Abstract
The human APOE4 allele is associated with an early age of onset and increased risk of Alzheimer’s disease (AD). Apolipoprotein E is secreted as part of a high-density lipoprotein-like particle by glial cells in the brain for the primary purpose of transport of lipophilic compounds involved in the maintenance of synapses. Previous studies examining synaptic integrity in the amygdala of human apoE targeted replacement (TR) mice showed a decrease in spontaneous excitatory synaptic activity, dendritic arbor, and spine density associated with apoE4 compared with apoE3 and apoE2 in adult male mice. In the present study, we assessed how APOE genotype affects synaptic integrity of amygdala neurons by comparing electrophysiological and morphometric properties in human apoE3, E4, and E2/4 TR mice at the age of 18–20 months. In contrast to adult mice, we found that aged apoE4 TR mice exhibited the highest level of excitatory synaptic activity compared with other cohorts. Additionally, apoE4 mice had significantly greater spontaneous inhibitory activity than all other cohorts. Taken together, there was a significant interaction between genotypes when comparing inhibition relative to excitation; there was a simple main effect of frequency type with an imbalance toward inhibition in apoE4 mice but not in apoE3 or apoE2/4 mice. These results suggest that apoE isoforms differentially influence synaptic transmission throughout the life span, where aging coupled with apoE4 expression, results in an imbalance in maintaining integrity of synaptic transmission.
Keywords: Apolipoprotein E, Synaptic, Amygdala, Alzheimer’s dementia
1. Introduction
Humans are the only species to contain 3 alleles in the APOE gene, designated APOE2, APOE3, and APOE4. Genetic studies consistently show that human APOE4 carriers have the highest risk for developing late onset Alzheimer’s disease (AD) compared with APOE3 (neutral risk) and APOE2 carriers (reduced risk) (Rubinsztein and Easton, 1999) (Ward et al., 2012). Each APOE allele encodes an apoE isoform that differs by a single amino acid, which results in significant differences in receptor affinity, protein stability, lipid homeostasis, and inflammation (Laskowitz et al., 1997).
The loss of synapses or decline in synaptic function is a strong correlate of cognitive decline in AD (Terry et al., 1991). Previous studies using 7 months apoE targeted replacement (TR) mice showed that mice expressing apoE4 displayed reduced excitatory synaptic transmission, dendritic arborization, and spine density in the lateral amygdala compared with apoE3, and that these reductions occurred in the absence of any pathologic hallmarks such as gliosis, amyloid deposition, or neurofibrillary tangles (Wang et al., 2005). Similar outcomes were also present in 1-month-old apoE4 TR mice (Klein et al., 2010). In support of these findings, additional studies show that spine density was reduced in cortical layers II/III of apoE4 mice versus apoE3 mice and becomes progressively worse with age (Dumanis et al., 2009).
The amygdala is one of the earliest structures to undergo neurodegeneration in AD (Hamann et al., 2002) (Cuenod et al., 1993). It is also involved in the regulation of neural processes that influence affective states such as depression and anxiety, which commonly occur in AD (Molosh et al., 2013). For example, Hamann et al. (2002) found significant impairment in fear conditioning in AD patients, suggesting a loss of synaptic integrity in the amygdala. Behavioral studies in mice have also shown that apoE differentially impacts multiple measures of anxiety (Raber, 2007; Siegel et al., 2010).
There is an emerging hypothesis that maintaining balance in neuronal networks (i.e., excitatory vs. inhibitory [E/I] ratio) is critical to avoiding both “normal” cognitive decline (Machulda et al., 2011) as well as AD (Palop and Mucke, 2010; Palop et al., 2006) (Huang and Mucke, 2012). Recent studies in prodromal AD patients show both hyper- and hypo-excitable states of neuronal transmission can lead to a degenerative phenotype (Minami et al., 2010; Palop and Mucke, 2010), suggesting the importance of maintaining E/I balance. By the age of 7 months, the apoE4 TR mice already showed reduced excitatory activity and unchanged inhibitory activity compared with apoE3 mice (Wang et al., 2005) suggesting the presence of an imbalance toward inhibition. To address the hypothesis that this progressive decline in synaptic transmission would worsen with age and further drive an imbalance toward inhibition, in the present study we measured how APOE genotype affects excitatory and inhibitory activity and morphometric properties of amygdala neurons in aged (18–20 months) human apoE3, E4, and E2/4 TR mice.
