Abstract
B-type lamins (lamins B1 and B2) have been considered to be essential for many crucial functions in the cell nucleus (e.g., DNA replication and mitotic spindle formation). However, this view has been challenged by the observation that an absence of both B-type lamins in keratinocytes had no effect on cell proliferation or the development of skin and hair. The latter findings raised the possibility that the functions of B-type lamins are subserved by lamins A and C. To explore that idea, we created mice lacking all nuclear lamins in keratinocytes. Those mice developed ichthyosis and a skin barrier defect, which led to death from dehydration within a few days after birth. Microscopy of nuclear-lamin-deficient skin revealed hyperkeratosis and a disordered stratum corneum with an accumulation of neutral lipid droplets; however, BrdU incorporation into keratinocytes was normal. Skin grafting experiments confirmed the stratum corneum abnormalities and normal BrdU uptake. Interestingly, the absence of nuclear lamins in keratinocytes resulted in an interspersion of nuclear/endoplasmic reticulum membranes with the chromatin. Thus, a key function of the nuclear lamina is to serve as a “fence” and prevent the incursion of cytoplasmic organelles into the nuclear chromatin.
INTRODUCTION
The B-type lamins, which are expressed in virtually all cells from the earliest stages of development, have been assumed to play vital functions in the cell nucleus (1–8). Multiple studies contributed to this view. One group found lamin B at sites of DNA replication during S-phase and suggested that it was important for DNA replication (1). Disruption of nuclear lamin organization with a dominant negative lamin B mutant in Xenopus egg extracts inhibited DNA replication (2, 3), mitotic spindle assembly (4), and nuclear envelope assembly in interphase (5). An RNA interference (RNAi) knockdown of lamin B1 and lamin B2 was reported to lead to mitotic arrest and apoptosis (6). Other studies reported that B-type lamins were important for chromatin organization and proper gene expression (7, 8).
The view that B-type lamins play crucial roles in the cell nucleus likely dampened enthusiasm for testing the importance of B-type lamins in knockout mice. Ultimately, however, Yang et al. (9) created conditional knockout alleles for both Lmnb1 and Lmnb2 and used those alleles to generate keratinocyte-specific Lmnb1 Lmnb2 knockout mice. Remarkably, a complete absence of both lamin B1 and lamin B2 in keratinocytes had no discernible effect on cell growth or the complex developmental programs involved in the formation of the epidermis, hair, and nails. By electron microscopy, the nuclear envelope and heterochromatin distribution appeared normal in Lmnb1 Lmnb2-deficient keratinocytes (9). Similarly, hepatocyte-specific Lmnb1 Lmnb2 knockout mice had normal liver histology and normal liver function tests (10). These studies cast doubt on the notion that B-type lamins are essential for DNA replication and mitosis but did not exclude the possibility that these functions were simply subserved by lamins A and C. Recently, a paper in Cell Research (11) reported that mouse embryonic stem cells proliferate normally in the absence of all nuclear lamins. At face value, that paper refuted the idea that nuclear lamins are crucial for mitosis and DNA replication, at least in stem cells, but many questions remain. For example, are nuclear lamins important for the vitality and growth of differentiated cell types in vivo? Would an absence of nuclear lamins impair the structural integrity of the nuclear envelope? Would an absence of nuclear lamins lead to apoptotic cell death and tissue dysfunction?
To address these questions, we bred mice that lacked all nuclear lamins in keratinocytes. We chose to investigate nuclear lamin deficiency in keratinocytes because these cells proliferate rapidly and undergo complex differentiation programs. We reasoned that if nuclear lamins were required for DNA replication, mitosis, and complex gene regulation programs, the consequences of nuclear lamin deficiency would be very pronounced in keratinocytes.
In addition to investigating the consequences of a complete absence of nuclear lamins in keratinocytes, we investigated the consequences of a complete deficiency of all farnesylated nuclear lamins. Prelamin A, lamin B1, and lamin B2 have a CaaX motif at their carboxyl termini and undergo farnesylation of the carboxyl-terminal cysteine (the “C” of the CaaX motif) followed by release of the “aaX” and methylation of the farnesylcysteine (12, 13). In vitro studies have suggested that these modifications are important for their function (14–18). In the case of the B-type lamins, the carboxyl-terminal farnesylcysteine methyl ester is retained in the mature protein. In the case of prelamin A, the carboxyl terminus (including the farnesylcysteine methyl ester) is clipped off, releasing mature lamin A (19, 20). In earlier studies, Coffinier et al. (21) created “mature lamin A-only mice” that produced mature lamin A directly (bypassing prelamin A synthesis and protein farnesylation). Remarkably, those mice were fertile and healthy. Those authors proposed that the cells and tissues in these mice might have been protected from pathology by their capacity to produce two other farnesylated nuclear lamins (lamins B1 and B2). To assess the consequences of a complete deficiency of farnesylated lamin proteins, we bred mature lamin A-only mice that lacked both lamin B1 and lamin B2 in keratinocytes.
MATERIALS AND METHODS
Immunofluorescence microscopy.
Freshly harvested skins were embedded in OCT (optimum cutting temperature) compound (Sakura Finetek) and sectioned (10 μm) with a cryostat. The sections were fixed either in ice-cold methanol (followed by acetone rinse) or 4% paraformaldehyde (PFA), washed with 0.1% Tween 20 in Tris-buffered saline (TBS), and incubated with M.O.M. mouse Ig blocking reagent (Vector Laboratories). For BODIPY staining, 5-μm skin sections were fixed in 4% PFA, washed with phosphate-buffered saline (PBS), and air dried for 5 min. For BrdU staining, skin sections were fixed in ice-cold acetone, washed with 0.1% Tween 20 in TBS, and pretreated with 1 N and 2 N HCl to denature double-stranded DNA. After neutralization in 0.1 M sodium borate (pH 8.5), the sections were washed with 0.1% Tween 20 in TBS and incubated with M.O.M. mouse Ig blocking reagent. The following primary antibodies were used: a mouse monoclonal antibody against mature lamin A (1:400) (MAB3540; Millipore), a goat polyclonal antibody against lamin B1 (1:400) (sc-6217; Santa Cruz), a mouse monoclonal antibody against lamin B2 (1:100) (33-2100; Invitrogen), a rabbit polyclonal antibody against keratin 14 (1:800) (PRB-155P; Covance), a mouse monoclonal antibody against the amino-terminal region of lamin A/C (1:50) (LAZ) (22), a rabbit polyclonal antibody against lamin C (1:200) (LS-B2972; Life Span Biosciences), BODIPY 493/503 (1:1,000) (D-3922; Molecular Probes), a rat monoclonal antibody against BrdU (1:200) (ab6326; Abcam), a rabbit polyclonal antibody against caspase 3 (1:100) (559565; BD Biosciences), a mouse monoclonal antibody against LAP2β (1:400) (611000; BD Biosciences), a mouse monoclonal antibody against emerin (1:200) (NCL-EMERIN; Novocastra), a mouse monoclonal antibody against Lys-Asp-Glu-Leu (1:200) (SPA-827; Stressgen), and a rabbit polyclonal antibody against calnexin (1:200) (SPA-860; Stressgen). Biotinylated anti-mouse IgG M.O.M. reagent (Vector Laboratories) and Alexa Fluor-conjugated streptavidin (Invitrogen) were used to detect binding of mouse monoclonal primary antibodies; Alexa Fluor-labeled donkey antibodies against goat, rabbit, or rat IgG (Invitrogen) were used to detect binding of other primary antibodies. Confocal microscopy images were obtained with a Zeiss LSM700 laser-scanning microscope (Plan Apochromat 20×/0.80 numerical aperture [NA] [air] or 63×/1.4 NA [oil] objectives). Z-stacked images were generated with Zen 2010 software (Zeiss).
