Abstract
Neisseria meningitidis causes disease only in humans. An important mechanism underlying this host specificity is the ability of the organism to resist complement by recruiting the complement downregulator factor H (FH) to the bacterial surface. In previous studies, binding of FH to one of the major meningococcal FH ligands, factor H binding protein (FHbp), was reported to be specific for human FH. Here we report that sera from 23 of 73 rhesus macaques (32%) tested had high FH binding to FHbp. Similar to human FH, binding of macaque FH to the meningococcal cell surface inhibited the complement alternative pathway by decreasing deposition of C3b. FH contains 20 domains (or short consensus repeats), with domains 6 and 7 being responsible for binding of human FH to FHbp. DNA sequence analyses of FH domains 6 and 7 from macaques with high or low FH binding showed a polymorphism at residue 352 in domain 6, with Tyr being associated with high binding and His with low binding. A recombinant macaque FH 6,7/Fc fragment with Tyr352 showed higher binding to FHbp than the corresponding fragment with His352. In previous studies in human FH transgenic mice, binding of FH to FHbp vaccines decreased protective antibody responses, and mutant FHbp vaccines with decreased FH binding elicited serum antibodies with greater protective activity. Thus, macaques with high FH binding to FHbp represent an attractive nonhuman primate model to investigate further the effects of FH binding on the immunogenicity of FHbp vaccines.
INTRODUCTION
The four-component meningococcal serogroup B vaccine referred to as 4CMenB (Bexsero; Novartis Vaccines) contains factor H binding protein (FHbp) as one of its principal antigens (1). This vaccine is licensed in the European Union, Australia, and Canada. Another serogroup B vaccine that contains FHbp is in late-stage clinical development by Pfizer Vaccines (2). FHbp was referred to as GNA 1870 (3) or LP2086 (4) when our laboratories discovered that an important function of this protein was to bind complement factor H (FH) to the bacterial surface and downregulate complement activation (5). Further, binding of FH to FHbp was specific for human FH (6). Thus, the FHbp vaccine trials in humans were under way before it was recognized that the antigen bound to a host protein. Furthermore, the preclinical studies had been performed in animal models (primarily mice and rabbits) in which endogenous (or native) FH did not bind to the vaccine antigen.
In previous studies, we used a human FH transgenic mouse model to investigate the effects of human FH binding on the immunogenicity of FHbp vaccines (7–10). Collectively the results indicated that human FH decreased protective antibody responses to FHbp vaccines that bound human FH and that mutant FHbp antigens containing single amino acid substitutions that resulted in low FH binding elicited serum antibodies with greater complement-mediated bactericidal activity (reviewed in references 11 and 12).
The reported lack of binding of FH from species other than humans, including several species of nonhuman primates (6), has hampered efforts to investigate the effects of FH binding on FHbp immunogenicity in species more closely related to humans than the transgenic mouse model. With 93% genome sequence identity with humans, close similarity to the human immune system, and susceptibility to infectious diseases (13), rhesus macaques are an ideal nonhuman primate immunogenicity model (14). In the present study, we report that some rhesus macaques express FH with a polymorphism that results in binding of FH to meningococcal FHbp with an affinity similar to that of human FH. The results suggest that rhesus macaques whose FH binds well to FHbp may be a promising nonhuman primate model to investigate meningococcal vaccines that contain FHbp.
MATERIALS AND METHODS
Serum and blood samples.
The rhesus macaques were 2 to 3 months of age at the time of enrollment in three immunization protocols, conducted over a period of 4 years (total of 73 animals). The protocols were approved by the Institutional Animal Care and Use Committee at the California National Primate Research Center (Davis, CA). Details of one of the studies have been published (15). The macaques lived in outdoor social housing with their dams and extended families. The colony's founders and the genetic relationships of the descendant population have been described (16, 17).
Binding of FH to FHbp determined by ELISA.
The enzyme-linked immunosorbent assay (ELISA) was performed as described previously (7). In brief, wells of a microtiter plate were coated with FHbp 1 (2 μg/ml in phosphate-buffered saline [PBS]; 100 μl per well), which had been purified as described previously (18). The plate was incubated overnight at 4°C. After washing and blocking, serial 4-fold dilutions of macaque sera were added to the wells, starting at a dilution of 1:40. After overnight incubation at 4°C, bound FH was detected with goat anti-human FH antibody (1 μg/ml; Complement Technology, Inc.) that had been affinity purified over a human FH column. The bound goat IgG was detected with alkaline phosphatase (AP)-conjugated donkey anti-goat IgG (1:5,000; Sigma-Aldrich) (incubation for 1 h at room temperature).
Anti-FH capture ELISA to measure serum FH levels.
Microtiter wells were coated with a monoclonal antibody (MAb) to human complement factor H that cross-reacted with nonhuman primate FH (Quidel); 100 μl of a 3-μg/ml solution of the MAb was added to the wells, and the plate was incubated overnight at 4°C. The wells were washed and blocked, and serial 4-fold dilutions of macaque serum pools were added, starting at a dilution of 1:25. The subsequent detection steps were performed as described above for detection of FH binding with the FHbp capture ELISA.
