Abstract
The diagnosis of active tuberculosis (TB) disease remains a challenge, especially in high-burden settings. Cytokines and chemokines are important in the pathogenesis of TB. Here we investigate the usefulness of circulating and compartmentalized cytokines/chemokines for diagnosis of TB. The levels of multiple cytokines/chemokines in plasma, pleural fluid (PF), and cerebrospinal fluid (CSF) were determined by Luminex liquid array-based multiplexed immunoassays. Three of 26 cytokines/chemokines in plasma were significantly different between TB and latent tuberculosis infection (LTBI). Among them, IP-10 and MIG had the highest diagnostic values, with an area under the receiver operating characteristic curve (ROC AUC) of 0.92 for IP-10 and 0.86 for MIG for distinguishing TB from LTBI. However, IP-10 and MIG levels in plasma were not different between TB and non-TB lung disease. In contrast, compartmentalized IP-10 and MIG in the PF and CSF showed promising diagnostic values in discriminating TB and non-TB pleural effusion (AUC = 0.87 for IP-10 and 0.93 for MIG), as well as TB meningitis and non-TB meningitis (AUC = 0.9 for IP-10 and 0.95 for MIG). A longitudinal study showed that the plasma levels of IP-10, MIG, granulocyte colony-stimulating factor (G-CSF), and gamma interferon (IFN-γ) decreased, while the levels of MCP-1/CCL2 and eotaxin-1/CCL11 increased, after successful treatment of TB. Our findings provide a practical methodology for discriminating active TB from LTBI by sequential IFN-γ release assays (IGRAs) and plasma IP-10 testing, while increased IP-10 and MIG at the site of infection (PF or CSF) can be used as a marker for distinguishing pleural effusion and meningitis caused by TB from those of non-TB origins.
INTRODUCTION
Accurate diagnosis of active tuberculosis (TB) remains a challenge in clinical practice, especially in high-burden settings. Active pulmonary TB (PTB) presents a spectrum of clinical manifestations, which may complicate the diagnosis. However, none of the common TB symptoms are tuberculosis disease specific. Additionally, many patients, especially those identified during routine physical examination, do not display clear symptoms or at least some obvious manifestations. Although detection of Mycobacterium tuberculosis in sputum by use of bacteriological assays is a gold standard for active TB, the poor sensitivity of sputum smear microscopy, which may be as low as 35% in settings with high rates of TB and HIV coinfection, might lead to a missed diagnosis (1, 2). While culture of M. tuberculosis in sputum is substantially more sensitive (up to 60%) than smear microscopy, the Achilles heel of this approach is the long duration prior to results (10 to 14 days for liquid culture and 3 to 4 weeks for solid culture), which is a consequence of the long doubling time of M. tuberculosis (1). A new quantitative PCR test (Xpert MTB/RIF) has been reported to more sensitive than sputum smear microscopy, but the sensitivity of the Xpert MTB/RIF test was inversely correlated with bacillary burdens in sputum samples (3, 4). A recent report indicated a sensitivity of 86.1% for the Xpert MTB/RIF test and 69.4% for nested PCR for detection of M. tuberculosis during routine clinical practice in a geographical area with intermediate TB incidence (5). Notably, the diagnostic value of the Xpert MTB/RIF test was limited when it was used for diagnosis of extrapulmonary TB (patients without sputum samples), such as tuberculous pericarditis, with a sensitivity that was inferior even to that of unstimulated gamma interferon (IFN-γ) (6).
Recently, IFN-γ release assays (IGRAs) were developed to identify individuals infected with M. tuberculosis (7, 8). They have proved useful for the diagnosis of latent tuberculosis infection (LTBI) but less so for the diagnosis of active TB disease (7). Two systematic reviews of published studies showed that the two currently commercially available IGRAs, the QuantiFERON TB test (QFT) (Cellestis, Victoria, Australia) and the T-SPOT.TB test (Oxford Immunotec, Abington, United Kingdom), have limited power to distinguish active TB from LTBI (8, 9). As a consequence, identification of biomarkers that can accurately distinguish individuals with active TB from those with LTBI has been designated an important priority in TB research (10, 11).
Active TB manifests as excessive inflammatory responses to persistent M. tuberculosis infection due to inadequate host immunity. A whole-blood, 393-transcript signature of active TB, which was dominated by a neutrophil-driven, interferon-inducible gene profile, was identified to distinguish active TB from LTBI, an asymptomatic M. tuberculosis infection without overwhelming inflammation due to effective containment of infected M. tuberculosis by host immunity (12). Additionally, a whole-blood, 86-gene transcriptional signature of active TB is distinct from those of other diseases (12). Cytokines and chemokines have been recognized to play important roles in shaping immunity against TB through polarizing T cell subset responses, regulating immune cell trafficking, and, consequently, regulating inflammatory responses (13). Therefore, it was not surprising that patients with active pulmonary TB had significantly higher concentrations of in vitro M. tuberculosis antigen-induced IFN-γ, IP-10, MIG, tumor necrosis factor alpha (TNF-α), and interleukin-2 (IL-2) than did healthy controls and other pulmonary disease controls (14). However, measurement of antigen-induced cytokines/chemokines by use of peripheral blood mononuclear cells (PBMC) is still labor-intensive and time-consuming, and therefore not convenient in clinical practice. On this basis, we speculated that cytokines/chemokines in circulation (plasma) and at the sites of disease (such as pleural fluid [PF] and cerebrospinal fluid [CSF]) can be used for diagnosis. To test this hypothesis, cytokines and chemokines previously reported to be involved in the host immune response and/or pathogenesis of TB were selected for evaluation. These included cytokines shaping host immunity (IL-1β, IL-4, IL-6, IL-9, IL-10, IL-12, IL-13, IL-17A, IFN-γ, IL-22, and granulocyte colony-stimulating factor [G-CSF]), chemokines regulating immune cell trafficking (monocyte chemoattractant protein 1 [MCP-1], MCP-2, MCP-3, eotaxin, macrophage inflammatory protein 3α [MIP-3α], 6Ckine, MDC, MIG, IP-10, CXCL-12, and CXCL-13), and cytokines regulating inflammatory responses (such as IL-1α, IL-6, TNF-α, IL-8, IL-11, and IL-1β) (13, 15, 16).
MATERIALS AND METHODS
Study subjects.
A total of 123 patients with TB and 91 patients with non-TB diseases were enrolled at Shenzhen Third People's Hospital from March 2011 to July 2012. A total of 16 healthy controls (HC) and 33 subjects with LTBI were recruited from household contacts of active TB patients, all of whom had no evidence of disease or history of TB. A previously described in-house M. tuberculosis antigen-specific IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay was used to diagnose LTBI (17, 18). The detailed clinical characteristics of patients are summarized in Table 1. All patients had a medical history taken and physical examination performed, with routine investigations, including HIV serology, chest radiography, and microbiological sputum examination, whenever possible. When clinically indicated, patients underwent aspiration of PF or CSF for biochemical, cytological, and detailed microbiological evaluation. Patients with HIV infection were excluded. All patients received the 2HRZE/4HR antituberculosis treatment regimen following the protocols of DOTS (directly observed therapy, short course). The study was approved by the Institutional Review Board of Shenzhen Third People's Hospital. Written informed consent was obtained from all participants.