Histology.
Freshly harvested skins were fixed in 10% formalin overnight at 4°C, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin. For oil red O staining, frozen skin sections (10 μm) were fixed in 1% formalin, washed with running tap water, rinsed with 60% isopropanol, and stained with oil red O solution (23); nuclei were counterstained with hematoxylin. Light microscopy was performed with a Leica MZ6 dissecting microscope (a Plan 0.5× objective, air) with a DFC290 digital camera (Leica) or a Nikon Eclipse E600 microscope (Plan Fluor 20×/0.50 NA or 40×/0.75 NA objective, air) with a DS-Fi2 camera (Nikon); the images were processed with Leica Application suite imaging software or NIS-Elements F (Nikon).
Studies with primary keratinocytes.
Primary keratinocytes were isolated from the skin of newborn mice as described previously (24). Briefly, the skin was floated on cold 0.25% trypsin (without EDTA) with the dermal side facing down and incubated overnight at 4°C. The next morning, the epidermis was gently separated from the dermis and was physically dissociated with a blade. Isolated keratinocytes were seeded on collagen I-coated plates and cultured in KGM-2 BulletKit medium (Lonza) at 37°C. After 4 to 6 days, cells were harvested, and the cell pellets were lysed in urea buffer (9 M urea, 10 mM Tris-HCl [pH 8.0], 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μM EDTA, 0.2% mercaptoethanol, and a Roche protease inhibitor cocktail tablet). Protein extracts were separated on 4 to 12% gradient polyacrylamide bis-Tris gels (Invitrogen) and transferred to a nitrocellulose membrane. The membranes were then incubated with the following primary antibodies: a goat polyclonal antibody against lamin A/C (1:400) (sc-6215; Santa Cruz); a goat polyclonal antibody against lamin B1 (1:400) (sc-6217; Santa Cruz); a mouse monoclonal antibody against lamin B2 (1:400) (33-2100; Invitrogen); a rabbit polyclonal antibody against keratin 14 (1:800) (PRB-155P; Covance); and a goat polyclonal antibody against actin (1:1,000) (sc-1616; Santa Cruz). Binding of primary antibodies was detected with infrared (IR)-dye-conjugated secondary antibodies (Rockland) and an Odyssey infrared scanner (Li-Cor).
For quantitative reverse transcription-PCR (qRT-PCR) analysis, total RNA was isolated with an RNeasy kit (Qiagen) according to the manufacturer's protocol. RNA was treated with DNase I (Ambion) and used to synthesize cDNA with random primers, oligo(dT), and SuperScript III (Invitrogen). qPCRs were performed with SYBR green PCR master mix (Bioline) on a 7900 fast real-time PCR system (Applied Biosystems). Transcript levels were calculated with the comparative cycle threshold method and were normalized to levels of cyclophilin A.
Skin permeability assay.
Toluidine blue staining to assess skin permeability was performed as described earlier (25). Briefly, newborn mice were euthanized; they were dehydrated in 25%, 50%, 75%, and 100% methanol serially for 2 min each and then rehydrated in 75%, 50%, and 25% methanol for 1 min each. After two washes in PBS for 3 min each, mice were incubated in 0.1% toluidine blue solution (in PBS) for 3 h at room temperature followed by destaining in PBS for 20 min.
Skin transplantation.
An oval piece of skin approximately 1 cm in length was excised from the dorsum of euthanized newborn mice (donors), punctured 5 or 6 times with a blade, and kept in cold PBS until the transplant surgery was performed. Athymic nude mice (recipients; NU/J; The Jackson Laboratory) were anesthetized with isoflurane and administered analgesic drug (carprofen) subcutaneously. Saline (intraperitoneally) and artificial tear ointment were also applied to prevent dehydration and eye dryness during the surgery. The flank area was sterilized, and an area of skin slightly larger than the donor skin was excised. Excess PBS was removed from the donor skin with sterile gauze, and the donor skin was attached to the recipient skin at the borders with 6 or 7 Prolene sutures. The graft region was bandaged, and the recipient mice were returned to their cage. The mice were monitored daily and were given carprofen and antibiotics (amoxicillin) as needed. All procedures involving animals, including the skin transplantation surgery, were approved by the institutional animal care and use committee (IACUC) of the University of California, Los Angeles.
Electron microscopy.
For electron microscopy, samples were fixed in 2% glutaraldehyde, 1% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, postfixed in 2% osmium tetroxide in the same buffer, en bloc stained with 2% aqueous uranyl acetate, dehydrated in acetone, infiltrated, and embedded in LX-112 resin (Ladd Research Industries, Burlington, VT). Samples were sectioned on a Reichert Ultracut S ultramicrotome and counterstained with 0.8% lead citrate. Grids were examined on a JEOL JEM-1230 transmission electron microscope (JEOL USA, Peabody, MA) and photographed with the Gatan Ultrascan 1000 digital camera (Gatan, Warrendale, PA).
RESULTS
Generation of mice lacking A- and B-type lamins in keratinocytes.
We generated keratinocyte-specific Lmna Lmnb1 Lmnb2 triple-knockout mice (Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ) by breeding Lmna knockout mice that were homozygous for floxed alleles of Lmnb1 and Lmnb2 and carried the keratin 14-Cre transgene (9).
A- and B-type lamins were undetectable in most keratinocytes from newborn Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice by immunohistochemistry (Fig. 1A and B). Protein extracts from primary keratinocyte preparations from newborn Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice expressed only trace amounts of lamin A/C, lamin B1, and lamin B2 (Fig. 1C). Lmna, Lmnb1, and Lmnb2 transcript levels were minimal (Fig. 1D). In line with an earlier report (26), however, we did find that the Lmna knockout allele yielded trace amounts of an internally truncated form of lamin A (detected with a monoclonal antibody against the amino-terminal region of lamin A/C [22]) (see Fig. S1 in the supplemental material).
FIG 1.
