Abstract
Macrophages regulate cell fate decisions during microbial challenges by carefully titrating signaling events activated by innate receptors such as dectin-1 or Toll-like receptors (TLRs). Here, we demonstrate that dectin-1 activation robustly dampens TLR-induced proinflammatory signature in macrophages. Dectin-1 induced the stabilization of β-catenin via spleen tyrosine kinase (Syk)-reactive oxygen species (ROS) signals, contributing to the expression of WNT5A. Subsequently, WNT5A-responsive protein inhibitors of activated STAT (PIAS-1) and suppressor of cytokine signaling 1 (SOCS-1) mediate the downregulation of IRAK-1, IRAK-4, and MyD88, resulting in decreased expression of interleukin 12 (IL-12), IL-1β, and tumor necrosis factor alpha (TNF-α). In vivo activation of dectin-1 with pathogenic fungi or ligand resulted in an increased bacterial burden of Mycobacteria, Klebsiella, Staphylococcus, or Escherichia, with a concomitant decrease in TLR-triggered proinflammatory cytokines. All together, our study establishes a new role for dectin-1-responsive inhibitory mechanisms employed by virulent fungi to limit the proinflammatory environment of the host.
INTRODUCTION
Activation of host pattern recognition receptors (PRRs) upon engagement by invading microbe or microbe-associated molecular patterns to elicit protective immune responses is one of the most well-studied physiological processes (1–4). However, dampening of the protective innate immune cell functions during multiple infections upon engagement of many PRRs constituting different families has not been explored. Dectin-1, a C-type lectin receptor (CLR) expressed on the surface of antigen-presenting cells, like macrophages and dendritic cells, are sensors of invading fungi and elicit predominantly Th17-mediated immune responses. For example, Candida albicans, a major opportunistic pathogen, is the cause of severe candidiasis affecting individuals under immunocompromised conditions (5, 6). In addition to this, several other fungi are shown to cause many dreadful diseases in immune-deficient individuals (7, 8). Significantly, in spite of driving the T cell responses toward the Th17 inflammatory phenotype through the dectin-1 receptor, the protection against severe candidiasis is not completely attained (9). Of note, recent reports have clearly implicated that engagement/activation of dectin-1 receptor by fungi or fungal counterparts intercepts the protective functional role of other PRRs, majorly Toll-like receptors (TLRs). Activation of dectin-1 by C. albicans has been shown to induce interleukin 10 (IL-10), which has an inhibitory effect over TLR by targeting MyD88 (10, 11). Similar studies have also shown that dampening of TLR-triggered protective immune responses is mediated by dectin-1-induced expression of suppressor of cytokine signaling 1 (SOCS-1) (12).
The dectin-1 signaling network is comprised of an immediate intracellular signaling kinase, spleen tyrosine kinase (Syk). Activation of Syk elicits the robust production of reactive oxygen species (ROS) and subsequently induces NF-κB-driven gene expression (13). However, the signaling intermediates bridging the potent secondary messenger-mediated activation and subsequent NF-κB-induced gene expression are not established.
Here, we demonstrate that activation of dectin-1 receptor by fungi or by its cognate ligand, curdlan, leads to the induced expression of protein inhibitors of activated STAT (PIAS-1) and SOCS-1. PIAS-1 and SOCS-1 are known to be the potential posttranslation protein modifiers that regulate the function of several cytoplasmic as well as nuclear proteins. PIAS molecules are shown to alter the protein stability and localization of several signaling molecules and epigenetic regulators. On the other hand, SOCS molecules with E3 ubiquitin ligase activity are shown to mediate proteasomal degradation of vital signaling adaptors to downregulate the receptor signal strength (14, 15). Elevated levels of ROS due to dectin-1 signaling stabilized β-catenin and collectively contributed to the expression of WNT5A. Subsequently, secreted WNT5A activates the calcium-activated calmodulin kinase (Ca2+/CAMKII) pathway to induced PIAS-1 and SOCS-1 expression through the TAK1–NF-κB axis. Importantly, we demonstrate that overexpression of PIAS-1 and SOCS-1 in macrophages leads to reduced protein levels of pivotal TLR adaptors, such as IRAK-1, IRAK-4, and MyD88. The dectin-1-dependent increased burden of the tested bacteria, in vivo as well as in vitro, in the presence of curdlan or C. albicans infection and lowered levels of tumor necrosis factor alpha (TNF-α), IL-12, and IL-1β cytokines upon infection with fungi further substantiated our findings. In summary, the study reinforces the existence of a counteracting effect among two PRR families during multiple infections. We propose that the invading pathogens exploit such mechanisms to evade protective responses and also drive the less pathogenic (commensal) bacteria to become opportunistic pathogens.
MATERIALS AND METHODS
Mice.
Thioglycolate-elicited peritoneal macrophages were isolated from wild-type (WT) (C3H/HeJ or C57BL/6J) mice or TLR2−/− or NCF1−/− mice in a C57BL/6J background that were maintained at the Central Animal Facility, Indian Institute of Science. The RAW 264.7 mouse macrophage cell line (obtained from National Centre for Cell Sciences, Pune, India) was cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco-Invitrogen, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, USA). The experiments with mouse macrophages were carried out after the approval from the Institutional Ethics Committee for animal experimentation as well as from the Institutional Biosafety Committee.
Reagents and antibodies.
Curdlan (100 μg/ml), PAM3CSK4 (1 μg/ml), R848 (1 μg/ml), flagellin (0.1 μg/ml), CpG DNA (10 μg/ml), lipopolysaccharide (LPS) (100 ng/ml), and laminarin (100 μg/ml) were obtained from Invivogen, USA. Anti-Ser9 phospho-glycogen synthase kinase 3β (pGSK-3β), anti-Ser-33/37/Thr41 phospho-β-catenin, anti-β-catenin, anti-Tyr402 phospho-PYK2, anti-Tyr323 phospho-Syk, anti-Tyr231 phospho-CAMKII, anti-Ser412 phospho-TAK1, anti-TAK1, anti-NF-κB p65, anti-WNT5A, anti-PIAS-1, anti-SOCS-1, anti-IRAK-1, anti-IRAK-4, anti-IRAK-M, and anti-MyD88 were purchased from Cell Signaling Technology (USA). Anti-rabbit horseradish peroxidase (HRP)-conjugated antibody, anti-mouse HRP-conjugated antibody, and anti-rabbit antibody–Alexa Fluor 647 were obtained from Jackson ImmunoResearch (USA). Anti-β-actin antibody conjugated to HRP was purchased from Sigma-Aldrich. Anti-mouse PCNA was purchased from Calbiochem (Germany). Anti-Myc and anti-Flag antibodies were obtained from Santa Cruz Biotechnology, Inc. (USA). 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) was from Sigma-Aldrich.
Fungal cultures.
C. albicans (MTCC 4748), Aspergillus flavus (MTCC 9064), and Aspergillus fumigatus (MTCC 1811) were obtained from Microbial Type Culture Collection, IMTECH, Chandigarh, India. C. albicans was grown on Sabouraud-dextrose agar (SDA) plates, and colonies were picked and subjected to liquid cultures. Log-phase cultures were harvested and enumerated for CFU. A. flavus and A. fumigatus were plated and subcultured on SDA plates and incubated for 4 days at 30°C. Conidia were harvested by gently scraping the surface of 4-day-old culture with an inoculation needle and collected in vials containing 5 ml phosphate-buffered saline (PBS), pH 7, and 0.01% (vol/vol) sterile polyoxyethylene sorbitan monooleate (Tween 20; Merck) solution. The concentration of conidia in different suspensions was determined by using a Neubauer hemocytometer. Serial dilutions were prepared in sterile PBS containing Tween 20. The viability of conidia was determined by a plate count technique on SDA plates.
Transient-transfection studies.
Murine RAW 264.7 macrophages were transiently transfected with respective target-specific small interfering RNA (siRNA) or with control nontargeting siRNA using low-molecular-weight polyethylenimine (Sigma-Aldrich). Similarly, transient transfection with various dominant negative or overexpression (OE) constructs was carried out along with vector controls. Mouse Wnt5a siRNA, Tak1 siRNA, β-catenin siRNA, and Dectin-1 siRNA were obtained from Dharmacon (USA) as siGENOME SMARTpool reagents, which contain a pool of four different double-stranded RNA oligonucleotides. Transient-transfection efficiency was found to be more than 50%, as estimated by counting the number of siGLO lamin A/C-positive cells in a microscopic field using fluorescence microscope. In all the cases, 48 h posttransfection, the cells were treated or infected as indicated and processed for analysis.
Treatment with pharmacological reagents.
In the experiments involving pharmacological reagents, cells were treated with respective inhibitors for 1 h prior to treatment with the ligand at the following concentrations: MG-132, 10 μM; Syk inhibitor, 10 μM; DPI, 10 μM; β-catenin inhibitor, 15 μM; LiCl, 5 mM; IWP-II, 5 mM; PTX, 200 ng; BAPTA-AM, 10 μM; KN-93, 10 μM; AG-17, 15 μM; and BAY 11-7082, 10 μM. All the pharmacological inhibitors were purchased from Calbiochem. Dimethyl sulfoxide (DMSO) at 0.1% concentration was used as a vehicle control. A tested concentration of each pharmacological reagent was identified by a careful titration experiment assessing the viability of macrophages using an MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide] assay prior to utilization in the experimental studies.
Immunofluorescence.
RAW 264.7 macrophages were seeded onto coverslips and treated as indicated. The cells were fixed with 3.7% paraformaldehyde for 15 min at room temperature. After blocking, cells were incubated with primary antibodies in saponin-containing diluent overnight at 4°C. Secondary antibody incubation was in the dark for 1 h at room temperature. Nuclear staining was performed with DAPI for 2 min. Coverslips with cells were then mounted on a slide with glycerol. Confocal images were taken on a Zeiss LSM 710 Meta confocal laser scanning microscope (Carl Zeiss AG, Germany) using a Plan-Apochromat 63× magnification/1.4-numerical-aperture oil differential inference contrast (DIC) objective (Carl Zeiss AG), and images were analyzed using ZEN 2009 software.