2. Methods
2.1. Preparation of animals
The TR mice were created by gene targeting as described previously (Sullivan et al., 1997). Briefly, the construction of the TR mice differ from other types of apoE transgenic mice in that human APOE genomic fragments were used to replace the mouse Apoe gene via homologous recombination. All lines of apoE TR mice contain chimeric genes consisting of mouse 5′ regulatory sequences continuous with mouse exon 1 (noncoding) followed by humans exons (and introns) 2–4. Thus, all 3 lines of apoE TR mice regulate apoE gene expression in the same fashion. The present study used mice that have been backcrossed to C57B16/J mice 8 times and therefore were >99.6% C57B16/J. The animals were genotyped using an allele-specific PCR approach based on Hixson and Vernier (1990). All mice are maintained on a normal chow diet consisting of 4.5% (wt/wt) fat and 0.2% (wt/wt) cholesterol (Prolab Isopro, Agway Inc, DeWitt, NY, USA). The animals were handled in accordance with guidelines approved by the Duke and VA Animal Care and Use Committee. All experiments were performed on 18 to 20-month-old males for each genotype. TR animals were either homozygous for APOE3 (3/3), APOE4 (4/4) or heterozygous for APOE2 and APOE4 (2/4).
2.2. Slice preparation
Acute coronal amygdala slices (350-µm thick) from the TR mice were prepared as described previously (Klein et al. 2010). Briefly, mice were anaesthetized with 200 mg/kg 2,2,2-tribromoethanol and decapitated. Brains were quickly removed and placed in ice-cold oxygenated artificial cerebral spinal fluid (ACSF) containing (mM): 126 NaCl, 2.5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1 NaH2PO4, 25 NaHCO3, and 10 glucose. Upon removal of the cerebellum, the brain was hemisected and the right hemisphere was glued to the Vibratome 1000 Plus (Vibratome, Campden, England) stage and immersed in the cooled oxygenated ACSF.
2.3. Electrophysiology
All experiments were conducted single-blinded, where the researcher performing the electrophysiological recordings and data analysis was unaware of the APOE genotype. After a 1-hour incubation period, single slices were transferred to the recording chamber. Whole-cell patch-clamp recordings were performed in lateral amygdala neurons using patch pipettes with resistances of 3–5 MΩfilled with internal solution (IS). For the recording of spontaneous excitatory synaptic currents (sEPSCs) the IS contained (in mM): 140 potassium gluconate, 0.5 CaCl2, 2 MgATP, 2 MgCl2, 5 EGTA, and 10 Hepes (pH 7.2–7.3). In addition, 0.3% biocytin (Sigma) was added to the IS on the day of the experiment. For the recording of spontaneous inhibitory synaptic currents (sIPSCs) the IS contained (in mM): 140 CsCl, 2 MgCl2, 0.5 EGTA, 4 MgATP, 0.5 Tris GTP, 10 Hepes, and 5 QX-314 (pH 7.2–7.3). Slices were superfused at room temperature (18 °C–22 °C) with ACSF. Neurons were clamped at −70 mV and recordings were acquired using an Axopatch 200B amplifier, filtered at 1 kHz, and sampled at 10 kHz using pClamp 10.1 software (Molecular Devices). Postsynaptic current intervals and amplitudes were measured using MiniAnalysis software (Synaptosoft, Inc Decatur, GA, USA). Firing properties and electrical excitability were assessed using whole-cell current-clamp mode. Hyper- and depolarizing step pulses were delivered at 1 second duration ranging from −0.2–0.2 nA in 25 pA increments.
2.4. Biocytin staining
At the end of the recording session, the patch-pipette was gently retracted from the neuron and the brain slice was immediately transferred to a solution containing 4% paraformaldehyde in phosphate buffered saline, pH 7.4 (USB Corp, Cleveland, OH, USA) and stored at 4 °C. Slices were incubated overnight with avidin-biotin-peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Inc, Burmingham, CA, USA) diluted in PBS containing 1% Triton X-100. Slices were then stained with DAB (Vector Labs), rinsed with deionized water and mounted on gelatin subbed slides, air-dried overnight and coverslipped using standard techniques. The morphology of the biocytin-filled neurons was examined using a Zeiss Axio ImagerD2 light microscope, traced with Neurolucida software under a 40× objective and analyzed with Neuroexplorer software (MicroBrightField, Colchester, VT, USA).
2.5. Statistics
Statistical analysis of electrophysiological and morphologic data was performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Averaged data are presented as mean ± standard error of the mean. Statistical significance (p < 0.05) was assessed using a 1-way analysis of variance followed by post hoc unpaired t tests (unless otherwise specified).
3. Results
3.1. Membrane properties in lateral amygdala neurons in apoE TR mice
Membrane properties of pyramidal neurons within the lateral amygdala of male apoE3/E3, apoE4/E4, and apoE2/E4 TR mice were measured using whole cell patch-clamp electrophysiology. Neurons with a resting membrane potential more negative than −55 mV and access resistance of less than 20 MΩwere included in the analysis. Neurons were identified as pyramidal-like based on morphology and on firing properties elicited in response to increasing depolarization current injection (Faber et al., 2001). Some neurons displayed spike frequency accomodation and others showed little or no accomodation. There were no significant genotype differences in resting membrane potential or spike frequency in non-accomodating neurons (data not shown). Genotype differences were observed, however, in the percentage of neurons with spike frequency accomodation where apoE2/E4 displayed the greatest percentage at 57% (n = 14), apoE3/E3 at 33% (n = 36), and apoE4/E4 at 23% (n = 27). These results indicate that human apoE isoforms may differentially influence the degree of spike frequency accomodation of lateral amygdala neurons in aged mice.
