Abstract
Given the resurgence of pertussis despite high rates of vaccination with the diphtheria-tetanus-acellular pertussis (DTaP) vaccine, a better understanding of vaccine-induced immune responses to Bordetella pertussis is needed. We investigated the antibody, cell-mediated, and cytokine responses to B. pertussis antigens in children who received the primary vaccination series (at 2, 4, and 6 months) and first booster vaccination (at 15 to 18 months) with 5-component acellular pertussis (aP) vaccine. The majority of subjects demonstrated a 4-fold increase in antibody titer to all four pertussis antigens (pertussis toxin [PT], pertactin [PRN], filamentous hemagglutinin [FHA], and fimbriae [FIM]) following the primary series and booster vaccination. Following the primary vaccine series, the majority of subjects (52 to 67%) mounted a positive T cell proliferative response (stimulation index of ≥3) to the PT and PRN antigens, while few subjects (7 to 12%) mounted positive proliferative responses to FHA and FIM. One month after booster vaccination (age 16 to 19 months), our study revealed significant increase in gamma interferon (IFN-γ) production in response to the PT and FIM antigens, a significant increase in IL-2 production with the PT, FHA, and PRN antigens, and a lack of significant interleukin-4 (IL-4) secretion with any of the antigens. While previous reports documented a mixed Th1/Th2 or Th2-skewed response to DTaP vaccine in children, our data suggest that following the first DTaP booster, children aged 16 to 19 months have a cytokine profile consistent with a Th1 response, which is known to be essential for clearance of pertussis infection. To better define aP-induced immune responses following the booster vaccine, further studies are needed to assess cytokine responses pre- and postbooster in DTaP recipients.
INTRODUCTION
Bordetella pertussis is a significant cause of morbidity and mortality worldwide, especially in young children (1, 2). Following widespread use of the whole-cell pertussis (wP) vaccines in the 1940s, the incidence of pertussis in the United States, which had previously exceeded 200,000 cases annually, declined dramatically (3, 4). Due to the relatively high rate of adverse local and systemic effects associated with wP vaccine, safer acellular pertussis (aP) vaccines replaced wP vaccine in the United States in the mid-1990s (5). The aP vaccine contains fewer antigens than the wP vaccine and lacks the reactogenic endotoxin (6). In the 1980s, the incidence of pertussis began to increase again, and despite high rates of immunization with the aP vaccine, over 48,000 cases of pertussis were reported in the United States by 2012, the highest incidence since 1955 (3). While infants continue to be at greatest risk for pertussis infection, there is evidence that the rate of pertussis has been increasing among adolescents and adults (3, 7). Moreover, older individuals play an important role in transmission of pertussis to young infants, who are at the highest risk of complications and mortality from infection (3, 7).
There are several theories that may explain the rise in cases of pertussis, including improved methods of detection such as PCR assays, vaccine-induced antigenic variation of the B. pertussis organism, poor or waning immunity conferred by the current aP vaccines, or some combination of these factors (1, 6, 8, 9). Given the resurgence in pertussis cases despite high vaccination rates, it is important to better characterize the mechanisms of immune protection against B. pertussis. While many human and mouse studies have examined the immune response to B. pertussis infection and vaccination, the exact mechanism of immunity and correlates of protection remain unclear (1, 10).
Several studies provide evidence for the roles of both antibody and cell-mediated immune (CMI) responses to B. pertussis (11–14) in prevention of disease and infection. Many human and mouse studies have investigated the relative contributions of Th1 (type 1 helper T cell) and Th2 (type 2 helper T cell) responses to pertussis infection and to both wP and aP vaccines (15–22). Most studies have found that natural pertussis infection and wP vaccine induce a predominantly Th1 response to pertussis antigens (15, 17–20). While the majority of studies with aP vaccine describe a mixed Th1/Th2 or Th2-predominant response (2, 12, 16, 18, 20), a few studies document a Th1-predominant response (21, 22). Furthermore, there are various results regarding which of the B. pertussis antigens are the most or least effective at inducing antibody and cell-mediated responses and cytokine production. In order to gain better understanding of vaccine-induced immune responses, our study aimed to investigate the antibody, cell-mediated, and cytokine responses to B. pertussis antigens in children under 2 years of age who received their primary series and first booster vaccination with multicomponent aP vaccine.
MATERIALS AND METHODS
Study design overview.
This was an open-label, single-arm, single-center, descriptive study designed to assess antibody and cell-mediated immune (CMI) responses to pertussis antigens in children who received the primary aP vaccine series and first booster. Subjects were enrolled from a local pediatric practice in Madison, TN, from September 2005 to February 2006. This study was approved by both the Western Institutional Review Board and Vanderbilt Institutional Review Board. Informed consent was obtained from the parents or legal guardians of all participants.
Vaccine.
The vaccine (Pentacel), manufactured by Sanofi Pasteur Limited, is a combination product. Each 0.5-ml dose contains 15 flocculation units (Lf) diphtheria toxoid, 5 Lf tetanus toxoid, and the following acellular pertussis antigens: 20 μg detoxified pertussis toxin (PT), 20 μg filamentous hemagglutinin (FHA), 3 μg pertactin (PRN), and 5 μg fimbria types 2 and 3 (FIM). It also includes inactivated poliovirus (IPV) (40 D-antigen units [DU] of type 1, 8 DU of type 2, and 32 DU of type 3 poliovirus) and 10 μg purified capsular polysaccharide of Haemophilus influenzae type b (Hib) covalently bound to 24 μg of tetanus toxoid. The vaccine contains 1.5 mg aluminum phosphate as the adjuvant.
