Abstract
A subunit vaccine candidate was produced from Brucella suis 145 (biovar 4; expressing both the A antigen of Brucella abortus and the M antigen of Brucella melitensis). The preparation consisted mostly of polysaccharide (PS; >90% [wt/wt]; both cell-associated PS and exo-PS were combined) and a small amount of protein (1 to 3%) with no apparent nucleic acids. Vaccinated mice were protected (these had a statistically significant reduction in bacterial colonization compared to that of unvaccinated controls) when challenged with representative strains of three Brucella species most pathogenic for humans, i.e., B. abortus, B. melitensis, and B. suis. As little as 1 ng of the vaccine, without added adjuvant, protected mice against B. suis 145 infection (5 × 105 CFU), and a single injection of 1 μg of this subunit vaccine protected mice from B. suis 145 challenge for at least 14 months. A single immunization induced a serum IgG response to Brucella antigens that remained elevated for up to 9 weeks. The use of heat (i.e., boiling-water bath, autoclaving) in the vaccine preparation showed that it was thermostable. This method also ensured safety and security. The vaccine produced was immunogenic and highly protective against multiple strains of Brucella and represents a promising candidate for further evaluation.
INTRODUCTION
The Brucella species are the most common zoonotic infectious agents worldwide (1), have been weaponized in previous biological warfare programs (2, 3), and are possible terrorist biothreats (4). Despite its obvious importance, brucellosis has been described as a neglected zoonotic disease (5). Reasons for it being overlooked are the lack of recognition of its impact on world health, it being underreported and misdiagnosed as other diseases (5), the mortality after infection is only about 2% (6), and attenuated vaccines for animal use exist (7). For the latter, an obstacle for the use of live vaccines in developing countries is the lack of cold-chain refrigeration (8).
In regard to eliminating the need for cold-chain refrigeration, we previously showed that a polysaccharide (PS) preparation, extracted from Brucella abortus 1119-3 with dilute acetic acid and heat, was an effective vaccine against Brucella. It was protective against B. abortus strains 2308 and 30 in female mice (9), B. abortus strain 2308 in pregnant guinea pigs (10), and a field strain of Brucella suis in swine (11). For the latter study, the PS, freeze-dried, stored at room temperature for a decade, and sent by regular mail to investigators in Venezuela, did not require cold-chain storage. It was equally protective whether given orally (i.e., needle-free) or injected intramuscularly. A single dose of PS, whether extracted from B. abortus 1119-3 or B. suis 1330, and without added adjuvant, provided swine protection from a field strain of B. suis. The assessment of PS as a vaccine was also encouraging in that it gave results similar to that of the live attenuated B. abortus RB51 vaccine. Both groups of vaccinated sows, challenged with B. suis, had litter sizes about twice that of those unvaccinated, had long-term protection of several months, and had similar serology with antibody titers (B. suis cells as antigen) either negative or at the lower limit of detection (11).
With the noted results, PS was extracted in a similar manner, with dilute acetic acid and high heat, from B. suis 145 (biovar 4; having both the A antigen of B. abortus and the M antigen of Brucella melitensis) (12). Evidence is now given on the efficacy of this single thermostable PS vaccine against representative strains of all three species most pathogenic for humans—B. abortus, B. melitensis, and B. suis.
MATERIALS AND METHODS
Biosafety.
The biosafety level 3 (BSL3) containment facilities at DRDC Suffield Research Centre had Public Health Agency of Canada (PHAC) and Canadian Food Inspection Agency (CFIA) approval for use certification. These facilities also had American CDC/NIH-NIAID inspection and approval for research. Phenol extracts, as the source of the subunit vaccine, were sterility tested before removal from the BSL3 laboratory for further vaccine preparation.
Animal use.
BALB/c female mice, 19 to 21 g and 35 days of age, were acquired from Charles River (St. Constant, QC, Canada). Animal care was in accordance with the Canadian Council for Animal Care (CCAC) guidelines. This was compliant with the American Office of Laboratory Animal Welfare (OLAW). Groups of 5 to 10 mice were used as specified.
Bacterial cultures.
Brucella spp. were acquired from CFIA-Nepean (Ontario). Verification of B. abortus 30, B. abortus 2308, B. melitensis 16M, and B. suis 145, as well as their expression of A and/or M antigens, were done by Janet Payeur and Darla Ewalt at the National Veterinary Services Laboratory (Ames, IA, USA). For storage, a loopful of bacteria grown on agar plates was transferred to cryovials containing 1 ml of brucella broth (BD, Sparks, MD, USA) and then kept at −70°C within the BSL3 facility.
Bacterial growth.
For bacterial challenge, the frozen bacterial stock was inoculated into brucella agar (Becton, Dickinson and Company, Sparks, MD) within a plate and incubated at 35°C, 90% humidity, 5% CO2, for 3 days. A loopful of bacteria from this plate was suspended in 10 ml of sterile 0.9% saline and adjusted with saline to an optical density at 600 nm (OD600) of 1.0 (Forma; Ultra-Spectrophotometer 1000), found to be 5 × 109 CFU/ml. For challenge, this suspension was diluted 1,000-fold in sterile saline and mice were each given 0.1 ml (5 × 105 CFU) intraperitoneally (i.p.).
For vaccine preparation, a loopful of the frozen stock was transferred to 100 ml of brucella broth in a 500-ml flask, shaken at 150 rpm, and incubated as described before for 2 days. A tenth of a milliliter of this culture was transferred to each of two 500-ml flasks with 100 ml fresh medium and incubated overnight (16 h). A half milliliter of the latter culture was used to inoculate 150-cm2 Corning tissue culture flasks (Fisher Scientific, Edmonton, Alberta, CA) containing 90 ml of sterile brucella agar. A dozen sterile 3-mm glass beads were rolled over the surface of the first flask to spread the inoculum, and then these beads were transferred to the next inoculated flask. The inoculated flasks had the tops secure but loosened and were incubated agar-side down for 1 week.
Vaccine preparation.
The bacterial layer from each tissue culture flask was removed by adding 10 ml phenol-saline (1% sterile saline, liquefied phenol added to 5% [vol/vol]), rolling sterile glass beads over the surface, removing the cell suspension, and then repeating. The pooled bacterial suspension was centrifuged at 10,000 × g and 4°C for 30 min to partition shed antigens (in the supernatant) and cells with associated antigens (the pellet).
