Abstract
JC polyomavirus (JCPyV) can cause progressive multifocal leukoencephalopathy (PML), a debilitating, often fatal brain disease in immunocompromised patients. JCPyV-seropositive multiple sclerosis (MS) patients treated with natalizumab have a 2- to 10-fold increased risk of developing PML. Therefore, JCPyV serology has been recommended for PML risk stratification. However, different antibody tests may not be equivalent. To study intra- and interlaboratory variability, sera from 398 healthy blood donors were compared in 4 independent enzyme-linked immunoassay (ELISA) measurements generating >1,592 data points. Three data sets (Basel1, Basel2, and Basel3) used the same basic protocol but different JCPyV virus-like particle (VLP) preparations and introduced normalization to a reference serum. The data sets were also compared with an independent method using biotinylated VLPs (Helsinki1). VLP preadsorption reducing ≥35% activity was used to identify seropositive sera. The results indicated that Basel1, Basel2, Basel3, and Helsinki1 were similar regarding overall data distribution (P = 0.79) and seroprevalence (58.0, 54.5, 54.8, and 53.5%, respectively; P = 0.95). However, intra-assay intralaboratory comparison yielded 3.7% to 12% discordant results, most of which were close to the cutoff (0.080 < optical density [OD] < 0.250) according to Bland-Altman analysis. Introduction of normalization improved overall performance and reduced discordance. The interlaboratory interassay comparison between Basel3 and Helsinki1 revealed only 15 discordant results, 14 (93%) of which were close to the cutoff. Preadsorption identified specificities of 99.44% and 97.78% and sensitivities of 99.54% and 95.87% for Basel3 and Helsinki1, respectively. Thus, normalization to a preferably WHO-approved reference serum, duplicate testing, and preadsorption for samples around the cutoff may be necessary for reliable JCPyV serology and PML risk stratification.
INTRODUCTION
Seroprevalence studies indicate that by early adulthood, JC polyomavirus (JCPyV) has infected approximately half of the general population (1, 2). Thereafter, JCPyV asymptomatically persists in renourinary tract and is intermittently shed into the urine (2–4). In immunocompromised patients, JCPyV can cause progressive multifocal leukoencephalopathy (PML), a demyelinating disease of the brain, with typically fatal outcome (5, 6). PML results from lytic JCPyV replication in subcortical oligodendrocytes that generate neuronal myelin sheaths. Progressive demyelination followed by neuronal dysfunction and cell death underlies the radiological and clinical features of PML (1, 5, 6). Despite some promising in vitro data (7), there is currently no specific antiviral therapy, and the outcome of PML depends largely on mounting JCPyV-specific immune functions that suppress JCPyV replication (1, 6, 8, 9). PML had been a frequent complication of HIV and AIDS patients in the era before combination antiretroviral therapy, affecting 1% to 8% of the patients at risk (10, 11). The availability of combination antiretroviral therapy (cART) has decreased the incidence of PML and significantly improved PML outcome (10, 12). Recently, an increasing number of PML cases were observed among multiple sclerosis (MS) patients treated with natalizumab. Natalizumab is a monoclonal antibody blocking α4β1 integrin and thereby homing of inflammatory cells to MS lesions (13–15). Practically all MS patients were found to be JCPyV seropositive at the time of natalizumab treatment, indicating that most, if not all, cases of PML were in fact caused by JCPyV reactivation (16). Thus, the risk of PML after 24 months of natalizumab therapy can be as high as 1:100 in JCPyV-seropositive patients but less than 1:10,000 in JCPyV-seronegative MS patients compared to less than 1:500,000 in the general population per year (1). Therefore, screening of MS patients for JCPyV antibodies may provide a relevant PML risk stratification tool and inform decisions regarding follow-up and treatment modalities (17, 18).
JCPyV antibodies can be detected by different techniques, including virus neutralization, hemagglutination inhibition of red blood cells, indirect immunofluorescence using JCPyV protein-expressing cells, and the enzyme-linked immunosorbent assay (ELISA) (1, 19). However, neutralization, while being functionally important, has some limitations, including the absence of a defined cutoff and the inability to detect specific, nonneutralizing antibodies. Hemagglutination inhibition assays generally show low sensitivity and do not allow reliable measurement of low antibody titers and detection of antibodies against JCPyV with typical PML-associated mutations in the sialic acid-binding region of the mutant VP1 gene (20, 21). While ELISA is the most widely used technique, the different assays vary in performance, serum dilutions, empirically derived cutoffs, and antigen preparations. Although the major viral capsid protein VP1 is frequently used, preparations of monomer, pentamer, or virus-like particles (VLPs) have been reported, which together with differences in serum dilutions and cutoffs are likely to affect reliability and commutability of results (1, 19). We previously investigated the seroprevalence of JCPyV antibodies in 400 healthy blood donors from Basel, Switzerland. Although our overall results corresponded well to reports from other studies (22), including those on MS patients (23), as reviewed in reference 1, the interpretation of results around the cutoff is usually difficult. Particularly the implications of a false-negative result for patient counseling regarding the PML risk under natalizumab therapy or conversely the withholding of therapy for a patient with a false-positive result prompted us to work out an improved protocol integrating the option of preadsorption reduction testing. The Basel assay was compared with an independent ELISA from Helsinki using biotinylated JC VP1 VLPs. The results indicate that more than 90% of sera can be reliably assayed and that approximately 10% of sera with IgG levels around the cutoff need confirmatory testing by preadsorption reduction assay.
MATERIALS AND METHODS
Study participants and samples.
Serum samples (n = 398) were obtained from citrate-anticoagulated blood collected from 398 healthy blood donors at the time of blood donation in Basel, Switzerland (IRB 267/06), and were described previously (2). The sera had been stored frozen at −20°C until thawed and aliquoted for retesting in both participating laboratories. At the time of blood donation, the donors tested negative for infections with human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and Treponema pallidum.
Isolation and purification of recombinant JCPyV VLPs.