Generation of mice lacking both A-type and B-type lamins in skin keratinocytes. (A and B) Skin sections from newborn Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice were stained with antibodies against keratin 14 (Krt14, red) and lamin B1 (green) along with an antibody against lamin A (white) (A) or lamin B2 (cyan) (B). DNA was visualized with DAPI (4′,6′-diamidino-2-phenylindole; blue). As expected, lamin B1 and lamin B2 expression was nearly absent in keratin 14-positive keratinocytes in the epidermis of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. However, in a subset of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, we did observe rare lamin B1- and lamin B2-positive keratinocytes, indicating that rare cells had escaped the Cre recombination event. Lamin A was not detected in keratinocytes or dermal fibroblasts (with a monoclonal antibody against amino acids 598 to 611 of lamin A). Scale bar, 50 μm. (C) Western blot analysis of lamin expression in primary keratinocytes. Lamins A, B1, B2, and C were nearly undetectable in protein extracts of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes. (D) qRT-PCR analysis of Lmna, Lmnb1, and Lmnb2 transcript levels in primary keratinocytes. Values are means ± SD. Lmna+/+ Lmnb1fl/fl Lmnb2fl/fl, n = 2; Lmna−/− Lmnb1fl/fl Lmnb2fl/fl, n = 2; Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ, n = 5; Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ, n = 5; Lmna−/− Lmnb1+/+ Lmnb2+/+, n = 4.
Defective skin barrier in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice.
Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice were born at the expected Mendelian frequency (Fig. 2A) and were similar in size to littermate control mice (either Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ or Lmna+/− Lmnb1Δ/Δ Lmnb2Δ/Δ) (Fig. 2B). However, the triple-knockout mice manifested ichthyosis (Fig. 2B and C), and none survived more than a few days after birth (Fig. 2A). They had defective skin barrier function, as judged by toluidine blue staining (Fig. 2D). In the triple-knockout mice, the dye penetrated the skin, resulting in darkly stained skin.
FIG 2.
Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice die soon after birth with a skin barrier defect. (A) Numbers of mice per genotype at P1 and at the time of weaning (3 to 4 weeks). (B and C) Photographs of newborn Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ, Lmna+/− Lmnb1Δ/Δ Lmnb2Δ/Δ, and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice had ichthyosis (scaly skin). (D) Skin barrier assay with toluidine blue demonstrating dye penetration in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. (E) Body weight loss after separation of newborn pups from the mother. Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice lost weight rapidly and were visibly dehydrated and near death after 7 h of observation. Values represent mean ± SD. Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ, n = 3; Lmna+/− Lmnb1Δ/Δ Lmnb2Δ/Δ, n = 5; and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, n = 4.
We suspected that the early death of triple-knockout mice was related to a defective skin barrier. To explore this idea, we compared epidermal water loss by measuring body weight changes after separating newborn pups from their mother. Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna+/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice lost less than 3% of their body weight over 7 h of observation. However, Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice lost more than 10% of their body weight, became obviously dehydrated, and died (Fig. 2E).
An abnormal stratum corneum in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice.
The stratum corneum is crucial for skin barrier function, and we suspected that it would be morphologically abnormal in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. Indeed, Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice had a thickened epidermis with a compressed stratum corneum. Similar findings have been observed in other forms of ichthyosis (27–29). Also, many cells within the stratum corneum retained a cell nucleus (parakeratosis) (Fig. 3A).
FIG 3.
Epidermal hyperplasia and an abnormal stratum corneum in newborn Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. (A) Hematoxylin and eosin (H&E) staining of skin sections from newborn Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ, Lmna+/− Lmnb1Δ/Δ Lmnb2Δ/Δ, and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. In Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, the epidermis (brackets) was thickened (epidermal hyperplasia) with a compressed stratum corneum. Also, nuclei were retained in the stratum corneum (parakeratosis) (yellow arrowheads). The layers of the stratum granulosum and stratum corneum were interspersed (black arrowheads). (B) Accumulation of numerous tiny neutral lipid droplets in the stratum corneum (brackets) of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. Skin sections from Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice were stained with BODIPY (green); DNA was visualized with DAPI (red).
Defects in lipid metabolism in the skin often result in ichthyosis and a defective skin barrier (30–32). For example, CGI-58 (comparative gene identification 58) deficiency impairs triglyceride hydrolysis in the skin, leading to an accumulation of neutral lipid droplets in the stratum corneum, a skin barrier defect, and ichthyosis (33, 34). To determine if lipid metabolism was perturbed in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, we stained sections from newborn mouse skin with BODIPY, a fluorescent dye that binds to neutral lipids (16) (Fig. 3B). We observed numerous lipid droplets within the stratum corneum of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice but none in the stratum corneum of control mice. Lipid droplets in the stratum corneum of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice were also evident by oil red O staining (see Fig. S2 in the supplemental material).
A deficiency of the lamin B receptor (LBR) also causes ichthyosis (35). The N-terminal domain of LBR binds to B-type lamins, while the C terminus has sterol Δ14-reductase activity. Because the Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice had ichthyosis, we considered the possibility that a deficiency of nuclear lamins impairs LBR function and leads to defective skin sterol metabolism and ichthyosis. However, we observed no significant differences in keratinocyte sterol levels in newborn Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice and littermate control mice (see Fig. S3 and Table S1 in the supplemental material). The levels of sterol intermediates of cholesterol biosynthesis (either before or after the Δ14-reductase-mediated step) were comparable in the epidermis of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ and littermate control mice.
A deficiency of nuclear lamins has little or no effect on cell proliferation.
Although morphological abnormalities were detectable in the stratum corneum of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, numbers of keratinocytes in the basal layer were normal (Fig. 3). To determine if a deficiency of nuclear lamins affected keratinocyte proliferation, we examined BrdU incorporation into keratinocytes in vivo (Fig. 4). Pregnant female mice were given an intraperitoneal injection of BrdU at embryonic day 18.5 (E18.5), and skin samples were harvested from embryos or newborn pups 2 h or 24 h later. At both time points, the numbers of BrdU-positive keratinocytes in the skin (and the intensity of the BrdU staining) were indistinguishable in Lmna+/− Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice.
FIG 4.
BrdU labeling of skin cells in Lmna+/− Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. Pregnant female mice were given an intraperitoneal injection of BrdU at E18.5, and skin biopsy specimens from embryos or newborn pups were collected 2 h or 24 h later. Skin sections were stained with antibodies against BrdU (green) and lamin B1 (red). DNA was visualized with DAPI (blue). The percentage of BrdU-positive cells was assessed in >350 epidermal keratinocytes/genotype in a blinded fashion; the results are shown in the bar graphs beneath the confocal images.
In follow-up studies, we generated mouse embryonic fibroblasts from Lmna−/− Lmnb1fl/fl Lmnb2fl/fl K14-Cre embryos. Unexpectedly, there was expression of the K14-Cre transgene in some cultured fibroblasts, resulting in recombination within Lmnb1 and Lmnb2 (converting the “floxed” alleles to “Δ” alleles) (see Fig. S4A in the supplemental material). In some immortalized cell lines, the extent of recombination was modest, whereas in others it was substantial. To achieve more complete recombination, we treated the fibroblasts with Cre adenovirus (or lacZ adenovirus as a control) (see Fig. S4A to C in the supplemental material). The Cre adenovirus markedly reduced lamin B1 and B2 expression (see Fig. S4A and B in the supplemental material). The levels of proliferation of Cre and LacZ adenovirus-treated cells were virtually identical (see Fig. S4C in the supplemental material), and we did not observe overgrowth of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ fibroblasts by the cells that had not undergone complete recombination (and therefore retained the capacity to produce lamins B1 and B2).