Recovery of crude exo-polysaccharide (exo-PS; fraction B1) followed the method published for B. melitensis (13), which sheds much of its outer membrane (14). Briefly, the previously noted supernatant was collected into a flask, and glacial acetic acid was added to 3% (vol/vol). The flask was placed in a boiling-water bath for 2 h with manual swirling every 15 min. The preparation was cooled to room temperature, a half-volume of 90% phenol was added, and the mixture was then magnetically stirred and heated to about 70°C (i.e., to a temperature at which the phenol-water mixture turned from opaque to transparent) for 30 min. The preparation was chilled at 4°C overnight, and the bottom phenol layer containing crude exo-PS was retained.
Recovery of the cell-associated PS (fraction B2) followed the method published for B. abortus (15). The cell pellet from the initial centrifugation was washed and then resuspended in 5 volumes (wt/vol) of acetic-saline (3% glacial acetic acid, 1% sodium chloride), heated in a boiling-water bath for 2 h, and centrifuged, and the supernatant was extracted with phenol as described before. Additional PS (fraction B3) was harvested by resuspending the cell pellet in 5 volumes of acetic-saline, autoclaving (121°C, 15 lb/in2, 2 h), centrifuging, and performing a 90% hot phenol extraction on the supernatant as noted before.
Fractions B1, B2, and B3 were processed separately. The PS were precipitated and the phenol was removed with three washings of 5 volumes of chilled (4°C) methanol-acetate (1% [wt/vol] sodium acetate trihydrate in methanol) and centrifugation. The pellets were dissolved in and dialyzed against distilled water (1,000-molecular-weight [MW]-cutoff Spectrum Spectra/Por 6 dialysis tubing; Fisher Scientific). Much of the protein was removed by the addition of 1/10 of the volume of 2 M trichloroacetic acid (TCA) and centrifugation. The supernatant was dialyzed against distilled water to remove TCA (100-fold volume, 24 h, 4°C, magnetic stirring, done 3 times) before freeze-drying (VirTis Model Advantage XL.70; Gardiner, NY, USA) and then dispensed at 1 mg/0.5 ml into cryovials before storage at −70°C. Fractions B1, B2, and B3 were either used separately for their characterization or combined for PS vaccine efficacy studies.
Vaccine characterization.
Assays were done on B. suis 145 PS vaccine at 2 mg/ml and dilutions. Nucleic acid content was determined by absorbance at 260 and 280 nm. Protein content was assessed with the Bio-Rad protein microassay as outlined by the manufacturer (Bio-Rad Laboratories, Mississauga, Ontario, Canada). Proteins were also assessed qualitatively with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) that was then stained with either Coomassie blue, silver nitrate (Bio-Rad), or SYPRO Ruby protein gel stain (Invitrogen, Burlington, Ontario, Canada). The carbohydrate content of the vaccine was assessed by the phenol-sulfuric acid method adapted to a microtiter plate (16). Standards were glucose, mannose, rhamnose, Yersinia enterocolitica O:9 PS (prepared from purified lipopolysaccharide [LPS] provided by Malcolm Perry, NRC, Ottawa, ON, Canada) and B. abortus 1119-3 PS prepared previously by our laboratory (17). Carbohydrates were also run on SDS-PAGE gels and assessed qualitatively by the lack of reaction with Pro-Q Emerald 300 staining kits for glycoproteins (i.e., discrete bands) or LPS (i.e., diffuse brightly stained columns) (Invitrogen). LPS content was estimated by its appearance on the Pro-Q Emerald 300-stained gel and by assaying for 2-keto-3-deoxyoctonic acid (KDO) (18).
Assessment of vaccine efficacy.
For vaccination, the content of a cryovial of vaccine (1 mg) was added to 100 ml of sterile 0.9% saline. A 1-ml tuberculin syringe with a needle was used to deliver vaccine (1 μg/0.1 ml/mouse) by the i.p. route or, for the long-term study, by the intramuscular (i.m.) (hind left leg) route. Mice were challenged (5 × 105 CFU/0.1 ml sterile saline by the i.p. route) 4 weeks later. One week after challenge, mice were weighed and terminated, their abdomens were rinsed with 70% ethanol, and their spleens were aseptically removed. Each spleen was manually crushed in a sterile Corning glass tissue grinder with 2 ml sterile saline. One milliliter of the crushed spleen suspension was transferred to a tube containing 9 ml sterile saline; serial dilutions were prepared and 0.1 ml of each dilution was plated onto each of two brucella agar plates that were incubated as described above.
For the long-term protection study, at time zero, 100 mice served as the controls by receiving 0.1 ml sterile saline i.m., and another 100 mice each received 1 μg vaccine in 0.1 ml saline i.m. At each time point (1 day; 2, 4, 6, 8, 10, and 12 weeks; 6, 9, and 14 months), 10 control mice and 10 vaccinates were then moved into BSL3 and challenged with B. suis 145. One week later, these were terminated, and their spleens were assessed for infection as noted above.
To confirm the hypothesis that the PS contributed to vaccine efficacy and did not depend on cell wall components or nucleic acid or protein content, 200 mg of vaccine, in 50 ml of 0.02 M Tris-HCl and 0.02% sodium azide, pH 7.5, was digested with 100 μg each of lysozyme, DNase, and RNase for 4 h at room temperature and continuous stirring and then 400 μg of proteinase for another 24 h (enzymes from Sigma Chemical Co., St. Louis, MO). The digested vaccine was purified as described before and tested for its protective efficacy against B. suis 145 challenge in mice.
As a second experiment to show PS contributed to vaccine efficacy, smooth LPS was purified from B. suis 145 cells as published for B. abortus LPS (17). Fifty milligrams of this LPS was dissolved in 10 ml of 3% acetic acid and hydrolyzed in a boiling-water bath for 2 h. The hydrolysis released lipid A, which aggregated and was removed by centrifugation, and soluble PS. The PS was dialyzed against distilled water and then lyophilized. Fifty milligrams of LPS was also dissolved in 10 ml of 0.2 M NaOH and hydrolyzed in a boiling-water bath for 2 h, and PS was prepared as described before.
Rotofor pH fractionation of B. suis 145 vaccine.
