Abstract
Influenza causes serious and sometimes fatal disease in individuals at risk due to advanced age or immunodeficiencies. Despite progress in the development of seasonal influenza vaccines, vaccine efficacy in elderly and immunocompromised individuals remains low. We recently developed a passive immunization strategy using an adeno-associated virus (AAV) vector to deliver a neutralizing anti-influenza antibody at the site of infection, the nasal airways. Here we show that young, old, and immunodeficient (severe combined immunodeficient [SCID]) mice that were treated intranasally with AAV9 vector expressing a modified version of the broadly neutralizing anti-influenza antibody FI6 were protected and exhibited no signs of disease following an intranasal challenge with the mouse-adapted H1N1 influenza strain A/Puerto Rico/8/1934(H1N1) (PR8) (Mt. Sinai strain). Nonvaccinated mice succumbed to the PR8 challenge due to severe weight loss. We propose that airway-directed AAV9 passive immunization against airborne infectious agents may be beneficial in elderly and immunocompromised patients, for whom there still exists an unmet need for effective vaccination against influenza.
INTRODUCTION
Influenza infections cause up to 49,000 deaths per year in the United States (1). The economic burden of annual influenza epidemics is estimated to be on the order of $87 billion per year, with more than one-half of these costs covering the hospital care required for almost 1 million patients, of whom 70% are elderly (>65 years of age) (2). Individuals with reduced capacity to mount an immune response upon infection have increased susceptibility to influenza infections and complications, which include fatal pneumonia and acute respiratory distress syndrome (ARDS) (3). Thymic aging precedes the aging of most other organs and contributes to the progressive age-related deterioration of the immune system known as immunosenescence (4). In addition, immunocompromised individuals, such as patients with HIV/AIDS, organ transplant recipients, and patients suffering from autoimmune diseases, account for almost 1% of the population and are considered to be at high risk for influenza infections. While influenza vaccine coverage in the United States has increased in the past decade, studies have demonstrated decreased efficacy of seasonal influenza vaccines in these high-risk patient populations (5).
Vectored delivery of antibodies against infectious viruses has been proposed as a novel strategy to achieve protection without requiring the mounting of an immune response, which is traditionally generated by active immunization. Adeno-associated virus (AAV) vectors are currently being developed as prophylactic treatment for HIV (6, 7), and recently Balazs et al. demonstrated that intramuscular injection of AAV8 expressing the broadly neutralizing influenza antibody F10 protected young mice (between 14 and 19 weeks of age), old mice (between 46 and 55 weeks of age), and immunodeficient mice from challenge with influenza (8). We previously demonstrated that localized expression of anti-influenza antibodies at the airway mucosal surface, where replication of airborne infectious viruses is initiated, is a safe and effective target against influenza. Expression of the broadly neutralizing anti-influenza antibody FI6 (9), via an AAV9 vector, in the airways of mice and ferrets protected the animals from lethal challenges with various influenza strains, including virus reconstructed from clinical material associated with the 1918 H1N1 pandemic, various H5N1 clinical isolates (10), and a clinical isolate of H7N9 (11).
This versatile approach may be especially beneficial for immunodeficient and immunocompromised patients, as it circumvents the need for host adaptive immunity while providing passive immunization. Here, we evaluated the efficacy of AAV9.FI6-IA to protect aged and immunodeficient mice, which model the high-risk elderly and immunocompromised patient populations, against influenza.
MATERIALS AND METHODS
Viral vector construction.
For the AAV9.201Ig-IA construct, the light and heavy chain sequences of an anti-SIVsmF236 antibody isolated from an infected macaque (12) were used to construct an immunoadhesin (IA) using macaque IgG secretion signal and Fc domains, as described previously (7). AAV9 vectors expressing either firefly luciferase (ffLuc) or the modified FI6 IA under the control of a hybrid cytomegalovirus (CMV) enhancer-chicken β-actin promoter were constructed and produced as described previously (10).
Animals.
