Abstract
Canine brucellosis is an infectious disease caused by the Gram-negative bacterium Brucella canis. Unlike conventional control programs for other species of the genus Brucella, currently there is no vaccine available against canine brucellosis, and preventive measures are simply diagnosis and isolation of infected dogs. New approaches are therefore needed to develop an effective and safe immunization strategy against this zoonotic pathogen. In this study, BALB/c mice were subcutaneously immunized with the following: (i) the recombinant Brucella Omp31 antigen formulated in different adjuvants (incomplete Freund adjuvant, aluminum hydroxide, Quil A, and Montanide IMS 3012 VGPR), (ii) plasmid pCIOmp31, or (iii) pCIOmp31 plasmid followed by boosting with recombinant Omp31 (rOmp31). The immune response and the protective efficacy against B. canis infection were characterized. The different strategies induced a strong immunoglobulin G (IgG) response. Furthermore, spleen cells from rOmp31-immunized mice produced gamma interferon and interleukin-4 (IL-4) after in vitro stimulation with rOmp31, indicating the induction of a mixed Th1-Th2 response. Recombinant Omp31 administered with different adjuvants as well as the prime-boost strategy conferred protection against B. canis. In conclusion, our results suggest that Omp31 could be a useful candidate for the development of a subcellular vaccine against B. canis infection.
INTRODUCTION
Canine brucellosis, caused by Brucella canis, is a worldwide bacterial disease that affects dogs and has been shown to constitute a risk for humans (1). Traditionally, the infection has been associated with kennels, but nowadays it has spread through various dog populations, including shelter and stray dogs (2). It causes mainly reproductive disorders, such as abortions and infertility. Furthermore, signs of canine brucellosis might not become apparent for many years in infected animals (3), making it difficult to implement measurements to avoid the spread of the disease to noninfected animals.
Methods to control the disease at this time are simply diagnostic tests, such as rapid slide agglutination test with 2-mercaptoethanol (2ME-RSAT), agar gel immunodiffusion test (AGID), or enzyme-linked immunosorbent assay (ELISA) (4, 5), and control actions to avoid the contagion of healthy animals (6). While control measures for brucellosis on other animal species include vaccination, at present, there is no available vaccine against B. canis. On the other hand, despite the continuous development of different serological techniques, diagnosis remains a complex issue that is not always reliable (7). Moreover, any ideal canine brucellosis control program should rely on a vaccine that contains protective antigens that do not cause misinterpretation of serological results between infected and vaccinated animals.
B. canis and Brucella ovis are the two natural rough species of the genus, a characteristic given by the lack of the O-polysaccharide chain of the lipopolysaccharide (8). This feature becomes relevant, since it has been demonstrated that the accessibility of critical outer membrane protein (OMP) epitopes to antibodies has implications in protective immunity, since antibody binding to OMP was demonstrated to be critical for protection against Brucella rough species (9, 10). Many studies have focused on the OMP properties as immunogens, not only to be used as vaccine candidates but also as diagnostic antigens (11, 12). Experiments on antibody binding capacity showed that Omp31 (13), Omp25 (14), and Omp2b (15) are displayed at high levels and exposed on the outer membranes of B. canis and B. ovis (16). In spite of significant variability in the surface phenotype, most of the epitopes of the OMP are conserved among the main pathogenic species of the genus Brucella (9, 17). Previous studies demonstrated that a high percentage of B. canis-infected dogs developed detectable titers of specific antibodies against recombinant Omp31 (rOmp31) from Brucella melitensis (18). Furthermore, the nucleotide sequence of this protein is quite conserved in the genus, and the B. canis Omp31 sequence displays only one nucleotide substitution in comparison with B. melitensis Omp31 (19). It has also been reported that the administration of a monoclonal antibody against a hydrophilic loop of Omp31 protected against B. ovis infection in mice (10, 16). Also, when Omp31 was evaluated as a vaccine candidate, it conferred protection similar to that of B. melitensis Rev.1 against B. ovis and B. melitensis infection, either as a recombinant protein or as DNA vaccine (pCIOmp31) (20, 21). On the other hand, rOmp31 also stimulated a strong cellular and humoral immune response in rams, which significantly reduced bacterial burden and lesions in organs after B. ovis infection (22).