Brucella suis 145 PS vaccine (243 mg) was dissolved in 4 M urea (48.6 ml). Bio-Rad 3/10 ampholyte (1 ml) was added, and the sample was loaded onto a Bio-Rad Rotofor. Electrophoresis was performed at 12 W for 3 h 40 min, using 0.1 M H3PO4 and 0.1 M NaOH as the electrode buffers. Fractions were harvested, the pH was measured, and fractions were dialyzed against distilled water at 4°C using 1,000-MW-cutoff tubing. Fractions were added sequentially to labeled vials and freeze-dried, and then 1-mg portions were added to recorded random-numbered tubes. These vaccine samples were tested by another research team member, in a blind fashion, for efficacy against B. suis 145 challenge.
Assessment of immune responses.
At each of the indicated time points, a set of unvaccinated control and vaccinated mice (housed in a BSL2 holding area) were bled from the tail vein. Individual blood samples were collected, and the sera were removed and stored at −20°C.
To determine anti-Brucella antibody titers, microtiter enzyme-linked immunosorbent assay (ELISA) plates were coated with killed B. suis 145 cells as the immobilized antigen (10 μg/ml, 0.1 ml per well). The negative-control serum consisted of a pool of naive, unvaccinated mice, and it was tested at a 1:10 dilution. The positive-control serum was a pool of sera from hyperimmune mice (given a total of 100 μg of killed B. abortus 2308 cells at 2 intramuscular sites and 2 subcutaneous sites at weeks 0, 1, and 5 and bled at week 10). This positive control was used at a 1:1,000 dilution. Indicator antibody (0.1 ml per well) was rabbit anti-mouse-antibody (IgG or IgM) conjugated to horseradish peroxidase (Miles-Yeda Ltd., Rehovoth, Israel), diluted 1:1,000 in wash buffer. Substrate (0.2 ml per well) was an equal mixture of 2,2′azino-bis-3-ethylbenzthizoline-6-sulfonic acid (ABTS) and hydrogen peroxide (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD). Plates were incubated 1 h at 37°C and then read at 405 nm (Molecular Devices, Sunnyvale, CA).
Capillary electrophoresis (CE) assessed mouse monoclonal antibodies (Yst9-3, Bm3-8, and Yst9-2 antibodies with affinity for Brucella A, M, and A/M/C antigens; NRC, Ottawa, ON, Canada) or sera binding to the vaccine or vaccine constituents. Five microliters of serum, or mouse monoclonal antibodies diluted 1:10 with deionized water to yield 0.3 mg antibody/ml, was coincubated with 5 μl of vaccine (1 mg/ml deionized water) at room temperature for 30 min. Nine microliters of this was used for CE analysis (P/ACE system; Beckman Coulter Canada Inc., Mississauga, ON, Canada). The run buffer was 50 mM boric acid (Fluka Chemicals, Switzerland) and 2% polyethylene glycol 600 (Kodak, Rochester, NY) in distilled water, with pH adjusted to 7.0 with 1 M NaOH. Separation was done on a 37-cm (length) by 50-μm (internal diameter) uncoated column (Polymicro Technologies, Phoenix, AZ) using 20 kV, sample injection of 20 s, run time of 5.5 to 15 min, and absorbance of 214 nm.
Statistics.
Statistical analysis and data graphing were done with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Much of the analysis was done between two groups (e.g., a vaccinated group and an unvaccinated group that were challenged) using nonparametric unpaired t tests with 95% confidence levels, two-tailed P values, and standard errors of the mean (SEM). For group comparisons (e.g., all groups given different vaccine fractions compared to those given saline before challenge), analysis of variance (ANOVA) was used. To determine the clearance rates of B. suis 145 bacterial challenge in control unvaccinated and vaccinated mice, the formula used was m = (ln CFU2 – ln CFU1)/(t2 – t1), where m is the slope, ln is the natural logarithm, CFU is the CFU at the latter (2) or earlier time point (1), and t is the number of weeks at the latter (2) or earlier (1) time point.
RESULTS
Characterization of B. suis 145 vaccine subunit components.
Exo-PS (from the supernatant of a cell suspension and cell washings), cell-associated PS (released with 3% acetic acid and a boiling-water bath), and additional cell-associated PS (released with 3% acetic acid and autoclaving) were assessed. The total yield of these components per 100 g (wet weight) of cells increased from 0.184 g to 0.808 g, or about 5-fold, if B. suis 145 had first been passaged through a mouse (i.e., bacterial cells, taken from a plate used to assess splenic CFU of an infected animal, were used to inoculate brucella broth and subsequently brucella agar in tissue culture flasks). Characterization was done on PS fractions from the latter because of the larger amounts available. The amount of nucleic acid was less than 0.1%, protein was about 1 to 3%, KDO was 0.007 to 0.08%, and LPS was present in the exo-PS preparation (37%) but not in the cell-associated PS (Table 1; see also Fig. S1 in the supplemental material). In regard to carbohydrate content, the presence of LPS in fraction B1 interfered with the assay, and the value of 60% for non-LPS carbohydrate was determined by difference. Fraction B2 had a carbohydrate content of 99% when mannose was used as the standard. B3 had a value of 14% carbohydrate when mannose was used as the standard but about 90% when B. abortus 1119-3 PS, prepared in the same way, was used as the standard.
TABLE 1.
Characterization of the B. suis 145 subunit vaccine fractions
| Fractiona | Yield (g) from 100 g (wet wt) cells |
% |
|||||
|---|---|---|---|---|---|---|---|
| Grown on Brucella agar | First passaged through a mouse | Nucleic acids | Protein | Free carbohydrate | KDO | LPS | |
| B1 (exo-PS) | 0.052 | 0.488 | <0.1 | 3.1 | 60 | 0.06 | 37 |
| B2 (cell-associated PS) | 0.028 | 0.104 | <0.1 | 0.8 | 99 | 0.08 | Not detected |
| B3 (cell-associated PS) | 0.104 | 0.216 | <0.1 | 0.8 | 90 | 0.007 | Not detected |
Exopolysaccharide fraction B1 was from the supernatant and washings of cells suspended in 5% phenol and 1% saline (see Materials and Methods). The preparation had 3% acetic acid added and was heated 2 h in a boiling-water bath. Cell-associated polysaccharide fraction B2 was released from washed cells suspended in 3% acetic acid and heated 2 h in a boiling water bath. Fraction B3 was released from these same cells that were washed, suspended in 3% acetic acid and autoclaved for 2 h (121°C).
