Abstract
A MarR-like transcriptional repressor (RcrR) and two predicted ABC efflux pumps (RcrPQ) encoded by a single operon were recently shown to be dominant regulators of stress tolerance and development of genetic competence in the oral pathogen Streptococcus mutans. Here, we focused on polar (ΔrcrR-P) and nonpolar (ΔrcrR-NP) rcrR mutants, which are hyper- and nontransformable, respectively, to dissect the mechanisms by which these mutations impact competence. We discovered two open reading frames (ORFs) in the 3′ end of the rcrQ gene that encode peptides of 27 and 42 amino acids (aa) which are also dramatically upregulated in the ΔrcrR-NP strain. Deletion of, or start codon mutations in, the ORFs for the peptides in the ΔrcrR-NP background restored competence and sensitivity to competence-stimulating peptide (CSP) to levels seen in the ΔrcrR-P strain. Overexpression of the peptides adversely affected competence development. Importantly, overexpression of mutant derivatives of the ABC exporters that lacked the peptides also resulted in impaired competence. FLAG-tagged versions of the peptides could be detected in S. mutans, and FLAG tagging of the peptides impaired their function. The competence phenotypes associated with the various mutations, and with overexpression of the peptides and ABC transporters, were correlated with the levels of ComX protein in cells. Collectively, these studies revealed multiple novel mechanisms for regulation of competence development by the components of the rcrRPQ operon. Given their intimate role in competence and stress tolerance, the rcrRPQ-encoded peptides may prove to be useful targets for therapeutics to diminish the virulence of S. mutans.
INTRODUCTION
The ability of bacteria to survive and persist in dynamic and often hostile environments in the host is intimately associated with their pathogenic potential. For Streptococcus mutans, the major etiological agent of human dental caries, its establishment, persistence, and virulence are tightly integrated with the pathways that control the development of genetic competence. Competence affords organisms the ability to internalize exogenous DNA and, in some cases, to assimilate that DNA into their gene repertoire, contributing to genome plasticity and consequently to survival and evolution of bacterial populations (1–4). Natural competence for DNA transformation is tightly regulated. In streptococci, including S. mutans, competence is transiently induced and occurs in only a subpopulation of the bacteria. It is widely accepted that the primary control point for development of the state of competence in many Firmicutes is the alternative sigma factor ComX, sometimes called SigX, which activates transcription of late competence genes encoding constituents for DNA uptake and metabolism (2). Importantly, the mechanisms governing comX gene expression, as well as the amount and activity of ComX in cells, differ substantially between species. Thus, dissecting how comX expression and ComX activity are regulated is central to understanding how the decision to commit to competence is determined and how virulence traits of S. mutans, such as biofilm formation and acid tolerance, are integrated with the competence regulatory system (5–10).
One common feature of regulation of comX expression is that secreted peptides, sometimes referred to as pheromones, trigger regulatory systems to activate expression of the comX gene. To date, two unrelated systems, ComCDE and ComRS, have been shown to activate comX expression, depending on the species of bacteria. The ComC peptide is secreted by the ComAB ATP binding cassette (ABC) transporter complex and processed to its mature form, competence-stimulating peptide (CSP). When extracellular CSP accumulates in sufficient concentrations, usually in the nM range, it can activate the expression of early CSP-induced genes, including comC, comDE, comAB, and comX, through the ComDE two-component system (TCS) via a positive-feedback loop. Interestingly, it appears as though the genes encoding ComCDE in Streptococcus pneumoniae and some related oral streptococci arose from a different lineage than the genes annotated as comC and comDE in S. mutans. In fact, in contrast to the pneumococcus, the ComE protein of S. mutans does not directly activate comX expression and S. mutans ComABCDE are more closely related to components of the bacteriocin signaling system BlpABCRH of S. pneumoniae (11). Consistent with this observation, CSP of S. mutans is a very effective signal for direct activation by ComE of a repertoire of genes encoding bacteriocins. Notably, sufficient quantities of CSP are able to induce comX transcription in S. mutans, but it is not yet clear how this occurs.
The ComRS system was discovered more recently, and ComR directly activates the expression of comX in many streptococci (8). The primary signaling molecule for the ComRS system is XIP (SigX-inducing peptide). XIP is a secreted 7-amino-acid (aa) peptide derived from the C terminus of its 17-aa precursor, ComS, although the secretion and maturation processes for XIP have not been characterized. Once secreted, XIP is imported into the cell by the Opp (Ami) oligopeptide transport system, where it acts as an allosteric activator of ComR, a transcriptional regulator of the Rgg family. Activated ComR enhances transcription of comX by binding directly to the promoter region of comX, as well as to the comS promoter region, to create a positive-feedback loop. In S. mutans, XIP signaling and CSP signaling are highly dependent on growth phase and environment (12, 13). Depending on the conditions, XIP activation of ComX occurs uniformly throughout the population, whereas CSP activates comX in only a subpopulation of cells (9, 14). Of note, physiologically high levels of XIP or CSP can also induce cell death (10, 13–15), some of which occurs as a result of the presence of endogenously produced bacteriocins or autolytic enzymes (15–18). Evidence is accumulating that there are multiple other control points modulating competence, transformation efficiency, and XIP- or CSP-mediated cell death in S. mutans, including, but not limited to, CiaRH, HtrA, IrvRA, and HdrRM (5, 8, 15, 19–22). However, the interrelationship of these systems with ComCDE or ComRS and how they may integrate information about the physiological state of the cells with decisions to commit to competence or cell death are not well understood.
The rcrRPQ operon in S. mutans UA159 (originally annotated in OralGen as SMu0835-7 and in GenBank as SMU.921-3) plays a crucial role linking competence with cellular physiology, including acid and oxidative stress tolerance. Also of note, inactivation of rcrR caused downregulation of the relP gene encoding the (p)ppGpp synthase responsible for the bulk of (p)ppGpp during exponential growth, and inactivation of relP caused downregulation of genes in the rcrRPQ operon (23). The rcrR gene encodes a MarR family transcriptional regulator that negatively regulates expression of the rcrRPQ operon (23). The rcrP and rcrQ genes encode separate transmembrane ATP-binding cassette (ABC) transporters with the ATP-binding cassette and transmembrane domains located on a single polypeptide. Both RcrP and RcrQ are type 6 transporters, which include ABC transporters that typically function as exporters (Transporter Classification Database [http://www.tcdb.org/]) (23). Notably, when the rcrR gene was replaced with a nonpolar antibiotic resistance marker (ΔrcrR-NP), rcrPQ were overexpressed and this mutant strain could not be transformed with exogenous DNA, regardless of whether XIP or CSP was added to cultures. In contrast, when rcrR was replaced with a polar antibiotic resistance cassette (ΔrcrR-P), the strain expressed 100-fold-lower levels of the rcrPQ transcripts than the ΔrcrR-NP strain and the polar mutant strain was constitutively hypertransformable, displaying approximately 104-fold-higher transformation efficiency than the parental strain in the absence of exogenous CSP or XIP. Consistent with the transformation phenotypes, the comYA gene, a target of ComX and the first gene in a late competence operon encoding proteins required for transformation, was downregulated nearly 15-fold in the nontransformable ΔrcrR-NP strain but upregulated more than 100-fold in the hypertransformable ΔrcrR-P strain compared with the UA159 parental strain. Thus, the rcrRPQ-encoded system is a dominant regulator of the commitment to competence in S. mutans. These genes are also highly conserved in all S. mutans isolates and in many related organisms (23).
The objective of this study was to begin to understand the mechanisms by which the ΔrcrR-P and ΔrcrR-NP mutations influence competence development. We provide direct evidence that the effects of the rcrR mutations on competence development are strongly correlated with ABC transporter expression levels. We also show that two previously undiscovered peptides encoded as part of the rcrRPQ operon serve as effectors that link the rcr system to competence control. Collectively, these data reveal previously undisclosed regulatory components and novel mechanisms by which S. mutans controls the decision to commit to competence.