TABLE 1.
Demographic characteristics of study populations
| Study part | Group | No. of cases | Patient age (yr) (mean ± SEM) | Patient sex (no. of males/no. of females) | No. (%) of positive sputum cultures | No. (%) of positive PF or CSF cultures | No. (%) of positive ELISPOT results | Type of sample | Relevant figure or comment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | LTBI | 17 | 28.88 ± 3.11 | 11/6 | NAa | NA | 17 (100) | Plasma | 1 |
| PTB | 20 | 24.60 ± 2.71 | 14/6 | 20 (100) | NA | 16 (80) | Plasma | ||
| 2 | TBP | 61 | 29.80 ± 1.18 | 41/20 | 10 (16.7) | 17 (27.87) | 54 (88.51) | PF and/or plasmad | 2 |
| Non-TBPb | 36 | 57.31 ± 2.94 | 21/15 | 0 (0) | 0 (0) | 5 (13.89) | PF | ||
| 3 | TBM | 16 | 27.38 ± 8.26 | 8/8 | 2 (12.5) | 4 (25) | 11 (68.75) | CSF | 3 |
| Non-TBMc | 40 | 33.70 ± 2.47 | 23/17 | 0 (0) | 0 (0) | 4 (10) | CSF | ||
| 4 | PTB | 8 | 35.70 ± 3.42 | 4/4 | 8 (100) | NA | 5 (62.50) | Plasma | 4 |
| 5 | HC | 16 | 26.75 ± 1.50 | 12/4 | NA | NA | 0 (0) | Plasma | Samples for ELISA |
| LTBI | 16 | 33.81 ± 2.32 | 15/1 | NA | NA | 16 (100) | Plasma | ||
| PTB | 18 | 34.83 ± 2.61 | 11/7 | 18 (100) | NA | 16 (72.22) | Plasma | ||
| Pneumonia | 15 | 34.47 ± 2.58 | 10/4 | 0 (0) | NA | 2 (13.24) | Plasma |
NA, not applicable.
Cases of non-TBP included lung cancer (n = 12), parapneumonic effusions (n = 14), and liver cirrhosis (n = 10).
Cases of non-TBM included syphilitic meningitis (n = 14), viral meningitis (n = 3), cryptococcus encephalitis (n = 2), and bacterial meningitis (n = 21).
For 10 of 61 TBP cases, the levels of chemokines/cytokines in pleural fluid and parallel plasma samples were compared.
Case definitions.
The diagnosis of pulmonary TB (PTB) was based on a compatible clinical presentation and sputum bacterium examination by use of the Bactec TB 960 culture system per the manufacturer's instructions. All patients with pulmonary TB recruited in this study were sputum culture positive for M. tuberculosis. The diagnosis of bacterial pneumonia was based on clinical manifestations, sputum bacterium examination, and a clinical response to antibiotic treatment. The diagnosis of tuberculous pleuritis (TBP) was confirmed if a patient had an exudative pleural effusion, was culture positive for M. tuberculosis (using pleural fluid, a pleural biopsy specimen, or sputum), and/or had pleural biopsy specimens positive for granulomatous inflammation with acid-fast bacilli (AFB) present (19, 20). Nontuberculous pleuritis (non-TBP) patients were defined on the basis of having no microbiological or histological evidence of TB, confirmation of an alternative diagnosis, or lack of progression to TB disease over 6 months of follow-up without TB treatment (20). The diagnosis of tuberculous meningitis (TBM) was based on a combination of nuchal rigidity and abnormal CSF. Confirmation of the cause of meningitis was based on clinical presentations, cytochemistry of the CSF, smears and cultures of the CSF, pathogen DNA PCR assay of the CSF, or intracranial lesions in computed tomography (CT) or brain magnetic resonance imaging (MRI). The clinical criterion for diagnosis of TBM was over 2 weeks of fever, headache, and neck stiffness. Diagnosis of TBM was confirmed if a patient had microbial and/or cytochemistry evidence of TB in CSF and a clinical response to anti-TB treatment (21). Nontuberculous meningitis (non-TBM) was defined as clinical meningitis with no evidence of TB and a confirmation of an alternative cause.
Sample collection and preparation.
For measurement of cytokines and chemokines, 2 ml of peripheral whole blood and/or PF or CSF was collected. After centrifugation at 300 × g for 5 min, the plasma and supernatant in the PF and CSF were collected and stored at −80°C. Unless otherwise indicated, the plasma, PF, and CSF samples were collected before initiation of anti-TB treatment. For longitudinal evaluation of cytokine levels during anti-TB treatment, plasma was collected from pulmonary TB patients 3, 6, and 12 months after initiation of anti-TB treatment.
Luminex assay.
A total of 26 soluble immunological molecules, including 15 cytokines (IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-17A, IFN-γ, TNF-α, IL-22, and G-CSF) and 11 chemokines (MCP-1, MCP-2, MCP-3, eotaxin, MIP-3α, 6Ckine, MDC, MIG, IP-10, CXCL-12, and CXCL-13), were measured using human cytokine/chemokine panels (MPXHCYTO-60K-17, MPXHCYP2-62K-6, MPXHCYP3-63K-5, and HTH17MAG-14K-3; Millipore). Analytes were quantified using a Magpix analytical test instrument, which utilized xMAP technology (Luminex), and xPONENT 4.2 software (Luminex). xMAP technology used fluorescently coded magnetic microspheres coated with analyte-specific capture antibodies to simultaneously measure multiple analytes in a specimen. Assays were performed according to the manufacturers' instructions. Concentrations of cytokines (pg/ml) were determined on the basis of the fit of a standard curve for mean fluorescence intensity versus pg/ml. Two quality controls were run with each assay (control 1, low level; and control 2, high level).
Measurement of IP-10 by ELISA.
The levels of IP-10 and MIG in plasma samples from patients with pulmonary TB and those with bacterial pneumonia were measured by enzyme-linked immunosorbent assay (ELISA) following the manufacturer's protocol (Quantikine ELISA kit; R&D Systems Co. Ltd.).
Statistical analysis.
GraphPad Prism 5 (GraphPad software) was used to generate plots and to perform statistical analyses of the data. The median cytokine or chemokine levels (interquartile ranges [IQR]) of the TB and control groups were compared using the nonparametric Mann-Whitney U test, since simultaneous testing has the potential to inflate type 1 errors. However, multiple testing corrections necessarily inflate type 2 errors, increasing the chance of false-negative conclusions. To balance the risks of type 1 and type 2 errors, the Bonferroni correction was used to correct the individual alpha errors for multiple comparison, using the following significance level for all biomarker analyses: α = 0.05/26 = 0.002. An adjusted P value of <0.002 was considered significant (22). Differences of cytokine/chemokine levels between plasmas and paired pleural fluid samples were compared using the paired t test. The dynamic changes of cytokine/chemokine levels before and after anti-TB treatment were compared using the paired t test. Receiver operator characteristic (ROC) curves for the selected biomarkers were then constructed by plotting the number of truly positive samples (sensitivity) against the number of false-positive samples (1 − specificity) for each possible cutoff point. Areas under the curve (AUC), along with their 95% confidence intervals (95% CI), were calculated by using a nonparametric approach. Cutoffs for antigen-specific IP-10, MIG, IL-6, and SDF-1 were estimated at various sensitivities and specificities. To avoid false-positive results, a cutoff value corresponding to the maximum Youden index, defined as sensitivity + specificity − 1, was retained (23). Optimal cutoff levels for differentiating individual groups were determined by ROC curve analysis, based on the highest likelihood ratio.