The preparation of JCPyV VLP in Basel was described previously (2). Briefly, the JCPyV Mad-1 VP1 gene was inserted into the respective site of the baculovirus using the Bac-to-Bac baculovirus expression system (Invitrogen, Basel, Switzerland) and transfected into Spodoptera frugiperda Sf9 cells (American Type Culture Collection [ATCC], Manassas, VA) in suspension according to the manufacturer's protocol. Pellets of infected Sf9 cells were resuspended in phosphate-buffered saline (PBS) containing 0.1 mM CaCl2 and complete EDTA-free protease inhibitors (Roche, Basel, Switzerland), followed by sonication and centrifugation at 10,000 × g for 10 min to separate cytoplasmic VLPs in supernatants from the cell nuclei pellet. The supernatant was filtered with a 0.45-μm-pore paper filter and run on a 40% sucrose cushion at 70,000 × g for 3 h. The pellet was resuspended in PBS containing complete EDTA-free protease inhibitors and 0.25% deoxycholic acid, incubated in a water bath at 37°C for 1 h, and then chilled on ice for 5 min. Then an equal volume of 4 M NaCl with 0.1 mM CaCl2 was added, and the mixture was incubated on ice for 30 min and centrifuged afterwards at 3,220 × g for 20 min. Intranuclear VLPs were isolated by resuspension of the nuclear pellet in a mixture of 10 mM Tris-HCl (pH 8), 500 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, and 0.1% NP-40 and homogenization with glass mortar and pestle. After sonication and centrifugation at 17,000 × g for 1 h 30 min, VLPs were purified by CsCl ultracentrifugation at 150,000 × g for 24 h (Optima XPN-90 ultracentrifuge with an SW55 Ti rotor; Beckmann Coulter, Indianapolis, IN). Fractions were collected using the Beckman Coulter fraction recovery system, and those containing VLPs were pooled and ultracentrifuged at 300,000 × g for 2 h (SW55 Ti rotor) and stored in buffer A (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 1.0 mM CaCl2) at −80°C. The three-dimensional structure and purity of VLPs were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining as well as transmission electron microscopy.
The preparation of the JCPyV VLPs in Helsinki was also based on the JCPyV Mad-1 DNA sequence, but the major virus capsid protein VP1 coding region (nucleotides [nt] 1469 to 2533) was chemically synthesized and optimized for S. frugiperda usage (GenScript). VLPs were produced in insect cells using the Bac-to-Bac system (Invitrogen, Carlsbad, CA) as recommended by the manufacturer's instructions. Sf9 cells were infected and harvested at 4 to 5 days after infection by a 2-step lysis procedure (24), resuspending the cell pellet in buffer 1 (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton) at +4°C for 10 min, followed by 10 min of centrifugation at 10,000 × g. The supernatant was removed, and the pellet was resuspended in sonication buffer (10 mM Tris-HCl [pH 8.0], 500 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton) supplemented with a proteinase inhibitor cocktail (complete EDTA free; Roche, Mannheim, Germany). After three 20-s sonications and centrifugation at 15,000 × g for 40 min, the supernatant diluted in 20 mM Tris (pH 7.5) was layered on top of 1.52 g/cm3 cesium chloride. After ultracentrifugation (Beckman L-70) in a Beckman SW28 Ti rotor at 23,000 × g at 10°C for 4 h, fractions were studied by SDS-PAGE. Those rich in VP1 and VLPs by electron microscopy (EM) were pooled and dialyzed against PBS. For use as an antigen, the VLPs were biotinylated with the EZ-Link Sulfo-NHS-LC-biotinylation kit (Pierce, Rockford, IL) as described previously (24).
ELISA.
The Basel ELISA was essentially done as described previously (2). In brief, microtiter plates were coated with JCPyV VLPs (100 ng/well) overnight at 4°C followed by being washed five times with washing buffer (0.1% Tween 20). The wells were then incubated with blocking buffer (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 1 mM CaCl2, 4% bovine serum albumin [BSA], 0.1% Tween 20) for 2 h at 25°C and then washed five times with washing buffer. Serum samples were diluted 1:400 in blocking buffer, and 100 μl was added per well. After 1 h of incubation at 25°C, the wells were washed again (5 times) and incubated for 1 h at 25°C with 100 μl/well of 1:10,000-diluted Fc-specific goat anti-human IgG conjugated with peroxidase (Sigma-Aldrich, Buchs, Switzerland). The wells were washed five times and incubated at 25°C for 30 min with 100 μl of freshly prepared 0.4 mg/ml o-phenylenediamine (Sigma-Aldrich, Buchs, Switzerland). The color reaction was stopped by adding 50 μl of 1 M sulfuric acid per well, and the optical density at 492 nm (OD492) was measured using a Safire II plate reader (Tecan, Maennedorf, Switzerland). All results were recorded as blank well subtraction. The cutoff defining a positive serologic response was an OD492 of 0.110, as reported previously (2, 25, 26).
Normalization of IgG activities (normalized OD [nOD]) was obtained by parallel testing of an internal laboratory reference serum at a dilution yielding an OD of close to 1.0 on every ELISA plate. The nOD was obtained by dividing the OD of the patient sample by the OD of the reference serum.
The Helsinki JCPyV IgG ELISA was conducted essentially as described for the human polyomaviruses MCPyV and TSPyV (24, 27). Briefly, streptavidin plates (Thermo Fisher Scientific, Waltham, MA) with the biotinylated VLPs attached (120 ng/well) were coated with a sample diluent (Ani Labsystems). The serum samples diluted 1:200 were applied in duplicate, and the absorbances at 492 nm were recorded with blank-plate subtraction. The cutoff values were calculated according to samples (n = 170) with absorbances below a provisional threshold of 0.150. Utilizing the mean (0.048) and standard deviation (SD) (0.036), the cutoffs for JCPyV IgG presence and absence were set at mean + 4 SD (0.192) and mean + 3 SD (0.156), respectively.
In summary, the most significant differences between assays from Basel and Helsinki1, respectively, were the type and concentration of coating antigen (nonmodified VLPs at 100 ng/well versus biotinylated VLPs at 120 ng/well) and the serum dilution (400-fold versus 200-fold).
JCPyV VLP preadsorption assay.
ELISA plates were coated with 25 ng/well JCPyV VLPs overnight at 4°C. The serum samples were diluted 1:100 in blocking buffer (nonpreadsorbed) or in blocking buffer containing 25 ng JCPyV VLPs per 100 μl (preadsorbed). After incubating without agitation at 25°C for 1 h, 100 μl of diluted serum or serum-VLP mixture was transferred into previously blocked (for 2 h at 25°C) JCPyV VLP-coated wells, and the standard ELISA protocol was followed (described above). Mock and preadsorbed samples were tested in parallel. The results were reported as the percentage of IgG OD activity reduction after preadsorption with JCPyV VLPs according to [(ODnonpreadsorbed − ODpreadsorbed)/ODnonpreadsorbed] × 100. To investigate the possible contribution of BKPyV antibodies (cross-reactivity), the preadsorption assay was performed in parallel with BK VP1 VLPs using 14 JCVPyV-positive sera (0.08 < nOD < 0.25 and preadsorption inhibition of ≥35%).
Study design and statistical analysis.