As mentioned, prevention of B. canis infection is dependent on sustained screening of dogs. Repeated experience in brucellosis control has shown that the spread of the disease in any animal species can be prevented or reduced only by the use of vaccines (23). Unfortunately, efforts to develop an effective vaccine against B. canis in dogs have been unsuccessful thus far. Since Carmichael's seminal work in the 1980s, there has been no further research in this matter. In that work, a less-mucoid strain (M-) of B. canis was used to infect dogs. The results demonstrated that the M- variant met some of the criteria for an immunizing agent (24). Nevertheless, the study failed to provide unequivocal assurance of acceptable attenuation, and later communications demonstrated the zoonotic nature of the strain (25, 26).
Subcellular vaccines may represent an alternative, since they can be designed to include only the immunogens required for protective immunity, and therefore are safer than whole inactivated or live attenuated vaccines (27). Yet, despite these advantages, recombinant proteins tend to be poorly immunogenic in vivo (28, 29). Thus, the use of potent immunomodulating compounds or suitable delivery systems to stimulate specific strong immune responses is required (30). The appropriate selection of adjuvants is essential in the formulation of novel and efficacious vaccines (31).
We have demonstrated that rOmp31 formulated in incomplete Freund adjuvant (IFA) induced protection against B. ovis and B. melitensis in mice when injected intraperitoneally (20, 21). Both the use of IFA and the route of immunization are common for experimental immunizations but are not recommended for domestic animals. As we decided to investigate the immunogenicity and protective capacity of Omp31 against B. canis infection in mice, we carefully chose three different safe adjuvants approved for use in dogs: aluminum hydroxide gel, Quil A saponin, and Montanide IMS3012 VGPR (Seppic, France). Also, more-appropriate routes of injection were employed. Here, we present the results of this study.
MATERIALS AND METHODS
Animals.
BALB/c mice (6 to 8 weeks old) obtained from Universidad de Buenos Aires were acclimated and randomly distributed into experimental groups. Mice were kept in conventional animal facilities with filtered air and handled following international guidelines required for animal experiments under our Faculty Animal Welfare Commission (Acta 087/02, Facultad de Ciencias Veterinarias [FCV], Universidad Nacional del Centro de la Provincia de Buenos Aires [UNCPBA], Tandil, Argentina; http://www.vet.unicen.edu.ar).
Bacterial strains.
B. canis ATCC RM6/66 and B. canis less-mucoid strain (M-) were obtained from our Brucella culture collection. B. canis RM6/66 was used as the challenge strain after two serial passages in BALB/c mice and reisolation from spleens. Bacterial suspension was prepared as previously described (32). Briefly, this strain was grown on brucella agar (Britania, Argentina) for 24 h at 37°C. For infection, the cells were harvested and spectrophotometrically adjusted in phosphate-buffered saline (PBS) so that an optical density at 600 nm (OD600) of 0.165 equals approximately 109 CFU/ml. The exact numbers of cells were assessed retrospectively by dilution and spreading on the required medium (33). A suspension of heat-killed B. canis (HKBC) was prepared under the same conditions and was inactivated for 1 h at 80°C.
Antigen production.
Recombinant Omp31 (rOmp31) from B. melitensis was cloned, expressed in Escherichia coli BL21(DE3) (Stratagene), and purified as previously described (18). Briefly, to purify the soluble protein from the inclusion bodies in urea solution, a nickel-chelated resin (HisLink; Promega) was used following the manufacturer′s instructions, in batch format and denaturing conditions. The presence and purity of rOmp31 in eluates were checked by SDS-PAGE and Coomassie blue staining. Eluates containing the purified protein were dialyzed overnight against deionized water with 1 mM phenylmethylsulfonyl fluoride (PMSF) and stored at −70°C. Protein concentration was determined by the bicinchoninic acid assay (BCA) with bovine serum albumin as the standard (Pierce, Rockford, IL).
DNA vaccine coding for Omp31 was expressed and purified as previously described (34). E. coli JM109 cells were transformed with pCI-neo vector (Promega, Madison, WI) containing the Omp31 gene. The plasmid was amplified and isolated using “megaprep” plasmid isolation columns (GenElute; Sigma). The purity and concentration of DNA were determined by spectrophotometry at 260/280 nm.
Adjuvants and preparation of the immunogens.
Aluminum hydroxide (AH) gel was prepared as described previously (35). To adsorb the antigen, the aluminum hydroxide suspension was mixed with an equal volume of rOmp31 in PBS and incubated for 30 min at room temperature. The AH-adsorbed rOmp31 antigen was washed, and the final pellet was resuspended in PBS. Incomplete Freund adjuvant (IFA) was prepared mixing Marcol 52 (kindly provided by Biogenesis, Argentina) with 10% of Arlacel (Sigma, St. Louis, MO, USA) in order to facilitate emulsification with the immunogen. Montanide IMS 3012 VGPR (MON) (Seppic, France) and Quil A (Brenntag Biosector, Denmark) were used according to the manufacturer's instructions.