RESULTS
Characteristics of the study populations.
The participants recruited for this study included the following four groups: healthy controls (HC), those with LTBI, those with active TB disease, and disease controls (Table 1). The TB group consisted of 46 PTB cases, 61 TBP cases, and 16 TBM cases. Non-TB patients included 15 patients with bacterial pneumonia, 36 patients with non-TBP, and 40 patients with non-TBM (Table 1).
Different plasma cytokine/chemokine patterns in active TB and LTBI.
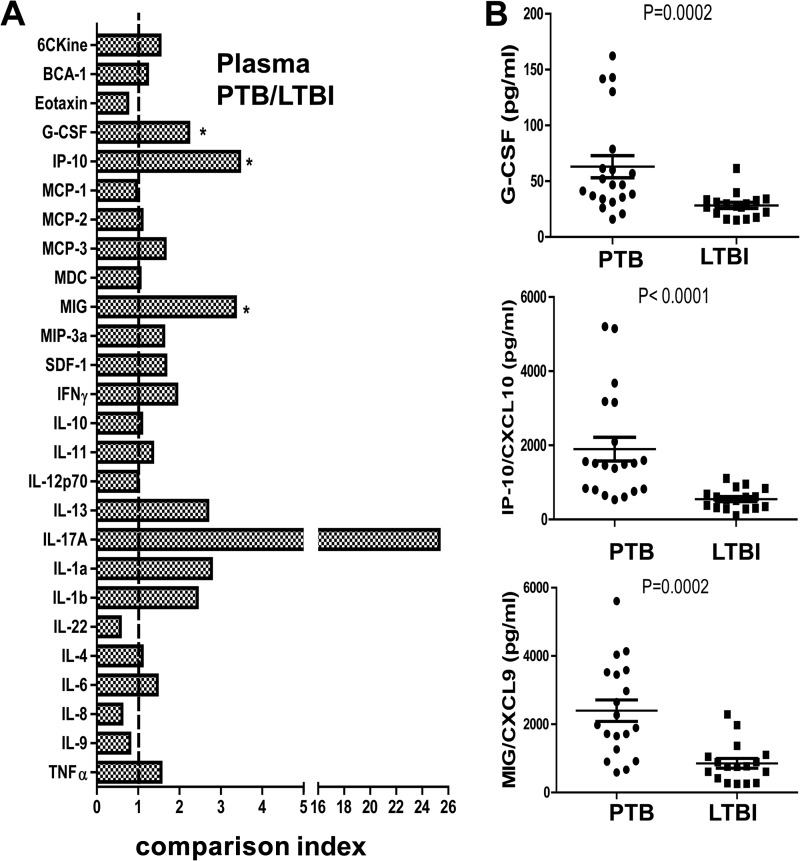
To identify biomarkers that discriminate active TB from LTBI, we measured the plasma levels of 26 chemokines/cytokines in active TB patients (n = 20) and LTBI subjects (n = 17) by using Luminex assays. The median levels of three chemokines/cytokines (IP-10, MIG, and G-CSF) were significantly (P < 0.002) higher in the active TB group than in the LTBI group after Bonferroni correction (Fig. 1A). Of those, IP-10 and MIG displayed the least amount of overlap between active TB patients and LTBI subjects. Individually, IP-10 and MIG had the highest diagnostic values, with an area under the ROC curve (AUC) of 0.92 for IP-10 and 0.86 for MIG for distinguishing active TB from LTBI (Table 2). Combining IP-10 with other cytokines/chemokines did not increase the diagnostic accuracy (data not shown). No difference in IP-10 and MIG levels in the plasma, determined by ELISA, was revealed between HC and LTBI subjects (data not shown). Furthermore, there was no difference in IP-10 and MIG levels determined by ELISA in plasma samples from patients with active TB and those with bacterial pneumonia (data not shown), indicating that the increase of these chemokines/cytokines circulating in the plasma was not pathogen specific but instead reflected an inflammatory response to infection.
FIG 1.
Differential plasma cytokine/chemokine expression patterns between LTBI and TB. (A) The levels of 26 cytokines/chemokines in plasma were detected by Luminex assay. The comparison index was calculated by dividing the mean plasma level of each cytokine/chemokine for PTB (n = 20) by that for LTBI (n = 17). The nonparametric Mann-Whitney U test was used to compare the differences between groups. *, P < 0.002. (B) The plasma levels of G-CSF, IP-10/CXCL10, and MIG/CXCL9 in PTB (n = 20) and LTBI (n = 17) subjects were significantly different. Each dot represents one individual; horizontal lines and error bars represent mean values ± standard errors of the means (SEM). The nonparametric Mann-Whitney U test was used to compare the differences between groups. P values are indicated.
TABLE 2.
Diagnostic values of cytokines/chemokines in discriminating LTBI and PTB
| Cytokine/chemokine | AUC (95% CI) | P value | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Likelihood ratio | Cutoff (pg/ml) |
|---|---|---|---|---|---|---|
| IP-10 | 0.92 (0.78–1.05) | 0.0001 | 0.88 (0.47–0.99) | 0.92 (0.62–0.99) | 10.5 | 1,008 |
| MIG | 0.86 (0.74–0.98) | 0.0002 | 0.53 (0.29–0.76) | 0.94 (0.71–0.99) | 8.95 | 1,976 |
| G-CSF | 0.83 (0.68–0.97) | 0.0002 | 0.75 (0.51–0.91) | 0.88 (0.64–0.99) | 6.37 | 34.83 |
IFN-γ, MIG, and IP-10 levels in pleural fluid accurately discriminate TBP from non-TBP patients.