All 398 healthy blood donor sera were investigated independently in two research centers: in Basel (according to a previously published ELISA protocol [Basel1]), with a new VLP preparation [Basel2] and an improved protocol with an OD normalization step [Basel3]) and in Helsinki (Helsinki1). The results of all four testing series were compared as intralaboratory intra-assay (Basel1 versus Basel2), intralaboratory interassay (Basel2 versus Basel3), and interlaboratory interassay (Basel2/Basel3 versus Helsinki1) comparisons. Discordant results were further investigated in preadsorption experiments, wherein the serum dilutions were tested in parallel by ELISA either directly or after prior preincubation with VLPs. Samples showing a decrease in IgG activity of ≥35% were regarded as JCPyV-seropositive sera. For the statistical analysis, GraphPad Prism 6.0 was used. Data with a nonnormal distribution as indicated by Kolmogorov-Smirnov Z test were analyzed by using the Kruskal-Wallis test or Mann-Whitney U test. Categorical data were analyzed using Pearson's χ2 test. Evaluation of agreement between different ELISA measurements was performed using Bland-Altman analysis. The specificity and sensitivity of the assays were calculated with MedCalc easy-to-use statistical software (www.medcalc.org). A two-sided P value of <0.05 was considered statistically significant.
RESULTS
Intralaboratory comparison of JCPyV VLP-based IgG serology.
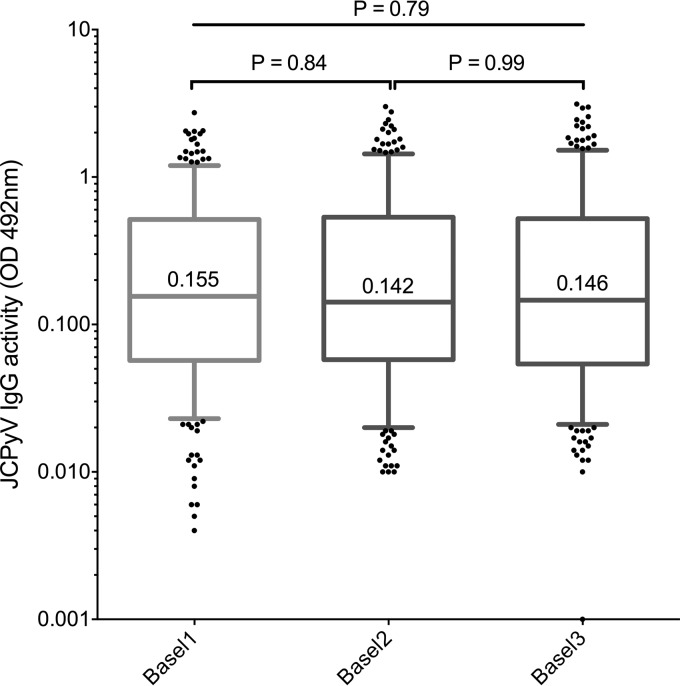
For an intralaboratory comparison, 398 stored sera from 400 healthy blood donors that had been tested and reported previously (Basel1) (2) were compared with a second single measurement using a newly prepared batch of JCPyV VLPs (Basel2). As shown in Fig. 1, there was no significant difference in the overall distribution of the IgG activity (P = 0.79) or in the median values. Applying the previously established cutoff of 0.110, 58.0% were higher and called seropositive in the data set Basel1, 54.5% in Basel2, and 54.8% in Basel3 (Table 1). Thus, there were slightly more JCPyV-seropositive sera by Basel1 than by Basel2 or Basel3, but the difference was statistically not significant (P = 0.95, χ2 test).
FIG 1.
Intralaboratory comparison of JCPyV VLP IgG activity (OD492) by ELISA in 398 health blood donors. Basel1 shows the IgG activity results as reported previously; Basel2 shows results of retesting using a newly prepared VLP batch; Basel3 shows results of retesting together with the internal normalization control using reference serum (Basel3). IgG activity corresponds to an optical density at 492 nm without or with normalization (OD492/nOD492) as described in Materials and Methods. Boxes span the interquartile range (IQR) with the 25th and the 75th percentiles. The number in the box indicates the median, the whiskers indicate the 5th and 95th percentile range, and dots indicate outliers below and above the range. P values were calculated by the Kruskal-Wallis test and Mann-Whitney U test.
TABLE 1.
Intralaboratory comparison of qualitative JCPyV IgG resultsa
| Assay | No. of sera with JCPyV IgG serostatusb: |
|
|---|---|---|
| Seropositive | Seronegative | |
| Basel1 | 231 | 167 |
| Basel2 | 221 | 177 |
| Basel3 | 218 | 180 |
Sera from 398 healthy blood donors were tested. The cutoff of 0.110 was used for JCPyV serostatus determination.
P = 0.95, calculated by χ2 test.
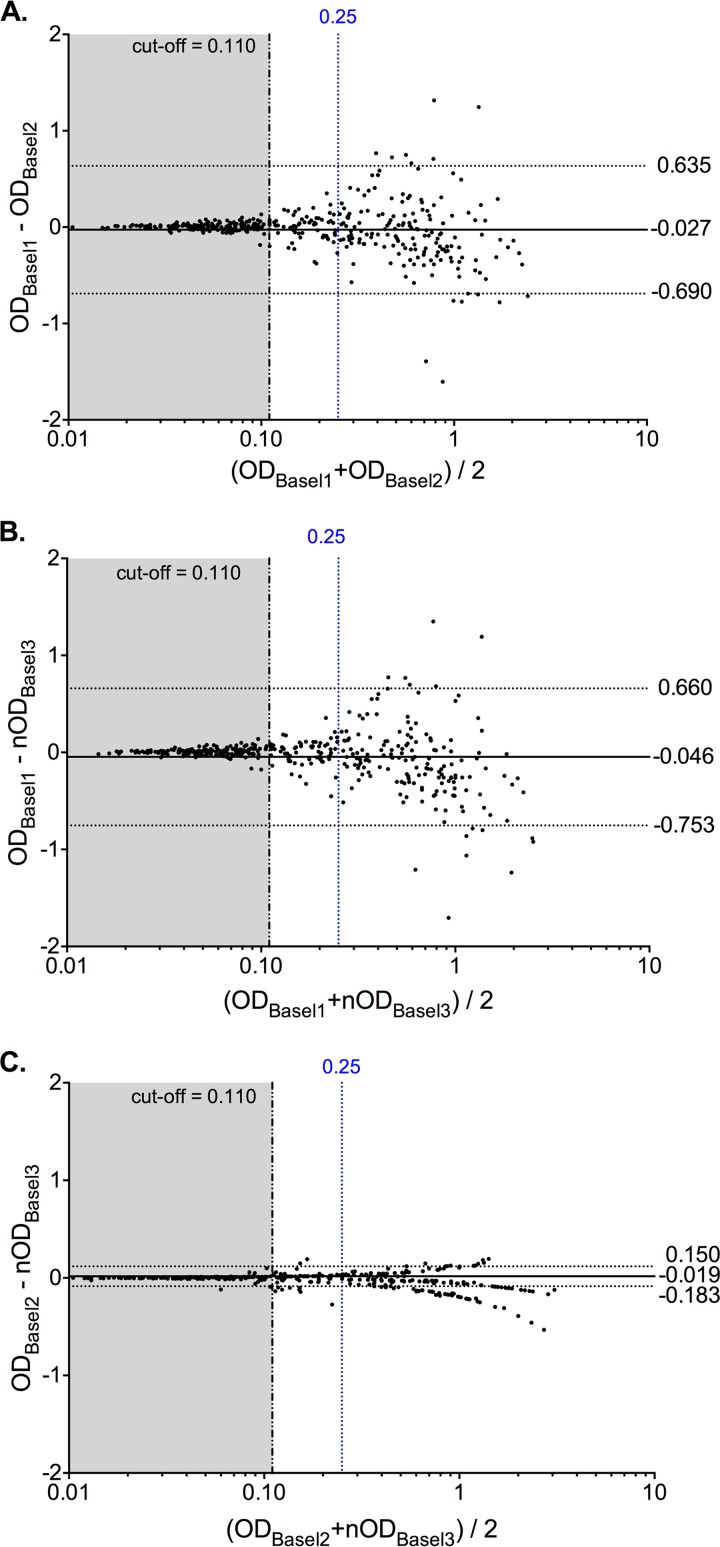
