Abstract
Infectious hemorrhagic fevers caused by the Marburg and Ebola filoviruses result in human mortality rates of up to 90%, and there are no effective vaccines or therapeutics available for clinical use. The highly infectious and lethal nature of these viruses highlights the need for reliable and sensitive diagnostic methods. We assembled a protein microarray displaying nucleoprotein (NP), virion protein 40 (VP40), and glycoprotein (GP) antigens from isolates representing the six species of filoviruses for use as a surveillance and diagnostic platform. Using the microarrays, we examined serum antibody responses of rhesus macaques vaccinated with trivalent (GP, NP, and VP40) virus-like particles (VLP) prior to infection with the Marburg virus (MARV) (i.e., Marburg marburgvirus) or the Zaire virus (ZEBOV) (i.e., Zaire ebolavirus). The microarray-based assay detected a significant increase in antigen-specific IgG resulting from immunization, while a greater level of antibody responses resulted from challenge of the vaccinated animals with ZEBOV or MARV. Further, while antibody cross-reactivities were observed among NPs and VP40s of Ebola viruses, antibody recognition of GPs was very specific. The performance of mucin-like domain fragments of GP (GP mucin) expressed in Escherichia coli was compared to that of GP ectodomains produced in eukaryotic cells. Based on results with ZEBOV and MARV proteins, antibody recognition of GP mucins that were deficient in posttranslational modifications was comparable to that of the eukaryotic cell-expressed GP ectodomains in assay performance. We conclude that the described protein microarray may translate into a sensitive assay for diagnosis and serological surveillance of infections caused by multiple species of filoviruses.
INTRODUCTION
The first recorded outbreak of Marburg virus (MARV) took place in 1967 in Germany and Yugoslavia and was traced to infected African green monkeys from Uganda (1), while the first outbreaks of Ebola virus were documented in Sudan and the Democratic Republic of Congo in 1976 (2, 3). Cycles of filovirus outbreaks continue to be a major concern from a biodefense and public health perspective as no licensed therapeutic agents or vaccines are available. Filoviral hemorrhagic fever is characterized by rapid disease onset and mortality rates of up to 90% (4). Following an incubation period that can range from 2 to 21 days, infected patients commonly develop nonspecific flulike symptoms of fever, vomiting, loss of appetite, headache, abdominal pain, fatigue, and diarrhea, while bleeding occurs in a smaller number of infections (1, 3, 5). Case fatalities are associated with reduced adaptive immune responses (6, 7) and the release of high levels of immune response mediators (8–10) that contribute to vascular dysfunction, coagulation disorders, shock, and eventual multiorgan failure (2).
There is a persistent need for sensitive and reliable serological approaches for examining filoviral infections. Because genetic material from the pathogen is often missing, antibody detection methods are indispensable, especially for examining nonviremic patients and for disease surveillance. While enzyme-linked immunosorbent assays (ELISAs) for detecting specific IgG and IgM based on live virus preparation were previously developed (11–13), the need for biosafety level 4 (BSL4) labs and associated safety issues are major limitations. Serological assays based on recombinant filovirus antigens are alternatives that do not require infectious agents, and several ELISAs were reported (14–18). For example, Nakayama and coworkers developed a glycoprotein (GP)-based ELISA representative of all six species of filoviruses and analyzed human patient sera from Ebola and Marburg virus outbreaks (15). However, these previous methods have only addressed a limited number of antigens and species of filoviruses. The Filoviridae family includes one species of Marburg virus (Marburg marburgvirus) and five species of Ebola virus {Sudan ebolavirus [Sudan virus (SEBOV)], Zaire ebolavirus [Zaire virus (ZEBOV)], Reston ebolavirus [Reston virus (REBOV)], Bundibugyo ebolavirus [Bundibugyo virus (BEBOV)], and Tai Forest ebolavirus [Tai Forest virus (TAFV)]}, each of which can cause severe hemorrhagic fevers in primates, including humans (2). Further complicating assay development, the single-stranded, negative-sensed RNA genome (∼19 kb) encodes seven structural proteins (1, 19, 20) that are all potential antigens: the nucleoprotein (NP), virion protein 35 (VP35), VP40, glycoprotein (GP), VP30, VP24, and RNA-dependent RNA polymerase (L). The major functions of each component of the viral proteome were previously characterized. The RNA genome is encapsulated by the NP, and the ribonucleoprotein complex is associated with VP35, VP30, and L (21, 22). Transcription and replication of the viral genome requires L, NP, and VP35 (23), while transcription for Ebola virus but not for Marburg virus requires VP30 as an additional cofactor (24, 25). VP40 is a matrix protein critical for virion assembly as well as budding from infected cells (26, 27), and VP24 appears to play a role in nucleocapsid assembly and inhibition of interferon signaling (28–30). Unlike Marburg GP, Ebola GP is expressed following RNA editing, while the unedited transcript encodes a soluble GP that is released from infected cells (31, 32). Further, trimeric GP complexes on the virion surface are receptors for fusion and entry into the host cell (33–35).
Here, we adopted a protein microarray strategy for detection of filovirus antibodies in sera. The microarray is composed of NP, GP, and VP40 from all Ebola and Marburg virus species, as well as several control proteins. We evaluated the performance of this assay by using sera collected from rhesus macaques that were treated with filovirus vaccines and challenged with Ebola and Marburg viruses. We further examined the potential use of antigens that were expressed exclusively in Escherichia coli.