Immunopulldown.
Immunopulldown assays were carried out using a protocol with certain modifications provided by Millipore, USA. In brief, macrophages were gently suspended and lysed in ice-cold RIPA buffer. Obtained cell lysates were incubated with anti-Flag or anti-Myc antibodies (in respective experimental setup) or with rabbit preimmune sera at 4°C for 2 h in shaking conditions. The immune complexes were captured upon addition of protein A (Bangalore Genei, India)-agarose beads at 4°C for 2 h. The beads were separated, washed, and boiled in 5× Laemmli buffer for 10 min. These samples were analyzed for respective target molecules upon separation by SDS-PAGE followed by an immunoblotting technique.
Intracellular ROS detection.
A total of 1 × 106 peritoneal macrophages or RAW 264.7 macrophages transfected with respective constructs were treated with curdlan (100 μg/ml) for various time points. After treatment, cells were washed with fresh medium and then incubated with fresh DMEM containing 10 μm 2′,7′-dichlorofluorescein diacetate (DCFDA) to the final concentration for 30 min, and then cells were washed with PBS to remove excess DCFDA. The cells were analyzed for fluorescence emitted due to ROS from DCFDA by using fluorescence spectrophotometer with an excitation wavelength of 495 nm and emission wavelength of 529 nm.
RNA isolation and quantitative real-time RT-PCR.
Total RNA from infected macrophages was isolated by the TRIzol method (Sigma-Aldrich, USA) as per the manufacturer's protocol and treated with RNase-free DNase (Promega, USA). The cDNA synthesis kit (Bioline, United Kingdom) was used for reverse transcription (RT) according to the manufacturer's protocol. A real-time PCR amplification (Applied Biosystems, USA) using SYBR green PCR mix (Kappa Biosystems, USA) was performed for quantification of target gene expression with the following conditions: 45 cycles of 94°C for 20 s, 60°C for 30 s, and 72°C for 30 s. All reactions were repeated at least three times independently to ensure the reproducibility of the results. Amplification of the Gapdh housekeeping gene was used as an internal control. Primer sequences used in the study are as follows: Gapdh forward, 5′-GAGCCAAACGGGTCATCATCT-3′; Gapdh reverse, 5′-GAGGGGCCATCCACAGTCTT-3′; Wnt5a forward, 5′-TGCGGAGACAACATCGACTAT-3′; Wnt5a reverse, 5′-TCCATGACACTTACAGGCTACA-3′; Pias-1 forward, 5′-ACGCAAACACGAACTTCTTACA-3′; Pias-1 reverse, 5′-TCCGCAGGCGTCATAATTTTC-3′; Socs-1 forward, 5′-CTGCGGCTTCTATTGGGGAC-3′; Socs-1 reverse, 5′-AAAAGGCAGTCGAAGGTCTCG-3′; Dectin-1 forward, 5′-GACTTCAGCACTCAAGACATCC-3′; Dectin-1 reverse, 5′-TTGTGTCGCCAAAATGCTAGG-3′.
Nuclear fractionation.
Cells were treated as indicated, harvested by centrifugation, and gently resuspended in ice-cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). After incubation on ice for 15 min, cell membranes were disrupted with 10% NP-40, and the nuclear pellets were recovered by centrifugation at 4,000 rpm for 15 min at 4°C. The supernatants from this step were used as cytosolic extracts. Nuclear pellets were lysed with ice-cold buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1 mM PMSF), and nuclear extracts were collected after centrifugation at 13,000 rpm for 20 min at 4°C.
In vivo bacterial challenge and bacterial viability assays.
Each experimental set (in vivo) consisted of 4 mice. Each mouse was intravenously or intraperitoneally injected with 500 μg of curdlan, and after 6 h, mice were intravenously or intraperitoneally challenged with respective bacteria (1 × 106) obtained from MTCC. After 48 to 72 h of challenge, mice were sacrificed. In the case of intravenous challenge, bacterial CFU were enumerated from spleen and lymph node, and in the case of intraperitoneal challenge, peritoneal exudates were isolated and bacterial CFU were enumerated. Similarly, bacterial CFU were enumerated after peritoneal macrophages were infected (0.5 × 106/ml) in vitro or nontargeting (NT)/Dectin-1 siRNA-transfected RAW 264.7 macrophages were infected (0.3 × 106/ml) with respective bacteria (1:10 multiplicity of infection [MOI]) for 18 h in the presence or absence of curdlan (100 μg/ml) or C. albicans coinfection.
Immunoblotting.
Cells were washed with ice-cold PBS and scraped off the culture dish and collected by centrifugation. Cell pellets were lysed in RIPA buffer consisting of 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 μg/ml of aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF and incubated on ice for 30 min. Whole-cell lysate was collected by centrifuging lysed cells at 13,000 rpm for 10 min at 4°C. Protein concentration in each cell lysate was determined by Bradford's method of protein detection. Equal protein amounts from each cell lysate were subjected to SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore) by using the semidry Western blotting (Bio-Rad, USA) method. Nonspecific binding was blocked with 5% nonfat dry milk powder in TBST (20 mM Tris-HCl [pH 7.4], 137 mM NaCl, and 0.1% Tween 20) for 60 min. The blots were incubated overnight at 4°C with primary antibodies diluted in TBST with 5% bovine serum albumin (BSA). After being washed with TBST, blots were incubated with anti-rabbit or anti-mouse IgG secondary antibody conjugated to HRP diluted in TBST with 5% BSA for 2 h. After further washing in TBST, the immunoblots were developed with the enhanced chemiluminescence detection system (PerkinElmer, USA) as per the manufacturer's instruction.
Cytokine detection.
Cell-free supernatants were obtained from various experimental setups and were analyzed for TNF-α, IL-12, and IL-1β by enzyme-linked immunosorbent assay (ELISA) by utilizing kits procured from BD Biosciences (USA), according to the manufacturer's protocol.
Statistical analysis.
Levels of significance for comparison among the samples were determined by Student t test distribution and one-way analysis of variance (ANOVA). The represented graphical data are expressed as the means ± standard errors of the means (SEM) of 4 to 5 values from 3 independent experiments unless mentioned otherwise. P values of <0.05 were considered to be significant. Prism 5.0 software (GraphPad Software, Inc., USA) was used for all the statistical analysis.
RESULTS
Dectin-1 signaling intercepts TLR inflammatory signature.
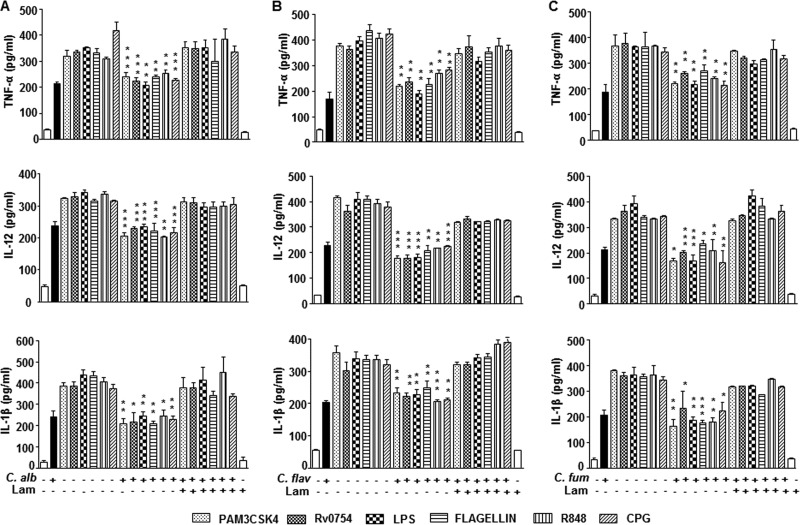
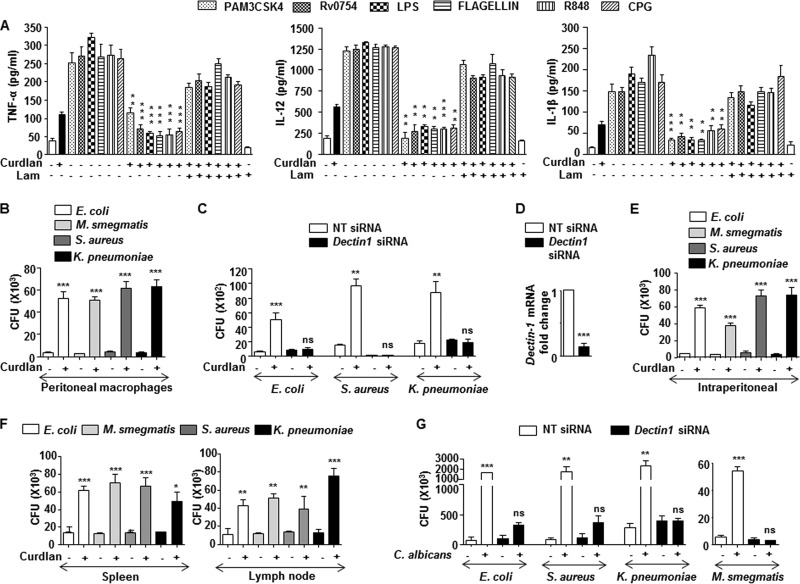
Several studies indicated the possible cross-regulation of dectin-1–TLR pathways (16). However, the mechanistic details of such interactions during multiple infections are scanty. In this regard, we chose to study the effect of dectin-1 signaling on the TLR pathways. Prior activation of dectin-1 receptor in macrophages upon infection with C. albicans, A. flavus, and A. fumigatus inhibited TLR (TLR1, -2, -4, -5, -7, -8, -9)-induced expression of inflammatory cytokines, such as TNF-α, IL-12, and IL-1β (Fig. 1A to C). Further, utilization of cognate ligand to the curdlan dectin-1 receptor also exhibited similar inhibitory effect (Fig. 2A). Laminarin, a competitive ligand to dectin-1 receptor with no signaling attributes, completely blocked the inhibitory potential of dectin-1 over TLR functions (Fig. 1 and Fig. 2A). Importantly, treatment of macrophages with laminarin alone did not have any effect on cytokine production. These results underscore the role of dectin-1 receptor signaling during fungus-mediated downregulation of immune responses. Additionally, the inhibitory effect of dectin-1 signaling on the immune protective functions of TLR was directly correlated with the increased bacterial viability. While treatment of curdlan prior to infection with Escherichia coli, Mycobacterium smegmatis, Staphylococcus aureus, or Klebsiella pneumoniae led to enhanced survival of bacteria in peritoneal macrophages (Fig. 2B) and nontargeting siRNA-transfected macrophages (Fig. 2C and D), we found significant decreases in the bacterial CFU in Dectin-1 siRNA-transfected macrophages despite the curdlan treatment (Fig. 2C and D). This suggests that curdlan-dependent enhanced bacterial survival is specifically dectin-1 mediated. Further establishing this observation in vivo, the intraperitoneal inoculation of mice with curdlan prior to the challenge of mice with bacteria via the same route significantly enhanced the survival of bacteria in macrophages of the peritoneal exudate (Fig. 2E). Similar results were obtained when assessed for bacterial CFU in the spleens (Fig. 2F, left) and lymph nodes (Fig. 2F, right) of mice challenged with bacteria after treatment with curdlan. RNA interference of Dectin-1 significantly reduced the ability of C. albicans to protect the tested bacteria, E. coli, M. smegmatis, S. aureus, or K. pneumoniae, from clearance in the macrophages compared to that in macrophages transfected with nontargeting siRNA (Fig. 2G). Together, these results imply that there exists a counterregulation by dectin-1 on TLR receptors.
