Abstract
Lymphatic filariasis is known to be associated with diminished CD4+ Th1 and elevated CD4+ Th2 responses to parasite-specific antigens. The roles of cytokine-expressing CD8+ T cells in immune responses to filarial infections are not well defined. To study the roles of CD8+ T cells expressing type 1, type 2, and type 17 cytokines in filarial infections, we examined the frequencies of these cells in clinically asymptomatic, patently infected (INF) individuals, directly ex vivo and in response to parasite or nonparasite antigens; these frequencies were compared with the results for individuals with filarial lymphedema (i.e., clinical pathology [CP]) and those without active infection or pathology (i.e., endemic normal [EN]). INF individuals exhibited significant decreases in the frequencies of CD8+ T cells expressing tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin-22 (IL-22) at baseline and/or in response to filarial antigens, compared with CP and EN individuals. In contrast, the same individuals exhibited significant increases in the frequencies of CD8+ T cells expressing IL-4, IL-5, IL-9, IL-13, and IL-21, compared with CP and/or EN individuals. Curative treatment resulted in significantly increased frequencies of CD8+ T cells expressing IL-2 and significantly decreased frequencies of CD8+ T cells expressing type 2 cytokines. Finally, the regulation of these responses appears to be independent of IL-10 and transforming growth factor β (TGF-β), since blockade of IL-10 or TGF-β signaling did not significantly alter the frequencies of type 1 or type 2 cytokine-expressing CD8+ T cells. Our findings suggest that alterations in the frequencies of cytokine-expressing CD8+ T cells are characteristic features of lymphatic filarial infections.
INTRODUCTION
Lymphatic filariasis (LF) can manifest with a variety of clinical outcomes (1). One of the main characteristics of filarial infections is the ability to alter host immune responses to establish both long-lived and relatively quiescent (at least immunologically) infections (1). This typically establishes a clinically asymptomatic state in which the host is mostly free of signs and symptoms of infection but continues to harbor high densities of viable parasites. While the majority of the 120 million infected individuals have subclinical infections, a significant minority of individuals (∼40 million) develop lymphatic pathology following infection (2). The most common pathological manifestations of lymphatic filariasis are hydroceles and lymphedema (leading to elephantiasis) (2). Most individuals in areas in which the disease is endemic, however, are completely free of infection (i.e., endemic normal [EN]). It is still not clear whether the endemic normal state is due to the ability of these individuals to mount effective immune responses against the parasite, lack of exposure to the parasite, or other nonimmunological mechanisms of resistance (1).
Although the immune responses to filarial parasites have been well studied with respect to natural history, diagnosis, and treatment (3), there is a relative paucity of information on the mechanisms underlying susceptibility to disease and the development of pathology. The pathogenesis of filarial infections and lymphatic filarial disease is influenced by multiple factors, of both parasite and host origins (4). Maintenance of the asymptomatic state is now recognized to reflect an immunoregulatory environment involving multiple levels of host cells and cytokines, and a breakdown in this state could lead to pathological manifestations (4, 5). In terms of host immunity, both arms of the host immune system, innate and adaptive, are thought to play major roles in the regulation of active infections and the development of pathology (6). Subclinical filarial infections are known to be associated with the downregulation of Th1, Th17, and Th22 responses, T cell proliferation, and expanded Th2 responses (6). Moreover, both the downregulation of CD4+ Th1 responses and T cell proliferation are at least partially mediated by interleukin-10 (IL-10) and transforming growth factor β (TGF-β), since blockade of these cytokines results in reversal of this immune modulation (7).
While the nature of immune responses to parasite antigens has been well characterized in terms of CD4+ T cell responses, very little data exist on the role of CD8+ T cells in filarial infections and disease. Therefore, we, examined cytokine expression patterns of CD8+ T cells in patients with filarial lymphedema, asymptomatic infected individuals, and uninfected individuals at homeostasis and following stimulation with parasite and control antigens. We show that human filarial infections are characterized by profound alterations in both spontaneously expressed and parasite antigen-specific frequencies of CD8+ T cell subsets; unlike findings observed for CD4+ cells, however, neither IL-10 nor TGF-β is significantly involved in regulating the expression patterns of CD8+ T cell subsets.
MATERIALS AND METHODS
Study population.