Immunizations and experimental design.
Mice were randomly separated into groups (n = 10). Each group received different antigens according to the vaccination schedule. Mice immunized with pCIOmp31 were injected three times (days 0, 15, and 30) by the intramuscular (i.m.) route (100 μg in 100 μl of PBS). Mice in the prime-boost group (pCIOmp31 plus boost [pCIOmp31+boost]) were immunized by the same plasmid schedule followed by a final subcutaneous (s.c.) booster (fourth injection) performed with the rOmp31-IFA formulation (30 μg in 200 μl). Recombinant Omp31 formulated in the different adjuvants was administered two times (days 30 and 45) by the s.c. route (30 μg in 200 μl).
As a positive-control vaccine, HKBC B. canis emulsified in IFA (1 × 109 CFU in IFA) was administered twice subcutaneously (days 30 and 45) according to our previous work (28). In addition, a PBS-injected group was also included (negative control). All schedules were synchronized in order to inject simultaneously the last boost in all groups.
Animals were examined by a veterinarian to evaluate general status and local adverse reactions at the injection site.
Indirect ELISAs.
Mice were bled by submandibular puncture every 2 weeks before and after the challenge. Serum reactivity to rOmp31 was determined by indirect ELISA. The plates were sensitized with 0.1 μg of rOmp31 in 100 μl of PBS (pH 7.2) at 4°C overnight. Blocking was done with PBS containing 0.05% Tween 20 and 3% skim milk. Mouse sera were diluted 1/100 in PBS containing 0.05% Tween 20 and 1% skim milk and incubated for 1 h at 37°C. Bound antibodies were detected by a goat anti-mouse IgG (whole molecule) conjugated to horseradish peroxidase (Sigma, Germany) diluted in the same buffer. The reaction was developed by adding 2,2′-azino-bis(3-athylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) (Sigma, Germany) (1 mM) in citrate-phosphate buffer containing 0.03% H2O2. The absorbance was determined using a microplate reader (Multiskan EX; LabSystems). The cutoff value for the assay was calculated as the mean of the specific optical density plus 3 standard deviations (SD) for 20 sera obtained from nonimmunized mice and assayed at dilutions of 1:100. The titer of each serum was calculated as the last serum dilution yielding a specific optical density higher than the cutoff value.
Cytokine production.
To evaluate and characterize the cellular immune response induced by the immunization strategies, five mice per group were sacrificed 30 days after the last immunization. The spleens were aseptically removed and homogenized in RPMI 1640 (Gibco) supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 50 μg of streptomycin per ml, and 10% fetal calf serum. Cells were cultured at 4 × 106/ml in duplicate wells with Omp31 (5 μg/ml) or concanavalin A (ConA; 2.5 μg/ml) (Sigma) or with culture medium alone. Cell cultures were incubated for a period of 48 h at 37°C in a humidified atmosphere of 5% CO2 in air. At the end of the incubation, cell culture supernatants were collected, aliquoted, and frozen at −70°C until analyzed for gamma interferon (IFN-γ) and interleukin-4 (IL-4) production by sandwich ELISA using paired cytokine-specific monoclonal antibodies according to the manufacturer's instructions (Pharmingen, San Diego, CA).
Protection experiments.
Thirty days after the last immunization, five mice per group were challenged by intraperitoneal (i.p.) inoculation with 5.5 × 105 CFU of B. canis RM6/66 in 200 μl of PBS. Mice were sacrificed by cervical dislocation 30 days after being challenged, and their spleens were removed aseptically, weighed, and kept at −20°C until processed. To determine the infection level, the spleens were thawed, individually homogenized using an appropriate volume of PBS in sterile plastic bags, and serially diluted (10-fold), and each dilution was seeded onto two plates of Trypticase soy agar supplemented with yeast extract, 0.5% (TSAYE medium). After 4 days of incubation, the number of CFU was counted and expressed by the log10 CFU per spleen value as previously described (32, 33).
Statistical analysis of data.
The CFU data were normalized by log transformation and evaluated by analysis of variance (ANOVA) followed by Dunnett's posthoc test. The Kruskal-Wallis test and ANOVA were used to compare antibody and cellular responses, respectively. Graphs were performed using Graph Pad software, version 4.0, San Diego, CA.