MATERIALS AND METHODS
Culture conditions.
Escherichia coli DH10B was grown in Luria broth, and S. mutans UA159 and its derivatives were grown in brain heart infusion (BHI) broth (Difco). For selection of antibiotic-resistant colonies after genetic transformation, erythromycin (300 μg ml−1 for E. coli, 10 μg ml−1 for S. mutans), kanamycin (Km; 50 μg ml−1 for E. coli, 1 mg ml−1 for S. mutans), or spectinomycin (Sp; 50 μg ml−1 for E. coli, 1 mg ml−1 for S. mutans) was added to media, when needed. Overnight cultures of S. mutans were grown in BHI broth at 37°C in a 5% CO2 aerobic atmosphere. For growth assays, overnight cultures were diluted 1:100 in fresh medium and the optical density at 600 nm (OD600) was monitored using a Bioscreen C Lab system (Helsinki, Finland) as detailed elsewhere (24). Growth was monitored in the absence or presence of 400 nM synthetic competence-stimulating peptide (sCSP) (25), with sterile mineral oil (0.05 ml per well) placed on top of the cultures to avoid the inhibitory effects of oxygen on growth of S. mutans (24, 26).
Construction of mutant strains.
Standard DNA manipulation techniques were used to engineer plasmids and strains (5, 27). S. mutans mutants were created using a PCR ligation mutagenesis approach (28) to replace nearly the entire target open reading frame (ORF) with a nonpolar kanamycin marker (NPKm). Transformants were selected on BHI agar containing appropriate antibiotics. Double-crossover recombination into each gene was confirmed by PCR and sequencing to ensure that no mutations were introduced into flanking genes. For overexpression studies, the genes of interest were amplified from UA159 and cloned into a pIB184 shuttle expression plasmid (29), kindly provided by I. Biswas (University of Kansas Medical Center). A full list of strains used and constructed in this study is provided in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| S. mutans strains | ||
| UA159 | Wild type | |
| ΔrcrR-NP | ΔrcrR::NPKmr | 23 |
| ΔrcrR-P | ΔrcrR::ΩKmr | 23 |
| SAB4 | ΔcomX::NPEmr | 5 |
| SAB204 | Δ837pep12::NPEmr | This study |
| SAB231 | Δ837pep12::NPEmr/ΔrcrR-NP | This study |
| SAB232 | Δ837pep12::NPEmr/ΔrcrR-P | This study |
| SAB338 | 837pep1::2T>C | This study |
| SAB342 | 837pep1::2T>C/ΔrcrR-NP | This study |
| SAB346 | 837pep2::2T>C | This study |
| SAB347 | 837pep12::2T>C | This study |
| SAB348 | 837pep2::2T>C/ΔrcrR-NP | This study |
| SAB349 | 837pep12::2T>C/ΔrcrR-NP | This study |
| SJ410 | UA159 carrying pIB184::837pep1 | This study |
| SJ422 | UA159 carrying pIB184 | 13 |
| SJ425 | ΔrcrR-P carrying pIB184 | This study |
| SJ434 | UA159 carrying pIB184::rcrPQ/837pep12 | This study |
| SJ484 | UA159 carrying pIB184::837pep12 | This study |
| SJ485 | UA159 carrying pIB184::837pep2 | This study |
| SJ491 | ΔrcrR-P carrying pIB184::rcrPQ/837pep12 | |
| SJ507 | UA159 carrying pIB184::rcrPQ lacking pep | This study |
| SJ509 | ΔrcrR-P carrying pIB184::rcrPQ lacking pep | This study |
| SJ510 | ΔrcrR-P carrying pIB184::837pep12 | This study |
| Pep1-C-FLAG | UA159 tagged with FLAG at the C terminus of 837pep1 | This study |
| Pep2-C-FLAG | UA159 tagged with FLAG at the C terminus of 837pep2 | This study |
| Plasmids | ||
| pDL278 | E. coli-Streptococcus shuttle vector, Spr | 47 |
| pIB184 | Shuttle expression plasmid with the constitutive P23 promoter, Emr | 29 |
NP, nonpolar; P, polar; Em, erythromycin.
Transformation assays.
Cells were grown to an OD600 of 0.15 in a 5% CO2 aerobic atmosphere, and 200 ng of purified pDL278, which harbors a spectinomycin resistance (Spr) gene, was added to the culture. After 2.5 h of incubation at 37°C, transformants and total CFU were enumerated by plating appropriate dilutions on BHI agar plates with and without 1 mg ml−1 spectinomycin, respectively. CFU were enumerated after 48 h of incubation. When desired, purified sCSP was added to reach a final concentration of 400 nM.
Gene expression analysis.
To measure the expression of genes using Real-time reverse transcription-PCR (RT-PCR), S. mutans UA159 and its derivatives were grown in BHI broth in the absence or presence of 400 nM sCSP and cells were harvested in mid-exponential phase (OD600 of 0.4). Extraction of RNA, RT-PCR, Real-time RT-PCR, and data analysis were performed as described elsewhere (19). 16S rRNA was used as an internal reference. All assays were performed in triplicate with RNA isolated from three independent biological replicates.
Fractionation of S. mutans cells.
Fractionation of S. mutans strains carrying a C-terminal FLAG tag on candidate peptides was performed as previously described (30, 31), with minor modifications. Briefly, cells were grown in 5 ml of BHI broth to an OD600 of 0.4. Cultures were centrifuged at 3,200 × g for 10 min at 4°C to obtain culture supernatants (S) and cell pellets. Supernatants were filtered through 0.22-μm-pore-size syringe filters (Millipore), and proteins were precipitated with 100 μl of 100% (wt/vol) trichloroacetic acid (TCA; Fisher Scientific), collected by centrifugation, washed twice with 300 μl of acetone (Fisher Scientific), and resuspended in 50 μl buffer A (0.5 M sucrose; 10 mM Tris-HCl, pH 6.8; 10 mM MgSO4) containing 17 μg ml−1 of phenylmethanesulfonyl fluoride (PMSF) (ICN Biomedicals Inc.). Cell pellets were washed once with 1 ml buffer A, collected by centrifugation, and resuspended in 1 ml buffer A. To obtain cell wall (CW) fractions and protoplasts, the cell pellets were incubated at 37°C overnight following the addition of 25 μl of 10 mg ml−1 lysozyme (Sigma) and 2 μl of 1.0 × 104 U ml−1 of mutanolysin (Sigma). Protoplasts and digested cell wall (CW) fractions were separated by centrifugation at 3,200 × g at 4°C, and the supernatant fluids (CW) were filtered through 0.22-μm-pore-size syringe filters and precipitated with TCA as described above. The protoplasts were washed twice with 1 ml of buffer A, resuspended in 1 ml of osmotic lysis buffer (50 mM Tris [pH 7.5], 10 mM MgSO4, 0.8 M NaCl), and lysed by 3 cycles of sonication for 10 s each time on a setting of 5 with an W-370 Ultrasonicator (Heat Systems-Ultrasonics Inc.). After the addition of 10 μl of 1 mg ml−1 DNase (Promega) and 1 mg ml−1 RNase (Sigma), the protoplasts were incubated for 30 min at room temperature. The unlysed protoplasts were discarded by centrifugation at 3,200 × g for 5 min at 4°C. To separate the cytoplasmic (C) and membrane (M) fractions, supernatant fluids were transferred into 1.5-ml ultracentrifuge tubes (Beckman Coulter) and centrifuged at 100,000 × g for 1 h at 4°C in an Optima Ultracentrifuge (Beckman Coulter). After centrifugation, the supernatants were transferred to new 1.5-ml tubes as the cytoplasmic (C) fraction and the pellets were resuspended in 50 μl of buffer A as the membrane (M) fraction. For the preparation of whole-cell lysates (WL), cell pellets were resuspended in 1 ml of buffer A supplemented with 25 μl of 10 mg ml−1 lysozyme and 2 μl of 10,000 U ml−1 of mutanolysin and incubated at 37°C overnight, and then 160 μl of 5 M NaCl was added and the cells were disrupted by sonication. The lysed cells were then treated with 10 μl of 1 mg ml−1 DNase and 1 mg ml−1 RNase for 30 min at room temperature and centrifuged at 3,210 × g for 5 min at 4°C. The resulting supernatants (WL) were transferred into new tubes, and TCA was precipitated. Protein concentrations were determined using the bicinchoninic acid (BCA) assay (Thermo Scientific) with purified bovine serum albumin as the standard.