We studied a group of 32 clinically asymptomatic, filaria-infected (INF) individuals, 23 individuals with filarial lymphedema (i.e., with clinical pathology [CP]), and 15 uninfected individuals with endemic normal (EN) status in an area in which LF is endemic, in Tamil Nadu, South India (Table 1). All CP individuals tested negative for circulating filarial antigen with both the ICT filarial antigen test (Binax, Portland, ME) and the TropBio Og4C3 enzyme-linked immunosorbent assay (ELISA) (TropBio Pty., Ltd., Townsville, Queensland, Australia), indicating a lack of current active infection. The diagnosis of prior filarial infection was made on the basis of history and clinical examination results, as well as positive Brugia malayi antigen (BmA)-specific IgG4 results. BmA-specific IgG4 and IgG ELISAs were performed exactly as described previously (8). All INF individuals tested positive for active infection with both the ICT filarial antigen test and the TropBio Og4C3 ELISA and had not received any antifilarial treatment prior to this study. All INF individuals were treated with standard doses of diethylcarbamazine (DEC) and albendazole, and follow-up blood samples were obtained 1 year later. A subset of individuals (n = 7) who became circulating antigen negative following treatment were used for posttreatment analysis. We also used 7 of the 32 INF individuals exclusively for performing cytokine blocking studies. All EN individuals were circulating filarial antigen negative and without any signs or symptoms of infection or disease. There were no differences between the groups in terms of demographic characteristics or socioeconomic status. All individuals were examined as part of clinical protocols approved by the institutional review boards of both the National Institute of Allergy and Infectious Diseases and the National Institute for Research in Tuberculosis (ClinicalTrials.gov identifiers NCT00375583 and NCT00001230), and written informed consent was obtained from all participants.
TABLE 1.
Characteristics of the study population
| Characteristic | INFa (n = 32) | EN (n = 15) | CP (n = 23) |
|---|---|---|---|
| Age (median [range]) (yr) | 37 (19–65) | 36 (24–65) | 42 (27–65) |
| No. male/no. female | 22/10 | 9/6 | 17/6 |
| Lymphedema/elephantiasis | No | No | Yes |
| ICT card test result | Positive | Negative | Negative |
| W. bancrofti circulating antigen levels (geometric mean [range]) (U/ml) | 1,409 (138–22,377) | <32b | <32 |
INF, individuals with asymptomatic filarial infections; EN, uninfected individuals; CP, individuals with filarial pathology.
Below the limits of detection.
Parasite and control antigens.
Saline extracts of B. malayi adult worms (yielding BmA) and microfilariae (Mf) were used for parasite antigens, and mycobacterial purified protein derivative (PPD) (Serum Statens Institute, Copenhagen, Denmark) was used as the control antigen. The final concentration was 10 μg/ml for BmA, Mf, and PPD. Endotoxin levels in the BmA preparation were <0.1 endotoxin unit (EU)/ml, as determined using the QCL-1000 Chromogenic LAL test kit (BioWhittaker). Phorbol myristoyl acetate (PMA) and ionomycin, at concentrations of 12.5 ng/ml and 125 ng/ml, respectively, were used as the positive-control stimuli.
In vitro cultures.
Whole-blood cell cultures were performed to determine the intracellular levels of cytokines. Briefly, whole blood was diluted 1:1 with RPMI 1640 medium supplemented with penicillin-streptomycin (100 U and 100 mg/ml, respectively), l-glutamine (2 mM), and HEPES (10 mM) (all from Invitrogen, San Diego, CA) and was placed in 12-well tissue culture plates (Costar, Corning Inc., NY). The cultures were then stimulated with BmA, Mf, PPD, PMA-ionomycin, or medium alone, in the presence of the costimulatory reagent CD49d/CD28 (BD Biosciences), for 6 h at 37°C. FastImmune brefeldin A solution (10 μg/ml; BD Biosciences) was added after 2 h. After 6 h, whole blood was centrifuged and washed using cold phosphate-buffered saline (PBS), and then 1× FACS lysing solution (BD Biosciences, San Diego, CA) was added. The cells were fixed using Cytofix/Cytoperm buffer (BD Biosciences, San Diego, CA), frozen, and stored at −80°C until use. For cytokine neutralization experiments (n = 7), whole blood from INF individuals was cultured in the presence of anti-IL-10 (5 μg/ml), anti-TGF-β (5 μg/ml), or isotype control antibody (5 μg/ml) (R&D Systems) for 1 h, BmA and brefeldin A were then added, and blood was cultured for an additional 5 h.
Intracellular cytokine staining.
The cells were thawed, washed first with PBS and then with PBS with 1% bovine serum albumin (BSA), and stained with surface antibodies for 30 to 60 min. The surface antibodies used were CD3-Amcyan, CD4-allophycocyanin (APC)-H7, and CD8-phycoerythrin (PE)-Cy7 (all from BD Biosciences). The cells were washed and permeabilized with BD Perm/Wash buffer (BD Biosciences) and were stained for intracellular cytokines for an additional 30 min before washing and data acquisition. The cytokine antibodies used were gamma interferon (IFN-γ)-PE, IL-4-fluorescein isothiocyanate (FITC), IL-5-APC, IL-13-PE, IL-10-APC, and CD4-APC-Cy7 (all from BD Pharmingen); tumor necrosis factor alpha (TNF-α)-FITC, IL-17-FITC, and CD3-Amcyan (all from BD Biosciences); IL-2-APC, IL-9-PE, and IL-21-Alexa Fluor 647 (all from e-Biosciences); and IL-22-APC (R&D Systems). Flow cytometry was performed with a FACSCanto II flow cytometer with FACSDiva software (version 6; Becton Dickinson). The lymphocyte gating was set by forward and side scatter, and data for 100,000 gated lymphocyte events were acquired. Data were collected and analyzed using FlowJo software (TreeStar, Ashland, OR). All data are depicted as the frequency of CD8+ T cells expressing cytokines. Values recorded following medium stimulation are depicted as the baseline frequency, while frequencies following stimulation with antigens or PMA-ionomycin are depicted as net frequencies (with baseline values subtracted).
