Abstract
Subunit recombinant vaccines against classical swine fever virus (CSFV) are a promising alternative to overcome practical and biosafety issues with inactivated vaccines. One of the strategies in evaluation under field conditions is the use of a new marker E2-based vaccine produced in the milk of adenovirally transduced goats. Previously we had demonstrated the efficacy of this antigen, which conferred early protection and long-lasting immunity in swine against CSFV infection. Here, we have used a simpler downstream process to obtain and formulate the recombinant E2 glycoprotein expressed in the mammary gland. The expression levels reached approximately 1.7 mg/ml, and instead of chromatographic separation of the antigen, we utilized a clarification process that eliminates the fat content, retains a minor amount of caseins, and includes an adenoviral inactivation step that improves the biosafety of the final formulation. In a vaccination and challenge experiment in swine, different doses of the E2 antigen contained within the clarified whey generated an effective immune response of neutralizing antibodies that protected all of the animals against a lethal challenge with CSFV. During the immunization and after challenge, the swine were monitored for adverse reactions related to the vaccine or symptoms of CSF, respectively. No adverse reactions or clinical signs of disease were observed in vaccinated animals, in which no replication of CSFV could be detected after challenge. Overall, we consider that the simplicity of the procedures proposed here is a further step toward the introduction and implementation of a commercial subunit vaccine against CSF.
INTRODUCTION
Classical swine fever (CSF) is a highly contagious, often fatal, notifiable disease which is responsible for significant losses in the swine industry worldwide (1, http://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2014). The etiological agent is an enveloped RNA virus (CSF virus [CSFV]) that belongs to the Pestivirus genus of the family Flaviviridae (2). Vaccination of pigs with lapinized Chinese vaccines is still practiced in some regions of the world in which the virus is enzootic, in order to prevent and control the spread of the disease. However, such vaccines are not safe enough and do not allow differentiation of infected from vaccinated animals (DIVA). Attenuated CSFV vaccines can potentially influence CSFV evolution through recombination with wild viruses, suggesting that it is necessary to avoid their excessive use (3). Despite their accepted advantages, these vaccines have one major disadvantage: serological DIVA is impossible (4). Subunit recombinant marker vaccines derived from viral proteins have been considered safer and cheaper alternatives against CSFV (5).
The E2 glycoprotein is the major antigen that induces neutralizing and protective antibodies in CSFV-infected pigs. This glycoprotein is exposed as a homodimer on the outer surface of the virus and mediates the viral entry into the target cells (6, 7). Several marker vaccines based on E2 have been generated so far for the induction of a protective immune response against CSFV (8–12). However, the structural complexity of E2 has demonstrated the necessity to produce this glycoprotein in superior production cell systems in order to enhance its immunogenicity and protective capacity (12). Recently, we developed a new marker subunit vaccine based on E2-CSFV, which is produced in the mammary glands of genetically transformed goats. This formulation has consistently shown an early and elevated protective activity in vaccinated swine (13, 14). Despite these characteristics, the new goat milk-derived marker vaccine has a complex downstream process, which involves separation of fat milk and whey, as well as an additional immobilized-metal affinity chromatography (IMAC) step (15).
Here, we demonstrate that the protective capacity of the goat milk-derived E2 vaccine remains invariable when the formulation is generated by using the E2 antigen present within the whey, without the necessity of purifying the glycoprotein by a chromatography step. A simple and effective viral inactivation step was also considered in the proposed process, which has improved the vaccine biosafety. This is a significant advantage toward the commercial introduction of the new vaccine due to the potential reduction in antigen production costs and simplicity of the separation and formulation processes.
MATERIALS AND METHODS
E2-CSFV antigen production.
The E2 antigen was produced in the mammary glands of goats adenovirally transduced with Ad-E2 as previously described (15). The studies involving experimentation with goats were in accordance with guidelines and recommendations from the Guide for the Care and Use of Laboratory Animals (current edition) and policies from the Chilean Biosafety Manual from Fondecyt-Conicyt. The experimental protocols were drafted by the authors and approved by the Ethics Committee of the Universidad de Concepción, Chile. In all cases, supervision of veterinary authorities from the School of Biological Sciences, Universidad de Concepción, Chile, was guaranteed.