Western blotting.
The FLAG-tagged versions of candidate peptides were detected by Western blotting as described previously (30), with minor modifications. Briefly, 10 μg of protein from the various protein fractions described above was mixed with 5× SDS sample buffer (200 mM Tris-HCl [pH 6.8], 10% [vol/vol] SDS, 20% [vol/vol] glycerol, 10% [vol/vol] β-mercaptoethanol, 0.02% [vol/vol] bromophenol blue). The protein samples were boiled for 5 min and then separated by SDS-PAGE in a 12% acrylamide gel using a Mini-Protean 3 cell system (Bio-Rad Laboratories). Following electrophoresis, the proteins were transferred from the gel to Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore) using a TE series Transphor electrophoresis unit (Hoefer Scientific Instruments) in transfer buffer (25 mM Tris, 192 mM glycine, 10% [vol/vol] methanol). The membranes were treated with a primary monoclonal anti-FLAG M2 antibody (Sigma) (1:1,000 dilution) and a secondary peroxidase-labeled, goat anti-mouse IgG antibody (Kirkegaard & Perry Laboratories) (1:10,000 dilution). For detection of ComX, whole-cell lysates of the strains were prepared by bead disruption and subjected to Western blotting using polyclonal anti-ComX antisera, recently raised against recombinant ComX protein (J. Kaspar, S.-J. Ahn, and R. A. Burne, unpublished data). The detection of FLAG-tagged proteins and ComX was performed using a SuperSignal West Pico chemiluminescent substrate kit (Thermo Scientific) and visualized with a FluorChem 8900 imaging system (Alpha Innotech).
RESULTS
rcrPQ expression levels strongly influence competence-related phenotypes.
Given our previous finding that the ΔrcrR-NP strain expresses roughly 100-fold more rcrPQ mRNA than the ΔrcrR-P or wild-type strains, we hypothesized that the level of expression of the rcrPQ genes might play a role in defining the phenotypic behaviors of the two strains independently of RcrR (23). To directly test this, we created strain SJ434, in which the rcrPQ genes were overexpressed under the control of the P23 promoter present on the shuttle plasmid pIB184 (29) in the wild-type genetic background. The transcript levels of rcrPQ in SJ434 were about 10-fold higher than in the wild-type strain carrying the empty plasmid (SJ422) (see Fig. S1 in the supplemental material). Notably, a dramatic decrease in transformation efficiency was observed in SJ434 in the absence (data not shown) or presence (Fig. 1A) of sCSP compared to the results seen with SJ422 carrying only pIB184.
FIG 1.
Analysis of the rcrPQ-overexpressing strains. (A) Qualitative transformation efficiency of S. mutans strains overexpressing rcrPQ in the wild-type and ΔrcrR-P genetic backgrounds. Cells were transformed with 200 ng of purified pDL278, which harbors a spectinomycin resistance (Spr) gene, in the presence of sCSP (400 nM). The photograph of the plates was taken after 48 h of incubation and is representative of duplicate biological replicates (refer to Materials and Methods for additional experimental details). (B) Growth curves of S. mutans strains overexpressing rcrPQ in the wild-type and ΔrcrR-P genetic backgrounds in the presence of 400 nM sCSP. SJ422, wild-type strain harboring pIB184; SJ434, wild-type strain overexpressing rcrPQ; SJ425, ΔrcrR-P strain harboring pIB184; SJ491, ΔrcrR-P strain overexpressing rcrPQ. For comparison, growth of the ΔrcrR-NP and ΔrcrR-P strains under identical conditions is shown in Fig. S2 in the supplemental material. Optical density at 600 nm was monitored every 30 min at 37°C using the Bioscreen C Lab system. The results are representative of those from three independent experiments performed in triplicate.
We previously noted that the hypertransformable ΔrcrR-P strain exhibited a longer doubling time and lower final OD600 values in the presence of sCSP than the wild-type strain whereas the ΔrcrR-NP mutant displayed an almost complete alleviation of sensitivity to sCSP (see Fig. S2 in the supplemental material). While the concentration of sCSP used to observe growth inhibition here (400 nM) was substantially lower than that previously shown to trigger cell death via lysis (2 μM), one cannot exclude the possibility that the apparent inhibition of the growth of certain S. mutans strains and mutants by sCSP is attributable to slower growth coupled with lysis or growth arrest of subpopulations. Notwithstanding such speculation, rcr mutant strains that displayed defects in the ability to be transformed always grew faster and had higher final yields than the wild-type or highly transformable rcr mutant strains. Since CSP sensitivity is easily measured by growth rate and yield inhibition and strongly correlated with transformability, we evaluated CSP sensitivity as a function of overexpression of rcrPQ in the wild-type genetic background, strain SJ434. Consistent with the decrease in transformability shown by SJ434, there was a marked alleviation of sensitivity to sCSP (Fig. 1B) in this strain, with faster growth (doubling time of 43 min) and much higher final optical density (0.73) than the wild-type strain harboring the empty vector (SJ422; doubling time of 148 min, final OD of ∼0.51). Notably, the changes in transformability and CSP sensitivity were not accompanied by significant reductions in the expression level of comX or comYA, as assessed by quantitative RT-PCR (qRT-PCR) (see Fig. S3).
Interestingly, an even more pronounced effect of rcrPQ overexpression was observed in the ΔrcrR-P genetic background (strain SJ491) than in the wild-type genetic background. High rcrPQ expression in the ΔrcrR-P background almost completely alleviated sensitivity to sCSP, even in comparison to the degree observed in the nontransformable ΔrcrR-NP strain (Fig. 1B). SJ491 also displayed remarkably reduced transformation efficiency in the absence (data not shown) or presence (Fig. 1A) of sCSP compared to the results seen with the ΔrcrR-P strain carrying only the empty vector (SJ425). Therefore, we conclude that enhanced expression of the rcrPQ genes, as was noted previously for the nonpolar rcrR mutant, is a major contributor to the competence phenotypes. Moreover, the data show that RcrPQ levels can exert an impact on competence even in a strain carrying an intact copy of the rcrR gene. It should also be noted that the transformation efficiency of the rcrPQ-overexpressing strains could be enhanced by CSP treatment, in contrast to what was seen with the ΔrcrR-NP strain (23). We propose that this is related to the fact that the ΔrcrR-NP mutant produces roughly 10-fold more rcrPQ mRNA than is expressed by S. mutans SJ491 harboring the plasmid-borne rcrPQ genes, further supporting the hypothesis that the level of expression of the ABC transporters strongly influences competence and CSP sensitivity.
Two previously undiscovered peptides regulate competence development.
While constructing various mutants in the rcrPQ genes, we noted that the strains behaved differently depending on the 3′ endpoint of deletions in the rcrQ gene (data not shown). When we inspected the 3′ region of rcrQ, we discovered two small open reading frames (ORFs). One overlapped the 3′ end of rcrQ by 1 nucleotide (nt) and was predicted to encode a 27-aa peptide that we designated 837pep1. The ORF for the second peptide overlapped the 3′ end of rcrQ by 53 nt and was predicted to encode a 42-aa peptide that we designated 837pep2 (Fig. 2A). The two peptides and RcrQ are encoded in three different reading frames. When measured by Real-time qPCR, the transcription of the ORFs was dramatically increased in the ΔrcrR-NP strain (data not shown), so it was likely that transcription of these putative ORFs arose from the rcrR promoter and was under the control of RcrR. Consistent with this idea, we were unable to detect promoter activity using lacZ or cat fusions to a 0.2-kbp fragment amplified from the 3′ end of rcrQ (data not shown). Also, prior analysis of rcrRPQ gene transcription in various polar and nonpolar mutants did not provide evidence for the presence of promoters within the rcrRPQ structural genes (23).