Statistical analysis.
Data analyses were performed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). Geometric means were used for measurements of central tendency. Statistically significant differences among the three groups were analyzed using the Kruskal-Wallis test with Dunn's correction for multiple comparisons.
RESULTS
Diminished frequencies of antigen-specific type 1 cytokine-expressing CD8+ T cells in INF individuals.
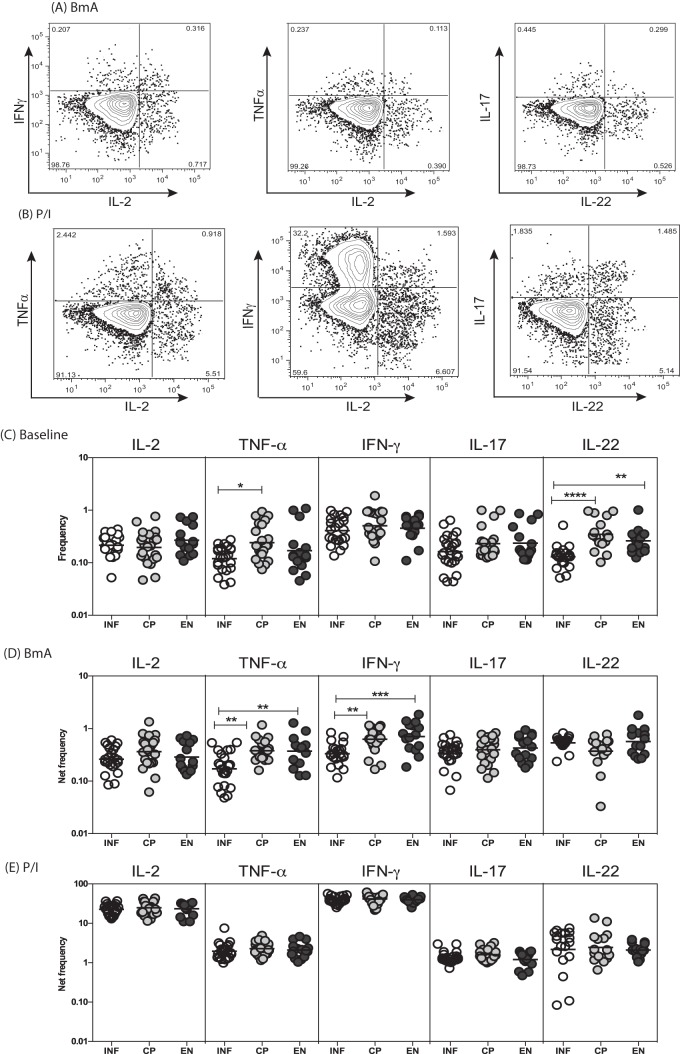
To determine the spontaneously expressed and antigen-stimulated frequencies of CD8+ T cells expressing type 1 and type 17 cytokines, we used flow cytometry to measure the frequencies of IFN-γ-, TNF-α-, IL-2-, IL-17-, and IL-22-expressing CD8+ T cells in INF, CP, and EN individuals, using the strategy presented in Fig. 1A. As shown in Fig. 1B, INF individuals exhibited significantly lower spontaneous frequencies of CD8+ T cells expressing TNF-α, in comparison with CP individuals, and IL-22, in comparison with both CP and EN individuals. Similarly, INF individuals exhibited significantly lower frequencies of BmA-stimulated CD8+ T cells expressing TNF-α and IFN-γ, in comparison with both CP and EN individuals. Moreover, as shown in Fig. S1A and B in the supplemental material, INF individuals also had significantly lower frequencies of Mf antigen-stimulated but not PPD antigen-stimulated CD8+ T cells expressing IL-2, TNF-α, and IFN-γ, in comparison with both CP and EN individuals. In contrast, INF individuals exhibited no significant differences in the frequencies of CD8+ T cells expressing type 1 and type 17 cytokines in response to PMA and ionomycin. Thus, filarial infection is characterized by diminished frequencies of filarial antigen-specific type 1 cytokine-expressing CD8+ T cells.
FIG 1.