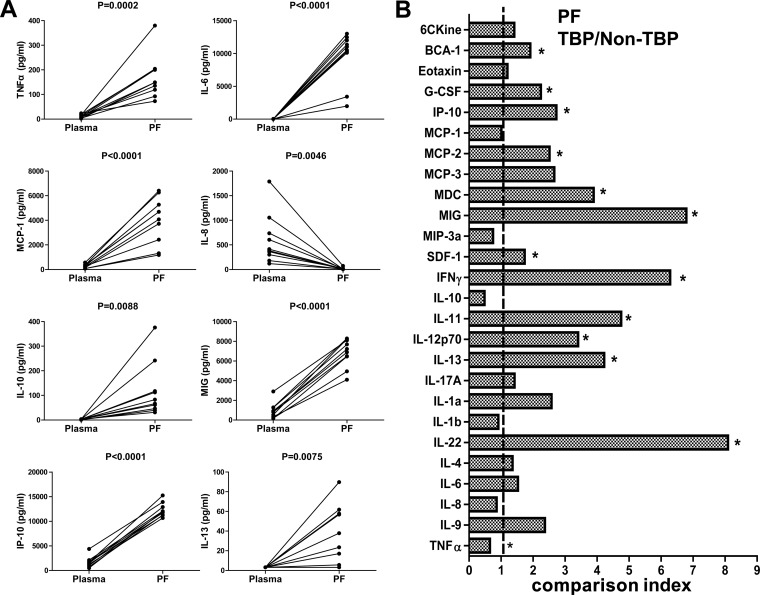
Chemokines and cytokines play important roles in recruiting immune cells to sites of infection and activating these cells to eliminate microbes, albeit at the potential cost of tissue damage that contributes to morbidity and mortality. Chemokines and cytokines are enriched at the site of infection, providing concentration gradients for cell recruitment and leukocyte activation. The compartmentalized immune response at sites of disease also offers a potential for greater diagnostic accuracy of cytokine/chemokine measurements than that obtained with plasma. To investigate this potential for TB, we first compared levels of 26 chemokines/cytokines in pleural fluid and parallel plasma samples from patients with TBP. Of the 26 cytokines/chemokines measured, 8 (TNF-α, IL-6, IL-13, MCP-1, IL-8, IL-10, MIG, and IP-10) had significantly higher levels in pleural fluid than in matched plasma (Fig. 2A). In contrast, one chemokine (eotaxin-1) had a lower level in pleural fluid than in parallel plasma (median [IQR], 88.6 [64.2, 120.2] versus 360.1 [191.6, 485.0]), but the difference was not statistically significant after Bonferroni correction (data not shown).
FIG 2.
Characterization of cytokine and chemokine expression in TBP and non-TBP patients. (A) The levels of TNF-α, IL-6, IL-13, MCP-1, IL-8, IL-10, MIG, and IP-10 in plasmas and paired pleural fluid samples from TBP patients (n = 10) were determined by Luminex assay. The paired t test was used for comparison, and P values are indicated. (B) The levels of 26 cytokines/chemokines in pleural fluid were detected by Luminex assay. The comparison index was calculated by dividing the mean level of each cytokine/chemokine in pleural fluid from TBP patients (n = 51) by that for non-TBP patients (n = 36). The nonparametric Mann-Whitney U test was used to compare the differences between groups. *, P < 0.002.
A comparison index was used to demonstrate the differences in cytokine/chemokine expression between TB and non-TBP patients (Fig. 2B). The comparison index was defined as the ratio of the median concentration of a cytokine/chemokine in the pleural fluid for the TBP group to that observed for the non-TBP group. As such, a ratio of >1 was observed for 20 cytokines/chemokines. Among these, 13 chemokine/cytokine (IFN-γ, MIG, IP-10, MCP, IL-13, MDC, TNF-α, SDF-1, BCA-1, G-CSF, IL-12p70, IL-22, and IL-11) median levels were significantly (P < 0.002) higher in the TBP group than in the non-TBP group after Bonferroni correction (Fig. 2B). Next, we analyzed the diagnostic values of these compartmentalized chemokines/cytokines individually and in combination. Consistent with previous reports (24–26), IFN-γ alone showed high accuracy for distinguishing TBP from non-TBP, with an AUC of 0.95 (Table 3). Likewise, IFN-γ, MIG, and IP-10 were also useful for discriminating TBP from non-TBP, with an AUC of 0.93 for MIG and 0.87 for IP-10 (Table 3). Age had a minimal effect, if any, on IP-10 levels in the pleural fluid, in both patients with TBP and those with non-TB disease (see Fig. S1 in the supplemental material). Combinations of the measured cytokines/chemokines indeed increased the diagnostic sensitivity but compromised the specificity.
TABLE 3.
Diagnostic values of cytokines/chemokines in discriminating TBP from non-TBP
| Cytokine/chemokine | AUC (95% CI) | P value | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Likelihood ratio | Cutoff (pg/ml) |
|---|---|---|---|---|---|---|
| IFN-γ | 0.95 (0.88–1.02) | <0.0001 | 0.96 (0.87–0.99) | 0.94 (0.81–0.99) | 17.29 | 37.51 |
| MIG/CXCL9 | 0.93 (0.86–0.99) | <0.0001 | 0.96 (0.87–0.99) | 0.89 (0.74–0.97) | 8.65 | 4,009 |
| IP-10/CXCL10 | 0.87 (0.78–0.96) | <0.0001 | 0.80 (0.67–0.90) | 0.86 (0.71–0.95) | 5.79 | 7,857 |
| MCP-2/CCL8 | 0.89 (0.76–0.95) | <0.0001 | 0.86 (0.74–0.94) | 0.83 (0.67–0.94) | 5.18 | 100.1 |
| IL-13 | 0.86 (0.77–0.95) | <0.0001 | 0.76 (0.63–0.87) | 0.92 (0.78–0.98) | 9.18 | 3.575 |
| MDC/CCL22 | 0.85 (0.76–0.93) | <0.0001 | 0.67 (0.52–0.79) | 0.86 (0.71–0.95) | 4.8 | 1,669 |
| TNF-α | 0.83 (0.72–0.94) | <0.0001 | 0.78 (0.65–0.89) | 0.83 (0.66–0.93) | 4.58 | 39.1 |
| SDF-1/CXCL12 | 0.76 (0.65–0.86) | <0.0001 | 0.61 (0.46–0.74) | 0.86 (0.71–0.95) | 4.38 | 2,472 |
| BCA-1/CXCL13 | 0.74 (0.63–0.85) | 0.0002 | 0.49 (0.35–0.63) | 0.86 (0.71–0.95) | 3.53 | 130.2 |
| G-CSF | 0.73 (0.62–0.84) | 0.0003 | 0.67 (0.52–0.79) | 0.75 (0.58–0.88) | 2.67 | 157.1 |
| IL-12p70 | 0.72 (0.61–0.83) | 0.0004 | 0.57 (0.42–0.71) | 0.81 (0.64–0.92) | 2.92 | 2.24 |
| IL-22 | 0.72 (0.62–0.83) | 0.0004 | 0.51 (0.37–0.65) | 0.94 (0.81–0.99) | 9.18 | 16.45 |
| IL-11 | 0.68 (0.57–0.80) | 0.0039 | 0.47 (0.33–0.62) | 0.89 (0.74–0.97) | 4.24 | 3.152 |
Compartmentalized MIG and IP-10 in CSF accurately discriminate TBM from non-TBM patients.