FIG 2.
Two peptides identified in the rcrRQ operon. (A) Sequence of the peptides, designated 837pep1 (gray amino acid sequence) and 837pep2 (black amino acid sequence). The TGA stop codon of rcrQ and ATG start codon of the gene downstream of rcrQ, tpx, are shown in bold and italics. (B) Growth phenotype of strains lacking the peptides in the wild-type (SAB204), ΔrcrR-NP (SAB231), and ΔrcrR-P (SAB232) genetic backgrounds when grown in the presence of 400 nM sCSP. Growth of the ΔrcrR-NP and ΔrcrR-P strains under identical conditions is shown in Fig. S2 in the supplemental material. Optical density at 600 nm was monitored every 30 min at 37°C using the Bioscreen C Lab system. The results are representative of those from three independent experiments performed in triplicate.
To determine whether these predicted ORFs contributed to rcr-mediated effects on competence development, an erythromycin resistance cassette was used to replace part of the coding sequences of the two ORFs in strain UA159, without disrupting the rcrQ gene, to create strain SAB204 (Δ837pep1 Δ837pep2). When SAB204 was grown in the presence of sCSP (Fig. 2B), it behaved identically to the wild-type strain, and there was no significant change in the ability of SAB204 to be transformed compared to the wild-type strain results (data not shown). However, when we disrupted the ORFs for the peptides in the ΔrcrR-NP genetic background (SAB231, Δ837pep1 Δ837pep2 ΔrcrR-NP), the nontransformable ΔrcrR-NP strain became hypersensitive to sCSP (Fig. 2B; see also S2 in the supplemental material) and hypertransformable (data not shown), thus acquiring a phenotype more similar to that of the ΔrcrR-P mutant (see Fig. S2). When the ORFs for the peptides were deleted in the ΔrcrR-P genetic background (SAB232, Δ837pep1 Δ837pep2 ΔrcrR-P), the strains grew at a rate, and with an extended lag time (Fig. 2B), that was similar to that seen with the ΔrcrR-P mutant strain (see Fig. S2), and the strain remained hypertransformable. These results provide support for the idea of the involvement of the predicted peptides in the competence phenotypes observed in RcrR-deficient mutants, most likely with one or both serving as negative regulators of competence. It is particularly noteworthy that aberrantly high expression of the ABC transporters, as occurs in the ΔrcrR-NP genetic background, was required to observe alterations in competence and in the sensitivity to sCSP in strains lacking the peptides. Thus, one possible interpretation of these results is that the peptides themselves, or targets with which the peptides interact, are substrates for the ABC exporters.
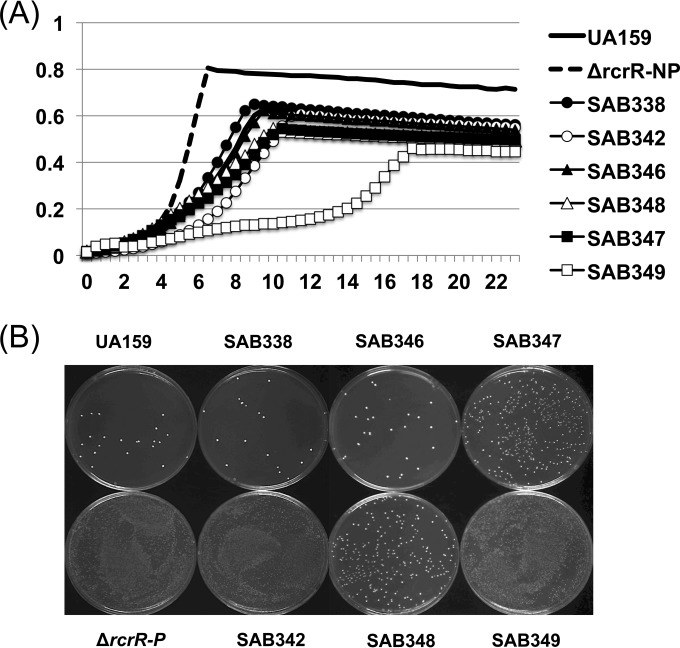
To analyze the involvement of individual peptides in the observed phenotypes, the start codon of the first peptide and of the second peptide and of both peptides was mutated from ATG to ACG in the wild-type background, creating strains SAB338 (pep1tc), SAB346 (pep2tc), and SAB347 (pep1tc/pep2tc), respectively. The mutations were introduced directly into the chromosome by site-directed mutagenesis using a PCR product generated by overlap extension PCR (32). Potential mutants were screened using mismatch amplification mutation analysis (MAMA) PCR (33), as previously described (34, 35). Note that the mutations in the coding sequence of 837pep1 did not alter the amino acid sequence of 837pep2 or RcrQ and that mutation of the 837pep2 start codon did not affect the amino acid sequence of RcrQ or 837pep1. The growth of the individual point mutant strains in the presence of sCSP was comparable to that of the wild-type strain (Fig. 3A). However, the strain lacking both peptides (SAB347; pep1tc and pep2tc) grew slightly slower and achieved a lower final yield in the presence of sCSP than did the other strains (Fig. 3A). Consistent with the growth phenotypes, deficiency of either peptide had no influence on the transformation efficiency of S. mutans, whereas loss of both peptides resulted in enhanced transformation efficiency (Fig. 3B). The observation that there were no phenotypic changes in the deletion replacement mutant (SAB204) in the wild-type genetic background but that there were significant changes when the peptides were completely eliminated using point mutationss may be associated with the fact that the deletion mutant had the capacity to produce a truncated form of 837pep2 that may have retained some activity.
FIG 3.
Analysis of the 837pep1 or -pep2 start codon mutant strains. (A) Growth curves in the presence of 400 nM sCSP. For comparison, growth of the ΔrcrR-NP and ΔrcrR-P strains under identical conditions is shown in Fig. S2 in the supplemental material. The results are representative of those from three independent experiments performed at least in triplicate. (B) Qualitative transformation efficiency (see the text for details). Cells were transformed with 200 ng of purified pDL278, which harbors a spectinomycin resistance (Spr) gene, in the absence of sCSP. The photograph of the plates was taken after 48 h of incubation and is representative of duplicate biological replicates (refer to Materials and Methods for experimental details). UA159, wild type; ΔrcrR-P, rcrR polar mutant; SAB338, 837pep1tc; SAB342, 837pep1tc/ΔrcrR-NP; SAB346, 837pep2tc; SAB348, 837pep2tc/ΔrcrR-NP; SAB347, 837pep12tc; SAB342, 837pep12tc/ΔrcrR-NP.
We also created the same point mutants in the ΔrcrR-NP genetic background by first introducing the point mutations into the wild-type strain and then introducing the ΔrcrR-NP mutation. These strains were designated SAB342 (pep1tc ΔrcrR-NP), SAB348 (pep2tc ΔrcrR-NP), and SAB349 (pep1tc pep2tc ΔrcrR-NP). Interestingly, in the ΔrcrR-NP genetic background, mutation of the individual peptide resulted in different patterns of sensitivity to sCSP, including a longer lag phase with SAB342, lacking 837pep1, but a greater apparent doubling time with SAB348, lacking 837pep2 (Fig. 3A). Also of interest, SAB342 displayed a hypertransformable phenotype, identical to that of SAB231 (Δ837pep1 Δ837pep2 ΔrcrR-NP) (data not shown), whereas SAB348 could be transformed, albeit not nearly as efficiently as the wild-type or hypertransformable strains. Importantly, when the start codons of both peptides were mutated in the ΔrcrR-NP genetic background, the SAB349 strain became sensitive to sCSP to an extent similar to that seen with the ΔrcrR-P strain (Fig. 1B; see also Fig. S2 in the supplemental material) and became hypertransformable (Fig. 3B), adding further evidence that these peptides negatively regulate progression to competence and modulate sensitivity to CSP.
rcrPQ expression levels and the peptides can individually influence competence.