3.2. Aged apoE4/E4 and E2/E4 TR mice display increased excitatory transmission in lateral amygdala (LA) neurons
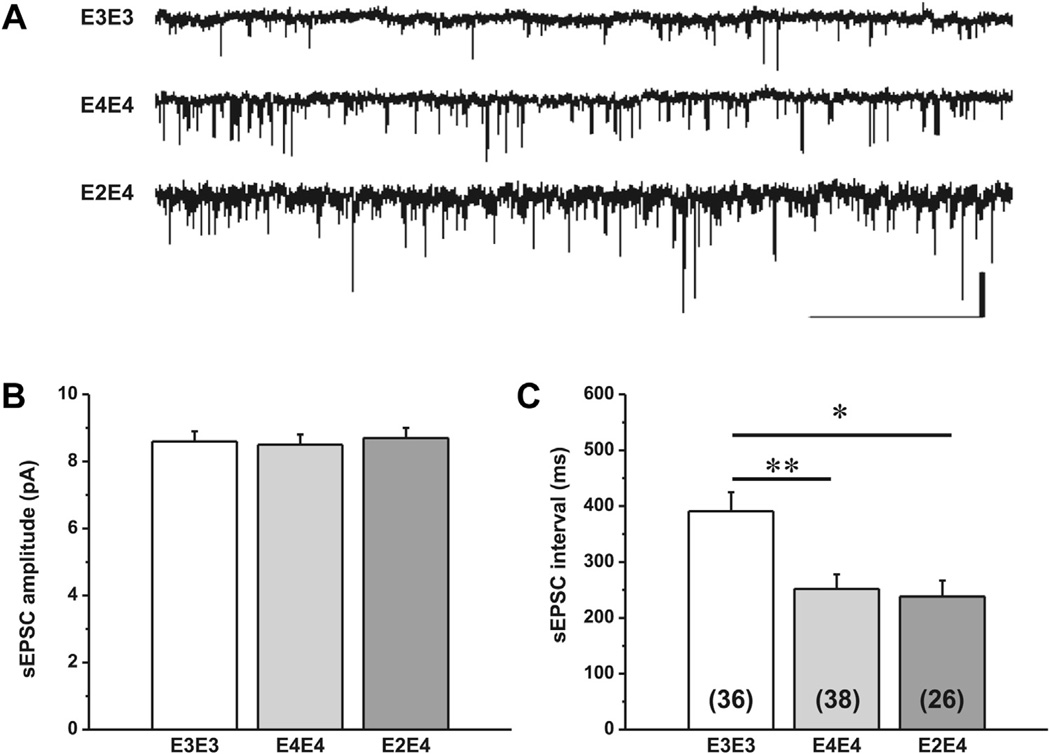
The sEPSC amplitudes and inter-event intervals were analyzed from 2 minutes continuous recordings from at least 2–4 neurons from 12–15 mice per genotype. Representative traces and the mean sEPSC amplitude and interval from each genotype are presented in Fig. 1. There was a significant main effect of genotype on sEPSC interval, F (2,96) = 9.54, p ≤ 0.0002 but there was no effect on sEPSC amplitude. Post hoc t tests revealed a significant decrease in the mean sEPSC interval between apoE3/E3 and both, apoE4/E4 and E2/E4 mice, t (71) = 3.7, p = 0.0004 and t (60) = 3.354, p = 0.0014, respectively. There was no significant difference in sEPSC interval between apoE4/E4 and apoE2/E4. Further, there was no significant difference in sEPSC interval between accommodating and non-accomodating neurons within each genotype and thus the data includes both populations of pyramidal neurons.
Fig. 1.
Spontaneous excitatory postsynaptic currents (sEPSCs) in aged apoE TR mice. (A) Representative traces of whole-cell patch-clamp recordings illustrating spontaneous excitatory postsynaptic currents (sEPSCs) in lateral amygdala neurons from apoE3 and apoE4 TR mice. Scale bar is 10 pA, 2 ms. Comparison of mean ± SEM amplitude (B) and interval (C) of sEPSCs. Data were collected from 12–15 mice per genotype and the number of neurons is indicated in parentheses. One-way ANOVA showed a significant effect of genotype on sEPSC interval (p < 0.005) but not amplitude. Post hoc t tests revealed a significant decrease in sEPSC interval for apoE4/E4 and apoE2/E4 compared with apoE3/E3 (p < 0.0005). Abbreviations: ANOVA, analysis of variance; apoE, apolipoprotein; SEM, standard error of the mean; TR, targeted replacement.