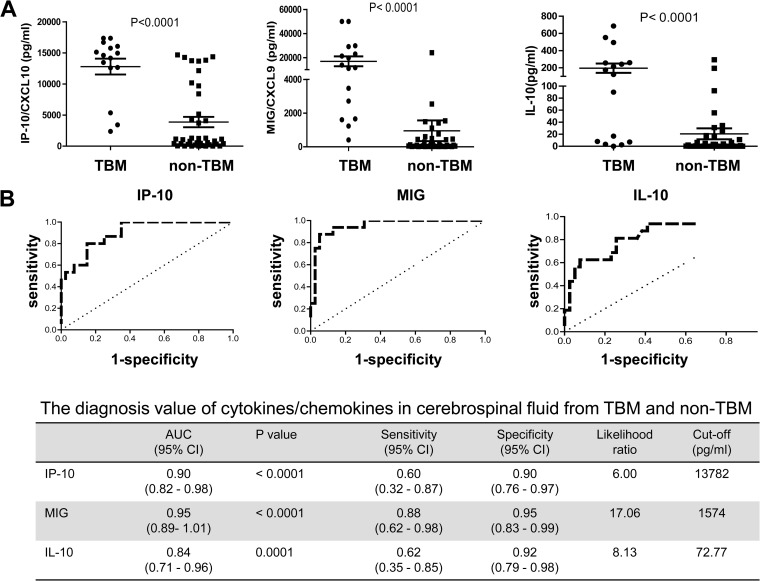
TBM is the most severe clinical manifestation of TB, with a high mortality. Early diagnosis of TBM remains challenging for clinicians but is vital, because early diagnosis and treatment can reduce mortality (27). To investigate whether compartmentalized cytokines/chemokines can be used as diagnostic markers as for TBP, the concentrations of 26 cytokines/chemokines in CSF from TBM and non-TBM patients were compared. Among them, the levels of IP-10, MIG, and IL-10, but not IFN-γ, in CSF were significantly different between TBM and non-TBM patients (Fig. 3A). Similarly, MIG and IP-10 showed the most promising potential for discriminating TBM from non-TBM, with AUC of 0.95 and 0.90, respectively (Fig. 3B). Thus, in pleural fluid and in CSF, compartmentalized MIG and IP-10 were useful diagnostic biomarkers.
FIG 3.
Expression of IP-10, MIG, and IL-10 in CSF from TBM and non-TBM patients. (A) The levels of IP-10, MIG, and IL-10 in CSF from TBM (n = 16) and non-TBM (n = 40) patients were determined by Luminex assay. Each dot represents one individual; horizontal lines and error bars represent mean values ± SEM. The nonparametric Mann-Whitney U test was used to compare the differences between groups. P values are indicated. (B) ROC curves analyzing the diagnostic values of IP-10, MIG, and IL-10 in CSF for discriminating TBM and non-TBM.
Effects of anti-TB treatment on plasma chemokine/cytokine levels.
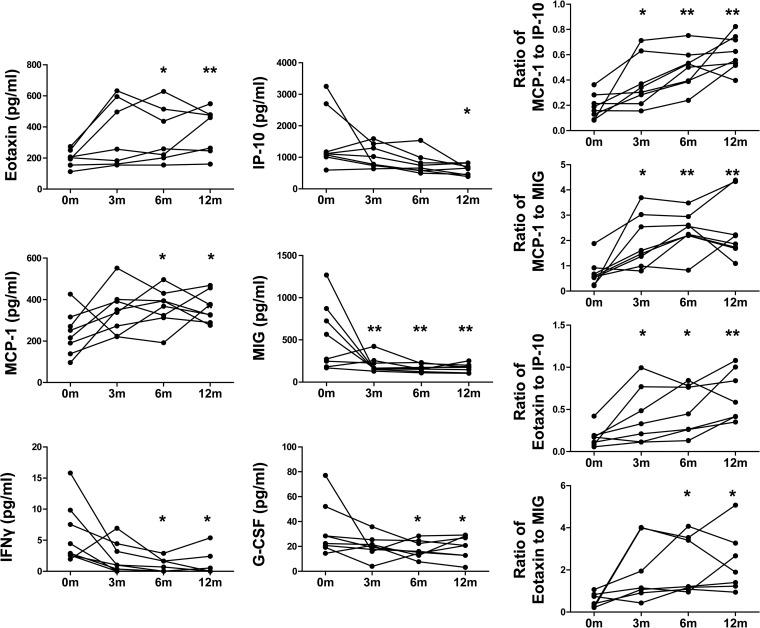
To investigate the effects of anti-TB treatment on the pattern of chemokine/cytokine expression, we measured the chemokine/cytokine levels in plasma before (0m) and 3 (3m), 6 (6m), and 12 (12m) months after initiation of anti-TB treatment. As shown in Fig. 4A, the mean plasma levels of MIG, IP-10, G-CSF, and IFN-γ were decreased after anti-TB treatment (6m), although the overall levels of IFN-γ and G-CSF were extremely low and some patients had low levels at 0m, so no trend could be established. The decrease was sustained from 6 months after anti-TB treatment (12 months after initiation of anti-TB treatment). Note that the decrease in MIG was observed from 3 months after initiation of anti-TB treatment in those patients where it was most elevated at 0m. In contrast, the levels of MCP-1 and eotaxin-1 were increased 6 months after anti-TB treatment and continued to increase at the end of 12 months of follow-up. While the levels of MIG and IP-10 reflect the Th1 response, the level of MCP-1 is important for recruitment and activation of macrophages, which are involved in the clearance of apoptotic neutrophils and subsequent resolution of acute inflammation caused by bacterial infection (28). Additionally, MCP-1 promotes tissue repair by enhancing the production of hepatocyte growth factor. Thus, the ratios of MCP-1 to MIG and IP-10 might better reflect the switch of the host response from fighting against M. tuberculosis to resolving the inflammatory battleground as well as repairing the inflammation-mediated tissue injury, since most bacteria had been cleared by extensive anti-TB drug treatment. We therefore calculated the ratios of MCP-1 to IP-10 and MIG. As shown in Fig. 4B, we found that the ratios of MCP-1 to IP-10 and MCP-1 to MIG were significantly increased after 3 months of anti-TB treatment. A similar result was observed for the ratio of eotaxin-1 to IP-10, a marker reflecting the balance of the Th1 and Th2 responses (29). Thus, the ratio of MCP-1 or eotaxin-1 to IP-10 might be more sensitive than IP-10 alone in monitoring the efficacy of anti-TB treatment. In line with this, the IP-10/MCP-1 ratio in CSF was a useful diagnostic marker for neuropsychiatric lupus patients (30).
FIG 4.
Dynamic changes of cytokine/chemokine expression in plasma before and after anti-TB treatment. (A) The levels of eotaxin-1, IP-10, G-CSF, IFN-γ, MCP-1, and MIG in the plasmas of PTB patients (n = 8) before anti-TB treatment and 3, 6, and 12 months after initiation of anti-TB treatment were detected by Luminex assay. (B) The MCP-1/IP-10, MCP-1/MIG, eotaxin-1/IP-10, and eotaxin-1/MIG ratios in plasmas of PTB patients (n = 8) before anti-TB treatment and 3, 6, and 12 months after initiation of anti-TB treatment were calculated. The paired t test was used for comparison between different times points, and P values are indicated. *, P < 0.05; **, P < 0.005. 0m, before anti-TB treatment; 3m, 3 months after initiation of anti-TB treatment; 6m, 6 months after initiation of anti-TB treatment; 12m, 12 months after initiation of anti-TB treatment.