Fifty milligrams (dry weight) of purified LPS from washed B. suis 145 cells yielded 27 mg of PS after acid hydrolysis and 31 mg of PS after alkaline hydrolysis. Purity of these PS preparations (formamido-mannopyranose, >90%) was confirmed by David Bundle, Alberta Glycomics Centre, University of Alberta. Also, it was found that different LPS had different sensitivities to acid hydrolysis and heat (boiling-water bath). Brucella suis 145 cell-associated LPS, B. abortus 1119-3 LPS, and Escherichia hermannii LPS hydrolyzed at about 2 h in 3% acetic acid; Yersinia enterocolitica O:9 LPS hydrolyzed at about 4 h in 6% acetic acid; B. melitensis 16M exo-LPS hydrolyzed at about 6 h in 10% acetic acid.
Efficacy of B. suis 145 vaccine subunit components.
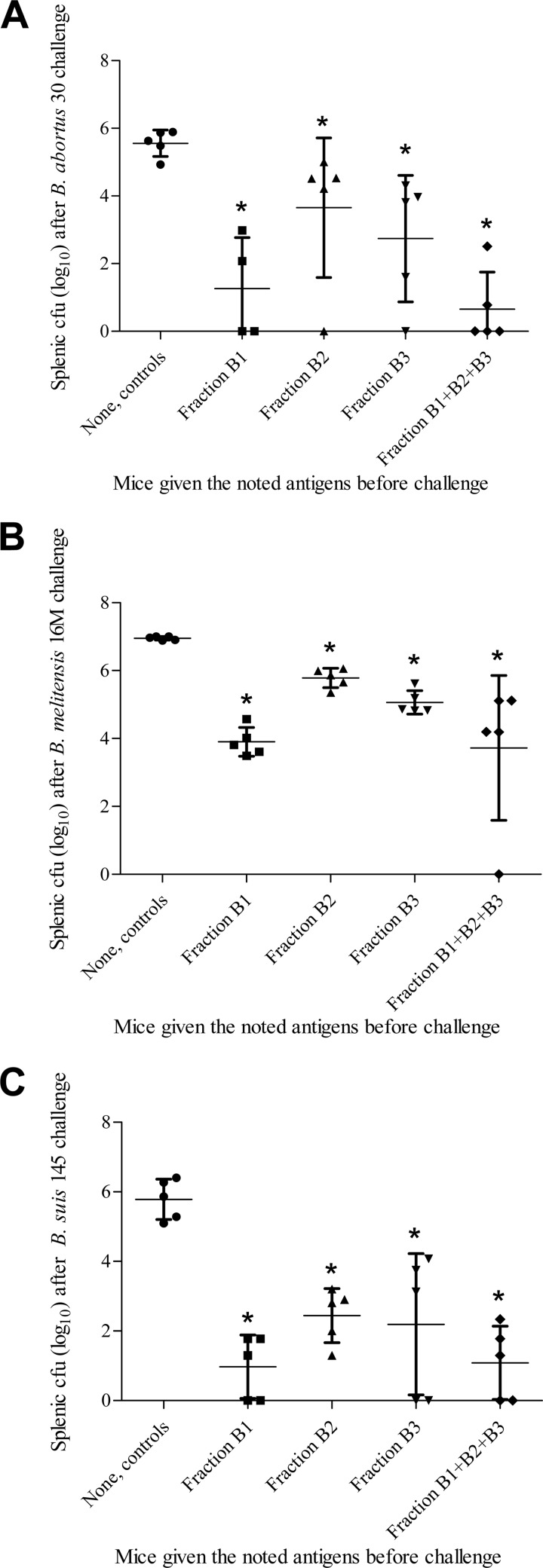
Brucella suis 145 fraction B1, B2, and B3, administered in a single 1-μg dose, reduced splenic colonization by B. abortus, B. melitensis, and B. suis. Immunization with the different fractions significantly reduced (about 1 to 3 log10) splenic CFU counts compared to those of controls given saline (Fig. 1). Although the protective efficacies of these fractions were not significantly different between each other (P values between 0.06 and 0.33), fraction B1, the exo-PS, appeared to reduce colonization more than B2 and B3, the cell-associated PS. Immunization with 1 μg of combined B1-B2-B3 produced results similar to those of 1 μg of B1. Subsequently, the term “B. suis 145 subunit vaccine” used in this report refers to the preparation that combined B1-B2-B3.
FIG 1.
(A) Splenic bacterial loads in mice immunized with 1 μg of B. suis 145 subunit vaccine fractions (i.p.) and challenged with B. abortus strain 30 (i.p.). Mice vaccinated with fractions B1, B2, B3, and B1-B2-B3 had splenic CFU counts significantly different from that of unvaccinated mice, with P values of <0.02, <0.02, <0.01, and <0.01, respectively, as indicated by asterisks within the figure. (B) Splenic bacterial loads in mice immunized with 1 μg of B. suis 145 subunit vaccine fractions (i.p.) and challenged with B. melitensis strain 16M (i.p.). Mice vaccinated with fractions B1, B2, B3, and B1-B2-B3 had splenic CFU counts significantly different from that of unvaccinated mice, with all having P values of <0.0001, as indicated by asterisks within the figure. (C) Splenic bacterial loads in mice immunized with 1 μg of B. suis 145 subunit vaccine fractions (i.p.) and challenged with B. suis strain 145 (i.p.). Mice vaccinated with fractions B1, B2, B3, and B1-B2-B3 had splenic CFU counts significantly different from that of unvaccinated mice, with all having P values of <0.05, as indicated by asterisks within the figure.
If, instead of separating the different fractions and then combining them, the B. suis 145 phenol-saline suspension (containing both whole cells and exo-PS) was processed in its entirety, having 3% acetic acid added followed by autoclaving for 2 h, the efficacy of this PS preparation (fraction A) was similar to that of fractions B2 and B3 rather than that of fraction B1 (data not shown).
The TCA precipitated fraction, discarded during the preparation of B. suis 145 subunit vaccine, was briefly assessed. Its dry weight amount was equivalent to that of fractions B1, B2, and B3 combined, it was 34% protein and 64% carbohydrate, and 1 μg delivered i.p. reduced splenic colonization by B. suis 145 (also given by the i.p. route, groups of 10 mice for controls and vaccinates), similar to that observed for fraction B1 or B1-B2-B3 (data not shown). The B. suis 145 subunit vaccine extensively digested by DNase, RNase, lysozyme, and proteinase K and then repurified provided highly significant protection to mice compared to that of controls (P < 0.001 for lowered splenic CFU) and no significant difference from the original B. suis 145 PS vaccine (P > 0.15 for splenic CFU). The PS recovered after acid and alkaline hydrolysis of purified B. suis 145 LPS, when used as vaccines, significantly reduced splenic colonization against B. suis 145 challenge compared to that of unvaccinated mice. There was no difference between the groups receiving B. suis 145 subunit vaccine, PS recovered from LPS by acid hydrolysis, and PS recovered from LPS by alkaline hydrolysis.