3.3. Aged apoE4/E4 and E2/E4 TR mice display increased inhibitory transmission in LA neurons
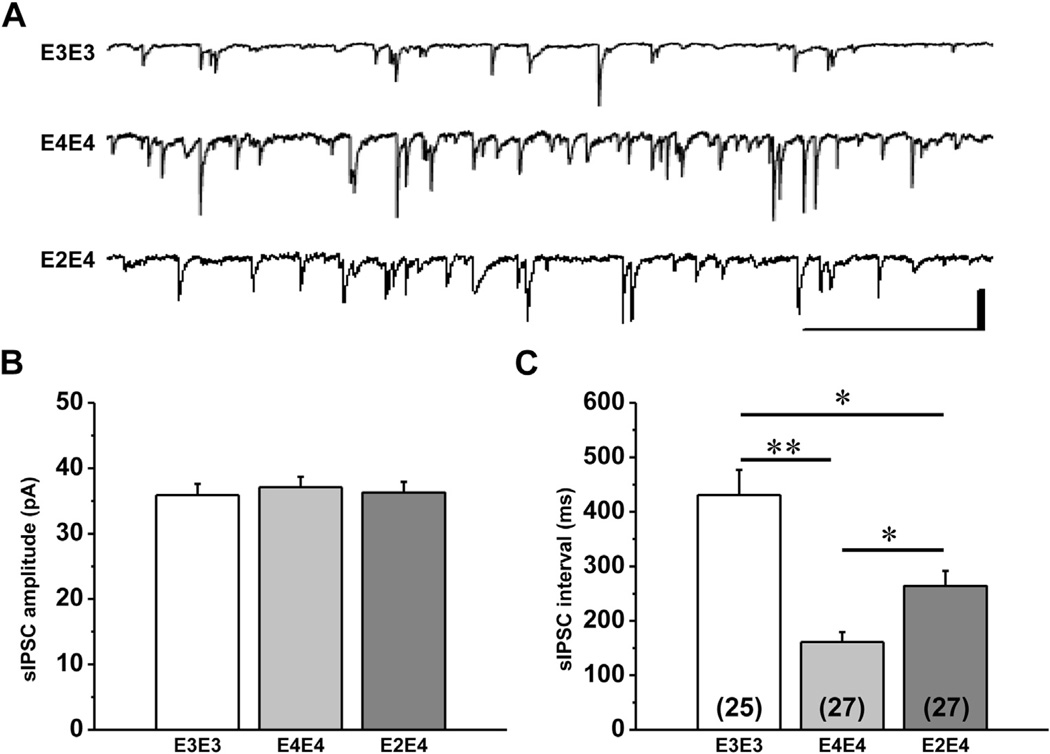
To explore a potential mechanism for the increased excitatory transmission observed in mice expressing apoE4, we also measured inhibitory activity to determine if there was loss of GABAergic activity to account for this effect. The sIPSC amplitudes and inter-event intervals were analyzed from 2 minutes continuous recordings from at least 2–4 neurons from 8–10 mice per genotype. Representative traces and the mean sIPSC amplitude and interval from each genotype are presented in Fig. 2. There was a significant main effect of genotype on sIPSC interval, F (2,75) = 17.40, p < 0.0001 but there was no effect on sIPSC amplitude. Post hoc t tests revealed a significant decrease in the mean sIPSC interval in apoE4/E4 and apoE2/E4 mice compared with apoE3/E3, t (49) = 5.54, p ≤ 0.0001 and t (50) = 3.15, p = 0.0027, respectively. There was also a significant difference in sIPSC interval between apoE4/E4 and apoE2/E4 mice, t (51) = 3.057, p = 0.0036.
Fig. 2.
Spontaneous inhibitory postsynaptic currents (sIPSCs) in aged apoE TR mice. (A) Representative traces of whole-cell patch-clamp recordings illustrating spontaneous inhibitory post-synaptic currents (sIPSCs) in lateral amygdala neurons from apoE TR mice. Scale bar is 50 pA, 2 ms. Comparison of mean ± SEM amplitude (B) and interval (C) of sIPSCs. One-way ANOVA showed a significant effect of genotype on sIPSC interval (p < 0.005) but not amplitude. Post hoc t tests revealed a significant decrease in sIPSC interval for apoE4/E4 and apoE2/E4 compared with apoE3/E3 (p < 0.0005). There was also a significant difference between apoE4/E4 and apoE2/E4 (p < 0.01). Abbreviations: ANOVA, analysis of variance; apoE, apolipoprotein; SEM, standard error of the mean; TR, targeted replacement.