DISCUSSION
Due to the shortage of available methods for diagnosis of TB, IGRAs have increasingly been used as an auxiliary diagnostic tool for active TB in clinical practice in China (18, 31–33). Indeed, it has been reported that detection of IFN-γ in pleural fluid mononuclear cells is useful for diagnosis of tuberculous pleurisy (31, 32). Additionally, IGRAs have improved the sensitivity for diagnosis of pulmonary TB (18), pediatric TB (34), and osteoarticular TB (33). However, specificity is the Achilles heel of IGRAs for diagnosis of active TB, as IGRAs cannot effectively discriminate active TB from LTBI (9). This is particularly the case in China, a country with a high prevalence of LTBI due to a high burden of TB. Hence, it is crucial to effectively differentiate active TB from LTBI. In this study, we found that the plasma levels of IP-10, MIG, and G-CSF were significantly increased in patients with active TB and could discriminate active TB from LTBI, with AUC of >0.75 individually. Notably, IP-10 alone had an AUC of 0.92 for discriminating active TB from LTBI. In contrast, both unstimulated and M. tuberculosis antigen-stimulated IP-10 in QFT-IT supernatant could not differentiate active TB from LTBI (35, 36), although M. tuberculosis antigen-stimulated IP-10 might be more robust for diagnosis of LTBI in young children and in HIV-infected individuals with low CD4 T cell counts (35, 37). Since current diagnostic methods, including IGRAs and antigen-specific IP-10 detection, perform well at identifying M. tuberculosis-infected from uninfected people, combining IGRAs or antigen-specific IP-10 detection with plasma IP-10 measurement might have greater utility for the diagnosis of active TB.
Elevated cytokines/chemokines in plasma are measured in the absence of antigen stimulation and thus can be elevated nonspecifically by other disorders. As an example, IP-10 was reported to be upregulated in a diverse spectrum of non-TB inflammatory diseases (38–40). It was therefore not surprising that the plasma levels of IP-10 and MIG, which could effectively differentiate active TB from LTBI, were not different between patients with active TB and those with bacterial pneumonia, as well as between HC and LTBI subjects. On the other hand, previous reports showed that cytokines/chemokines measured in pleural fluid, such as IFN-γ, IP-10, SDF-1, IL-6, and IL-10, were significantly higher in patients with TB than in patients with non-TBP (6, 41–43). IFN-γ alone showed a high accuracy in distinguishing TBP from non-TBP (24), with a sensitivity close to 100%, and the combination of IP-10, IL-6, and IL-10 resulted in 96% correct classification of TBP (42). Our results confirmed previous reports that IFN-γ and IP-10 individually have high and moderate accuracies, respectively, for diagnosis of TBP. Besides IFN-γ and IP-10, we found that other cytokines/chemokines, including MIG, MCP-2, IL-13, MDC, TNF-α, and SDF-1, also had the potential to discriminate TBP from non-TBP individually, with AUC of >0.75. In contrast to a previous report (42), we found that IL-10 was not useful for diagnosis of TBP, despite being enriched in pleural fluid of TB patients, possibly reflecting genetic differences in the study cohorts. Notably, we revealed for the first time that MIG had a high accuracy, comparable to that of IFN-γ, for diagnosis of TBP. The usefulness of compartmentalized MIG was further supported by our finding that its level in CSF was significantly higher in patients with TBM than in those with non-TBM. MIG alone had a sensitivity of 88% and a specificity of 95% for diagnosis of TBM. Taken together, these results indicate that chemokines/cytokines compartmentalized at the site of disease, unlike circulating plasma levels, form a relatively disease-specific pattern which may be employed as a disease-specific marker for diagnostics. Validation of these results with a larger cohort will aid in the development of new and improved TBP/TBM diagnostics.
It is widely accepted that the plasma IP-10 concentration is a reflection of TB disease activity (44–46). Moreover, several studies have reported that plasma IP-10 levels decrease upon successful treatment of active TB and suggest that measuring IP-10 could help in monitoring disease activity and treatment responses for prognosticating TB relapse (44, 47, 48). Our results confirmed the decrease of plasma IP-10 levels after completion of anti-TB treatment. In addition, we found that MIG, IFN-γ, and G-CSF levels also decreased after anti-TB treatment. The median level of plasma MIG was significantly decreased 3 months after initiation of anti-TB treatment, while the level of IP-10 was statistically insignificant until 12 months after initiation of treatment, suggesting that MIG might be superior to IP-10 for monitoring the efficacy of anti-TB therapy. Nevertheless, the decrease of MIG was most pronounced in 4 of 7 patients, and a larger-cohort study is warranted to valid this observation.
Unlike the case for the other cytokines/chemokines studied, plasma levels of MCP-1 and eotaxin-1 were lower in TB patients before anti-TB treatment than in LTBI subjects and significantly increased after successful treatment. Our results were consistent with a report by Djoba Siawaya et al. (47) but in contrast with those of Alessandri et al., who found that the concentration of MCP-1 in plasma was elevated in TB patients compared to controls and remained elevated throughout the treatment (49). The reasons for these contrasting results are unknown but may reflect genetic differences in the populations studied and/or differential responses to locally prevalent M. tuberculosis clades (50). MCP-1 was shown to play an important role in protective immunity against M. tuberculosis (51, 52). However, MCP-1 might increase susceptibility to M. tuberculosis infection (53, 54), and increased MCP-1 plasma levels correlate with TB disease severity (55). Eotaxin-1 plays a critical role in eosinophil recruitment during inflammation (56), but its role in TB remains largely unknown. Eotaxin-1 is a profibrotic chemokine (57), so it might contribute to the fibrosis that is a characteristic feature of healed TB lesions. Riffo-Vasquez et al. (58) reported that M. tuberculosis inhibited eotaxin-1 production by macrophages in vitro. Thus, the increase of eotaxin-1 might result directly from disarming the inhibitory activity of M. tuberculosis through chemotherapy. Regardless of the increase or decrease of plasma cytokines/chemokines in patients after treatment, the levels of IP-10, MIG, MCP-1, and eotaxin returned to levels comparable to those in uninfected individuals (data not shown), reinforcing the potential roles played by these cytokines/chemokines in TB pathogenesis. Taken together, our findings show that the dynamic changes of MCP-1, eotaxin-1, IP-10, MIG, IFN-γ, and G-CSF provide a new direction for developing potential biomarkers for monitoring the efficacy of anti-TB treatment, either individually or as a pattern.
Supplementary Material
ACKNOWLEDGMENTS
Financial support for this work was provided by the Twelve-Fifth Mega-Scientific Project on Prevention and Treatment of AIDS, Viral Hepatitis, and Other Infectious Diseases (grant 2012ZX10003002) and by the Natural Science Foundation of China (grants 81171535 and 81172732).
Footnotes
Published ahead of print 1 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00499-14.