Surface plasmon resonance.
Macaque serum FH concentrations were measured by surface plasmon resonance (SPR) using a Biacore X100 Plus instrument (GE Life Sciences), with purified human FH as a standard. Purified recombinant FHbp peptide ID 1 (1,900 response units) was covalently immobilized on a CM5 chip (GE Life Sciences) using an amine coupling kit (GE Life Sciences). Purified human FH (0 to 10 μg/ml) or dilutions (1:50 and 1:100) of a human serum pool or individual macaque sera were injected over the surface, and the data were analyzed with the concentration analysis module of the Biacore X100 evaluation software. Kinetic analysis was performed with the same chip. Macaque sera were diluted to a starting FH concentration of 4.7 μg/ml (31.6 nM), and five dilutions from 31.6 to 0.316 nM were tested. The data were analyzed with Biacore X100 evaluation software, using a 1:1 binding model.
Binding of macaque FH to live meningococci.
The flow cytometric assay for measuring binding of FH to the surface of live bacteria was described previously (19). In the present study, we used serogroup B strain H44/76 (B:15:P1.7,16), which was grown to mid-log phase in Franz medium (20) supplemented with 4 mM lactate. All rhesus macaque sera were heated for 30 min at 56°C to inactivate heat-labile complement components; 107 CFU/ml of bacteria was added to macaque serum pools (10% [vol/vol]), and the mixture was incubated for 30 min at room temperature. After washing, bound FH was detected with polyclonal sheep antibody to human FH (1:1,000; Abcam) and donkey anti-sheep IgG antibody conjugated to Alexa Fluor 488 (1:1,000; Invitrogen). The labeled bacteria were fixed with 0.5% formaldehyde, and fluorescence (80,000 events) was read on a BD Fortessa flow cytometer. Data were analyzed with FlowJo software, version X (TreeStar).
In some experiments, we investigated whether the addition of soluble recombinant human FH domains 6 and 7 fused to human IgG1 Fc (FH 6,7/Fc) or FH domains 18, 19, and 20 (FH 18–20/Fc) inhibited binding of macaque FH to FHbp. The recombinant fragments were similar to those described previously (21) except that mouse IgG2a Fc had been replaced by human IgG1 Fc (22). For inhibition of FH binding, the recombinant fragments (50 μg/ml) in Dulbecco's PBS (DPBS) containing 1% bovine serum albumin (BSA) together with macaque serum (1:10 dilution) were incubated with bacteria for 30 min at room temperature. After washing, bound macaque FH was detected with a murine monoclonal antibody to human FH (1:500 dilution; Quidel) that also reacts with macaque FH, followed by goat anti-mouse IgG F(ab′)2-specific antibody conjugated to Alexa Fluor 488 (1:1,000 dilution; Jackson ImmunoResearch).
FH regulation of complement C3b deposition on live meningococci.
We used flow cytometry to measure the effects of macaque FH on downregulation of rat alternative pathway-mediated C3b deposition on bacteria, as described previously (6). In brief, bacteria (strain H44/76) were grown to mid-log phase and resuspended to a density of ∼108 CFU/ml in DPBS-BSA containing 10 mM Mg2+-EGTA. The bacteria were mixed with different dilutions of heat-inactivated macaque or human serum as a source of FH and 30% infant rat serum as a complement source. Rat FH does not effectively inhibit the alternative pathway in strain H44/76 (23), which permitted us to evaluate the efficacy of primate or human FH in attenuating complement deposition in this strain. The reaction mixtures were incubated for 10 min at room temperature. After washing, rat C3b bound to the bacteria was detected with fluorescein isothiocyanate (FITC)-conjugated goat IgG antibody to rat complement C3 (1:100 dilution; MP Biomedicals), diluted in DPBS-BSA.
Genomic DNA preparation, PCR amplification, and sequencing.
Genomic DNA was prepared from 100 μl of anticoagulated whole macaque blood using the DNeasy blood and tissue kit (Qiagen). The following oligonucleotide primers were used to amplify the exons encoding FH short consensus repeat domains 6 or 7: GACCTAGAAACCCTAATGGAATGTGT (rheSCR6F), AGTGCCCTCCTCTCTTCGATCTTT (rheSCR6R), GTCACTTCAGTTCGTCTCCAGTCA (rheSCR7F), and ATGCTGGGATCTCAGAGCAGTGTA (rheSCR7R). The PCR cycling conditions were initial denaturation at 95°C for 3 min; 30 cycles of denaturation at 95°C for 15 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s; and final extension at 72°C for 5 min. PCR products were purified using a PCR purification kit (Qiagen) and quantified by measuring the absorbance at 260 nm (NanoDrop 1000; Thermo Scientific). DNA sequencing was performed by a commercial service (Davis Sequencing), using the forward and/or reverse PCR primers.