To further explore the contribution of the peptides to phenotypes exhibited by the ΔrcrR-NP mutant and rcrPQ-overexpressing strains, we next determined whether enhanced expression of the peptides alone could impact the development of competence. We overexpressed both peptides under the control of the P23 promoter in the pIB184 shuttle expression plasmid (29) in the wild-type genetic background to create strain SJ484 (Table 1). Higher expression of both peptides resulted in elevated sensitivity to sCSP, particularly when the cells entered the mid-exponential phase of growth (Fig. 4A). Nevertheless, there were no significant differences in the transformation efficiencies of the strain in the absence (data not shown) or presence (Fig. 4B) of sCSP. Subsequently, we created strains overexpressing only 837pep1 (SJ410) or 837pep2 SJ485 (Table 1). For the construction of SJ485, the structural gene for 837pep2 was PCR amplified from SAB338 (837pep1tc). Interestingly, higher expression of each peptide individually resulted in elevated sensitivity to sCSP, but the effects were more pronounced when 837pep1 was overexpressed (Fig. 4A). Also of note, enhanced expression of the individual peptides adversely affected transformation efficiency in the absence (data not shown) or presence (Fig. 4B) of sCSP, unlike the results seen when both peptides were overexpressed. A decrease in transformation efficiency was also observed when the individual peptides were overexpressed in the ΔrcrR-P genetic background (data not shown). Thus, the peptides are capable of influencing sensitivity to sCSP and transformability, but individual overexpressed peptides or combinations of overexpressed peptides do not always have equivalent influences on these two phenotypes.
FIG 4.
Analysis of strains overexpressing peptides produced from the rcrRPQ operon. (A) Growth curves of S. mutans strains overexpressing 837pep1 or 837pep2 or both in the wild-type genetic background in the presence of 400 nM sCSP. SJ422, wild-type strain harboring pIB184; SJ410, the strain overexpressing 837pep1 alone; SJ484, the strain overexpressing both 837pep1 and 837pep2; SJ485, the strain overexpressing 837pep2 alone. Optical density at 600 nm was monitored every 30 min at 37°C using the Bioscreen C Lab system. The results are representative of those from three independent experiments performed at least in triplicate. (B) Qualitative transformation efficiency of S. mutans wild-type strains overexpressing 837pep1 or 837pep2 or both. Cells were transformed with 200 ng of purified pDL278, which harbors a spectinomycin resistance (Spr) gene, in the presence of 400 nM sCSP. The photograph of the plates was taken after 48 h of incubation and is representative of duplicate biological replicates (refer to Materials and Methods for experimental details).
In light of the discovery of the peptides and their effects on competence, and in recognition of the fact that the effects of overexpression of rcrPQ, as detailed above and in Fig. 4, could be due to the combination of overexpression of the ABC transporters and of the peptides, we engineered rcrPQ-overexpressing derivatives in which the start codons of the peptides were mutated. The modified rcrPQ genes (rcrPQ lacking pep) were introduced into the wild-type and ΔrcrR-P genetic backgrounds on plasmid pIB184 to create strains SJ507 and SJ509, respectively (Table 1). Both strains showed almost complete alleviation of the growth-inhibitory effects of sCSP (data not shown) to as great an extent as that seen in the ΔrcrR-NP strain (see Fig. S2). Likewise, both strains showed a dramatic reduction in transformation efficiency (data not shown). Therefore, the RcrPQ porters can dominantly influence competence development in a way that does not require deletion of rcrR or expression of the 837pep1 and 837pep2 peptides. This observation lends support to the hypothesis that the 837 peptides are not substrates for the ABC transporters.
FLAG tagging of the peptides confirms expression and functionality.
To further confirm that the peptides could be expressed, we fused a FLAG epitope (DYKDDDDK) to the C terminus of 837pep1 and 837pep2 expressed from their native chromosomal loci using overlapping extension PCR (32) and named the strains Pep1-C-FLAG and Pep2-C-FLAG, respectively. Our initial attempts to detect the peptides from cell lysates prepared by homogenizing cells in the presence or absence of SDS were not successful. However, when the cells were treated with lysozyme and mutanolysin and then lysed by sonication, both 837pep1 and 837pep2 were detected by Western blotting with an anti-FLAG antibody (Fig. 5). The peptides were not detected in protein fractions from the wild-type strain that did not express the FLAG tag. The 837pep1 and 837pep2 FLAG-tagged peptides were detected in the cell wall (CW) and cytoplasmic (CP) fractions but not in the membrane (CM) or supernatant (S) fractions (data not shown). While the FLAG tag certainly could alter the targeting of the peptides to their normal location(s), it is clear that both peptides can be expressed in S. mutans. It should also be noted that the failure to detect the peptides in cells using a treatment (homogenization) that did not first enzymatically compromise the cell wall raises the possibility that envelope stress regulates expression of the rcrRPQ operon.
FIG 5.

Detection of 837pep1 and 837pep2 by Western blotting using an anti-FLAG antibody. The FLAG-tagged strains, Pep1-C-FLAG (left) and Pep2-C-FLAG (right), were grown exponentially and total cell lysates were generated as described in Materials and Methods. Proteins from clarified whole-cell lysates were separated by SDS-PAGE in a 12% acrylamide gel. The experiment was performed twice with similar results. See the text for details on which cellular fractions contained detectable FLAG-tagged peptides. WT, wild type.
Since the peptides were hypothesized to serve as negative regulators of progression to competence, we investigated whether the FLAG tag could interfere with the function of the peptides. For this, we engineered expression of the FLAG-tagged peptides in the ΔrcrR-NP genetic background and found that the strain no longer behaved like the nonpolar rcrR mutant. Instead, these strains became hypertransformable and sensitive to sCSP (data not shown), in similarity to the effects that were observed when the ORFs for the peptides were disrupted in the ΔrcrR-NP genetic background (Fig. 2B). Thus, FLAG tagging of the peptides either rendered them nonfunctional or altered their localization in a way that meant that they could no longer exert effects on competence development and sensitivity to sCSP. These results add further support to the hypothesis that the peptides themselves have essential functions in the regulation of competence development.
Insights into how the peptides and RcrPQ block competence development.
To begin to probe how the peptides and ABC exporters might impact competence, the levels of expression of comX and comYA were measured in selected strains showing marked changes in transformation efficiency as a result of mutation of the peptides or of overproduction of the peptides or ABC transporters (see Fig. S3 and S4 in the supplemental material). While there was a clear correlation of the ability of the selected recombinant strains to become competent with late competence gene (comYA) expression and CSP sensitivity, we postulated that the peptides may influence the stability or activity of ComX. Thus, lysates of the strains were separated by SDS-PAGE and subjected to Western blotting with an antiserum elicited to a purified recombinant S. mutans ComX protein (Fig. 6). The hypertransformable rcrR polar mutant produced elevated levels of ComX, and the nonpolar, nontransformable rcrR mutant produced no detectable ComX, in similarity to the results seen with a comX deletion mutant. Importantly, there was a strong correlation between the levels of ComX protein and the competence and CSP sensitivity phenotypes of the strains. For example, when the peptides were mutated in the rcrR-NP strain and competence was restored, ComX levels were restored to the levels seen in the rcrR-P strain. Further, strains that lost the ability to be transformed as a result of overexpression of the peptides or the ABC transporters, with or without the peptides, all displayed a loss of ComX from the cells. Thus, the mechanism by which the ABC transporters and peptides modulate competence is that of influencing ComX levels in the cells.
