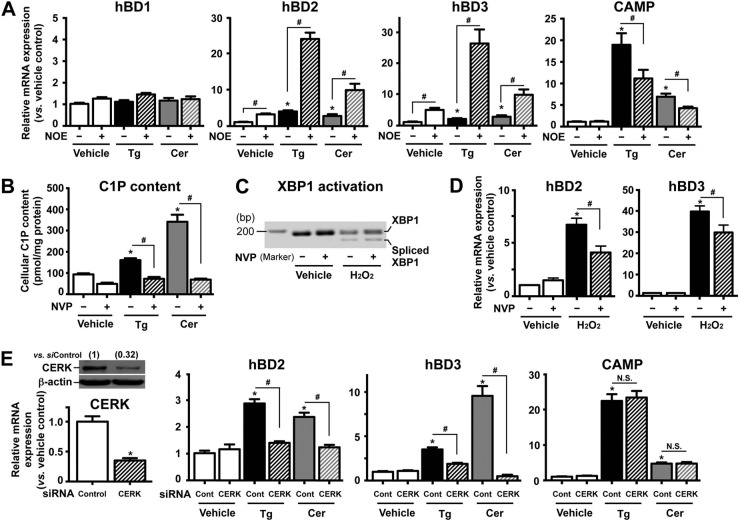
FIG 1.
C1P signals to stimulate hBD2 and hBD3 (but not hBD1 and CAMP) mRNA expression in response to ER stress. Human KC pretreated with or without ceramidase inhibitor (NOE [25 μM]) (A) or ceramide kinase inhibitor (NVP [50 nM]) (B to D) for 30 min or transfected with scrambled siRNA or CERK siRNA for 24 h (E) were incubated with or without thapsigargin (Tg [0.1 μM]), C2Cer (7.5 μM), or H2O2 (500 μM) for 24 h. The mRNA levels were assessed by qRT-PCR. (C) XBP1 mRNA splicing was assessed by PCR. (E) Levels of CERK expression were assessed by Western blotting and qRT-PCR. Similar results were obtained when the experiment was repeated (triplicate) using different cell preparations. Data are means ± standard deviation (SD) (n = 3). *, P < 0.01 versus vehicle control or scrambled siRNA-treated cells; #, P < 0.01 versus TG, Cer, or H2O2 without inhibitor or siRNA. N.S., not significant.
We next assessed whether Cer itself or one of its metabolites accounts for the ER stress-induced increase in hBD2 and hBD3 production. KC first were pretreated with N-oleoylethanolamine (NOE), a specific inhibitor of ceramidase, the enzyme that hydrolyzes Cer to sphingosine (the immediate precursor of S1P), followed by exposure of these cells to either Tg or C2Cer. While NOE pretreatment significantly attenuated the expected increase in CAMP expression, both the Tg- and C2Cer-induced increases in hBD2 and hBD3 production accelerated in such NOE-treated cells (Fig. 1A). In addition, exogenous S1P did not increase either hBD2 or hBD3 production (Fig. 2A). Thus, S1P does not account for the ER stress- or C2Cer-induced upregulation of hBD2 and hBD3. Rather, Cer itself or another Cer metabolite likely is responsible for the ER stress-induced increases in hBD2 and hBD3 expression.
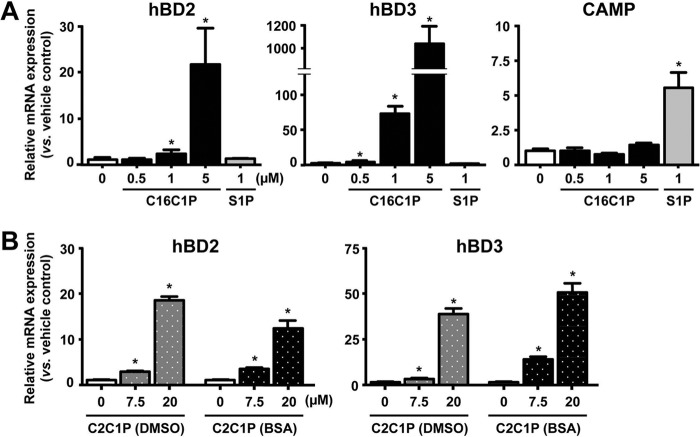
FIG 2.
C1P, but not S1P, stimulates hBD2 and hBD3 mRNA expression. Human KC were treated with C16C1P (A), C2C1P (B), or S1P (A and B) at the indicated concentration for 24 h. C16C1P and S1P were dissolved in dodecane-ethanol (A). C2C1P was delivered into cells as a DMSO solution or BSA conjugates (B). Data are means ± SD (n = 3). *, P < 0.01 versus vehicle control.
In addition to S1P, another Cer downstream metabolite, C1P, also functions as a signaling lipid. To assess whether C1P stimulates hBD2 and hBD3 expression, we first inhibited the ceramide kinase (CERK) that converts Cer to C1P, using a specific pharmacological inhibitor of CERK, NVP-231. While both Tg and C2Cer treatment increased cellular C1P content, NVP-231 pretreatment significantly suppressed the Tg- or C2Cer-induced increase in C1P production (Fig. 1B). Moreover, NVP-231 treatment significantly attenuated the expected Tg-induced increase in hBD2 and hBD3 but not CAMP production (Table 1). To further ascertain the role of C1P in hBD2 and hBD3 upregulation, cells were transfected with siRNA against CERK. Consistent with NVP-231 treatment, silencing of CERK significantly attenuated both the Tg- and C2Cer-induced increases in hBD2 and hBD3, without altering levels of CAMP production (Fig. 1E). Prior studies demonstrated that UVB irradiation increases both hBD2 and hBD3 production in both cultured human KC and human skin (4, 31), while we also showed UVB irradiation induces ER stress and increases hBD production (32). We further demonstrated that another oxidative stressor induced by exogenous H2O2 also induced ER stress (assessed by formation of the mature [spliced] form of XBP1, a universal indicator of ER stress) (Fig. 1C) and significantly increased production of both hBD2 and hBD3 (Fig. 1D). This increased hBD production was significantly suppressed by inhibition of CERK (Fig. 1D), giving further support to C1P as a signal to increase hBD2 and hBD3 expression in response to ER stress.
TABLE 1.