3.4. Aged apoE4/E4 TR mice display an imbalance toward inhibitory transmission in LA neurons
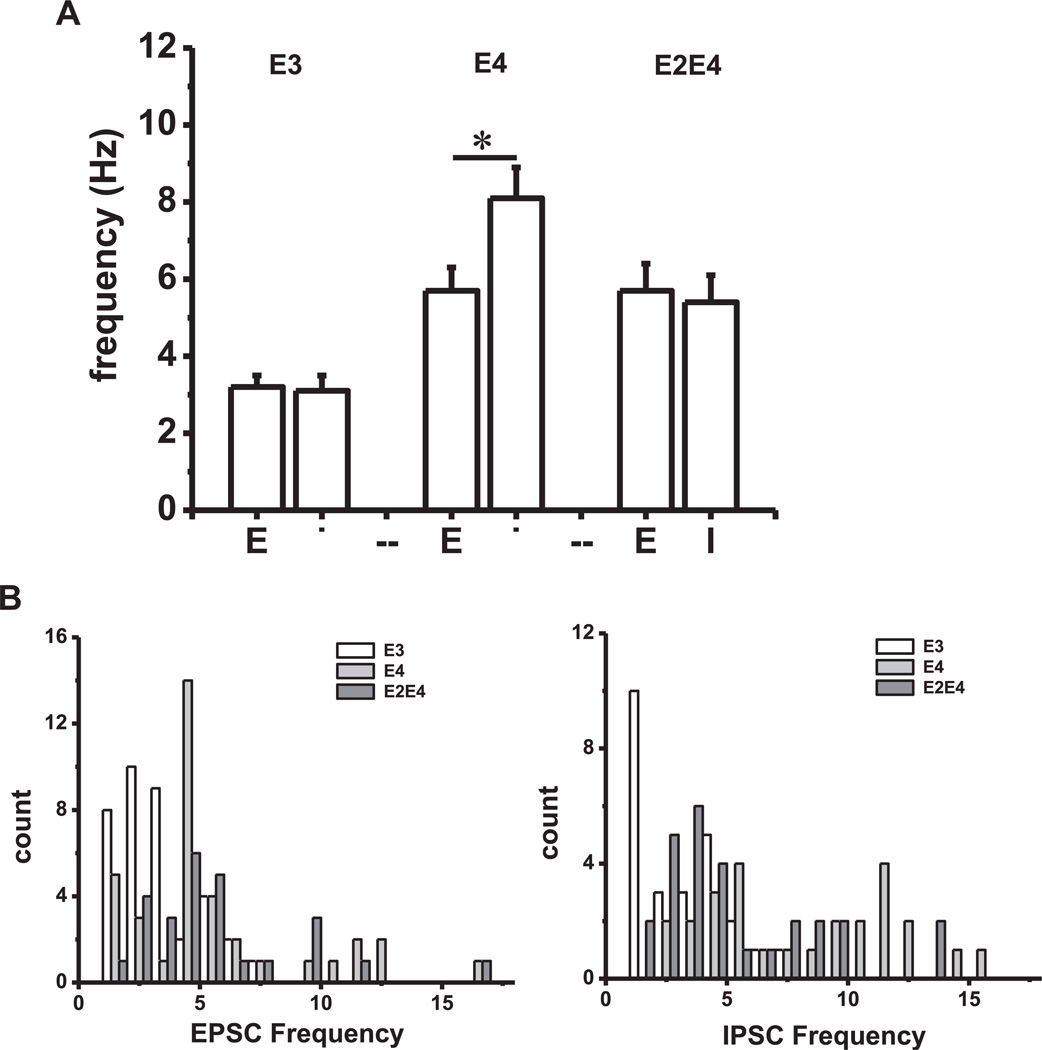
Given the unexpected increase in inhibitory activity in mice expressing apoE4, we compared sEPSC and sIPSC frequencies to determine the relative contribution of each as a function of genotype. As shown in Fig. 3A, a 3 × 2 analysis of variance showed a significant interaction between genotypes when comparing inhibition relative to excitation, F (2,171) = 3.74, p = 0.03. There was a simple main effect of frequency type in apoE4/E4 mice t (61) = 2.56, p = 0.006 but no significant simple main effect of frequency in apoE3/E3 or apoE2/E4 mice. In addition, frequency distribution plots presented in Fig. 3B also revealed a skewed distribution of both sEPSC and sIPSC frequencies in apoE4/E4 and apoE2/E4 mice. This data shows that most of the neurons in apoE3/E3 mice display sEPSC and sIPSC frequencies between 2 and 4 Hz with none exceeding 8 Hz, whereas mice expressing apoE4 contain neurons with both sEPSC and sIPSC frequencies greater than 8 Hz.
Fig. 3.
Comparisons of sEPSC and sIPSC frequencies. (A) Data represent the mean ± SEM frequency of sEPSCs (E) and sIPSCs (I) for each genotype. A 2 × 2 ANOVA showed significantly greater inhibition relative to excitation in apoE4/E4 mice compared with apoE3/E3 and apoE2/E4 mice (p < 0.001). (B) Frequency distribution histograms of data presented in Figs. 1 and 2 illustrating a skewed distribution of neurons with high frequencies of EPSCs and IPSCs in apoE4/E4 and apoE2/E4 mice in contrast to apoE3 mice. Abbreviations: ANOVA, analysis of variance; SEM, standard error of the mean; sEPSC, spontaneous excitatory postsynaptic currents; sIPSC, spontaneous inhibitory postsynaptic currents; TR, targeted replacement.