FIG 6.

Effect of RcrPQ porters or peptides on ComX production shown by Western blotting assays. Whole-cell lysates were separated by SDS-PAGE in a 12% acrylamide gel and transferred to a polyvinylidene difluoride (PDVF) membrane, and then ComX was detected using polyclonal anti-ComX antisera raised against full-length recombinant ComX protein. (A) Effect of overexpression of either RcrPQ porters (SJ509) or peptides (SJ510) or both (SAB 491) on ComX production in the ΔrcrR-P genetic background. (B) Effect of peptide deletion (SAB231) on ComX production in the ΔrcrR-NP genetic background. WT, wild-type strain; ΔcomX, negative-control strain. As a positive control, the wild-type strain was grown in the presence of sCSP (400 nM), because ComX is not detected in the absence of sCSP under the Western blot conditions used in this study. The experiment was performed twice with similar results.
DISCUSSION
The goal of this study was to begin to understand the mechanisms underlying the dramatic differences in competence development by the hypertransformable ΔrcrR-P and nontransformable ΔrcrR-NP mutants of S. mutans. We previously proposed that the levels of the RcrPQ porters and uncharacterized effectors, possibly substrates for RcrPQ, accounted for the changes in transformation efficiency (23). Testing of this hypothesis here has provided direct experimental evidence that the RcrPQ porters play crucial roles in the regulation of development of genetic competence. Importantly, unique peptide regulators of the competence cascade were also discovered to be encoded by the rcr operon. Therefore, the findings presented here add critical new elements to the evolving model of how S. mutans may control the transition to the competent state at the genetic, biochemical, and physiological levels. Also of note, although there are apparent homologs of the rcrRPQ operon distributed in many streptococci, including S. gordonii, S. pneumoniae, S. pyogenes, S. salivarius, S. sanguinis, and S. thermophilus (23), we were able to find homologues of 837pep1 and 837pep2 only in the rcrQ genes of S. mutans strains and could not find the peptides elsewhere in existing databases. Consequently, the rcrRPQ peptides and the factors with which they may interact could prove to be attractive targets in efforts to reduce the establishment, persistence, and virulence of S. mutans without adversely affecting beneficial organisms.
This report provides the first direct demonstration that the level of expression of the RcrPQ porters plays a critical role in competence development independently of RcrR. In particular, the block in competence and resistance to CSP that occurs in the ΔrcrR-NP strain could be almost completely reproduced by simply overexpressing rcrPQ in the wild-type genetic background and even in the ΔrcrR-P genetic background. Importantly, loss of competence and resistance to CSP were also induced in the rcrPQ strain lacking pep, which overexpresses rcrPQ but does not express the peptides (837pep1 and 837pep2). Therefore, the rcrPQ overexpression experiments confirm our proposal that the levels of production of the RcrPQ porters do indeed contribute to the phenotypic differences observed between the ΔrcrR-NP and ΔrcrR-P and wild-type strains. The simplest explanation for these observations could be that RcrPQ peptides export an as-yet-undefined effector(s) that enhances the production or stability of ComX (Fig. 7), thus explaining the inability to detect ComX in strains overexpressing rcrPQ, with or without the 837 peptides. Consistent with this idea, we recently showed that overexpression of comX in the ΔrcrR-NP strain restored the ability of the strain to be transformed (S.-J. Ahn and R. A. Burne, unpublished data). Consequently, these findings place the RcrPQ porters as a central control point not only for competence but also for the cell death and lysis pathways induced by CSP, which have been shown to be dependent on the presence of an intact comX gene (15, 36).
FIG 7.
Working model for the regulation of competence development by the Rcr system of S. mutans. (A) On the left is a representation of the transcriptional activity of the rcrPQ genes that include the coding sequences for the 837 peptides. In the hypertransformable ΔrcrR-P (top) strain or in the wild-type strain treated with peptides that stimulate competence (not shown), expression of rcrPQ and expression of peptide (837pep1 and 837pep2; red triangles and diamonds) were low. This allowed a positively acting factor (blue boxes with “+” signs) that is able to be extruded by the RcrPQ porters to accumulate in cells and block the degradation of ComX by the MecA-Clp complex (yellow). ComX was then free to activate comYA expression, and the cells were able to progress to competence. In contrast, the nontransformable ΔrcrR-NP (bottom) strain expressed very high levels of the RcrPQ porters and the 837 peptides. Overproduction of the ABC transporters led to export of the positively acting factor(s) that is required for stabilization of ComX. Further, the overproduction of the peptides stimulated ComX degradation and/or interfered with its ability to activate late competence genes, consistent with the inability to detect ComX and the low levels of comYA mRNA in this nonpolar mutant. The working model also incorporates the hypothesis that the 837 peptides may be able to antagonize the association of XIP with ComR, inhibiting expression of comX. (B) A schematic showing the inverse correlation of rcrRPQ expression levels with the ability of cells to progress to competence. It is proposed that rcrRPQ expression is impacted by increases in the presence of certain stressors (e.g., oxidative stress) and (p)ppGpp levels (see the introduction and Discussion for more detail). As the level of expression of the RcrPQ transporters and the 837 peptide levels increases, the capacity of the cells to develop competence and perhaps to induce programmed cell death becomes impaired. This entanglement of the components of the Rcr system with comX expression and ComX protein allows the cells to finely tune competence development depending on the environment and physiological state of the cells.
Results presented here also clearly show that the peptides encoded at the 3′ end of rcrQ (837pep1 and 837pep2) are expressed and can negatively regulate competence. A key finding related to the role and regulation of the 837 peptides is that deletion of the peptides in the ΔrcrR-NP genetic background induced behaviors similar to those seen with the polar (ΔrcrR-P) strain, converting the nontransformable, CSP-sensitive, nonpolar rcrR mutant to a CSP-hypersensitive and -hypertransformable strain. Mutating of the start codon of each peptide, or of both peptides, in the wild-type or ΔrcrR-NP genetic background confirmed that the phenotypes were associated with differential expression of the peptides and not perturbation of rcrQ. Of note, mRNA levels of the rcrRPQ genes were not influenced by deletion or start codon mutations of the peptides, as measured by Real-time qPCR (data not shown). Note also that the effect of the peptides on competence and CSP sensitivity was highly dependent on the level of production of the RcrPQ porters, since there were no obvious phenotypes associated with the deletion of the peptides in either the wild-type or ΔrcrR-P genetic background. Since the rcrPQ genes are expressed in the latter strains at levels 100- to 1,000-fold lower than those seen in the ΔrcrR-NP mutant, these findings reinforce not only the idea that the production of the proper amount of the RcrPQ efflux pumps is critical to competence but also the idea that balancing production of the ABC transporters with the levels of the 837 peptides is critical for proper regulation of competence development. Encoding of the peptides within the rcr operon likely allows the cells to achieve this balance, since all evidence supports the hypothesis that the peptides and rcrQ are transcribed from the same mRNA. It should also be mentioned that the dramatically lower levels of expression of rcrPQ in the wild-type and ΔrcrR-P genetic backgrounds probably explain why deletion of these peptides in these genetic backgrounds did not result in a change in the competence phenotype, which indicates that some threshold level of the peptides and porters may be needed to influence competence.