RESULTS
Prime-boost strategy and recombinant Omp31-based vaccines developed significant specific IgG responses.
To evaluate the humoral immune response elicited by the different strategies of immunization, anti-Omp31 IgG antibodies were measured by specific indirect ELISA in sera from immunized and control mice. Sera from mice injected with PBS and heat-killed B. canis (HKBC) which served as controls for the protection experiments were included. pCIOmp31+boost strategy, rOmp31-AH gel, rOmp31-IFA, or rOmp31-Quil A formulations elicited a strong specific IgG response after the second boost (P < 0.01) (Fig. 1). In contrast, pCIOmp31, rOmp31-Montanide, and HKBC induced a weak humoral immune responses against rOmp31 (P > 0.05). Thirty days after the i.p. challenge with B. canis RM6/66, specific anti-Omp31 antibody levels increased significantly in groups immunized with plasmid vaccine, pCIOmp31+boost, or Omp31-Quil A (Fig. 1). In contrast, B. canis challenge was unable to boost the response of mice immunized with rOmp31-HA or rOmp31-IFA. Neither the animals injected with PBS nor the HKBC-immunized animals showed anti-Omp31 antibodies. These results are consistent with our previous reports in which we tested different Omp31 strategies against another rough species of the genus such as B. ovis (21, 34). Anyway, antibody response against B. canis antigens other than Omp31 was observed in all groups after challenge, as indicated by rapid slide agglutination test (RSAT)-positive results (not shown).
FIG 1.
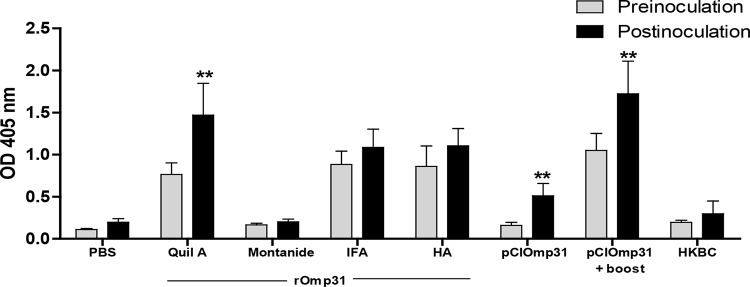
Antibody response against recombinant Omp31 in mice immunized by using different strategies. Mice were immunized as described in Materials and Methods. IgG-specific antibodies against rOmp31 were evaluated by indirect ELISA preinoculation (30 days after last immunization) and postinoculation (30 days after challenge with B. canis RM6/66). Values are means plus standard deviations (SD) (error bars) for 10 and 5 mice preinoculation and postinoculation, respectively. The figure shows the results of a representative experiment from two experiments performed with similar results. Values that are significantly different (P < 0.01) preinoculation and postinoculation are indicated with two asterisks.
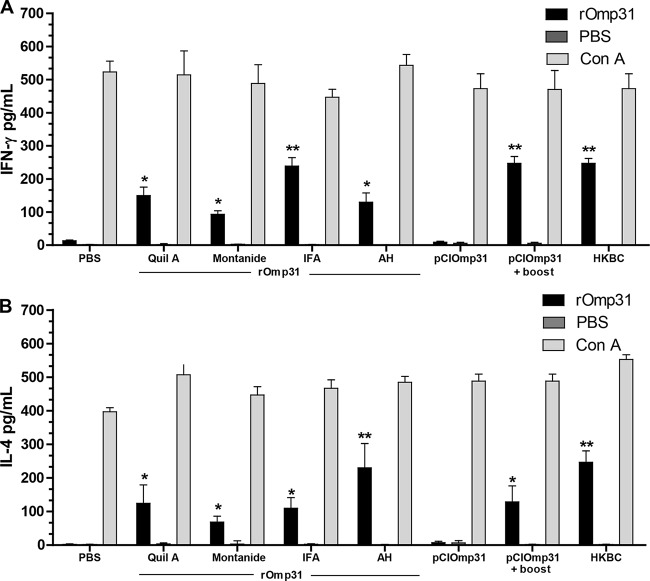
Recombinant Omp31-based vaccines induced specific cellular immune responses.