Potency of the B. suis 145 subunit vaccine.
Different amounts of the subunit vaccine were given to mice (groups of 10, given 0.1 ml vaccine i.p.; controls were given sterile saline) 4 weeks before B. suis 145 challenge (also i.p.). The groups that received vaccine doses of 1 ng to 100 μg exhibited significant reductions of splenic colonization. The greatest statistical significance between controls and vaccinated mice (P < 0.0001) was found in mice given 100 ng to 100 μg. Their splenic bacterial loads 1 week after B. suis 145 challenge were about 200- to 450-fold (log10 2.20 to 2.65) lower than those of the unvaccinated controls (see Table S1 in the supplemental material).
Clearance.
Groups of 10 mice, either controls given sterile saline or mice vaccinated with B. suis 145 subunit vaccine, cleared much of the B. suis 145 challenge over 1 to 8 weeks. The striking difference was 1 week after challenge, when the unvaccinated group had 26% of the bacteria remaining and the vaccinated group had only 0.014% remaining (over a 1,000-fold difference). Afterward, the rate of bacterial clearance from the spleens averaged about 74% of the splenic CFU per week for unvaccinated controls and 46% per week for vaccinates (Fig. 2).
FIG 2.
Splenic bacterial loads in unvaccinated controls and mice given 1 μg (i.p.) B. suis 145 subunit vaccine (10 mice per group) following challenge of B. suis 145 (5 × 105 CFU i.p.). There was no difference at time zero, as both groups received 5 × 105 CFU of B. suis 145. The statistical differences between the unvaccinated controls and vaccinated mouse groups at 1 and 2 weeks after challenge were P = 0.0001 and P < 0.03, respectively, as indicated by asterisks within the figure. For the groups at 4 and 8 weeks, there was no statistical difference between controls and vaccinated mice, as the P values for both were >0.05.
Long-term efficacy of a single dose of B. suis 145 subunit vaccine.
A single dose of the subunit vaccine (without adjuvant) reduced splenic colonization of B. suis 145 challenge in mice over the entire length of a 14-month study. The unvaccinated controls had a large variation in colony counts because of a few outliers within the groups (*, **, and *** denote 1, 2, and 3 mice, respectively, in the groups of 10), while this was not observed in the vaccinated groups (see Table S2 in the supplemental material).
Isoelectric points.
Brucella suis 145 PS, having charged groups such as the formamido component, was fractionated by isoelectric focusing. Most fractions provided highly significant protection compared to that of the saline controls (P < 0.001), with those that caused the lowest splenic CFU having either acidic (pH 4.3) or alkaline (pH 11.4) pI (see Fig. S2 in the supplemental material). Two exceptions where the fractions were not protective and differed from the other fractions (ANOVA, P = 0.02) but not the saline control (ANOVA, P > 0.8) were those with a pI of 8.5 and 9.2.
Antibody response of the B. suis 145 subunit vaccine.
In the 14-month long-term study, sera from vaccinated or control mice were collected before challenge and assessed by ELISA. Anti-Brucella (killed B. suis 145 cells as the antigen) antibodies were not detected in negative (unvaccinated and unchallenged)-control mouse sera. For the groups that received B. suis 145 subunit vaccine, anti-Brucella IgM was present predominantly from weeks 1 to 4 and anti-Brucella IgG from weeks 5 to 9, although some of the mice in the different groups had very low antibody titers at later time points (Fig. 3). Some of the mouse sera did not have detectable levels of anti-Brucella IgM or IgG, which contributed to the large variation observed for the mean group titers.
FIG 3.
Serum antibody titers (killed B. suis 145 cells as immobilized antigen) in mice that received B. suis 145 subunit vaccine. Note that a positive-control mouse anti-Brucella serum diluted 1:1,000 gave an A405 of 1.0 for IgM and 2.5 for IgG and that the sera of vaccinated mice noted in the graph were diluted 1:10. Error bars are standard errors of the mean (SEM).
Capillary electrophoresis.
The A and/or M antigenicity of B. suis 145 exo-PS or cell-associated PS was identified by the change of CE elution times when these were first coincubated with mouse monoclonal antibodies (Yst9-3 or anti-A, Bm3-8 or anti-M, and Yst9-2 or anti-A/M/C antigens). These bound to B. suis 145 cell-associated PS fractions B2 and B3 (e.g., monoclonal antibody Yst9-2 eluted at 5.3 min, Yst9-2 coincubated with B2 or B3 eluted at 4.9 min). These did not appear to bind to fraction B1 exo-PS (i.e., there was no change in elution time). Autoimmune antibodies were detected by CE by separately eluting antiserum of vaccinates (diluted 1:10), mouse monoclonal YsT9-3 (1 mg/ml), and both incubated together. Their 214-nm absorbances at 3.8 min were 0.001, 0.004, and 0.012, respectively. The antiserum's absorbance peak at 5.3 min shifted to 5.5 min when it was coincubated with YsT9-3 (see Fig. S3 in the supplemental material). CE results differed if B. suis 145 PS vaccine was coincubated with coagulated (see Fig. S4 in the supplemental material) or heparin-treated uncoagulated vaccinated mouse serum (see Fig. S5 in the supplemental material). For the latter, a component in vaccinate serum bound more to vaccine lots of greater protective efficacy than others (i.e., B. suis 145 PS vaccine lot 4 and lot 5 both protected mice from B. suis 145 challenge, but mice given the latter had lower splenic colonization).
DISCUSSION
As a defense against bacterial and viral infectious diseases, there has been much progress in the development of live attenuated strains as vaccines. There have also been successes on the use of their components to induce immunity, namely, key proteins, but some successful PS subunit vaccines have been those against Haemophilus influenzae, Neisseria meningitidis, Salmonella enterica serovar Typhi, and Streptococcus pneumoniae (19, 20). The PS of these vaccines have been conjugated to proteins to enhance antibody titers (21) and T-cell memory responses (22).