Study population.
Criteria for enrollment were as follows: healthy infants 42 to 84 days of age, at least 37 weeks gestational age at delivery, free of obvious health problems as determined by medical history and clinical examination before entering the study, with no known or suspected impairment of immunologic function, and with no contraindication to the vaccine. Subjects with recent fever (less than 72 h prior to the visit) or with a history of having received the diphtheria-tetanus-aP (DTaP), DTwP, Hib conjugate, poliovirus, or pneumococcal conjugate vaccine prior to enrollment were excluded.
Study schedule and procedures.
Four doses of the combination study vaccine were administered at approximately 2, 4, 6, and 15 to 18 months of age. Other standard vaccines were given as recommended by the American Academy of Pediatrics (AAP) (5) (Table 1). Blood samples were collected for analysis of B. pertussis antigen-specific antibody and T cell proliferation prior to the first dose of Pentacel (at 2 months of age, pre-primary series), 1 month after the third dose (7 months, post-primary series), prior to the fourth dose (15 to 18 months, prebooster) and 1 month after the fourth dose (16 to 19 months, postbooster). Antigen-specific cytokine production was measured only in postbooster samples.
TABLE 1.
Overview of study schedule and procedures
| Parameter | Action at estimated age: |
||||||
|---|---|---|---|---|---|---|---|
| 2 mo (43–84 days) | 4 mo (90–152 days) | 6 mo (182–208 days) | 7 mo (209–237 days) | 12 mo (365–414 days) | 15–18 mo (439–537 days) | 16–19 mo (469–566 days) | |
| Sampling point | Pre-primary series | Post-primary series | Prebooster | Postbooster | |||
| Enrollment | X | ||||||
| Administration of Pentacel | X | X | X | X | |||
| Administration of standard vaccinesa | Prevnar, Hep B | Prevnar | Prevnar, Hep B | M-M-RII, Varivax, Prevnar | |||
| Blood sample for antibody and T cell response | X | X | X | X | |||
| Blood sample for cytokine level | X | ||||||
| Adverse event monitoring | X | X | X | X | X | X | X |
The first dose of hepatitis B (Hep B) vaccine was given between birth and 1 month of age. Influenza vaccine, if indicated, was given to subjects as recommended by the American Academy of Pediatrics after 6 months of age (5). Hep B vaccine (Recombivax HB), Merck & Co., Inc.; Prevnar, Lederle Laboratories, Pearl River, NY; M-M-RII, Merck & Co., Inc., West Point, PA; Varivax, Merck & Co., Inc., West Point, PA.
Serum antibody determinations.
Sera were tested for anti-PT, -FHA, -PRN, and -FIM immunoglobulin G (IgG) titers by enzyme-linked immunosorbent assay (ELISA) using a standardized protocol as previously described (23–25). ELISA units were assigned based on the U.S. Food and Drug Administration human reference pertussis antisera (lot 3). Immulon 2 plates were coated with optimized antigen concentrations of 1 μg/ml of PT, 2 μg/ml of FHA, 2 μg/ml of PRN, or 0.5 μg/ml of FIM. The lower limits of detection of IgG antibody were 2 EU/ml for PT, 3 EU/ml for FHA, and 5 EU/ml for FIM. Sequential serum samples from each subject were run simultaneously in the same assay. Serial 2-fold dilutions starting at 1:60 were performed for each sample. ELISA calculations were performed based on a log linear model using SoftMax Pro (Molecular Devices). Seroconversion to B. pertussis antigen was defined as a 4-fold increase in antibody titers from baseline (pre-primary series dose).
Lymphocyte sample processing.
Blood samples were collected, held at room temperature, and processed within 2 h. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Sigma-Aldrich) density gradient centrifugation and resuspended at a concentration of 1 × 107 cells/ml in freezing medium containing 90% fetal bovine serum (Invitrogen Life Technologies) and 10% dimethyl sulfoxide (DMSO). The cells were then transferred to cryogenic vials, placed in Nalgene cryofreezing containers (Nalge Nunc International), and stored at −80°C. Frozen specimens were transferred to a liquid nitrogen freezer and stored in the vapor phase. At the time of analysis, cryopreserved cells were thawed in a 37°C water bath, incubated with 20 μg/ml DNase (Roche), and washed twice. Viability was determined by trypan blue exclusion. The lymphocyte proliferation and cytokine assays were performed from January to May 2007.
Lymphocyte proliferation assay.
The capacity of PBMCs to respond to pertussis antigens was measured by antigen-specific proliferation. Triplicate cultures of PBMC suspension (2 × 105 cells/well) were incubated with 1 μg of heat-inactivated PT/ml, 10 μg FHA/ml, 10 μg FIM/ml, or 10 μg PRN/ml. PBMC cultures without stimulus were used as a negative control, and PBMC cultures stimulated with pokeweed mitogen (PWM) (1 μg/ml) were used as a positive control. After 5 days of incubation at 37°C in an atmosphere of 5% CO2, [3H]thymidine (0.5 μCi/well) was added to the cultures, and incorporated radioactivity was measured by scintillation counting 16 h later. The results were expressed as mean counts per minute (cpm) from triplicate wells. In the event that there were insufficient PBMCs to test all the pertussis antigens, we tested individual antigens in the following order of priority: PT, FIM, PRN, and then FHA. Only samples with viability of 70% or greater were used for analysis (average viability was 89.8%). A T cell proliferative response was defined to be positive when the B. pertussis antigen-induced proliferation was at least 3-fold higher than the spontaneous proliferation (stimulation index [SI] ≥ 3). As a basic quality criterion, the proliferative response of PBMCs to PWM had to be at least 10-fold higher than spontaneous proliferation (SI ≥ 10).