Briefly, the adenoviral vector Ad-E2 contains the extracellular domain of E2-CSFV glycoprotein with a hexahistidine tag in the C′ terminus preceded by the tissue plasminogen signal peptide. The Ad-E2 vector was infused through the nipple channel at a concentration of 1 × 109 gene transfer units (GTU)/ml until the udder volume was replenished. Four female goats (Saanen), 1.5 years old and in the second month of lactation, were used in the experiment. Twenty-four hours after adenovirus instillation, udders were extensively milked to remove the infused solution. A further udder washing step at 24 h postinoculation, consisting of phosphate saline buffer instillation, was carried out prior to milk collection. Milk sampling started 48 h after adenovirus infusion, and it was maintained for the subsequent 20 days. The E2 expression levels obtained in the samples of whey were quantified by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (12).
Viral inactivation and milk processing.
Fresh milk samples collected each day were first titrated for the presence of the E2 adenoviral vector. Titration experiments were conducted in three independent experiments as previously described (16), based on the infection capacity of viable vectors in the HEK-293 cell line, and expressed as gene transfer units (GTU). The photosensitizer agent methylene blue was then utilized at a concentration of 50 μM for inactivation assays, which were conducted with overnight stirring at 4°C. Samples were subjected to white light irradiation for 1 h at a dose of 6,000 lx, using a slide projector with a 360-W Apollo Orizon EYB 71 lamp as light source. After treatment, the milk samples were retitrated to analyze the adenoviral vector reduction. In addition, the E2 antigen integrity was evaluated by Western blotting.
Afterward, the milk was processed by 4-fold serial dilutions in a milk-separating buffer (10 mM Tris-HCl, 10 mM CaCl2, pH 8.0) and chilled on ice for 30 min. The mix was separated by centrifugation at 10,000 rpm for 30 min at 4°C. The fatty layer was discarded, and the serum milk containing the E2 antigen was separated from the casein precipitate. Three filtration steps were carried out using 0.8- and 0.4-μm membranes (Sartorius, Germany) for goat whey clarification. The total amount of proteins in serum samples was determined by the bicinchoninic acid method (Pierce, USA).
E2 formulation.
The E2 antigen, goat whey derived without purification, was dialyzed against 20 volumes of phosphate buffer (10 mM NaH2PO4, pH 7.4) and was sterilized by filtration (0.2-μm filter; Sartorius, Germany). The oil-based adjuvant Montanide 888 (SEPPIC, France) was mixed in sterile mineral oil (Sigma, USA) at a ratio of 1:9. A water-in-oil emulsion was produced in and Ultra-Turrax T25 basic homogenizer (IKA Works Inc., USA) at a proportion of 40% oil phase and 60% aqueous phase.
Starting from goat milk samples containing E2 at a concentration of 1 g per liter, different dilutions for immunizations were considered. Serum samples clarified as described above were diluted to contain 100, 50, and 25 μg of E2 in 2-ml formulation doses. The preparations were stored in nonpyrogenic 50-ml centrifuge tubes (Corning, USA) with 7 doses (14 ml) each. As a placebo, formulation samples of goat whey diluted in phosphate buffer, without the E2 antigen, were emulsified and stored under the same conditions.
Swine immunization trial and challenge.
The experiments with pigs were conducted in accordance with guidelines and recommendations from the Guide for the Care and Use of Laboratory Animals (current edition) and policies from the Cuban Society of Laboratory Animal Science (SCCAL). The experimental protocols were drafted by the authors and approved by the Animal Welfare Commission of the Center for Genetic Engineering and Biotechnology. In all cases, supervision of veterinary authorities from the Institute for Veterinary Medicine, Havana, Cuba, was guaranteed. Pigs were housed in individual rooms, and appropriate feeding, water supply, and health monitoring were permanently provided. Animals that were euthanized were humanly handled.
Crossbred Dubroc/Yorkshire swine 6 weeks old, weighing about 20 kg, serologically negative for CSFV, and belonging to a nonvaccinated and CSF-free herd were used in the experiment. Thirty animals were divided into five groups. All groups, of six animals each, were housed in separate experimental rooms and were handled according to international guidelines for experimentation with animals. The groups were immunized with formulations of clarified whey containing E2 antigen at 100 μg (group A), at 50 μg (group B), and at 25 μg (group C). As a positive control, animals were injected with 25 μg of E2 purified from whey as described previously (15). Animals from the placebo group were immunized with goat whey in Montanide 888 at a dilution equivalent to that for group A.
Animals were vaccinated 3 weeks apart and challenged 1 week after the last immunization. Challenges were conducted by intramuscular injection of 105 50% pig lethal doses (PLD50) of homologous CSFV Margarita strain. All animals were euthanized at 2 weeks postchallenge (p.c.).
Reactogenicity, clinical study, and detection of CSFV-neutralizing antibodies.