We examined BrdU incorporation in four lines of MEFs (two Lmna+/− Lmnb1fl/fl Lmnb2fl/fl K14-Cre lines and two Lmna−/− Lmnb1fl/fl Lmnb2fl/fl K14-Cre lines) (see Fig. S4D in the supplemental material). In one of the Lmna−/− Lmnb1fl/fl Lmnb2fl/fl K14-Cre lines, recombination was modest and some cells retained the ability to produce lamin B1 (see Fig. S4D, middle, in the supplemental material). In the other line of Lmna−/− Lmnb1fl/fl Lmnb2fl/fl K14-Cre fibroblasts, the extent of recombination was greater, and only small numbers of cells retained the ability to produce lamin B1 (see Fig. S4D [bottom] in the supplemental material). In the subset of cells from Lmna−/− Lmnb1fl/fl Lmnb2fl/fl K14-Cre lines that lacked all nuclear lamins, one-half of the cells (153/306) were stained positively for BrdU (see Fig. S4E in the supplemental material). Thus, BrdU uptake is robust in fibroblasts lacking all nuclear lamins. In the subset of Lmna+/− Lmnb1fl/fl Lmnb2fl/fl K14-Cre fibroblasts that expressed lamin C but lacked B-type lamins, 46.7% of the cells (206/441) were stained positively for BrdU (see Fig. S4E in the supplemental material).
Defective skin development with hypotrophic hair follicles in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ skin grafts.
Because Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice died within a few days after birth, our ability to assess the development of the skin and hair was limited. For that reason, we transplanted the skin from newborn Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice (and littermate control mice) onto nude mice (Fig. 5). By postsurgery day 5, the skin from Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ donors appeared pink and healthy, but the skin from Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ donors appeared less robust and dry. At day 10, the hair was visible in most Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ skin grafts but was nearly absent in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ skin grafts (Fig. 5A). As determined by microscopy, the stratum corneum of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ skin grafts was compressed, and many cells within the stratum corneum retained a cell nucleus (Fig. 5B). However, keratinocyte proliferation was not impaired in lamin-deficient skin grafts (as judged by BrdU incorporation) (see Fig. S5 in the supplemental material), and there was little evidence of cell death (see Fig. S6 in the supplemental material).
FIG 5.
Abnormal skin development and hypotrophic hair follicles in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ skin grafts. An oval piece of skin from the dorsum of newborn Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice was grafted onto the flanks of immunodeficient nude mice. (A) Images of skin grafts 5 and 10 days after the surgery. Hair had emerged from the Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ skin grafts by day 10 (yellow arrowheads). (B) H&E staining of skin sections from skin grafts at day 10. The stratum corneum of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ skin grafts was compressed, and pyknotic nuclei were observed in the stratum corneum (blue arrowheads).
An absence of nuclear lamins causes intrusion of nuclear and endoplasmic reticulum membranes into the nuclear chromatin.
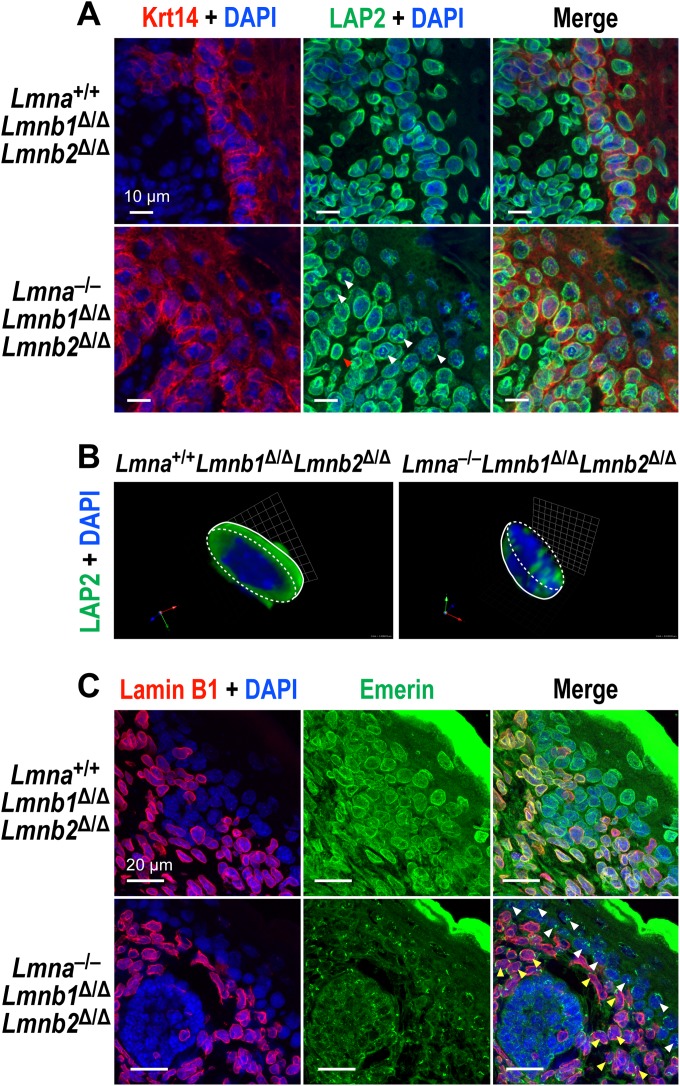
We assessed whether an absence of all nuclear lamins affects the localization of nuclear membrane proteins that interact with lamins. LAP2β, the largest isoform of LAP2 (lamina-associated polypeptide 2), is an integral protein of the inner nuclear membrane (INM). In Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, LAP2β was positioned at the nuclear rim (Fig. 6A). However, in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, LAP2β was often present in a patchy distribution within the nucleoplasm, interspersed with the chromosomal DNA (Fig. 6A and B). LAP2β localization was normal in the dermal fibroblasts of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice (where B-type lamins are expressed normally) (Fig. 6A).
FIG 6.
Abnormal distribution of nuclear membrane proteins in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes. (A) Immunofluorescence microscopy of newborn skin sections stained for Krt14 (red) and LAP2β (green). DNA was stained with DAPI (blue). In Krt14-positive keratinocytes in the epidermis of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, LAP2β was often mislocalized in a patchy distribution within the nucleoplasm (white arrowheads). In the Krt14-negative dermal fibroblasts from the same mice (where B-type lamins are expressed), LAP2β staining was mainly at the nuclear rim (red arrowhead). (B) Three-dimensional (3D) images of “half-cut-open” nuclei stained for LAP2β (green). Images were captured along the z axis, and 3D images were constructed with Volocity 5.4. The boundaries of nuclei are outlined with dashed lines. (C) Mislocalization of emerin within the nucleoplasm in the absence of nuclear lamins. Skin sections from newborn Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice were stained with antibodies against lamin B1 (red) and emerin (green); DNA was visualized with DAPI (blue). In keratinocytes from Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, some of the emerin was mislocalized to the ER, as expected, but we also frequently observed patches of emerin within the nucleoplasm (white arrowheads), particularly in epidermal keratinocytes. In dermal fibroblasts of the same mice (where B-type lamins are expressed but A-type lamins are absent), emerin was mislocalized to the ER (yellow arrowheads).