Cytokine assays.
Different CD4+ helper T cell subsets have distinct patterns of cytokine secretion (26). Th1 cells produce interleukin-2 (IL-2) as well as gamma interferon (IFN-γ), which regulates the cell-mediated immune response and helps macrophages kill engulfed intracellular pathogens (20, 22, 27). Th2 cells secrete IL-4, IL-5, and IL-13, which mediate defense against helminths and drive allergic disease (26, 27). Tumor necrosis factor alpha (TNF-α) has been measured as a Th1 cytokine (28) and as a control cytokine (29) which is produced by unstimulated, nonproliferating cells. IL-10 is secreted by various cells, including Th1, Th2, regulatory T cells, and innate immune cells (26, 30). For this study, we considered significant IL-2 and IFN-γ production to be consistent with a Th1 response and IL-4 and IL-5 to represent a Th2 response. IL-10 and TNF-α were not categorized as a predominately Th1 or Th2 response.
To determine antigen-specific cytokine production, a 100-μl aliquot of supernatant from cultures established for the measurement of lymphocyte proliferation was harvested at 48 h, quick-frozen, and stored at −80°C. Cultures were replenished with medium and kept until day 6 as described above. Cytokine bead arrays (CBA) detecting IFN-γ, TNF-α, IL-2, IL-4, IL-5, and IL-10 (Becton Dickinson) were used according to the manufacturer's instructions (31); intra- and interassay imprecisions of the CBA for all six cytokines have been previously evaluated by Tarnok et al. (32). The detection limits were 3.3 pg/ml for IFN-γ, 1.1 pg/ml for TNF-α, 1.1 pg/ml for IL-2, 1.3 pg/ml for IL-4, 1.3 pg/ml for IL-5, and 1.4 pg/ml for IL-10.
Statistical analysis.
The geometric mean titers (GMTs) of anti-PT, anti-FHA, anti-PRN, and anti-FIM antibody concentrations were calculated using the log transformation of concentrations and taking the antilog of the mean of these transformed values. The magnitudes of changes in the T cell proliferative response between the pre- and post-primary series time points and between the post-primary series and prebooster time points were compared using the Wilcoxon signed-rank test. The difference in cytokine production in response to pertussis antigen and in nonstimulated cultures was compared by Wilcoxon signed-rank test. For all analyses, a two-tailed P value of ≤0.05 was considered to be significant. All analyses were performed using R version 3.0.2 (www.r-project.org).
RESULTS
Study population.
A total of 50 subjects were enrolled and evaluated for antibody, proliferative, and cytokine responses. The mean age at enrollment was 62 days (43 to 84 days), the mean birth weight was 3.4 kg, and the mean gestational age was 39 weeks; 54% were female, and 74% were categorized as white, 16% as black, and 10% as “other” race. One subject dropped out of the study following the third vaccine, prior to the collection of the post-primary series vaccination sample, because the family relocated.
Humoral response.
Table 2 demonstrates serum antibody concentrations against the four B. pertussis antigens (PT, FHA, PRN, and FIM) and the percentages of subjects with seroconversion at the pre-primary series, post-primary series, prebooster, and postbooster sampling points. The trend in antibody response to each B. pertussis antigen at each sampling point is presented in Fig. 1. One month following the third vaccine dose (post-primary series, at approximately 7 months of age), the GMT to each pertussis antigen was significantly higher than the prevaccination levels. At the prebooster sampling point (15 to 18 months), the serum antibody titers to each B. pertussis antigen had declined significantly but remained higher than prevaccination levels. One month following the booster vaccination, antibody titers rose significantly to concentrations higher than post-primary series levels. In addition, the proportion of subjects with seroconversion to B. pertussis antigens increased from 81 to 92% post-primary series to 96 to 98% postbooster, depending on the specific B. pertussis antigen (Table 2).
TABLE 2.
Serum antibody responses to B. pertussis antigensa
| Sample (n) | PT |
FHA |
PRN |
FIM |
||||
|---|---|---|---|---|---|---|---|---|
| GMT (CI) | % with SC | GMT (CI) | % with SC | GMT (CI) | % with SC | GMT (CI) | % with SC | |
| Pre-primary series (48) | 2.3 (2.2–2.5) | 3.9 (3.5–4.5) | 2.8 (2.1–3.8) | 8.9 (7.6–10.3) | ||||
| Post-primary series (49) | 25.2 (20.6–30.5) | 92 | 49.3 (40.5–59.8) | 92 | 39.7 (30–51.8) | 81 | 157.0 (123.2–201.9) | 85 |
| Prebooster (47) | 5.3 (4.3–6.6) | 33 | 11.9 (9.4–15.5) | 42 | 8.2 (6.3–11.2) | 44 | 28.1 (22.0–37.4) | 33 |
| Postbooster (48) | 58.0 (46.3–72.3) | 98 | 97.6 (78.9–124.0) | 96 | 136.3 (96.6–185.5) | 96 | 427.7 (313.8–576.9) | 98 |
PT, pertussis toxin; FHA, filamentous hemagglutinin; PRN, pertactin; FIM, fimbria types 2 and 3. Concentrations of antibody specific to B. pertussis antigens (PT, FHA, PRN, and FIM) are reported as geometric mean titer (GMT) with 95% bootstrap confidence intervals (CI), and the percentages of evaluated subjects with seroconversion (SC) are shown.