Ten days after immunizations, local reactogenicity associated with the inoculation site, rectal temperature, and food intake variability were closely monitored. Then, after challenge, parameters such as CSF clinical signs, depression, fever, and food intake variability were scored daily. Serum samples for neutralizing peroxidase-linked assay (NPLA) were taken at day 0 before the first immunization and each week until challenge. Samples for NPLA were also taken weekly after challenge and at the time of euthanasia.
Postmortem analysis.
After sacrifice, animals were subjected to an exhaustive necropsy in which the presence of pathological lesions in different organs and tissues was evaluated. Examination of gross pathological lesions on spleen, kidneys, tonsils, small intestine, and brain was conducted postmortem in all animals. For observation of lymphocyte perivascular infiltration, brain samples were fixed in 10% formalin, embedded in paraffin, cut, and stained with hematoxylin-eosin by standard procedures.
Viral isolation.
Heparinized blood samples were collected on days 0, 2, 4, 6, 8, 10, 12, and 14 p.c. for viral isolation assay. Each sample was inoculated in 8 wells from 96-well microplates (Costar, USA) containing monolayers of PK-15 cells growing in Dulbecco modified Eagle medium (DMEM) and 5% fetal bovine serum (free of pestivirus and pestivirus antibodies). After 1 h of incubation at 37°C in 5% CO2, the monolayers were washed twice, the medium was replaced, and plates were incubated for 3 days. Plates were washed twice with phosphate-buffered saline (PBS), fixed, and stored at 4°C until the immunoperoxidase monolayer assay. Immunodetection of viral antigen was performed with the 1G6 anti-E2-CSFV monoclonal antibody followed by a goat anti-mouse–horseradish peroxidase-conjugated IgG (Sigma, USA) as the secondary antibody. Those wells containing at least one spot were considered positive. The viremia level was expressed on a scale from 1 to 8 according to the number of positive wells, regardless of the number of spots per well.
Statistical analysis.
For the analysis of the results, NPLA titers were compared by analysis of variance (ANOVA) followed by the Newman-Keuls multiple-comparison test. Statistical processing was performed with the program GraphPad Prism V6.0.
RESULTS
Characterization and formulation of the recombinant whey-derived E2 antigen.
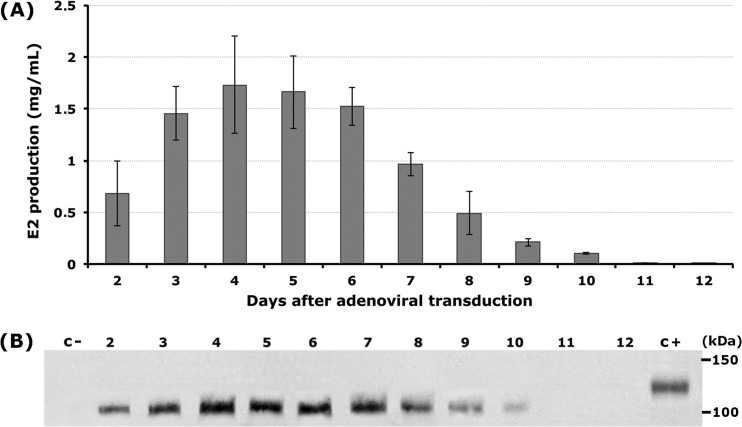
The E2 antigen was first detected by Western blotting and ELISA in the milk of goats adenovirally transduced with the Ad-E2 vector. High-level expression was achieved, according to quantifications performed by ELISA in samples of clarified whey containing E2 devoid of fat content and showing a reduced amount of caseins. In the Western blot, the expression pattern of the milk-derived E2 antigen showed a band with a molecular weight smaller than that for E2 derived from PK-15 cultured cells, as expected from their different glycosylation patterns (12, 17). The expression levels ranged from a maximum yield of about 1.7 mg/ml of milk to minor recoveries of approximately 0.8 mg/ml in a time period of 8 days. After this time, E2 expression levels started to decline, reaching 0.05 mg/ml on day 12 (Fig. 1A and B).
FIG 1.
Classical swine fever virus E2 antigen expression in the milk of adenovirally transduced goats. (A) Time course expression determined by ELISA in samples from day 2 to 12 of milking. (B) Western blot assay from samples in panel A after 10% SDS-PAGE under nonreducing conditions. Lanes: c−, milk serum from a nontransduced goat (negative control); 2 to 12, milk serum samples after Ad-E2His adenovirus transduction; c+, E2 positive control.
