Abstract
We studied the immunoglobulin (Ig) response to causative serotype-specific capsular polysaccharides in adult pneumococcal pneumonia patients. The serotypes were grouped according to their degree of encapsulation and invasive potential. Seventy patients with pneumococcal pneumonia, 20 of whom were bacteremic, were prospectively studied. All pneumococcal isolates from the patients were serotyped, and the Ig titers to the homologous serotype were determined in acute- and convalescent-phase sera using a serotype-specific enzyme-linked immunosorbent assay. The Ig titers were lower in bacteremic cases than in nonbacteremic cases (P < 0.042). The Ig titer ratio (convalescent/acute titer) was ≥2 in 33 patients, 1 to 1.99 in 20 patients, and <1 in 17 patients. Patients ≥65 years old had a lower median Ig titer ratio than did younger patients (P < 0.031). The patients with serotypes with a thin capsule (1, 4, 7F, 9N, 9V, and 14) and medium/high invasive potential (1, 4, 7F, 9N, 9V, 14, and 18C) had higher Ig titer ratios than did patients with serotypes with a thick capsule (3, 6B, 11A, 18C, 19A, 19F, and 23F) and low invasive potential (3, 6B, 19A, 19F, and 23F) (P < 0.05 for both comparisons after adjustment for age). Ig titer ratios of <1 were predominantly noted in patients with serotypes with a thick capsule. In 8 patients with pneumococcal DNA detected in plasma, the three patients with the highest DNA load had the lowest Ig titer ratios. In conclusion, a high antibody response was associated with serotypes with a thin capsule and medium/high invasive potential, although a low antibody response was associated with serotypes with a thick capsule and a high pneumococcal plasma load.
INTRODUCTION
Streptococcus pneumoniae is the most important cause of community-acquired pneumonia (CAP) in adults, and it is associated with significant morbidity and mortality (1). The pneumococcal capsule constitutes an important virulence factor and provides protection against the host immune response (2). More than 90 known encapsulated serotypes have been identified (3) based on the composition of their capsular polysaccharides (CPS). Pneumococcal infections show a broad spectrum of clinical presentations, from nasopharyngeal colonization to invasive pneumococcal disease (IPD), depending on host factors and the causative serotype (4). In vaccination studies, a significant type-specific antibody response has been found to confer protection against pneumococcal disease (5–7), but in studies with patients with pneumococcal infections, the anti-CPS antibody responses vary considerably (8–11). Therefore, the association between the immunogenicity of pneumococcal CPS and disease should be investigated further.
Heavily encapsulated serotypes, as determined by fluorescein isothiocyanate (FITC)-dextran exclusion, were recently shown by Weinberger et al. (12) to be associated with resistance to neutrophil-mediated killing and an increased prevalence in nasopharyngeal carriage. Nasopharyngeal acquisition of pneumococci has been observed to generate elevated antibody levels in unvaccinated individuals (13, 14). If the degree of encapsulation correlates with immunogenicity in pneumococcal disease, we hypothesized that that heavily encapsulated serotypes would induce higher antibody responses in pneumococcal pneumonia than would serotypes with a low degree of encapsulation. Furthermore, in a meta-analysis by Brueggemann et al. (15), an inverse correlation was found between carriage prevalence and the invasive potential for serogroups/serotypes, with the most prevalent carried serotypes being less likely to cause invasive disease. Thus, we hypothesized that serotypes with a low invasive potential induce a high anti-CPS antibody response in pneumococcal pneumonia, which protects against invasive disease.
In this prospective study of adult patients with pneumococcal pneumonia, we aimed to study the anti-CPS antibody response to the causative pneumococcal serotype, with the serotypes grouped according to their degree of encapsulation and invasive potential. We also aimed to study if this antibody response is correlated with the pneumococcal DNA load in the bloodstream.
(Data from the manuscript were presented at the ISPPD-8, Iquaçu Falls, Brazil, 11 to 15 March 2012, and at the 23rd European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, 27 to 30 April 2013.)
MATERIALS AND METHODS
Study population and controls.
Between November 1999 and April 2002, 235 adult patients with CAP admitted to the Department of Infectious Diseases at Örebro University Hospital, Sweden, were enrolled in a prospective study. The inclusion criteria and population characteristics were described previously (16). CAP was defined as an acute onset of illness, with new radiological signs of pulmonary consolidation and ≥2 of the following signs or symptoms: fever of ≥38°C, dyspnea, cough, pleuritic chest pain, or abnormal lung auscultation. During the same period, 113 adult controls were enrolled who were hospitalized for skin infections (n = 14), urinary tract infections (n = 14), arthritis or spondylitis (n = 6), or planned orthopedic or urologic surgery (n = 79) (16). None of the controls had been hospitalized for any reason during the preceding month or had received any antibiotic treatment during the preceding week. Respiratory symptoms were absent in all controls at admission.
Culture for S. pneumoniae and serotyping.
Samples from blood, sputum, and the nasopharynx (nasopharyngeal aspirate [NpA] and nasopharyngeal swabs [NpS]) in CAP patients, and from the blood, NpA, and NpS in the controls were collected at admission. A Bactec blood culturing system (Becton Dickinson, MD, USA) was used for blood culture. The sputum, NpA, and NpS specimens were cultured according to standard microbiological methods (17). A sputum sample was considered representative for the lower airways if >5 neutrophils per squamous epithelial cell were found (18). A detection limit of >105 CFU/ml was used for the sputum cultures. All isolates of S. pneumoniae were stored at −70°C and transported in a frozen state to the Statens Serum Institut (SSI) in Copenhagen, Denmark, for serotyping by the Quellung reaction (19).
ELISA for pneumococcal antibodies in paired sera.
Paired sera collected within 2 days after admission (acute-phase serum) and after approximately 4 weeks (convalescent-phase serum) were obtained in CAP patients and controls and stored at −70°C until transportation to SSI under frozen conditions. The sera were thawed in 2003 to measure the type-specific total immunoglobulin antibodies (Ig measurement) against the homologous pneumococcal serotypes by enzyme-linked immunosorbent assay (ELISA). At the time of this study, the World Health Organization (WHO) consensus guidance protocol for using the ELISA to test the immune response to pneumococcal CPS, measured in μg/ml, was not yet established (20–22). Instead, we used the ELISA described and evaluated by Konradsen (21) and Konradsen, Sørensen, and Henrichsen (23). As in the WHO ELISA, the American Type Culture Collection (ATCC) polysaccharides were used for coating, and all sera were adsorbed by adding cell wall polysaccharides (CWPS) to inhibit nonspecific binding (23, 24). A pool of postvaccination serum samples from 43 healthy adults, who had been vaccinated with a 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23 [PPV23]), was used as standard serum. This in-house standard serum correlated with the international standard serum 89SF (20–22).