REFERENCES
- 1.Dorman SE. 2010. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin. Infect. Dis. 50(Suppl 3):S173–S177. 10.1086/651488. [DOI] [PubMed] [Google Scholar]
- 2.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009–1021. 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.Shenai S, Amisano D, Ronacher K, Kriel M, Banada PP, Song T, Lee M, Joh JS, Winter J, Thayer R, Via LE, Kim S, Barry CE, 3rd, Walzl G, Alland D. 2013. Exploring alternative biomaterials for diagnosis of pulmonary tuberculosis in HIV-negative patients by use of the GeneXpert MTB/RIF assay. J. Clin. Microbiol. 51:4161–4166. 10.1128/JCM.01743-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. 2014. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 1:CD009593. 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CH, Woo H, Hyun IG, Kim C, Choi JH, Jang SH, Park SM, Kim DG, Lee MG, Jung KS, Hyun J, Kim HS. 2014. A comparison between the efficiency of the Xpert MTB/RIF assay and nested PCR in identifying Mycobacterium tuberculosis during routine clinical practice. J. Thorac. Dis. 6:625–631. 10.3978/j.issn.2072-1439.2014.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandie S, Peter JG, Kerbelker ZS, Meldau R, Theron G, Govender U, Ntsekhe M, Dheda K, Mayosi BM. 2014. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-gamma in a high burden setting: a prospective study. BMC Med. 12:101. 10.1186/1741-7015-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon HW, Hur M. 2013. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection: an updated review. Ann. Clin. Lab. Sci. 43:221–229. [PubMed] [Google Scholar]
- 8.Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, Lange C, Losi M, Markova R, Migliori GB, Nienhaus A, Ruhwald M, Wagner D, Zellweger JP, Huitric E, Sandgren A, Manissero D. 2011. Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur. Respir. J. 37:88–99. 10.1183/09031936.00115110. [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, Pai M. 2011. Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J. Infect. Dis. 204(Suppl 4):S1120–S1129. 10.1093/infdis/jir410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. 2011. Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 11:343–354. 10.1038/nri2960. [DOI] [PubMed] [Google Scholar]
- 11.Wallis RS, Kim P, Cole S, Hanna D, Andrade BB, Maeurer M, Schito M, Zumla A. 2013. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect. Dis. 13:362–372. 10.1016/S1473-3099(13)70034-3. [DOI] [PubMed] [Google Scholar]
- 12.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:973–977. 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slight SR, Khader SA. 2013. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor Rev. 24:105–113. 10.1016/j.cytogfr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Jiang J, Cao Z, Yang B, Zhang J, Cheng X. 2012. Diagnostic performance of multiplex cytokine and chemokine assay for tuberculosis. Tuberculosis (Edinb.) 92:513–520. 10.1016/j.tube.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Kapina MA, Shepelkova GS, Avdeenko VG, Guseva AN, Kondratieva TK, Evstifeev VV, Apt AS. 2011. Interleukin-11 drives early lung inflammation during Mycobacterium tuberculosis infection in genetically susceptible mice. PLoS One 6:e21878. 10.1371/journal.pone.0021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, Clayberger C. 2008. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin. Immunol. 126:202–210. 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Wang H, Liao M, Chen X, Graner M, Zhu X, Zhang J, Yang Q, Lu H, Zhou B, Chen X. 2010. Diagnosis of latent tuberculosis infection in bacille Calmette-Guerin vaccinated subjects in China by interferon-gamma ELISpot assay. Int. J. Tuberc. Lung Dis. 14:1556–1563. [PubMed] [Google Scholar]
- 18.Chen X, Yang Q, Zhang M, Graner M, Zhu X, Larmonier N, Liao M, Yu W, Deng Q, Zhou B. 2009. Diagnosis of active tuberculosis in China using an in-house gamma interferon enzyme-linked immunospot assay. Clin. Vaccine Immunol. 16:879–884. 10.1128/CVI.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopi A, Madhavan SM, Sharma SK, Sahn SA. 2007. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest 131:880–889. 10.1378/chest.06-2063. [DOI] [PubMed] [Google Scholar]
- 20.Sahn SA, Huggins JT, San Jose ME, Alvarez-Dobano JM, Valdes L. 2013. Can tuberculous pleural effusions be diagnosed by pleural fluid analysis alone? Int. J. Tuberc. Lung Dis. 17:787–793. 10.5588/ijtld.12.0892. [DOI] [PubMed] [Google Scholar]
- 21.Botha H, Ackerman C, Candy S, Carr JA, Griffith-Richards S, Bateman KJ. 2012. Reliability and diagnostic performance of CT imaging criteria in the diagnosis of tuberculous meningitis. PLoS One 7:e38982. 10.1371/journal.pone.0038982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushner LE, Wendelboe AM, Lazzeroni LC, Chary A, Winters MA, Osinusi A, Kottilil S, Polis MA, Holodniy M. 2013. Immune biomarker differences and changes comparing HCV mono-infected, HIV/HCV co-infected, and HCV spontaneously cleared patients. PLoS One 8:e60387. 10.1371/journal.pone.0060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubbo PA, Nagot N, Le Moing V, Brabet M, Bourdin A, Nogue E, Bollore K, Vendrell JP, Van De Perre P, Tuaillon E. 2012. Multicytokine detection improves latent tuberculosis diagnosis in health care workers. J. Clin. Microbiol. 50:1711–1717. 10.1128/JCM.00117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J, Shi HZ, Liang QL, Qin SM, Qin XJ. 2007. Diagnostic value of interferon-gamma in tuberculous pleurisy: a metaanalysis. Chest 131:1133–1141. 10.1378/chest.06-2273. [DOI] [PubMed] [Google Scholar]
- 25.Supriya P, Chandrasekaran P, Das SD. 2008. Diagnostic utility of interferon-gamma-induced protein of 10 kDa (IP-10) in tuberculous pleurisy. Diagn. Microbiol. Infect. Dis. 62:186–192. 10.1016/j.diagmicrobio.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Kalantri Y, Hemvani N, Chitnis DS. 2011. Evaluation of real-time polymerase chain reaction, interferon-gamma, adenosine deaminase, and immunoglobulin A for the efficient diagnosis of pleural tuberculosis. Int. J. Infect. Dis. 15:e226–e231. 10.1016/j.ijid.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Marx GE, Chan ED. 2011. Tuberculous meningitis: diagnosis and treatment overview. Tuberc. Res. Treat. 2011:798764. 10.1155/2011/798764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. 2004. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J. Immunol. 172:398–409. 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- 29.Rastogi D, Wang C, Lendor C, Rothman PB, Miller RL. 2006. T-helper type 2 polarization among asthmatics during and following pregnancy. Clin. Exp. Allergy 36:892–898. 10.1111/j.1365-2222.2006.02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto H, Iikuni N, Kamitsuji S, Yoshio T, Minota S, Kamatani N. 2006. IP-10/MCP-1 ratio in CSF is an useful diagnostic marker of neuropsychiatric lupus patients. Rheumatology (Oxford) 45:232–234. 10.1093/rheumatology/kei233. [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Gao M, Zhang X, Du F, Jia H, Yang X, Wang Z, Zhang L, Ma L, Wu X, Xie L, Zhang Z. 2013. Interferon-gamma release assay performance of pleural fluid and peripheral blood in pleural tuberculosis. PLoS One 8:e83857. 