FIG 1.
Trend for antibody response to each B. pertussis antigen during the vaccination series. Antibody titers are reported as geometric mean titer (GMT) with 95% confidence intervals.
Proliferative response.
Table 3 demonstrates T cell proliferative responses to B. pertussis antigens. Prior to vaccination, none of the B. pertussis antigens induced a positive proliferative response. Following the primary vaccination series, only the PT and PRN antigens induced positive proliferative responses, with a median SI of ≥3. The frequency of post-primary series positive proliferative response was highest for PT (67% of subjects) and PRN (52%) and lowest for FHA (7%) and FIM (12%).
TABLE 3.
T-cell proliferative responses to B. pertussis antigens
| Sample | PT |
FHA |
PRN |
FIM |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | SIa | Pb | % CMI+c | n | SI | P | % CMI+ | n | SI | P | % CMI+ | n | SI | P | % CMI+ | |
| Pre-primary series | 34 | 0.9, 1.0, 1.2 | 0 | 28 | 0.1, 0.2, 0.3 | 0 | 27 | 1.0, 1.5, 2.5 | 15 | 25 | 0.6, 0.8, 1.0 | 0 | ||||
| Post-primary series | 33 | 2.5, 3.9, 5.8 | <0.001 | 67 | 29 | 0.4, 0.7, 1.5 | 0.008 | 7 | 29 | 1.9, 3.0, 5.5 | 0.002 | 52 | 24 | 1.1, 1.3, 1.6 | <0.001 | 12 |
| Prebooster | 43 | 1.2, 1.7, 3.2 | 0.032 | 37 | 34 | 0.3, 0.6, 1.4 | 0.984 | 9 | 31 | 1.4, 2.0, 2.8 | 0.058 | 19 | 27 | 0.8, 1.1, 1.7 | 1 | 0 |
| Postbooster | 37 | 1.3, 3.3, 5.1 | 54 | 29 | 0.3, 0.9, 2.1 | 14 | 21 | 1.2, 1.7, 2.5 | 24 | 18 | 0.7, 1.1, 1.5 | 0 | ||||
SI is presented as median with interquartile range (lower quartile, median, upper quartile).
The magnitudes of T cell proliferative responses were compared between the pre- and post-primary series time points and between the post-primary series and prebooster time points by using the Wilcoxon signed-rank test. A P value of ≤0.05 is considered statistically significant.
Percentage of subjects with a positive cell-mediated immune response (i.e., SI ≥ 3).
The proliferative response to PT decreased significantly by the prebooster sampling point compared to the post-primary series response. Following the booster vaccine, the proliferative response to PT antigen increased from a median SI of 1.7 to 3.3, and the proportion of subjects with positive PT-specific proliferative response increased from 37% to 54%. However, the postbooster proliferative response to FHA, PRN, and FIM antigens did not increase; the median SI was <3 for each of these antigens.
Overall, the proliferative response to FIM was very poor, with a minority of subjects mounting a significant proliferative response post-primary series and none of the evaluable subjects mounting a positive proliferative response at the pre- or postbooster time point. Of note, at the postbooster sampling point, there were fewer evaluable samples for the FIM antigen than for the other antigens (n = 18 for FIM, compared to n = 21 to 37 for other antigens).
Cytokine profile.
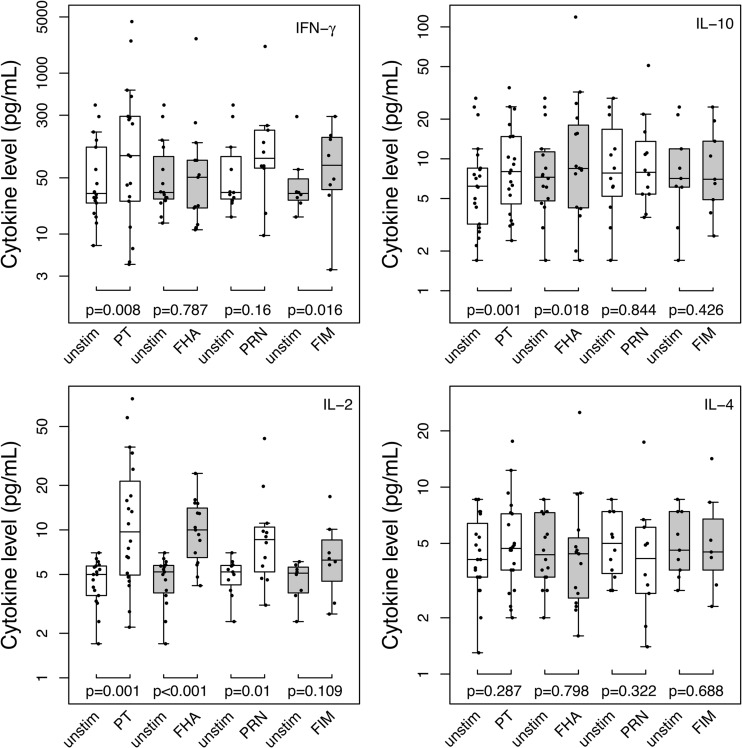
Cytokine secretion by antigen-stimulated PBMCs postbooster is summarized in Fig. 2. After comparing B. pertussis antigen-induced cytokine production with cytokine levels without antigen stimulation, a significant increase in IFN-γ secretion in response to PT and FIM was noted (P = 0.008 and 0.016, respectively). There was also a significant increase in IL-2 production in response to the PT, FHA, and PRN antigens (P = 0.001, P < 0.001, and P = 0.01, respectively). There was no statistically significant increase in IL-4 secretion in response to any studied antigen. We were unable to perform statistical analysis of IL-5 production because too few subjects' PBMCs secreted detectable amounts of IL-5 both under unstimulated conditions and in response to antigen stimulation. Subjects did produce IL-5 in response to mitogen stimulation, indicating that the assay conditions for cytokine measurement were satisfactory. There was significant increase in IL-10 production in response to the PT and FHA antigens (P = 0.01 and 0.018, respectively). TNF-α production did not increase significantly from baseline in response to any of the pertussis antigens.