MATERIALS AND METHODS
Cloning.
Full-length genes for NP and VP40 and the GP mucin-like domain fragment (GP mucin) for six filovirus species, Reston ebolavirus, Bundibugyo ebolavirus, Zaire ebolavirus, Sudan ebolavirus, Tai Forest ebolavirus, and Marburg marburgvirus were cloned into the pENTR/TEV/D-TOPO vector (Life Technologies, Grand Island, NY) and sequence verified. The nucleotide substitutions found in the cloned sequence compared with that in the reference sequence from GenBank are summarized in Table S1 in the supplemental material. All entry vector clones were shuttled into destination E. coli expression vectors via the LR reaction (LR Clonase II; Life Technologies). Specifically, VP40 and GP mucin open reading frames (ORFs) were shuttled into pDEST-HisMBP (Addgene plasmid 11085) containing an N-terminal His-tagged maltose-binding protein (HisMBP) tag, while all NP ORFs were shuttled into pDEST17 (Life Technologies) containing an N-terminal His tag.
Protein expression and purification.
Proteins were expressed in either BL21-AI cells (Life Technologies) or Rosetta 2(DE3) cells (EMD Millipore, Billerica, MA). Expression for pDEST-HisMBP constructs was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), while expression for the pDEST17 constructs was induced with 0.2% l-arabinose. Pelleted cells from overnight cultures grown at 18°C were lysed using B-PER reagent (Thermo Scientific, Rockford, IL) supplemented with 2× Halt protease and phosphatase inhibitor cocktail, EDTA-free (Thermo Scientific), 0.2 mg/ml lysozyme, 50 to 100 U/ml DNase I (Thermo Scientific), and 2 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were separated into supernatant and insoluble pellet fractions by centrifugation, and induced protein expression was confirmed through Western blotting or mass spectrometry and Coomassie staining. HisMBP-tagged VP40s and GP mucins were soluble and present in the supernatant fraction. With the exception of ZEBOV NP (Zaire NP), all His-tagged NPs were insoluble and predominantly in the pellet fraction. Supernatants containing expressed VP40s were loaded onto HisTrap HP columns (GE Healthcare, Piscataway, NJ) preequilibrated with 20 mM sodium phosphate, 0.5 M NaCl, 40 mM imidazole (pH 7.4). VP40 fractions were collected by applying an imidazole step elution. All GP mucins except MARV GP mucin (Marburg GP mucin) were purified using HisTrap HP columns. Binding and washing steps were conducted with 25 mM HEPES, 0.5 M NaCl, 25 mM imidazole (pH 8), and bound GP mucins were eluted using an imidazole gradient. Marburg GP mucin was purified using an MBPTrap HP column preequilibrated with 25 mM HEPES, 0.2 M NaCl, 1 mM EDTA (pH 7.4). Bound protein was eluted using 25 mM HEPES, 0.2 M NaCl, 1 mM EDTA, 10 mM maltose (pH 7.4). NPs were purified through on-column refolding on HisTrap HP columns. Briefly, NP pellets were resolubilized in 25 mM HEPES, 0.2 M NaCl, 25 mM imidazole, 1 mM β-mercaptoethanol, 6 M guanidine hydrochloride (pH 8). Proteins were bound to columns under denaturing conditions and refolded using a 6 to 0 M urea gradient over a 30-column volume range. Refolded proteins were eluted using an imidazole gradient. Although Zaire NP was found in the supernatant, the protein did not appear to bind to the HisTrap column under the conditions used for VP40 purification. This may have been due to a hidden His tag, and, thus, guanidine hydrochloride was added directly to the Zaire NP supernatant to a final concentration of 6 M in order to expose the His tag. Denatured Zaire NP was processed in a manner similar to that for the other resolubilized NPs. The purities and concentrations of collected fractions were measured by an Agilent protein 230 kit (Agilent Technologies). All purified proteins were stored at −20°C in their respective elution buffers with glycerol added to a final concentration of 25%.
Microarray printing.
The purified recombinant proteins were spotted (see Fig. S1 in the supplemental material) on nitrocellulose-coated FAST slides (KeraFAST, Boston, MA), using a contactless inkjet microarray printer (ArrayJet, Edinburgh, Scotland). The microarray included a total of 34 proteins: (i) E. coli-expressed filovirus antigens, (ii) insect cell-expressed ZEBOV and Sudan ebolavirus and Marburg virus (Angola) GP ectodomain (ΔTM) (IBT Bioservices, Gaithersburg, MD), (iii) mammalian cell-expressed Marburg virus (Musoke) GP ΔTM (IBT Bioservices), (iv) human, monkey, mouse, rabbit, and goat IgG (Rockland Immunochemicals, Gilbertsville, PA), (v) human, monkey, and rabbit IgM (Rockland Immunochemicals), (vi) HisMBP (ProteinOne, Rockville, MD), (vii) dengue virus serotype 2 (dengue2) and 3 (dengue3) nonstructural protein 1 (NS1), and (viii) bovine serum albumin (BSA) (Thermo Scientific). The purified dengue virus proteins were previously described (36). Briefly, the proteins were expressed with a HisMBP tag in E. coli and purified via immobilized metal affinity chromatography. Each protein was printed in triplicate. All purified proteins were diluted to 200 ng/μl in printing buffer (25 mM HEPES, 0.5 M NaCl, 25% glycerol, 1 mM dithiothreitol [DTT] [pH 8]). Alexa Fluor 647-conjugated streptavidin (Life Technologies) was diluted 1:50 in printing buffer and included in the microarray as a reference marker. The buffer served as an empty placeholder on the microarray. Printed slides were desiccated overnight under vacuum and stored at −20°C.