FIG 1.
Fungus-activated dectin-1 limits TLR-induced cytokines. (A to C) Mouse macrophages from C57BL/6J mice were infected with C. albicans (C. alb), A. flavus (A. flav), or A. fumigatus (A. fum), respectively, at an MOI of 1:10 for 6 h followed by treatment with TLR ligands for an additional 18 h in the presence or absence of 100 μg/ml laminarin (Lam). Lam treatment alone served as a control. Cell-free supernatants were analyzed for TNF-α, IL-12, and IL-1β by ELISA. All data represent the means ± SEM from 3 independent experiments. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
FIG 2.
Interception of protective TLR-induced cytokines by dectin-1 engagement favors bacterial survival. (A) Mouse macrophages from C57BL/6J mice were treated with dectin-1 ligand, curdlan (100 μg/ml), for 6 h, followed by treatment with TLR ligands for an additional 18 h in the presence or absence of Lam. Lam treatment alone served as a control. Cell-free supernatants were analyzed for TNF-α, IL-12, and IL-1β by ELISA. (B and C) Mouse peritoneal macrophages (B) or nontargeting (NT) or Dectin-1 siRNA-transfected RAW 264.7 macrophages (C) were treated with curdlan for 6 h followed by infection with the indicated bacteria at an MOI of 1:10 for 18 h. Cells were lysed and enumerated for bacterial CFU. (D) Validation of Dectin-1 siRNA action in RAW 264.7 cells. (E) Intraperitoneal injection of mice with curdlan for 6 h followed by intraperitoneal challenge with the indicated bacteria (1 × 106 CFU) for 18 h. Peritoneal macrophages were isolated and enumerated for bacterial CFU. (F) Intravenous infusion of mice with curdlan for 6 h followed by intravenous challenge of mice with the indicated bacteria (1 × 106 CFU) for 18 h. Spleen and lymph nodes were isolated and enumerated for bacterial CFU. (G) RAW 264.7 cells transiently transfected with NT or Dectin-1 siRNA were infected with C. albicans for 6 h prior to 18 h of infection with the indicated bacteria. Cells were lysed and enumerated for bacterial CFU. All data represent the means ± SEM from 3 independent experiments. *, P < 0.05; **, P < 0.005; ***, P < 0.001; ns, not significant.
Dectin-1-induced PIAS-1 and SOCS-1 negatively influence TLR signaling by targeting IRAK-1, IRAK-4, and MyD88.
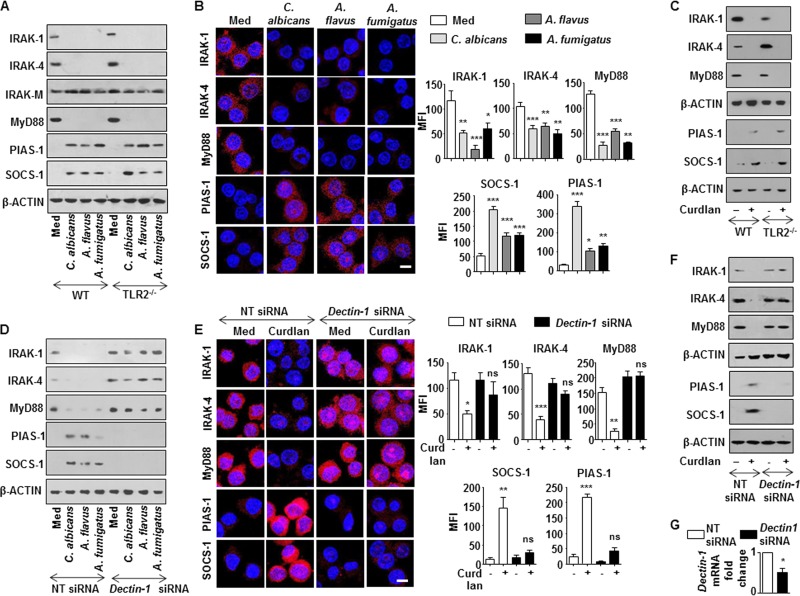
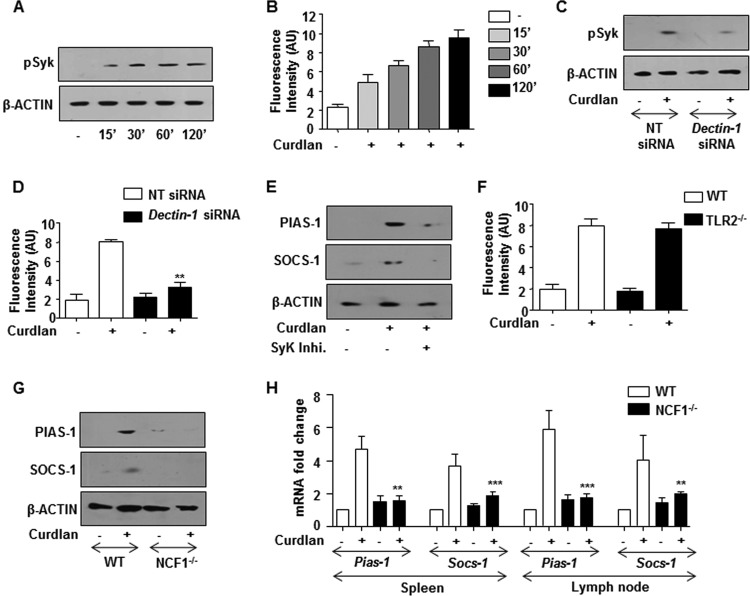
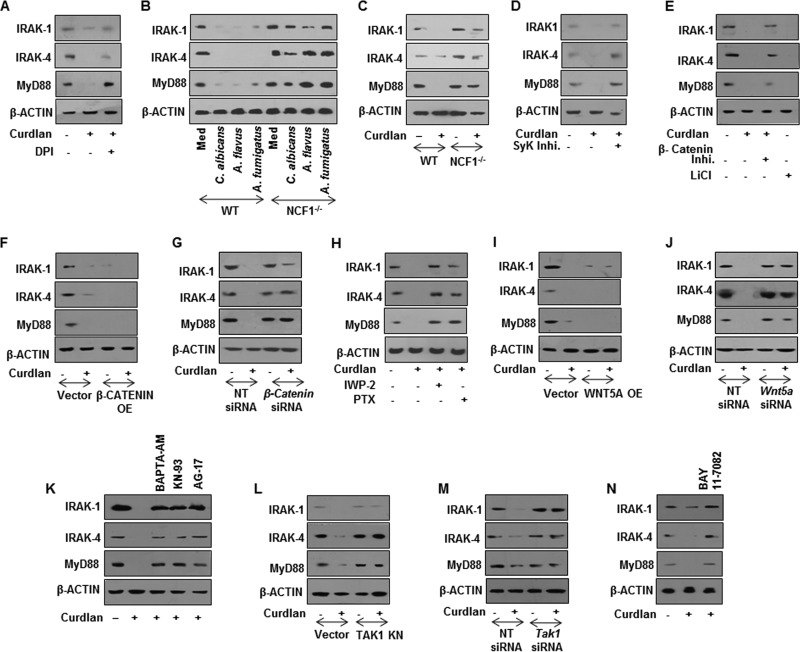
Impaired TLR signaling can be attributed to several molecular processes, including the downregulation of TLR signaling adaptors, induction of SOCS, and inhibitory IRAK, such as IRAK-M (17–19). As shown in Fig. 3A and B, infection of macrophages with C. albicans, A. flavus, or A. fumigatus abrogated the expression of the TLR signaling adaptors, IRAK-1, IRAK-4, and MyD88. However, no change in the expression levels of inhibitory IRAK-M was observed with infection of macrophages with the tested fungi (Fig. 3A). Though the existing literature emphasized the requirement of TLR2 for effector functions of dectin-1 (20, 21), surprisingly, we observed that infection of macrophages from TLR2-null mice with the tested fungi or curdlan (Fig. 3C) followed a similar trend. This suggests that dectin-1 can function independent of TLR2. However, siRNA-mediated knockdown of dectin-1 compromised the ability of these fungi (Fig. 3D) or curdlan (Fig. 3E to G) to downregulate IRAK-1, IRAK-4, and MyD88.