FIG 2.
Cytokine secretion by antigen-stimulated PBMCs, measured 1 month following aP booster. Cytokine (IFN-γ, IL-2, IL-10, and IL-4) production in response to pertussis antigens (PT, FHA, PRN, and FIM) and under unstimulated conditions (unstim) was compared by using the Wilcoxon signed-rank test. Cytokine levels are plotted as box-and-whisker plots. The bottom and top of the box represent the first and third quartiles, respectively, and the horizontal band inside the box represents the median. The ends of the whiskers represent the minimum and maximum values, excluding outliers. A two-tailed P value of ≤0.05 was considered to represent a significant increase in cytokine production in response to the tested antigen.
DISCUSSION
The majority of our study subjects demonstrated significant increases in antibody responses to all four B. pertussis antigens following the primary DTaP vaccination series. Antibody titers declined prior to the fourth dose (booster) but then increased significantly after the fourth dose, with higher antibody titers achieved than after the primary vaccine series. The rapid decline in antibody titers prior to the booster dose has been illustrated in many studies (13, 22, 33) and supports the importance of a pertussis vaccine booster dose in the second year of life.
Although there is conflicting evidence regarding which B. pertussis antigens are considered most important for protection against disease (6, 34, 35), there is evidence that optimal anti-FIM antibody concentrations reduce the short-term risk of pertussis in young children (36, 37). While PT, a key protective B. pertussis antigen, is a component of all current aP vaccines, FIM antigen is not present in all aP vaccines used globally (1, 9, 38, 39). Given recent evidence that PRN-deficient strains of B. pertussis are now circulating widely in the United States (40) and since our study revealed that the FIM-containing aP vaccine was effective in inducing an anti-FIM humoral response, the inclusion of immunogenic FIM in vaccine preparations may be important for enhanced protection. Further studies examining the anti-FIM antibody response are needed.
In our cohort, when comparing post-primary to pre-primary vaccination series samples, the proliferative response to PT and PRN antigens was positive in the majority of subjects, while only a minority of subjects mounted an adequate proliferative response to FHA and FIM. In contrast, Zepp et al. investigated proliferative responses at 1 month after a primary series of a 3-component (PT, FHA, and PRN) DTaP vaccine given at 3, 4, and 5 months and reported a strong T cell proliferative response for all 3 pertussis antigens (PT, FHA, and PRN) (22). Unlike in two previous studies (13, 22) reporting stable or even increased T cell proliferative responses measured at 12 to 14 months of age following a primary vaccination series with 3-component aP (13, 22), the children in our cohort revealed a decrease in proliferative responses to PT and PRN prior to the booster series. Unexpectedly, following the booster vaccination at 15 to 18 months in our cohort, only a PT-specific response remained significant (median SI ≥ 3), while poor proliferative responses to the other B. pertussis antigens were observed. The differences in T cell proliferative response to various antigens observed between studies may be explained by various antigen concentrations within the aP vaccines and slightly differing vaccination and sampling protocols.
Our analysis of the pattern of cytokine secretion in young infants is unique in that we investigated cytokine responses after the fourth dose of DTaP (postbooster, age 16 to 19 months), while other studies measured cytokine responses at various other time points. While interpreting cytokine secretion profiles, it is important to note that the cytokine response to purified antigens may not exactly reflect the response to whole bacteria in B. pertussis-infected patients. Our study results suggest preferential induction of Th1 cytokines, as evidenced by a significant increase in IFN-γ production in response to the PT and FIM antigens and a significant increase in IL-2 production in response to the PT, FHA, and PRN antigens. The lack of a significant increase in IL-4 secretion with any of the B. pertussis antigens and the lack of IL-5 production under unstimulated and B. pertussis antigen-stimulated conditions suggest that our subjects lacked a significant Th2 response. This Th1 cytokine pattern is similar to that seen with wP and natural infection and has been shown in humans and mice to be critical for clearance of pertussis infection (17, 19, 41). Studies in older children between 4 and 6 years of age (who had received 3-component primary aP vaccination) reported higher levels of the Th1 cytokines IFN-γ and IL-2 than of Th2 cytokines (11, 29). These authors suggested that given the relatively high exposure to B. pertussis in this Italian cohort, subclinical pertussis infection over time may have affected the immune response in these subjects. Other investigators (Zepp et al.) who noted a Th1-predominant cytokine profile in response to DTaP vaccine in infants used IL-10 as the sole marker for a Th2 profile (21, 22). However, while IL-10 was previously considered a Th2 cytokine (particularly in mice), it is now known that in humans, IL-10 is not secreted by all Th2 cells and is produced by various cell types, including Th1, Th2, regulatory T cells, and innate immune cells (26, 30). Since IL-10 is not an exclusive Th2 cytokine, conclusions about Th2 predominance cannot be made based on the lack of significant IL-10 production in the studies by Zepp et al. (21, 22) or the presence of a significant IL-10 in response to the PT and FHA antigens observed in our cohort.