Briefly, polystyrene microtiter plates (MaxiSorp; Nunc A/S, Denmark) were coated overnight with 100 μl of ATCC polysaccharide (phosphate-buffered saline [PBS] [pH 7.2] plus 0.1% polysaccharide, to a final concentration of 2.5 μg/ml). The control wells were applied with 100 μl of PBS. The next day, the plates were washed three times with 300 μl of washing buffer (PBS [pH 7.4] plus 0.1% Tween 20). A 2-fold dilution series from 1:200 to 1:25,600 of the standard serum was added to each ELISA plate. For each patient sample, a dilution series of 1:100, 1:300, 1:900, and 1:2,700 was made. The plates were incubated at room temperature for 1.5 h and washed three times. One hundred microliters of Ig conjugate (Dako P0212 Ig diluted 1:7,000 in PBS [pH 7.2] plus 1% bovine serum albumin [BSA] plus 0.5% Tween 20) was added to each well. The plates were incubated for another 1.5 h, washed four times, and 100 μl of substrate (TMB Plus; Kem-En-Tec Diagnostics A/S, Denmark) was added to each well. Finally, the plates were incubated on a microplate shaker at low speed. The reaction was stopped after 30 min by adding 100 μl of 1 M H2SO4 to each well. The optical densities at 490 nm were measured using an automatic ELISA reader (Sunrise; Tecan Schweiz AG, Switzerland). By comparing the optical density (OD) data from the dilution series of the patient serum with those of the standard serum, an antibody titer in arbitrary units (AU) was calculated for the patient serum (21, 23). On each plate, the OD of the substrate was measured in blank wells, without antigen or serum, with OD×1,000 values of <100 AU. Also, a low, medium, and high titer standard serum was added to each plate and compared between the plates by their OD to demonstrate that no plate-to-plate variation occurred (25).
PCR for detecting pneumococcal DNA in plasma.
Plasma was collected in EDTA tubes at admission and was stored at −70°C before being thawed for DNA extraction with an automatic NucliSENS easyMAG instrument (bioMérieux, France). The purified DNA was then examined by real-time quantitative PCR for Spn9802 DNA, as described previously (26).
Serotype groups.
All included patients were grouped according to the isolated pneumococcal serotype with respect to sampling site (blood, sputum, or nasopharynx), degree of encapsulation (low or high), and invasive potential (low, medium, or high) (12, 15). Based on Fig. 2B in the study by Weinberger et al. (12), a mean area of 500 pixels for the zone of FITC-dextran exclusion was used for dividing serotypes into equally large groups for low (1, 4, 7F, 9N, 9V, and 14) and high (3, 6B, 11A, 18C, 19A, 19F, and 23F) degrees of encapsulation. We included serotype 3 in the group of heavily encapsulated serotypes, even though no zone area was presented in the reference study, as it was considered to have already-identified extensive capsule production. Based on Fig. 3 in the study by Brueggemann et al. (15), pooled estimate odds ratios of <0.25, 0.25 to 2, and >2 were used for dividing the serotypes into low (3, 6B, 19A, 19F, and 23F), medium (4, 9N, 9V, 14, and 18C), and high (1 and 7F) invasive potentials, respectively.
FIG 2.
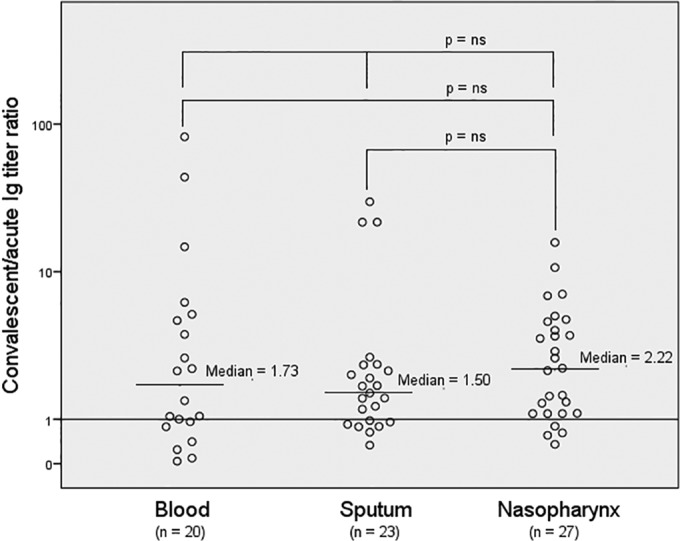
Serotype-specific immunoglobulin (Ig) response (convalescent/acute Ig titer ratio) to the causative serotype in pneumococcal pneumonia patients with respect to culture site. Only one site per patient is presented. The patient was included in the blood category if the blood culture was positive, the sputum category if the sputum culture was positive and blood culture was negative, and the nasopharynx category if the culture from nasopharyngeal secretions was positive and no blood culture or sputum culture was positive. ns, nonsignificant.
FIG 3.