Filarial infection is associated with altered baseline and filarial antigen-stimulated frequencies of type 1 cytokine-expressing CD8+ T cells. (A and B) Representative flow cytometry dot plots of CD8+ T cells expressing type 1 cytokines (IL-2, TNF-α, and IFN-γ) and type 17 cytokines (IL-17 and IL-22) following BmA (A) or PMA-ionomycin (P/I) (B) stimulation in an INF individual. (C) Scatter plots of spontaneously expressed frequencies of CD8+ T cells expressing type 1 and type 17 cytokines in INF (n = 25), CP (n = 23), and EN (n = 15) individuals. (D) Scatter plots of BmA-stimulated frequencies of CD8+ T cells expressing type 1 and type 17 cytokines in INF, CP, and EN individuals. (E) Scatter plots of PMA-ionomycin-stimulated frequencies of CD8+ T cells expressing type 1 and type 17 cytokines in INF, CP, and EN individuals. Horizontal lines, geometric means. Antigen-stimulated frequencies are shown as net frequencies, with the baseline levels subtracted. P values were calculated using the Kruskal-Wallis test with Dunn's correction for multiple comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Enhanced frequencies of antigen-specific type 2 cytokine-expressing CD8+ T cells in INF individuals.
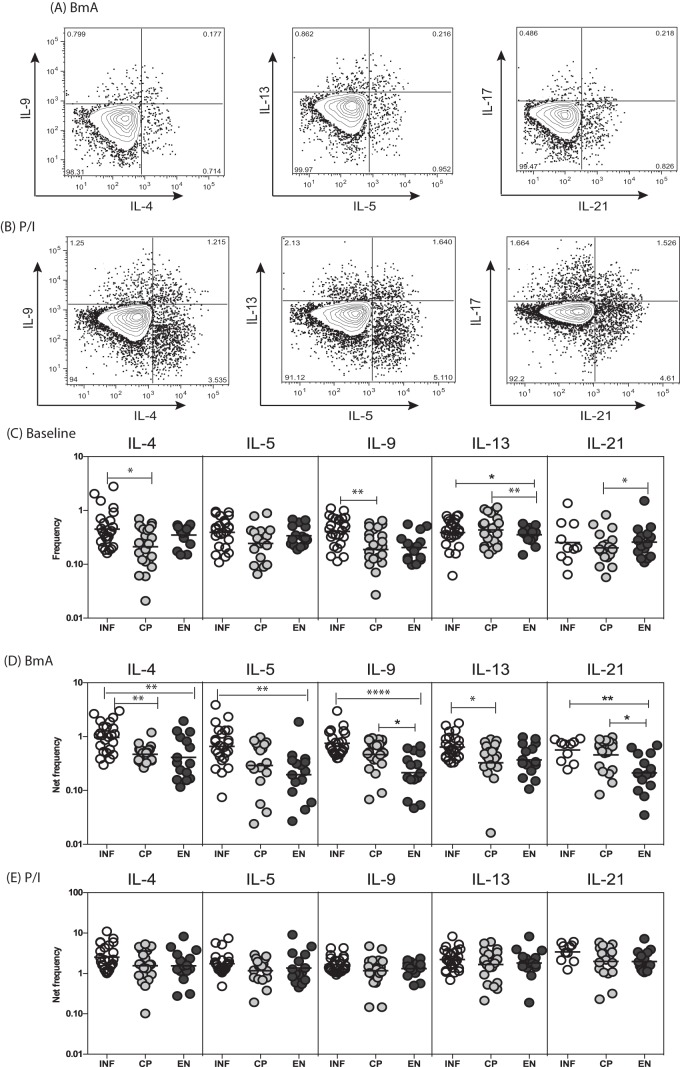
To determine the spontaneously expressed and antigen-stimulated frequencies of CD8+ T cells expressing type 2 cytokines, we used flow cytometry to measure the frequencies of IL-4-, IL-5-, IL-9-, IL-13-, and IL-21-expressing CD8+ T cells in INF, CP, and EN individuals (Fig. 2A). As shown in Fig. 2B, INF individuals had significantly higher spontaneous frequencies of CD8+ T cells expressing IL-13, in comparison with EN but not CP individuals. Similarly, INF individuals had significantly higher frequencies of BmA-stimulated CD8+ T cells expressing IL-4 and IL-13, in comparison with CP individuals, and IL-4, IL-9, IL-13, and IL-21, in comparison with EN individuals. Moreover, as shown in Fig. S1A and B in the supplemental material, INF individuals also exhibited significantly lower frequencies of Mf antigen-stimulated but not PPD antigen-stimulated CD8+ T cells expressing IL-4, IL-5, IL-9, and IL-21, in comparison with CP and/or EN individuals. In contrast, INF individuals had no significant differences in the frequencies of CD8+ T cells expressing type 2 cytokines in response to PMA-ionomycin. Thus, filarial infection is characterized by enhanced frequencies of filarial antigen-specific type 2 cytokine-expressing CD8+ T cells.
FIG 2.