Microarray processing.
All microarray processing steps were performed under 21°C conditions, and each antibody or serum sample was processed in duplicate microarrays. Printed microarrays were incubated for 1 h in 1× Biacore Flexchip blocking buffer (GE Healthcare) with 2% normal goat serum (Vector Laboratories, Burlingame, CA) or 2% normal rabbit serum. Microarrays were washed 3 times for 5 min each with wash buffer (1× Tris-buffered saline [TBS], 0.2% Tween 20, 3% BSA), which was used in all subsequent wash steps. Microarrays were incubated with the primary antibody diluted 1:1,000 or a serum sample diluted 1:150 in probe buffer (1× TBS, 0.1% Tween 20, 3% BSA). After a 1-h incubation in the primary antibody or sera, microarrays were washed and incubated for 1 h with Alexa Fluor 647-conjugated secondary antibodies diluted 1:2,000 in probe buffer. Microarrays were washed and then rinsed with water before analysis.
Vaccinations and infections.
Rhesus macaque sera were obtained from two separate vaccine studies for ZEBOV and MARV (our unpublished data). The vaccine trials were similar in design and procedure to a study previously described by Warfield et al. (37). Briefly, for the ZEBOV study, five animals were vaccinated with ZEBOV virus-like particles (VLP) and MARV VLP. The vaccinated animals were subsequently challenged with ZEBOV. Three serum samples (naive, postimmunization, and postchallenge) were collected for each animal. The Marburg virus study was conducted in a similar manner, except that the animals were vaccinated with MARV VLP and challenged with MARV.
Antibodies.
Rabbit polyclonal anti-ZEBOV NP (anti-Zaire NP; 0301-012), mouse monoclonal anti-SEBOV GP (anti-Sudan GP; 0202-029), mouse monoclonal anti-SEBOV VP40 (anti-Sudan VP40; 0202-018), mouse monoclonal anti-ZEBOV GP (anti-Zaire GP; 0201-020), rabbit polyclonal anti-ZEBOV VP40 (anti-Zaire VP40; 0301-010), mouse monoclonal anti-Marburg virus (Musoke) GP (anti-Marburg GP; 0203-023), and mouse monoclonal anti-Marburg virus (Musoke) VP40 (anti-Marburg VP40; 0203-012) antibodies were purchased from IBT Bioservices. Alexa Fluor 647-conjugated goat anti-mouse IgG (A21237) and goat anti-rabbit IgG (A21244) antibodies were purchased from Life Technologies. Alexa Fluor 647-conjugated rabbit anti-monkey IgG (bs-00335R-A647) and rabbit anti-monkey IgM (bs-0336R-A647) antibodies were purchased from Bioss (Woburn, MA).
Data acquisition and analysis.
Processed slides were scanned at a 635-nm wavelength using a GenePix 4400A scanner (Molecular Devices, Sunnyvale, CA). Acquired images were analyzed with GenePix Pro 7 software. Any defective or missing spots were removed from further analysis. The median fluorescence intensity for each microarray spot was corrected through local background subtraction on GenePix Pro 7. Subsequent analysis was done in Microsoft Excel and R. The resulting background-corrected fluorescence intensities were averaged across replicate spots and quantile normalized for each serum group (naive, immunized, and challenged). A paired t test was conducted to compare each antigen-antibody signal for naive versus immunized, naive versus challenged, and immunized versus challenged sera.
RESULTS
Filovirus protein microarray.
Taking into consideration the complexity of the viral proteome and previous data suggesting potential targets of host antibody responses (14–16, 38), we developed a microarray composed of a minimal set of proteins representative of all Marburg and Ebola virus species. The VP40s and NPs for Reston ebolavirus, Bundibugyo ebolavirus, Zaire ebolavirus, Sudan ebolavirus, and Tai Forest ebolavirus and MARV were expressed as full-length recombinant proteins in E. coli. Initially, we prepared GP ectodomain (ΔTM) constructs from all filovirus species for expression in E. coli. However, because the GP ΔTM proteins were not all stable in solution (data not shown), the coding sequences were truncated and expressed as more stable, GP mucin-like domain fragments (see Table S1 in the supplemental material), with HisMBP fusion tags (amino termini). The final GP protein design was supported by data from previous reports suggesting that the antibody responses to ZEBOV were directed at least in part against the GP mucin-like domain (39–42).