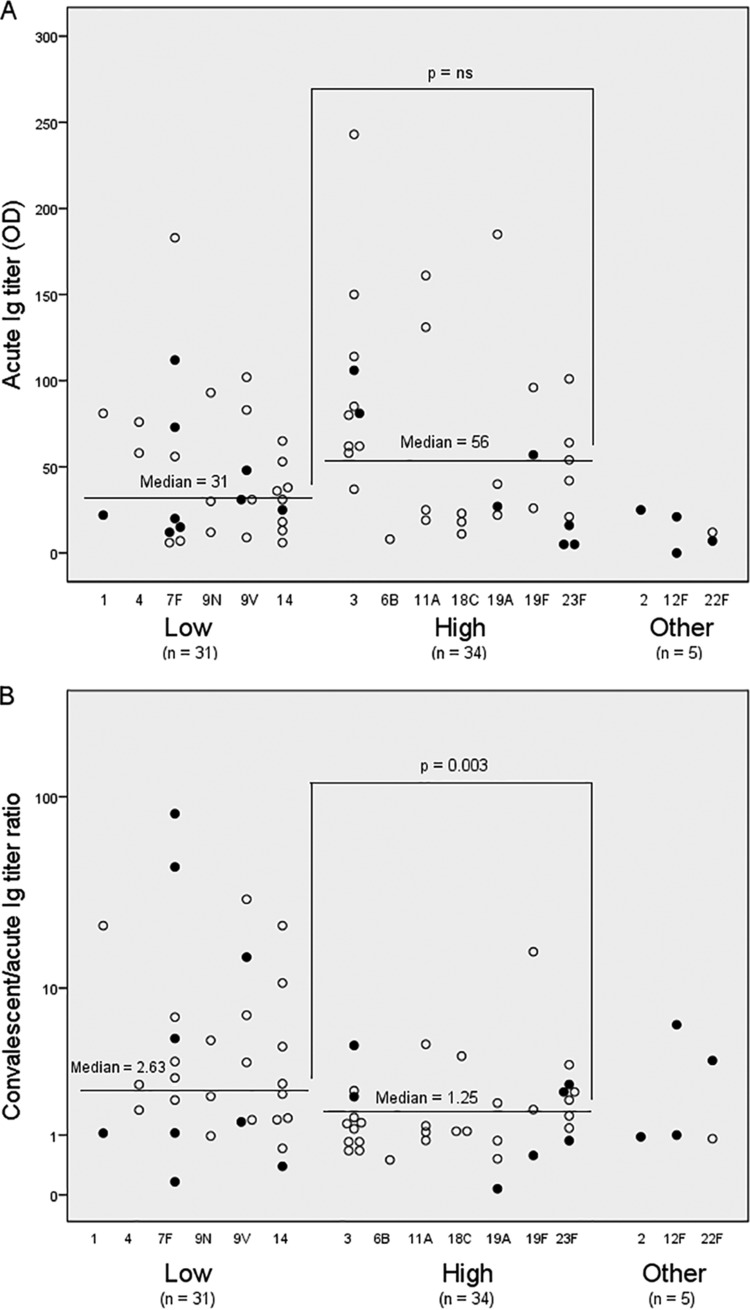
Serotype-specific immunoglobulin (Ig) titers at admission (A) and response (convalescent/acute Ig titer ratio) (B) to the causative serotype in pneumococcal pneumonia patients, with respect to the degree of encapsulation of the serotype. In 65 of the 70 included patients in the study, a low (1, 4, 7F, 9N, 9V, and 14) or high (3, 6B, 11A, 18C, 19A, 19F, and 23F) degree of encapsulation of the serotype was defined, based on Fig. 2B in the study by Weinberger et al. (12). ns, nonsignificant.
Statistical analysis.
To describe the patient characteristics with respect to acute Ig titer, the patients were divided into two groups with acute Ig titers of <40 AU and ≥40 AU. This cutoff was used as an estimation for patients with nonprotective levels of antibodies, based on guidelines for pneumococcal revaccination with PPV23 (21). To analyze the antibody response in the patients, the Ig titer ratios between convalescent- and acute-phase sera were calculated. To describe the patient characteristics with respect to the Ig titer ratio, the patients were divided into three groups, with Ig titer ratios of <1, 1 to 1.99, and ≥2. A titer ratio of ≥2 was considered a significant response.
Differences in the results between any three groups were examined by a one-way Kruskal-Wallis nonparametric analysis of variance (ANOVA) test and by the Mann-Whitney U test between any two groups. A two-tailed P value of <0.05 was considered statistically significant, except for multiple comparisons, for which the P values were adjusted using Holm's method (27). In order for each factor to be considered significant, the smallest P value must be <0.0125 (0.05/4), the second <0.0167 (0.05/3), the third <0.025 (0.05/2), and the fourth <0.05 (0.05/1). Linear regression was used to adjust for a potentially confounding factor. Nonnormal distributed data were first transformed by taking the log10 of the values before the regression analyses were performed. For groups of proportions, Pearson's chi-square test was used, or Fisher's exact test was used for small numbers. All statistical analyses were performed with a statistical software package (IBM SPSS Statistics, version 21).
Ethics.
The study was approved by the ethics committee of the Örebro County Council (686-1999). All participants provided their informed consent.
RESULTS
Of 235 CAP patients, 70 (35%) with pneumococcal CAP were included in the study (Fig. 1). A total of 16 serotypes were identified in 132 isolates, with multiple isolates in 47 (67%) patients (Table 1). In two patients, two isolates with different serotypes were identified. In one patient, the Ig titer ratios were 3.52 for serotype 23F (NpA) and 0.59 for serotype 11A (NpS). In the other patient, the Ig titer ratios were 0.58 for serotype 19F (blood) and 0.75 for serotype 14 (NpS). None of the patients included died within 30 days after admission.
FIG 1.
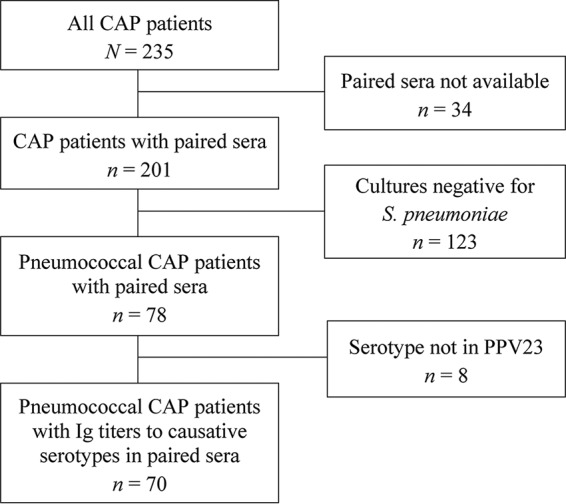
Flow chart of community-acquired pneumonia (CAP) patients included in the study. In 70 pneumococcal CAP patients, the causative serotypes belonged to the serotypes of the 23-valent pneumococcal polysaccharide vaccine (PPV23), for which serotype-specific immunoglobulin titers were measured.
TABLE 1.