Previously, we investigated PS from Brucella as a subunit vaccine candidate against brucellosis in three animal models, namely, mice, guinea pigs, and swine (9–11). The vaccine had the potential for being useful in developing countries, as it was highly effective in small doses without adjuvant, thermostable (extracted with dilute acetic acid and heat and had been stored lyophilized at room temperature for a decade before use), and sterile and did not require cold-chain refrigeration. In the findings now presented, it was investigated if PS from B. suis 145, a strain that expressed both A and M antigens, could give generic protection against Brucella species most pathogenic for humans, namely, B. abortus, B. melitensis, and B. suis that expressed the A, M, or A/M antigens.
Most of the PS of B. melitensis 16M is part of shed blebs (14), while that of B. abortus 1119-3 is part of LPS on the cell (15). As B. suis 145 expresses both, exo-PS (fraction B1) and cell-associated PS were extracted (fraction B2 was prepared from washed cells treated with diluted acetic acid and a boiling-water bath, and fraction B3 was additional PS from these same cells treated with diluted acetic acid and autoclaving). Nucleic acids in these fractions were below the level of detection (<0.1%), and protein was also very low (1 to 3%) (Table 1). These results were as expected given that nucleic acids sequester into the upper water layer of a phenol-water extraction, while Brucella PS sequesters into the lower phenol layer, and that the use of TCA precipitation and centrifugation removed proteins. All three fractions had KDO (0.007 to 0.08%), suggesting the presence of LPS, but Brucella PS contains KDO, a remnant of the core polysaccharide region that remains on the PS after LPS is hydrolyzed with acid and heat (23). Exo-PS fraction B1 did have LPS (about 37%), which was puzzling, as it had been subjected to the same conditions used to extract cell-associated PS fraction B2 and for the hydrolysis of purified B. suis 145 LPS to produce purified PS for comparison. We have observed that different LPS differ in the amount of acetic acid and time for hydrolysis in a boiling-water bath (B. abortus LPS < Y. enterocolitica O:9 LPS < B. melitensis LPS), and perhaps B. suis 145 shed some LPS that differed from cell-associated LPS.
The polysaccharide-containing fractions extracted from B. suis 145 reduced splenic colonization by B. abortus 30, B. melitensis 16M, and B. suis 145 in a mouse model (Fig. 1). Also, PS, released from B. suis 145 purified LPS hydrolyzed with either acid or base and heat, produced results similar to those of fractions B2 and B3, confirming that PS was the active component of the subunit vaccine. As noted before, exo-PS fraction B1 did appear different from the cell-associated fractions, with the former providing lower splenic bacterial colonization after challenge. Cole et al. (24) have reported that although the protective component of a Francisella tularensis subunit vaccine was the PS of its LPS, its protective efficacy was lost when the LPS was cleaved to release PS. Perhaps the same occurred for the B. suis 145 extracellular fraction in that some of its LPS resisted acid-heat hydrolysis, protecting a PS antigen that provided additional vaccine efficacy. Such an antigen could be a different A or M antigen similar to different A antigens observed for B. abortus and Yersinia enterocolitica O:9 (25), an additional “soluble supernatant substance” or “S antigen” proposed by researchers in the 1930s (26), or more recently the reported novel PS antigens of B. suis (27, 28). These possibilities could also explain how CE showed that anti-A, anti-M, and anti-A/M/C monoclonal antibodies bound to cell-associated PS fractions B2 and B3 but not the extracellular fraction B1 and how the subunit vaccine fractionated by pH had the two most effective fractions at the lowest and highest pI. As the extracellular and cell-associated fractions all reduced colonization by the noted three species of Brucella and were statistically highly different from saline but not from each other, and testing all fractions in all the efficacy studies reported was beyond our capability, these fractions were combined to form the B. suis 145 subunit vaccine used in further studies.
Our results show that very small amounts of our B. suis 145 subunit vaccine (without adjuvant) reduced mouse splenic colonization by the same strain (see Table S1 in the supplemental material) for 14 months (see Table S2 in the supplemental material). Anti-Brucella IgM and IgG titers in the sera of vaccinated mice were predominant at weeks 1 to 4 and weeks 5 to 9, respectively, although very small amounts of these antibodies were detected at longer times in some of the mice (Fig. 3). These results are similar to those reported for LPS extracted from F. tularensis live vaccine strain. In that study, long-term protection against tularemia was given to mice vaccinated by as little as 0.1 ng of LPS, and their immunity was induced by the PS component and correlated with anti-LPS antibody near the lower limit of detection (24). One nanogram or less of the B. suis 145 thermostable subunit vaccine in mice suggests that perhaps only a few μg would be needed for large animals or humans, a dose similar to that of protective antigen within anthrax vaccine adsorbed, used to protect or treat people against anthrax (29). For resource-poor countries, the amount of vaccine produced, and hence the reduction in cost, could be greatly enhanced by first passaging B. suis 145 through a mouse (a 5-fold increase in PS yield) and by not using TCA to remove glycoproteins (a 2-fold increase in PS yield). Alternatively, perhaps the most attractive approach would be for a pharmaceutical to produce PS synthetically (30), in bulk and without requiring containment or high security.
Further support would be needed to determine if the noted very small amounts of the B. suis 145 subunit vaccine would work in different animal models against a wide range of other smooth species and strains of Brucella. With regard to rough species and strains, it is unlikely that the PS vaccine would protect against those lacking PS on the surface. However, even for this situation, protection against Brucella has been identified as residing in the core polysaccharide of rough LPS (31). As part of this core region remains on the released polysaccharide after smooth LPS has been hydrolyzed, a PS vaccine against rough strains of Brucella should not be dismissed without investigation.
We acknowledge that, in our studies, large variations in individual CFU counts were observed within particular groups of mice. In the vaccinated groups, this variability may have been in part due to their very low, and in some cases no, splenic bacterial counts. This very low level of colonization after vaccination is striking 1 week after challenge, when mice had about 1,000-fold less splenic CFU than the nonvaccinated controls (Fig. 2).