In the absence of a “gold standard,” the results were further investigated using the Bland-Altman analysis (Fig. 2). For Basel1 versus Basel2, overall slightly higher values were seen for Basel2, yielding a bias value of ΔOD of −0.027 (Fig. 2A). The 95% confidence interval (CI) was large, ranging from −0.690 to 0.635 and mostly due to differences in the higher-IgG-activity sera. Below an OD of 0.25 (arbitrarily defined by 2× the cutoff of 0.110 + the mean of the blank), and particularly below the cutoff OD of 0.110, the difference was minimal. The data suggest that variations in higher-titer sera with an OD of >0.25 contributed significantly to the intralaboratory variability. Restricting the Bland-Altman analysis to OD values of <0.25 revealed a mean bias of ΔOD of 0.033 and a more narrow 95% CI (−0.219 to 0.286 [data not shown]). Thus, variability appeared to be less for OD values below 0.25, but they had an impact on the qualitative interpretation of when to call a serum JCPyV seropositive.
FIG 2.
Intralaboratory comparison of JCPyV serology results using Bland-Altman analysis. (A) Basel1 versus Basel2. (B) Basel1 versus Basel3. (C) Basel2 versus Basel3. In each panel, the solid horizontal line represents the overall median bias, and dashed horizontal lines indicate the 95% confidence interval. The black vertical dashed line indicates the assay cutoff at an OD of 0.110, and the blue vertical dashed line indicates an OD of 0.25.
Therefore, the intralaboratory discordance of the qualitative outcomes of both assays was examined (Table 2). Among 48 discordant results (12% of 398 sera), a majority of 33 (69%) sera were close to the cutoff and therefore likely to result from assay variations. Conversely, 15 were found to be highly discordant (with one positive result equal to or higher than an OD of 0.25) and unlikely to reflect mere assay variations. Independent retesting of these sera indicated that they most likely resulted from technical errors, including mislabeling or pipetting (data not shown).
TABLE 2.
Discordant results in intralaboratory testing of 398 healthy blood donors
| Discordant resulta | No. of discordant samples |
||
|---|---|---|---|
| Basel1 vs Basel2 | Basel1 vs Basel3 | Basel2 vs Basel3 | |
| −/+ | 8 | 9 | 12 |
| −/++ | 9 | 10 | 1 |
| +/− | 25 | 27 | 11 |
| ++/− | 6 | 6 | 1 |
| Total | 48 | 52 | 25 |
−, seronegative at an OD of <0.110; +, seropositive at 0.11 < OD < 0.25; ++, seropositive at an OD of >0.25.
To assess the reliability of the assay further, all 398 sera were tested again in single measurements, but in the presence of a JCPyV-positive reference serum yielding an OD value of close to 1.0 for normalization (data set Basel3). The overall distribution of the normalized OD values was not significantly different from those of Basel1 and Basel2 (Fig. 1), but the qualitative results seemed closer to those of Basel2 (Table 1). The Bland-Altman analysis of Basel1 versus Basel3 was similar to that of Basel1 versus Basel2, with a bias of ΔOD of −0.046 and a large 95% CI from −0.753 to 0.660 (Fig. 2B). There were 52 (13% of 398 sera) discordant results between Basel1 and Basel3, of which 36 (69%) were close to the cutoff (Table 2). In contrast, the Bland-Altman analysis of Basel2 versus Basel3 showed very good agreement with a bias of ΔOD of −0.019 and a narrower 95% CI from −0.183 to 0.150 (Fig. 2C). Only 25 results (6% of 398 sera) were qualitatively discordant, 23 (92%) of them being close to the cutoff (Table 2). Restricting the Bland-Altman analysis to an OD of <0.25 confirmed that the intralaboratory variation around the cutoff was also reduced (not shown). The results indicated that the intralaboratory variability was significantly improved by standardized testing and normalization and that the results close to the cutoff (e.g., below an OD of 0.25) would benefit from independent confirmatory testing.
Interlaboratory comparison of serological IgG reactivity to JCPyV VLPs.