Distribution of samples with isolated serotypes in pneumococcal CAP patientsa
| Serotype | No. of patients | No. of samples fromb: |
|||
|---|---|---|---|---|---|
| Blood | Sputum | NpA | NpS | ||
| 1 | 2 | 1 | 2 | 2 | 1 |
| 2 | 1 | 1 | 1 | 1 | 0 |
| 3 | 11 | 2 | 4 | 7 | 5 |
| 4 | 2 | 0 | 1 | 2 | 1 |
| 6B | 1 | 0 | 1 | 1 | 0 |
| 7F | 9 | 5 | 1 | 5 | 2 |
| 9N | 3 | 0 | 1 | 2 | 3 |
| 9V | 6 | 2 | 3 | 4 | 5 |
| 11A | 4 | 0 | 2 | 4 | 1 |
| 12F | 2 | 2 | 0 | 1 | 1 |
| 14 | 9 | 1 | 4 | 4 | 6 |
| 18C | 3 | 0 | 0 | 2 | 1 |
| 19A | 4 | 1 | 2 | 2 | 2 |
| 19F | 3c | 1 | 1 | 1 | 3 |
| 22F | 2 | 1 | 2 | 2 | 1 |
| 23F | 8d | 3 | 5 | 6 | 2 |
| Total | 70 | 20 | 30 | 46 | 34 |
CAP, community-acquired pneumonia.
NpA, nasopharyngeal aspirate; NpS, nasopharyngeal swab.
One patient with serotype 19F in blood had serotype 14 in NpS (not included in the table).
One patient with serotype 23F in NpA had serotype 11A in NpS (not included in the table).
Type-specific antibody titers in all patients.
Table 2 shows the patient characteristics in all patients, divided into two groups of patients with acute Ig titers of <40 AU (n = 37 [53%]) and ≥40 (n = 33 [47%]). There were no significant differences between the two groups with respect to the patient characteristics. For the total group of patients, the median acute Ig titer was 36 AU (range, 0 to 243 AU), and in 11 (16%) patients, the acute Ig titer was >100 AU (range, 101 to 243 AU). As noted in Table 3, the median acute Ig titer was significantly lower in patients with pneumococcal bacteremia than that in patients without bacteremia (24 versus 94 AU; P = 0.042).
TABLE 2.
Characteristics and type-specific Ig titers to the causative serotype in pneumococcal CAP patientsa
| Characteristicb | All | Data for patients with: |
||||
|---|---|---|---|---|---|---|
| Acute Ig titer (AU) of: |
Convalescent/acute Ig titer ratio of: |
|||||
| <40 | ≥40 | <1 | 1–1.99 | ≥2 | ||
| n | 70 | 37 | 33 | 17 | 20 | 33 |
| Mean age (yr) | 66.3 | 67.8 | 64.6 | 74.5 | 63.3 | 63.8 |
| Age ≥ 65 yr | 40 (57) | 23 (62) | 17 (52) | 13 (77) | 10 (50) | 17 (52) |
| Female | 32 (46) | 16 (43) | 16 (48) | 8 (47) | 10 (50) | 14 (42) |
| Comorbidityc | 36 (51) | 20 (54) | 16 (48) | 10 (59) | 8 (40) | 18 (55) |
| Smoking | 22 (31) | 9 (24) | 13 (39) | 3 (18) | 11 (55) | 8 (24) |
| CRB-65 score of 2–4 | 24 (34) | 12 (32) | 12 (36) | 9 (53) | 5 (25) | 10 (30) |
| PSI class IV-V | 28 (40) | 15 (41) | 13 (39) | 8 (47) | 6 (30) | 14 (42) |
| Bacteremia | 20 (29) | 14 (38) | 6 (18) | 6 (35) | 4 (20) | 10 (30) |
| Serotype with high degree of encapsulationd | 34/65 (52) | 14/32 (44) | 20/33 (61) | 11/15 (73) | 12/19 (63) | 11/31 (35) |
| Serotype with medium/high invasive potentiale | 34/61 (56) | 21/30 (70) | 13/31 (42) | 4/14 (29) | 9/17 (53) | 21/30 (70) |
| Onset of illness to acute-phase serum (median [range]) (days) | 3 (0–36) | 3 (0–36) | 4 (0–21) | 5 (0–36) | 4.5 (0–11) | 2 (0–21) |
| Acute- to convalescent-phase serum (median [range]) (days) | 30 (20–96) | 29 (20–96) | 30 (22–81) | 31 (25–82) | 28 (22–58) | 30 (20–96) |
| Acute Ig titer (median OD [range]) | 36 (0–243) | 20 (0–38) | 81 (40–243) | 35 (8–243) | 56 (0–161) | 30 (5–183) |
| Convalescent Ig titer (median OD [range]) (days) | 84 (0–1,754) | 30 (0–1,230) | 118 (12-1,754) | 24 (2–215) | 77 (0–197) | 144 (11-1,754) |
| Convalescent/acute Ig titer ratio (median OD [range]) | 1.68 (0.07–82) | 2.33 (0.07–82) | 1.43 (12–23) | 0.77 (0.07–0.98) | 1.29 (1.00–1.90) | 4.00 (2.00–82) |
Ig, immunoglobulin; CAP, community-acquired pneumonia.
All data are presented as number (%) of patients, unless otherwise specified. CRB-65, confusion, respiratory rate, blood pressure, age of >65 years; PSI, pneumonia severity index; OD, optical density in arbitrary units.
One or more of the following chronic conditions: chronic obstructive pulmonary disease, heart disease, diabetes mellitus, liver disease, renal insufficiency, neoplasm, and immunosuppression.
Serotypes with low (1, 4, 7F, 9N, 9V, and 14) or high (3, 6B, 11A, 18C, 19A, 19F, and 23F) degrees of encapsulation (Weinberger et al. [12]) were based on 65/70 cases with defined degrees of encapsulation.
Serotypes with low (3, 6B, 19A, 19F, and 23F), medium (4, 9A, 9V, 14, and 18C), or high (1 and 7F) invasive potential (Brueggemann et al. [15]) were based on 61/70 cases with a defined invasive potential.
TABLE 3.