Construction and expression of rhesus macaque FH 6,7/Fc fusion proteins.
To investigate the role of His or Tyr polymorphisms at residue 352 in the binding of rhesus macaque FH to FHbp, we generated rhesus macaque FH domains 6 and 7 fused in frame to the N terminus of the Fc fragment of murine IgG2a. FH domains 6 and 7 were amplified from rhesus macaque liver cDNA (Biogen) using the following primers: 5′-GGCGCGCCTTCCTTGAGACCTTGTGATTATCCA-3′ (rhesus FH SCR6 AscI) and 5′-GCGGCCGCGATGCATCTGGGAGTAGGAGACC-3′ (FH SCR7 NotI). The PCR products were cloned into the AscI and NotI sites of pcDNA3 (Invitrogen) encoding mouse IgG2a Fc (24). The rhesus H352Y substitution was created using the QuikChange site-directed mutagenesis kit, according to the manufacturer's instructions (Stratagene), and the primer, 5′-ACTTTCCAGTACCTGTAGGAAAATATTTCTCCTATTACTGTGATG-3′. The sequences of the constructs were verified by DNA sequencing.
The amino acid sequences of rhesus macaque FH domains 6 and 7 inferred from rhesus macaque cDNA (Biogen) were identical to the amino acid sequences inferred from DNA from the rhesus macaques from the California primate center that showed low-level binding to FHbp, with the exception of one residue at position 387 (Arg instead of Gly) in domain 7. For reasons that are not clear, efforts to create macaque FH 6,7/Fc with Gly at position 387 were not successful. Therefore, we used macaque FH 6,7/Fc proteins that contained Arg at position 387 for our studies of the effects of the H352Y point mutation on the binding of rhesus FH to FHbp. Transfection of CHO cells and protein purification using protein A-Sepharose were performed as described previously (21).
RESULTS
Binding of rhesus macaque FH to FHbp.
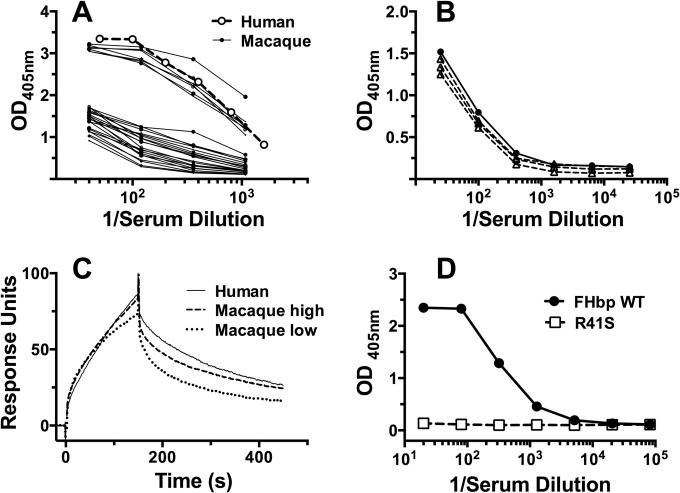
We used an ELISA to test the binding of FH to FHbp in sera from three groups of rhesus macaques. In one study, seven of 28 sera (25%) showed high levels of FH binding and 21 sera showed low levels of binding (Fig. 1A). Of the sera with high binding, six had binding similar to that of human control serum and one showed even higher binding. In the second and third studies, seven of 20 sera (35%) and nine of 25 sera (36%) from additional groups of animals showed high binding of FH to FHbp. Thus, a total of 23 of 73 macaque sera tested (32% [95% confidence interval, 21 to 43%]) had high FH binding. One possible explanation for low binding of FH to FHbp in the macaque sera would be low concentrations of FH. As determined with an anti-FH capture ELISA, however, three serum pools from seven animals each with low FH binding to FHbp and one serum pool from seven animals with high FH binding to FHbp had similar total FH concentrations (Fig. 1B). In these experiments, the sera were pooled because of the limited volumes of serum available from individual animals.
FIG 1.
Binding of rhesus macaque FH to FHbp. (A) Binding of rhesus macaque FH to FHbp, as determined by ELISA. Of 28 macaque sera tested, 7 had high binding of FH to FHbp, and 21 had low binding. For comparison, data are shown for a control human serum with 470 μg/ml of FH. (B) Concentrations of FH in macaque sera, as measured by an anti-FH MAb capture assay. One serum pool from animals with high FH binding to FHbp (●) and three pools from animals with low binding of FH to FHbp (△) were tested. (C) Binding of macaque serum FH to FHbp, as measured by surface plasmon resonance (SPR). Serum FH concentrations were measured by SPR using purified human FH as a standard. Kinetic analyses were performed using five serum dilutions, corresponding to 0.316 to 31.6 nM FH. The data for 31.6 nM FH are shown. (D) Binding of macaque serum FH to R41S mutant FHbp, as determined by ELISA. A serum pool from animals with high binding of FH to FHbp showed no detectable binding to the mutant FHbp adsorbed to wells of a microtiter plate. There was high binding of macaque FH to the wild-type (WT) FHbp. OD, optical density.