In regard to the nonvaccinated control mice, some of the variance might have been due to the bacterium adapting to the host, enhancing its virulence and hence resulting in high but different CFU for different animals in the group. Another contributing factor to the large variation of splenic CFU for unvaccinated mice may be the nonspecific immunity of the host. It is accepted that not all in a herd will develop brucellosis when exposed to Brucella. Instead, the infection rates are usually 50 to 80% (32). In a laboratory incident where staff were exposed to B. melitensis, 8 of 26 microbiologists developed brucellosis (an attack rate of only 31%), and of these only a few showed mild or subclinical symptoms (33). In this study, for the unvaccinated control group challenged at 6 months (see Table S2 in the supplemental material), 3 of the 10 mice had very low splenic CFU. Upon assessing these 3 mice separately from their 7 littermates and comparing the former with the vaccinated group, the 3 unvaccinated mice had splenic CFU statistically significant from that of their littermates (P < 0.05) but not from that of the vaccinated group (P > 0.4). Perhaps the unvaccinated 3 mice that resisted Brucella challenge had been exposed to agents that engendered cross-protective immunity, as suggested several decades ago (34). Regardless of the mechanism by which livestock or people resist a Brucella infection, it would be worthwhile to develop a serological assay using surrogate markers that could assess their immune status against Brucella infection, be these anti-Brucella antibodies (Fig. 3) (35, 36), autoimmune anti-antibody produced during brucellosis (37), cytokines (38, 39), or other immune components (40–43) (see Fig. S3 to S5 in the supplemental material). Such an assay would be valuable to determine whether prize livestock at risk, or first responders facing a terrorist biothreat, are in need of vaccination or do not require this due to natural immunization that resulted in protective immunity.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Defence Research and Development Canada (DRDC) through its medical countermeasures program and Vaccine Development Initiative.
We thank the Canadian Food Inspection Agency—Nepean, specifically John Stevens, for the Brucella cultures, and the National Research Council of Canada—Ottawa, especially the late Malcolm Perry, David Bundle, and Margaret Gidney, for antigens and monoclonal antibodies. Our appreciation goes to Janet Payeur and Darla Ewalt of the U.S. Department of Agriculture, National Veterinary Services Laboratories, for the characterization and certification of Brucella species and strains used. Additional technical assistance was provided by students Monique L. van Hoek, Philip Wu, Deana Sabuda, Kris Hubler, Cindy Dykstra, Kamil Lotfali, Christopher A. Sikora, William Mauro, and Amy Turnbull. We thank Junfei Yin for creating some of the figures from the data. We kindly acknowledge the large number of BSL3 support staff, animal services, trades specialists, commissionaires, and managers at DRDC, Suffield Research Centre, who directly or indirectly contributed to this study.
All authors contributed to the presented data and contributed to the writing of the manuscript. All authors confirm that we have no commercial affiliations, consultancies, stock or equity interests, or patent licensing arrangements that could be considered to pose a conflict of interest regarding the submitted manuscript.
Footnotes
Published ahead of print 15 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00447-14.
REFERENCES
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91–99. 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.Domaradskij IV, Orent W. 2006. Achievements of the Soviet biological weapons programme and implications for the future. Rev. Sci. Tech. Off. Int. Epiz. 25:153–161. [DOI] [PubMed] [Google Scholar]
- 3.Pappas G, Panagopoulou P, Christou L, Akritidis N. 2006. Brucella as a biological weapon. Cell. Mol. Life Sci. 63:2229–2236. 10.1007/s00018-006-6311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doganay GD, Doganay M. 2013. Brucella as a potential agent of bioterrorism. Recent Pat. Antiinfect. Drug Discov. 8:27–33. 10.2174/1574891X11308010006. [DOI] [PubMed] [Google Scholar]
- 5.Mableson HE, Okello A, Picozzi K, Welburn SC. 2014. Neglected zoonotic diseases—the long and winding road to advocacy. PLoS Negl. Trop. Dis. 8(6):e2800. 10.1371/journal.pntd.0002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams E. 1988. Brucellosis in humans: its diagnosis and treatment. APMIS Suppl. 3:21–25. [PubMed] [Google Scholar]
- 7.Ficht TA, Kahl-McDonagh MM, Arenas-Gambos AM, Rice-Ficht AC. 2009. Brucellosis: the case for live, attenuated vaccines. Vaccine 27(Suppl 4 ):D40–D43. 10.1016/j.vaccine.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Kristensen D. 2009. Opportunities and challenges of developing thermostable vacines. Expert Rev. Vaccines 8:547–557. 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- 9.Cherwonogrodzky JW, Di Ninno VL. 1995. A polysaccharide vaccine to enhance immunity against brucellosis. Arch. Med. Vet. (Chile) 27:29–37. [Google Scholar]
- 10.Cherwonogrodzky JW, Wong JP, Di Ninno VL, Lord VR, Marino OC. 1996. A polysaccharide vaccine to enhance immunity against Brucella species, abstr 101.019, p 233 Abstr. Int. Congr. Infect. Dis. [Google Scholar]
- 11.Lord VR, Cherwonogrodzky JW, Schurig GG, Lord RD, Marcano MJ, Meléndez GE. 1998. Venezuelan field trials of vaccines against brucellosis in swine. Am J. Vet. Res. 59:546–551. [PubMed] [Google Scholar]
- 12.Asbakk K, Gall D, Stuen S. 1999. A screening ELISA for brucellosis in reindeer. Zentralbl. Veterinarmed. B 46:649–657. [DOI] [PubMed] [Google Scholar]
- 13.Bundle DR, Cherwonogrodzky JW, Perry MB. 1987. Structural elucidation of the Brucella melitensis M antigen by high-resolution NMR at 500 MHz. Biochemistry 26:8717–8726. 10.1021/bi00400a034. [DOI] [PubMed] [Google Scholar]