Acute type-specific Ig titer and Ig response to the causative serotype in subpopulations of pneumococcal CAP patientsa
| Subpopulation factorb | No. with factor/no. without factor | Median acute Ig titer (range) (AU): |
P valuec | Median convalescent/acute Ig titer ratio (range): |
P valuec | ||
|---|---|---|---|---|---|---|---|
| With factor | Without factor | With factor | Without factor | ||||
| Age ≥65 yr | 40/30 | 31 (0–185) | 48 (6–243) | 0.62 | 1.38 (0.07–22) | 2.06 (0.16–82) | 0.031 |
| Female | 32/38 | 39 (5–243) | 31 (0–183) | 0.84 | 1.47 (0.16–16) | 1.91 (0.07–82) | 0.39 |
| Comorbidityd | 36/34 | 31 (5–185) | 39 (0–243) | 0.74 | 1.84 (0.07–44) | 1.56 (0.16–82) | 0.64 |
| Smoking | 22/48 | 59 (0–243) | 31 (5–185) | 0.48 | 1.41 (0.16–30) | 2.13 (0.07–82) | 0.82 |
| CRB-65 score, 2–4 | 24/46 | 40 (0–150) | 31 (5–243) | 0.32 | 1.56 (0.07–22) | 1.95 (0.16–82) | 0.14 |
| PSI class IV-V | 28/42 | 36 (0–185) | 35 (6–243) | 0.72 | 1.91 (0.07–44) | 1.59 (0.16–82) | 0.85 |
| Bacteremia | 20/50 | 24 (0–112) | 94 (6–243) | 0.042 | 1.73 (0.07–82) | 1.68 (0.5–30) | 0.73 |
| Serotype with high degree of encapsulatione | 34/31 | 56 (5–243) | 31 (6–183) | 0.20 | 1.25 (0.07–16) | 2.63 (0.16–82) | 0.003 |
| Serotype with low invasive potentialf | 27/34 | 60 (5–243) | 31 (6–183) | 0.058 | 1.31 (0.07–16) | 2.61 (0.16–82) | 0.006 |
Ig, immunoglobulin; CAP, community-acquired pneumonia.
CRB-65, confusion, respiratory rate, blood pressure, age of >65 years; PSI, pneumonia severity index.
Values in bold type represent statistically significant differences at a P value of <0.05.
One or more of the following chronic conditions: chronic obstructive pulmonary disease, heart disease, diabetes mellitus, liver disease, renal insufficiency, neoplasm, and immunosuppression.
Serotypes (n = 65) with low (1, 4, 7F, 9N, 9V, and 14) or high (3, 6B, 11A, 18C, 19A, 19F, and 23F) degree of encapsulation (Weinberger et al. [12]).
Serotypes (n = 61) with low (3, 6B, 19A, 19F, and 23F), medium (4, 9A, 9V, 14, and 18C), or high (1 and 7F) invasive potential (Brueggemann et al. [15]).
The median convalescent Ig titer was 84 AU (range, 0 to 1,754 AU) in the total group of patients (Table 2), 30 (43%) of whom had convalescent titers of >100 AU.
With respect to the antibody responses in all patients, Table 2 shows the patient characteristics divided into three groups, with Ig titer ratios of <1 (n = 17 [24%]), 1 to 1.99 (n = 20 [29%]), and ≥2 (n = 33 [47%]). Although there were no significant differences between the three groups with respect to patient characteristics, the serotype distributions differed (Table 2). Serotypes with a high degree of encapsulation were noted in 73% of the cases with an Ig titer ratio of <1 and in 35% of the cases with an Ig titer ratio of ≥2 (P = 0.016). Serotypes with medium/high invasive potential were noted in 29% of the cases with an Ig titer ratio of <1 and in 70% of the cases with an Ig titer ratio of ≥2 (P = 0.02). As noted in Table 3, patients ≥65 years old had significantly lower median Ig titer ratios than did patients <65 years (1.38 versus 2.06; P = 0.031). When patients with pneumococci isolated from blood, sputum, and the nasopharynx were compared, no difference was observed in the antibody responses (Fig. 2).
Type-specific antibody titers related to bloodstream pneumococcal DNA load.
EDTA plasma was collected in 30 (43%) patients, 10 of whom were bacteremic. PCR for Spn9802 DNA was positive in eight of 30 samples, including four patients with pneumococcal bacteremia (Table 4). The three patients with the highest loads of pneumococcal DNA had the lowest antibody titer ratios.
TABLE 4.
Characteristics of pneumococcal CAP patients with pneumococcal DNA (Spn9802) detected in plasma at admissiona
| No. | Age (yr)/sexb | Comorbidity | Smoking | PSI scorec | Blood culture positivity | Days from disease onset to acute-phase serum | Days from acute- to convalescent-phase serum | Serotype/degree of encapsulation | Spn9802 DNA copies/ml | Ig titer ratio (convalescent/acute)d |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83/F | No | 93 | Yes | 6 | 26 | 19F/high | 1.0 × 1010 | 0.58 (33/57) | |
| 2 | 79/F | No | 97 | Yes | 8 | 24 | 9V/low | 3.0 × 109 | 1.33 (64/48) | |
| 3 | 89/F | Lung disease | No | 79 | Yes | 7 | 58 | 7F/low | 1.8 × 104 | 1.05 (21/20) |
| 4 | 23/M | No | 78 | No | 11 | 34 | 7F/low | 6.2 × 103 | 2.89 (528/183) | |
| 5 | 54/M | Liver disease, lung disease | Yes | 115 | Yes | 4 | 31 | 3/high | 4.5 × 103 | 2.12 (172/81) |
| 6 | 58/M | Yes | 88 | No | 10 | 34 | 3/high | 5.8 × 102 | 1.45 (122/84) | |
| 7 | 31/M | No | 31 | No | 4 | 29 | 7F/low | 4.0 × 102 | 6.86 (48/7) | |
| 8 | 74/M | Heart disease, tumor | No | 124 | No | 2 | 25 | 3/high | 1.8 × 102 | 2.35 (87/37) |
CAP, community-acquired pneumonia.
F, female; M, male.
PSI, pneumonia severity index.
Ig, immunoglobulin.
Type-specific antibody response with respect to degree of encapsulation.
Sixty-five (93%) patients were grouped with respect to a low or high degree of encapsulation of the causative serotype, according to Weinberger et al. (12). Figure 3A shows that the acute Ig titers did not differ between the two groups. A high acute Ig titer (>100 AU) was observed in 11 patients, including three patients with serotypes with a low degree of encapsulation and eight patients with serotypes with a high degree of encapsulation. Interestingly, Ig titer ratios of ≥2 were noted in 3/3 cases with a low degree of encapsulation and in 1/8 cases with a high degree of encapsulation (P = 0.024).
As shown in Fig. 3B, serotypes with a low degree of encapsulation had a significantly higher median Ig titer ratio between paired sera compared with those of serotypes with a high degree of encapsulation (2.63 versus 1.25; P = 0.003). Also, after a log10 transformation of the Ig titer ratios was performed, the patients with serotypes with a low degree of encapsulation had a 2.4-fold (95% confidence interval [CI], 1.4 to 4.4; P = 0.003) higher mean Ig titer ratio than that of patients with serotypes with a high degree of encapsulation, and after adjustment for age, this difference was still 2.2-fold higher (95% CI, 1.2 to 3.8; P = 0.01).
Type-specific antibody response with respect to invasive potential.