Filarial infection is associated with altered baseline and filarial antigen-stimulated frequencies of type 2 cytokine-expressing CD8+ T cells. (A and B) Representative flow cytometry dot plots of CD8+ T cells expressing type 2 cytokines (IL-4, IL-5, IL-9, IL-13, and IL-21) following BmA (A) or PMA-ionomycin (B) stimulation in an INF individual. (C) Scatter plots of spontaneously expressed frequencies of CD8+ T cells expressing type 2 cytokines in INF (n = 25), CP (n = 23), and EN (n = 15) individuals. (D) Scatter plots of BmA-stimulated frequencies of CD8+ T cells expressing type 2 cytokines in INF, CP, and EN individuals. (E) Scatter plots of PMA-ionomycin-stimulated frequencies of CD8+ T cells expressing type 2 cytokines in INF, CP, and EN individuals. Horizontal lines, geometric means. Antigen-stimulated frequencies are shown as net frequencies, with the baseline levels subtracted. P values were calculated using the Kruskal-Wallis test with Dunn's correction for multiple comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Regulation of the frequencies of type 1 and type 2 cytokine-expressing CD8+ T cells in INF individuals after treatment of filarial infections.
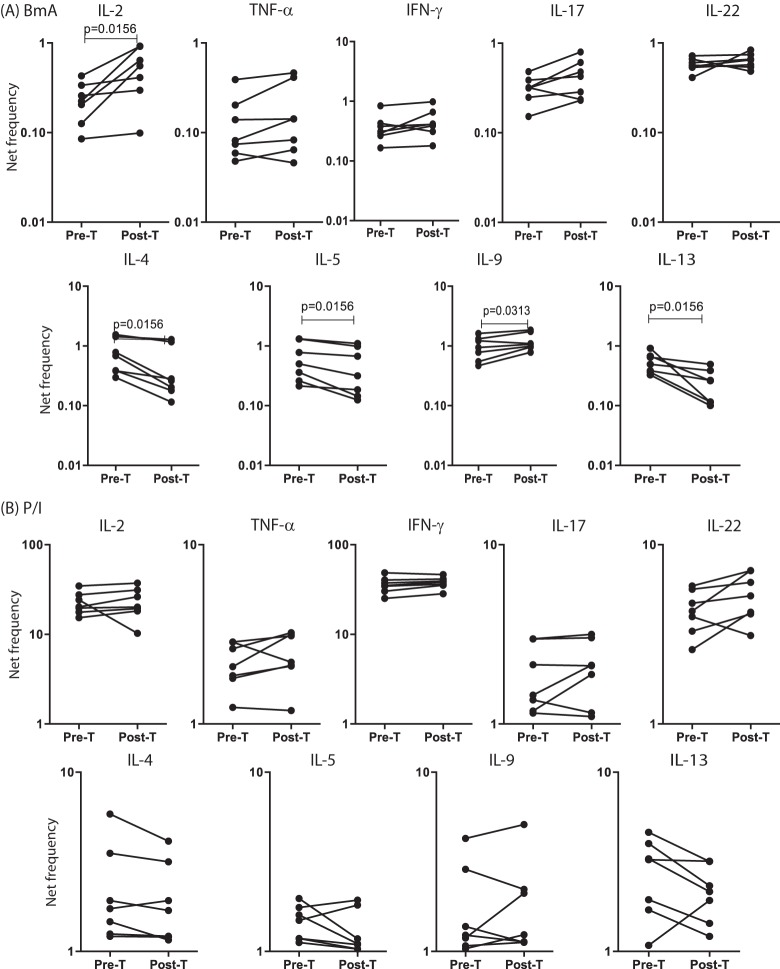
To determine the role of antigen persistence in the maintenance of CD8+ T cells expressing type 1, type 2, and type 17 cytokines in filarial infections, we measured the frequencies of CD8+ T cells expressing these cytokines in a subset of INF individuals (n = 7) who had been treated with antifilarial chemotherapy and, as a result, had eliminated infection (as demonstrated by the absence of circulating filarial antigen). As shown in Fig. 3A, treatment of filarial infection and consequent cure resulted in significantly increased frequencies of CD8+ T cells expressing IL-2 and IL-9 and significantly decreased frequencies of CD8+ T cells expressing IL-4, IL-5, and IL-13 following BmA stimulation, whereas treatment and consequent cure had no significant effect on the frequencies of CD8+ T cells expressing these cytokines in response to PMA-ionomycin (Fig. 3B). In addition, Mf antigen but not PPD stimulation resulted in significantly increased frequencies of CD8+ T cells expressing IL-2, TNF-α, and IL-9 and significantly decreased frequencies of CD8+ T cells expressing IL-4, IL-5, and IL-13 (see Fig. S2 in the supplemental material). Thus, the downmodulation of CD8+ type 1 responses and upregulation of CD8+ type 2 responses in filarial infections are dependent on the presence of active infection.
FIG 3.
Treatment of filarial infection is associated with altered frequencies of filarial antigen-specific CD8+ T cells expressing type 1 and type 2 cytokines. (A) Frequencies of CD8+ T cells expressing type 1 and type 2 cytokines following stimulation with BmA, before (Pre-T) and after (Post-T) treatment with standard doses of DEC and albendazole, in a subset of INF individuals (n = 7). (B) Frequencies of CD8+ T cells expressing type 1 and type 2 cytokines following stimulation with PMA-ionomycin, before and after treatment with standard doses of DEC and albendazole, in a subset of INF individuals (n = 7). Antigen-stimulated frequencies are shown as net frequencies, with the baseline levels subtracted. Each line represents a single individual. P values were calculated using the Wilcoxon signed-rank test.