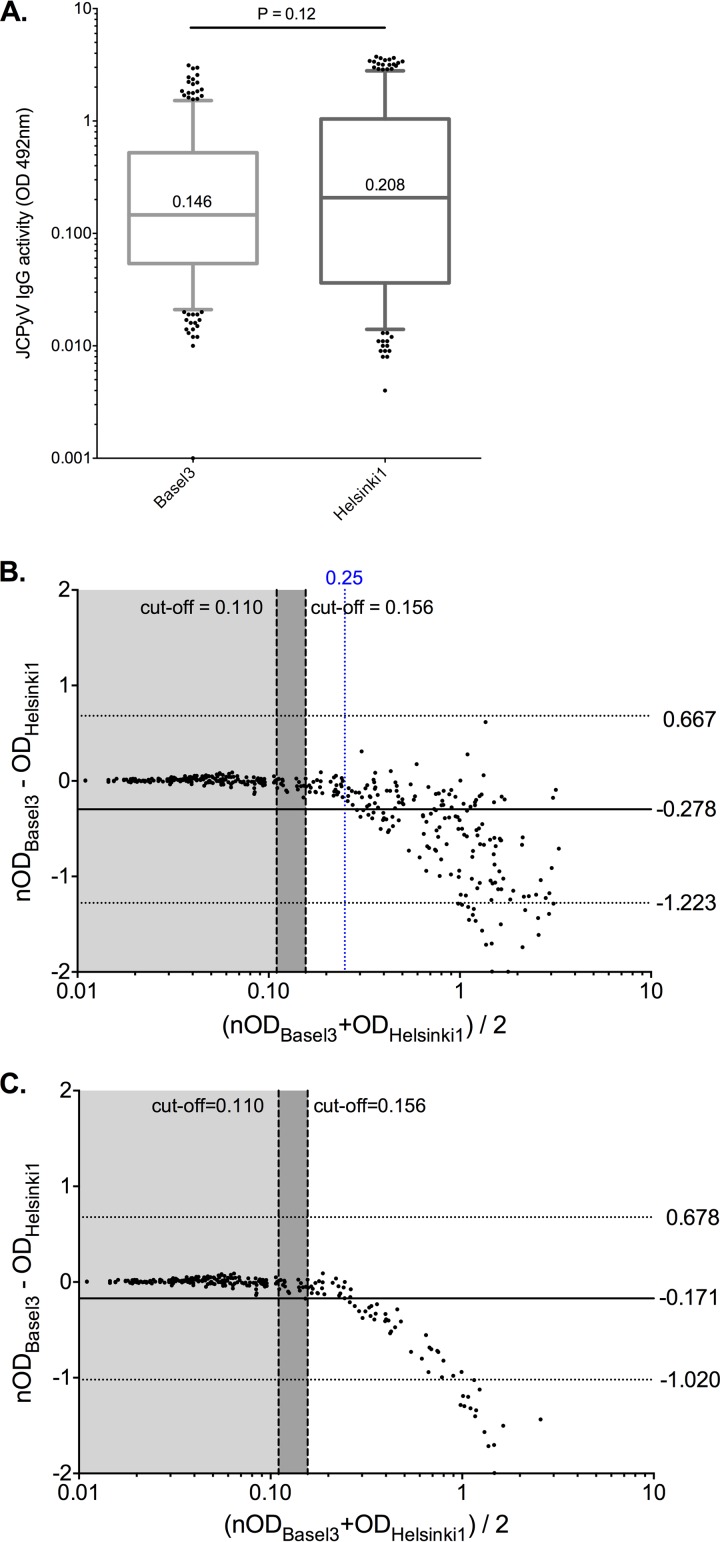
For an independent interlaboratory interassay comparison, the 398 sera were tested in another laboratory using independently prepared, codon-optimized and biotinylated JCPyV VLPs (see Materials and Methods). The overall distribution of OD results of data set Helsinki1 was not significantly different from that from Basel3 (median, 0.208; P = 0.12) (Fig. 3A). Using the cutoff OD of 0.156 determined for this assay, the JCPyV IgG seroprevalence in Helsinki1 was 53.5% and not significantly different from seroprevalence in Basel2 (54.5%) and Basel3 (54.8%) (P = 0.92, calculated by χ2 test).
FIG 3.
JCPyV serology results using the biotinylated VLPs (Helsinki1). (A) Overall serology results of 398 healthy blood donors (Helsinki1) as described in Materials and Methods and in the legend to Fig. 1. (B) Bland-Altman analysis comparing Basel3 versus Helsinki1. (C) Bland-Altman analysis comparing Basel3 versus Helsinki1 restricted to data points of an OD of <0.25 in the Basel3 assay. The solid horizontal line represents the overall median bias, and dashed horizontal lines indicate the 95% confidence interval. The left vertical black dashed line indicates the assay cutoff OD of 0.110 of the Basel assays, the right vertical black dashed line indicates the assay cutoff OD of 0.156 of the Helsinki1 assay, and the blue vertical dashed line indicates the OD of 0.25.
The Bland-Altman analysis of Basel3 versus Helsinki1 revealed, however, a large bias of ΔOD of −0.278 and a 95% CI from −1.223 to 0.667 (Fig. 3B). This indicated that the overall OD values were higher for Helsinki1, which appeared to result mostly from sera with higher JCPyV IgG titers leading to a corresponding downward shape of the data points (Fig. 3B). Restriction of the Bland-Altman analysis to data points of an OD of <0.25 supported the notion that the differences were smaller around the critical cutoffs of 0.110 and 0.156 of either assay (Fig. 3C). Only 15 (4%) of 398 samples were discordant, 14 of them (93%) being close to the respective cutoff (Table 3). This emphasized the role of assay standardization and the need for an independent confirmatory assay for results close to the respective cutoff.
TABLE 3.
Discordant results in interlaboratory testing of 398 healthy blood donors
| Discordant resulta | No. of discordant samples |
|
|---|---|---|
| Basel2 vs Helsinki1 | Basel3 vs Helsinki1 | |
| −/+ | 14 | 5 |
| −/++ | 4 | 0 |
| +/− | 19 | 9 |
| ++/− | 3 | 1 |
| Total | 40 | 15 |
−, seronegative at an OD of <0.110; +, seropositive at 0.11 < OD < 0.25; ++, seropositive at an OD of >0.25.
Preadsorption assay to determine serostatus of discordant samples.