Inhibition of ceramide kinase diminished ER stress-induced hBD2 and hBD3 (but not CAMP) mRNA expression in human KC
| Treatmenta | Relative mRNA expression vs vehicle controlb |
||
|---|---|---|---|
| hBD2 | hBD3 | CAMP | |
| Vehicle | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| NVP-231 | 1.2 ± 0.1 | 1.5 ± 0.1 | 2.0 ± 0.2* |
| Tg | 12.0 ± 1.1* | 6.2 ± 0.3* | 93.9 ± 11.0* |
| Tg + NVP-231 | 8.1 ± 0.7# | 3.8 ± 0.4# | 91.9 ± 10.8* |
Normal human KC pretreated with or without ceramide kinase inhibitor (NVP-231 [50 nM]) for 30 min were incubated with an ER stressor, thapsigargin (Tg [0.1 μM]).
Values are means ± SD (n = 3). *, P < 0.01 versus vehicle control; #, P < 0.01 versus Tg alone.
To exclude the possibility that one or more additional Cer metabolites (e.g., glucosylceramide), the predominant glycosphingolipid species (>95%) in epidermis (33) and/or sphingomyelin (SM), stimulate hBD2 and hBD3 expression under similar conditions, we next cotreated KC with Tg plus a specific inhibitor of glucosylceramide synthase, deoxynojirimycin (DNM). Instead of attenuating AMP production, DNM cotreatment further enhanced the Tg-induced increase in hBD2, hBD3, and CAMP expression (Table 2). If SM signals to stimulate hBD production, exogenous sphingomyelinase, which hydrolyzes SM to Cer, significantly decreases cellular SM content (34) and results in suppression of hBD production following ER stress. Yet, both hBD2 expression and hBD3 expression were still increased in cells incubated with exogenous sphingomyelinase (Table 2). Together, these results suggest that C1P, but neither Cer nor other Cer metabolites (i.e., S1P, glucosylceramide, or SM), mediates the ER stress-induced enhancement of hBD2 and hBD3 production.
TABLE 2.
Neither glucosylceramide nor sphingomyelin stimulates hBD2, hBD3, and CAMP mRNA expression in human KC
| Treatmenta | Relative mRNA expression vs vehicle controlb |
||
|---|---|---|---|
| hBD2 | hBD3 | CAMP | |
| Vehicle | 1.0 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.1 |
| DNM | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.2 |
| Tg | 3.7 ± 0.2* | 3.4 ± 0.2* | 22.3 ± 1.9* |
| Tg + DNM | 4.8 ± 0.3# | 4.0 ± 0.2# | 8.3 ± 2.2# |
| Vehicle | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| SMase | 3.0 ± 0.2* | 7.8 ± 1.0* | 3.2 ± 0.3* |
Normal human KC pretreated with or without glucosylceramide synthase inhibitor (DNM [10 μM]) for 30 min were incubated with thapsigargin (Tg [0.1 μM]) or exogenous sphingomyelinase (SMase [0.1 U/ml]) for 24 h.
Values are means ± SD (n = 3). *, P < 0.01 versus vehicle control; #, P < 0.01 versus Tg alone.
We next directly assessed whether exogenous C1P stimulates hBD2 and hBD3 production. Treatment of KC with exogenous N-palmitoyl-ceramide-1-phosphate (C16C1P) produced a dose-dependent increase in hBD2 and hBD3 production (Fig. 2A) but altered neither CAMP nor hBD1 production (not shown). Moreover, exogenous C2C1P delivered into cells, as a BSA conjugate or in DMSO, significantly increased both hBD2 and hBD3 production (Fig. 2B), while as expected, S1P increased CAMP but not hBD2 or hBD3 production (Fig. 2A). Similar to a prior study showing more potent effects of C1P in dodecane-ethanol signaling on altering cellular function (35), C16C1P delivery into cells using dodecane-ethanol showed a higher induction of hBD production compared with other delivery systems. Consistent with increases in mRNA expression, production of both hBD2 and hBD3 proteins was increased (hBD2, vehicle, 6.88 ± 0.00 pg/mg protein, and C1P, 16.35 ± 1.3 pg/mg protein [P < 0.01]; hBD3, vehicle, 8.21 ± 1.6 pg/mg protein, and C1P, 587.9 ± 6.19 pg/mg protein [P < 0.01], versus vehicle control). These results further demonstrate that C1P stimulates hBD2 and hBD3 (but not CAMP) production. Neither C2C1P in DMSO, BSA conjugate, nor C16C1P (dissolved in dodecane-ethanol) altered cell viability under our experimental conditions (not shown). We employed C16C1P at concentrations of 1 μM in most of the studies described below.
Finally, we investigated whether the C1P-induced increases in hBD2 and hBD3 production occur not only in cultured KC but also in other epithelial cells (i.e., human cervical carcinoma [HeLa] and bronchogenic carcinoma [A549] cells). hBD2 and hBD3 mRNA production also increased significantly in these epithelial cells in response to C16C1P treatment (Table 3). These results indicate that C1P stimulates hBD2 and hBD3 production in a variety of epithelial cells through a C1P-dependent signal.
TABLE 3.
C1P stimulates hBD2 and hBD3 mRNA expression through STAT1 and STAT3 activation in extracutaneous epithelial cells
| Treatmenta | Relative mRNA expression versus vehicle controlb |
|||
|---|---|---|---|---|
| HeLa cells |
A549 cells |
|||
| hBD2 | hBD3 | hBD2 | hBD3 | |
| Vehicle | 1.0 ± 0.1 | 1.0 ± 0.4 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| Fludarabine | 1.1 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.8 |
| STA-21 | 1.3 ± 0.1 | 0.7 ± 0.5 | 1.0 ± 0.2 | 1.6 ± 0.4 |
| C16C1P | 2.1 ± 0.2* | 4.0 ± 0.7* | 2.5 ± 0.3* | 8.9 ± 1.2* |
| C16C1P + fludarabine | 0.9 ± 0.3# | 1.0 ± 0.3# | 0.7 ± 0.1# | 3.3 ± 0.7# |
| C16C1P + STA-21 | 1.2 ± 0.2# | 1.2 ± 0.2# | 1.3 ± 0.3# | 4.3 ± 1.1# |
HeLa cells or A549 cells were pretreated with or without STAT1 inhibitor (fludarabine [10 μM]) or STAT3 inhibitor (STA-21 [2 μM]) for 30 min and then were incubated with C16C1P (1 μM) for 24 h.
Values are means ± SD (n = 3). *, P < 0.01 versus vehicle control; #, P < 0.01 versus C1P alone.
cPLA2a and COX1/2 activation accounts for C1P-mediated stimulation of hBD2 and hBD3 production.