More commonly, a Th2 or mixed Th1/Th2 cytokine profile has been reported with aP vaccination (16, 18, 20, 42) at various time points, including 2 months after primary 2-component (PT and FHA) aP vaccination (16), 1 month following primary 3-component (PT, FHA, and PRN) aP vaccination (42), and 2 to 4 years after primary 5-component (PT, FHA, PRN, and FIM 2/3) aP vaccination (20). Studies also show that a DTaP booster administered between 4 and 6 years of age in children previously primed with DTaP induced a Th2 or mixed Th1/Th2 cytokine profile (20, 43, 44). A potential explanation for the difference in cytokine profile observed in our study population compared with other studies may be that cellular immunity during infancy may vary with age. Rowe et al. (45) analyzed tetanus-specific and polyclonal cytokine responses in infants from age 2 to 18 months. They found that the Th2 cytokine response peaks at 12 months and then declines. Meanwhile, IFN-γ production (Th1) initially develops rapidly, declines around 6 months, remains low through age 12 months, and then resurges between 12 and 18 months. Since we measured cytokine responses at the postbooster period (16 to 19 months), it is possible that the cytokine profile observed in our subjects reflects the normal age-related variability of cellular immunity in infants. Furthermore, the significant levels of spontaneous IFN-γ secretion in this population may indicate an intrinsic ability of PBMCs to secrete IFN-γ at this stage.
Our study has a number of limitations. We analyzed cytokine profiles only following the booster vaccine, and we do not have prebooster sample analysis to serve as a control. It would be important to measure cytokine secretion prebooster in order to discriminate between responses specifically due to the vaccine booster (i.e., adaptive immune responses related to memory immune cells) versus a nonspecific immune response. Therefore, our data do not rule out a nonspecific immune response (perhaps age related) that is not due to the vaccine itself. Further study is needed, measuring cytokine production both pre- and postbooster. In addition, the cytokine profile observed in our study may have been affected by antigens within vaccines coadministered with DTaP (e.g., IPV and Hib). As the AAP recommends that DTaP, IPV, and Hib vaccinations be given at approximately the same time point, it may be impractical to administer only the DTaP vaccination without the other components of the Pentacel vaccine. Studies of nonvaccinated control subjects would not have been ethical since DTaP vaccines are recommended for all children. The interpretation of data for T cell proliferative response and cytokine production is limited by the fact that many samples were not evaluable due to the limited quantity of PBMCs recovered from some of the subjects, and priority for analysis was given first to PT, followed by the FIM, PRN, and FHA antigens. It was particularly difficult to interpret cell-mediated and cytokine responses to FIM because there were significantly fewer evaluable samples for the FIM antigen. Although we did not specifically test for pertussis infection in this cohort, it is unlikely that the Th1 cytokine profile was due to subclinical pertussis infection during the study. From the post-primary series to prebooster sampling points, only four subjects had an increase in antibody titer to FHA only, one had a slightly increased titer to PT, and one had increased titers to all four antigens. While PT is a B. pertussis-specific antigen, FHA antigen is also found in Bordetella parapertussis and nonencapsulated Haemophilus influenzae strains (46–48). Therefore, while it is possible that two subjects may have experienced subclinical pertussis during the study period, this is unlikely to fully explain our findings.
Our study has several strengths. Although it is often difficult to obtain sufficient blood samples for studies of infants, we were able to collect blood from a substantial number of children, including those younger than 6 months. Our study investigated the immune response to the 5-component aP vaccine and examined the immune response to four pertussis antigens, including FIM, which is often excluded in other studies. We measured several different Th1 and Th2 cytokines, thus allowing more complete examination of the pattern of cytokine secretion. We also examined Th1 and Th2 cytokines at a unique time point (1 month after the booster vaccination administered at 15 to 18 months), thus providing insight into infants' immune response at an important stage in the pertussis vaccine schedule, as children do not receive their next aP vaccination until 4 to 6 years of age.
While it has been suggested that the cell-mediated immune response may be a more reliable correlate of protection from pertussis infection than the humoral response (22), the generally weaker T cell proliferative response to booster vaccination in our subjects supports the notion that the relative importance of each arm of the adaptive immune response may depend partly on the specific pertussis antigen against which the response is directed (49). It is often postulated that the failure of aP vaccine to induce a strong Th1 response is one explanation for the increasing incidence of pertussis infection (1). The Th1-consistent cytokine profile following aP booster vaccination in our subjects supports the importance of a fourth vaccine dose at this age. This study suggests that the immune response induced by aP likely depends on several factors, including the age of recipients, the vaccination schedule, the balance of antigens within vaccines, and the individual host's propensity for a Th1 versus Th2 response. Recent animal studies indicate that another CD4+ T helper cell subset, Th17 cells, may also be important for controlling B. pertussis infection (2, 50). Larger studies are needed that investigate, among children primed with aP, a broad spectrum of aP-induced cytokines, including IL-17, at various time points, including both pre- and postbooster. In addition, further studies are needed to determine the roles of various T cell subsets (Th1, Th2, and Th17) in protecting against human pertussis infection, as well as which antigens in the pertussis vaccine are most effective at eliciting protective immune response against pertussis.