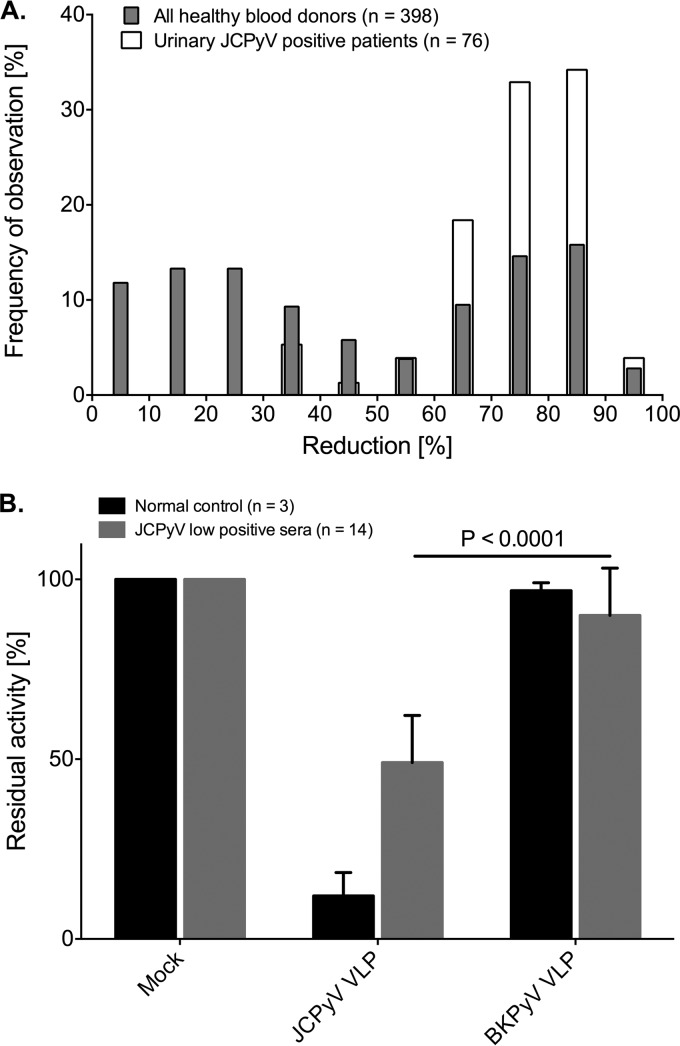
Discordant samples were tested using VLP preadsorption as a competition step to distinguish JCPyV-specific IgG-positive samples from those with unspecific antibodies (see Materials and Methods). The preincubation with soluble JCPyV VLPs is expected to bind JCPyV-specific antibodies and to reduce the remaining activity of the OD in the following ELISA. To estimate the specific reduction, the serum samples of the 398 healthy blood donors, which included 76 individuals with documented urinary JCPyV shedding detected by PCR as reported previously (2), were compared in the preadsorption assay. OD values obtained for nonpreadsorbed sera served as the reference to calculate reduction of IgG activity. Examination of the distribution of results for the 76 JCPyV-positive donors with proven urinary JCPyV shedding showed that preadsorption reduced the OD activity by ≥35% (Fig. 4A). Therefore, a reduction of ≥35% was chosen to define the JCPyV-seropositive status for sera between an OD of 0.08 (cutoff − blank OD, 0.110 to 0.03) and an OD of 0.25.
FIG 4.
Distribution of OD reductions in healthy blood donors and 76 patients shedding JCPyV in urine after preincubation with JCPyV VLPs and contribution of cross-reactive BKPyV antibodies to JCPyV-specific activity. (A) The histogram indicates the percentage of individuals with the corresponding degree of reduction of IgG activity after preadsorption with JCPyV VLPs as described in Materials and Methods. The results for 76 patients with JCPyV DNA-positive urine samples are shown (white bars), as are the results for 398 healthy blood donors (gray bars). Assuming that all urinary JCPyV DNA-positive patients are also JCPyV seropositive, a 35% reduction was determined for definition of serum samples with JCPyV-specific antibody titers. (B) A bar chart presents the remaining activity of JCPyV-specific antibodies for 14 low-JCPyV sera after preincubation with either JCPyV or BKPyV VLPs. The P value was calculated by t test.
To investigate how much cross-reactivity of BKPyV antibody contributed to the OD values close to the cutoff, a set of 14 JCPyV-positive sera with activity of 0.08 < nOD < 0.25 and preadsorption inhibition of ≥35% were also preadsorbed with BKPyV VLPs. The results revealed an average reduction by BK VLP preadsorption of only 7.87%, which is significantly different from the 50.96% for JCPyV VLPs (Fig. 4B) (P < 0.0001, t test).
Preadsorption testing of the samples discordant between Basel3 and Helsinki1 indicated false-positive results for 1 (0.05%) and 4 (1.00%) donors, respectively, as well as false-negative results for 1 (0.05%) and 9 (2.26%) cases (Tables 4 and 5). The numbers of true-positive and true-negative results, respectively, were 217 and 179 in Basel3 and 209 and 176 in Helsinki1. Accordingly, the specificities of the Basel3 and Helsinki1 assays were 99.44% and 97.78%, respectively, whereas the sensitivities corresponded to 99.54% and 95.87%, respectively.
TABLE 4.
Determination of JCPyV serological resultsa
| Basel3 result | Helsinki1 result | Preadsorption result | Final serostatus | False-positive result | False-negative result |
|---|---|---|---|---|---|
| Positive | Positive | Positive | Positive | ||
| Positive | Negative | Positive | Positive | Helsinki1 | |
| Positive | Negative | Negative | Negative | Basel3 | |
| Negative | Negative | Negative | Negative | ||
| Negative | Positive | Negative | Negative | Helsinki1 | |
| Negative | Positive | Positive | Positive | Basel3 |
Samples were defined as positive if the OD was above the cutoff (0.11 for Basel3 and 0.156 for Helsinki1) and if preadsorption reduced the OD by ≥35% for an OD of ≥0.08.
TABLE 5.
Summary of JCPyV serological results
| Result | No. of results for: |
|
|---|---|---|
| Basel3 | Helsinki1 | |
| True positive | 217 | 209 |
| False positive | 1 | 4 |
| True negative | 179 | 176 |
| False negative | 1 | 9 |
| Total | 398 | 398 |
DISCUSSION
Serological studies of JCPyV infection have recently gained increasing interest as predictors of the risk of developing PML in MS patients treated with natalizumab. In fact, a positive JCPyV serostatus, together with other factors, such as treatment history and duration of natalizumab exposure, has been proposed for identification of MS patients with a 100-fold-increased risk of PML. Given this impact of JCPyV serology, we critically reviewed the intra- and interlaboratory variation of two independently developed assays on a previously described population of Swiss healthy blood donors. The results demonstrate that the overall test performance of VLP-based ELISAs is good for describing the seroprevalence of this population from an epidemiological point of view. Approximately 54% to 58% of healthy adults are JCPyV seropositive, in line with other studies, including those done in MS patients (reviewed in detail recently in reference 1). Although no statistically significant differences in intra-assay and interassay variability were revealed on the population level, qualitative differences were seen in the identification of the individual JCPyV serostatus. The present study demonstrated that both the intra- and interassay variability caused approximately 10% discordant results. Two different sources of discordance became apparent. The first consisted typically of discordance at ODs higher than 0.25 in one of the comparators, was almost exclusively due to technical errors, such as labeling and pipetting, and could be resolved by retesting. In practice, this pitfall occurs typically when testing large series by single measurements and can be tackled by testing samples in duplicates or triplicates.
