Abstract
The present study investigated the simultaneous oxidation of pyruvate and amino acids during H2-evolving growth of the hyperthermophilic archaeon Thermococcus kodakarensis. The comparison of mass balance between a cytosolic hydrogenase (HYH)-deficient strain (the ΔhyhBGSL strain) and the parent strain indicated that NADPH generated via H2 uptake by HYH was consumed by reductive amination of 2-oxoglutarate catalyzed by glutamate dehydrogenase. Further examinations were done to elucidate functions of three enzymes potentially involved in pyruvate oxidation: pyruvate formate-lyase (PFL), pyruvate:ferredoxin oxidoreductase (POR), and 2-oxoisovalerate:ferredoxin oxidoreductase (VOR) under the HYH-deficient background in T. kodakarensis. No significant change was observed by deletion of pflDA, suggesting that PFL had no critical role in pyruvate oxidation. The growth properties and mass balances of ΔporDAB and ΔvorDAB strains indicated that POR and VOR specifically functioned in oxidation of pyruvate and branched-chain amino acids, respectively, and the lack of POR or VOR was compensated for by promoting the oxidation of another substrate driven by the remaining oxidoreductase. The H2 yields from the consumed pyruvate and amino acids were increased from 31% by the parent strain to 67% and 82% by the deletion of hyhBGSL and double deletion of hyhBGSL and vorDAB, respectively. Significant discrepancies in the mass balances were observed in excess formation of acetate and NH3, suggesting the presence of unknown metabolisms in T. kodakarensis grown in the rich medium containing pyruvate.
INTRODUCTION
The euryarchaeal order Thermococcales, composed of two major genera, Thermococcus and Pyrococcus, comprises hyperthermophiles capable of growing at temperatures around 100°C (1, 2). Many members of the Thermococcales are anaerobic sulfur-reducing heterotrophs preferring proteinaceous substrates and utilize elemental sulfur (S0) as a terminal electron acceptor evolving H2S. In the absence of S0, some of these hyperthermophiles can grow on carbohydrates and related compounds, evolving hydrogen gas (H2) by using protons as a terminal electron acceptor (3).
The energy-conserving metabolism of carbohydrates associated with H2 evolution in the order Thermococcales has been well studied for Thermococcus kodakarensis and Pyrococcus furiosus (4). Pyruvate, added into the medium or generated from carbohydrates, is oxidized to acetyl coenzyme A (acetyl-CoA) by pyruvate:ferredoxin (Fd) oxidoreductase (POR) (5–8) and subsequently is converted to acetate concomitant with ATP synthesis by ADP-forming acetyl-CoA synthetases (9, 10). In addition to pyruvate oxidation, peptides and amino acids also are dissimilated simultaneously (11). Amino acids, which are directly incorporated into the cells or formed by degradation of peptides, are deaminated to the corresponding 2-oxoacids using 2-oxoglutarate as an amino acceptor by a number of aminotransferases with different substrate specificities (12–15). Glutamate formed by the transamination is regenerated to 2-oxoglutarate by alanine aminotransferase-mediated transfer of the amino group to pyruvate (15), resulting in the formation of alanine as one of the end products (15, 16). Although it is also possible that glutamate dehydrogenase (GDH) participates in the regeneration of 2-oxoglutarate through oxidative deamination of glutamate, GDH was suggested to act in the direction of glutamate formation when T. kodakarensis was grown with excess pyruvate (17). The amino acid-derived 2-oxoacids then are oxidized to acyl-CoAs by a set of 2-oxoacid (2-ketoacid):Fd oxidoreductases (KORs), followed by conversion to the corresponding carboxylic acids concomitant with ATP synthesis. This is similar to the pyruvate oxidation pathway, and the final step is catalyzed by NDP-forming acyl-CoA synthetases, including ADP-forming acetyl-CoA synthetases, composed of possible 10 combinations of five α-subunits and two β-subunits (18–20).