Prior studies showed that C1P binds directly to cytosolic phospholipase A2a (cPLA2a), leading to increased prostanoid generation by enhancing its catalytic activity (28, 29). Hence, we next assessed whether cPLA2a activation serves as a downstream signal of hBD2 and hBD3 production. While a specific cPLA2a inhibitor, pyrrophenone (Pyr), itself did not alter basal expression of hBD2 and hBD3, it significantly diminished C16C1P-induced upregulation of hBD2 and hBD3 (Fig. 3A).
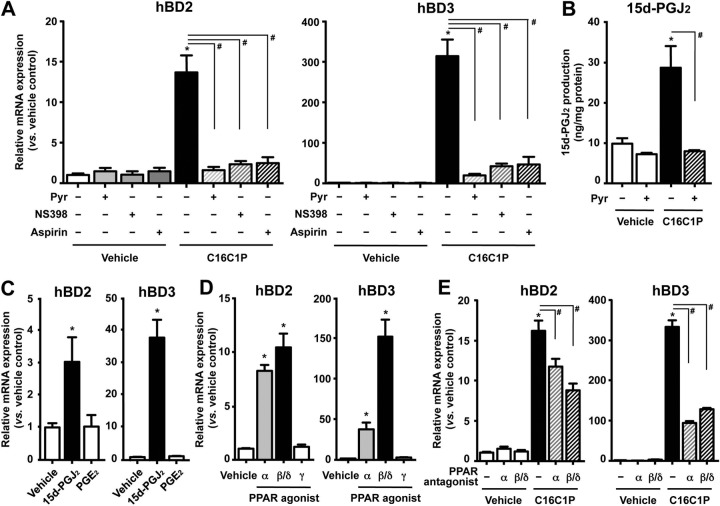
FIG 3.
C1P-mediated increases in PGJ2 followed by PPARα or PPARβ/δ (but not PPARγ) activation are responsible for a downstream signal of C1P-induced hBD2 and hBD3 production. Normal human KC were treated with or without C16C1P (1 μM) (A, B, and E), prostanoids (15d-PGJ2 [2.5 μM] or PGE2 [10 μM]) (C), or a PPAR agonist (PPARα, GW9578 [5 μM], PPARβ/δ, GW0742 [5 μM]; PPARγ, GW1929 [10 μM]) (D) for 24 h. Cells were pretreated with cPLA2 inhibitor (Pyr [0.01 μM]), COX inhibitor (NS398 [1 μM] or aspirin [100 μM]) (A and B), or PPAR antagonist (PPARα, GW6471 [1 μM]; or PPARβ/δ, GSK0660 [1 μM]) (E) for 30 min. Data are means ± SD (n = 3). *, P < 0.01 versus vehicle control; #, P < 0.01 versus C1P alone.
The arachidonic acid released by cPLA2a is immediately catabolized to prostaglandin H2 by either cyclooxygenase 1 (COX-1) or COX-2. To further elucidate the downstream pathway whereby C1P signals increased hBD2 and hBD3 production, we next cotreated KC with either acetylsalicylic acid (aspirin), which inhibits both COX-1 and COX-2, or NS398, a specific inhibitor of COX-2. Both aspirin and NS398 significantly suppressed the expected C16C1P-induced increase in hBD2 and hBD3 mRNA production (Fig. 3A). Prior studies showed that C1P (10 μM), which displays cellular toxicity in dodecane-ethanol, showed a nonspecific cPLA2 activation (36). As described above, cell toxicities were not evident under our experimental conditions. Moreover, C1P delivered as a BSA conjugate and in DMSO into cells also stimulates hBD production (Fig. 2). Together, these results indicate that C1P-induced activation of cPLA2a is required for the COX1/2-mediated conversion of arachidonic acid to prostaglandin H2, leading to increased hBD2 and hBD3 production.
15d-PGJ2 is required for the C1P enhancement of hBD2 and hBD3 production.
Prior studies demonstrated that PGD2 and its metabolite, 15d-PGJ2, but neither PGE2, PGF2α, iloprost (a stable analog of prostaglandin I2 [PGI2]), nor U-46619 (a thromboxane A2 receptor agonist), increase hBD2 and hBD3 production (10). Hence, to identify which prostanoid is responsible for the C1P-induced stimulation of hBD2 and hBD3 production, we next measured 15d-PGJ2 levels in KC following treatment with exogenous C16C1P. Exogenous C16C1P significantly increased 15d-PGJ2 levels (Fig. 3B), while the cPLA2a inhibitor Pyr diminished the expected C16C1P-induced increase in 15d-PGJ2 levels. Accordingly, exogenous 15d-PGJ2 significantly increased hBD2 and hBD3 production, while PGE2 treatment had no effect on either hBD2 or hBD3 production (Fig. 3C). Because an increase in arachidonate production by activation of cPLA2 is required for prostanoid generation, these results further support C1P stimulation of the catalytic activity of cPLA2. These results suggest that the C1P-mediated increase in levels of 15d-PGJ2 likely accounts for the stimulation of hBD2 and hBD3 production.
15d-PGJ2 is a promiscuous activator (ligand) of the PPARα, PPARβ/δ, and PPARγ (37). Hence, we next investigated which PPAR isoform or isoforms are required for the C1P-induced increase in hBD2 and/or hBD3 production. KC were treated with specific agonists of PPARα (GW9578), PPARβ/δ (GW0742), or PPARγ (GW1929). While treatment with GW9578 or GW0742 significantly increased hBD2 and hBD3 production, GW1929 (PPARγ agonist) had no effect on either hBD2 or hBD3 production (Fig. 3D). Pertinently, blockade of PPARα and PPARβ/δ activation by pretreatment with specific PPARα or PPARβ/δ antagonists (i.e., GW6471 or GSK0660, respectively), significantly suppressed C16C1P-induced stimulation of hBD2 and hBD3 production (Fig. 3E). Together, these results indicate that C1P-mediated activation of PPARα and/or PPARβ/δ but not PPARγ accounts for increased hBD2 and hBD3 production.
C1P enhances hBD2/3 production through STAT1 and STAT3 activation.