Regulation of competence by RcrPQ levels and the 837 peptides is without precedent and perhaps unique to S. mutans, but valuable insights into mechanistic aspects of how these gene products exert their influence on competence may be gained from the paradigm organisms S. pneumoniae and Bacillus subtilis, where modulation of ComX and ComK stability, respectively, is a central control point for competence development. Thus, one could propose a model in which the ABC transporters are able to extrude a factor(s) that is essential for the activity or stability of ComX (Fig. 7A). However, because the rcrPQ-overexpressing strains could significantly reduce transformation efficiency regardless of whether the 837 peptides were present, this factor must be distinct from 837pep1 or 837pep2. In B. subtilis, the master regulator that triggers competence is sequestered in a ComK-MecA-ClpC complex but can be activated when released from the complex by ComS, which in turn prevents proteolysis of ComK (12, 37). Thus, the proposed substrate(s) for the ABC transporters could diminish the susceptibility of ComX to MecA/Clp-dependent degradation in a manner similar to that seen with ComS of B. subtilis. Therefore, we propose a working model in Fig. 7A in which there is a factor(s), likely a protein/peptide, that promotes the stability or activity of ComX. Under normal conditions in the wild-type strain treated with CSP or XIP or in the rcrP strain (Fig. 7A), low levels of expression of rcrPQ would allow accumulation of this positively acting factor and stabilization of ComX.
The model (Fig. 7) also predicts that the 837 peptides can function to antagonize competence. Recently, it was reported that the activity or stability of ComX in S. pneumoniae and S. thermophilus and ComK in B. subtilis was controlled at the posttranslational level (38–42). In those studies, an adapter protein, such as ComW or MecA, was demonstrated to play a role in antagonizing ComX activation of late competence genes or in stimulating the degradation of ComX via the Clp protease pathways. Our model predicts that the 837 peptides enhance the susceptibility of ComX to MecA/Clp-dependent degradation, which was recently shown to modulate ComX stability in S. mutans (43, 44). As depicted in the model, the peptides would be present at low levels in the wild-type strain (not shown) or in the rcrP strain (Fig. 7A), leading to stable ComX production and the development of competence. In contrast, in the ΔrcrR-NP strain (Fig. 7A), the positively acting factor is predicted to be depleted via extrusion by the overproduced RcrPQ porters and the 837 peptides are predicted to accumulate as a result of rcrPQ overexpression, leading to ComX degradation. Mechanistically, the effects of the 837 peptides could be exerted by direct interactions of the peptides with ComX or by complexing of the 837 peptides with the positively acting factor to interfere with its inhibition of ComX degradation. Thus, the 837 peptides may have some similarities with anti-sigma factor control of ComX activity (39, 42, 45). Importantly, the working model is consistent with the observation that restoration or loss of competence and CSP sensitivity correlated perfectly with the presence or absence, respectively, of ComX in the various mutants and overexpressing strains examined here (Fig. 6). Note that the model also would accommodate the findings showing that the rcrRPQ genes integrate stress and competence. Stress-induced and/or (p)ppGpp-induced overexpression of rcrRQP would lead to higher levels of RcrPQ and 837 peptide production (Fig. 7B), allowing the cells to downregulate competence if environmental conditions were not favorable to committing substantial cellular biosynthetic capacity to produce the DNA uptake machinery or perhaps to avoid cell death.
There was one observation made here that is not entirely congruent with the model, although it may have been due to confounding factors arising from entanglement of regulatory circuits. Specifically, if the peptides alone are capable of promoting ComX degradation, then one might predict that the peptide-overexpressing strains would be uniformly nontransformable. However, when the peptides were overexpressed together in the wild-type genetic background, there was an increase in sensitivity to sCSP without a concurrent effect on competence. However, individual expression of the peptides in the wild-type background also increased CSP sensitivity while decreasing transformation efficiency. While these are unusual behaviors that appear to be at odds with the model, there are a number of related considerations. For example, the level of overexpression and the level of stability of the peptides in these backgrounds may not be similar to those in the ΔrcrR-NP strain. It must also be considered that expression of rcrRPQ could be influenced by overexpression of the 837 peptides, perhaps by their interaction with RcrR, leading to alterations in the amount of RcrPQ or of other factors. Consistent with these ideas, when the peptides were overexpressed in the hypertransformable ΔrcrR-P strain, where rcrPQ levels could not be influenced by alterations in RcrR binding, elevated levels of the peptides consistently caused a profound decrease in transformation efficiency. Another critical observation that should not be overlooked is that overexpression of the peptides causes phenotypes where CSP sensitivity and competence become uncoupled. We are not aware of any other case where this has been observed, but it is a potentially significant finding. In particular, the 837 peptides may function in a manner that assists cells in committing to development of competence and/or in selecting whether subpopulations can undergo programmed cell death. Experiments to explore this possibility are under way using microfluidic methods described elsewhere (9) to analyze the behavior of individual cells carrying certain rcrRPQ mutations or overexpressing particular constituents of the operon, including the 837 peptides.
It is also important to take into account the potential involvement of the peptides and other rcrRPQ gene products in ComRS-dependent activation of comX (Fig. 7A). Given that activation of gene expression by ComR is strongly dependent on its interaction with the XIP pheromone, the 837 peptides that are significantly induced in the ΔrcrR-NP genetic background may antagonize the association of XIP with ComR. Along these lines, we have recently discovered that the comX promoter, which is highly dependent on the presence of the ComR-XIP complex, is not active in the ΔrcrR-NP strain (J. Kaspar, S.-J. Ahn, and R. A. Burne, submitted for publication). The recent findings that ComS sequences diverge between species (46) and that the interactions of ComR with XIP can be antagonized by nonspecific oligopeptides present in the growth medium (9) raise the possibility that the 837 peptides, or targets of these peptides, act at the level of ComR-dependent activation of comX. The finding that mutating the peptides restores ComX protein levels is consistent with this idea (Fig. 6); however, there were no major changes in comX gene expression in these strains as assessed by qRT-PCR. Again, these results are not necessarily at odds with the model, since mutation of the peptides alone clearly does not result in the spectrum of changes induced by a nonpolar insertion in the rcrR gene, e.g., overexpression of rcrPQ. Clearly, understanding how these newly identified peptides are localized and with what components they interact is an essential next step for future studies.
In summary, the picture emerging from our data and recent literature is that comX transcription and ComX stability and activity are regulated in S. mutans more stringently and require far more inputs than previously appreciated. The results of this study represent a considerable advance in understanding the mechanisms linking RcrRPQ with competence and stress tolerance. However, the findings also present the opportunity to put forth some other hypotheses. For example, it is possible that the peptides are able to function as allosteric regulators of RcrR and thus govern autogenous regulation of the rcr operon. In fact, 837pep1 is very hydrophobic and multiple allosteric effectors of MarR-like transcriptional regulators are small hydrophobic peptides. Of note, we recently found that even small changes in the level of the rcrRPQ operon can profoundly impact competence (48), so this hypothesis is attractive from the perspective of RcrRPQ serving as a cellular thermostat to integrate physiologic status with decisions to commit to competence (Fig. 7B). Likewise, one can hypothesize that differential secretion of factors by the RcrPQ porters could influence population behavior by controlling the availability of a quorum-sensing molecule(s). While we have not been able to induce changes in the transformability of the wild-type strain by cocultivation of that strain with, or with supernatants from, the rcrR nonpolar mutant (data not shown), we still cannot exclude the possibility that one aspect of the Rcr system involves cell:cell signaling. Finally, given that we have been able to find apparent homologues of the 837 peptides only in isolates of S. mutans, where they are very highly conserved, these peptides or their derivatives could function as unique signals for intercellular communication by S. mutans and thus could serve as attractive therapeutic targets. Ongoing studies are directed at disclosing the interacting partners for the 837 peptides, identifying the substrates for the ABC transporters, and determining whether RcrRPQ peptides are connected to competence in other streptococci.
Supplementary Material
ACKNOWLEDGMENTS
We thank Indranil Biswas (University of Kansas Medical Center) for providing the pIB184 shuttle expression plasmid.
This work was supported by grants R01 DE13239 and T90 DE21990 from the NIH/NIDCR.
Footnotes
Published ahead of print 18 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01942-14.