FIG 3.
Dectin-1 signaling downregulates the expression of IRAK-1, IRAK-4, and MyD88 and upregulates PIAS-1 and SOCS-1. (A) Immunoblot analysis of IRAK-1, IRAK-4, MyD88, PIAS-1, and SOCS-1 in macrophages from C57BL/6J WT and TLR2−/− mice infected with respective fungi for 12 h. (B) RAW 264.7 macrophages were infected with the indicated fungi for 12 h. Representative immunofluorescence images (left) and MFI (right) for IRAK-1, IRAK-4, MyD88, PIAS-1, and SOCS-1 are shown. (C) Peritoneal macrophages from C57BL/6J WT or TLR2−/− mice were treated with curdlan for 12 h. Whole-cell lysates were analyzed for the indicated genes by immunoblotting. (D) Murine RAW 264.7 macrophages were transiently transfected with Dectin-1 siRNA followed by infection with respective fungi at an MOI of 1:10. Whole-cell lysates were analyzed for IRAK-1, IRAK-4, MyD88, PIAS-1, and SOCS-1 by immunoblotting. Immunofluorescence (E) and immunoblotting (F) analysis of IRAK-1, IRAK-4, MyD88, PIAS-1, and SOCS-1 in RAW 264.7 macrophages (E) or peritoneal macrophages from C3H/HeJ mice (F) transiently transfected with Dectin-1 siRNA followed by treatment with curdlan for 12 h. (G) Validation of Dectin-1 siRNA action in peritoneal macrophages. All blots are representative of 3 independent experiments, and all data represent the means ± SEM from 3 independent experiments. *, P < 0.05; **, P < 0.005; ***, P < 0.001; ns, not significant; Med, medium; WT, wild type; NT, nontargeting. Bar, 5 μm.
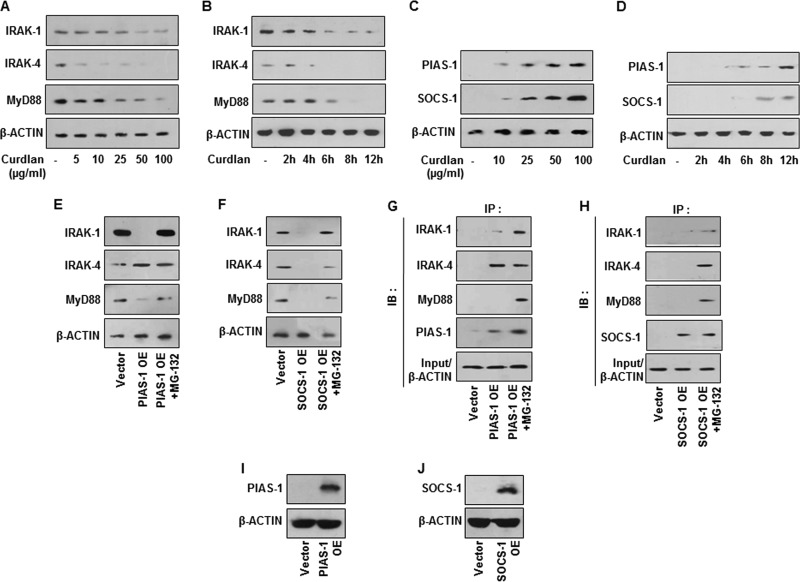
Previous reports have suggested the crucial role for posttranslational modifications of IRAK-1 (22), IRAK-4 (23), and MyD88 (24) to dampen TLR responses. Additionally, SOCS-1 was also found to modulate TLR responses (25). We thus assessed the role for posttranslational modifications by PIAS-1 and SOCS-1 in targeting TLR signaling adaptors like IRAK-1, IRAK-4, and MyD88. Interestingly, engagement of dectin-1 with respective fungi (Fig. 3A and B) or curdlan (Fig. 3C) induced the expression of PIAS-1 and SOCS-1 independent of TLR2, further potentiating the independent role for dectin-1. We also observed the essential role of dectin-1 receptor in fungus (Fig. 3D)- or curdlan (Fig. 3E to G)-induced expression of PIAS-1 and SOCS-1. Concurrently, while expression of IRAK-1, IRAK-4, and MyD88 was reduced after treatment with curdlan with dose (Fig. 4A) and time (Fig. 4B), PIAS-1 and SOCS-1 levels were found to be upregulated (Fig. 4C and D). Next, to establish the role of dectin-1-responsive PIAS-1 and SOCS-1 in targeting TLR2 adaptor molecules, immune pulldown assays were performed. Flag/Myc-immunoprecipitates from cells transfected with PIAS-1-Flag or SOCS-1-Myc OE constructs were probed for IRAK-1, IRAK-4, and MyD88. As illustrated in Fig. 4E to J, while SOCS-1 targeted IRAK-1, IRAK-4, and MyD88 for proteasomal degradation, PIAS-1 was found to target IRAK-1 and MyD88.
FIG 4.
Dectin-1 signaling targets IRAK-1, IRAK-4, and MyD88 by inducing PIAS-1 and SOCS-1. (A and B) Immunoblot analyses of IRAK-1, IRAK-4, and MyD88 in cell lysates of macrophages treated with different concentrations of curdlan for 12 h (A) or with 100 μg/ml curdlan for the indicated time intervals (B). (C and D) Peritoneal macrophages were treated with different concentrations of curdlan (C) or with 100 μg/ml curdlan for various time intervals (D). Whole-cell lysates were analyzed for PIAS-1 and SOCS-1 by immunoblotting. (E and F) Transient transfection of RAW 264.7 macrophages with PIAS-1 OE or SOCS-1 OE construct, respectively, in the presence or absence of MG-132 followed by immunoblotting analysis of IRAK-1, IRAK-4, and MyD88. (G and H) Immunopulldown (IP) assay with anti-Flag (G) or anti-Myc (H) antibodies in cell lysates of RAW 264.7 macrophages transfected with PIAS-1 OE (G) or SOCS-1 OE (H), followed by immunoblotting analysis of IRAK-1, IRAK-4, and MyD88. (I and J) Validation of PIAS-1 OE (I) and SOCS-1 OE (J) constructs using immunoblotting with respective antibodies. All blots are representative of 3 independent experiments. Med, medium; WT, wild type; OE, overexpression construct; IB, immunoblot; NT, nontargeting.
The Syk–ROS–β-catenin axis mediates dectin-1-induced expression of PIAS-1 and SOCS-1.
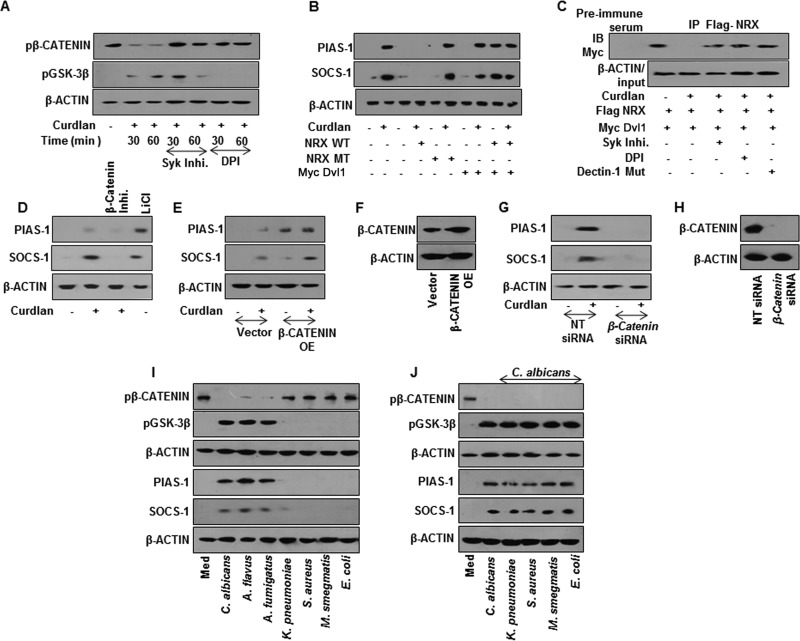
The signaling cohorts associated with the dectin-1 receptor axis are Syk, ROS, and NF-κB, the transcription factor-regulating gene expression (26–28). ROS, being one of the potent second messengers, is shown to regulate signaling pathways either by stabilization of intermediates or destabilization of negative regulators of signaling pathways. One such pathway shown to be modulated by the redox state of the cell is the WNT signaling pathway (29). Additionally, emerging reports have clearly emphasized the decisive role for the WNT pathway in immune cell fate decisions during pathogenic infections (30, 31). In the off state, phosphorylation of β-catenin by GSK-3β leads to its proteosomal degradation and suppresses the WNT pathway (32). Disheveled (Dvl1), an upstream negative regulator, modulates catalytic activity of GSK-3β, which itself is under the control of a cytosolic redox sensor, nucleoredoxin (NRX). NRX, in a reduced state, holds the activity of Dvl1, as a result of which GSK-3β is functionally active and subjects β-catenin to proteasomal degradation. An increase in ROS oxidizes NRX, by which Dvl1 is relieved, and blocks the activity of GSK-3β and stabilizes β-catenin independent of the WNT ligand. We investigated the existence of such mechanisms in dectin-1-induced expression of PIAS-1 and SOCS-1. Activation of dectin-1 receptor with curdlan led to an increase in phosphorylation of Syk and subsequently induced production of ROS (Fig. 5A to D) and led to the expression of PIAS-1 and SOCS-1 (Fig. 5E). Further, utilization of macrophages from TLR2-null mice implicated the TLR2-independent role for dectin-1 in executing its functions (Fig. 5F). The role of ROS in the dectin-1-mediated signaling pathway was further potentiated by utilizing macrophages from NCF1−/− (ROS−/−) mice. We demonstrated that the dectin-1-induced expression of PIAS-1 and SOCS-1 is significantly reduced in macrophages deficient in ROS production in vitro (Fig. 5G). Similarly, intravenous inoculation of mice with curdlan and further analysis of PIAS-1 and SOCS-1 in spleen and lymph node implicated the role for ROS in dectin-1-responsive PIAS-1 and SOCS-1 expression (Fig. 5H). Pharmacological intervention of dectin-1 signaling by the Syk inhibitor or inhibition of ROS production by DPI showed the pronounced effect on stabilization of β-catenin (Fig. 6A). Curdlan-induced expression of PIAS-1 and SOCS-1 was brought down by the overexpression of wild-type NRX, which resulted in increased sequestration of Dvl1 and subsequent abrogation of WNT signaling (Fig. 6B). In such a scenario, curdlan-induced ROS was insufficient to rescue PIAS-1 and SOCS-1 levels. The overexpressed mutant form of NRX was unable to induce PIAS-1 and SOCS-1. Also, overexpressed Dvl1 bypassed the requirement of both NRX and ROS, constitutively activating WNT signaling and in turn inducing PIAS-1 and SOCS-1 (Fig. 6B). We further potentiated the contribution of ROS in stabilization of β-catenin and induced expression of PIAS-1 and SOCS-1 by immunopulldown assay and observed that intervention of ROS induced by curdlan prevents Dvl1-NRX interaction, subsequently leading to the stabilization of β-catenin. Correspondingly, inhibition of ROS by SyK inhibitor, DPI, or a dectin-1 mutant construct promoted Dvl1-NRX interaction, resulting in β-catenin degradation (Fig. 6C). Blocking of GSK-3β by LiCl (Fig. 6D) or overexpression of β-catenin (Fig. 6E and F) or transient knockdown of β-catenin (Fig. 6G and H) implicated a role for β-catenin in dectin-1 signaling axis-mediated expression of PIAS-1 and SOCS-1. Supporting these observations, unlike the three bacteria, infection of macrophages with C. albicans, A. flavus, and A. fumigatus induced robust activation of WNT signaling and increased expression of PIAS-1 and SOCS-1 (Fig. 6I). These results are in accordance with our previous data, which showed that E. coli, M. smegmatis, and S. aureus do not activate WNT signaling in the infected macrophages (31). Further coinfection of C. albicans with the tested bacteria did not alter the ability of the fungi to induce the activation of the WNT pathway or expression of PIAS-1 and SOCS-1 (Fig. 6J).