While PPAR response element (PPRE) sequences have not been identified in either promoter of hBD2 or hBD3 (38), the promoters of both hBDs contain binding sequences for numerous transcription factors, including STAT1, STAT3, and NF-κB. Moreover, activation of both STAT and NF-κB stimulates hBD2 and hBD3 transcription (39). Therefore, we next investigated the role of STAT1, STAT3, and NF-κB in C1P-induced hBD2 and hBD3 upregulation. While either of the specific pharmacological inhibitors fludarabine (STAT1) and STA-21 (STAT3) alone did not alter the basal expression of either hBD2 nor hBD3, pretreatment with these inhibitors significantly attenuated the expected C16C1P-induced increases in hBD2 and hBD3 production (Fig. 4A). Moreover, consistent with a pharmacological intervention of STAT1 or STAT3 activity, siRNA against STAT1 or STAT3 significantly suppressed both hBD2 and hBD3 mRNA expression (Fig. 4B and C) but neither hBD1 nor CAMP expression (data not shown). In addition, both fludarabine and STA-21 also diminished the PPARα- or PPARβ/δ-agonist-induced stimulation of hBD2 and hBD3 production (Table 4). In contrast to the involvement of STATs, pretreatment of KC with the NF-κB inhibitor BAY 11-7085 altered neither hBD2 nor hBD3 production following C16C1P treatment (hBD2 mRNA results: vehicle, 1.0 ± 0.2; BAY 11-7085, 1.3 ± 0.1; C1P, 7.6 ± 1.1 [P < 0.01]; and C1P plus BAY 11-7085, 7.8 ± 0.9 [P < 0.01]; hBD3 mRNA results: vehicle, 1.0 ± 0.1; BAY 11-7085, 0.9 ± 0.2; C1P, 144.8 ± 17.3 [P < 0.01]; and C1P plus BAY 11-7085, 140.9 ± 15.0 [P < 0.01]; P values are versus vehicle control). Finally, inhibitors of STAT1 or STAT3 diminished the C16C1P-induced increases in hBD2 and hBD3 production in HeLa and A549 cells, as in KC (Table 3). These results suggest that C1P stimulates hBD2 and hBD3 production in epithelial cells through C1P→STAT1 and STAT3 activation.
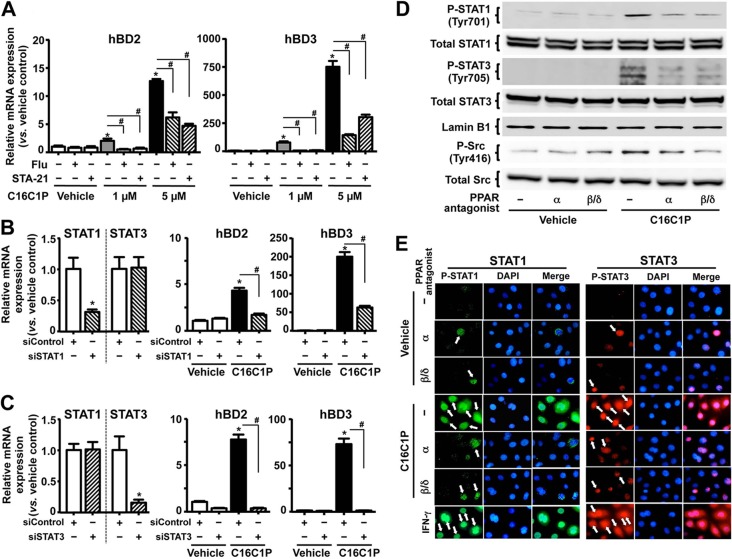
FIG 4.
PPAR-mediated activation of STAT1 and STAT3 is responsible for a transcriptional activation of C1P-induced hBD2 and hBD3 mRNA expression. Normal human KC pretreated with STAT1 inhibitor (fludarabine [10 μM]) or STAT3 inhibitor (STA-21 [2 μM]) (A) or transfected with scrambled siRNA, STAT1 siRNA (B), or STAT3 siRNA (C) were treated with C16C1P for 24 h. The mRNA levels of hBD2 and hBD3 were assessed by qRT-PCR. (D and E) Western blot analysis (D) and immunohistochemistry (E) of phosphorylation of STAT1 (Tyr-701) or STAT3 (Tyr-705). The PPAR antagonists were as follows: PPARα, GW6471 (5 μM); PPARβ/δ, GSK0660 (5 μM). Cells were also treated with a known STAT1 and STAT3 activator, interferon gamma (IFN-γ [20 ng/ml]) (56), which was used as a positive control for STAT1 and STAT3 activation. Activated STAT1 or STAT3 by phosphorylation at a tyrosine (Tyr) residue of STAT1 (Tyr-701) and STAT3 (Tyr-705) was translocated to the nucleus (indicated by arrows).
TABLE 4.
STAT1 and STAT3 account for PPARα or PPARβ/δ-mediated stimulation of hBD2 and hBD3 mRNA expression in human KC
| Treatmenta | Relative mRNA expression vs vehicle controlb |
|
|---|---|---|
| hBD2 | hBD3 | |
| Vehicle | 1.0 ± 0.1 | 1.0 ± 0.0 |
| Fludarabine | 0.4 ± 0.1 | 2.1 ± 0.3 |
| STA-21 | 0.5 ± 0.2 | 1.7 ± 0.2 |
| PPARα agonist | 4.1 ± 0.3* | 30.1 ± 6.4* |
| PPARα + fludarabine | 1.9 ± 0.3# | 13.1 ± 1.5# |
| PPARα + STA-21 | 1.2 ± 0.5# | 14.4 ± 3.3# |
| PPARβ/δ agonist | 5.2 ± 0.6* | 103.9 ± 13.1* |
| PPARβ/δ + fludarabine | 0.8 ± 0.4# | 38.1 ± 4.5# |
| PPARβ/δ + STA-21 | 0.9 ± 0.0# | 11.7 ± 1.9# |
Normal human KC were pretreated with an inhibitor of STAT1 (fludarabine [10 μM]) or STAT3 (STA-21 [2 μM]) for 30 min and then were incubated with or without a PPARα agonist (GW9578 [5 μM]) or PPARβ/δ agonist (GSK0742 [5 μM]) for 24 h.
Values are means ± SD (n = 3). *, P < 0.01 versus vehicle control; #, P < 0.01 versus each agonist alone.
Activation of STAT1 or STAT3 requires phosphorylation at a tyrosine (Tyr) residue of STAT1 (Tyr-701) and STAT3 (Tyr-705), leading to their dimerization and translocation to the nucleus, where they activate transcription of target genes (40). Thus, we next assessed whether either STAT1 and/or STAT3 is phosphorylated following C16C1P treatment. Western blot analyses revealed that C16C1P treatment increased both Tyr-701-phosphorylated STAT1 and Tyr-705-phosphorylated STAT3 levels, while antagonists of either PPARα (GW6471) or PPARβ/δ (GSK0660) significantly diminished C16C1P-induced phosphorylation of STAT1 and STAT3 (Fig. 4D). Finally, antagonists of both PPARα and PPARβ/δ significantly attenuated the C16C1P-induced nuclear translocation of these transcription factors (Fig. 4E). Together, these results indicate that C1P activates PPARα and/or PPARβ/δ, followed by activation of STAT1 and STAT3, leading to stimulation of hBD2 and hBD3 production.