REFERENCES
- 1.Claverys JP, Martin B, Havarstein LS. 2007. Competence-induced fratricide in streptococci. Mol. Microbiol. 64:1423–1433. [DOI] [PubMed] [Google Scholar]
- 2.Claverys JP, Martin B, Polard P. 2009. The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiol. Rev. 33:643–656. [DOI] [PubMed] [Google Scholar]
- 3.Johnsborg O, Havarstein LS. 2009. Regulation of natural genetic transformation and acquisition of transforming DNA in Streptococcus pneumoniae. FEMS Microbiol. Rev. 33:627–642. [DOI] [PubMed] [Google Scholar]
- 4.Johnsborg O, Eldholm V, Bjornstad ML, Havarstein LS. 2008. A predatory mechanism dramatically increases the efficiency of lateral gene transfer in Streptococcus pneumoniae and related commensal species. Mol. Microbiol. 69:245–253. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SJ, Wen ZT, Burne RA. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 74:1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspiras MB, Ellen RP, Cvitkovitch DG. 2004. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol. Lett. 238:167–174. [DOI] [PubMed] [Google Scholar]
- 7.Mair RW, Senadheera DB, Cvitkovitch DG. 2012. CinA is regulated via ComX to modulate genetic transformation and cell viability in Streptococcus mutans. FEMS Microbiol. Lett. 331:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mashburn-Warren L, Morrison DA, Federle MJ. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son M, Ahn SJ, Guo Q, Burne RA, Hagen SJ. 2012. Microfluidic study of competence regulation in Streptococcus mutans: environmental inputs modulate bimodal and unimodal expression of comX. Mol. Microbiol. 86:258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenderska IB, Lukenda N, Cordova M, Magarvey N, Cvitkovitch DG, Senadheera DB. 2012. A novel function for the competence inducing peptide, XIP, as a cell death effector of Streptococcus mutans. FEMS Microbiol. Lett. 336:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin B, Quentin Y, Fichant G, Claverys JP. 2006. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 14:339–345. [DOI] [PubMed] [Google Scholar]
- 12.Claverys JP, Prudhomme M, Martin B. 2006. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 60:451–475. [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Ahn SJ, Kaspar J, Zhou X, Burne RA. 2013. Growth phase and pH influence peptide signaling for competence development in Streptococcus mutans. J. Bacteriol. 196:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I. 2011. Subpopulation-specific transcriptome analysis of competence-stimulating-peptide-induced Streptococcus mutans. J. Bacteriol. 193:1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry JA, Jones MB, Peterson SN, Cvitkovitch DG, Levesque CM. 2009. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol. Microbiol. 72:905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufour D, Cordova M, Cvitkovitch DG, Levesque CM. 2011. Regulation of the competence pathway as a novel role associated with a streptococcal bacteriocin. J. Bacteriol. 193:6552–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreth J, Merritt J, Shi W, Qi F. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol. Microbiol. 57:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinas GE, Cortes PR, Orio AG, Echenique J. 2008. Acidic stress induces autolysis by a CSP-independent ComE pathway in Streptococcus pneumoniae. Microbiology 154:1300–1308. [DOI] [PubMed] [Google Scholar]
- 19.Ahn SJ, Lemos JA, Burne RA. 2005. Role of HtrA in growth and competence of Streptococcus mutans UA159. J. Bacteriol. 187:3028–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okinaga T, Niu G, Xie Z, Qi F, Merritt J. 2010. The hdrRM operon of Streptococcus mutans encodes a novel regulatory system for coordinated competence development and bacteriocin production. J. Bacteriol. 192:1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi F, Merritt J, Lux R, Shi W. 2004. Inactivation of the ciaH Gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 72:4895–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan R, Rukke HV, Ricomini Filho AP, Fimland G, Arntzen MO, Thiede B, Petersen FC. 2012. Extracellular identification of a processed type II ComR/ComS pheromone of Streptococcus mutans. J. Bacteriol. 194:3781–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaton K, Ahn SJ, Sagstetter AM, Burne RA. 2011. A transcriptional regulator and ABC transporters link stress tolerance, (p)ppGpp, and genetic competence in Streptococcus mutans. J. Bacteriol. 193:862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn SJ, Burne RA. 2007. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J. Bacteriol. 189:6293–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn SJ, Wen ZT, Burne RA. 2007. Effects of oxygen on virulence traits of Streptococcus mutans. J. Bacteriol. 189:8519–8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Ftritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 28.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193–205. [DOI] [PubMed] [Google Scholar]
- 29.Biswas I, Jha JK, Fromm N. 2008. Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology 154:2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baev D, England R, Kuramitsu HK. 1999. Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy. Infect. Immun. 67:4510–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yother J, White JM. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. [DOI] [PubMed] [Google Scholar]
- 33.Cha RS, Zarbl H, Keohavong P, Thilly WG. 1992. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 2:14–20. [DOI] [PubMed] [Google Scholar]
- 34.Zeng L, Burne RA. 2010. Seryl-phosphorylated HPr regulates CcpA-independent carbon catabolite repression in conjunction with PTS permeases in Streptococcus mutans. Mol. Microbiol. 75:1145–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng L, Das S, Burne RA. 2010. Utilization of lactose and galactose by Streptococcus mutans: transport, toxicity, and carbon catabolite repression. J. Bacteriol. 192:2434–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry JA, Cvitkovitch DG, Levesque CM. 2009. Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol. Lett. 299:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamoen LW, Venema G, Kuipers OP. 2003. Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology 149:9–17. [DOI] [PubMed] [Google Scholar]
- 38.Boutry C, Wahl A, Delplace B, Clippe A, Fontaine L, Hols P. 2012. Adaptor protein MecA is a negative regulator of the expression of late competence genes in Streptococcus thermophilus. J. Bacteriol. 194:1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo P, Li H, Morrison DA. 2004. Identification of ComW as a new component in the regulation of genetic transformation in Streptococcus pneumoniae. Mol. Microbiol. 54:172–183. [DOI] [PubMed] [Google Scholar]
- 40.Msadek T, Dartois V, Kunst F, Herbaud ML, Denizot F, Rapoport G. 1998. ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol. Microbiol. 27:899–914. [DOI] [PubMed] [Google Scholar]
- 41.Piotrowski A, Luo P, Morrison DA. 2009. Competence for genetic transformation in Streptococcus pneumoniae: termination of activity of the alternative sigma factor ComX is independent of proteolysis of ComX and ComW. J. Bacteriol. 191:3359–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung CK, Morrison DA. 2005. Two distinct functions of ComW in stabilization and activation of the alternative sigma factor ComX in Streptococcus pneumoniae. J. Bacteriol. 187:3052–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian XL, Dong G, Liu T, Gomez ZA, Wahl A, Hols P, Li YH. 2013. MecA protein acts as a negative regulator of genetic competence in Streptococcus mutans. J. Bacteriol. 195:5196–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong G, Tian XL, Gomez ZA, Li YH. 2014. Regulated proteolysis of the alternative sigma factor SigX in Streptococcus mutans: implication in the escape from competence. BMC Microbiol. 14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Zuber P. 1998. A molecular switch controlling competence and motility: competence regulatory factors ComS, MecA, and ComK control sigmaD-dependent gene expression in Bacillus subtilis. J. Bacteriol. 180:4243–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fontaine L, Goffin P, Dubout H, Delplace B, Baulard A, Lecat-Guillet N, Chambellon E, Gardan R, Hols P. 2013. Mechanism of competence activation by the ComRS signalling system in streptococci. Mol. Microbiol. 87:1113–1132. [DOI] [PubMed] [Google Scholar]
- 47.LeBlanc DJ, Lee LN, Abu-Al-Jaibat A. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130–145. [DOI] [PubMed] [Google Scholar]
- 48.Seaton K, Ahn S-J, Burne RA. Regulation of competence and gene expression in Streptococcus mutans by the RcrR transcriptional regulator. Mol. Oral Microbiol., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.