- 14.Avila-Calderon ED, Lopez-Merino A, Jain N, Peralta H, Lopez-Villegas EO, Sriranganathan N, Boyle SM, Witonsky S, Contreras-Rodriguez A. 2012. Characterization of outer membrane vesicles from Brucella melitensis and protection induced in mice. Clin. Dev. Immunol. 2012:352493. 10.1155/2012/352493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caroff M, Bundle DR, Perry MB, Cherwonogrodzky JW, Duncan JR. 1984. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 46:384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S-I, Lee YC. 2005. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 339:69–72. 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Cherwonogrodzky JW, Dubray G, Moreno E, Mayer H. 1990. Antigens of Brucella, p 19–64 In Nielsen K, Duncan JR. (ed), Animal brucellosis. CRC Press, Boca Raton, FL. [Google Scholar]
- 18.Lee CH, Tsai CM. 1999. Quantification of bacterial lipopolysaccharides by the Purpald assay: measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal. Biochem. 267:161–168. 10.1006/abio.1998.2961. [DOI] [PubMed] [Google Scholar]
- 19.Berti F, Adamo R. 2013. Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem. Biol. 8:1653–1663. 10.1021/cb400423g. [DOI] [PubMed] [Google Scholar]
- 20.Anwar E, Goldberg E, Fraser A, Acosta CJ, Paul M, Leibovici L. 2014. Vaccines for preventing typhoid fever. Cochrane Database Syst. Rev. 1:CD001261. 10.1002/14651858.CD001261.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Thiem VD, Lin FYC, Canh DG, Son NH, Anh DD, Mao ND, Chu C, Hunt SW, Robbins JB, Shneerson R, Szu SC. 2011. The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG Anti-Vi, and is compatible with routine infant vaccines. Clin. Vaccine Immunol. 18:730–735. 10.1128/CVI.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuberan B, Linhardt RJ. 2000. Carbohydrate based vaccines. Curr. Org. Chem. 4:653–677. 10.2174/1385272003376111. [DOI] [Google Scholar]
- 23.Zygmunt MS, Dubray G, Bundle DR, Perry MP. 1988. Purified native haptens of Brucella abortus B19 and B. melitensis 16M reveal the lipopolysaccharide origin of the antigens. Ann. Inst. Pasteur Microbiol. 139:421–433. [DOI] [PubMed] [Google Scholar]
- 24.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, Vogel SN. 2009. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc. Natl. Acad. Sci. U. S. A. 106:4343–4348. 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen K, Smith P, Yu W, Nicoletti P, Jungersen G, Stack J, Godfroid J. 2006. Serological discrimination by indirect enzyme immunoassay between the antibody response to Brucella sp. and Yersinia enterocolitica O:9 in cattle and pigs. Vet. Immunol. Immunopathol. 109:69–78. 10.1016/j.vetimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Wilson GS, Miles AA. 1948. Topley and Wilson's principles of bacteriology and immunity, 3rd ed, vol 1, p 825, 833, 834 Edward Arnold & Co., London, United Kingdom. [Google Scholar]
- 27.Zaccheus MV, Ali T, Cloeckaert A, Zygmunt MS, Wintraub A, Iriarte M, Moriyón I, Widmalm G. 2013. The epitopic and structural characterization of Brucella suis biovar 2 O-polysaccharide demonstrates the existence of a new M-negative C-negative smooth Brucella serovar. PLoS One 8:e53941. 10.1371/journal.pone.0053941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zygmunt MS, Jacques I, Bernardet N, Cloeckaert A. 2012. Lipopolysaccharide heterogeneity in the atypical group of novel emerging Brucella species. Clin. Vaccine Immunol. 19:1370–1373. 10.1128/CVI.00300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine. 2001. CDC anthrax vaccine safety and efficacy research program: interim report, p 14 National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 30.Guiard J, Paszkiewicz E, Dadowska J, Bundle DR. 2013. Design and synthesis of a universal antigen to detect brucellosis. Angew Chem. Int. Ed. Engl. 52:1–6. 10.1002/anie.201209858. [DOI] [PubMed] [Google Scholar]
- 31.Monreal D, Grilló MJ, González D, Marin CM, De Miguel MJ, López-Goñi I, Blasco JM, Cloeckaert A, Moriyón I. 2003. Characterization of Brucella abortus O-polysaccharide and core lipopolysaccharide mutants and demonstration that a complete core is required for rough vaccines to be efficient against Brucella abortus and Brucella ovis in the mouse model. Infect. Immun. 71:3261–3271. 10.1128/IAI.71.6.3261-3271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aparicio ED. 2013. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev. Sci. Tech. Off. Int. Epiz. 32:53–60. [PubMed] [Google Scholar]
- 33.Staszkiewicz J, Lewis CM, Colville J, Zervos M, Band J. 1991. Outbreak of Brucella melitensis among microbiology laboratory workers in a community hospital. J. Clin. Microbiol. 29:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrell ID, Robertson L, Hinchliffe PM. 1975. Serum antibody response in acute brucellosis. J. Hyg. Camb. 74:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherwonogrodzky JW, Nielsen KH. 1988. Brucella abortus 1119-3 O-chain polysaccharide to differentiate sera from B. abortus S-19-vaccinated and field-strain-infected cattle by agar gel immunodiffusion. J. Clin Microbiol. 26:1120–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu W, Gu D, Yuan Y, Hamit T. 1993. Research on anti-idiotypic antibodies to bovine abortus. Wei Sheng Wu Xue Bao 33:378–382. [PubMed] [Google Scholar]
- 37.Yumuk Z, Afacan G, Calişkan S, Irvem A, Arslan U. 2007. Relevance of autoantibody detection to the rapid diagnosis of brucellosis. Diagn. Microbiol. Infect. Dis. 58:271–273. 10.1016/j.diagmicrobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin CL, Goenka R. 2006. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit. Rev. Immunol. 26:407–442. 10.1615/CritRevImmunol.v26.i5.30. [DOI] [PubMed] [Google Scholar]
- 39.Skendros P, Boura P. 2013. Immunity to brucellosis. Rev. Sci. Tech. 32:137–147. [DOI] [PubMed] [Google Scholar]
- 40.Gao L, Shang S, Zhang C, Tong M, Chen Y. 2013. Lower mannose-binding lectin contributes to deleterious H1N1 2009 infection in children. APMIS 122:136–139. 10.1111/apm.12111. [DOI] [PubMed] [Google Scholar]
- 41.Niyonsaba F, Nagaoka I, Ogawa H. 2006. Human defensins and cathelicidins in the skin: beyond direct antimicrobial properties. Crit. Rev. Immunol. 26:545–576. 10.1615/CritRevImmunol.v26.i6.60. [DOI] [PubMed] [Google Scholar]
- 42.Ishag HZ, Li C, Huang L, Sun MX, Wang F, Ni B, Malik T, Chen PY, Mao X. 2013. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 158:349–358. 10.1007/s00705-012-1489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viriyakosol S, Jimenez Mdel P, Gurney MA, Ashbaugh ME, Fierer J. 2013. Dectin-1 is required for resistance to coccidioidomycosis in mice. mBio 4(1):e00597-12. 10.1128/mBio.00597-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.