We also examined emerin localization (Fig. 6C). In the setting of Lmna deficiency, emerin is mislocalized to the endoplasmic reticulum (ER) (36). In Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, emerin was mainly located at the nuclear rim. In dermal fibroblasts of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, emerin was mislocalized to the ER. However, in the keratinocytes of the same mice, emerin was often observed in a patchy distribution within the nucleoplasm (Fig. 6C).
The nucleoplasmic distribution of emerin in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes (in contrast to ER mislocalization in the setting of Lmna deficiency) led us to suspect that the nuclear lamina functions as a “fence,” preventing interspersion of chromatin and ER membranes. To explore this idea, we examined skin sections from newborn mice by immunohistochemistry with two ER markers (KDEL and calnexin) (Fig. 7). In Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, both markers were frequently found within the nucleoplasm (Fig. 7).
FIG 7.
Mislocalization of ER membrane proteins within the nucleoplasm in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes. (A) Immunofluorescence microscopy of newborn-mouse skin sections stained with antibodies against KDEL (green), lamin B1 (magenta), and Krt14 (red). DNA was stained with DAPI (blue). In Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, KDEL was often present within the nucleoplasm (yellow arrowheads); in dermal fibroblasts from the same mice, KDEL was mainly in the ER (red arrowheads), and the nucleus was free of patches of KDEL staining. (B) Immunofluorescence microscopy of newborn-mouse skin sections stained for calnexin (red), lamin B1 (cyan), and lamin A (green). In Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, we observed patches of calnexin staining within the nucleoplasm (yellow arrowheads); in lamin B1-expressing dermal fibroblasts, calnexin was mainly in the ER (white arrowheads). DNA was visualized with DAPI (blue).
We also examined Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes by electron microscopy (Fig. 8). In line with the immunofluorescence microscopy findings, keratinocytes in the stratum granulosum and stratum spinosum of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice had ER membranes within the nucleoplasm (Fig. 8A and B). We also observed intrusion of cytoplasmic fibers into the nucleus and invagination of nuclear membranes. Heterochromatin distribution was often abnormal (Fig. 8A). We found occasional examples of keratinocytes from Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice with morphologically normal cell nuclei (see Fig. S7A in the supplemental material). In the skin of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, the stratum granulosum was thickened and interspersed with the stratum corneum (Fig. 8C; also, see Fig. S7B in the supplemental material). We did find some examples of lamellar bodies in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes (Fig. 8C; also, see Fig. S7B in the supplemental material).
FIG 8.
Electron micrographs of keratinocytes in the skin from Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ and Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice. (A) Images of keratinocytes in the stratum granulosum and stratum spinosum. In Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes, nuclear membranes were occasionally invaginated into the nucleoplasm (yellow arrowheads). ER membranes, including rough ER, were also found within the nucleoplasm (blue arrowheads). (B) Higher-power image of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes showing intrusion of ER membranes into the nucleoplasm (arrowheads). (C) Lower-power images of the stratum corneum and stratum granulosum. In Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, the stratum granulosum was thickened (brackets). It was possible to identify lamellar bodies in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes (arrowheads).
Additional electron micrographs of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ cells demonstrating incursion of nuclear membranes into the chromatin are available in File S2 of the supplemental material. Incursion of membranes into the chromatin was never observed in keratinocytes from Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ mice.
Normal skin and hair in LmnaLAO/LAO Lmnb1Δ/Δ Lmnb2Δ/Δ mice.
Prelamin A, lamin B1, and lamin B2 are farnesylated proteins. Cell culture studies have suggested that the farnesylation of nuclear lamins is important for targeting those proteins to the nuclear envelope (14–18), and an absence of lamin B1 farnesylation causes severe neurodevelopmental abnormalities (37). One potential explanation for the normal skin in Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ mice is that those mice retained the capacity to produce a farnesylated nuclear lamin (prelamin A). Prelamin A is evanescent in cells because of its rapid conversion to mature lamin A, but the best evidence suggests that farnesyl-prelamin A exists in the cell nucleus (38). To test the idea that the ability to produce farnesyl-prelamin A in Lmna+/+ Lmnb1Δ/Δ Lmnb2Δ/Δ mice explained their normal skin, we bred mature lamin A-only mice (21) lacking lamins B1 and B2 in keratinocytes (LmnaLAO/LAO Lmnb1Δ/Δ Lmnb2Δ/Δ). These mice were healthy and had no abnormalities in the skin or hair, indicating that the synthesis of a farnesylated nuclear lamin is not important for keratinocytes (see Fig. S8 in the supplemental material). As determined by immunohistochemistry, the mature lamin A in LmnaLAO/LAO Lmnb1Δ/Δ Lmnb2Δ/Δ keratinocytes was located predominantly at the nuclear rim.
DISCUSSION
More than 30 years after the discovery of nuclear lamins (39, 40), the physiological importance of these proteins remains unclear. For much of this time, the B-type lamins were considered to be crucial for a variety of key functions in the cell nucleus, for example, DNA replication and the formation of the mitotic spindle (1–8). That view began to unravel with the discovery that keratinocyte-specific Lmnb1 Lmnb2 mice were healthy and free of gross, microscopic, or ultrastructural pathology in the skin or hair (9). In the current study, we tested whether a deficiency of all nuclear lamins in keratinocytes would be equally well tolerated. Our studies yielded three important findings. First, an absence of nuclear lamins in keratinocytes led to ichthyosis, a skin barrier defect, dehydration, and early death. Second, the loss of all nuclear lamins impaired the development of the stratum corneum and led to hypotrophic hair follicles, but there was little, if any, impact on DNA synthesis (as judged by BrdU uptake by keratinocytes). Third, an absence of nuclear lamins resulted in a striking abnormality in cell morphology—interspersion of nuclear membranes and endoplasmic reticulum with the nuclear chromatin. Thus, the nuclear lamina serves as a “fence,” preventing incursion of cytoplasmic structures into the cell nucleus.
The fact that keratinocytes and fibroblasts were able to survive and proliferate in the absence of nuclear lamins was intriguing, given the reports claiming essential roles for nuclear lamins in the nucleus (1–8). On the other hand, our findings were consistent with a recent study reporting that mouse embryonic stem (ES) cells proliferate normally in the absence of nuclear lamins (11). Interestingly, the nuclear lamin-deficient ES cells, when injected into nude mice, formed teratomas containing endodermal, mesodermal, and ectodermal structures. The latter observation suggested that nuclear lamins might be dispensable for complex developmental programs. Our studies showed that this is not the case. A deficiency of nuclear lamins in keratinocytes led to a disorganized epidermis and hypotrophic hair follicles, which in turn led to a skin barrier defect and early death.