IL-10- and TGF-β-independent regulation of cytokine-expressing CD8+ T cells in INF individuals.
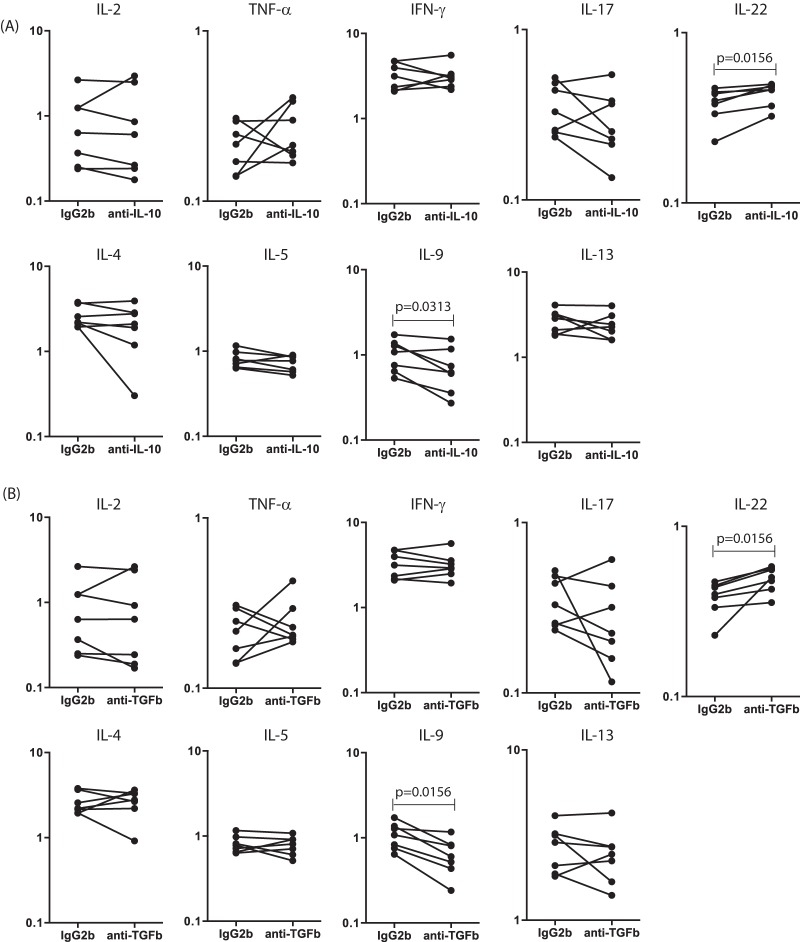
To determine the roles of IL-10 and TGF-β in the modulation of antigen-specific CD8+ T cell type 1 and type 2 responses in INF individuals, we measured the frequencies of CD8+ T cells expressing these cytokines in the presence of anti-IL-10 or anti-TGF-β neutralizing antibodies or an isotype control in a subset of INF individuals (n = 7). As shown in Fig. 4A, IL-10 neutralization resulted in significantly increased frequencies of CD8+ T cells expressing IL-22 and significantly decreased frequencies of CD8+ T cells expressing IL-9 but not the other type 1, type 2, or type 17 cytokines in INF individuals. Similarly, as shown in Fig. 4B, TGF-β blockade resulted in insignificantly increased frequencies of CD8+ T cells expressing IL-22 and significantly decreased frequencies of CD8+ T cells expressing IL-9 but not the other type 1, type 2, or type 17 cytokines in INF individuals. Thus, neither IL-10 nor TGF-β plays an important role in the modulation of CD8+ T cell cytokine responses in filarial infections.
FIG 4.
IL-10 and TGF-β do not affect the frequencies of CD8+ T cells expressing type 1 and type 2 cytokines in filarial infections. (A) Frequencies of CD8+ T cells expressing type 1 and type 2 cytokines following stimulation with BmA, after IL-10 neutralization, in a subset of INF individuals (n = 7). IL-10 neutralization significantly increases the frequency of CD8+ T cells expressing IL-22 and significantly decreases the frequency of CD8+ T cells expressing IL-9. (B) Frequencies of CD8+ T cells expressing type 1 and type 2 cytokines following stimulation with BmA, after TGF-β neutralization, in a subset of INF individuals (n = 7). TGF-β neutralization significantly increases the frequency of CD8+ T cells expressing IL-22 and significantly decreases the frequency of CD8+ T cells expressing IL-9. Antigen-stimulated frequencies are shown as net frequencies, with the baseline levels subtracted. Each line represents a single individual. P values were calculated using the Wilcoxon signed-rank test.