By surface plasmon resonance, the high binding macaque FH had similar kinetics of binding to immobilized FHbp, in comparison with FH in human serum. In two macaque sera with high binding, the equilibrium dissociation constants of FH for FHbp were 21 nM and 8 nM, similar to the value for human serum FH (17 nM). The binding kinetics for FH from a macaque with low binding showed slower association and faster dissociation than the high binding macaque or human FH. Representative data for one of the two macaque sera with high binding, one with low binding, and a representative human serum sample are shown in Fig. 1C.
In human FH transgenic mice, mutant FHbp vaccines with low FH binding elicited greater titers of serum bactericidal antibodies than did control FHbp vaccines that bound human FH (7–9, 25). One of the FHbp mutants tested had a single amino acid substitution (serine for arginine at position 41, i.e., R41S), which decreased the affinity of the binding of human FH to FHbp by ∼100-fold (7). Similar to human FH, the R41S substitution greatly decreased the binding of the macaque high binding FH to the mutant FHbp, as tested by ELISA (Fig. 1D).
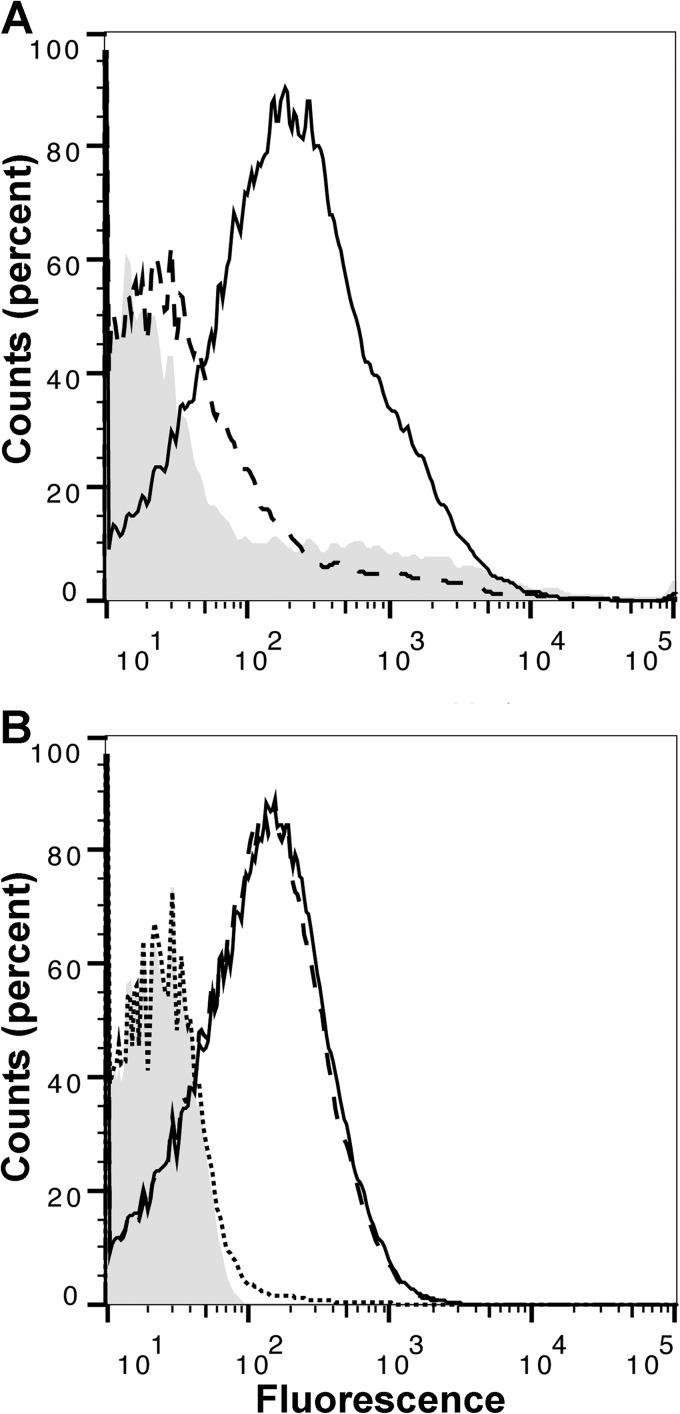
Binding of macaque FH to the surface of live meningococci.