In Thermococcales hyperthermophiles, two kinds of [NiFe] hydrogenase, membrane-bound hydrogenase (MBH) and cytosolic hydrogenase (HYH), are considered to participate in H2 metabolism (21–28). MBH reduces protons to evolve H2 using reduced ferredoxin (Fdred), generated via catabolism of growth substrates, as an electron donor. It also exhibited proton-pumping activity during H2 evolution, and the resulting proton-motive force was coupled with ATP synthesis driven by the membrane-bound ATP synthase directly or by being converted to sodium-motive force (21–24). It has been demonstrated genetically that MBH-lacking strains of T. kodakarensis and P. furiosus showed severe growth impairment under the H2-evolving growth conditions (26–28). HYH in these hyperthermophiles acted in H2 uptake rather than H2 evolution for the recovery of reducing equivalents from H2 in the form of NADPH (25–28).
As mentioned above, 2-oxoacids, including pyruvate, are oxidatively decarboxylated to the corresponding CoA derivatives in an Fd-dependent manner by multiple KORs (29). Four kinds of KORs have been purified from P. furiosus and Thermococcus litoralis, and the following substrate specificities have been revealed: POR specifically catalyzes oxidation of pyruvate (5–8), and 2-oxoisovalerate:Fd oxidoreductase (VOR) shows activity with preference to 2-oxoacids derived from branched-chain amino acids (30, 31). Indolepyruvate:Fd oxidoreductase (IOR) recognizes 2-oxoacids from aromatic amino acids (32, 33), and 2-oxoglutarate:Fd oxidoreductase (OGOR) is specific to 2-oxoglutarate (34). POR is widely distributed in the three domains, whereas VOR, IOR, and OGOR have been found only in Archaea (29). Recently, it was demonstrated that OGOR from T. kodakarensis specifically was responsible for Glu/Gln degradation, as succinate production no longer was observed in the strain lacking OGOR (17). However, the details of functions and physiological roles of the set of KORs have been not thoroughly investigated to date.
Whole-genome analysis of T. kodakarensis has identified pflD (35), encoding a homolog of pyruvate formate-lyase (PFL) that catalyzes CoA-dependent cleavage of pyruvate to formate and acetyl-CoA (36). In addition, pflA, encoding a homolog of PFL-activating enzyme that probably activates PFL by forming the stable radical form (37), was identified as a neighbor of pflD on the T. kodakarensis chromosome. The presence of pflDA is rather rare in Archaea, and it is found in a few Thermococcus species (T. kodakarensis, T. litoralis, and T. sibiricus) but not in Pyrococcus spp. among the order Thermococcales. In mesophilic facultative anaerobes, such as Escherichia coli, PFL has an important role in anaerobic metabolism, and formate generated by PFL is degraded to H2 and CO2 by the formate hydrogen-lyase (FHL) complex (38, 39). Although no obvious homolog of the FHL complex was identified in T. kodakarensis, the general function of PFL raises the possibility that PflDA in cooperation with an unknown formate oxidoreductase establishes a bypass pathway for pyruvate oxidation in this hyperthermophile.
In this study, we initially confirmed and assessed in detail the effects of gene deletion for HYH and then investigated the functions of POR, VOR, and PFL in the pyruvate/amino acid oxidation during H2-evolving growth by T. kodakarensis on the basis of changes in mass balance caused by the deletion of the corresponding genes.
MATERIALS AND METHODS
Microorganisms and culture conditions.
E. coli DH5α was used for general DNA manipulation, and E. coli strains were cultivated at 37°C in Luria-Bertani (LB) medium containing 100 μg/ml ampicillin. T. kodakarensis strains and plasmids used in this study are listed in Table 1. T. kodakarensis strains were grown anaerobically at 85°C in a nutrient-rich medium (ASW-YT) composed of a 0.8-fold dilution of artificial seawater (40), 10 g/liter yeast extract, and 5.0 g/liter tryptone with either 5.0 g/liter S0 (ASW-YT-S0) or 5.0 g/liter sodium pyruvate (ASW-YT-Pyr). Minimal medium (ASW-AA-S0) containing the nutritionally required amino acids plus trace minerals, vitamins, and 5.0 g/liter elemental sulfur was prepared as described previously (40).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| T. kodakarensis strains | ||
| KOD1 | Wild type | 45 |
| KUW1 | ΔpyrF ΔtrpE (uracil and tryptophan auxotroph) | 42 |
| KUW1Δhyh | KUW1 derivative, ΔhyhBGSL | This study |
| KUW1ΔΔhyh_pfl | KUW1 derivative, ΔhyhBGSL ΔpflDA | This study |
| KUW1ΔΔhyh_por | KUW1 derivative, ΔhyhBGSL ΔporDAB | This study |
| KUW1ΔΔhyh_vor | KUW1 derivative, ΔhyhBGSL ΔvorDAB | This study |
| Plasmids | ||
| pUC118 | Ampr, general cloning vector | TaKaRa Bio |
| pUD3 | pUC118 derivative, pyrF marker cassette | 41 |
| pUD3-Δhyh | pUD3 derivative, hyhBGSL homologous regions | This study |
| pUD3-Δpfl | pUD3 derivative, pflDA homologous regions | This study |
| pUD3-Δpor | pUD3 derivative, porDAB homologous regions | This study |
| pUD3-Δvor | pUD3 derivative, vorDAB homologous regions | This study |
Target gene disruption in T. kodakarensis.