In order to obtain further information on the type of immune response induced by the different immunization protocols at the time of bacterial challenge, we used ELISA to investigate cytokine secretion in rOmp31-stimulated spleen cell cultures from the different immunization groups. Recombinant Omp31 significantly stimulated the production of IFN-γ and IL-4 in splenocytes from mice immunized with rOmp31 formulated in the different adjuvants and from pCIOmp31+boost-vaccinated and HKBC-immunized mice (P < 0.01). In contrast and as reported previously (21), pCIOmp31 immunization did not induce IFN-γ and IL-4 production. Splenocytes from mice immunized by pCIOmp31+boost, rOmp31-IFA, and HKBC produced significantly (P < 0.01) higher levels of IFN-γ than cells from mice given rOmp31-AH, rOmp31-MON, or rOmp31-Quil A (P < 0.05). Also, significantly higher levels of IL-4 were detected in groups immunized with rOmp31-HA and HKBC (P < 0.01). In contrast, specific secretion of IL-4 was comparable between the other groups of immunized mice (Fig. 2). Cells from PBS-immunized mice did not secrete IFN-γ οr IL-4 when stimulated with rOmp31. Spleen cells from all immunized mice produced both cytokines in response to ConA, with no significant differences observed among the groups. These results indicate that rOmp31 in different adjuvants injected subcutaneously induced a mixed Th1-Th2 cytokine response.
FIG 2.
Determination of IFN-γ (A) and IL-4 (B) levels in supernatants of spleen cell cultures from mice immunized by using different strategies. Values are the numbers of cells producing IFN-γ or IL-4 after stimulation with rOmp31 as described in Materials and Methods. Spleen cells (4 × 106/ml) were stimulated with complete medium RPMI 1640 or rOmp31 (5 μg/ml) for 48 h. The levels of IFN-γ (A) and IL-4 (B) in the cell supernatants were quantified (in picogram per milliliter) by monoclonal antibody capture ELISA. Values are means plus SD of the response of spleen cells from five individual mice from duplicate experiments. Values that are significantly different are indicated as follows: *, P < 0.05; **, P < 0.01.
The different recombinant Omp31-based strategies protect BALB/c mice against B. canis infection.
Thirty days after the last immunization, the mice were challenged by an i.p. injection of 5.65 × 105 CFU of B. canis RM6/66. Thirty days later, the mice were sacrificed, and their spleens were removed and processed to determine the bacterial burden. B. canis growth was significantly inhibited (P < 0.05) in groups immunized with rOmp31 with every adjuvant and the pCIOmp31+boost strategy compared to the PBS control (Table 1). Plasmid pCIOmp31 was the only vaccine formulation that failed to give any level of protection against B. canis infection. As previously reported by our group when using heat-killed whole bacterial cells (21, 34), the control vaccine HKBC in IFA induced the highest protection level (3.48 log units of protection).
TABLE 1.
Protection against B. canis in mice immunized with Omp31 by using different strategies of immunization
| Vaccine (n = 5) | Adjuvant | Log10 B. canis in the spleena | Log unit of protection |
|---|---|---|---|
| PBS | 6.18 ± 0.11 | ||
| rOmp31 | Quil A | 4.14 ± 0.68 | 1.86b |
| Montanide | 4.63 ± 0.50 | 1.42b | |
| IFA | 4.37 ± 0.36 | 1.66b | |
| HA | 4.37 ± 0.82 | 1.65b | |
| pCIOmp31 | 5.67 ± 0.66 | 0.66 | |
| pCIOmp31+boost | IFA | 4.53 ± 0.92 | 1.50b |
| HKBC | IFA | 2.25 ± 0.58 | 3.48c |
The content of bacteria in spleens is represented as the mean log CFU ± SD per group.
Significantly different (P < 0.05) from the value for PBS-immunized mice by Dunnett's t test.
Significantly different (P < 0.01) from the value for PBS-immunized mice by Dunnett's t test.
All mice immunized with rOmp31 or HKBC emulsified in IFA developed large nonseptic abscesses at the injection site. This lesion persisted several weeks, and the mice also exhibited local hair loss. None of the other strategies induced local or systemic adverse reactions (not shown).
DISCUSSION
Traditional approaches to Brucella vaccine development employ whole-cell vaccines which are composed of suspensions of whole killed or attenuated cells (36). Nowadays, approved vaccines for use in ruminants for preventing brucellosis are based on attenuated strains (37). While these vaccines have reduced virulence for animals, they are pathogenic for humans, and they are resistant to antibiotics used to treat human brucellosis (36). Therefore, these vaccines have restricted use in animals because they can induce abortion in pregnant females (36). In view of these risks, many researchers have investigated alternative vaccination strategies for brucellosis, including the use of subunit vaccines based on recombinant proteins or DNA (27). Alternatively, the use of adjuvants in combination with antigens might be an alternative to enhance vaccination efficacy. Owing to the lack of suitable strategies to protect animals and humans against canine brucellosis, our goal is to explore different approaches to develop and test an appropriate vaccine against B. canis.