Young (6- to 8-week-old) female BALB/c (BALB/cByJ) mice and severe combined immunodeficient (SCID) (CBySmn.CB17-Prkdcscid/J) mice on a BALB/cByJ background were purchased from the Jackson Laboratory (Bar Harbor, ME). Old (13-month-old) female BALB/c mice (retired breeders) were purchased from the Jackson Laboratory. Mice were housed at the animal facility of the Translational Research Laboratories at the University of Pennsylvania. Experiments were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Mouse studies.
For vector injection, mice were anesthetized by intraperitoneal injection of a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine in phosphate-buffered saline (PBS), suspended by their dorsal incisors with their hind limbs supported on a platform, and given 1011 genome copies (GC) of AAV9 vector in a total volume of 50 μl of PBS, administered intranasally (i.n.) as three 17-μl aliquots, in alternate nares. For influenza challenge, 2 weeks after vector treatment, vaccinated and naïve mice were weighed, their tails were color coded, they were anesthetized as described above, and they were given challenge virus, i.e., strain A/Puerto Rico/8/1934(H1N1) (PR8), in a total volume of 50 μl of PBS, administered i.n., as described above.
Mice were then weighed daily and monitored for signs of disease or distress. Animals that exhibited behavioral signs of distress or lost 30% of their initial body weight were euthanized by CO2 asphyxiation. Six days after the PR8 challenge, three mice per group were sacrificed, their lungs were harvested, and lung homogenates were prepared for viral load assays. Mice that survived the challenge were sacrificed on day 28, and bronchoalveolar lavage fluid (BALF) samples were isolated as described previously (13).
Challenge studies conducted in young and old BALB/c mice were repeated three times, whereas challenge studies conducted in SCID mice were repeated twice, to ensure the accuracy and reproducibility of the experimental findings. Results were consistent between studies, and representative results are presented.
Viral plaque assay.
Confluent monolayers of MDCK cells grown in 6-well plates were incubated with serial 10-fold dilutions of lung homogenates for 1 h at 37°C in 5% CO2. The medium was replaced with Dulbecco's modified Eagle's medium (DMEM) containing 0.3% agarose, 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 2 μg/ml l-1-tosylamido-2-phenylethylchloromethyl ketone-treated trypsin (Sigma-Aldrich Co., St. Louis, MO), and the cells were incubated for another 72 h. The cells were then fixed with 4% paraformaldehyde in PBS (Sigma-Aldrich Co., St. Louis, MO) for 1 h at room temperature. The agarose overlay was carefully removed, and the individual plaques were stained with crystal violet (Sigma-Aldrich Co., St. Louis, MO) and quantified.
Imaging.
For ffLuc expression, mice were anesthetized by intraperitoneal injection of a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine in PBS. Mice were then injected i.n. with 50 μl of 15 mg/ml-1 d-luciferin (Caliper, Princeton, NJ) and, after 5 min, were imaged using an IVIS Xenogen imaging system. Bioluminescence was quantified using a designated region of interest (ROI), with a unique ROI for each area (nose or lungs), using Living Image 2.50.1 software.
ELISA.
Plates (96 wells) were coated with the H1N1 antigen HA(ΔTM)(A/Beijing/01/2009) (Immune Technology Corp., New York, NY) at 2 μg/ml in PBS at 4°C overnight. Plates were washed with PBS plus 0.05% Tween 20 (PBS-T) and blocked with 5% FBS in PBS for 1 h. Samples were heat inactivated (56°C for 1 h) and added to the enzyme-linked immunosorbent assay (ELISA) plates at various dilutions. Known amounts of the affinity-purified FI6/370 IA were used as standards. After the 1-h incubation, plates were washed with PBS-T and blocked with 5% FBS in PBS for 1 h, and Bio-SP-conjugated AffiniPure goat anti-human IgG was then added at a 1:10,000 dilution in PBS for 1 h at room temperature. Plates were washed with PBS-T and incubated with streptavidin (1:30,000 dilution) for 1 h at room temperature. Plates were then washed with PBS, and 3,3′,5,5′-tetramethylbenzidine (TMB) was added to the dried wells for 30 min in the dark. The color reaction was stopped with the addition of H2SO4 stop solution. Plates were read at 450 nm and 540 nm (used to measure background levels).
Statistical analysis.
Analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). Statistical significance was set at P = 0.05 and statistical power at 0.80. Results are presented as mean ± standard error of the mean (SEM). The Mantel-Cox test was used to test the survival distributions for differences; the Mann-Whitney test was used to determine differences between two groups, and Tukey's test was used to test pairwise comparisons between groups.
RESULTS AND DISCUSSION
Previously, we harnessed both the ability of AAV9 to transduce airway cells following noninvasive nasal delivery and its promising safety profile in mice and macaques (13, 14) to develop a passive immunization vaccine to express an anti-influenza antibody in the airway surface liquid layer. In a recent study, we used AAV9 to express FI6, a broadly neutralizing human antibody that neutralizes group 1 and 2 influenza A strains (9), and demonstrated that the AAV9.FI6-IA vaccine protected young (6- to 8-week-old) BALB/c mice against lethal challenges with various clinical isolates of H1N1 and H5N1, as well as mouse-adapted H1N1 (PR8) (10), while an AAV9 vector expressing an irrelevant transgene (ffLuc) did not protect mice against PR8 challenge (10).
Elderly subjects (adults over the age of 65 years) are susceptible to serious complications caused by seasonal influenza, and annual vaccination is highly recommended for this age group, although it is not always effective. In a recent report, only 9% of elderly subjects were protected against influenza infection following influenza vaccination (15). The number of elderly subjects in the United States is expected to expand from 40 million in 2010 to 72 million by 2030, and globally the number is projected to triple by 2050 (http://www.un.org/esa/population/publications/worldageing19502050). This rate of population aging is unprecedented and affects both developed nations and countries with poor health care infrastructures.
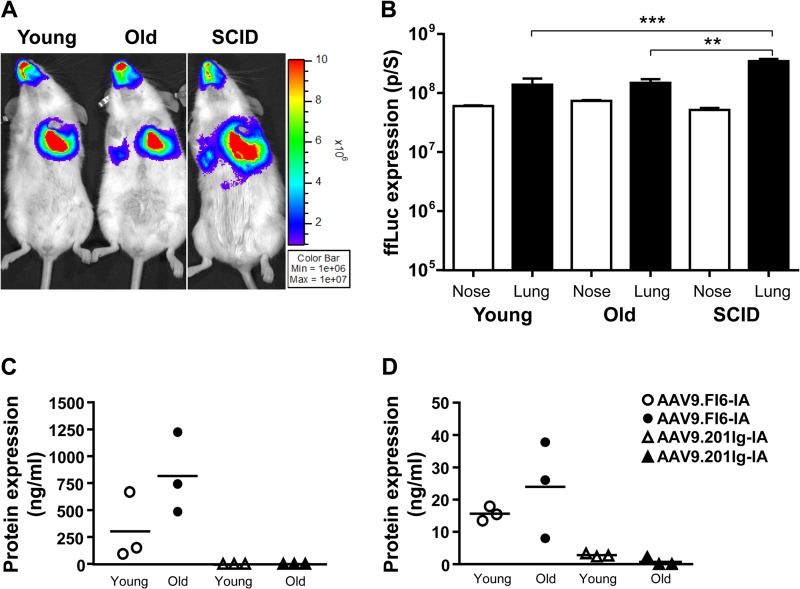
The effects of age and immune status on the efficiency of airway-directed AAV-mediated gene transfer are unclear. We investigated the transduction profile of the AAV9 vector in old and immunodeficient (SCID) mice, to determine whether the nasal and lung airways undergo transduction at levels similar to those achieved with AAV9 in young mice. Young (6- to 8-week-old) and old (13-month-old) BALB/c mice and young (6- to 8-week-old) SCID mice were given i.n. 1011 genome copies (GC) of AAV9.ffLuc in 50 μl. Fourteen days later, ffLuc expression was assessed (Fig. 1A) and quantitated (Fig. 1B). Expression was observed in the nasal and lung airways, and no significant differences in the levels of expression in the nasal and lung airways in young and old BALB/c mice were observed. In SCID mice, however, transduction in the lungs was significantly higher than levels in young (P < 0.001, Tukey's test) and old (P < 0.01, Tukey's test) mice (Fig. 1B). In parallel studies, the effects of age and immunodeficiency on the deposition of AAV9 vector genome in the lungs were evaluated at the time of necropsy. We observed no differences in the numbers of AAV9 vector genomes in the lungs of old, young, and SCID mice (data not shown).