Because tyrosine kinases, such as Janus kinase (JAK) and Src kinase, phosphorylate STAT (41), we next assessed the role of these kinases in the C1P-mediated stimulation of hBD2 and hBD3 expression. A specific JAK inhibitor, AG490, did not attenuate the C16C1P-induced increase in hBD2 and hBD3 production (hBD2 mRNA results: vehicle, 1.0 ± 0.0; AG490, 1.1 ± 0.0; C1P, 13.5 ± 1.2 [P < 0.01]; and C1P plus AG490, 13.0 ± 2.4 [P < 0.01]; hBD3 mRNA results: vehicle, 1.0 ± 0.0; AG490, 1.1 ± 0.3; C1P, 232.0 ± 20.1 [P < 0.01]; and C1P plus AG490, 236.8 ± 12.4 [P < 0.01]; P values are versus vehicle control). In contrast, either of two Src-specific inhibitors, PP2 or KB-SRC4, which is a derivative of PP2 eliminating c-Abl kinase inhibition (42), significantly blocked C16C1P-induced hBD2 and hBD3 production (Table 5). Furthermore, these Src inhibitors also attenuated the PPARα- or PPARβ/δ-agonist-induced increases in hBD2/3 production (Table 5). Since the promoter region of Src contains PPRE (43), we next assessed whether the Src protein level is increased in cells and results in increasing catalytic activity following C1P treatment. Yet, neither C1P (Fig. 4D), PPARα, nor PPARβ/δ agonists (data not shown) affected Src protein levels. Therefore, we next studied activation of Src kinase by its phosphorylation. C16C1P markedly increased Tyr-416 phosphorylation of Src, while either the PPARα or PPARβ/δ antagonist significantly diminished C16C1P-induced increases (Fig. 4D). Together, these data indicate that STAT1/3 phosphorylation by Src kinase is required for the C1P-induced upregulation of hBD2 and hBD3 production.
TABLE 5.
Inhibition of Src kinase diminished C1P-induced hBD2 and hBD3 mRNA expression in human KC
| Treatmenta | Relative mRNA expression vs vehicle controlb |
|
|---|---|---|
| hBD2 | hBD3 | |
| Vehicle | 1.0 ± 0.0 | 1.0 ± 0.1 |
| KB-SRC4 | 1.3 ± 0.4 | 1.5 ± 0.1 |
| PP2 | 1.2 ± 0.3 | 0.6 ± 0.1 |
| C16C1P | 19.3 ± 0.9* | 394.4 ± 24.7* |
| C16C1P + KB-SRC4 | 1.2 ± 0.4# | 5.1 ± 0.1# |
| C16C1P + PP2 | 2.6 ± 0.6# | 2.6 ± 0.1# |
| PPARα agonist | 3.2 ± 0.2* | 3.6 ± 0.4* |
| PPARα + KB-SRC4 | 1.3 ± 0.4# | 1.1 ± 0.4# |
| PPARα + PP2 | 0.7 ± 0.2# | 0.6 ± 0.1# |
| PPARβ/δ agonist | 3.4 ± 0.1* | 17.5 ± 0.9* |
| PPARβ/δ + KB-SRC4 | 1.2 ± 0.1# | 6.3 ± 0.3# |
| PPARβ/δ + PP2 | 1.3 ± 0.3# | 6.7 ± 0.4# |
Normal human KC were pretreated with Src kinase inhibitors (PP2 or KG-SRC4 [1 μM]) for 30 min and then were incubated with or without C16C1P (1 μM), PPARα agonist (GW9578 [5 μM]), or PPARβ/δ agonist (GW0742 [5 μM]) for 24 h.
Values are means ± SD (n = 3). *, P < 0.01 versus vehicle control; #, P < 0.01 versus C1P or each agonist alone.
C1P signaling enhances antimicrobial activity against a virulent pathogen.
We next examined the potential importance of the C1P-driven mechanism by determining whether C1P-induced hBD3 production enhances defense against a virulent strain of S. aureus (Note that hBD2 demonstrates little killing activity against this pathogen [44, 45].) Conditioned medium from KC previously exposed to C16C1P (1 μM) showed killing activity against S. aureus that was comparable to that of synthetic LL-37 (the positive control) (Table 6). In contrast, conditioned medium from KC cotreated with C16C1P plus either of the two STAT inhibitors (fludarabine and STA-21) attenuated the expected C16C1P-induced increase in antimicrobial activity. Notably, neither the STAT1 nor STAT3 inhibitor alone altered virulent S. aureus viability. Finally, we assessed the hBD3 content of conditioned medium following treatment with C16C1P. While the expected increase in the hBD3 content of conditioned medium occurred following C16C1P treatment, this increase was blocked when KC were cotreated with either inhibitors of STAT1 (fludarabine) or STAT3 (STA-21) (Table 6). Since C16C1P did not alter CAMP production (as described above), the S. aureus killing activity observed here can be attributed to C1P stimulation of hBD3 production. Together, these results show that C1P-driven stimulation of hBD3 production via STAT1 and STAT3 represents a potentially important antimicrobial mechanism against microbial pathogens.
TABLE 6.
C1P signal stimulates sufficient levels of hBD3 production to kill Staphylococcus aureus
| Treatmenta | Antimicrobial activity (% of vehicle control)b | hBD3 secretion (pg/mg protein)b,c |
|---|---|---|
| Vehicle | 100.0 ± 9.9 | 8.21 ± 1.6 |
| Fludarabine | 104.7 ± 9.8 | 8.96 ± 0.6 |
| STA-21 | 103.8 ± 18.8 | 9.86 ± 3.9 |
| C16C1P | 62.3 ± 2.8* | 587.93 ± 61.9 |
| C16C1P + fludarabine | 99.1 ± 15.8 | 76.18 ± 25.4 |
| C16C1P + STA-21 | 109.4 ± 1.6 | 71.09 ± 47.9 |
| LL-37 (positive control) | 5.9 ± 3.3* |
Normal human KC were incubated with STAT1 inhibitor (fludarabine [10 μM]), STAT3 inhibitor (STA-21 [2 μM]), and/or C16C1P (1 μM). S. aureus killing activity in the conditioned medium of cells was assessed.