ACKNOWLEDGMENTS
We thank Kathryn M. Edwards and Michael T. Rock for reviewing our manuscript, monitoring study procedures, and giving input on the Materials and Methods section of the manuscript. We are also grateful to Catherine Dundon, Goodlettsville Pediatrics, and the study subjects and their families for participating in this study.
This work was supported by an investigator-initiated grant provided by Sanofi Pasteur. The project publication described was supported by CTSA award no. UL1TR000445 from the National Center for Advancing Translational Sciences.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Published ahead of print 24 September 2014
REFERENCES
- 1.Higgs R, Higgins SC, Ross PJ, Mills KH. 2012. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 5:485–500. 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- 2.Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. 2013. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 9:e1003264. 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2012. Pertussis: surveillance & reporting. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/pertussis/surv-reporting.html. [Google Scholar]
- 4.Guris D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin. Infect. Dis. 28:1230–1237. 10.1086/514776. [DOI] [PubMed] [Google Scholar]
- 5.Pickering LK. (ed). 2012. Red book, 29th ed, p 553–566 American Academy of Pediatrics, Elk Grove Village, IL. [Google Scholar]
- 6.Cherry JD. 2013. Pertussis: challenges today and for the future. PLoS Pathog. 9:e1003418. 10.1371/journal.ppat.1003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamberger ES, Srugo I. 2008. What is new in pertussis? Eur. J. Pediatr. 167:133–139. 10.1007/s00431-007-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA, Skoff TH. 2013. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 131:e1047–e1052. 10.1542/peds.2012-1928. [DOI] [PubMed] [Google Scholar]
- 9.Edwards KM. 2014. Unraveling the challenges of pertussis. Proc. Natl. Acad. Sci. U. S. A. 111:575–576. 10.1073/pnas.1321360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schure RM, Hendrikx LH, de Rond LG, Ozturk K, Sanders EA, Berbers GA, Buisman AM. 2013. Differential T- and B-cell responses to pertussis in acellular vaccine-primed versus whole-cell vaccine-primed children 2 years after preschool acellular booster vaccination. Clin. Vaccine Immunol. 20:1388–1395. 10.1128/CVI.00270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ausiello CM, Lande R, Urbani F, Di Carlo B, Stefanelli P, Salmaso S, Mastrantonio P, Cassone A. 2000. Cell-mediated immunity and antibody responses to Bordetella pertussis antigens in children with a history of pertussis infection and in recipients of an acellular pertussis vaccine. J. Infect. Dis. 181:1989–1995. 10.1086/315509. [DOI] [PubMed] [Google Scholar]
- 12.Ausiello CM, Urbani F, La Sala A, Lande R, Piscitelli A, Cassone A. 1997. Acellular vaccines induce cell-mediated immunity to Bordetella pertussis antigens in infants undergoing primary vaccination against pertussis. Dev. Biol. Stand. 89:315–320. [PubMed] [Google Scholar]
- 13.Cassone A, Ausiello CM, Urbani F, Lande R, Giuliano M, La Sala A, Piscitelli A, Salmaso S. 1997. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. The Progetto Pertosse-CMI Working Group. Arch. Pediatr. Adolesc. Med. 151:283–289. 10.1001/archpedi.1997.02170400069013. [DOI] [PubMed] [Google Scholar]
- 14.Rieber N, Graf A, Belohradsky BH, Hartl D, Urschel S, Riffelmann M, Wirsing von Konig CH, Liese J. 2008. Differences of humoral and cellular immune response to an acellular pertussis booster in adolescents with a whole cell or acellular primary vaccination. Vaccine 26:6929–6935. 10.1016/j.vaccine.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 15.Mahon BP, Sheahan BJ, Griffin F, Murphy G, Mills KH. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med. 186:1843–1851. 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascart F, Hainaut M, Peltier A, Verscheure V, Levy J, Locht C. 2007. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 25:391–398. 10.1016/j.vaccine.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Mascart F, Verscheure V, Malfroot A, Hainaut M, Pierard D, Temerman S, Peltier A, Debrie AS, Levy J, Del Giudice G, Locht C. 2003. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J. Immunol. 170:1504–1509. 10.4049/jimmunol.170.3.1504. [DOI] [PubMed] [Google Scholar]
- 18.Ryan M, Gothefors L, Storsaeter J, Mills KH. 1997. Bordetella pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice. Dev. Biol. Stand. 89:297–305. [PubMed] [Google Scholar]
- 19.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills KH. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 175:1246–1250. 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 20.Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Oymar K, Miller E, Storsaeter J, Mills KH. 1998. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93:1–10. 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zepp F, Knuf M, Habermehl P, Schmitt HJ, Meyer C, Clemens R, Slaoui M. 1997. Cell-mediated immunity after pertussis vaccination and after natural infection. Dev. Biol. Stand. 89:307–314. [PubMed] [Google Scholar]
- 22.Zepp F, Knuf M, Habermehl P, Schmitt JH, Rebsch C, Schmidtke P, Clemens R, Slaoui M. 1996. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect. Immun. 64:4078–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherry JD, Heininger U, Christenson PD, Eckhardt T, Laussucq S, Hackell JG, Mezzatesta JR, Stehr K. 1995. Surrogate serologic tests for the prediction of pertussis vaccine efficacy. Ann. N. Y. Acad. Sci. 754:359–363. 10.1111/j.1749-6632.1995.tb44470.x. [DOI] [PubMed] [Google Scholar]