FIG 1.
AAV9-mediated ffLuc expression in mouse airways. (A) Imaging of ffLuc expression. Young, old, and SCID BALB/c mice were dosed i.n. with 1011 GC of AAV9.ffLuc vector and underwent imaging of ffLuc expression 14 days later. Luminescence was observed in both the nasal and lung airways. (B) Quantification of levels of luminescence in the airways (nose and lung) of young, old, and SCID mice (n = 3/group). **, P < 0.01; ***, P < 0.001, Tukey's test. (C and D) Assessment of FI6 expression in lavage fluid samples. At day 14, FI6 expression was assessed in BALF (C) and NLF (D) samples from young and old mice (n = 3/group) treated with 1011 GC of AAV9.FI6-IA. As controls for nonspecific expression, BALF and NLF samples from mice injected i.n. with 1011 GC of AAV.201Ig-IA (a vector expressing an antibody irrelevant to influenza; n = 3/group) were analyzed.
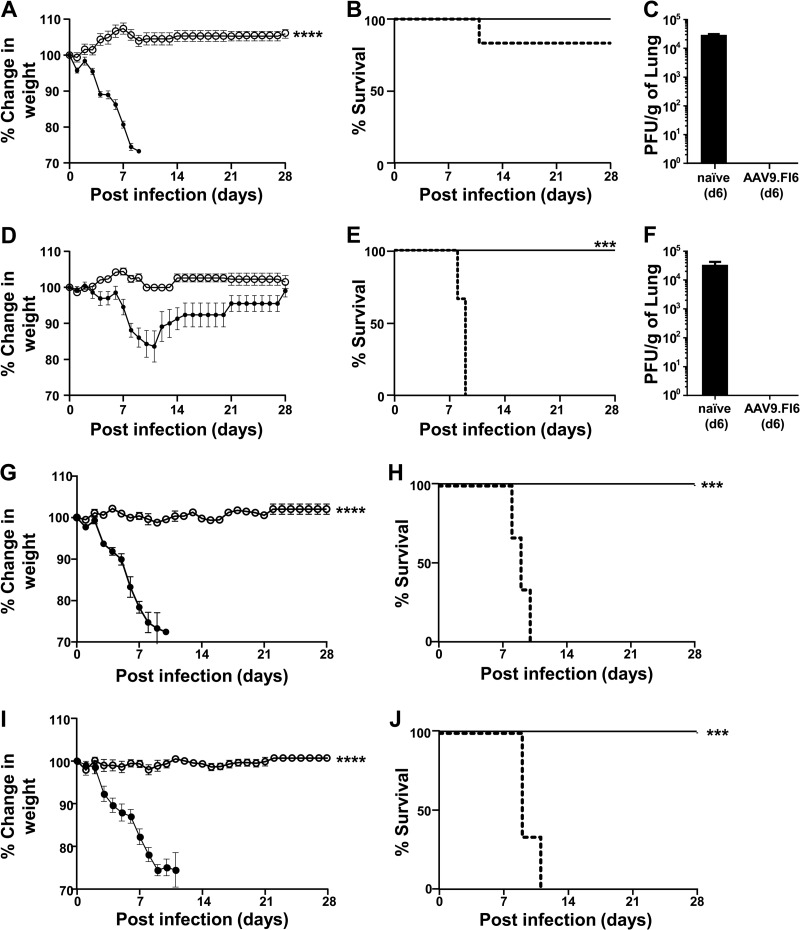
Given that AAV9 transduced well the airways of young, old, and SCID mice, we next examined the effects of age on the protective efficacy of AAV9.FI6-IA against challenge with PR8. BALB/c mice of two different ages, i.e., young and old, were utilized. Mice were dosed i.n. with 1011 GC of AAV9.FI6-IA. Fourteen days later, mice were challenged i.n. with 30 PFU of PR8. The weights of mice were monitored daily for 28 days (Fig. 2A and D), and any symptoms of behavioral distress were noted. At day 6, three mice from each group were sacrificed, and the lungs were processed to determine viral loads. In contrast to the lungs of vaccinated mice, high viral loads were noted in the lungs of nonvaccinated mice (Fig. 2C and F). Vaccination with AAV9.FI6-IA conferred complete survival of young and old mice against challenge with PR8, and mice did not exhibit any signs of disease or experience weight loss throughout the study (Fig. 2A, B, D, and E). In contrast, upon PR8 challenge of untreated mice, significant weight loss and signs of behavioral distress were observed and mice were euthanized by day 11. To determine whether vaccination with the AAV9 vector conferred a nonspecific survival advantage against challenge with influenza, control young and old mice were vaccinated with AAV9.201Ig-IA, an unrelated antibody molecule that does not neutralize influenza. The level of FI6 