FIG 5.
Dectin-1 activates Syk and produces ROS to induce the expression of PIAS-1 and SOCS-1. (A and B) Immunoblot analysis of pSyk (A) and detection of ROS by DCFDA (B) in mouse peritoneal macrophages treated with curdlan at the indicated time points. −, no curdlan treatment. (C and D) RAW 264.7 macrophages are transiently transfected with Dectin-1 siRNA followed by treatment with curdlan for 60 min. Immunoblotting analysis of pSyk in whole-cell lysates (C) and ROS production (D) was analyzed by DCFDA. (E) Mouse peritoneal macrophages were pretreated with Syk inhibitor for 60 min and then treated with curdlan for 12 h. Total cell lysates were analyzed for PIAS-1 and SOCS-1 by immunoblotting. (F) ROS detection in macrophages from WT or TLR2−/− mice which are treated with curdlan for 120 min. (G) Peritoneal macrophages isolated from C57BL/6J WT and NCF1−/− mice were treated with curdlan for 12 h. Lysates were analyzed for PIAS-1 and SOCS-1 by immunoblotting. (H) C57BL/6J WT or NCF1−/− mice were intravenously challenged with curdlan for 12 h. Spleen and lymph nodes were isolated and analyzed for PIAS1 and SOCS-1 expression by quantitative real-time RT-PCR. All blots are representative of 3 independent experiments, and all data represent the means ± SEM from 3 independent experiments. *, P < 0.05; **, P < 0.005; ***, P < 0.001; Med, medium; WT, wild type; NCF1−/−, p47phox−/− (mice deficient in ROS production).
FIG 6.
SyK-ROS-stabilized β-catenin mediates dectin-1 ligand-induced expression of PIAS-1 and SOCS-1. (A) Immunoblotting analysis of pβ-catenin and pGSK-3β in whole-cell lysates of mouse macrophages pretreated with Syk inhibitor or DPI for 60 min followed by treatment with curdlan. (B) Expression analysis of PIAS-1 and SOCS-1 by immunoblotting in RAW 264.7 macrophages transiently transfected with the indicated OE constructs followed by treatment with curdlan for 12 h. (C) Immunopulldown (IP) with anti-Flag antibody followed by immunoblot analysis for Myc in RAW 264.7 macrophages transiently transfected with respective constructs in the presence or absence of SyK inhibitor or DPI. (D) Mouse peritoneal macrophages were pretreated with β-catenin inhibitor for 60 min or with LiCl alone for 4 h and then treated with curdlan for 12 h. Expression of PIAS-1 and SOCS-1 was analyzed by immunoblotting. (E) RAW 264.7 macrophages were transiently transfected with β-catenin OE followed by treatment with curdlan. Total cell lysates were analyzed for PIAS-1 and SOCS-1 by immunoblotting. (F) Validation of β-catenin OE construct. (G and H) Peritoneal macrophages from C3H/HeJ mice were transiently transfected with β-catenin siRNA followed by treatment with curdlan for 12 h. Lysates were analyzed for PIAS-1 and SOCS-1 expression (G) and β-catenin levels (H) by immunoblotting. (I and J) Mouse peritoneal macrophages from C3H/HeJ mice were infected with the indicated fungi and bacteria (I) or coinfected with C. albicans followed by the tested bacteria (J) for 60 min to assess pβ-catenin and pGSK-3β levels and for 12 h to assess PIAS-1 and SOCS-1. All blots are representative of 3 independent experiments. WT, wild type; MT, mutant; NT, nontargeting; IB, immunoblot; OE, overexpression construct.
Dectin-1-induced expression of PIAS-1 and SOCS-1 requires WNT5A-Ca2+/CAMKII signaling.
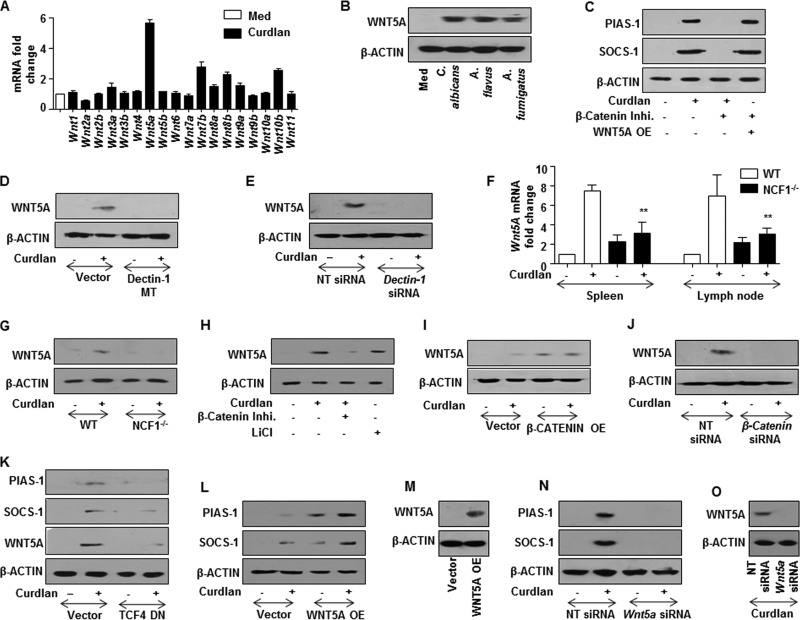
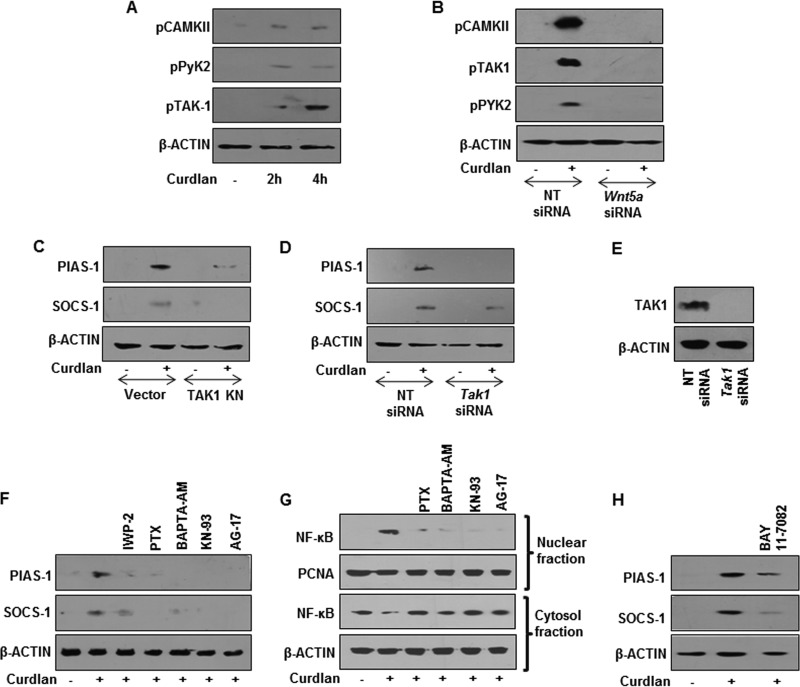
A screen for WNT ligands identified WNT5A as a significant dectin-1-responsive ligand that arbitrates the WNT signaling pathway (Fig. 7A and B). However, as shown earlier, stabilization of β-catenin led to the expression of PIAS-1 and SOCS-1 (Fig. 6G). Intriguingly, we observed the induction of PIAS-1 and SOCS-1 in WNT5A-transfected RAW 264.7 macrophages even in the presence of β-catenin inhibitor (Fig. 7C). This suggests that PIAS-1 and SOCS-1 are not the direct targets of β-catenin-mediated transcription but are WNT5A dependent. Concordant with our previous observation, we found that dectin-1 receptor was essential for curdlan-induced expression of WNT5A (Fig. 7D and E). Further, there was a significant reduction in the curdlan-induced expression of WNT5A in mice deficient for ROS production (Fig. 7F and G). Additionally, stabilization of β-catenin led to induced expression of WNT5A (Fig. 7H to K). The pivotal role of WNT5A in dectin-1-induced expression of PIAS-1 and SOCS-1 was validated by overexpression or transient knockdown of WNT5A. We demonstrated that overexpression of WNT5A led to augmented expression of curdlan-induced PIAS-1 and SOCS-1 (Fig. 7L and M). Further, knockdown of WNT5A by siRNA reduced the expression of PIAS-1 and SOCS-1 (Fig. 7N and O). Of note, it was recently demonstrated that WNT5A-mediated, calcium-activated calmodulin kinase (Ca2+/CAMKII), with several signaling intermediates, plays a crucial role in immune cell functions (33–35). Therefore, we investigated the involvement of the WNT5A-Ca2+/CAMKII axis in the dectin-1-mediated signaling pathway. The WNT5A-Ca2+/CAMKII pathway is subsequently known to drive NF-κB-mediated gene expression through TGF-β-activated kinase (TAK1) and proline-rich tyrosine kinase (Pyk2) (36, 37). Dectin-1-induced expression of WNT5A led to the activation of CAMKII, TAK1, and Pyk2, which was further validated by genetic knockdown of WNT5A by specific siRNA (Fig. 8A and B). Inhibition of TAK1, either by the kinase-negative mutant construct (Fig. 8C) or siRNA (Fig. 8D and E), and pharmacological intervention of activity of CAMKII and Pyk2 (Fig. 8F) implicated the involvement of WNT5A-Ca2+/CAMKII-Pyk2 and the TAK1 axis in dectin-1-induced expression of PIAS-1 and SOCS-1. Next, we demonstrated that there is increased nuclear translocation of NF-κB in macrophages upon engagement of dectin-1 receptor with curdlan (Fig. 8G) via the WNT-Ca2+/CAMKII-TAK1 axis. Pharmacological intervention of NF-κB translocation by BAY 11-7082 further implicated the role for NF-κB in dectin-1-induced expression of PIAS-1 and SOCS-1 (Fig. 8G and H). All together, we demonstrated that dectin-1-induced expression of PIAS-1 and SOCS-1 required ROS-stabilized β-catenin and WNT5A production.