On day 1 posttransduction, udders were milked and washed to remove the infused solution prior to milk collection. Subsequently, viral inactivation in a group of milk samples using 50 μM methylene blue combined with white light irradiation resulted in a significant reduction of the adenoviral vector charge starting on day 2 posttransduction. Complete adenoviral vector inactivation was achieved in the treated milk samples from day 3 posttransduction onwards (Fig. 2). An additional udder wash, performed after milking at 24 h postinstillation, contributed to the drop in adenoviral vector titers detected in milk samples collected at 48 h and afterwards. For E2 antigen preparation, whey samples milked from 48 h (day 3 posttransduction) until the eighth day were used. In these preparations, no Ad-E2 vector was detected in the titration assays, and the E2 recombinant protein remained in its homodimeric native form (Fig. 2).
FIG 2.
Adenoviral detection and E2 antigen characterization in milk samples subjected to viral inactivation. Adenoviral titers were determined by transduction in HEK-293A cells using samples from goat milk before and after treatment with methylene blue (50 μM), used as viral inactivation agent. Positive standard deviation bars are shown. Statistically significant differences (P < 0.01) between treated and untreated samples at 48 h posttransduction are also indicated. Inset, Western blot analysis of E2 antigen present in goat milk samples after the methylene blue viral inactivation treatment. Lane 1, milk serum corresponding to day 1 posttransduction, in which samples were taken from udders but no inactivation treatment was conducted. Lane 2, milk serum treated with methylene blue. Lane c−, milk serum from a nontransduced goat (negative control). Lane c+, E2 from PK-15 adenovirally transduced cells.
In order to evaluate the immunogenicity of the antigen without further purification steps, final concentrations of E2 at 100 μg/dose, 50 μg/dose, and 25 μg/dose were prepared using the clarified whey from inactivated milk samples. In such preparations, the antigen could be easily detected by SDS-PAGE and Western blot analysis. The total amount of proteins per preparation was 520 μg (1×), 1.04 mg (2×), and 2.08 mg (4×) (Fig. 3). The E2 antigen quantified by ELISA corresponded to 4.8% of the total proteins in every dilution. The corresponding immunogens were all emulsified in Montanide 888 for immunization experiments in swine.
FIG 3.
Classical swine fever virus E2 antigen vaccine formulation consisting of the recombinant protein contained within the clarified, inactivated whey from the milk of transduced goats. (A) Ten percent SDS-PAGE under nonreducing conditions of whey samples containing 25 μg (lane 1×), 50 μg (lane 2×), and 100 μg (lane 4×) of the E2 antigen. Lane C+ corresponds to E2 chromatographically purified antigen from goat milk (25 μg), and lane C− corresponds to whey from an untreated goat. (B) Western blot analysis of samples in panel A. Molecular mass markers are shown in both panels.
The recombinant E2 antigen in the whey of goats elicits high levels of CSFV-neutralizing antibodies in swine.
Vaccination of swine was carried out with the formulations containing 100, 50, or 25 μg/dose of E2 antigen contained in the whey of goats. Antibodies with the capacity to neutralize the CSFV in vitro were detected as soon as 7 days postvaccination in the groups of animals that received the purified E2 antigen and also in those pigs injected with the whey-derived antigen. Fourteen days after the first inoculation, specific antibodies with values over four logarithmic units, expressed as the logarithm of neutralizing titers, were measured in all the vaccinated groups. After the second dose was administered, at day 21, neutralizing titers kept rising until they reached maximum values of over 10 logarithmic units. During the whole evaluation period, no statistically significant differences (P ≤ 0.05) in the titer values were encountered when comparing the formulations assayed (Fig. 4). After challenging the animals with CSFV in the fourth week, the specific neutralizing antibody titers remained at similar levels in all of those animals that received the E2 antigen. No CSFV-specific neutralizing antibodies were detected in pigs from the placebo group.
FIG 4.
Time course of CSFV-specific neutralizing antibodies in the groups of vaccinated pigs and body temperature changes after challenge. (A) Pigs were immunized with increasing concentrations of the E2 antigen contained within the clarified whey from the milk of goats. The first dose was followed by a boost 3 weeks later. All the groups were challenged at week 4 with 105 PLD50 of the homologous CSFV Margarita strain by intramuscular injection. The animals from the placebo group remained unresponsive during the time prior to challenge. (B) Time course of body temperature changes in pigs challenged 1 week after the last dose administered. Fever was considered a rectal temperature of >40°C. Animals from the placebo group were euthanized at day 14 p.c. to avoid suffering. No statistically significant differences (P ≤ 0.05) in the titer values were encountered when comparing the formulations assayed, according to the Newman-Keuls multiple-comparison test.