expression was assessed in BALF and nasal lavage fluid (NLF) samples from vaccinated young and old mice (n = 3 per group) prior to challenge with PR8 (Fig. 1C and D). The large intragroup variation in the levels of FI6 is an inherent limitation of BALF sampling. No statistical difference in the expression of FI6 in the lungs and nasal airways of young and old mice was observed. Fourteen days later, mice were challenged with PR8. Young mice were challenged with 30 PFU, and the dose of PR8 used to challenge old mice was increased to 300 PFU for this study. Vaccination with AAV9.FI6-IA conferred full protection of both young and old mice against challenge with PR8, and mice did not exhibit any signs of disease or experience weight loss throughout the study (Fig. 2G, H, I, and J). However, mice vaccinated with the irrelevant AAV9 vector (AAV9.201Ig-IA) exhibited significant weight loss starting at day 4, and mice were euthanized by day 11 (Fig. 2H and J).
FIG 2.
AAV9.FI6-IA protection of old BALB/c mice from influenza challenge. (A and D) Young vaccinated (○) and nonvaccinated (●) mice (n = 8/group) (A) and old vaccinated (○) and nonvaccinated (●) mice (n = 8/group) (D) were challenged i.n. with PR8 (30 PFU) and weighed daily. Percent changes in weight were calculated based on the weight on the day of the challenge. (B and E) Young (B) and old (E) mice were euthanized when they appeared in distress or their body weight declined >30%, as depicted in the Kaplan-Meier plots. Solid lines, vaccinated mice; dashed lines, nonvaccinated mice. (C and F) Young (C) and old (F) mice (n = 3) from the vaccinated and nonvaccinated groups were necropsied at day 6 (d6) to quantify viral loads in the lung. (G and I) Young (G) and old (I) mice (n = 5/group) were vaccinated with either AAV9.FI6 (○) or an irrelevant vaccine (AAV9.201Ig-IA) (●), challenged i.n. with PR8 (30 PFU), and weighed daily. Percent changes in weight were calculated based on the weight on the day of the challenge. (H and J) Young (H) and old (J) mice were euthanized when they appeared in distress or their body weight declined >30%. Solid lines, mice vaccinated with AAV9.FI6; dashed lines, mice vaccinated with the irrelevant vector. ****, P ≤ 0.0001, Mann-Whitney test; ***, P ≤ 0.001, Mantel-Cox test.