- 24.Deen JL, Mink CA, Cherry JD, Christenson PD, Pineda EF, Lewis K, Blumberg DA, Ross LA. 1995. Household contact study of Bordetella pertussis infections. Clin. Infect. Dis. 21:1211–1219. 10.1093/clinids/21.5.1211. [DOI] [PubMed] [Google Scholar]
- 25.Meyer CU, Zepp F, Decker M, Lee M, Chang SJ, Ward J, Yoder S, Bogaert H, Edwards KM. 2007. Cellular immunity in adolescents and adults following acellular pertussis vaccine administration. Clin. Vaccine Immunol. 14:288–292. 10.1128/CVI.00364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ. 2012. Helper T cell diversity and plasticity. Curr. Opin. Immunol. 24:297–302. 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirahara K, Vahedi G, Ghoreschi K, Yang XP, Nakayamada S, Kanno Y, O'Shea JJ, Laurence A. 2011. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology 134:235–245. 10.1111/j.1365-2567.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieber N, Graf A, Hartl D, Urschel S, Belohradsky BH, Liese J. 2011. Acellular pertussis booster in adolescents induces Th1 and memory CD8+ T cell immune response. PLoS One 6:e17271. 10.1371/journal.pone.0017271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ausiello CM, Lande R, Urbani F, la Sala A, Stefanelli P, Salmaso S, Mastrantonio P, Cassone A. 1999. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect. Immun. 67:4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. 1993. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J. Immunol. 150:353–360. [PubMed] [Google Scholar]
- 31.Rock MT, Yoder SM, Talbot TR, Edwards KM, Crowe JE., Jr 2004. Adverse events after smallpox immunizations are associated with alterations in systemic cytokine levels. J. Infect. Dis. 189:1401–1410. 10.1086/382510. [DOI] [PubMed] [Google Scholar]
- 32.Tarnok A, Hambsch J, Chen R, Varro R. 2003. Cytometric bead array to measure six cytokines in twenty-five microliters of serum. Clin. Chem. 49:1000–1002. 10.1373/49.6.1000. [DOI] [PubMed] [Google Scholar]
- 33.Tomoda T, Ogura H, Kurashige T. 1991. Immune responses to Bordetella pertussis infection and vaccination. J. Infect. Dis. 163:559–563. 10.1093/infdis/163.3.559. [DOI] [PubMed] [Google Scholar]
- 34.Robbins JB, Schneerson R, Keith JM, Miller MA, Kubler-Kielb J, Trollfors B. 2009. Pertussis vaccine: a critique. Pediatr. Infect. Dis. J. 28:237–241. 10.1097/INF.0b013e31818a8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe M, Connelly B, Weiss AA. 2006. Characterization of serological responses to pertussis. Clin. Vaccine Immunol. 13:341–348. 10.1128/CVI.13.3.341-348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallander HO, Ljungman M, Jahnmatz M, Storsaeter J, Nilsson L, Gustafsson L. 2009. Should fimbriae be included in pertussis vaccines? Studies on ELISA IgG anti-Fim2/3 antibodies after vaccination and infection. APMIS 117:660–671. 10.1111/j.1600-0463.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 37.Storsaeter J, Hallander HO, Gustafsson L, Olin P. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907–1916. 10.1016/S0264-410X(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. 1997. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 46:1–25. [PubMed] [Google Scholar]
- 39.WHO. 2010. Pertussis vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:385–400. [PubMed] [Google Scholar]
- 40.Pawloski LC, Queenan AM, Cassiday PK, Lynch AS, Harrison MJ, Shang W, Williams MM, Bowden KE, Burgos-Rivera B, Qin X, Messonnier N, Tondella ML. 2014. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin. Vaccine Immunol. 21:119–125. 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills KH, Barnard A, Watkins J, Redhead K. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. 1997. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 65:2168–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan EJ, Nilsson L, Kjellman N, Gothefors L, Mills KH. 2000. Booster immunization of children with an acellular pertussis vaccine enhances Th2 cytokine production and serum IgE responses against pertussis toxin but not against common allergens. Clin. Exp. Immunol. 121:193–200. 10.1046/j.1365-2249.2000.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheifele DW, Ochnio JJ, Halperin SA. 2009. Cellular immunity as a potential cause of local reactions to booster vaccination with diphtheria and tetanus toxoids and acellular pertussis antigens. Pediatr. Infect. Dis. J. 28:985–989. 10.1097/INF.0b013e3181a9cc2a. [DOI] [PubMed] [Google Scholar]
- 45.Rowe J, Macaubas C, Monger T, Holt BJ, Harvey J, Poolman JT, Loh R, Sly PD, Holt PG. 2001. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic Th1 function. J. Infect. Dis. 184:80–88. 10.1086/320996. [DOI] [PubMed] [Google Scholar]
- 46.Barenkamp SJ, Leininger E. 1992. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect. Immun. 60:1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Q, Viljanen MK, Arvilommi H, Aittanen B, Mertsola J. 1998. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA 280:635–637. 10.1001/jama.280.7.635. [DOI] [PubMed] [Google Scholar]
- 48.Isacson J, Trollfors B, Taranger J, Lagergard T. 1995. Acquisition of IgG serum antibodies against two Bordetella antigens (filamentous hemagglutinin and pertactin) in children with no symptoms of pertussis. Pediatr. Infect. Dis. J. 14:517–521. 10.1097/00006454-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Mills KH, Ryan M, Ryan E, Mahon BP. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. U. S. A. 111:787–792. 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]