Not unexpectedly, the second and more frequent cause of discordance resulted from sera with a low IgG activity close to the cutoff of the assays. As indicated in this study, approximately 10% of sera were in this IgG activity range of ODs of less than 0.250 to 0.080. In this critical range, the overall assay variability was small according to the Bland-Altman analysis, but it nevertheless resulted in qualitatively different calls of the serostatus. Repeat testing is therefore unlikely to satisfactorily resolve this discordance, and other testing formats are needed. As shown here, introduction of normalization to a reference serum improved the overall assay performance by reducing the rate of discordance. Therefore, the use of reference sera for normalization seems to be a valuable option for assay standardization. Since discordances in this low OD range could still be caused by false-positive (e.g., cross-reactive) or false-negative (e.g., low IgG activities) results, the role of introducing a preadsorption step was investigated. By examining 398 sera with or without JCPyV VLP preadsorption, a 35% reduction was defined as a specific confirmatory value for JCPyV-seropositive sera. Preadsorption with BKPyV VLPs reduced the OD values by only 7.87%, indicating that cross-reactive antibodies to BKPyV contributed little, in agreement with earlier observations (2). Therefore, discordances between the Basel3 and Helsinki1 data sets could be resolved by preadsorption reduction, indicating the specificities of the Basel3 and Helsinki1 assays as 99.44% and 97.78%, respectively, and the sensitivities as 99.54% and 95.87%.
The investigation of JCPyV infection can employ detection of viral DNA or measurement of anti-JCPyV antibodies. However, previous studies revealed that in some cases, JCPyV DNA PCR is neither a sensitive indicator of JCV infection nor a specific predictor of PML, as demonstrated by some PML patients who had no detectable viral DNA in urine or blood samples (28, 29 [reviewed in detail recently in reference 1]). More recently, HLA class II DRB1*04:01 was reported to be associated with low JCPyV-specific T-cell responses and absence of detectable urinary viral shedding, albeit with normal JCPyV-specific antibodies (30). In another study, HLA-DRB1*15 was found to be associated with a negative JCPyV serostatus in MS patients, as measured by a commercial assay (31). The authors propose that serological assays might underestimate JCPyV infection status, with potential consequences for MS patients falsely identified as not exposed to JCPyV (32). We conclude that an improved serological assay with preadsorption for antibody activities around the cutoff is a critical first analysis to classify patients concerning JCPyV exposure. Further studies are needed to establish whether HLA-DRB typing is required to better stratify MS patients with critical HLA types.
Despite the obvious advantages of neutralization, ELISA has become the most versatile method for detection of anti-JCPyV antibodies (19, 20). It can utilize VP1 in the form of monomers, pentamers, or VLPs (1). However, ELISA with VLPs as the coating antigen has been shown to be more sensitive and specific than VP1 in the monomer or pentamer form (25, 33). For MS patients, there is a commercially available STRATIFY JCV DxSelect kit based on a second-generation ELISA for detection of JCPyV antibodies in humans (34). Clearly, screening, normalization with widely accepted reference sera, and preadsorption testing for specific reduction are important issues. In this context, it should be emphasized that a WHO-approved international reference serum would be most valuable, as has been shown for other infections and vaccination serologies.
In summary, our findings demonstrate that serological assays for JCPyV IgG need to take into account whether epidemiological questions or individual risk assessments are to be addressed. No significant statistical differences were seen in the overall characteristics of four independent test series of 398 sera from healthy blood donors. Qualitatively, approximately 90% of the results were concordant between the data sets. For 10% of results, confirmatory testing was needed.
According to the results described here, the following points deserve consideration in laboratory testing of sera with unknown JCPyV serostatus by VLP-based ELISA. (i) At least duplicate testing should be performed to avoid technical errors. (ii) Normalization using a reference serum may improve IgG activity measurement. (iii) There should be repeat testing in preadsorption assay of sera in the low OD range around the cutoff (e.g., from OD 0.08 to 0.25 for the assay described here). (iv) There should be quality assessment programs with appropriate training of laboratory personnel involved in ELISA testing of patient samples. (v) Finally, clinical interpretation of negative JCPyV serology data may require the knowledge of the patients' HLA types.
ACKNOWLEDGMENTS
The expert technical assistance of Jacqueline Samaridis, TCV Laboratory, Basel, with the preparation of the JCPyV VLPs and the ELISA is gratefully acknowledged.
The work at Helsinki was supported by the Academy of Finland (project 1122539), the Sigrid Jusélius Foundation, and the Helsinki University Central Hospital Research and Education, and Research and Development Funds. The Basel group was supported by an institutional grant from the University of Basel to H.H.H.
P.K. expressed and purified JC polyomavirus VP1 virus-like particles, carried out the ELISA, analyzed the data, and wrote the manuscript. M.S., instructed by T.C., expressed, purified, and biotinylated the Helsinki JC-VP1 VLPs, analyzed the data, and participated in the manuscript writing. F.W. expressed and purified JC polyomavirus VP1 virus-like particles and carried out the ELISA. L.H. accounted for the Helsinki serodiagnostics, designing and carrying out the ELISA and analyzing the data. E.A. conceived the collaborative work and participated in the Helsinki assay design and data analysis. K.H. conceived and coordinated the study in Helsinki and participated in data analysis and manuscript writing. H.H.H. designed the study, supervised the procedures, analyzed the data, interpreted the data, and wrote the manuscript.