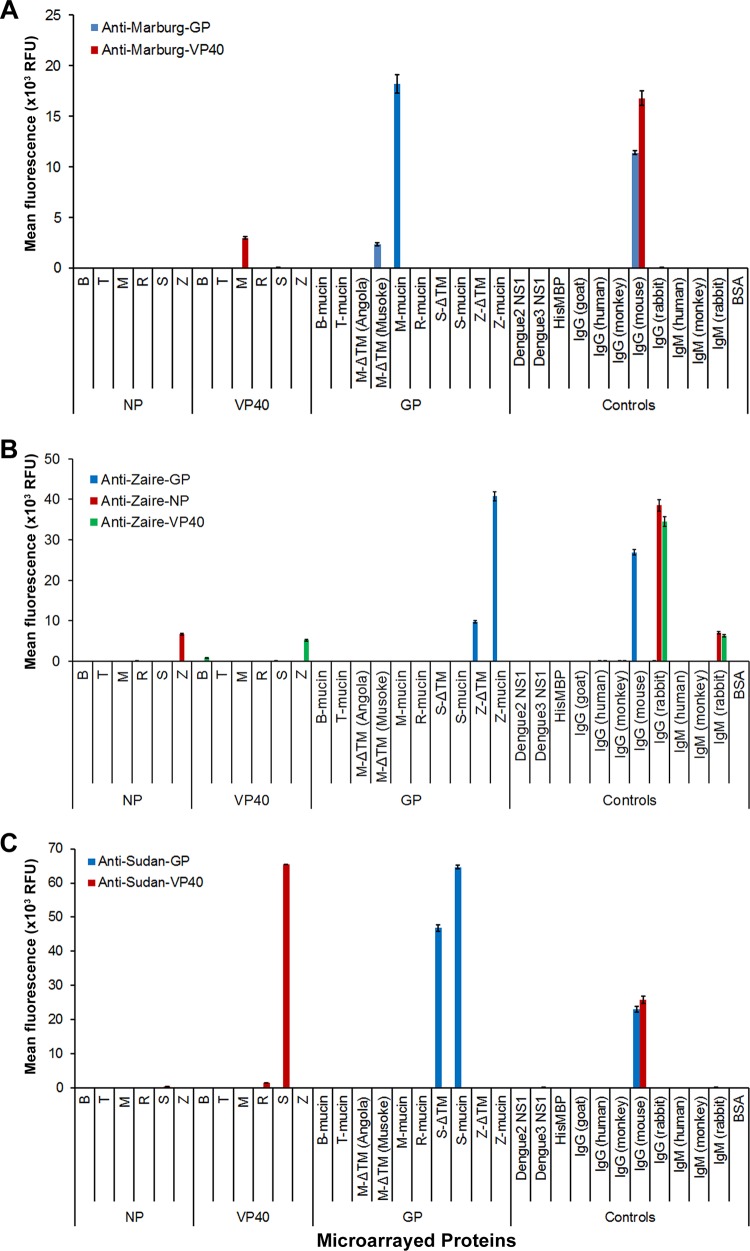
The recombinant filovirus antigens purified from E. coli, along with the control proteins, were printed in 120- to 130-μm diameter spots in a 12 × 12 format (see Fig. S1 in the supplemental material) on slides covered with a thin layer of nitrocellulose. Additionally, GP ΔTM produced in eukaryotic host cells were included in the microarray for comparison with the E. coli-produced GP mucins. IgGs (monkey, human, rabbit, goat, and mouse) and IgMs (human, monkey, and rabbit), HisMBP, BSA, and dengue virus proteins served as controls. For quality control purposes and to validate our assay design, printed microarrays were probed with anti-His antibody and with a panel of purified filovirus antibodies. Probing with anti-His antibody showed that all His-tagged proteins were successfully spotted and adsorbed onto the nitrocellulose-coated microarray slides (data not shown). Anti-Marburg VP40, anti-Marburg GP, anti-Sudan VP40, anti-Sudan GP, anti-Zaire VP40, anti-Zaire NP, and anti-Zaire GP were bound by their target antigens with a high degree of specificity (Fig. 1A, B, and C). Minor cross-reactivities between REBOV VP40 and Sudan VP40 and between BEBOV VP40 and ZEBOV VP40 were observed when microarrays were probed with anti-SEBOV VP40 and anti-ZEBOV VP40, respectively (Fig. 1B and C). Combined, data from these control antibodies indicate that the filovirus microarrays performed correctly under idealized test conditions.
FIG 1.
Validation of filovirus microarrays using control antibodies. A panel of antibodies against Marburg virus (A), Zaire ebolavirus (B), and Sudan ebolavirus (C) proteins were tested on printed microarrays. All antibodies are mouse monoclonal except for anti-Zaire NP and VP40, which are both rabbit polyclonal. Bound antibodies were detected fluorescently on a microarray scanner. Background-corrected fluorescence intensities were averaged across technical replicates on the microarrays. The bars represent mean fluorescence (relative fluorescence units [RFU]) ± standard error of the mean (SEM). All GP ΔTMs were expressed in insect cells except for Marburg GP ΔTM (Musoke), which was expressed in mammalian cells. B, Bundibugyo; T, Tai Forest; M, Marburg; R, Reston; S, Sudan; Z, Zaire.
Analysis of sera from ZEBOV and MARV challenge studies.