One remarkable finding in our current studies is that nuclear lamins form a barrier that prevents incursion of cytoplasmic organelles into the nuclear chromatin. Most often, the nuclear lamina is described as an intermediate filament meshwork that provides a structural scaffolding for the nucleus, or it is described as a structure that binds and organizes the nuclear chromatin. However, no one has emphasized a simpler and rather obvious function for the nuclear lamina—serving as a “border fence” that excludes cytoplasmic contents from the cell nucleus. In lamin-deficient keratinocytes, we found, by confocal microscopy, intermixing of nuclear membrane proteins and ER markers with the chromatin. By electron microscopy, we found rough ER intermixed with nuclear chromatin. A bizarre consequence of this situation was mis-mislocalization of emerin. Normally, an absence of lamins A and C causes emerin to be mislocalized to the ER (36), but when all lamins were absent, patches of emerin were found within the cell nucleus, surrounded by chromatin. We also observed fibrous structures, likely keratin fibers, within the nucleoplasm. Intranuclear membrane structures have been observed in some cultured cell lines, rat hepatocytes, and cells overexpressing a fusion protein, LBR-GFP, at high levels (41, 42). However, the extent of invaginations was limited and most likely related to nucleocytoplasmic transport or the unnatural accumulation of fusion proteins.
Our mice developed ichthyosis, hyperkeratosis, and parakeratosis, and accumulated neutral lipid droplets in the stratum corneum. Unfortunately, these findings are rather nonspecific, and the link between these findings and nuclear lamin deficiency is not clear. Ichthyosis can be caused by dozens of different genetic abnormalities in the skin—many involving defects in lipid biosynthetic proteins but others due to deficiencies in structural proteins (e.g., keratins and filaggrin) (31, 32, 43–45). Hyperkeratosis and triglyceride droplet accumulation can occur with genetic abnormalities in triglyceride hydrolysis, but they also occur in cases of run-of-the-mill dandruff. Recently, Adeyo et al. (46) found lipid droplet accumulation with a deficiency of SLURP1, a secreted peptide of keratinocytes. Triglyceride hydrolysis in the epidermal keratinocytes is known to be important for producing the lipids for lamellar bodies, which play key roles in skin barrier function (47–50). We were able to identify lamellar bodies in the keratinocytes of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, but we suspect that they were ineffective in establishing a normal skin barrier, given the disorganization in the upper layers of the epidermis.
In contemplating the skin pathology in our mice, we were intrigued by the fact that a deficiency of the lamin B receptor (LBR) causes ichthyosis (35). The amino-terminal domain of LBR binds to B-type lamins, while the carboxyl terminus contains a sterol Δ14-reductase domain. Initially, the loss of LBR was thought to cause ichthyosis by interfering with sterol metabolism (51, 52). This explanation seemed plausible, given that ichthyosis can be caused by a defect in sterol metabolism (e.g., cholesterol sulfatase deficiency) (31, 32, 43). Subsequently, Wassif et al. (53) reported that a deficiency of LBR alone does not perturb sterol metabolism because of the activity of a redundant sterol Δ14-reductase enzyme, TM7SF2. They concluded that the LBR-associated ichthyosis was not due to defective sterol metabolism and instead represented a “laminopathy.” Our data appear consistent with their interpretation. We found no evidence that a deficiency of nuclear lamins impairs sterol metabolism in keratinocytes; the levels of sterol intermediates (both before and after the Δ14-reductase step) were comparable in the epidermis of Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ and littermate control mice.
At this point, we cannot point to a simple biochemical mechanism for the skin and hair abnormalities in Lmna−/− Lmnb1Δ/Δ Lmnb2Δ/Δ mice, but we suggest two possibilities. The first is that an absence of nuclear lamins adversely affects chromatin structure and gene expression, leading to defective development of the epidermis and hair. The second possibility is more prosaic—that the interspersion of cytoplasmic organelles into the nucleus makes keratinocytes “sick,” resulting in impaired keratinocyte differentiation. Regardless of which mechanism applies, it is important to emphasize that the impaired skin and hair differentiation in lamin-deficient keratinocytes was by no means complete. Lamin-deficient keratinocytes retained the ability to differentiate into hair follicles and to form the different layers of the epidermis (but not well enough to prevent a skin barrier defect and early death).
The ability of nuclear lamin-deficient keratinocytes to proliferate and differentiate (albeit abnormally) stands in contrast to our experience with lamin deficiency in forebrain neurons (54). When Lmnb1 and Lmnb2 were inactivated in forebrain neurons during development, the result was death of all neurons and atrophy of the forebrain. Thus, the consequences of lamin deficiency in keratinocytes and neurons are very different, but we would hasten to add that these are very different cell types. Keratinocytes proliferate rapidly but then undergo apoptosis and are shed, with a turnover time of ∼8 to 12 days (55). In contrast, cortical neurons are postmitotic cells that are required to migrate from the ventricular zone to the cortical plate—a developmental process that subjects the cell nucleus to considerable strain (56, 57). In the setting of lamin B1 or lamin B2 deficiencies, the strain on the cell nucleus in migrating neurons leads to striking abnormalities in nuclear shape (54, 58). A key lesson from our observations is that one cannot generalize about the relevance of the nuclear lamina in different cell types. In the past, there has been a tendency to assume that the lamina plays similar functions—and is similarly important—for all cell types. Our studies show that the physiologic importance of the nuclear lamina can be quite different in different cells. A second lesson is that sorting out the relevance of nuclear lamins in different tissues requires genetically modified mice. In our opinion, additional studies with genetically modified mice are likely to add to our understanding of nuclear lamin function. It would be interesting, for example, to define the consequences of nuclear lamin deficiency in lymphocytes, hepatocytes, and adipocytes (59).
Over the past few years, there has been considerable interest in the importance of lamin posttranslational modifications (59, 60). Three of the nuclear lamins (prelamin A, lamin B1, and lamin B2) are modified by a farnesyl lipid. In the case of lamin B1, this lipid modification is crucial for the migration of neurons in the developing brain (37). For other cell types, for example, keratinocytes, the importance of lamin farnesylation is unclear. In the current study, we created mice in which the keratinocytes produced mature lamin A but lacked the ability to produce any farnesylated nuclear lamins. Interestingly, the skin and hair of these mice was normal (indistinguishable from those of wild-type mice), and the mature lamin A was largely positioned at the nuclear rim. These studies indicate that, at least in certain cell types, farnesylated lamins are dispensable.
In our study, we used a Lmna knockout allele, originally produced by Sullivan et al. (36), that has been used extensively by many laboratories. After breeding mice for this study, Jahn et al. (26) reported that this particular Lmna knockout allele produces an internally truncated lamin A, but there was little evidence that this protein is functional—or that it has toxic gain-of-function properties. In our studies, we detected the truncated lamin A in keratinocytes on Western blots, but the level of the protein was very low. In considering our findings, there is a formal possibility that the trace amounts of the truncated lamin A are sufficient to overcome a defect in DNA synthesis and mitosis and thereby allow keratinocytes to proliferate and differentiate. However, this possibility seems very remote, particularly given the ability of lamin-deficient ES cells to proliferate and differentiate into teratomas (11). In the latter ES cell studies, the Lmna knockout was achieved with a gene-targeting strategy distinct from the one used by Sullivan and coworkers.