3.5. Morphology
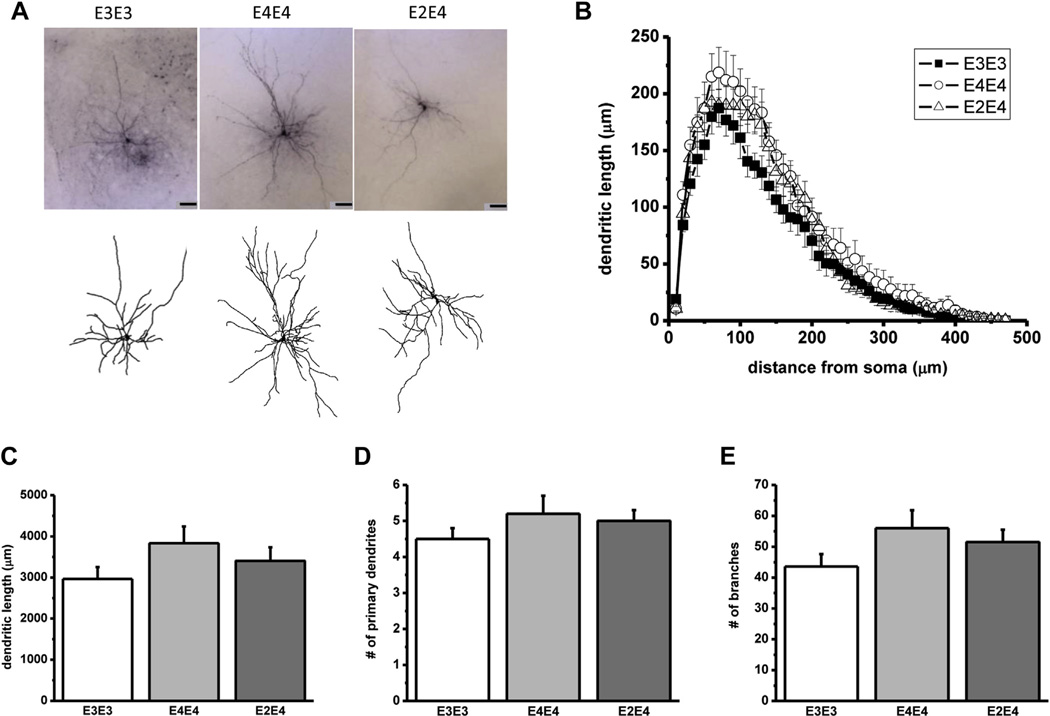
Neurons originating from the sEPSC studies were filled with biocytin and assessed for differences in morphologic properties. Representative photomicrographs shown in Fig. 4 illustrate the pyramidal-like morphology of the neurons examined and summarizes the quantitative analysis of the morphology in each genotype. Despite the effects of APOE genotype differences in electrophysiological properties, there was no concomitant significant interaction between genotypes on dendritic length or any other morphologic properties measured.
Fig. 4.
Dendritic arborization in lateral amygdala neurons in aged apoE TR mice. (A) High power images (top) and camera lucida reconstructions (bottom) corresponding to representative pyramidal-like lateral amygdala neurons from the designated apoE TR mice (scale bar, 50 µm). (B) Sholl analysis of averaged lateral amygdala neurons from the indicated apoE cohorts representing the dendritic length and the number of dendritic intersections crossing each 10-µm radius progressively more distal to the soma. (C–E) Average dendritic length, primary dendrites, and branches from each genotype. Although there was a trend toward an increase in dendritic arborization in apoE4/E4 mice, a 1-way ANOVA failed to show a significant interaction between genotypes. Data represent the mean ± SEM from the following number of neurons: apoE3/E3 (n = 11), apoE4/E4 (n = 10), apoE2/E4 (n = 11). Abbreviations: ANOVA, analysis of variance; apoE, apolipoprotein; SEM, standard error of the mean; TR, targeted replacement.
4. Discussion
In the present study, we assessed the effects of apoE isoforms on synaptic integrity within lateral amygdala neurons in aged (>18 months) human apoE TR mice. Our data show that the presence of apoE4 was associated with an increase in both excitatory (sEPSCs) and inhibitory (sIPSCs) transmission compared with apoE3. However, in the aged apoE4 TR amygdala there was also an increase in the imbalance between excitatory and inhibitory tone, in that the frequency of sIPSCs was significantly greater than that of sEPSCs. In both the aged apoE3 and E2/4 TR mice, the frequencies were roughly equal. There were no differences in the amplitudes of sEPSCs between any of the apoE TR cohorts. No significant differences in dendritic arborization were found between APOE genotypes. ApoE2/4 mice were comparable to apoE4/4 mice in sEPSCs but showed an intermediate effect in sIPSCs and displayed greater spike frequency adaptation.