Values are means ± SD (n = 3). *, P < 0.01 versus vehicle control.
hBD3 content in the conditioned medium was measured by ELISA (n = 2).
DISCUSSION
Many types of external perturbations, including microbial infections, increase hBD2, hBD3, and CAMP production in epithelial cells (2, 5, 41), but the mechanisms responsible have not yet been fully characterized. When investigating how these perturbants stimulate AMP production, we recently found that these various unrelated forms of external perturbations induce ER stress, which in turn increases production of Cer and S1P (8). The latter downstream metabolite then stimulates production of a major AMP (i.e., CAMP [7]). While VDR-dependent stimulation of CAMP production has been well characterized (46), we found that VDR transactivation instead is suppressed in cells under similar ER-stressed conditions, suggesting that VDR- and S1P-dependent pathways maintain CAMP production under basal versus stressed conditions, respectively (8). We now show here that another ER stress-induced Cer metabolite, C1P, upregulates two other key AMP (i.e., hBD2 and hBD3) in epithelial cells exposed to similar conditions. Our prior studies, as well as the present study, suggest that these ER stress-induced Cer metabolites (S1P and C1P) serve as sensors of external perturbations that in turn stimulate divergent defensive mechanisms that enhance epithelial antimicrobial defense via production of diverse innate immune elements (i.e., CAMP, hBD2, and hBD3).
To ensure that the C1P-dependent increase in hBD2 and hBD3 is relevant for antimicrobial defense, we assessed whether activation of the C1P-initiated pathway suffices to combat virulent S. aureus. While this pathogen is not susceptible to hBD2, elevations in hBD2 increase hBD3-stimulated killing of S. aureus (44). Hence, the costimulation of hBD2 and hBD3 by C1P likely enhances defense against a broad array of microbial pathogens that could attack the host when external-facing epithelia, such as the epidermis and other mucosal epithelia, are compromised. These studies also suggest that pharmacological modulation of the C1P signal could provide a new mechanism-driven therapeutic approach to prevent or treat selected microbial pathogen invasion. Since C1P alone stimulates this mechanism, this approach could be useful not only under stressed conditions but also under basal conditions, perhaps providing effective preventive therapy as well.
Since we have used (primary) cultured normal human KC from different donors, with somewhat different responses to chemicals and stimuli, including C1P, hBD2 and hBD3 expression levels in cell in response to C1P, there were variations between experiments (hBD2 mRNA, 3- to ∼15-fold, and hBD3 mRNA, 75- to ∼300-fold, versus the vehicle control). Nevertheless, C1P reproducibly increased production of both hBD3 and hBD2, and increases in hBD3 were higher than hBD2 in all cells, suggesting that C1P is a ubiquitous signaling Cer metabolite to stimulate hBD2 and hBD3 synthesis.
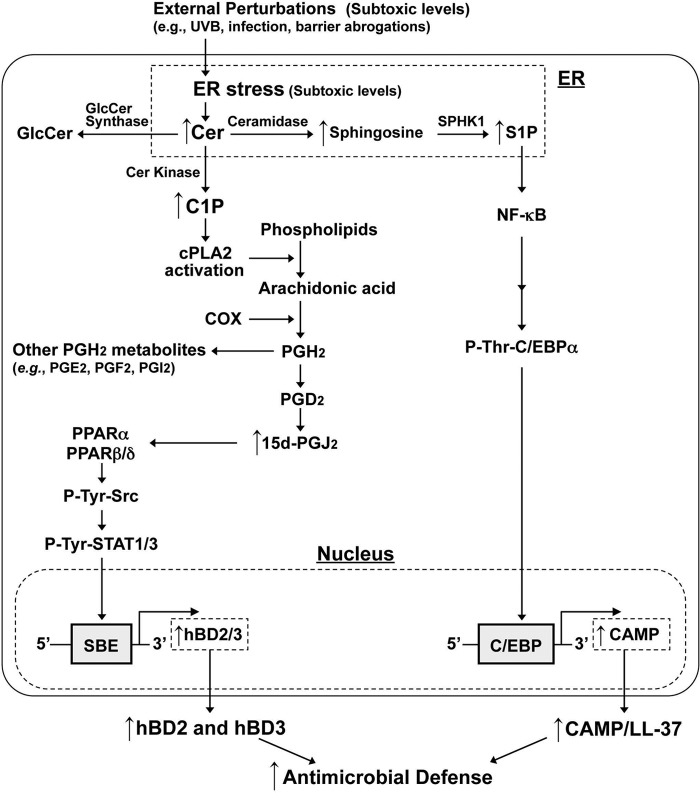
Although the biological roles of C1P are still not fully characterized, we elucidated here an important role for this Cer metabolite in regulating innate immunity, as well as the responsible metabolic and transcriptional mechanisms (Fig. 5). An increase in C1P activates cPLA2a, which results in increasing PGJ2 production, which in turn activates PPARα and PPARβ/δ. Increased Src kinase synthesis, which is transcriptionally regulated by PPARα and β/δ (43), is likely responsible for STAT1 and STAT3 activation through their phosphorylation (47–49). An increase in C1P activates cPLA2a, which results in increasing PGJ2 production, leading to activation of PPARα and PPARβ/δ followed by Src kinase activation. Prior studies demonstrated that PPAR-dependent Src kinase activation occurs through increases in transcriptional regulation of Src or Src phosphorylation (43, 50). Our results indicate that PPAR stimulated Src phosphorylation rather than Src production, leading to STAT activation (47, 51). In addition, a recent study showed that PPARβ/δ activation enhances mRNA expression of CERK, increasing C1P levels in response to external perturbations, such as barrier disruption, resulting in enhanced epithelial cell survival (52). Since we demonstrated here that ER stress increases production of C1P, leading to activation of PPARα and PPARβ/δ, increased C1P-PPAR signaling likely stimulates a feed-forward mechanism that enhances antimicrobial defense through upregulation of hBD2 and hBD3 production.
FIG 5.
Proposed mechanism of C1P-mediated induction of hBD2 and hBD3 in human KC.