In summary, we tested the in vivo functional importance of the nuclear lamins by breeding mice lacking all nuclear lamins in keratinocytes. Keratinocytes without nuclear lamins proliferated in a robust fashion, as judged by BrdU uptake. However, lamin-deficient keratinocytes yielded a morphologically and functionally abnormal epidermis and hypotrophic hair follicles. Interestingly, the absence of nuclear lamins resulted in an interspersion of nuclear and ER membranes with the chromatin, implying that a key function of the nuclear lamina is to prevent incursion of cytoplasmic organelles into the nucleus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jeffrey G. McDonald and David W. Russell (University of Texas Southwestern Medical Center) for sterol measurements, Xiu-Da Shen (University of California, Los Angeles) for advice on skin transplantation, David T. Woodley (University of Southern California) for discussions of skin graft histology, and Jinny Wong (University of California, San Francisco) for electron microscopy.
This work was supported by National Institutes of Health grants HL86683 and HL089781 (to L.G.F.) and AG035626 (to S.G.Y.).
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 13 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00997-14.
REFERENCES
- 1.Moir RD, Montag-Lowy M, Goldman RD. 1994. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J. Cell Biol. 125:1201–1212. 10.1083/jcb.125.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moir RD, Spann TP, Herrmann H, Goldman RD. 2000. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J. Cell Biol. 149:1179–1192. 10.1083/jcb.149.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis DJ, Jenkins H, Whitfield WG, Hutchison CJ. 1997. GST-lamin fusion proteins act as dominant negative mutants in Xenopus egg extract and reveal the function of the lamina in DNA replication. J. Cell Sci. 110:2507–2518. [DOI] [PubMed] [Google Scholar]
- 4.Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y. 2006. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 311:1887–1893. 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Soler RI, Moir RD, Spann TP, Stick R, Goldman RD. 2001. A role for nuclear lamins in nuclear envelope assembly. J. Cell Biol. 154:61–70. 10.1083/jcb.200101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. 2001. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 114:4557–4565. [DOI] [PubMed] [Google Scholar]
- 7.Tang CW, Maya-Mendoza A, Martin C, Zeng K, Chen S, Feret D, Wilson SA, Jackson DA. 2008. The integrity of a lamin-B1-dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J. Cell Sci. 121:1014–1024. 10.1242/jcs.020982. [DOI] [PubMed] [Google Scholar]
- 8.Belmont AS, Zhai Y, Thilenius A. 1993. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J. Cell Biol. 123:1671–1685. 10.1083/jcb.123.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, de Jong PJ, Fong LG, Young SG. 2011. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum. Mol. Genet. 20:3537–3544. 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang SH, Jung HJ, Coffinier C, Fong LG, Young SG. 2011. Are B-type lamins essential in all mammalian cells? Nucleus 2:562–569. 10.4161/nucl.2.6.18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Zheng X, Zheng Y. 2013. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res. 23:1420–1423. 10.1038/cr.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies BS, Coffinier C, Yang SH, Barnes RH, II, Jung HJ, Young SG, Fong LG. 2011. Investigating the purpose of prelamin A processing. Nucleus 2:4–9. 10.4161/nucl.2.1.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies BS, Fong LG, Yang SH, Coffinier C, Young SG. 2009. The posttranslational processing of prelamin A and disease. Annu. Rev. Genomics Hum. Genet. 10:153–174. 10.1146/annurev-genom-082908-150150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennekes H, Nigg EA. 1994. The role of isoprenylation in membrane attachment of nuclear lamins. A single point mutation prevents proteolytic cleavage of the lamin A precursor and confers membrane binding properties. J Cell Sci. 107:1019–1029. [DOI] [PubMed] [Google Scholar]
- 15.Krohne G, Waizenegger I, Hoger TH. 1989. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J. Cell Biol. 109:2003–2011. 10.1083/jcb.109.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtz D, Tanaka RA, Hartwig J, McKeon F. 1989. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell 59:969–977. 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- 17.Kitten GT, Nigg EA. 1991. The CaaX motif is required for isoprenylation, carboxyl methylation, and nuclear membrane association of lamin B2. J. Cell Biol. 113:13–23. 10.1083/jcb.113.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izumi M, Vaughan OA, Hutchison CJ, Gilbert DM. 2000. Head and/or CaaX domain deletions of lamin proteins disrupt preformed lamin A and C but not lamin B structure in mammalian cells. Mol. Biol. Cell 11:4323–4337. 10.1091/mbc.11.12.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, Mohr A, Meta M, Genant H, Jiang Y, Wisner ER, Van Bruggen N, Carano RA, Michaelis S, Griffey SM, Young SG. 2002. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. U. S. A. 99:13049–13054. 10.1073/pnas.192460799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodriguez F, Tryggvason K, Lopez-Otin C. 2002. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31:94–99. 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- 21.Coffinier C, Jung HJ, Li Z, Nobumori C, Yun UJ, Farber EA, Davies BS, Weinstein MM, Yang SH, Lammerding J, Farahani JN, Bentolila LA, Fong LG, Young SG. 2010. Direct synthesis of lamin A, bypassing prelamin a processing, causes misshapen nuclei in fibroblasts but no detectable pathology in mice. J. Biol. Chem. 285:20818–20826. 10.1074/jbc.M110.128835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger SK, Bar H, Ehlermann P, Walde S, Rutschow D, Zeller R, Ivandic BT, Zentgraf H, Katus HA, Herrmann H, Weichenhan D. 2008. Incomplete nonsense-mediated decay of mutant lamin A/C mRNA provokes dilated cardiomyopathy and ventricular tachycardia. J. Mol. Med. (Berl.) 86:281–289. 10.1007/s00109-007-0275-1. [DOI] [PubMed] [Google Scholar]
- 23.Lillie RD, Ashburn LL. 1943. Supersaturated solutions of fat stains in dilute isopropanol for demonstration of acute fatty degeneration not shown by Herxheimer's technique. Arch. Pathol. 36:432–440. [Google Scholar]
- 24.Lichti U, Anders J, Yuspa SH. 2008. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 3:799–810. 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch PJ, de Viragh PA, Scharer E, Bundman D, Longley MA, Bickenbach J, Kawachi Y, Suga Y, Zhou Z, Huber M, Hohl D, Kartasova T, Jarnik M, Steven AC, Roop DR. 2000. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J. Cell Biol. 151:389–400. 10.1083/jcb.151.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahn D, Schramm S, Schnolzer M, Heilmann CJ, de Koster CG, Schutz W, Benavente R, Alsheimer M. 2012. A truncated lamin A in the Lmna −/− mouse line: implications for the understanding of laminopathies. Nucleus 3:463–474. 10.4161/nucl.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmuth M, Martinz V, Janecke AR, Fauth C, Schossig A, Zschocke J, Gruber R. 2013. Inherited ichthyoses/generalized Mendelian disorders of cornification. Eur. J. Hum. Genet. 21:123–133. 10.1038/ejhg.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson JW. 2013. Practical skin pathology: a diagnostic approach. Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 29.Calonje E, McKee PH. 2012. McKee's pathology of the skin: with clinical correlations, 4th ed. Elsevier/Saunders, Edinburgh, United Kingdom. [Google Scholar]