The safety of the vaccine preparations was also corroborated during the vaccination time period. Local reactogenicity and associated parameters were monitored for 10 days, following the administration of both the first dose and the boost at day 21. It was observed that methylene blue, added for adenoviral vector inactivation, as well as whey contaminants remaining in the preparations, did not provoke adverse clinical signs such as fever or associated reactions. A variable food intake-related behavior was observed in some animals from different groups (Table 1). These findings did not differ from those observed in animals vaccinated with chromatographically purified E2.
TABLE 1.
Evaluation of adverse reactions and CSF clinical signs after an immunization-and-challenge trial in pigs and of visible pathological lesions after postmortem examinationa
| Group | Pig | Postimmunization |
Postchallenge |
Postmortem |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR | FD | FI | FD | FI | Dep | LPI | HS | KP | NI | NT | ||
| A (E2, 100 μg) | 4 | − | 0 | N | 0 | V | − | − | − | − | − | − |
| 7 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 10 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 18 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 19 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 22 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| B (E2, 50 μg) | 3 | − | 0 | N | 0 | N | − | − | − | − | − | − |
| 8 | − | 0 | N | 0 | V | − | − | − | + | − | − | |
| 20 | − | 0 | V | 0 | N | − | − | − | − | − | − | |
| 23 | − | 0 | N | 0 | N | − | + | − | − | − | − | |
| 28 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 29 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| C (E2, 25 μg) | 11 | − | 0 | N | 0 | N | − | − | − | + | − | − |
| 12 | − | 0 | N | 0 | V | − | − | − | − | − | − | |
| 15 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 24 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 26 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 30 | − | 0 | N | 0 | V | − | − | − | − | − | − | |
| D (purified E2, 25 μg) | 1 | − | 0 | N | 0 | N | − | − | − | − | − | − |
| 9 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 13 | − | 0 | N | 0 | N | − | − | − | + | − | − | |
| 21 | − | 0 | N | 0 | N | − | − | − | − | − | − | |
| 25 | − | 0 | N | 0 | V | − | − | − | − | − | − | |
| 27 | − | 0 | V | 0 | N | − | − | − | − | − | − | |
| E (placebo) | 2 | − | 0 | N | 8 | L | + | + | + | + | − | + |
| 5 | − | 0 | N | 10 | L | + | + | − | + | + | − | |
| 6 | − | 0 | N | 8 | L | + | + | + | + | + | + | |
| 14 | − | 0 | N | 10 | V | + | − | + | − | − | − | |
| 16 | − | 0 | V | 10 | L | + | + | + | + | + | + | |
| 17 | − | 0 | N | 8 | L | + | − | + | + | + | + | |
Pigs were immunized with different doses of E2-CSFV formulated within the whey of adenovirally transduced goats. Groups A to C received vaccine preparations containing 100, 50, and 25 μg of E2, respectively, in an oil-based adjuvant. Group D was immunized with 25 μg of E2 purified from the milk, while group E was injected with adjuvanted goat whey without E2 antigen. Two weeks after challenge, all animals were euthanized for necropsy and tissue analysis. Abbreviations: LR, local reactogenicity; FI, food intake (N, normal; V, variable; L, low); Dep, depression; PLL, perivascular lymphocyte infiltration in the brain; HS, hemorrhage in spleen; KP, kidney petechiae; NI, necrotic ileum; NT, necrotic tonsil; FD, number of days with fever (rectal temperature above 40°C).
Whey proteins do not impair the full protection conferred by the E2 antigen against CSFV in swine.
Four weeks after the first immunization, the protective capacities of the vaccine preparations formulated as described were evaluated through challenge of the immunized pigs, using a dose of 105 PLD50 of the homologous CSFV highly pathogenic Margarita strain. The time course of the rectal temperatures measured showed that no animals from the four groups vaccinated with the E2 antigen in the whey developed fever after challenge (Fig. 4). These animals did not show clinical signs that are typical of CSF infection, such as anorexia (low food intake) or prostration (depression). In contrast, some animals from the group injected with the placebo formulation were sacrificed at 10 days after challenge to avoid suffering, due to a deterioration of their general state. This included rectal temperatures above 40°C detected only 3 days after challenge. The temperatures increased to over 41.5°C after the seventh day in all of these animals. Notably, two animals died at day 12 postchallenge. Afterward, the necropsy of animals from the placebo group showed both visible and microscopic pathological lesions compatible with CSF in internal organs such as the tonsils, spleen, kidney, intestinal mucosa, and brain. The lesions included perivascular lymphocyte infiltration of brain tissue, marginal spleen infarcts, petechiae in kidney, and necrosis in tonsils and ileum. The animals from the vaccinated groups remained completely healthy more than 15 days after challenge, when the experiment was concluded. Only minor tissue damage was encountered in isolated animals from groups B to D during postmortem analysis, which were not considered relevant (Table 1).