Our current finding that aged apoE4 mice have increased excitatory activity by 18 months was unexpected, because our previously published studies demonstrated apoE4 TR mice exhibited reduced synaptic integrity in very young (1-month-old) and adult (7-month-old) males, (although we must note that a clear limitation of these comparisons is that the earlier studies; [Klein et al., 2010; Wang et al., 2005] used separate cohorts of mice). We hypothesized that this synaptic deficit in transmission would persist, or become progressively worse with age because apoE4 is associated with synaptic deficits in both humans and animal models. However, we found that with aging, the spontaneous excitatory synaptic events increased in frequency in apoE4 TR mice. This finding is consistent with the recent report of an age-dependent seizure phenotype in apoE4 TR mice as measured by EEG recordings (Hunter et al., 2012). A similar phenomenon has been reported in human amyloid precursor protein transgenic mice, a model for AD neuropathology (Sanchez et al., 2012). In this model, increased epileptiform activity was specifically associated with a deficit in inhibitory neurotransmission (Sanchez et al., 2012) (Verret et al., 2012). The suggestion of increased excitatory activity underlying cognitive deficits has now been extended to studies of humans with mild cognitive impairment, a risk factor for subsequent development of AD. Functional magnetic resonance imaging measures in this population shows enhanced activity in the hippocampal formation, and cognitive function is improved when this activity is reduced by an antiepileptic medication (Bakker et al., 2012). Therefore, the synaptic profile of apoE4 TR mice in aged animals may in fact reflect the profile in humans that are at risk for AD. Furthermore, our study supports the idea that aberrant network (synaptic transmission) activity is not only very common but also possibly causative in AD (Palop and Mucke, 2010). It is not clear why the increased excitatory activity in the aged apoE4 mice is accompanied by an increase in inhibitory activity, as many cases of hyperexcitability are because of a loss or reduction in inhibition as seen in epilepsy. It has also been shown that the GABA interneurons decreased with age in the apoE TR mice (Andrews-Zwilling et al., 2010). We recently co-authored work showing that the aged apoE4 mice are more prone to epileptic seizures (Hunter et al., 2012). After the age of 5 months apoE4 mice exhibited spontaneous tonic-clonic seizures and had a significantly higher incidence of cortical sharp waves and other EEG abnormalities (Hunter et al., 2012). At minimum, we believe this epileptic phenotype observed in the apoE4 mice illustrates the inability of apoE4 to maintain E/I balance in neuronal networks. These findings are somewhat surprising considering that the only potential insult to these animals was age.
One possibility is that the enhanced inhibition may have developed as a compensatory change in response to the hyperexcitability. This would not necessarily exclude the idea that the increased excitatory activity seen in the apoE4 mice (compared with the apoE3 mice) results from a loss of inhibition from extrinsic afferent cortical inputs (Swanson and Petrovich, 1998). This loss of inhibitory signal may in fact be the result of a slow neurodegenerative process occurring in these cortical areas.
Our anatomic findings seemed initially surprising, as they suggest that apoE4 mice but not apoE3 mice show enhanced age-dependent dendritic growth. However, previous studies would indicate that age-dependent dendritic hypertrophy may reflect pathologic processes. Age-related dendritic hypertrophy has been observed previously in rats (Rubinow et al., 2009) and apoE4 mice may be particularly vulnerable. One possible mechanism contributing to the increase in synaptic transmission and dendritic morphology in the aged apoE4 TR mice may be because of a differential regulation of the HPA-axis. Both the magnitude and duration of stress hormone exposure and/or release have a significant impact on synaptic plasticity. The hippocampus, frontal cortex, and amygdala are particularly sensitive to the harmful effects of stress (Roozendaal et al., 2009; Tsolaki et al., 2009). While brief periods of stress can induce adaptive changes that enhance memory, prolonged exposure produces neuronal damage that leads to maladaptive changes. For example, chronic stress causes reversible neuronal atrophy in the hippocampus and frontal cortex, brain regions critical for memory, cognition, and executive function (Cerqueira et al., 2007; McEwen and Sapolsky, 1995). Conversely, irreversible neuronal hypertrophy is observed in the amygdala (Mitra and Sapolsky, 2008; Mitra et al., 2005; Vyas et al., 2002).
Recent clinical reports demonstrate that increased cortisol (the predominant glucocorticoid in humans) levels in AD patients occurs as a function of apoE genotype (Peskind et al., 2001) and that APOE4 carriers with high cortisol levels were more vulnerable to cognitive decline (Lee et al., 2008). Animal studies showed that apoE KO mice display more anxiety than wild-type controls, suggesting that apoE regulates glucocorticoid levels via an unknown feedback mechanism (Robertson et al., 2005), and that the apoE-associated response to stress is dependent upon the age of the animal and the duration of stress exposure (Raber, 2007; Zhou et al., 1998).