A serum pool from animals with high binding of FH to FHbp, as measured by ELISA, also showed high FH binding to the surface of live meningococci by flow cytometry (Fig. 2A, solid line). In contrast, a serum pool from animals with low binding to FHbp by ELISA had much lower FH binding to the bacteria (Fig. 2A, dashed line). FH contains 20 domains, and human FH domains 6 and 7 bind to FHbp (26). In macaque serum with high FH binding, the addition of a recombinant fragment containing human FH domains 6 and 7 fused to human IgG1 Fc (FH 6,7/Fc) inhibited binding of FH to FHbp (Fig. 2B, dotted line). In contrast, there was no inhibition of macaque FH binding by a control human FH 18–20/Fc fragment (Fig. 2B, dashed line), which does not bind to FHbp (21). These data indicate that the regions of human and rhesus macaque FH that interact with FHbp are similar (i.e., domains 6 and 7) and macaque and human FH bind to overlapping regions of FHbp.
FIG 2.
Binding of rhesus macaque serum FH to live meningococci, as determined by flow cytometry. (A) Binding of FH in a serum pool from macaques with high binding of FH to FHbp (solid line), a serum pool from animals with low FH binding (dashed line), and a no-serum control (gray shaded histogram). All sera were tested at a 1:10 dilution. (B) Inhibition of binding of macaque FH to live meningococci by recombinant human FH domains 6 and 7 fused to human IgG1 Fc (FH 6,7/Fc). Solid line, high binding macaque serum pool without inhibitor; dotted line, high binding macaque serum pool with recombinant FH 6,7/Fc; dashed line, high binding macaque serum with the negative control recombinant FH18–20/Fc; shaded gray histogram, bacteria with no serum or inhibitor. The serum pool was tested at a dilution of 1:10, and the inhibitors were tested at 50 μg/ml.
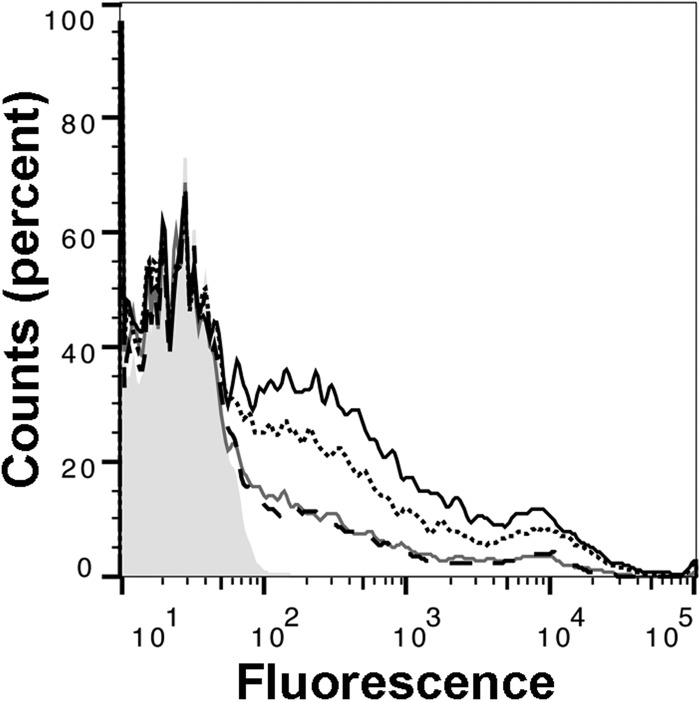
Binding of macaque FH to the bacterial surface downregulates the alternative complement pathway.
Rat FH does not regulate rat complement activation on several strains of Neisseria meningitidis, including H44/76 (21, 23). However, activation of rat complement, as measured by the deposition of rat C3 fragments on such meningococcal strains, can be downregulated by human FH (6). Therefore, we determined whether macaque FH that bound strain H44/76 downregulated rat C3 deposition. The experiments were performed in the presence of Mg2+ and EGTA, which chelates Ca2+ ions and blocks the complement classical pathway but permits alternative pathway activation. As observed previously (6), incubation of live N. meningitidis from serogroup B strain H44/76 in infant rat serum resulted in the deposition of high levels of rat C3 (Fig. 3, solid black line). The addition of a 1:720 dilution of a macaque serum pool from animals with high FH binding to FHbp decreased C3 deposition (Fig. 3, solid gray line) to an extent similar to that observed with the addition of the same dilution of human control serum (Fig. 3, dashed black line). In contrast, there was more rat C3 deposition (i.e., less inhibition) after the addition of a 1:720 dilution of a macaque serum pool from animals with low FH binding to FHbp (Fig. 3, dotted black line).
FIG 3.
Downregulation of rat complement C3b deposition on the surface of live meningococci by rhesus macaque serum FH. Live bacteria from meningococcal strain H44/76 were incubated with 30% infant rat serum as a source of complement in which rat FH did not bind to FHbp. Solid black line, no exogenous human or macaque FH; dotted black line, serum pool from macaques with low binding of FH to FHbp; solid gray line, serum pool from macaques with high binding of FH to FHbp; dashed black line, human serum control; shaded gray histogram, bacteria with no added serum. The data for the high binding macaque serum and the human serum are superimposed. All sera were tested at a 1:720 dilution.