FIG 7.
β-Catenin-mediated dectin-1-induced PIAS-1 and SOCS-1 expression requires WNT5A signaling. (A) Quantitative real-time RT-PCR analysis of WNT ligands in macrophages treated with curdlan for 12 h. (B) Peritoneal macrophages from C3H/HeJ mice were infected with the indicated fungi to estimate the expression of WNT5A by immunoblotting. (C) RAW 264.7 macrophages were transfected with WNT5A OE construct followed by pretreatment with β-catenin inhibitor. Cells were treated with curdlan for 12 h and then analyzed for PIAS-1 and SOCS-1 expression by immunoblotting. (D) RAW 264.7 macrophages were transfected with the dectin-1 MT construct and treated with curdlan for 4 h to analyze WNT5A expression by immunoblotting. (E) Immunoblotting analysis of WNT5A in peritoneal macrophages from C3H/HeJ mice transfected with Dectin-1 siRNA. (F) C57BL/6J WT or NCF1−/− mice were intravenously injected with curdlan for 18 h. Spleen and lymph nodes were isolated and analyzed for Wnt5a expression by quantitative real-time RT-PCR. (G) Immunoblotting analysis for WNT5A in macrophages from C57BL/6J WT or NCF1−/− mice treated with curdlan for 4 h. Macrophages were treated with β-catenin inhibitor or with LiCl (H) or transfected with the β-catenin OE construct (I) and then treated with curdlan for 4 h. Whole-cell lysates were analyzed for WNT5A expression by immunoblotting. (J) Immunoblotting analysis of WNT5A in peritoneal macrophages from C3H/HeJ mice transfected with β-catenin siRNA followed by treatment with curdlan for 4 h. (K to M) RAW 264.7 macrophages transfected with TCF4 DN (K) or WNT5A OE (L and M) were treated with curdlan for 12 h. Total cell lysates were analyzed for PIAS-1, SOCS-1 (K and L), and WNT5A (K and M) by immunoblotting. Immunoblotting analysis for PIAS-1, SOCS-1 (N), and WNT5A (O) in peritoneal macrophages from C3H/HeJ mice transfected with Wnt5a siRNA followed by treatment with curdlan. All blots are representative of 3 independent experiments, and all data represent the means ± SEM from 3 independent experiments. **, P < 0.005. Med, medium; MT, mutant; NT, nontargeting; WT, wild type; NCF1−/−, p47phox−/− (mice deficient in ROS production); OE, overexpression construct; DN, dominant negative.
FIG 8.
WNT5A-Ca2+/CAMKII signaling axis regulates dectin-1-induced PIAS-1 and SOCS-1 expression. (A) Immunoblotting analysis for pCAMKII, Pyk2, and pTAK1 in macrophages treated with curdlan for the indicated time intervals. (B) Total cell lysates from peritoneal macrophages transfected with Wnt5a siRNA and treated with curdlan were analyzed for pCAMKII, pPyk2, and pTAK1 levels. (C to E) RAW 264.7 macrophages transfected with the TAK1 KN construct (C) or Tak1 siRNA (D and E) were treated with curdlan for 12 h. Total cell lysates were analyzed for PIAS-1 and SOCS-1 (C and D) and TAK1 (E) by immunoblotting. Mouse peritoneal macrophages from C3H/HeJ mice were pretreated with the indicated pharmacological inhibitors followed by 12 h (F) or 60 min (G) of treatment with curdlan to estimate the expression of PIAS-1 and SOCS-1 from whole-cell lysate (F) or nuclear translocation of NF-κB from nuclear and cytosolic fractions (G). (H) Analysis of curdlan-induced PIAS-1 and SOCS-1 expression in macrophages pretreated with NF-κB inhibitor, BAY 11-7082. All blots are representative of 3 independent experiments. NT, nontargeting; KN, kinase negative.
The Syk-ROS-WNT-Ca2+/CAMKII axis effectuates dectin-1-induced downregulation of IRAK-1, IRAK-4, and MyD88.
The WNT5A-Ca2+/CAMKII axis regulated nuclear translocation of NF-κB and induced the expression of PIAS-1 and SOCS-1. The contribution of such a signaling axis in dectin-1-mediated impairment of TLR functions by degrading IRAK-1, IRAK-4, and MyD88 was validated. Experiments utilizing a pharmacological inhibitor of ROS or macrophages from NCF1-null mice clearly implicated the need for ROS in fungus- or curdlan-induced degradation of IRAK-1, IRAK-4, and MyD88 (Fig. 9A to C). Further, signaling perturbation studies by pharmacological agents or genetic knockdown procedures implicated the role for Syk (Fig. 9D), β-catenin (Fig. 9E to G), and WNT5A (Fig. 9H to J). Concordant results were obtained to demonstrate that Ca2+/CAMKII-Pyk2 (Fig. 9K), TAK1 (Fig. 9L and M), and NF-κB (Fig. 9N) played significant roles in dectin-1-induced degradation of IRAK-1, IRAK-4, and MyD88.
FIG 9.
Syk-ROS-WNT-Ca2+/CAMKII pathway mediates dectin-1-induced degradation of IRAK-1, IRAK-4, and MyD88. Immunoblotting analysis for IRAK-1, IRAK-4, and MyD88 in total cell lysates of peritoneal macrophages pretreated with DPI followed by 12 h curdlan treatment (A), peritoneal macrophages from C57BL/6J WT or NCF1−/− mice treated with respective fungi at an MOI of 1:10 for 12 h (B), peritoneal macrophages from C57BL/6J WT or NCF1−/− mice treated with curdlan for 12 h (C), peritoneal macrophages from C3H/HeJ mice pretreated with Syk inhibitor (D) or β-catenin inhibitor or LiCl (E) followed by treatment with curdlan for 12 h, RAW 264.7 macrophages transfected with β-catenin OE construct followed by 12 h of curdlan treatment (F), peritoneal macrophages from C3H/HeJ mice transfected with β-catenin siRNA followed by 12 h of curdlan treatment (G), peritoneal macrophages from C3H/HeJ mice pretreated with the indicated pharmacological inhibitors for 60 min followed by curdlan treatment for 12 h (H), RAW 264.7 macrophages (I) or peritoneal macrophages from C3H/HeJ mice (J) transiently transfected with the WNT5A OE construct (I) or Wnt5a siRNA (J) followed by treatment with curdlan for 12 h, peritoneal macrophages pretreated with the indicated pharmacological inhibitors followed by 12 h of curdlan treatment (K), RAW 264.7 macrophages transfected with the TAK1 KN construct (L) or Tak1 siRNA followed by 12 h of curdlan treatment (M) and peritoneal macrophages from C3H/HeJ mice pretreated with BAY 11-7082 followed by treatment of curdlan for 12 h (N). All blots are representative of 3 independent experiments. Med, medium; WT, wild type; NCF1−/−, p47phox−/− (mice deficient in ROS production); OE, overexpression construct; NT, nontargeting; KN, kinase negative.