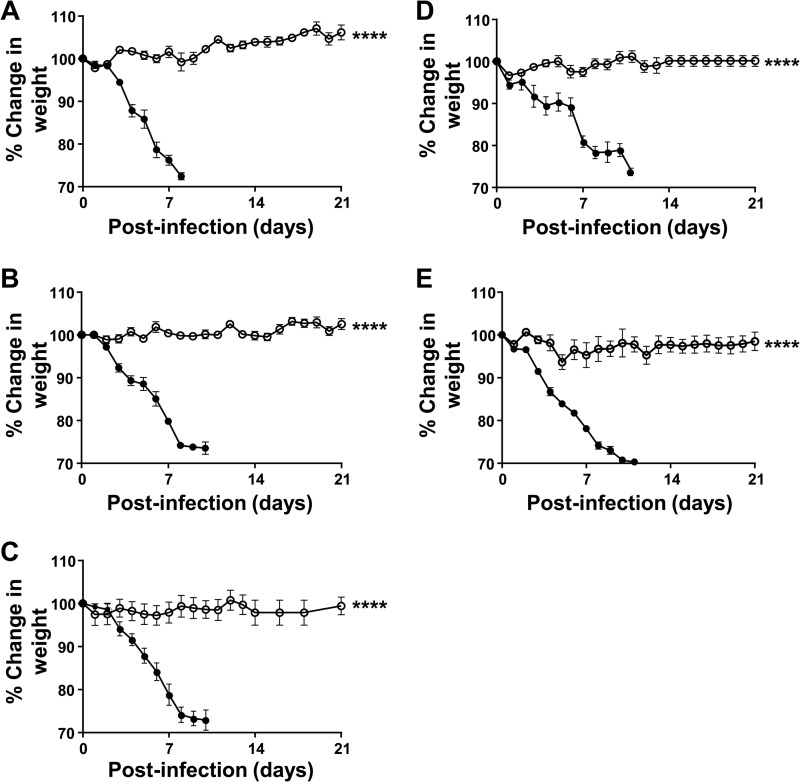
An important consideration for vaccine development is the durability of protection against infection with influenza. To address this concern, we designed a study that evaluated the duration of the protection conferred by a single dose of AAV9.FI6-IA vector. Mice (6- to 8-week-old) were given i.n. 1011 GC of AAV9.FI6-IA, and groups of vaccinated mice (n = 5) and age-matched nonvaccinated controls (n = 5) were challenged with 30 PFU of PR8 after 7 days, 14 days, 28 days, 3 months, or 6 months. The weights of the mice were monitored daily for 28 days following the challenge (Fig. 3), and any symptoms of behavioral distress were noted. Unlike the naïve mice, which succumbed to the challenge at each time point examined, vaccination with AAV9.FI6-IA conferred survival against the challenge with PR8 at all time points tested, and vaccinated mice did not exhibit any signs of disease or experience weight loss throughout the study (Fig. 3). These data demonstrate that AAV9.FI6 is effective in protecting mice against influenza for at least 6 months.
FIG 3.
Sustained protection against challenge with PR8. BALB/c mice were dosed i.n. with 1011 GC of AAV9.FI6-IA vector in 50 μl (n = 5 mice/group). Mice were challenged with PR8 (30 PFU) 7 days (A), 14 days (B), 28 days (C), 3 months (D), or 6 months (E) after the single instillation of vector. The weights of the animals were assessed daily, and mice were euthanized when they appeared in distress or they lost >30% of their initial body weight. Percent changes in weight were calculated based on the weight on the day of the challenge. ○, AAV9.FI6-IA vector-treated mice; ●, naïve mice. ****, P ≤ 0.0001, Mann-Whitney test.
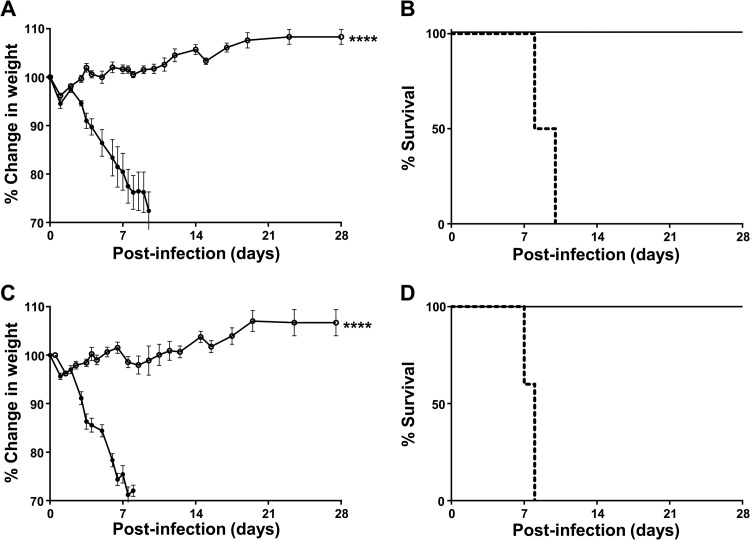
Individuals who suffer from immunodeficiency or are immunocompromised for medical reasons are vulnerable to influenza-related life-threatening complications. The development of effective vaccination strategies for these high-risk groups remains a priority. We examined the protective efficacy of the AAV9.FI6-IA vector against influenza in SCID mice, which lack B and T cells. SCID mice and immunocompetent BALB/c mice were treated i.n. with 1011 GC of AAV9.FI6-IA and were challenged 14 days later with 30 PFU of PR8. Vaccinated SCID and BALB/c mice maintained their weight, did not exhibit any signs of behavioral distress, and were fully protected against challenge with PR8 (Fig. 4A to D). Following the PR8 challenge, nonvaccinated BALB/c mice were euthanized by day 8, whereas nonvaccinated SCID mice were euthanized by day 10 (Fig. 4A and C). The difference in the onset of disease severity between SCID mice and BALB/c mice may reflect differences in host immune responses to influenza infection, as has been reported (16). Three vaccinated SCID mice and one nonvaccinated SCID mouse were necropsied at day 6, to quantify viral loads in the lung. A high viral load (4.3 × 105 PFU/g lung tissue) was found in the nonvaccinated SCID mouse at day 6, whereas no virus was detected in the vaccinated SCID mice. Similarly, at the conclusion of the experiment (day 28), no PR8 virus was detected in the lungs of vaccinated BALB/c or SCID mice.