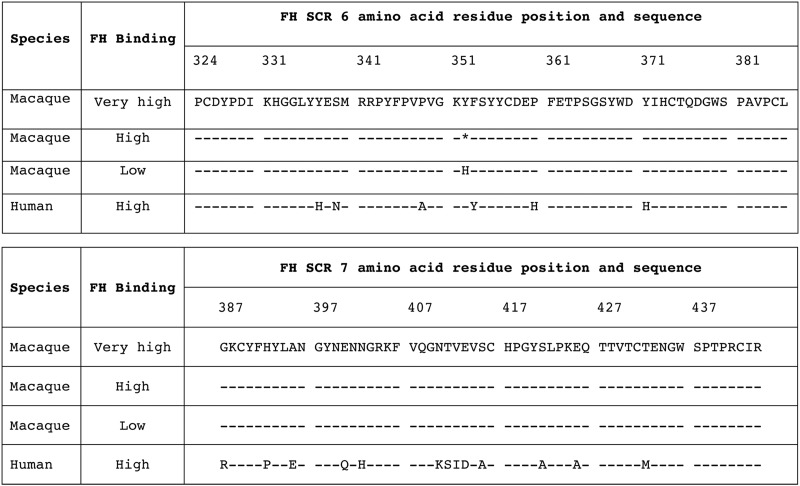
Tyrosine at position 352 in macaque FH domain 6 is associated with high binding to FHbp.
Since FHbp interacts with human FH via FH domains 6 and 7 (26), we reasoned that binding of macaque FH to FHbp also may be mediated by FH domains 6 and 7. Therefore, we sequenced the exons encoding these two domains from DNA extracted from whole blood from seven individual animals whose sera had high binding of FH to FHbp and seven animals whose sera showed low FH binding. In domain 7, the encoded amino acid sequences of high and low FH binders were identical (Fig. 4). In domain 6, each of the seven animals whose serum FH had low binding had two alleles encoding histidine (H) at residue 352. In contrast, each of the animals whose serum FH showed high binding to FHbp had at least one allele encoding tyrosine (Y); six animals had heterozygous Y/H and one animal (that showed the highest FH binding) (Fig. 1A) had homozygous Y/Y.
FIG 4.
Alignment of amino acid sequences of FH short consensus repeat (SCR) domains 6 and 7 inferred from DNA sequences of 7 animals with high or very high FH binding to FHbp and 7 animals with low binding of serum FH to FHbp. The macaque FH sequence from the one animal with very high binding is shown as the reference sequence. Asterisk at position 352, heterozygosity for Y/H. No sequence differences in FH domain 7 were seen among the macaques. The sequence data were based on samples from one animal with very high FH binding to FHbp, six animals with high binding, and seven animals with low binding.
Recombinant rhesus macaque FH fragment with Y352 has higher binding to FHbp than H352 fragment.
To test the hypothesis that Y352 in macaque FH contributes to high binding to FHbp, we generated recombinant macaque FH domains 6 and 7, containing either H or Y at position 352 (domain 6), fused to murine IgG2a Fc. The fragment with Y352 showed high binding to FHbp by ELISA, which was similar to that of a control human domain 6 and 7 fragment fused to mouse IgG2a Fc (Fig. 5). Macaque FH 6,7/Fc with H352 showed lower binding to FHbp. A negative-control human FH domain 18 to 20 fragment showed little binding.
FIG 5.
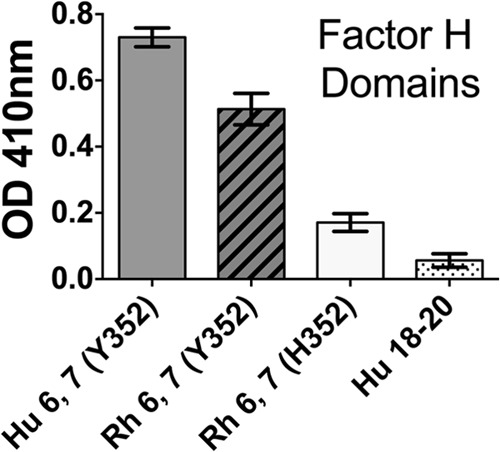
Binding of recombinant rhesus (Rh) macaque FH domains 6 and 7 to immobilized FHbp, as measured by ELISA. Fifty nanograms of each of the recombinant FH fragments fused to mouse IgG2a Fc was added to the wells, and binding was detected with anti-mouse IgG conjugated to alkaline phosphatase. As a positive control, we used human (Hu) FH 6,7/Fc containing Y352 in domain 6. As a negative control, we used human FH 18–20/Fc.
DISCUSSION
Binding of FH to FHbp was reported to be specific for human FH and, to a lesser extent, chimpanzee FH (6). To date, there are no known human FH polymorphisms that affect binding to FHbp. One common FH polymorphism in domain 7 (His to Tyr at position 402) affects susceptibility to age-related macular degeneration (27) but was reported not to affect the binding of human FH to FHbp (26).