Previous studies have shown that TLR2/6 or TLR3 ligand activation increases 15d-PGJ2 expression, which in turn induces hBD2 and hBD3 mRNA expression in primary human KC via NF-κB-dependent transcriptional regulation (10). Yet, 15d-PGJ2 is a potent inhibitor of the NF-κB signaling pathways: i.e., 15d-PGJ2 inhibits the IKK phosphorylation that is required for NF-κB activation (53, 54), while also inhibiting the NF-κB binding to DNA by alkylation of the NF-κB DNA-binding domain (55). Accordingly, we show here that an inhibitor of NF-κB did not alter C1P-induced hBD2 or hBD3 production in human epithelial cells. The differences between prior studies and ours could reflect pathways that regulate epithelial antimicrobial defense by divergent mechanisms: i.e., TLRs versus ER stress. Why TLR activation and ER stress induce different downstream pathways that stimulate hBD2 and hBD3 production is unclear, but the presence of such dual mechanisms could sustainably enhance antimicrobial defense in response to the multiple different forms of external perturbations that occur under in vivo conditions.
In summary, our present and recent studies have uncovered and characterized two antimicrobial defense mechanisms that are stimulated in epithelial cells in response to external perturbations through stimulation of an ER stress-initiated production of hBD2 and hBD3 and CAMP/LL-37, respectively, by C1P- and S1P-initiated signals.
ACKNOWLEDGMENTS
We thank Lillian Adame and Sally Pennypacker for technical support in cell culture. We thank Joan Wakefield for superb editorial assistance. We gratefully acknowledge the support of the Medical Research Services of the Veterans Affairs Medical Center, San Francisco.
Y.-I.K. was the recipient of Research Fellowship 2012 from the Honjo International Scholarship Foundation (Japan). This study was supported by the San Francisco Foundation, National Rosacea Society, and National Institutes of Health grants AR051077 and AR062025 (from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to Y.U.).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We state we have no conflicts of interest.
Footnotes
Published ahead of print 13 October 2014
REFERENCES
- 1.Lai Y, Gallo RL. 2009. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30:131–141. 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, Choi EH, Kim DK, Schroder JM, Feingold KR, Elias PM. 2008. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Invest. Dermatol. 128:917–925. 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258–15263. 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 4.Glaser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schroder JM, Schwarz A, Schwarz T. 2009. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J. Allergy Clin. Immunol. 123:1117–1123. 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, Lee SH, Elias PM, Choi EH. 2008. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J. Invest. Dermatol. 128:2880–2887. 10.1038/jid.2008.169. [DOI] [PubMed] [Google Scholar]
- 6.Mendez-Samperio P. 2010. The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections. Peptides 31:1791–1798. 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, Uchida Y. 2011. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J. Biol. Chem. 286:34121–34130. 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, Gallo RL, Saba J, Holleran WM, Uchida Y. 2013. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol. Cell. Biol. 33:752–762. 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond G, Kaiser V, Rhodes J, Russell JP, Bevins CL. 2000. Transcriptional regulation of beta-defensin gene expression in tracheal epithelial cells. Infect. Immun. 68:113–119. 10.1128/IAI.68.1.113-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard JJ, Gallo RL. 2010. Cyclooxygenase-2 enhances antimicrobial peptide expression and killing of Staphylococcus aureus. J. Immunol. 185:6535–6544. 10.4049/jimmunol.1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop-Bailey D, Hla T. 1999. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J. Biol. Chem. 274:17042–17048. 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 12.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. 1995. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 270:23975–23983. 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 13.Uchida Y. 2014. Ceramide signaling in mammalian epidermis. Biochim. Biophys. Acta 1841:453–462. 10.1016/j.bbalip.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, Hannun YA, Radin NS, Elias PM, Holleran WM. 2010. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J. Invest. Dermatol. 130:2472–2480. 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- 15.Mendelson K, Evans T, Hla T. 2014. Sphingosine 1-phosphate signalling. Development 141:5–9. 10.1242/dev.094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truman JP, Garcia-Barros M, Obeid LM, Hannun YA. 2014. Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochim. Biophys. Acta 1841:1174–1188. 10.1016/j.bbalip.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arana L, Ordonez M, Ouro A, Rivera IG, Gangoiti P, Trueba M, Gomez-Munoz A. 2013. Ceramide 1-phosphate (C1P) induces macrophage chemoattractant protein-1 release: involvement in C1P-stimulated cell migration. Am. J. Physiol. Endocrinol. Metab. 304:E1213–E1226. 10.1152/ajpendo.00480.2012. [DOI] [PubMed] [Google Scholar]
- 18.Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. 2012. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim. Biophys. Acta 1823:439–450. 10.1016/j.bbamcr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Gangoiti P, Granado MH, Arana L, Ouro A, Gomez-Munoz A. 2008. Involvement of nitric oxide in the promotion of cell survival by ceramide 1-phosphate. FEBS Lett. 582:2263–2269. 10.1016/j.febslet.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Munoz A, Duffy PA, Martin A, O'Brien L, Byun HS, Bittman R, Brindley DN. 1995. Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: antagonism by cell-permeable ceramides. Mol. Pharmacol. 47:833–839. [PubMed] [Google Scholar]
- 21.Granado MH, Gangoiti P, Ouro A, Arana L, Gomez-Munoz A. 2009. Ceramide 1-phosphate inhibits serine palmitoyltransferase and blocks apoptosis in alveolar macrophages. Biochim. Biophys. Acta 1791:263–272. 10.1016/j.bbalip.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Hoeferlin LA, Wijesinghe DS, Chalfant CE. 2013. The role of ceramide-1-phosphate in biological functions. Handb. Exp. Pharmacol. 215:153–166. 10.1007/978-3-7091-1368-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C, Schneider G, Abdel-Latif A, Mierzejewska K, Sunkara M, Borkowska S, Ratajczak J, Morris AJ, Kucia M, Ratajczak MZ. 2013. Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells—implications for tissue regeneration. Stem Cells 31:500–510. 10.1002/stem.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra P, Maceyka M, Payne SG, Lamour N, Milstien S, Chalfant CE, Spiegel S. 2007. Ceramide kinase regulates growth and survival of A549 human lung adenocarcinoma cells. FEBS Lett. 581:735–740. 10.1016/j.febslet.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Sassoli C, Formigli L, Bini F, Tani A, Squecco R, Battistini C, Zecchi-Orlandini S, Francini F, Meacci E. 2011. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J. Cell. Mol. Med. 15:2498–2511. 10.1111/j.1582-4934.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauer B, Vogler R, von Wenckstern H, Fujii M, Anzano MB, Glick AB, Schafer-Korting M, Roberts AB, Kleuser B. 2004. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J. Biol Chem. 279:38471–38479. 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- 27.Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B, Tran-Van-Nhieu J, Manin S, Poelstra K, Chun J, Carpentier S, Levade T, Mallat A, Lotersztajn S. 2007. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 21:2005–2013. 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- 28.Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. 2007. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J. Biol Chem. 282:20467–20474. 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE. 2005. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J. Biol. Chem. 280:17601–17607. 10.1074/jbc.M414173200. [DOI] [PubMed] [Google Scholar]