Outer membrane proteins of Brucella spp. have been characterized and studied as potential immunogenic or protective antigens (10, 16). In particular, recombinant Omp31-based vaccines (20, 21, 22), alone or associated with rough lipopolysaccharide conferred protection against B. ovis in mice (33) and rams (22). These results were encouraging for the testing of Omp31 delivery strategies against B. canis in mice.
B. canis, as any other Brucella species, is a facultative intracellular pathogen. Cell-mediated immunity plays a critical role in protection against virulent Brucella infection. However, previous studies have shown that specific antibodies bind to OMPs of rough Brucella microorganisms (10). Moreover, it has been shown that antibodies against Omp31 can mediate complement-dependent bacteriolysis of B. ovis (22). In vivo, this lytic mechanism could have a protective role during the bacteremic phase of B. ovis or B. canis infections before the entry of bacteria into their target cells. In this work, all rOmp31 administered with different adjuvants induced a vigorous IgG response as well as IL-4 and IFN-γ, suggesting the induction of a mixed Th2-Th1 immune response (20, 34). We speculate that differences in the magnitude of the immune response could be associated with the adjuvant and/or administration route used. Furthermore, the coordinated immune response against rOmp31 conferred protection against B. canis infection in mice independently of the adjuvant formulation used. Levels of protection were in the range of the ones obtained using Omp31 with the other rough strain of the genus (B. ovis) in the mouse model (20, 21, 34). However, the protection afforded was always significantly lower than the one provided by immunization with HKBC (control vaccine). In our experience, this is always the case when using whole dead cells or attenuated vaccines comprising the whole antigenic load of a microorganism (20, 31, 38). Anyway, most of these preparations interfere with diagnosis (37, 38). While the protection afforded could be improved using a multiple-subunit vaccine, it also remains possible that a more effective antigen or a better adjuvant might lead to a higher degree of protection with a monovalent subunit vaccine. Previously, we have demonstrated that the chimeric protein based on the addition to the N terminus of Brucella lumazine synthase (BLS) of a 27-mer peptide containing the exposed loop epitope of Omp31 (BLSOmp31) is able to develop strong humoral and cellular responses and confers protection against B. canis in mice (38).
When selecting immunization strategies for a trial with pets, the site of injection and the adjuvant to be used should be considered. Vaccines containing recombinant antigens may be less reactogenic but also less immunogenic, thus necessitating the inclusion of an adjuvant (28). However, the adjuvant should be chosen considering the benefits and risks for the target species. In this study, we selected three commercial adjuvants approved for use in dogs, along with IFA, since it has been used in previous works of Omp31 (20, 21, 34). In addition, the subcutaneous route was chosen as a common route for vaccine administration in dogs. As expected, the severity of local reaction occurring after IFA-emulsified vaccines in mice could rule out this adjuvant for future trials in dogs. Nevertheless, Omp31 formulated in the other adjuvants induced statistically similar levels of protection, which reinforces the potentiality of this immunogen to become an effective vaccine against B. canis in the susceptible host.
In conclusion, recombinant Omp31 could be a useful candidate for the development of a subunit vaccine against B. canis, since it elicits antigen-specific humoral and cellular responses and conferred protection in the mouse model.
ACKNOWLEDGMENTS
This work was supported by grant PICT2007-01189 from the Agencia Nacional de Promoción Científica Tecnológica (ANPCYT-Argentina) (to S.M.E.). M.C. and A.G.D. are recipients of a doctoral fellowship from CONICET (Argentina). S.M.E., G.H.G., and J.C. are members of the Research Career of CONICET. We thank Sergio Islas (CIC) and Fabián Amaya and Adrián Pérez (UNCPBA, Argentina) for animal care.