In our previous study reporting that FH binding was specific for human FH, there also may have been minimal binding of macaque FH (6). Since the macaque serum sample tested had been obtained commercially, the nature of the sample (individual or pooled) and the geographic source(s) of the animals were not known. In the present study, we found that rhesus macaques, which were of Indian and/or Chinese origin, showed heterogeneity in the binding of their serum FH to FHbp. Sera from a total of 73 macaques were tested and 23 (32%) had high binding of FH to FHbp. Previously, Schneider and colleagues noted six amino acid differences between human and rhesus macaque FH in domain 6, which clustered in the portion of human FH that interacted with FHbp (26). In the present study, we sequenced macaque exons encoding FH domains 6 and 7 from seven macaques with high binding of FH to FHbp and seven with low binding. While there were no differences in domain 7, all of the low binders were homozygous H/H at residue 352 in domain 6 and all of the high binders were either Y/Y (n = 1; very high binding) or H/Y (n = 6; high binding). Since we did not sequence exons encoding other FH domains, we cannot exclude the possibility that additional polymorphisms outside domains 6 and 7 also can affect the interaction of FH with FHbp. However, human FH domains 6 and 7 cover a large surface on FHbp (26), and the overall sequence identity of macaque and human FH is 88%, with 84% identity for domains 6 and 7 (Fig. 4).
This higher binding of FH from macaque homozygous Y352/Y352 or heterozygous Y352/H352 FH to FHbp, compared to macaque homozygous H352/H352 FH, was confirmed by ELISA and surface plasmon resonance (SPR) using immobilized FHbp and by flow cytometry using live bacteria. Since the total concentrations of FH in the high and low binding sera were similar, the higher binding is a result of higher affinity for FHbp, which was confirmed by SPR experiments. The high binding macaque serum FH showed similar downregulation of deposition of rat C3b on bacteria as human FH, and the activity of the high binding macaque serum FH was greater than that of macaque serum FH with lower affinity binding to FHbp. However, the low FH binding macaque sera downregulated rat C3b deposition to some degree. This result was not unexpected, since in our previous study low concentrations of human FH (i.e., 3 μg/ml, the lowest concentration tested) were sufficient to downregulate the rat alternative pathway and to increase the survival of strain H44/76 (28).
Because FH binding to FHbp has been thought to be specific for human FH (6), experimental studies investigating the effects of FH binding on FHbp vaccine immunogenicity have been limited to human FH transgenic mouse models (7–11, 25, 29). The relevance of these results to the prediction of vaccine responses of humans is unknown. Given the frequency of macaques with high binding of FH to FHbp, it should be feasible to investigate the effects of FH binding on FHbp immunogenicity in macaques. The utility of mutant FHbp antigens, such as R41S (Fig. 1D), with low binding to human and macaque FH in improving protective antibody responses also can be investigated.
A theoretical risk of administering a foreign antigen (i.e., FHbp) that binds to a host molecule (FH) is the development of autoantibodies to the host molecule, as recently observed in sera from two human FH transgenic mice immunized with a FHbp vaccine that bound human FH (10). Conceivably, the transgenic mouse model used in that study could be more susceptible to the development of FH autoantibodies than immunized humans. Although the macaque immunogenicity model is resource intensive, the results of investigating the development of anti-FH autoantibodies after FHbp immunization in macaques are likely to be more relevant for predicting human responses than the mouse model.
A recent study found that rhesus macaques can be colonized naturally by Neisseria species and demonstrated the feasibility of using the macaque model to investigate colonization and transmission of the organism (30). The results of the present study indicate that the macaque model also has the potential for investigation of the effects of FH binding on the immunogenicity and safety of meningococcal FHbp vaccines.
ACKNOWLEDGMENTS
This work was supported by grants AI054544, AI111728, AI046464, AI082263, and AI099125 from the National Institute of Allergy and Infectious Diseases, NIH, and grant OD011107 from the California National Primate Research Center. The work at Children's Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant C06 RR016226 from the National Center for Research Resources, NIH.
We are grateful to Emily Braga and Mike Cheng (Children's Hospital Oakland Research Institute) for expert technical assistance and to George Santos, formerly of Novartis Vaccines (Cambridge MA), for providing paired serum and blood samples from 28 of the rhesus macaques.