Sixty-one (87%) patients were grouped with respect to the low, medium, or high invasive potential of the causative serotype, according to Brueggemann et al. (15). Figure 4 shows that there was no significant difference in the Ig titer ratios when serotypes with low (1.31) invasive potential were compared with serotypes with medium (2.22; P = nonsignificant [NS]) or high (3.70; P = NS) invasive potential. However, the Ig titer ratio was lower in patients infected with serotypes with low invasive potential than that in patients infected with serotypes with medium/high invasive potential in combination (1.31 versus 2.61; P = 0.006) (Table 3). Also, after a log10 transformation of the Ig titer ratios was performed, the patients with serotypes with a medium/high invasive potential had a 2.4-fold (95% CI, 1.3 to 4.4; P = 0.006) higher mean Ig titer ratio than that of patients with serotypes with a low invasive potential, and after adjustment for age, this difference was still 1.9-fold higher (95% CI, 1.55 to 3.65; P = 0.036).
FIG 4.
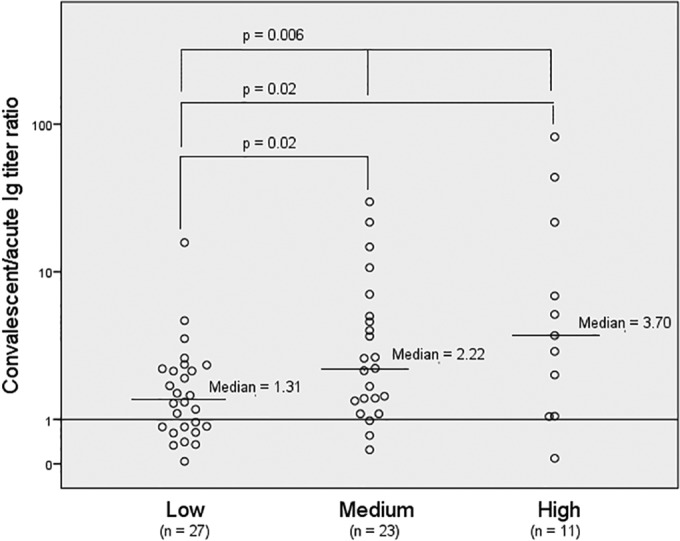
Serotype-specific immunoglobulin (Ig) response (convalescent/acute Ig titer ratio) to the causative serotype in pneumococcal pneumonia patients with respect to the invasive potential of the serotype. In 61 of the 70 included patients in the study, a low (3, 6B, 19A, 19F, and 23F), medium (4, 9N, 9V, 14, and 18C), or high (1 and 7F) invasive potential of the serotype was defined, based on Fig. 3 in the study by Brueggemann et al. (15).
Type-specific antibody level ratios in controls.
Paired sera were collected in 90 of 113 enrolled controls, four of whom (4.4%) (median age, 64 years; range, 39 to 75 years; 50% female) had S. pneumoniae isolated in the nasopharynx with serotypes covered by the PPV23. These four patients were admitted for elective surgery (n = 2; serotypes 3 and 11A), spondylitis (6B), and urinary tract infection (19F). The median time duration between acute- and convalescent-phase sera in the four controls was 46 days (range, 29 to 59 days), the median acute Ig titer was 101 AU (range, 15 to 226 AU), the median convalescent titer was 120 AU (range, 25 to 351 AU), and the median Ig titer ratio was 1.29 (range, 0.76 to 2.25). One control (11A), who developed airway symptoms between serum samplings, had an Ig titer ratio of 0.91. None of the other three controls developed respiratory symptoms during the hospital stay.
DISCUSSION
In this prospective study, we observed the greatest antibody response in pneumococcal CAP patients infected with serotypes with a low degree of encapsulation, as well as serotypes with medium/high invasive potential. These findings were opposite of our hypotheses. However, the results could not be explained by age or other patient characteristics. With few exceptions, these immunogenic serotypes, with thin capsules (1, 4, 7F, 9N, 9V, and 14) and medium/high invasiveness (1, 4, 7F, 9N, 9V, 14, and 18C), are the same serotypes.
Antibodies to CPS are naturally acquired through colonization and infection, with elevated titers often detected in healthy individuals (14, 28, 29). However, naturally acquired antibodies may lack in functionality, which might explain why such antibodies appear to lack in protection against disease (30). In the evaluations of the ELISA used in the present study (21, 23), the median Ig titer (geometric mean [GM] Ig titer to serotypes 1, 4, 7F, 14, 18C, and 19F) in 15 healthy individuals prior to vaccination was 32 AU (range, 8 to 61 AU), and 4 weeks after vaccination with the PPV23, the median Ig titer was 63 AU (range, 20 to 113 AU). In the present study of patients with pneumococcal pneumonia, we observed a median acute Ig titer of 36 AU, similar to that of the nonvaccinated population in the evaluation study, and a median convalescent titer of 84 AU after 4 weeks (Table 2). The WHO Collaborating Center for Reference and Research on Pneumococci at the SSI (Copenhagen, Denmark) previously used this ELISA for an evaluation of vaccine effectiveness and as a guide for pneumococcal revaccination (21, 23). Revaccination was recommended if the GM titer was <25 AU or <40 AU, with more than one of the six type-specific antibody levels being <25 AU. A GM titer of >40 AU was considered to be protective (21, 23). In our study of patients with pneumococcal pneumonia, we found that 47% (33/70) had acute Ig titers of ≥40 AU (Table 2). In comparison, Musher et al. (31) observed that one-third of patients with pneumococcal pneumonia were considered to have protective antibody titers at admission. However, we found no differences in the patient characteristics between the groups with Ig titers of <40 AU and ≥40 AU (Table 2).
Surprisingly, only 47% (33/70) of the patients responded with a ≥2-fold increase in Ig titer to their infective serotype, and as many as 24% (24/70) of the patients showed a decrease in Ig titer between paired sera (Ig titer ratio, <1; Table 2). In previous vaccination studies, repeated and high doses of bacterial antigens have caused hyporesponsiveness, presumably due to the depletion of the reactive B-cell pool (32). Accordingly, in adult patients with pneumococcal pneumonia, Coonrod and Drennan (9) noted a reduced and/or delayed production of anti-pneumococcal antibodies in cases with detected pneumococcal antigenemia, although a significant antibody response was often noted in cases without detected antigenemia. In the present study, the three patients with the highest levels of pneumococcal DNA in plasma had the lowest antibody responses (i.e., the lowest Ig titer ratios; Table 4). In addition, serotypes with a high degree of encapsulation were predominant in the group of patients with an Ig titer decrease between paired sera (Ig titer ratio, <1; Table 2). These results indicate that a high load of pneumococcal antigen, caused by infection with highly encapsulated serotypes or a large burden of S. pneumoniae cells, is associated with a low antibody response and/or decreasing antibody titers in pneumococcal pneumonia.