- 30.Shin HW, Kim D, Lee Y, Yoo HS, Lee BJ, Kim J S, Jang S, Lim H, Lee Y, Oh S. 2009. Alteration of sphingolipid metabolism and pSTAT3 expression by dietary cholesterol in the gallbladder of hamsters. Arch. Pharm. Res. 32:1253–1262. 10.1007/s12272-009-1911-9. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy Crispin M, Fuentes-Duculan J, Gulati N, Johnson-Huang LM, Lentini T, Sullivan-Whalen M, Gilleaudeau P, Cueto I, Suarez-Farinas M, Lowes MA, Krueger JG. 2013. Gene profiling of narrowband UVB-induced skin injury defines cellular and molecular innate immune responses. J. Invest. Dermatol. 133:692–701. 10.1038/jid.2012.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchida Y, Park K. 2013. Anti-microbial peptides in skin barrier functions. J. Skin Barrier Res. 15:1–8. [Google Scholar]
- 33.Uchida Y, Iwamori M, Nagai Y. 1988. Distinct differences in lipid composition between epidermis and dermis from footpad and dorsal skin of guinea pigs. Jpn. J. Exp. Med. 58:153–161. [PubMed] [Google Scholar]
- 34.Uchida Y, Murata S, Schmuth M, Behne MJ, Lee JD, Ichikawa S, Elias PM, Hirabayashi Y, Holleran WM. 2002. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J. Lipid Res. 43:1293–1302. 10.1194/jlr.M100442-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Wijesinghe DS, Subramanian P, Lamour NF, Gentile LB, Granado MH, Bielawska A, Szulc Z, Gomez-Munoz A, Chalfant CE. 2009. Chain length specificity for activation of cPLA2alpha by C1P: use of the dodecane delivery system to determine lipid-specific effects. J. Lipid Res. 50:1986–1995. 10.1194/jlr.M800367-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tauzin L, Graf C, Sun M, Rovina P, Bouveyron N, Jaritz M, Winiski A, Hartmann N, Staedtler F, Billich A, Baumruker T, Zhang M, Bornancin F. 2007. Effects of ceramide-1-phosphate on cultured cells: dependence on dodecane in the vehicle. J. Lipid Res. 48:66–76. 10.1194/jlr.M600399-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Forman BM, Chen J, Evans RM. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. U. S. A. 94:4312–4317. 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyrin-Biroulet L, Beisner J, Wang G, Nuding S, Oommen ST, Kelly D, Parmentier-Decrucq E, Dessein R, Merour E, Chavatte P, Grandjean T, Bressenot A, Desreumaux P, Colombel JF, Desvergne B, Stange EF, Wehkamp J, Chamaillard M. 2010. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc. Natl. Acad. Sci. U. S. A. 107:8772–8777. 10.1073/pnas.0905745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pita O, Leung DY, Howell MD. 2007. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J. Immunol. 179:984–992. 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 40.Bowman T, Garcia R, Turkson J, Jove R. 2000. STATs in oncogenesis. Oncogene 19:2474–2488. 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 41.Rane SG, Reddy EP. 2002. JAKs, STATs and Src kinases in hematopoiesis Oncogene. 21:3334–3358. 10.1038/sj/onc/1205398. [DOI] [PubMed] [Google Scholar]
- 42.Brandvold KR, Steffey ME, Fox CC, Soellner MB. 2012. Development of a highly selective c-Src kinase inhibitor. ACS Chem. Biol. 7:1393–1398. 10.1021/cb300172e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montagner A, Delgado MB, Tallichet-Blanc C, Chan JS, Sng MK, Mottaz H, Degueurce G, Lippi Y, Moret C, Baruchet M, Antsiferova M, Werner S, Hohl D, Saati TA, Farmer PJ, Tan NS, Michalik L, Wahli W. 2014. Src is activated by the nuclear receptor peroxisome proliferator-activated receptor beta/delta in ultraviolet radiation-induced skin cancer. EMBO Mol. Med. 6:80–98. 10.1002/emmm.201302666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. 2005. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 40:123–132. 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schroder JM. 2000. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am. J. Respir. Cell Mol. Biol. 22:714–721. 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 46.Gombart AF, Borregaard N, Koeffler HP. 2005. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 19:1067–1077. 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 47.Cirri P, Chiarugi P, Marra F, Raugei G, Camici G, Manao G, Ramponi G. 1997. c-Src activates both STAT1 and STAT3 in PDGF-stimulated NIH3T3 cells. Biochem. Biophys. Res. Commun. 239:493–497. 10.1006/bbrc.1997.7493. [DOI] [PubMed] [Google Scholar]
- 48.Smith PD, Crompton MR. 1998. Expression of v-src in mammary epithelial cells induces transcription via STAT3. Biochem. J. 331:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. 1998. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 18:2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardner OS, Dewar BJ, Earp HS, Samet JM, Graves LM. 2003. Dependence of peroxisome proliferator-activated receptor ligand-induced mitogen-activated protein kinase signaling on epidermal growth factor receptor transactivation. J. Biol. Chem. 278:46261–46269. 10.1074/jbc.M307827200. [DOI] [PubMed] [Google Scholar]
- 51.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, Kamens J, Grandis JR. 2003. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J. Biol. Chem. 278:31574–31583. 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 52.Tsuji K, Mitsutake S, Yokose U, Sugiura M, Kohama T, Igarashi Y. 2008. Role of ceramide kinase in peroxisome proliferator-activated receptor beta-induced cell survival of mouse keratinocytes. FEBS J. 275:3815–3826. 10.1111/j.1742-4658.2008.06527.x. [DOI] [PubMed] [Google Scholar]
- 53.Castrillo A, Diaz-Guerra MJ, Hortelano S, Martin-Sanz P, Bosca L. 2000. Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-Delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol. Cell. Biol. 20:1692–1698. 10.1128/MCB.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 403:103–108. 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 55.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 2000. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 97:4844–4849. 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qing Y, Stark GR. 2004. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J. Biol. Chem. 279:41679–41685. 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]