DISCUSSION
T cells play a major role in orchestrating the response to filarial infections. Both nude mice (which lack T cells) (9, 10) and severe combined immunodeficient (SCID) or Rag-deficient mice (which lack both T and B cells) (11, 12) are susceptible to infection with typically nonpermissive Brugia parasites, indicating that T cells are absolutely critical for elimination of infection. It appears, however, that CD4+ or CD8+ T cells can mediate resistance in nonpermissive animals. Thus, CD8+ T cells (at least in animal models) appear to play an important role in host defenses against filarial infections (6). Moreover, T cells are known to play an important role in the pathogenesis of filarial lymphedema in animal models (13). In human infections, it was first discovered that individuals with chronic lymphedema have increased frequencies of activated CD8+ T cells (expressing HLA-DR) in the peripheral blood (14). Later, it was shown that the frequencies of CD8+ T cells in tissues (including skin and subcutaneous tissues) were also increased (15). Moreover, T cell receptor (TCR) Vβ phenotyping revealed a biased TCR repertoire in the T cells infiltrating the affected tissues in individuals with disease (16). More recently, it was shown that there were very few differences in the frequencies of CD8+ T cells expressing type 1 and type 17 cytokines between filaria-infected and uninfected individuals at baseline, but that study did not examine the responses of CD8+ T cells to filarial antigens (17).
To examine the regulation of CD8+ T cells in chronic filarial infections and to help elucidate whether filaria-induced pathogenic reactions were caused by unchecked proinflammatory (type 1/type 17) CD8+ responses, we examined the contributions of CD8+ T cells expressing a variety of cytokines. Our study reveals that CD8+ T cells are present at altered frequencies in filaria-infected individuals, compared to both individuals with clinical pathology (CP) and individuals who are free of filarial infection (EN). Furthermore, the cytokine expression pattern of CD8+ T cells mirrors that described previously for CD4+ T cells, with a dampened type 1 response and a heightened Th2 response. Interestingly, CD8+ T cells expressing the classic type 17 cytokine IL-17 were not present at altered frequencies in INF individuals, suggesting that the contributions of CD8+ T cells to the altered type 17 response in filarial pathology are minimal. Following parasite antigen stimulation, there was clear induction of CD8+ type 1 and type 2 responses, which was not seen in response to the control antigen PPD. The roles of IL-9- and IL-22-expressing CD8+ T cells need to be examined further in future experiments. Finally, our data also demonstrate that the type 1 and type 2 CD8+ responses observed were not due to a cell-intrinsic defect in the INF group, as levels of mitogenic stimulation were equivalent across all three groups of subjects.
In addition to characterizing expression patterns, we examined some of the mechanisms regulating the expression of these cytokines. Since IL-10 and TGF-β are known to play roles in modulating CD4+ cytokine expression in chronic filarial infections (7), we examined the frequencies of CD8+ T cells expressing type 1, type 2, and type 17 cytokines following either IL-10 or TGF-β blockade during in vitro stimulation with filarial antigen. Our data, though preliminary due to the small sample size, interestingly show very little difference in the immune modulation of the CD8+ T cell cytokine repertoire by these two important regulatory cytokines. This is in marked contrast to the effects of these regulatory cytokines on CD4+ T cell expression of cytokines (18, 19).
Our finding that type 1 responses are dampened in patients with active infections but not those with filarial pathology has clear implications. IFN-γ and TNF-α are potent stimulators of nitric oxide and other proinflammatory mediators of inflammation in a variety of cell types and hence could contribute directly to development of pathology (20). CD8+ T cells expressing type 1 cytokines are known to induce the production of the lymphangiogenic growth factors vascular endothelial growth factor C (VEGF-C) and VEGF-D (21). Hence, increased induction of type 1 responses may promote lymphangiogenic activity and lead to progression of filarial pathology. Finally, type 1 cytokines (especially TNF-α) are known to promote pathology through their effects on inflammatory cell migration, fibroblast migration, proliferation, and activation, tissue modeling and resolution, and excess deposition of extracellular matrix components (22). Type 2 responses are considered to be fundamentally important in protection against the development of pathology both due to their ability to ameliorate Th1-induced inflammatory responses and due to their propensity to promote wound healing and tissue repair (23). For example, IL-5 and IL-13 have profibrotic activity, and IL-4 and IL-13 are critical mediators of alternative activation of macrophages and of the production of several proteins associated with tissue repair, such as arginase-1, matrix metallopeptidase-12 (MMP-12), and triggering receptor expressed on myeloid cells-2 (TREM2) (23).
Our study clearly shows that CD8+ T cells undergo a process of regulation upon antigen stimulation in chronic infections similar to that for CD4+ T cells. Finally, the importance of antigen persistence is clearly illustrated by our data on a small subset of individuals who cleared infections following treatment and therefore were proven to be filarial antigen negative. The follow-up data on those individuals indicate a clear reversal of the suppression of type 1 CD8+ responses and the expansion of type 2 CD8+ responses. To our knowledge, this is the first report on the regulation of CD8+ T cell subsets following the elimination of infection, and it clearly implicates the various T cell subsets in filarial pathogenesis.