FIG 4.
AAV9.FI6-IA protection of SCID mice from influenza challenge. (A) Vaccinated (○) and nonvaccinated (●) BALB/c mice were challenged i.n. with 30 PFU of PR8 and weighed daily. Percent changes in weight were calculated based on the weight on the day of the challenge. (B) BALB/c mice were euthanized when they appeared in distress or their body weight declined >30%, as depicted in the Kaplan-Meier plot. Solid lines, vaccinated mice; dashed lines, nonvaccinated mice. (C) Vaccinated (○) and nonvaccinated (●) SCID mice were challenged i.n. with 30 PFU of PR8 and weighed daily. Percent changes in weight were calculated based on the weight on the day of the challenge. (D) SCID mice were euthanized when they appeared in distress or their body weight declined >30%, as depicted in the Kaplan-Meier plot. Solid lines, vaccinated mice; dashed lines, nonvaccinated mice. ****, P ≤ 0.0001, Mann-Whitney test.
In this study, we demonstrated that, irrespective of their immune status and age, vaccinated mice survived the challenge and exhibited no signs of behavioral distress or loss of weight. The immune system of old mice is deficient in antigen presentation and B and T cell activation (17, 18). Moreover, old mice display aberrant interferon and cytokine responses, which are representative of immunosenescence in humans (19). The severity of disease following infection with influenza A virus has been reported to differ between young and aged mice and is dependent on the dose of influenza administered (20). In our studies, we found that the dose of PR8 had to be increased 10-fold to result in severe weight loss that necessitated euthanasia.
We have developed a noninvasive AAV9-based passive immunization strategy that protects old and immunodeficient mice against influenza challenge. Our strategy targets the airways, the site of initial influenza infection and replication, and may offer superior sterilizing protection, compared with systemically administered vector-based vaccines. A caveat associated with the use of a single vectored antibody against influenza is the possible generation of escape mutants. This is especially important in susceptible patient populations with diminished B and T cell responses. Increased safety and efficacy may be obtained by expressing two vectored anti-influenza antibodies to prevent escape mutants, as well as to broaden the range of influenza strains susceptible to the AAV9-based vaccine. This platform combines AAV9 and an antibody and offers the possibility of passive immunization of airways against other respiratory disease-causing agents that affect vulnerable populations, such as the respiratory syncytial virus (RSV).
ACKNOWLEDGMENTS
We thank Hui Peng and the staff of the Animal Models Core, Vector Core, Immunology Core, and Cell Morphology Core of the Gene Therapy Program at the University of Pennsylvania for invaluable assistance with these studies. We thank Jan Erikson (Wistar Institute) for providing the PR8-Mt. Sinai strain.