In conclusion, this study demonstrates that apoE isoforms differentially regulate structural and functional plasticity in lateral amygdala neurons in aged mice. In contrast to adults, the presence of human apoE4 in aged mice leads to an increase in both excitatory and inhibitory transmission compared with apoE3. The expression of apoE2 attenuates the effects of apoE4 on excitatory transmission in adults but not in aged animals. The expression of apoE2 did, however, attenuate the increase in sIPSCs seen in apoE4 mice. Taken together, the current data suggest that both physiological and structural components may contribute to the differences in synaptic plasticity across the life span of human apoE TR mice. We propose that each apoE isoform differentially impacts the E/I ratio of synaptic transmission that might be explained by inherent apoE structural differences and interactions with apoE receptors.
Acknowledgements
This work was supported NIH R01 AG025975-01 (Patrick M Sullivan), VA CDA 101BX007080 (Shawn K Acheson) a grant from the Durham VAMC Institute for Medical Research (Rebecca C Klein), and the Veterans Administration VISN 6 MIRECC (Rebecca C Klein, Scott D Moore).
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Syrota A, Boller F. Amygdala atrophy in Alzheimer’s disease. An in vivo magnetic resonance imaging study. Arch. Neurol. 1993;50:941–945. doi: 10.1001/archneur.1993.00540090046009. [DOI] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J. Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J. Neurophysiol. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer’s disease. Neuropsychologia. 2002;40:1187–1195. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JM, Cirrito JR, Restivo JL, Kinley RD, Sullivan PM, Holtzman DM, Koger D, Delong C, Lin S, Zhao L, Liu F, Bales K, Paul SM. Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Res. 2012;1467:120–132. doi: 10.1016/j.brainres.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Klein RC, Mace BE, Moore SD, Sullivan PM. Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2. Neuroscience. 2010;171:1265–1272. doi: 10.1016/j.neuroscience.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J. Neuroimmunol. 1997;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, Schwartz BS. Apolipoprotein E, genotype, cortisol, and cognitive function in community-dwelling older adults. Am. J. Psychiatry. 2008;165:1456–1464. doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, Boeve BF, Knopman DS, Petersen RC, Jack CR., Jr Effect of APOE epsilon4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch. Neurol. 2011;68:1131–1136. doi: 10.1001/archneurol.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr. Opin. Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Minami SS, Sung YM, Dumanis SB, Chi SH, Burns MP, Ann EJ, Suzuki T, Turner RS, Park HS, Pak DT, Rebeck GW, Hoe HS. The cytoplasmic adaptor protein X11alpha and extracellular matrix protein Reelin regulate ApoE receptor 2 trafficking and cell movement. FASEB J. 2010;24:58–69. doi: 10.1096/fj.09-138123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molosh AI, Sajdyk TJ, Truitt WA, Zhu W, Oxford GS, Shekhar A. NPY Y(1) receptors differentially modulate GABA(A) and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology. 2013;38:1352–1364. doi: 10.1038/npp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer’s disease: two faces of the same coin? Neuromolecular Med. 2010;12:48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56:1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Raber J. Role of apolipoprotein E in anxiety. Neural Plast. 2007;2007:91236. doi: 10.1155/2007/91236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J, Curley J, Kaye J, Quinn J, Pfankuch T, Raber J. apoE isoforms and measures of anxiety in probable AD patients and Apoe−/− mice. Neurobiol. Aging. 2005;26:637–643. doi: 10.1016/j.neurobiolaging.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Drogos LL, Juraska JM. Age-related dendritic hypertrophy and sexual dimorphism in rat basolateral amygdala. Neurobiol. Aging. 2009;30:137–146. doi: 10.1016/j.neurobiolaging.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Easton DF. Apolipoprotein E genetic variation and Alzheimer’s disease. A meta-analysis. Dement. Geriatr. Cogn. Disord. 1999;10:199–209. doi: 10.1159/000017120. [DOI] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, Mucke L. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol. Aging. 2010;33:345–358. doi: 10.1016/j.neurobiolaging.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tsolaki M, Kounti F, Karamavrou S. Severe psychological stress in elderly individuals: a proposed model of neurodegeneration and its implications. Am. J. Alzheimers Dis. Other Demen. 2009;24:85–94. doi: 10.1177/1533317508329813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wilson WA, Moore SD, Mace BE, Maeda N, Schmechel DE, Sullivan PM. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol. Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE) e4/4 among patients diagnosed with Alzheimer’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2012;38:1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Elkins PD, Howell LA, Ryan DH, Harris RB. Apolipoprotein-E deficiency results in an altered stress responsiveness in addition to an impaired spatial memory in young mice. Brain Res. 1998;788:151–159. doi: 10.1016/s0006-8993(97)01533-3. [DOI] [PubMed] [Google Scholar]