Footnotes
Published ahead of print 3 September 2014
REFERENCES
- 1. Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30(Suppl 2):B87–B97. 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McNeil LK, Zagursky RJ, Lin SL, Murphy E, Zlotnick GW, Hoiseth SK, Jansen KU, Anderson AS. 2013. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol. Mol. Biol. Rev. 77:234–252. 10.1128/MMBR.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799. 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088–2100. 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madico G, Welsch JA, Lewis LA, Mcnaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510. 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Granoff DM, Welsch JA, Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77:764–769. 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3606–3614. 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. 2012. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog. 8:e1002688. 10.1371/journal.ppat.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rossi R, Granoff DM, Beernink PT. 2013. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine 31:5451–5457. 10.1016/j.vaccine.2013.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa I, Pajon R, Granoff DM. 2014. Human Factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. mBio 5:e01625–14. 10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granoff DM, Ram S, Beernink PT. 2013. Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses? Clin. Vaccine Immunol. 20:1099–1107. 10.1128/CVI.00260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Granoff DM. 2014. Improving safety and efficacy of meningococcal vaccines. Microbe 9:321–327. [Google Scholar]
- 13. Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O'Brien WE, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234. 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 14. Haigwood NL, Walker CM. 2011. Comparison of immunity to pathogens in humans, chimpanzees and macaques, p 91–166 In Altevogt BM, Pankevich DE, Shelton-Davenport MK. (ed), Chimpanzees in biomedical and behavioral research: assessing the necessity. National Academy of Sciences Press, Washington, DC. [PubMed] [Google Scholar]
- 15. Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, Granoff DM. 2011. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 29:4728–4734. 10.1016/j.vaccine.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanthaswamy S, Kou A, Satkoski J, Penedo MC, Ward T, Ng J, Gill L, Lerche NW, Erickson BJ, Smith DG. 2010. Genetic characterization of specific pathogen-free rhesus macaque (Macaca mulatta) populations at the California National Primate Research Center (CNPRC). Am. J. Primatol. 72:587–599. 10.1002/ajp.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanthaswamy S, Kou A, Smith DG. 2010. Population genetic statistics from rhesus macaques (Macaca mulatta) in three different housing configurations at the California National Primate Research Center. J. Am. Assoc. Lab. Anim. Sci. 49:598–609. [PMC free article] [PubMed] [Google Scholar]
- 18. Konar M, Granoff DM, Beernink PT. 2013. Importance of inhibition of binding of complement factor H for serum bactericidal antibody responses to meningococcal factor H-binding protein vaccines. J. Infect. Dis. 208:627–636. 10.1093/infdis/jit239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giuntini S, Reason DC, Granoff DM. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect. Immun. 79:3751–3759. 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frasch C, Van Alphen L, Holst J, Poolman J, Rosenqvist E. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p 81–107 In Pollard AJ, Maiden MC. (ed), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 21. Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. 2013. Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. mBio 4:e00339–13. 10.1128/mBio.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shaughnessy J, Vu DM, Punjabi R, Serra-Pladevall J, Deoliveira RB, Granoff DM, Ram S. 2014. A fusion protein comprising factor H domains 6 and 7 and human IgG1 Fc as an anti-bacterial immunotherapeutic. Clin. Vaccine Immunol. 10.1128/CVI.00444-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis LA, Vu DM, Granoff DM, Ram S. 2014. Inhibition of the alternative pathway of nonhuman infant complement by porin B2 contributes to virulence of Neisseria meningitidis in the infant rat model. Infect. Immun. 82:2574–2584. 10.1128/IAI.01517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Visintin A, Halmen KA, Latz E, Monks BG, Golenbock DT. 2005. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J. Immunol. 175:6465–6472. 10.4049/jimmunol.175.10.6465. [DOI] [PubMed] [Google Scholar]
- 25. van der Veen S, Johnson S, Jongerius I, Malik T, Genovese A, Santini L, Staunton D, Ufret-Vincenty RL, Pickering MC, Lea SM, Tang CM. 2014. Nonfunctional variant 3 factor H binding proteins as meningococcal vaccine candidates. Infect. Immun. 82:1157–1163. 10.1128/IAI.01183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. 2009. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458:890–893. 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langford-Smith A, Keenan TD, Clark SJ, Bishop PN, Day AJ. 2014. The role of complement in age-related macular degeneration: heparan sulphate, a ZIP code for complement factor H? J. Innate Immun. 6:407–416. 10.1159/000356513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vu DM, Shaughnessy J, Lewis LA, Ram S, Rice PA, Granoff DM. 2012. Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis. Infect. Immun. 80:643–650. 10.1128/IAI.05604-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson S, Tan L, van der Veen S, Caesar J, Goicoechea De Jorge E, Harding RJ, Bai X, Exley RM, Ward PN, Ruivo N, Trivedi K, Cumber E, Jones R, Newham L, Staunton D, Ufret-Vincenty R, Borrow R, Pickering MC, Lea SM, Tang CM. 2012. Design and evaluation of meningococcal vaccines through structure-based modification of host and pathogen molecules. PLoS Pathog. 8:e1002981. 10.1371/journal.ppat.1002981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weyand NJ, Wertheimer AM, Hobbs TR, Sisko JL, Taku NA, Gregston LD, Clary S, Higashi DL, Biais N, Brown LM, Planer SL, Legasse AW, Axthelm MK, Wong SW, So M. 2013. Neisseria infection of rhesus macaques as a model to study colonization, transmission, persistence, and horizontal gene transfer. Proc. Natl. Acad. Sci. U. S. A. 110:3059–3064. 10.1073/pnas.1217420110. [DOI] [PMC free article] [PubMed] [Google Scholar]