During an episode of colonization, the immunogenic capsule may interact with the mucosal surface in the nasopharynx and, thereby, induce anti-CPS antibodies in healthy subjects (14, 28). These antibodies may be protective against IPD (4). Also, existing antibodies might allow the rapid elimination of circulating bacteria, resulting in negative blood cultures in pneumococcal disease. We observed that nonbacteremic patients had higher acute Ig titers than those of bacteremic patients (median titers, 24 versus 94 AU, respectively; P = 0.042) (Table 3), suggesting that previously acquired antibodies may provide protection against bacteremia.
In this study, we considered a positive nasopharyngeal culture for S. pneumoniae to be a possible true etiology of CAP, as recommended by Swedish guidelines (33). Cultures of blood and representative sputum are not sensitive enough to identify the etiology in the majority of cases of pneumococcal pneumonia (34). Thus, sensitive methods using nasopharyngeal samples for enhanced diagnostic yields might be useful (35). This approach is supported by a low pneumococcal carriage rate in healthy adult populations in northern Europe (36, 37), as well as in our control group (38). Furthermore, we found no difference in antibody responses with respect to the sampling site, as noted in Fig. 2.
We grouped the serotypes according to previous findings by Weinberger et al. and Brueggemann et al. (12, 15) to enable appropriate statistical analyses, as the numbers of each serotype were too small for individual comparisons. Therefore, the results of this study could not be transferred to individual serotypes but should be used for understanding common characteristics for groups of serotypes. We used the ELISA described by Konradsen (21), which was a validated method for pneumococcal antibody measurement prior to the establishment of the WHO ELISA (20–22). The guidance protocol of the ELISA used in the present study is similar to that of the WHO ELISA, using ATCC polysaccharides for coating and a standard serum that correlates with the international standard serum 89SF, and it was used for patient evaluation in another recent study (25). However, the presentation of antibody concentrations in AU instead of in μg/ml is a difference between the two guidance protocols, but it should not negatively affect the reliability of the results of the present study. Furthermore, in the ELISA, all sera were adsorbed with CWPS alone, but adsorption with 22F was not performed, as this was not the internationally accepted standard procedure at the time of the study. However, the 22F adsorption effect has been shown to be inferior to the CWPS adsorption effect (39). The absence of 22F adsorption was generalized in all sera in the study population, as well as in the standard serum; therefore, the impact of nonspecific binding should be equal in both groups, and the correlation between OD measurements is not likely to be influenced.
In conclusion, a high anti-CPS antibody response was noted in patients with pneumococcal pneumonia caused by serotypes with a low degree of encapsulation and/or medium/high invasive potential. A low antibody response and/or decreased antibody titers were associated with serotypes with a high degree of encapsulation and low invasive potential, as well as a high pneumococcal DNA load in plasma. Furthermore, patients with nonbacteremic pneumococcal pneumonia had higher antibody titers at admission than those of patients with bacteremic pneumococcal pneumonia, indicating that the antibodies are probably protective against invasive disease.
ACKNOWLEDGMENTS
This study was supported by grants from the Research Committee of Örebro County Council (OLL-137011).
We thank Eva Törnqvist and the Department of Laboratory Medicine, Microbiology, Örebro University Hospital, for excellent technical assistance.
We declare no conflicts of interest.
Footnotes
Published ahead of print 17 September 2014
REFERENCES
- 1. Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. 1996. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 275:134–141.8531309 [Google Scholar]
- 2. Watson DA, Musher DM, Verhoef J. 1995. Pneumococcal virulence factors and host immune responses to them. Eur. J. Clin. Microbiol. Infect. Dis. 14:479–490. 10.1007/BF02113425. [DOI] [PubMed] [Google Scholar]
- 3. Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, Nahm MH. 2012. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J. Biol. Chem. 287:27885–27894. 10.1074/jbc.M112.380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154. 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 5. Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187–195. [DOI] [PubMed] [Google Scholar]
- 6. Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Käyhty H, Karma P, Kohberger R, Siber G, Mäkelä PH, Finnish Otitis Media Study Group 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403–409. 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453–1460. 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 8. Kalin M, Lindberg AA. 1985. Antibody response against the type specific capsular polysaccharide in pneumococcal pneumonia measured by enzyme linked immunosorbent assay. Scand. J. Infect. Dis. 17:25–32. 10.3109/00365548509070416. [DOI] [PubMed] [Google Scholar]
- 9. Coonrod JD, Drennan DP. 1976. Pneumococcal pneumonia: capsular polysaccharide antigenemia and antibody responses. Ann. Intern. Med. 84:254–260. 10.7326/0003-4819-84-3-254. [DOI] [PubMed] [Google Scholar]
- 10. Zysk G, Bethe G, Nau R, Koch D, Gräfin von Bassewitz VCDH, Heinz HP, Reinert RR. 2003. Immune response to capsular polysaccharide and surface proteins of Streptococcus pneumoniae in patients with invasive pneumococcal disease. J. Infect. Dis. 187:330–333. 10.1086/367701. [DOI] [PubMed] [Google Scholar]
- 11. van Mens SP, Meijvis SC, Endeman H, van Velzen-Blad H, Biesma DH, Grutters JC, Vlaminckx BJ, Rijkers GT. 2011. Longitudinal analysis of pneumococcal antibodies during community-acquired pneumonia reveals a much higher involvement of Streptococcus pneumoniae than estimated by conventional methods alone. Clin. Vaccine Immunol. 18:796–801. 10.1128/CVI.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weinberger DM, Trzciński K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. 2009. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 5:e1000476. 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinberger DM, Dagan R, Givon-Lavi N, Regev-Yochay G, Malley R, Lipsitch M. 2008. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J. Infect. Dis. 197:1511–1518. 10.1086/587941. [DOI] [PubMed] [Google Scholar]