10.1371/journal.pone.0083857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao M, Yang Q, Zhang J, Zhang M, Deng Q, Liu H, Graner MW, Kornfeld H, Zhou B, Chen X. 2014. Gamma interferon immunospot assay of pleural effusion mononuclear cells for diagnosis of tuberculous pleurisy. Clin. Vaccine Immunol. 21:347–353. 10.1128/CVI.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia H, Pan L, Qin S, Liu F, Du F, Lan T, Zhang X, Wei R, Du B, Liu Z, Huang H, Zhang Z. 2013. Evaluation of interferon-gamma release assay in the diagnosis of osteoarticular tuberculosis. Diagn. Microbiol. Infect. Dis. 76:309–313. 10.1016/j.diagmicrobio.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Xiao J, Miao Q, Feng WX, Wu XR, Yin QQ, Jiao WW, Shen C, Liu F, Shen D, Shen AD. 2011. Interferon gamma release assay in diagnosis of pediatric tuberculosis: a meta-analysis. FEMS Immunol. Med. Microbiol. 63:165–173. 10.1111/j.1574-695X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 35.Ruhwald M, Dominguez J, Latorre I, Losi M, Richeldi L, Pasticci MB, Mazzolla R, Goletti D, Butera O, Bruchfeld J, Gaines H, Gerogianni I, Tuuminen T, Ferrara G, Eugen-Olsen J, Ravn P. 2011. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis (Edinb.) 91:260–267. 10.1016/j.tube.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Diao N, Lu C, Wu J, Gao Y, Chen J, Zhou Z, Huang H, Shao L, Jin J, Weng X, Zhang Y, Zhang W. 2012. Evaluation of the diagnostic potential of IP-10 and IL-2 as biomarkers for the diagnosis of active and latent tuberculosis in a BCG-vaccinated population. PLoS One 7:e51338. 10.1371/journal.pone.0051338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruhwald M, Aabye MG, Ravn P. 2012. IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Rev. Mol. Diagn. 12:175–187. 10.1586/erm.11.97. [DOI] [PubMed] [Google Scholar]
- 38.Geyer AI, Kraus T, Roberts M, Wisnivesky J, Eber CD, Hiensch R, Moran TM. 2013. Plasma level of interferon gamma induced protein 10 is a marker of sarcoidosis disease activity. Cytokine 64:152–157. 10.1016/j.cyto.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Reiberger T, Aberle JH, Kundi M, Kohrgruber N, Rieger A, Gangl A, Holzmann H, Peck-Radosavljevic M. 2008. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir. Ther. 13:969–976. [PubMed] [Google Scholar]
- 40.Sajadi SM, Khoramdelazad H, Hassanshahi G, Rafatpanah H, Hosseini J, Mahmoodi M, Arababadi MK, Derakhshan R, Hasheminasabzavareh R, Hosseini-Zijoud SM, Ahmadi Z. 2013. Plasma levels of CXCL1 (GRO-alpha) and CXCL10 (IP-10) are elevated in type 2 diabetic patients: evidence for the involvement of inflammation and angiogenesis/angiostasis in this disease state. Clin. Lab. 59:133–137. [DOI] [PubMed] [Google Scholar]
- 41.Dheda K, van Zyl-Smit RN, Sechi LA, Badri M, Meldau R, Meldau S, Symons G, Semple PL, Maredza A, Dawson R, Wainwright H, Whitelaw A, Vallie Y, Raubenheimer P, Bateman ED, Zumla A. 2009. Utility of quantitative T-cell responses versus unstimulated interferon-{gamma} for the diagnosis of pleural tuberculosis. Eur. Respir. J. 34:1118–1126. 10.1183/09031936.00005309. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland JS, Garba D, Fombah AE, Mendy-Gomez A, Mendy FS, Antonio M, Townend J, Ideh RC, Corrah T, Ota MO. 2012. Highly accurate diagnosis of pleural tuberculosis by immunological analysis of the pleural effusion. PLoS One 7:e30324. 10.1371/journal.pone.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y, Zhang Y, Hu S, Jin D, Chen X, Jin Q, Liu H. 2012. Different patterns of cytokines and chemokines combined with IFN-gamma production reflect Mycobacterium tuberculosis infection and disease. PLoS One 7:e44944. 10.1371/journal.pone.0044944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong JY, Lee HJ, Kim SY, Chung KS, Kim EY, Jung JY, Park MS, Kim YS, Kim SK, Chang J, Cho SN, Kang YA. 2014. Efficacy of IP-10 as a biomarker for monitoring tuberculosis treatment. J. Infect. 68:252–258. 10.1016/j.jinf.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, Benagiano M, D'Elios MM, Mantovani A, Del Prete G. 2005. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 7:1–8. 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Ruhwald M, Bjerregaard-Andersen M, Rabna P, Eugen-Olsen J, Ravn P. 2009. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res. Notes 2:19. 10.1186/1756-0500-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Djoba Siawaya JF, Beyers N, van Helden P, Walzl G. 2009. Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis. Clin. Exp. Immunol. 156:69–77. 10.1111/j.1365-2249.2009.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YC, Chin CH, Liu SF, Wu CC, Tsen CC, Wang YH, Chao TY, Lie CH, Chen CJ, Wang CC, Lin MC. 2011. Prognostic values of serum IP-10 and IL-17 in patients with pulmonary tuberculosis. Dis. Markers 31:101–110. 10.1155/2011/938794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alessandri AL, Souza AL, Oliveira SC, Macedo GC, Teixeira MM, Teixeira AL. 2006. Concentrations of CXCL8, CXCL9 and sTNFR1 in plasma of patients with pulmonary tuberculosis undergoing treatment. Inflamm. Res. 55:528–533. 10.1007/s00011-006-5136-9. [DOI] [PubMed] [Google Scholar]
- 50.Coussens AK, Wilkinson RJ, Nikolayevskyy V, Elkington PT, Hanifa Y, Islam K, Timms PM, Bothamley GH, Claxton AP, Packe GE, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Drobniewski FA, Mein CA, Bhaw-Rosun L, Nuamah RA, Griffiths CJ, Martineau AR. 2013. Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathog. 9:e1003468. 10.1371/journal.ppat.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. 2001. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 98:7958–7963. 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott HM, Flynn JL. 2002. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect. Immun. 70:5946–5954. 10.1128/IAI.70.11.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flores-Villanueva PO, Ruiz-Morales JA, Song CH, Flores LM, Jo EK, Montano M, Barnes PF, Selman M, Granados J. 2005. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J. Exp. Med. 202:1649–1658. 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussain R, Ansari A, Talat N, Hasan Z, Dawood G. 2011. CCL2/MCP-I genotype-phenotype relationship in latent tuberculosis infection. PLoS One 6:e25803. 10.1371/journal.pone.0025803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan Z, Jamil B, Khan J, Ali R, Khan MA, Nasir N, Yusuf MS, Jamil S, Irfan M, Hussain R. 2009. Relationship between circulating levels of IFN-gamma, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Scand. J. Immunol. 69:259–267. 10.1111/j.1365-3083.2008.02217.x. [DOI] [PubMed] [Google Scholar]
- 56.Lampinen M, Carlson M, Hakansson LD, Venge P. 2004. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy 59:793–805. 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 57.Puxeddu I, Bader R, Piliponsky AM, Reich R, Levi-Schaffer F, Berkman N. 2006. The CC chemokine eotaxin/CCL11 has a selective profibrogenic effect on human lung fibroblasts. J. Allergy Clin. Immunol. 117:103–110. 10.1016/j.jaci.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 58.Riffo-Vasquez Y, Coates AR, Page CP, Spina D. 2012. Mycobacterium tuberculosis chaperonin 60.1 inhibits leukocyte diapedesis in a murine model of allergic lung inflammation. Am. J. Respir. Cell Mol. Biol. 47:245–252. 10.1165/rcmb.2011-0412OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.