Sera from two separate animal studies were analyzed using our microarrays. In the ZEBOV study, rhesus macaques were vaccinated with a mixture of trivalent (GP, NP, and VP40) virus-like particles (VLP) for MARV and ZEBOV and subsequently challenged with ZEBOV. In the Marburg virus study, rhesus macaques were vaccinated with trivalent (GP, NP, and VP40) VLP for MARV and subsequently challenged with MARV. All vaccinated animals in the Zaire and Marburg studies survived the viral challenge. After application of the serum samples to the filovirus microarray, bound IgGs were detected using fluorescently labeled secondary antibodies (see Fig. S2 and S3 in the supplemental material).
For the ZEBOV study, the comparisons between sera from naive and immunized animals showed significant increases (P < 0.05) in IgGs against all vaccine antigens except for MARV NP (Marburg NP) (Fig. 2A). Cross-reactive IgGs against BEBOV, TAFV, REBOV, and SEBOV VP40 and BEBOV and TAFV NP were induced through vaccination (Fig. 2A). After animals were challenged, IgG signals against all Ebola virus NPs and VP40s and ZEBOV GP mucin (Zaire GP mucin) had significant increases (P < 0.005) in challenged sera compared to those in immunized sera (Fig. 2A). For the Marburg virus study, the microarrays detected significant increases (P < 0.05) in IgGs against Marburg NP, GP mucin, and VP40 in immunized sera compared to those in naive sera (Fig. 2B). Cross-reactive IgGs against all Ebola virus VP40s were detected in the immunized sera (Fig. 2B). We observed a cross-reactive signal against Zaire GP mucin which was statistically significant (P < 0.05) comparing naive and immunized sera but not naive and challenged sera (Fig. 2B). The comparison between naive and challenged sera showed significant increases (P < 0.05) in IgG responses for Marburg NP and GP mucin (Fig. 2B). However, the increase in IgG against Marburg VP40 was not statistically significant (Fig. 2B). The results from analysis of rhesus sera suggested that the microarray enabled detection of anti-GP antibodies in a species-specific manner. Further, the anti-GP antibodies were detected with minimal cross-reactivity toward other species for the cases of sera from the ZEBOV and MARV infections (Fig. 2C). We also examined IgM responses with sera from both animal studies, and representative data are provided in Fig. S4 in the supplemental material. Overall, minor IgM signals were detected against ZEBOV and MARV antigens using these convalescent-phase sera. The preliminary results indicate that the filovirus microarray may be used for IgM detection. An analysis of sera collected from time points closer to vaccination and the viral challenge will confirm the utility of measuring IgM responses by protein microarray.
FIG 2.
IgG antibody responses detected using filovirus microarrays. Naive, postimmunization (immunized), and postviral challenge (challenged) sera from Zaire ebolavirus (A) and Marburg virus (B) animal studies were applied to the assembled microarrays. Bound IgG antibodies were detected fluorescently on a microarray scanner. Following data preprocessing, normalized fluorescence signals were averaged across the five animals in each study. The bars represent normalized mean fluorescence (RFU) ± SEM. The cutoff line represents 2 standard deviations above the mean antibody signal observed in the naive sera. For each antigen-antibody response, paired t tests were done for naive versus immunized and naive versus challenged sera. Unless indicated with an asterisk, all immunized and challenged samples above the cutoff line were found to have significant antibody increases (P < 0.05) in comparison with the naive samples. (C) Side-by-side comparison of GP-specific IgG signals in challenged sera from Zaire ebolavirus and Marburg marburgvirus studies.
Comparison between E. coli and eukaryotic cell-expressed GP.
Both of the GP mucins produced in E. coli and GP ΔTMs produced in eukaryotic cells (insect or mammalian) were included in the printed microarray. Examining sera from the ZEBOV (Fig. 3A) and MARV (Fig. 3B) studies, we confirmed that the mucin domain was sufficient for capturing IgG responses to filoviruses. We observed slightly higher IgG signals from the Zaire GP mucin than from the Zaire GP ΔTM with sera from ZEBOV challenged animals (Fig. 3A and B), whereas antibody recognition of the Marburg GP mucin was comparable to that of the Marburg GP ΔTM (both Angola and Musoke) for sera obtained from animals challenged with MARV. Based on these microarray results, we concluded that the E. coli-produced GP mucin resulted in species specificity similar to that of the eukaryotic cell-expressed GP ΔTMs.
FIG 3.
Comparison of antibody signals between E. coli- and eukaryotic cell-expressed GPs. Data were acquired and analyzed in a manner similar to that for Fig. 2. The bars represent normalized mean fluorescence (RFU) ± SEM. All GP mucins were expressed in E. coli. All GP ΔTM were expressed in insect cells except for Marburg GP ΔTM (Musoke), which was expressed in mammalian cells. (A) Zaire ebolavirus study; (B) Marburg marburgvirus study.