In summary, our study examines the cytokine expression pattern of CD8+ T cells in filarial infection and disease. We show that CD8+ T cells play an important role in the regulation of immune responses to filarial antigens and this is dependent on the presence of persistent antigen but not IL-10 and TGF-β. Therefore, our study defines a potential role for CD8+ T cells in driving the inflammatory pathology in filarial disease and in preventing this pathological response in subclinical infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the Filariasis Clinic, Government General Hospital (Chennai, India), especially M. Sathiswaran and M. Yegneshwaran, and the NIRT Epidemiology Unit for assistance with patient recruitment.
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Published ahead of print 24 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00598-14.
REFERENCES
- 1.Nutman TB, Kumaraswami V. 2001. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 23:389–399. 10.1046/j.1365-3024.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 1992. Lymphatic filariasis: the disease and its control: fifth report of the WHO Expert Committee on Filariasis. World Health Organ. Tech. Rep. Ser. 821:1–71. [PubMed] [Google Scholar]
- 3.Taylor MJ, Hoerauf A, Bockarie M. 2010. Lymphatic filariasis and onchocerciasis. Lancet 376:1175–1185. 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 4.Babu S, Nutman TB. 2012. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 34:847–861. 10.1007/s00281-012-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maizels RM, Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733–744. 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 6.Babu S, Nutman TB. 2014. Immunology of lymphatic filariasis. Parasite Immunol. 36:338–346. 10.1111/pim.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CL, Mahanty S, Kumaraswami V, Abrams JS, Regunathan J, Jayaraman K, Ottesen EA, Nutman TB. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis: preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Invest. 92:1667–1673. 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lal RB, Ottesen EA. 1988. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J. Infect. Dis. 158:1034–1037. 10.1093/infdis/158.5.1034. [DOI] [PubMed] [Google Scholar]
- 9.Suswillo RR, Owen DG, Denham DA. 1980. Infections of Brugia pahangi in conventional and nude (athymic) mice. Acta Trop. 37:327–335. [PubMed] [Google Scholar]
- 10.Vincent AL, Sodeman WA, Winters A. 1980. Development of Brugia pahangi in normal and nude mice. J. Parasitol. 66:448. 10.2307/3280746. [DOI] [PubMed] [Google Scholar]
- 11.Nelson FK, Greiner DL, Shultz LD, Rajan TV. 1991. The immunodeficient scid mouse as a model for human lymphatic filariasis. J. Exp. Med. 173:659–663. 10.1084/jem.173.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu S, Shultz LD, Klei TR, Rajan TV. 1999. Immunity in experimental murine filariasis: roles of T and B cells revisited. Infect. Immun. 67:3166–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu S, Nutman TB. 2012. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 34:847–861. 10.1007/s00281-012-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lal RB, Kumaraswami V, Krishnan N, Nutman TB, Ottesen EA. 1989. Lymphocyte subpopulations in Bancroftian filariasis: activated (DR+) CD8+ T cells in patients with chronic lymphatic obstruction. Clin. Exp. Immunol. 77:77–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman DO, Horn TD, Maia e Silva CM, Braga C, Maciel A. 1995. Predominant CD8+ infiltrate in limb biopsies of individuals with filarial lymphedema and elephantiasis. Am. J. Trop. Med. Hyg. 53:633–638. [PubMed] [Google Scholar]
- 16.Freedman DO, Plier DA, de Almeida A, Miranda J, Braga C, Maia e Silva CM, Tang J, Furtado A. 1999. Biased TCR repertoire in infiltrating lesional T cells in human Bancroftian filariasis. J. Immunol. 162:1756–1764. [PubMed] [Google Scholar]
- 17.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Sanogo D, Doumbia SS, Traore SF, Mahanty S, Klion A, Nutman TB. 2010. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 184:5375–5382. 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anuradha R, George PJ, Chandrasekaran V, Kumaran PP, Nutman TB, Babu S. 2014. Interleukin 1 (IL-1)- and IL-23-mediated expansion of filarial antigen-specific Th17 and Th22 cells in filarial lymphedema. Clin. Vaccine Immunol. 21:960–965. 10.1128/CVI.00257-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anuradha R, George PJ, Hanna LE, Chandrasekaran V, Kumaran PP, Nutman TB, Babu S. 2014. Parasite-antigen driven expansion of IL-5− and IL-5+ Th2 human subpopulations in lymphatic filariasis and their differential dependence on IL-10 and TGFβ. PLoS Negl. Trop. Dis. 8:e2658. 10.1371/journal.pntd.0002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacMicking J, Xie QW, Nathan C. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323–350. 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 21.Numasaki M, Lotze MT, Sasaki H. 2004. Interleukin-17 augments tumor necrosis factor-α-induced elaboration of proangiogenic factors from fibroblasts. Immunol. Lett. 93:39–43. 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Wynn TA. 2007. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117:524–529. 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen JE, Wynn TA. 2011. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 7:e1002003. 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.