Finally, an additional parameter analyzed after challenge was the persistence in blood of detectable CSFV in the immunized groups. Repeated attempts at viral isolation utilizing blood leukocytes from vaccinated individuals failed to demonstrate circulation of CSFV, thus indicating an effective clearance of the virus, independently of the dose used and the amount of whey proteins present in the formulation. As expected, blood samples from pigs receiving the placebo formulation resulted in isolation of CSFV during the fifth, sixth, and seventh days after challenge.
DISCUSSION
At present, it is accepted that veterinary vaccines must comply with certain requirements such as low costs of production and an established efficacy to allow their availability in the marketplace (18). In the case of CSF vaccines, they should also ensure both an early and long-lasting protection combined with the possibility to differentiate infected from vaccinated animals (DIVA strategy) (19–21).
A biotechnological solution for the production of vaccines based on complex molecules could be, for instance, their expression in the mammary glands of genetically transformed ruminants. The mammary gland has certain advantages, such as high rates of secretion of recombinant proteins with structurally demanding requirements and a regular, steady regimen of production (22). Remarkably, ruminants are also prone to high production volumes of milk combined with relative low maintenance costs. In previous trials we have demonstrated the feasibility of using the CSFV E2 recombinant protein obtained from adenoviral transduced sources (such as cultured cell lines and the mammary glands of goats) to confer solid protection from the viral infection and disease in swine (12, 14, 15, 23). In the present study, we have extended the practical value of the E2 antigen by assessing its immunogenicity and protective efficacy after being formulated in the whey obtained from virus-inactivated goat milk, in a straightforward, scalable, biotechnological approach.
In addition to the advantages of recombinant antigen production in the mammary glands of livestock, here we have considered a significant reduction in the complexity of the downstream process directed toward the formulation of the veterinary vaccine. We have avoided the previously reported chromatography purification of the E2 antigen (15) by using a new simplified antigenic preparation based on a single step of milk clarification. This process involves a step of fat content elimination, casein precipitation, and viral inactivation of the whey raw material. This approach is able to render an immunogenic preparation in which the E2 antigen remains in its homodimeric and glycosylated form, thus eliciting neutralizing antibodies and a protective efficacy equivalent to that obtained with the chromatography-purified E2 antigen. The neutralizing-antibody titers, which rose to 1:128 (in the third week after the first immunization) and reached 1:512 following the boost at day 21, were in the same range as those previously recorded with the E2 antigen as formerly purified (12, 14, 15). Notably, independently of the fact that the doses administered were doubled or quadrupled in both the E2 antigen and the whey protein contaminants, no differences in NPLA titers were recorded during the time course of the immunization trial. This validates that goat whey-derived proteins still present in the new vaccine preparation do not hamper the induction of a potent humoral response specific to CSFV. The goat whey content appeared to be an appropriate vehicle for the delivery of vaccine preparations from recombinant antigens produced in the mammary gland.
Consequently, such neutralizing antibody levels correlated with a rapid and efficacious protection against lethal doses of CSFV. This protection was achieved as soon as those antibodies with neutralizing activity reached values over 1/160 (5, 14, 24). These levels obtained in this work are similar to those previously described in vaccination and challenge trials using the purified E2 antigen obtained either from transduced pig cultured cells or from the milk of goats (12). In spite of the use of four different E2 antigen preparations, an effective response against CSF clinical disease was obtained in all the vaccinated pigs, which included avoidance of CSFV infection after challenge. This was in accordance with the lack of viral replication encountered in previous vaccine preparations tested, based on E2 purified by a chromatographic approach (15).
In order to arrive at a biotechnologically feasible application, we considered the safety of the preparations by titrating the adenoviral vector contained within the whey derived from the milk of transduced goats. The combination of methylene blue and white light irradiation effectively inactivated the Ad-E2 vector initially present in the first samples of milk collected. With this approach, the use of inactivating agents such as the potentially toxic beta-propiolactone was avoided, in contrast with other procedures previously proposed (25, 26).