DISCUSSION
We developed a protein microarray composed of isolated recombinant antigens from the six species of Ebola and Marburg viruses and used this platform to examine the antibody responses of rhesus macaques to infection and vaccination. The florescence-based readout for the microarray is highly sensitive, and the assay only requires 1 to 2 μl of biological sample for full evaluation. The NP and GP antigens were most useful for distinguishing sera from ZEBOV in comparison to Marburg virus infection, while results from the Marburg virus study sera indicated that Marburg VP40 induced a cross-reactive VP40 antibody response against all Ebola viruses. We also observed a general antibody cross-reactivity among Ebola virus NP and VP40 proteins, in a manner similar to results from previously reported ELISA studies (43–45), while GP exhibited the highest level of antibody specificity. Supporting the value of the GP mucin domain as a serological marker of infection, E. coli-expressed GP mucins for Zaire and Marburg filoviruses displayed species-specific antibody recognition similar to that of the multidomain GPs (ΔTM) that were produced from eukaryotic cells, based on assay results from the ZEBOV and MARV studies. The microarray assay detected increases in IgG responses to specific filovirus antigens resulting from vaccination or viral challenge, and the relative levels of other antibody isotypes (IgM) could also be measured. We further noted that active infection stimulated a significant boost in immune responses primed by vaccination, as specific IgG levels in VLP-vaccinated macaques increased in response to aerosol challenges from either ZEBOV or MARV. The significant increase in ZEBOV- and MARV-specific IgG responses following viral challenge, as measured by the protein microarray, allows us to conclude that VLP vaccinations did not induce sterilizing immunity in the animals.
A portion of our results corroborate previously reported studies concerning antibody recognition of filovirus antigens. Antibody responses against NP and GP were detected in human patients by ELISAs (14–16) and Western blots (46). In another report, antibodies that recognized GP, NP, and VP40 were observed in sera from a SEBOV (Gulu) outbreak in 2000-2001 (38). These previous ELISA studies examined only select antigens from a single filovirus species or a single antigen from multiple filoviruses, whereas the microarray format supports a highly multiplex analysis of sera. Although only two species of virus were examined in our study, we propose that the protein microarray will also be useful for multiplexed examination of serological responses to most filovirus strains. Expanding the capabilities of these previously described methods, the protein microarray we describe may facilitate diagnosis and serological surveillance of infections caused by multiple species of the highly infectious filoviruses. Translating the laboratory test into a low-cost, point-of-care assay will greatly extend its practical utility. Care of patients with filoviral infections is challenging enough because of the resource-poor settings of outbreaks and the procedures that are required to prevent the spread of infections (47). Allaranga and coworkers proposed that an active epidemiological surveillance system, including surveillance of zoonotic infections, is vital for the early detection and effective response to filoviral hemorrhagic fever epidemics in Africa (48). We suggest that an optimized version of our microarray assay can be an important tool for epidemiological studies and potentially for diagnosis of infections. An expansion of the antigen probes used in the assay may also be a useful modification to consider. A recent report of hospital-based surveillance in Ghana suggested the importance of distinguishing infections caused by hepatitis viruses that produce symptoms that mimic viral hemorrhagic fevers from the infrequent infections caused by filoviruses (49). Further, the prevailing hypothesis concerning outbreaks of filoviral hemorrhagic fevers is that indigenous human populations occasionally make contact with animal reservoirs of Ebola and Marburg viruses, resulting in rapid spread of disease (50). Wildlife are often more severely affected than humans, as demonstrated by a 89% drop in chimpanzees and 50% decrease in gorilla populations as a result of one recorded Ebola virus outbreak (51). Observations concerning the zoonotic origin of filoviruses suggest that there may also be significant value in modifying our microarray assay to include the possibility of supporting serological surveillance of infections occurring within domestic or wildlife animal populations.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christine Pugh for providing the dengue NS1 proteins and Trevor Glaros for mass spectrometric analysis of the recombinant proteins used in this study.
This research was supported by the National Institute of Allergy and Infectious Diseases (R01AI096215) and the Defense Threat Reduction Agency (contract CB3948).
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army or the U.S. government.
Footnotes
Published ahead of print 17 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00484-14.
REFERENCES
- 1.Feldmann H, Klenk HD. 1996. Marburg and Ebola viruses. Adv. Virus Res. 47:1–52. 10.1016/S0065-3527(08)60733-2. [DOI] [PubMed] [Google Scholar]
- 2.Leroy EM, Gonzalez JP, Baize S. 2011. Ebola and Marburg haemorrhagic fever viruses: major scientific advances, but a relatively minor public health threat for Africa. Clin. Microbiol. Infect. 17:964–976. 10.1111/j.1469-0691.2011.03535.x. [DOI] [PubMed] [Google Scholar]