DISCUSSION
PRRs play a vital role in sensing the existing as well as the invading microbes to elicit protective immune responses (38). However, the cross-regulation among PRRs and the outcome of such cross talk during pathogenic infections is not dealt with in detail. In the present study, we identify a novel role for a developmental pathway such as the WNT signaling pathway in dictating such cross-regulations of PRR signaling in macrophages. Generally, activation of multiple PRRs takes place during infection in a cell due to the presence of complex cell wall components which serve as ligands for numerous PRRs (39). In the present context, we looked at the activation of dectin-1 receptor, a CLR family of PRR in macrophages. Though several reports implicate the necessary role for TLR2 in the execution of dectin-1-mediated gene expression (16), we report a TLR2-independent dectin-1 signaling axis. In order to address the signaling cascades involved in immune responses mediated by the dectin-1 pathway, we have utilized fungi, such as C. albicans, A. flavus, and A. fumigatus or a commercially available ligand, curdlan. Curdlan is a well-known selective dectin-1 agonist which is purified macroparticulate β-(1, 3)glucan from Alcaligenes faecalis (26, 40).
Activation of TLR and dectin-1 significantly abrogated the TLR-induced expression of inflammatory genes like those encoding TNF-α, IL-12, and IL-1β. Later on, we also demonstrated that the inhibitory role of dectin-1 over the TLR pathway is brought about by two negative modulators of cytokine as well as TLR signaling, namely, PIAS-1 and SOCS-1, in macrophages. Notably, to show the correlation of this study with the in vivo infection scenario, we analyzed the bacterial load in the curdlan-treated mice. Triggering of dectin-1 prior to TLR by curdlan significantly increased the bacterial CFU not only in macrophages in vitro but also in lymph node and spleen in vivo. These results underscore one of the possible pathogen-mediated evasion strategies to dampen the host immune responses.
Activation of host signaling pathways upon engagement of surface- or intracellular-expressed PRRs is shown to dictate the immune cell fate. Contribution of signaling pathways like Sonic Hedgehog (30), WNT (31), and NOTCH (41, 42) has been deciphered during various bacterial infections as well as during derailed inflammation (43–45). Here, we found significant contribution of WNT5A during dectin-1-mediated immune responses. WNT5A executes its function through FZD, a seven-pass transmembrane protein which functions similar to G-protein-coupled receptors (32). Interaction of WNT5A-FZD results in the activation of membrane-bound phospholipase, which eventually leads to release of intracellular Ca2+ (36). This in turn activates CAMKII and subsequently drives NF-κB-mediated gene expression through TAK1 and Pyk2 (35). In line with these observations, dectin-1-induced expression of WNT5A led to the activation of the Ca2+/CAMKII pathway and in turn regulated the expression of PIAS-1 and SOCS-1. TAK1 and Pyk2 are known regulators of B-cell receptor (BCR)-mediated B cell functions (46) and macrophage function (47). Our data clearly suggest the involvement of crucial kinases, like TAK1 and Pyk2, in mediating dectin-1-induced WNT5A-Ca2+/CAMKII signaling. NF-κB, a unique transcription factor, is shown to regulate numerous immune cell functions under various pathological conditions (42, 48). Regulation of NF-κB nuclear translocation by TAK1 has been reported in T-cell-mediated functions (49). We found that TAK1–NF-κB signaling regulated dectin-1-induced PIAS-1 and SOCS-1 expression.
As described earlier in several published reports, targeting the adaptor molecules of the TLR signaling pathway is one of the mechanisms involved in regulating TLR-mediated inflammatory gene expression (50). It has been shown that MyD88, one of the adaptor molecules associated with TLR signaling pathways, is targeted to proteosomal degradation by E3 ligases like Smurf (24). On the other hand, tripartite motif-containing (TRIM) proteins, another component of innate immune cell signaling pathways, are driven for proteosomal degradation by E3 ligases. Interestingly, though microRNA-mediated downregulation of TLR adaptors has been previously reported (43), no studies indicate roles for other posttranslational modifications to regulate their levels and subsequently inhibit TLR responses. In the present context, we observed the role for PIAS-1 and SOCS-1 in downregulating IRAK-1, IRAK-4, and MyD88 in macrophages in response to dectin-1 signaling. Interestingly, we found SOCS-1 mediated the dectin-1-responsive IRAK-1, IRAK-4, and MyD88 proteasomal degradation, and PIAS-1 action was limited to IRAK-1 and MyD88 degradation. These results emphasize the substrate specificity of E3-modifying enzymes like PIAS-1 and SOCS-1 in regulating the strength of innate immune responses upon activation of PRRs, such as TLRs. The SOCS family of genes is known to bring about ubiquitination-mediated degradation of target proteins such as STAT, thus downregulating IFN-γ response (51, 52). The PIAS family of proteins sumoylate several known immune modulators, thus relegating them to a new cellular location and resulting in a loss of function (53–55).
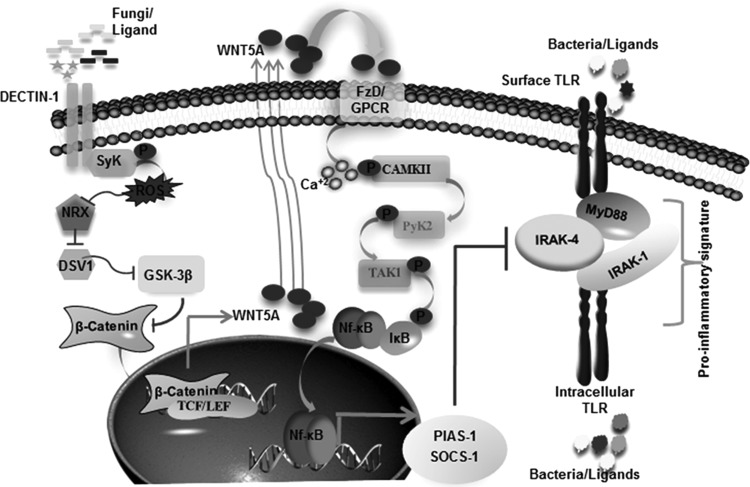
Our study suggests that activation of dectin-1 and TLR pathways in an infected cell by invading pathogens or during coinfections might bring down the inflammatory responses. All together, these results suggest that dectin-1-induced degradation of IRAK-1, IRAK-4, and MyD88 is mediated through PIAS-1 and SOCS-1 that require WNT5A-activated Ca2+/CAMKII–NF-κB activity (Fig. 10).
FIG 10.
Model depicting the molecular mechanism involved in counterregulation of TLR-induced inflammatory signature by dectin-1.
ACKNOWLEDGMENTS
We thank the Central Animal Facility, Indian Institute of Science (IISc), for providing mice for experimentation. We thank Gordon Brown (Institute of Medical Sciences, University of Aberdeen, Aberdeen, United Kingdom) for providing the mouse dectin-1 WT and mutant constructs. We thank Jun Nonomiya-Tsuji (North Carolina State University) for providing the TAK1 kinase-negative (KN) construct. We thank Akhihiko Yoshimura (School of Medicine, Keio University, Tokyo, Japan) for providing us the SOCS-1 OE construct. We thank Gary Owens (Cardiovascular Research Center, University of Virginia) for providing us the PIAS-1 OE construct. We thank Hiroaki Miki (RIMD, Osaka University, Japan) for NRX WT and Dsv1 constructs. We thank Roel Nusse (Department of Developmental Biology, Stanford University) for providing us WNT5A OE, β-catenin OE, and TCF4 DN constructs. Pannaga of the MCB imaging facility is acknowledged for her help. We acknowledge Pallavi Kakade for the timely help during the current course of investigation.
This study is supported by funds from the Department of Biotechnology (DBT), Department of Science and Technology (DST), Council for Scientific and Industrial Research (CSIR), and Indian Council of Medical Research (ICMR), Government of India, and the Indo-French Center for Promotion of Advanced Research (CEFIPRA). Infrastructure support from ICMR (Center for Advanced Study in Molecular Medicine), DST (FIST), and UGC (special assistance) (K.N.B.) and fellowships from CSIR (J.T. and, V.S.) and IISc (S.H., K.M., and P.P.) are acknowledged.