In recent years, various other vaccination approaches have been conducted in order to circumvent the intrinsic problems of live CSFV attenuated vaccines currently in use (5). Among them, the design of a tandem-repeat multiple-epitope vaccine (27), expression of the E2 protein in yeasts (28), and the development of a modified chimera vaccine (consisting of bovine viral diarrhea virus expressing the CSFV E2 protein) (29) are the most interesting alternatives, with varied potential for future licensing and field use. In such scenarios, the methodology of adenoviral transduction of the mammary gland for the expression of complex antigens at high levels arises as an innovative strategy able to overcome biosafety, potency, and DIVA-related issues. Taken together, our present proposal consists of adenoviral instillation for high-level E2 production followed by milk collection and a simplified step of viral inactivation and whey clarification. It constitutes a viable procedure for veterinary vaccine production with optimum results in terms of protection and great potential for field evaluations (in progress) and future licensing and implementation.
ACKNOWLEDGMENTS
We thank Paula Naranjo, Medicine Veterinary Institute, Havana, Cuba, for support with animals during the challenge trial.
This research was supported by FONDEF grant D09I1262 and FONDECYT grant 1110925, CONICYT, Chile.
Footnotes
Published ahead of print 1 October 2014
REFERENCES
- 1.Paton DJ, Greiser-Wilke I. 2003. Classical swine fever—an update. Res. Vet. Sci. 75:169–178. 10.1016/S0034-5288(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 2.Moennig V, Floegel-Niesmann G, Greiser-Wilke I. 2003. Clinical signs and epidemiology of classical swine fever: a review of new knowledge. Vet. J. 165:11–20. 10.1016/S1090-0233(02)00112-0. [DOI] [PubMed] [Google Scholar]
- 3.Ji W, Niu DD, Si HLi, Ding NZ, He CQ. 2014. Vaccination influences the evolution of classical swine fever virus. Infect. Genet. Evol. 25:69–77. 10.1016/j.meegid.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Feliziani F, Blome S, Petrini S, Giammarioli M, Iscaro C, Severi G, Convito L, Pietschmann J, Beer M, De Mia GM. 2014. First assessment of classical swine fever marker vaccine candidate CP7_E2alf for oral immunization of wild boar under field conditions. Vaccine 32:2050–2055. 10.1016/j.vaccine.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Huang YL, Deng MC, Wang FI, Huang CC, Chang CY. 2014. The challenges of classical swine fever control: modified live and E2 subunit vaccines. Virus Res. 179:1–11. 10.1016/j.virusres.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Thiel HJ, Stark R, Weiland E, Rümenapf T, Meyers G. 1991. Hog cholera virus: molecular composition of virions from a pestivirus. J. Virol. 65:4705–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiland F, Weiland E, Unger G, Saalmüller A, Thiel HJ. 1999. Localization of pestiviral envelope proteins E(rns) and E2 at the cell surface and on isolated particles. J. Gen. Virol. 80:1157–1165. [DOI] [PubMed] [Google Scholar]
- 8.Hulst MM, Westra DF, Wensvoort G, Moormann RJ. 1993. Glycoprotein E1 of hog cholera virus expressed in insect cells protects swine from hog cholera. J. Virol. 67:5435–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blome S, Meindl-Bohmer A, Loeffen W, Thuer B, Moennig V. 2006. Assessment of classical swine fever diagnostics and vaccine performance. Rev. Sci. Tech. 25:1025–1038. [PubMed] [Google Scholar]
- 10.van Rijn PA, Bossers A, Wensfort G, Moormann RJ. 1996. Classical swine fewer virus (CSFV) envelope glycoprotein E2 containing one structural antigenic unit protects pigs from lethal CSFV challenge. J. Gen. Virol. 77:2737–2745. 10.1099/0022-1317-77-11-2737. [DOI] [PubMed] [Google Scholar]
- 11.Moormann RJ, Bouma A, Kramps JA, Terpstra C, de Smit HJ. 2000. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet. Microbiol. 73:209–219. 10.1016/S0378-1135(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez O, Barrera M, Rodríguez MP, Frías MT, Figueroa NE, Naranjo P, Montesino R, Farnos O, Castell S, Venereo A, Ganges L, Borroto C, Toledo JR. 2008. Classical swine fever virus E2 glycoprotein antigen produced in adenovirally transduced PK-15 cells confers complete protection in pigs upon viral challenge. Vaccine 26:988–997. 10.1016/j.vaccine.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Barrera M, Sanchez O, Prieto Y, Castell S, Naranjo P, Rodriguez MP, Farnós O, Aguilera A, Frias M, Fonseca O, Figueredo JM, Aguero F, Montesino R, Toledo JR. 2009. Thermal stress treatment does not affect the stability and protective capacity of goat milk derived E2-marker vaccine formulation against CSFV. Vet. Immunol. Immunopathol. 127:325–331. 10.1016/j.vetimm.2008.10.330. [DOI] [PubMed] [Google Scholar]