- 14. Musher DM, Groover JE, Reichler MR, Riedo FX, Schwartz B, Watson DA, Baughn RE, Breiman RF. 1997. Emergence of antibody to capsular polysaccharides of Streptococcus pneumoniae during outbreaks of pneumonia: association with nasopharyngeal colonization. Clin. Infect. Dis. 24:441–446. 10.1093/clinids/24.3.441. [DOI] [PubMed] [Google Scholar]
- 15. Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203–1211. 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 16. Strålin K, Kaltoft MS, Konradsen HB, Olcén P, Holmberg H. 2004. Comparison of two urinary antigen tests for establishment of pneumococcal etiology of adult community-acquired pneumonia. J. Clin. Microbiol. 42:3620–3625. 10.1128/JCM.42.8.3620-3625.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. 1999. Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC. [Google Scholar]
- 18. Kalin M, Lindberg AA, Tunevall G. 1983. Etiological diagnosis of bacterial pneumonia by Gram stain and quantitative culture of expectorates. Leukocytes or alveolar macrophages as indicators of sample representativity. Scand. J. Infect. Dis. 15:153–160. [DOI] [PubMed] [Google Scholar]
- 19. Sørensen UB. 1993. Typing of pneumococci by using 12 pooled antisera. J. Clin. Microbiol. 31:2097–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quataert SA, Kirch CS, Wiedl LJ, Phipps DC, Strohmeyer S, Cimino CO, Skuse J, Madore DV. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konradsen HB. 1995. Quantity and avidity of pneumococcal antibodies before and up to five years after pneumococcal vaccination of elderly persons. Clin. Infect. Dis. 21:616–620. 10.1093/clinids/21.3.616. [DOI] [PubMed] [Google Scholar]
- 22. Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert SA, Hildreth S, Sikkema DJ, Käyhty H, Jonsdottir I, Nahm MH. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514–519. 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Konradsen HB, Sørensen UB, Henrichsen J. 1993. A modified enzyme-linked immunosorbent assay for measuring type-specific anti-pneumococcal capsular polysaccharide antibodies. J. Immunol. Methods 164:13–20. 10.1016/0022-1759(93)90270-H. [DOI] [PubMed] [Google Scholar]
- 24. Skovsted IC, Kerrn MB, Sonne-Hansen J, Sauer LE, Nielsen AK, Konradsen HB, Petersen BO, Nyberg NT, Duus JØ. 2007. Purification and structure characterization of the active component in the pneumococcal 22F polysaccharide capsule used for adsorption in pneumococcal enzyme-linked immunosorbent assays. Vaccine 25:6490–6500. 10.1016/j.vaccine.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 25. Hinge M, Ingels HA, Slotved HC, Mølle I. 2012. Serologic response to a 23-valent pneumococcal vaccine administered prior to autologous stem cell transplantation in patients with multiple myeloma. APMIS 120:935–940. 10.1111/j.1600-0463.2012.02922.x. [DOI] [PubMed] [Google Scholar]
- 26. Abdeldaim G, Herrmann B, Mölling P, Holmberg H, Blomberg J, Olcén P, Strålin K. 2010. Usefulness of real-time PCR for lytA, ply, and Spn9802 on plasma samples for the diagnosis of pneumococcal pneumonia. Clin. Microbiol. Infect. 16:1135–1141. 10.1111/j.1469-0691.2009.03069.x. [DOI] [PubMed] [Google Scholar]
- 27. Aickin M, Gensler H. 1996. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am. J. Public Health 86:726–728. 10.2105/AJPH.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soininen A, Pursiainen H, Kilpi T, Käyhty H. 2001. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J. Infect. Dis. 184:569–576. 10.1086/322794. [DOI] [PubMed] [Google Scholar]
- 29. Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, Melegaro A, Pebody R, George R, Soininen A, Edmunds J, Gay N, Käyhty H, Miller E. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J. Infect. Dis. 192:387–393. 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 30. Musher DM, Chapman AJ, Goree A, Jonsson S, Briles D, Baughn RE. 1986. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 154:245–256. 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 31. Musher DM, Phan HM, Watson DA, Baughn RE. 2000. Antibody to capsular polysaccharide of Streptococcus pneumoniae at the time of hospital admission for pneumococcal pneumonia. J. Infect. Dis. 182:158–167. 10.1086/315697. [DOI] [PubMed] [Google Scholar]
- 32. O'Brien KL, Hochman M, Goldblatt D. 2007. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect. Dis. 7:597–606. 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 33. Spindler C, Strålin K, Eriksson L, Hjerdt-Goscinski G, Holmberg H, Lidman C, Nilsson A, Ortqvist A, Hedlund J, Community Acquired Pneumonia Working Group of The Swedish Society of Infectious Diseases 2012. Swedish guidelines on the management of community-acquired pneumonia in immunocompetent adults–Swedish Society of Infectious Diseases 2012. Scand. J. Infect. Dis. 44:885–902. 10.3109/00365548.2012.700120. [DOI] [PubMed] [Google Scholar]
- 34. Ruiz-González A, Falguera M, Nogués A, Rubio-Caballero M. 1999. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? A microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am. J. Med. 106:385–390. [DOI] [PubMed] [Google Scholar]
- 35. Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. 2010. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 50:202–209. 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gunnarsson RK, Holm SE, Söderström M. 1998. The prevalence of potential pathogenic bacteria in nasopharyngeal samples from healthy children and adults. Scand. J. Prim. Health Care 16:13–17. 10.1080/028134398750003340. [DOI] [PubMed] [Google Scholar]
- 37. Palmu AA, Kaijalainen T, Saukkoriipi A, Leinonen M, Kilpi TM. 2012. Nasopharyngeal carriage of Streptococcus pneumoniae and pneumococcal urine antigen test in healthy elderly subjects. Scand. J. Infect. Dis. 44:433–438. 10.3109/00365548.2011.652162. [DOI] [PubMed] [Google Scholar]
- 38. Athlin S, Strålin K. 2013. The Binax NOW Streptococcus pneumoniae test applied on nasopharyngeal aspirates to support pneumococcal aetiology in community-acquired pneumonia. Scand. J. Infect. Dis. 45:425–431. 10.3109/00365548.2012.760843. [DOI] [PubMed] [Google Scholar]
- 39. Slotved HC, Guttmann C, Pedersen CD, Jacobsen JN, Krogfelt KA. 2009. Evaluation of the specificity of pneumococcal polysaccharide enzyme-linked immunosorbent assay and the effect of serum adsorption based on standard pneumococcal serogroup- or serotype-specific rabbit antisera. Clin. Vaccine Immunol. 16:1279–1284. 10.1128/CVI.00143-09. [DOI] [PMC free article] [PubMed] [Google Scholar]