Footnotes
Published ahead of print 22 September 2014
REFERENCES
- 1.Broz P, Monack DM. 2013. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 13:551–565. 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 2.Carty M, Reinert L, Paludan SR, Bowie AG. 2014. Innate antiviral signalling in the central nervous system. Trends Immunol. 35:79–87. 10.1016/j.it.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Rauta PR, Samanta M, Dash HR, Nayak B, Das S. 2014. Toll-like receptors (TLRs) in aquatic animals: signaling pathways, expressions and immune responses. Immunol. Lett. 158:14–24. 10.1016/j.imlet.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Zhou JM. 2013. Receptor-like kinases in plant innate immunity. J. Integr. Plant Biol. 55:1271–1286. 10.1111/jipb.12123. [DOI] [PubMed] [Google Scholar]
- 5.Brown GD. 2006. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6:33–43. 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho A, Giovannini G, De Luca A, D'Angelo C, Casagrande A, Iannitti RG, Ricci G, Cunha C, Romani L. 2012. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell. Mol. Immunol. 9:276–286. 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koller B, Muller-Wiefel AS, Rupec R, Korting HC, Ruzicka T. 2011. Chitin modulates innate immune responses of keratinocytes. PLoS One 6:e16594. 10.1371/journal.pone.0016594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladiator A, LeibundGut-Landmann S. 2013. Innate lymphoid cells: new players in IL-17-mediated antifungal immunity. PLoS Pathog. 9:e1003763. 10.1371/journal.ppat.1003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. 2014. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 40:117–127. 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, Walker L, Jansen T, Jacobs L, Tsoni V, Brown GD, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. 2007. Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 196:1565–1571. 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. 2011. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 208:369–381. 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberle ME, Dalpke AH. 2012. Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J. Immunol. 188:5644–5654. 10.4049/jimmunol.1103068. [DOI] [PubMed] [Google Scholar]
- 13.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. 2003. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 197:1119–1124. 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahyo T, Nishida T, Yasuda H. 2001. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol. Cell 8:713–718. 10.1016/S1097-2765(01)00349-5. [DOI] [PubMed] [Google Scholar]
- 15.Coccia EM, Stellacci E, Orsatti R, Benedetti E, Giacomini E, Marziali G, Valdez BC, Battistini A. 2002. Protein inhibitor of activated signal transducer and activator of transcription (STAT)-1 (PIAS-1) regulates the IFN-gamma response in macrophage cell lines. Cell. Signal. 14:537–545. 10.1016/S0898-6568(01)00272-8. [DOI] [PubMed] [Google Scholar]
- 16.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107–1117. 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. 2002. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17:583–591. 10.1016/S1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. 2002. SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677–687. 10.1016/S1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191–202. 10.1016/S0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 20.Yadav M, Schorey JS. 2006. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108:3168–3175. 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willcocks S, Offord V, Seyfert HM, Coffey TJ, Werling D. 2013. Species-specific PAMP recognition by TLR2 and evidence for species-restricted interaction with Dectin-1. J. Leukoc. Biol. 94:449–458. 10.1189/jlb.0812390. [DOI] [PubMed] [Google Scholar]
- 22.Murakami Y, Mizoguchi F, Saito T, Miyasaka N, Kohsaka H. 2012. p16(INK4a) exerts an anti-inflammatory effect through accelerated IRAK1 degradation in macrophages. J. Immunol. 189:5066–5072. 10.4049/jimmunol.1103156. [DOI] [PubMed] [Google Scholar]
- 23.Smith H, Peggie M, Campbell DG, Vandermoere F, Carrick E, Cohen P. 2009. Identification of the phosphorylation sites on the E3 ubiquitin ligase Pellino that are critical for activation by IRAK1 and IRAK4. Proc. Natl. Acad. Sci. U. S. A. 106:4584–4590. 10.1073/pnas.0900774106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YS, Park JS, Kim JH, Jung SM, Lee JY, Kim SJ, Park SH. 2011. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-beta1-induced degradation of MyD88 in TLR4 signalling. Nat. Commun. 2:460. 10.1038/ncomms1469. [DOI] [PubMed] [Google Scholar]
- 25.Oshansky CM, Krunkosky TM, Barber J, Jones LP, Tripp RA. 2009. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a Toll-like receptor pathway. Viral Immunol. 22:147–161. 10.1089/vim.2008.0098. [DOI] [PubMed] [Google Scholar]
- 26.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. 2008. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 112:4971–4980. 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Marshall JS. 2009. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology 214:321–330. 10.1016/j.imbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Fang J, Wang Y, Lv X, Shen X, Ni X, Ding K. 2012. Structure of a beta-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-kappaB signaling. Glycoconj. J. 29:365–377. 10.1007/s10719-012-9416-z. [DOI] [PubMed] [Google Scholar]
- 29.Arellanes-Robledo J, Reyes-Gordillo K, Shah R, Dominguez-Rosales JA, Hernandez-Nazara ZH, Ramirez F, Rojkind M, Lakshman MR. 2013. Fibrogenic actions of acetaldehyde are beta-catenin dependent but Wingless independent: a critical role of nucleoredoxin and reactive oxygen species in human hepatic stellate cells. Free Radic. Biol. Med. 65:1487–1496. 10.1016/j.freeradbiomed.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Holla S, Kurowska-Stolarska M, Bayry J, Balaji KN. 2014. Selective inhibition of IFNG-induced autophagy by Mir155- and Mir31-responsive WNT5A and SHH signaling. Autophagy 10:311–330. 10.4161/auto.27225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bansal K, Trinath J, Chakravortty D, Patil SA, Balaji KN. 2011. Pathogen-specific TLR2 protein activation programs macrophages to induce Wnt-beta-catenin signaling. J. Biol. Chem. 286:37032–37044. 10.1074/jbc.M111.260414. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Nusse R, Varmus H. 2012. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 31:2670–2684. 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seitz K, Dursch V, Harnos J, Bryja V, Gentzel M, Schambony A. 2014. Beta-arrestin interacts with the beta/gamma subunits of trimeric G-proteins and dishevelled in the Wnt/Ca(2+) pathway in xenopus gastrulation. PLoS One 9:e87132. 10.1371/journal.pone.0087132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang L, Xu L, Song Y, Li J, Mao J, Zhao AZ, He W, Yang J, Dai C. 2013. Calmodulin-dependent protein kinase II/cAMP response element-binding protein/Wnt/beta-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J. Biol. Chem. 288:23368–23379. 10.1074/jbc.M113.460394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Shi Y, Shu J, Gao J, Wu P, Tang SJ. 2013. Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of proinflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways. J. Biol. Chem. 288:13610–13619. 10.1074/jbc.M112.381046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. 2003. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 23:131–139. 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie J, Allen KH, Marguet A, Berghorn KA, Bliss SP, Navratil AM, Guan JL, Roberson MS. 2008. Analysis of the calcium-dependent regulation of proline-rich tyrosine kinase 2 by gonadotropin-releasing hormone. Mol. Endocrinol. 22:2322–2335. 10.1210/me.2008-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villena J, Kitazawa H. 2014. Modulation of intestinal TLR4-inflammatory signaling pathways by probiotic microorganisms: lessons learned from TL2937. Front. Immunol. 4:512. 10.3389/fimmu.2013.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22:240–273. 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin KR. 2012. Editorial: beta-glucans: going through GM-CSF to get to dectin. J. Leukoc. Biol. 91:521–524. 10.1189/jlb.1011508. [DOI] [PubMed] [Google Scholar]
- 41.Narayana Y, Balaji KN. 2008. NOTCH1 up-regulation and signaling involved in Mycobacterium bovis BCG-induced SOCS3 expression in macrophages. J. Biol. Chem. 283:12501–12511. 10.1074/jbc.M709960200. [DOI] [PubMed] [Google Scholar]
- 42.Kapoor N, Narayana Y, Patil SA, Balaji KN. 2010. Nitric oxide is involved in Mycobacterium bovis bacillus Calmette-Guerin-activated Jagged1 and Notch1 signaling. J. Immunol. 184:3117–3126. 10.4049/jimmunol.0903174. [DOI] [PubMed] [Google Scholar]
- 43.Ghorpade DS, Holla S, Kaveri SV, Bayry J, Patil SA, Balaji KN. 2013. Sonic hedgehog-dependent induction of microRNA 31 and microRNA 150 regulates Mycobacterium bovis BCG-driven Toll-like receptor 2 signaling. Mol. Cell. Biol. 33:543–556. 10.1128/MCB.01108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghorpade DS, Sinha AY, Holla S, Singh V, Balaji KN. 2013. NOD2-nitric oxide-responsive microRNA-146a activates Sonic hedgehog signaling to orchestrate inflammatory responses in murine model of inflammatory bowel disease. J. Biol. Chem. 288:33037–33048. 10.1074/jbc.M113.492496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaale K, Brandenburg J, Kispert A, Leitges M, Ehlers S, Reiling N. 2013. Wnt6 is expressed in granulomatous lesions of Mycobacterium tuberculosis-infected mice and is involved in macrophage differentiation and proliferation. J. Immunol. 191:5182–5195. 10.4049/jimmunol.1201819. [DOI] [PubMed] [Google Scholar]
- 46.Tse KW, Dang-Lawson M, Lee RL, Vong D, Bulic A, Buckbinder L, Gold MR. 2009. B cell receptor-induced phosphorylation of Pyk2 and focal adhesion kinase involves integrins and the Rap GTPases and is required for B cell spreading. J. Biol. Chem. 284:22865–22877. 10.1074/jbc.M109.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Racioppi L, Noeldner PK, Lin F, Arvai S, Means AR. 2012. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J. Biol. Chem. 287:11579–11591. 10.1074/jbc.M111.336032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bansal K, Elluru SR, Narayana Y, Chaturvedi R, Patil SA, Kaveri SV, Bayry J, Balaji KN. 2010. PE_PGRS antigens of Mycobacterium tuberculosis induce maturation and activation of human dendritic cells. J. Immunol. 184:3495–3504. 10.4049/jimmunol.0903299. [DOI] [PubMed] [Google Scholar]
- 49.Hirata Y, Sugie A, Matsuda A, Matsuda S, Koyasu S. 2013. TAK1-JNK axis mediates survival signal through Mcl1 stabilization in activated T cells. J. Immunol. 190:4621–4626. 10.4049/jimmunol.1202809. [DOI] [PubMed] [Google Scholar]
- 50.Ghorpade DS, Leyland R, Kurowska-Stolarska M, Patil SA, Balaji KN. 2012. MicroRNA-155 is required for Mycobacterium bovis BCG-mediated apoptosis of macrophages. Mol. Cell. Biol. 32:2239–2253. 10.1128/MCB.06597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. 2000. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J. Biol. Chem. 275:12848–12856. 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 52.Madonna S, Scarponi C, De Pita O, Albanesi C. 2008. Suppressor of cytokine signaling 1 inhibits IFN-gamma inflammatory signaling in human keratinocytes by sustaining ERK1/2 activation. FASEB J. 22:3287–3297. 10.1096/fj.08-106831. [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Ma Y, Qian L, Wang J. 2013. Sumoylation regulates nuclear localization and function of zinc finger transcription factor ZIC3. Biochim. Biophys. Acta 1833:2725–2733. 10.1016/j.bbamcr.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Li R, Pan Y, Shi DD, Zhang Y, Zhang J. 2013. PIAS1 negatively modulates virus triggered type I IFN signaling by blocking the DNA binding activity of IRF3. Antiviral Res. 100:546–554. 10.1016/j.antiviral.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Mascle XH, Lussier-Price M, Cappadocia L, Estephan P, Raiola L, Omichinski JG, Aubry M. 2013. Identification of a non-covalent ternary complex formed by PIAS1, SUMO1, and UBC9 proteins involved in transcriptional regulation. J. Biol. Chem. 288:36312–36327. 10.1074/jbc.M113.486845. [DOI] [PMC free article] [PubMed] [Google Scholar]