We all read and approved the final manuscript.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 22 October 2014
REFERENCES
- 1.Lucero NE, Corazza R, Almuzara MN, Reynes E, Escobar GI, Boeri E, Ayala SM. 2010. Human Brucella canis outbreak linked to infection in dogs. Epidemiol. Infect. 138:280–325. 10.1017/S0950268809990525. [DOI] [PubMed] [Google Scholar]
- 2.Hollett RB. 2006. Canine brucellosis: outbreaks and compliance. Theriogenology 66:575–587. 10.1016/j.theriogenology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Wanke MM. 2004. Canine brucellosis. Anim. Reprod. Sci. 82-83:195–207. 10.1016/j.anireprosci.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Mateu-de-Antonio EM, Martin M, Soler M. 1993. Use of indirect enzyme-linked immunosorbent assay with hot saline solution extracts of a variant (M-) strain of Brucella canis for diagnosis of brucellosis in dogs. Am. J. Vet. Res. 54:1043–1046. [PubMed] [Google Scholar]
- 5.Wanke MM, Delpino M, Baldi PC. 2002. Comparative performance of tests using cytosolic or outer membrane antigens of Brucella for the serodiagnosis of canine brucellosis. Vet. Microbiol. 88:367–375. 10.1016/S0378-1135(02)00152-9. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael LE, Joubert JC. 1988. Transmission of Brucella canis by contact exposure. Cornell Vet. 78:63–73. [PubMed] [Google Scholar]
- 7.Keid LB, Soares RM, Vasconcellos SA, Megid J, Salgado VR, Richtzenhain LJ. 2009. Comparison of agar gel immunodiffusion test, rapid slide agglutination test, microbiological culture and PCR for the diagnosis of canine brucellosis. Res. Vet. Sci. 86:22–26. 10.1016/j.rvsc.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Alton GG, Jones LM, Angus RD, Verger JM. 1988. Techniques for the brucellosis laboratory. INRA, Paris, France. [Google Scholar]
- 9.Bowden RA, Cloeckaert A, Zygmunt MS, Bernard S, Dubray G. 1995. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect. Immun. 63:3945–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden RA, Cloeckaert A, Zygmunt MS, Bernard S, Dubray G. 1995. Outer membrane protein and rough lipopolysaccharide specific monoclonal antibodies protect mice against Brucella ovis. J. Med. Microbiol. 43:344–347. 10.1099/00222615-43-5-344. [DOI] [PubMed] [Google Scholar]
- 11.Salhi I, Boigegrain R-A, Machold J, Weise C, Cloeckaert A, Rouot B. 2003. Characterization of new members of the group 3 outer membrane protein family of Brucella spp. Infect. Immun. 71:4326–4332. 10.1128/IAI.71.8.4326-4332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caro-Hernández P, Fernández-Lago L, de Miguel MJ, Martín-Martín AI, Cloeckaert A, Grilló M-J, Vizcaíno N. 2007. Role of the Omp25/Omp31 family in outer membrane properties and virulence of Brucella ovis. Infect. Immun. 75:4050–4061. 10.1128/IAI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vizcaíno N, Cloeckaert A, Zygmunt MS, Dubray G. 1996. Cloning, nucleotide sequence, and expression of the Brucella melitensis Omp31 gene coding for an immunogenic major outer membrane protein. Infect. Immun. 64:3744–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wergifosse P, Lintermans P, Limet JN, Cloeckaert A. 1995. Cloning and nucleotide sequence of the gene coding for the major 25-kilodalton outer membrane protein of Brucella abortus. J. Bacteriol. 177:1911–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ficht TA, Bearden SW, Sowa BA, Adams LG. 1989. DNA sequence and expression of the 36-kilodalton outer membrane protein gene of Brucella abortus. Infect. Immun. 57:3281–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden RA, Estein SM, Zygmunt MS, Dubray G, Cloeckaert A. 2000. Identification of protective outer membrane antigens of Brucella ovis by passive immunization of mice with monoclonal antibodies. Microbes Infect. 2:481–488. 10.1016/S1286-4579(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 17.Cloeckaert A, de Wergifosse P, Dubray G, Limet JN. 1990. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect. Immun. 58:3980–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassataro J, Pasquevich K, Bruno L, Wallach JC, Fossati CA, Baldi PC. 2004. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough brucellae. Clin. Diagn. Lab. Immunol. 11:111–114. 10.1128/CDLI.11.1.111-114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vizcaíno N, Kittelberger R, Cloeckaert A, Marín CM, Fernández-Lago L. 2001. Minor nucleotide substitutions in the omp31 gene of Brucella ovis result in antigenic differences in the major outer membrane protein that it encodes compared to those of the other Brucella species. Infect. Immun. 