- 3.Hartman AL, Towner JS, Nichol ST. 2010. Ebola and Marburg hemorrhagic fever. Clin. Lab. Med. 30:161–177. 10.1016/j.cll.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Borio L, Inglesby T, Peters C, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O'Toole T. 2002. Hemorrhagic fever viruses as biological weapons. JAMA 287:2391–2405. 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann H, Geisbert TW. 2011. Ebola haemorrhagic fever. Lancet 377:849–862. 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng I, Duku O, Gillo A. 1978. Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull. World Health Org. 56:247–270. [PMC free article] [PubMed] [Google Scholar]
- 7.Baize S, Leroy EM, Georges-Courbot M-C, Capron M, Lansoud-Soukate J, Debré P, Fisher-Hoch SP, McCormick JB, Georges AJ. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423–426. 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 8.Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. 2010. Human fatal Zaire Ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl. Trop. Dis. 4:e837. 10.1371/journal.pntd.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ. 1999. Markedly elevated levels of interferon (IFN)-γ, IFN-α, interleukin (IL)-2, IL-10, and tumor necrosis factor-α associated with fatal Ebola virus infection. J. Infect. Dis. 179:S188–S191. 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 10.Gupta M, Mahanty S, Ahmed R, Rollin PE. 2001. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with Ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology 284:20–25. 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- 11.Macneil A, Reed Z, Rollin PE. 2011. Serologic cross-reactivity of human IgM and IgG antibodies to five species of Ebola virus. PLoS Negl. Trop. Dis. 5:e1175. 10.1371/journal.pntd.0001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ksiazek TG, Rollin PE, Williams AJ, Bressler DS, Martin ML, Swanepoel R, Burt FJ, Leman PA, Khan AS, Rowe AK, Mukunu R, Sanchez A, Peters CJ. 1999. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 179:S177–S187. 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 13.Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. 1999. ELISA for the detection of antibodies to Ebola viruses. J. Infect. Dis. 179:S192–S198. 10.1086/514313. [DOI] [PubMed] [Google Scholar]
- 14.Prehaud C, Hellebrand E, Coudrier D, Volchkov VE, Volchkova VA, Feldmann H, Le Guenno B, Bouloy M. 1998. Recombinant Ebola virus nucleoprotein and glycoprotein (Gabon 94 strain) provide new tools for the detection of human infections. J. Gen. Virol. 79:2565–2572. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama E, Yokoyama A, Miyamoto H, Igarashi M, Kishida N, Matsuno K, Marzi A, Feldmann H, Ito K, Saijo M, Takada A. 2010. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin. Vaccine Immunol. 17:1723–1728. 10.1128/CVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saijo M, Niikura M, Morikawa S, Ksiazek TG, Meyer RF, Peters CJ, Kurane I. 2001. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J. Clin. Microbiol. 39:1–7. 10.1128/JCM.39.1.1-7.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikegami T, Saijo M, Niikura M, Miranda ME, Calaor AB, Hernandez M, Manalo DL, Kurane I, Yoshikawa Y, Morikawa S. 2003. Immunoglobulin G enzyme-linked immunosorbent assay using truncated nucleoproteins of Reston Ebola virus. Epidemiol. Infect. 130:533–539. 10.1017/S0950268803008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groen J, van den Hoogen BG, Burghoorn-Maas CP, Fooks AR, Burton J, Clegg CJSC, Zeller H, Osterhaus ADME. 2003. Serological reactivity of baculovirus-expressed Ebola virus VP35 and nucleoproteins. Microbes Infect. 5:379–385. 10.1016/S1286-4579(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez A, Kiley MP, Holloway BP, Auperin DD. 1993. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 29:215–240. 10.1016/0168-1702(93)90063-S. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann H, Mühlberger E, Randolf A, Will C, Kiley MP, Sanchez A, Klenk H-D. 1992. Marburg virus, a filovirus: messenger RNAs, gene order, and regulatory elements of the replication cycle. Virus Res. 24:1–19. 10.1016/0168-1702(92)90027-7. [DOI] [PubMed] [Google Scholar]
- 21.Becker S, Rinne C, Hofsäss U, Klenk H-D, Mühlberger E. 1998. Interactions of Marburg virus nucleocapsid proteins. Virology 249:406–417. 10.1006/viro.1998.9328. [DOI] [PubMed] [Google Scholar]
- 22.Elliott LH, Kiley MP, McCormick JB. 1985. Descriptive analysis of Ebola virus proteins. Virology 147:169–176. 10.1016/0042-6822(85)90236-3. [DOI] [PubMed] [Google Scholar]
- 23.Mühlberger E, Lotfering B, Klenk HD, Becker S. 1998. Three of the four nucleocapsid proteins of Marburg virus, NP, VP35, and L, are sufficient to mediate replication and transcription of Marburg virus-specific monocistronic minigenomes. J. Virol. 72:8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mühlberger E, Weik M, Volchkov VE, Klenk HD, Becker S. 1999. Comparison of the transcription and replication strategies of Marburg virus and Ebola virus by using artificial replication systems. J. Virol. 73:2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weik M, Modrof J, Klenk HD, Becker S, Muhlberger E. 2002. Ebola virus VP30-mediated transcription is regulated by RNA secondary structure formation. J. Virol. 76:8532–8539. 10.1128/JVI.76.17.8532-8539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. U. S. A. 97:13871–13876. 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75:5205–5214. 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. 2006. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 80:5156–5167. 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Xu L, Sun Y, Nabel GJ. 2002. The assembly of Ebola virus nucleocapsid requires virion-associated proteins 35 and 24 and posttranslational modification of nucleoprotein. Mol. Cell 10:307–316. 10.1016/S1097-2765(02)00588-9. [DOI] [PubMed] [Google Scholar]