Footnotes
Published ahead of print 24 September 2014
REFERENCES
- 1. Hirsch HH, Kardas P, Kranz D, Leboeuf C. 2013. The human JC polyomavirus (JCPyV): virological background and clinical implications. APMIS 121:685–727. 10.1111/apm.12128. [DOI] [PubMed] [Google Scholar]
- 2. Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199:837–846. 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 3. Polo C, Perez JL, Mielnichuck A, Fedele CG, Niubo J, Tenorio A. 2004. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin. Microbiol. Infect. 10:640–644. 10.1111/j.1469-0691.2004.00882.x. [DOI] [PubMed] [Google Scholar]
- 4. Chesters PM, Heritage J, McCance DJ. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676–684. 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 5. Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, Major EO. 2012. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 25:471–506. 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan CS, Broge TA, Jr, Seung E, Vrbanac V, Viscidi R, Gordon J, Tager AM, Koralnik IJ. 2013. Detection of JC virus-specific immune responses in a novel humanized mouse model. PLoS One 8:e64313. 10.1371/journal.pone.0064313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gosert R, Rinaldo CH, Wernli M, Major EO, Hirsch HH. 2011. CMX001 (1-O-hexadecyloxypropyl-cidofovir) inhibits polyomavirus JC replication in human brain progenitor-derived astrocytes. Antimicrob. Agents Chemother. 55:2129–2136. 10.1128/AAC.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khanna N, Wolbers M, Mueller NJ, Garzoni C, Du Pasquier RA, Fux CA, Vernazza P, Bernasconi E, Viscidi R, Battegay M, Hirsch HH. 2009. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J. Virol. 83:4404–4411. 10.1128/JVI.02657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alstadhaug K, Croughs T, Henriksen S, Leboeuf C, Sereti I, Hirsch H, Rinaldo CH. 2014. Treatment of progressive multifocal leukoencephalopathy with interleukin. JAMA Neurol. 71:1030–1035. 10.1001/jamaneurol.2014.825. [DOI] [PubMed] [Google Scholar]
- 10. Khanna N, Elzi L, Mueller NJ, Garzoni C, Cavassini M, Fux CA, Vernazza P, Bernasconi E, Battegay M, Hirsch HH, for the Swiss HIV Cohort Study 2009. Incidence and outcome of progressive multifocal leukoencephalopathy in 20 years of the Swiss HIV Cohort Study. Clin. Infect. Dis. 48:1459–1466. 10.1086/598335. [DOI] [PubMed] [Google Scholar]
- 11. Berger JR, Kaszovitz B, Post MJ, Dickinson G. 1987. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann. Intern. Med. 107:78–87. [DOI] [PubMed] [Google Scholar]
- 12. Clifford DB, Yiannoutsos C, Glicksman M, Simpson DM, Singer EJ, Piliero PJ, Marra CM, Francis GS, McArthur JC, Tyler KL, Tselis AC, Hyslop NE. 1999. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 52:623–625. 10.1212/WNL.52.3.623. [DOI] [PubMed] [Google Scholar]
- 13. Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353:375–381. 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 14. Kleinschmidt-DeMasters BK, Tyler KL. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353:369–374. 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 15. Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353:362–368. 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 16. Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. 2012. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 366:1870–1880. 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 17. Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, Hartung HP, Havrdova E, Hillert J, Hohlfeld R, Kremenchutzky M, Lyon-Caen O, Miller A, Pozzilli C, Ravnborg M, Saida T, Sindic C, Vass K, Clifford DB, Hauser S, Major EO, O'Connor PW, Weiner HL, Clanet M, Gold R, Hirsch HH, Radu EW, Sorensen PS, King J. 2011. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 10:745–758. 10.1016/S1474-4422(11)70149-1. [DOI] [PubMed] [Google Scholar]
- 18. Sorensen PS, Bertolotto A, Edan G, Giovannoni G, Gold R, Havrdova E, Kappos L, Kieseier BC, Montalban X, Olsson T. 2012. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult. Scler. 18:143–152. 10.1177/1352458511435105. [DOI] [PubMed] [Google Scholar]
- 19. Cinque P, Dumoulin A, Hirsch HH. 2010. Diagnosis of polyomavirus infection, replication and disease, p 401–424 In Jerome K. (ed), Laboratory diagnosis of viral infections, 4th ed. Informa Healthcare, New York, NY. [Google Scholar]
- 20. Hamilton RS, Gravell M, Major EO. 2000. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J. Clin. Microbiol. 38:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gorelik L, Reid C, Testa M, Brickelmaier M, Bossolasco S, Pazzi A, Bestetti A, Carmillo P, Wilson E, McAuliffe M, Tonkin C, Carulli JP, Lugovskoy A, Lazzarin A, Sunyaev S, Simon K, Cinque P. 2011. Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J. Infect. Dis. 204:103–114. 10.1093/infdis/jir198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viscidi RP, Khanna N, Tan CS, Li X, Jacobson L, Clifford DB, Nath A, Margolick JB, Shah KV, Hirsch HH, Koralnik IJ. 2011. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin. Infect. Dis. 53:711–715. 10.1093/cid/cir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorelik L, Lerner M, Bixler S, Crossman M, Schlain B, Simon K, Pace A, Cheung A, Chen LL, Berman M, Zein F, Wilson E, Yednock T, Sandrock A, Goelz SE, Subramanyam M. 2010. Anti-JC virus antibodies: implications for PML risk stratification. Ann. Neurol. 68:295–303. 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- 24. Chen T, Hedman L, Mattila PS, Jartti T, Ruuskanen O, Soderlund-Venermo M, Hedman K. 2011. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J. Clin. Virol. 50:125–129. 10.1016/j.jcv.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 25. Bodaghi S, Comoli P, Boesch R, Azzi A, Gosert R, Leuenberger D, Ginevri F, Hirsch HH. 2009. Antibody responses to recombinant polyomavirus BK large T and VP1 proteins in pediatric kidney transplant patients. J. Clin. Microbiol. 47:2577–2585. 10.1128/JCM.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ginevri F, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, Bodaghi S, Salotti V, Rinieri A, Botti G, Perfumo F, Locatelli F, Comoli P. 2007. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am. J. Transplant. 7:2727–2735. 10.1111/j.1600-6143.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 27. Kumar A, Kantele A, Jarvinen T, Chen T, Kavola H, Sadeghi M, Hedman K, Franssila R. 2012. Trichodysplasia spinulosa-associated polyomavirus (TSV) and Merkel cell polyomavirus: correlation between humoral and cellular immunity stronger with TSV. PLoS One 7:e45773. 10.1371/journal.pone.0045773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL. 1999. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology 52:253–260. 10.1212/WNL.52.2.253. [DOI] [PubMed] [Google Scholar]
- 29. Tornatore C, Berger JR, Houff SA, Curfman B, Meyers K, Winfield D, Major EO. 1992. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann. Neurol. 31:454–462. 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- 30. Jelcic I, Aly L, Binder TM, Jelcic I, Bofill-Mas S, Planas R, Demina V, Eiermann TH, Weber T, Girones R, Sospedra M, Martin R. 2013. T cell epitope mapping of JC polyomavirus-encoded proteome reveals reduced T cell responses in HLA-DRB1*04:01+ donors. J. Virol. 87:3393–3408. 10.1128/JVI.02803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sundqvist E, Buck D, Warnke C, Albrecht E, Gieger C, Khademi M, Lima Bomfim I, Fogdell-Hahn A, Link J, Alfredsson L, Sondergaard HB, Hillert J, Oturai AB, Hemme B, Kockum I, Olsson T. 2014. JC polyomavirus infection is strongly controlled by human leucocyte antigen class II variants. PLoS Pathog. 10:e1004084. 10.1371/journal.ppat.1004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berger JR, Houff SA, Gurwell J, Vega N, Miller CS, Danaher RJ. 2013. JC virus antibody status underestimates infection rates. Ann. Neurol. 74:84–90. 10.1002/ana.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang D, Fung CY, Ou WC, Chao PC, Li SY, Wang M, Huang YL, Tzeng TY, Tsai RT. 1997. Self-assembly of the JC virus major capsid protein, VP1, expressed in insect cells. J. Gen. Virol. 78:1435–1439. [DOI] [PubMed] [Google Scholar]
- 34. Lee P, Plavina T, Castro A, Berman M, Jaiswal D, Rivas S, Schlain B, Subramanyam M. 2013. A second-generation ELISA (STRATIFY JCV DxSelect) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J. Clin. Virol. 57:141–146. 10.1016/j.jcv.2013.02.002. [DOI] [PubMed] [Google Scholar]