69:7020–7028. 10.1128/IAI.69.11.7020-7028.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassataro J, Estein SM, Pasquevich KA, Velikovsky CA, de la Barrera S, Bowden RA, Fossati CA, Giambartolomei GH. 2005. Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection. Infect. Immun. 73:8079–8088. 10.1128/IAI.73.12.8079-8088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassataro J, Velikovsky CA, de la Barrera S, Estein SM, Bruno L, Bowden R, Pasquevich KA, Fossati CA, Giambartolomei GH. 2005. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect. Immun. 73:6537–6546. 10.1128/IAI.73.10.6537-6546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estein SM, Cheves PC, Fiorentino MA, Cassataro J, Paolicchi FA, Bowden RA. 2004. Immunogenicity of recombinant Omp31 from Brucella melitensis in rams and serum bactericidal activity against B. ovis. Vet. Microbiol. 102:203–213. 10.1016/j.vetmic.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Olsen SC, Stoffregen WS. 2005. Essential role of vaccines in brucellosis control and eradication programs for livestock. Expert Rev. Vaccines 4:915–928. 10.1586/14760584.4.6.915. [DOI] [PubMed] [Google Scholar]
- 24.Carmichael LE, Zoha SJ, Flores-Castro R. 1984. Biological properties and dog response to a variant (M-) strain of Brucella canis. Dev. Biol. Stand. 56:649–656. [PubMed] [Google Scholar]
- 25.Zoha SJ, Carmichael LE. 1982. Properties of cell wall antigens of virulent Brucella canis and a less mucoid variant of reduced pathogenicity. Am. J. Vet. Res. 43:171–174. [PubMed] [Google Scholar]
- 26.Wallach JC, Giambartolomei GH, Baldi PC, Fossati CA. 2004. Infection with M- strain of Brucella canis. Emerg. Infect. Dis. 10:146–148. 10.3201/eid1001.020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira S, Giambartolomei G, Cassataro J. 2011. Confronting the barriers to develop novel vaccines against brucellosis. Expert Rev. Vaccines 10:1291–1305. 10.1586/erv.11.110. [DOI] [PubMed] [Google Scholar]
- 28.Meeusen EN, Walker J, Peters A, Pastoret P-P, Jungersen G. 2007. Current status of veterinary vaccines. Clin. Microbiol. Rev. 20:489–510. 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strugnell R, Zepp F, Cunningham A, Tantawichien T. 2011. Vaccine antigens. Perspect. Vaccinol. 1:61–88. 10.1016/j.pervac.2011.05.003. [DOI] [Google Scholar]
- 30.Ott G, Van Nest G. 2007. Development of vaccine adjuvants: a historical perspective. Vaccine adjuvants and delivery systems. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 31.Garçon N, Leroux-Roels G, Cheng W-F. 2011. Vaccine adjuvants. Perspect. Vaccinol. 1:89–113. 10.1016/j.pervac.2011.05.004. [DOI] [Google Scholar]
- 32.Clausse M, Estein SM. 2011. Evaluation of a mouse infection model to evaluate homologue and heterologue vaccines against Brucella canis in mouse model. InVet 13:53–60 (In Spanish.) [Google Scholar]
- 33.Estein SM, Cassataro J, Vizcaíno N, Zygmunt MS, Cloeckaert A, Bowden RA. 2003. The recombinant Omp31 from Brucella melitensis alone or associated with rough lipopolysaccharide induces protection against Brucella ovis infection in BALB/c mice. Microbes Infect. 5:85–93. 10.1016/S1286-4579(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 34.Cassataro J, Velikovsky CA, Bruno L, Estein SM, de la Barrera S, Bowden R, Fossati CA, Giambartolomei GH. 2007. Improved immunogenicity of a vaccination regimen combining a DNA vaccine encoding Brucella melitensis outer membrane protein 31 (Omp31) and recombinant Omp31 boosting. Clin. Vaccine Immunol. 14:869–874. 10.1128/CVI.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margni RA. 1996. Inmunología e inmunoquímica fundamentos, 5th ed. Editorial Médica Panamericana, Buenos Aires, Argentina. [Google Scholar]
- 36.Nicoletti PL. 1990. Vaccination, p 283–299 In Nielsen KH, Duncan JR. (ed), Animal brucellosis. CRC Press, Boca Raton, FL. [Google Scholar]
- 37.Schurig GG, Sriranganathan N, Corbel MJ. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90:479–496. 10.1016/S0378-1135(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 38.Clausse M, Díaz AG, Ghersi G, Zylberman V, Cassataro J, Giambartolomei GH, Goldbaum FA, Estein SM. 2013. The vaccine candidate BLSOmp31 protects mice against Brucella canis infection. Vaccine 31:6129–6135. 10.1016/j.vaccine.2013.07.041. [DOI] [PubMed] [Google Scholar]