- 30.Hoenen T, Groseth A, Kolesnikova L, Theriault S, Ebihara H, Hartlieb B, Bamberg S, Feldmann H, Ströher U, Becker S. 2006. Infection of naïve target cells with virus-like particles: implications for the function of Ebola virus VP24. J. Virol. 80:7260–7264. 10.1128/JVI.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. U. S. A. 93:3602. 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volchkov VE, Becker S, Volchkova VA, Ternovoj VA, Kotov AN, Netesov SV, Klenk HD. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214:421–430. 10.1006/viro.1995.0052. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez A, Yang ZY, Xu L, Nabel GJ, Crews T, Peters CJ. 1998. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J. Virol. 72:6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldmann H, Will C, Schikore M, Slenczka W, Klenk HD. 1991. Glycosylation and oligomerization of the spike protein of Marburg virus. Virology 182:353–356. 10.1016/0042-6822(91)90680-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonezawa A, Cavrois M, Greene WC. 2005. Studies of Ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J. Virol. 79:918–926. 10.1128/JVI.79.2.918-926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez S, Cisney ED, Tikhonov AP, Schweitzer B, Putnak RJ, Simmons M, Ulrich RG. 2011. Antibody recognition of the dengue virus proteome and implications for development of vaccines. Clin. Vaccine Immunol. 18:523–532. 10.1128/CVI.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. 2007. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J. Infect. Dis. 196:S430. 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 38.Sobarzo A, Perelman E, Groseth A, Dolnik O, Becker S, Lutwama JJ, Dye JM, Yavelsky V, Lobel L, Marks RS. 2012. Profiling the native specific human humoral immune response to Sudan Ebola virus strain Gulu by chemiluminescence enzyme-linked immunosorbent assay. Clin. Vaccine Immunol. 19:1844–1852. 10.1128/CVI.00363-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowling W, Thompson E, Badger C, Mellquist JL, Garrison AR, Smith JM, Paragas J, Hogan RJ, Schmaljohn C. 2007. Influences of glycosylation on antigenicity, immunogenicity, and protective efficacy of Ebola virus GP DNA vaccines. J. Virol. 81:1821–1837. 10.1128/JVI.02098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson JA, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn AL, Hart MK. 2000. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287:1664−1666. 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 41.Shahhosseini S, Das D, Qiu X, Feldmann H, Jones SM, Suresh MR. 2007. Production and characterization of monoclonal antibodies against different epitopes of Ebola virus antigens. J. Virol. Methods 143:29–37. 10.1016/j.jviromet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Martinez O, Tantral L, Mulherkar N, Chandran K, Basler CF. 2011. Impact of Ebola mucin-like domain on antiglycoprotein antibody responses induced by Ebola virus-like particles. J. Infect. Dis. 204(Suppl 3):S825–S832. 10.1093/infdis/jir295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Changula K, Yoshida R, Noyori O, Marzi A, Miyamoto H, Ishijima M, Yokoyama A, Kajihara M, Feldmann H, Mweene AS, Takada A. 2013. Mapping of conserved and species-specific antibody epitopes on the Ebola virus nucleoprotein. Virus Res. 176:83–90. 10.1016/j.virusres.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucht A, Grunow R, Möller P, Feldmann H, Becker S. 2003. Development, characterization and use of monoclonal VP40-antibodies for the detection of Ebola virus. J. Virol. Methods 111:21–28. 10.1016/S0166-0934(03)00131-9. [DOI] [PubMed] [Google Scholar]
- 45.Niikura M, Ikegami T, Saijo M, Kurata T, Kurane I, Morikawa S. 2003. Analysis of linear B-cell epitopes of the nucleoprotein of Ebola virus that distinguish Ebola virus subtypes. Clin. Vaccine Immunol. 10:83–87. 10.1128/CDLI.10.1.83-87.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leroy EM, Baize S, Volchkov VE, Fisher-Hoch SP, Georges-Courbot MC, Lansoud-Soukate J, Capron M, Debré P, McCormick JB, Georges AJ. 2000. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 355:2210–2215. 10.1016/S0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 47.Roddy P, Howard N, Van Kerkhove MD, Lutwama J, Wamala J, Yoti Z, Colebunders R, Palma PP, Sterk E, Jeffs B, Van Herp M, Borchert M. 2012. Clinical manifestations and case management of Ebola haemorrhagic fever caused by a newly identified virus strain, Bundibugyo, Uganda, 2007-2008. PLoS One 7:e52986. 10.1371/journal.pone.0052986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allaranga Y, Kone ML, Formenty P, Libama F, Boumandouki P, Woodfill CJ, Sow I, Duale S, Alemu W, Yada A. 2010. Lessons learned during active epidemiological surveillance of Ebola and Marburg viral hemorrhagic fever epidemics in Africa. East Afr. J. Public Health 7:30–36. [PubMed] [Google Scholar]
- 49.Bonney JHK, Osei-Kwasi M, Adiku TK, Barnor JS, Amesiya R, Kubio C, Ahadzie L, Ölschläger S, Lelke M, Becker-Ziaja B, Pahlmann M, Günther S. 2013. Hospital-based surveillance for viral hemorrhagic fevers and hepatitides in Ghana. PLoS Negl. Trop. Dis. 7:e2435. 10.1371/journal.pntd.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mbonye A, Wamala J, Winyi-Kaboyo, Tugumizemo V, Aceng J, Makumbi I. 2012. Repeated outbreaks of viral hemorrhagic fevers in Uganda. Afr. Health Sci. 12:579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leroy EM, Rouquet P, Formenty P, Souquiere S, Kilbourne A, Froment JM, Bermejo M, Smit S, Karesh W, Swanepoel R, Zaki SR, Rollin PE. 2004. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 303:387–390. 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.