- 30.Radner FP, Grond S, Haemmerle G, Lass A, Zechner R. 2011. Fat in the skin: Triacylglycerol metabolism in keratinocytes and its role in the development of neutral lipid storage disease. Dermatoendocrinology 3:77–83. 10.4161/derm.3.2.15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard G. 2004. Molecular genetics of the ichthyoses. Am. J. Med. Genet. C Semin. Med Genet. 131C:32–44. 10.1002/ajmg.c.30032. [DOI] [PubMed] [Google Scholar]
- 32.Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. 2008. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J. Lipid Res. 49:697–714. 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, Preiss-Landl K, Lass A, Zimmermann R, Hoefler G, Zechner R, Haemmerle G. 2010. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J. Biol. Chem. 285:7300–7311. 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrop M, Prud'homme JF, Fischer J. 2001. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 69:1002–1012. 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shultz LD, Lyons BL, Burzenski LM, Gott B, Samuels R, Schweitzer PA, Dreger C, Herrmann H, Kalscheuer V, Olins AL, Olins DE, Sperling K, Hoffmann K. 2003. Mutations at the mouse ichthyosis locus are within the lamin B receptor gene: a single gene model for human Pelger-Huet anomaly. Hum. Mol. Genet. 12:61–69. 10.1093/hmg/ddg003. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. 1999. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147:913–920. 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung HJ, Nobumori C, Goulbourne CN, Tu Y, Lee JM, Tatar A, Wu D, Yoshinaga Y, de Jong PJ, Coffinier C, Fong LG, Young SG. 2013. Farnesylation of lamin B1 is important for retention of nuclear chromatin during neuronal migration. Proc. Natl. Acad. Sci. U. S. A. 110:E1923–E1932. 10.1073/pnas.1303916110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrowman J, Hamblet C, George CM, Michaelis S. 2008. Analysis of prelamin A biogenesis reveals the nucleus to be a CaaX processing compartment. Mol. Biol. Cell. 19:5398–5408. 10.1091/mbc.E08-07-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dwyer N, Blobel G. 1976. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J. Cell Biol. 70:581–591. 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerace L, Blum A, Blobel G. 1978. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J. Cell Biol. 79:546–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fricker M, Hollinshead M, White N, Vaux D. 1997. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 136:531–544. 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. 1997. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 138:1193–1206. 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akiyama M. 2011. Updated molecular genetics and pathogenesis of ichthiyoses. Nagoya J. Med. Sci. 73:79–90. [PMC free article] [PubMed] [Google Scholar]
- 44.Kalinin AE, Kajava AV, Steinert PM. 2002. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays 24:789–800. 10.1002/bies.10144. [DOI] [PubMed] [Google Scholar]
- 45.Homberg M, Magin TM. 2014. Beyond expectations: novel insights into epidermal keratin function and regulation. Int. Rev. Cell. Mol. Biol. 311:265–306. 10.1016/B978-0-12-800179-0.00007-6. [DOI] [PubMed] [Google Scholar]
- 46.Adeyo O, Allan BB, Barnes RH, II, Goulbourne CN, Tatar A, Tu Y, Young LC, Weinstein MM, Tontonoz P, Fong LG, Beigneux AP, Young SG. 2014. Palmoplantar keratoderma along with neuromuscular and metabolic phenotypes in Slurp1-deficient mice. J. Investig. Dermatol. 134:1589–1598. 10.1038/jid.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feingold KR. 2009. The outer frontier: the importance of lipid metabolism in the skin. J. Lipid Res. 50(Suppl):S417–S422. 10.1194/jlr.R800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz G, Muller G. 1991. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 32:1539–1570. [PubMed] [Google Scholar]
- 49.Fartasch M. 2004. The epidermal lamellar body: a fascinating secretory organelle. J. Investig. Dermatol. 122:XI–XII. 10.1111/j.0022-202X.2004.22541.x. [DOI] [PubMed] [Google Scholar]
- 50.Pappas A. 2009. Epidermal surface lipids. Dermatoendocrinology 1:72–76. 10.4161/derm.1.2.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waterham HR, Koster J, Mooyer P, Noort Gv G, Kelley RI, Wilcox WR, Wanders RJ, Hennekam RC, Oosterwijk JC. 2003. Autosomal recessive HEM/Greenberg skeletal dysplasia is caused by 3 beta-hydroxysterol delta 14-reductase deficiency due to mutations in the lamin B receptor gene. Am. J. Hum. Genet. 72:1013–1017. 10.1086/373938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olins AL, Rhodes G, Welch DB, Zwerger M, Olins DE. 2010. Lamin B receptor: multi-tasking at the nuclear envelope. Nucleus 1:53–70. 10.4161/nucl.1.1.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wassif CA, Brownson KE, Sterner AL, Forlino A, Zerfas PM, Wilson WK, Starost MF, Porter FD. 2007. HEM dysplasia and ichthyosis are likely laminopathies and not due to 3beta-hydroxysterol Delta14-reductase deficiency. Hum. Mol. Genet. 16:1176–1187. 10.1093/hmg/ddm065. [DOI] [PubMed] [Google Scholar]
- 54.Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, II, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, Fong LG, Young SG. 2011. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol. Biol. Cell. 22:4683–4693. 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potten CS, Saffhill R, Maibach HI. 1987. Measurement of the transit time for cells through the epidermis and stratum corneum of the mouse and guinea-pig. Cell Tissue Kinet. 20:461–472. [DOI] [PubMed] [Google Scholar]
- 56.Solecki DJ, Govek EE, Tomoda T, Hatten ME. 2006. Neuronal polarity in CNS development. Genes Dev. 20:2639–2647. 10.1101/gad.1462506. [DOI] [PubMed] [Google Scholar]
- 57.Tsai LH, Gleeson JG. 2005. Nucleokinesis in neuronal migration. Neuron 46:383–388. 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 58.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. 2010. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc. Natl. Acad. Sci. U. S. A. 107:5076–5081. 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies BS, Coffinier C, Yang SH, Jung HJ, Fong LG, Young SG. 2011. Posttranslational processing of nuclear lamins, p 21–41 In Tamanoi F, Hrycyna CA, Bergo MO. (ed), The enzymes, vol 29 Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 60.Young SG, Yang SH, Davies BS, Jung HJ, Fong LG. 2013. Targeting protein prenylation in progeria. Sci. Transl. Med. 5:171ps173. 10.1126/scitranslmed.3005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.