This work was supported in part by NIH grant P30-DK047757 (to J.M.W.) and by DOD/Defense Advanced Research Projects Agency grant 64047-LS-DRP.01 (to J.M.W.).
J.M.W. is an advisor to ReGenX Biosciences and Dimension Therapeutics and is a founder of, holds equity in, and receives grants from ReGenX Biosciences and Dimension Therapeutics; is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies; and holds a patent on AAV clades (US patent 7,906,111B2), with pending continuation (application number 13/023,918). J.M.W. and M.P.L. have a pending patent application for AAV-mediated passive immunization with airborne pathogens (application number PCT/US2012/034355). The other authors declare no competing financial interests.
Footnotes
Published ahead of print 10 September 2014
REFERENCES
- 1.Centers for Disease Control and Prevention. 2010. Estimates of deaths associated with seasonal influenza: United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 59(33):1057–1062. [PubMed] [Google Scholar]
- 2. Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. 2007. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25:5086–5096. 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 3. Oliveira EC, Lee B, Colice GL. 2003. Influenza in the intensive care unit. J. Intensive Care Med. 18:80–91. 10.1177/0885066602250368. [DOI] [PubMed] [Google Scholar]
- 4. Dorshkind K, Montecino-Rodriguez E, Signer RA. 2009. The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 9:57–62. 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 5. Ljungman P. 2012. Vaccination of immunocompromised patients. Clin. Microbiol. Infect. 18(Suppl 5):S93–S99. 10.1111/j.1469-0691.2012.03971.x. [DOI] [PubMed] [Google Scholar]
- 6. Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. 2012. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84. 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. 2009. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 15:901–906. 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balazs AB, Bloom JD, Hong CM, Rao DS, Baltimore D. 2013. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat. Biotechnol. 31:647–652. 10.1038/nbt.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856. 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 10. Limberis MP, Adam VS, Wong G, Gren J, Kobasa D, Ross TM, Kobinger GP, Tretiakova A, Wilson JM. 2013. Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. Sci. Transl. Med. 5:187ra172. 10.1126/scitranslmed.3006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Limberis MP, Racine T, Kobasa D, Li Y, Gao GF, Kobinger G, Wilson JM. 2013. Vectored expression of the broadly neutralizing antibody FI6 in mouse airway provides partial protection against a new avian influenza A virus, H7N9. Clin. Vaccine Immunol. 20:1836–1837. 10.1128/CVI.00545-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glamann J, Burton DR, Parren PW, Ditzel HJ, Kent KA, Arnold C, Montefiori D, Hirsch VM. 1998. Simian immunodeficiency virus (SIV) envelope-specific Fabs with high-level homologous neutralizing activity: recovery from a long-term-nonprogressor SIV-infected macaque. J. Virol. 72:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Limberis MP, Wilson JM. 2006. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U. S. A. 103:12993–12998. 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nieto K, Stahl-Hennig C, Leuchs B, Muller M, Gissmann L, Kleinschmidt JA. 2012. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Hum. Gene Ther. 23:733–741. 10.1089/hum.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson MG. 2013. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb. Mortal. Wkly. Rep. 62(07):119–123. [PMC free article] [PubMed] [Google Scholar]
- 16. Wu H, Haist V, Baumgartner W, Schughart K. 2010. Sustained viral load and late death in Rag2−/− mice after influenza A virus infection. Virol. J. 7:172. 10.1186/1743-422X-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garraud O, Borhis G, Badr G, Degrelle S, Pozzetto B, Cognasse F, Richard Y. 2012. Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond. BMC Immunol. 13:63. 10.1186/1471-2172-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee N, Shin MS, Kang I. 2012. T-cell biology in aging, with a focus on lung disease. J. Gerontol. A Biol. Sci. Med. Sci. 67:254–263. 10.1093/gerona/glr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang J, Fisher EM, Murasko DM. 2011. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res. Rev. 10:422–427. 10.1016/j.arr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pica N, Langlois RA, Krammer F, Margine I, Palese P. 2012. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge. J. Virol. 86:10293–10301. 10.1128/JVI.01131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]