- 14.Barrera M, Sánchez O, Farnós O, Rodríguez MP, Domínguez P, Tait H, Frías M, Avila M, Vega E, Toledo JR. 2010. Early onset and long lasting protection in pigs provided by a classical swine fever E2-vaccine candidate produced in the milk of goats. Vet. Immunol. Immunopathol. 133:25–32. 10.1016/j.vetimm.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Toledo JR, Sánchez O, Montesino R, Farnos O, Rodríguez MP, Alfonso P, Oramas N, Rodríguez E, Santana E, Vega E, Ganges L, Frias MT, Cremata J, Barrera M. 2008. Highly protective E2-CSFV vaccine candidate produced in the mammary gland of adenoviral transduced goats. J. Biotechnol. 133:370–376. 10.1016/j.jbiotec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Armendáriz-Borunda J, Bastidas-Ramírez BE, Sandoval-Rodríguez A, González-Cuevas J, Gómez-Meda B, García-Bañuelos J. 2011. Production of first generation adenoviral vectors for preclinical protocols: amplification, purification and functional titration. J. Biosci. Bioeng. 112:415–421. 10.1016/j.jbiosc.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Montesino R, Gil J, González LJ, Zamora Y, Royle L, Rudd PM, Dwek RA, Harvey DJ, Cremata JA. 2010. The N-glycosylation of classical swine fever virus E2 glycoprotein extracellular domain expressed in the milk of goat. Arch. Biochem. Biophys. 500:169–180. 10.1016/j.abb.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Meeusen ENT, Walker J, Peters A, Pastoret PP, Jungersen G. 2007. Current status of veterinary vaccines. Clin. Microbiol. Rev. 20:489–510. 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewulf J, Laevensa H, Koenenc F, Vanderhallenc H, Mintiensa K, Deluykerd H, de Kruif A. 2000. An experimental infection with classical swine fever in E2 sub-unit marker-vaccine vaccinated and in non-vaccinated pigs. Vaccine 19:475–482. 10.1016/S0264-410X(00)00189-4. [DOI] [PubMed] [Google Scholar]
- 20.de Smit AJ, Bouma A, de Kluijver EP, Terpstra C, Moormann RJ. 2001. Duration of the protection of an E2 subunit marker vaccine against classical swine fever after a single vaccination. Vet. Microbiol. 78:307–317. 10.1016/S0378-1135(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 21.Beer M, Reimann I, Hoffmann B, Depner K. 2007. Novel marker vaccines against classical swine fever. Vaccine 25:5665–5670. 10.1016/j.vaccine.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Houdebine LM. 2000. Transgenic animal bioreactors. Transgenic Res. 9:305–320. 10.1023/A:1008934912555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toledo JR, Sánchez O, Barrera M, Figueroa N, Prieto Y, Rodríguez MP, Frias MT, Borroto C. April 2013. US patent 8,409,562 B2.
- 24.Terpstra C, Wensvoort G. 1988. The protective value of vaccine-induced neutralizing antibody titres in swine fever. Vet. Microbiol. 16:123–128. 10.1016/0378-1135(88)90036-3. [DOI] [PubMed] [Google Scholar]
- 25.Logrippo GA. 1960. Investigations of the use of beta-propiolactone in virus inactivation. Ann. N. Y. Acad. Sci. 83:578–594. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez ES, Pose AG, Moltó MP, Espinoza AS, Zamora PA, Pedroso MS. 2012. Biosafety evaluation of recombinant protein production in goat mammary gland using adenoviral vectors: preliminary study. Biotechnol. J. 8:1049–1053. 10.1002/biot.201100455. [DOI] [PubMed] [Google Scholar]
- 27.Tian H, Hou X, Wu J, Chen Y, Shang Y, Yin S, Zhang K, Liu X. 2014. A promising multiple-epitope recombinant vaccine against classical swine fever virus. Vet. Immunol. Immunopathol. 157:59–64. 10.1016/j.vetimm.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Cheng CY, Wu CW, Lin GJ, Lee WC, Chien MS, Huang C. 2014. Enhancing expression of the classical swine fever virus glycoprotein E2 in yeast and its application to a blocking ELISA. J. Biotechnol. 174:1–6. 10.1016/j.jbiotec.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Blome S, Gabriel C, Schmeiser S, Meyer D, Meindl-Böhmer A, Koenen F, Beer M. 2014. Efficacy of marker vaccine candidate CP7_E2alf against challenge with classical swine fever virus isolates of different genotypes. Vet. Microbiol. 169:8–17. 10.1016/j.vetmic.2013.12.002. [DOI] [PubMed] [Google Scholar]