DNA manipulation was carried out by standard procedures, and the sequences of oligonucleotide primers used in this study are listed in Table S1 in the supplemental material. PCR was carried out with KOD-Plus-ver.2 DNA polymerase (Toyobo, Osaka, Japan). Plasmids for gene disruption of hyhBGSL (pUD3-Δhyh), pflDA (pUD3-Δpfl), porDAB (pUD3-Δpor), and vorDAB (pUD3-Δvor) in T. kodakarensis were constructed based on pUD3 harboring a pyrF marker cassette (41). pUD3-Δhyh and pUD3-Δvor were constructed as follows. DNA fragments containing the respective target genes, hyhBGSL (tk2072-tk2069)and vorDAB (tk1979-tk1981), together with the flanking regions (approximately 800 bp), were amplified from T. kodakarensis KOD1 genomic DNA with primer sets hyhBGSL800–5′/hyhBGSL800–3′ and vorD800–5′/vorB800–3′, respectively. The amplified DNA fragments were inserted into pUD3 at the HincII site. Inverse PCRs with primers hyhBGSL-inv-5′/hyhBGSL-inv-3′ and vor-inv-5′/vor-inv-3′ then were carried out to amplify the respective homologous regions along with the plasmid backbone, thereby removing the coding regions. The amplified fragments were self-ligated after 5′-phosphorylation to obtain pUD3-Δhyh and pUD3-Δvor.
pUD3-Δpor was constructed similarly, with slight changes. A DNA fragment containing porDAB (tk1982-tk1984) together with the flanking regions was amplified with a primer set of porD800–5′/porB800–3′ and inserted into pUC118 at the HincII site. The plasmid was digested by ClaI and NdeI to remove the coding regions of porDAB and subsequently was self-ligated after blunting of the ends. The fragment of the connected homologous regions was excised from the vector by EcoRV and inserted into pUD3 at the HincII site.
pUD3-Δpfl was constructed by the insertion of a fragment of upstream and downstream regions of pflDA (tk0289-tk0290) into pUD3 at the HincII site. The fragment was prepared by fusion PCR with the individual fragments of the upstream region amplified using pflD800–5′/pflD800-fusion-3′ primers and the downstream region amplified using pflA800-fusion-5′/pflA800–3′ primers.
Transformation of T. kodakarensis KUW1 (ΔpyrF ΔtrpE) with the constructed vectors, and selection of transformants formed by pop-in/pop-out recombination based on uracil prototrophy of pyrF+ strains and resistance of pyrF mutant strains to 5-fluoroorotic acid monohydrate (5-FOA), were performed according to the procedure described previously (42). In the case of vorDAB deletion, ASW-YT-Pyr plate medium containing 0.75 g/liter 5-FOA and 5.0 g/liter sodium pyruvate was used for isolation of the pop-out strain. The successful recombination was verified by PCR using appropriate primer sets (see Fig. S1 in the supplemental material).
Determination of growth properties and measurement of H2 and CO2 in gas phase.
T. kodakarensis strains were precultured in 100 ml of ASW-YT-Pyr at 85°C for 14 h, and the cells were inoculated into 120 ml of the same medium (1%, vol/vol) in a 100-ml bottle (40 ml headspace) with an Omnifit GL45 cap having two valve ports (Diva Industries, Danbury, CT). The bottle was incubated in an oil bath at 85°C, a portion of the broth was taken through one valve port every 2 h, and the optical density at 600 nm (OD600) was measured by using an S1200 diode array spectrophotometer (Biochrom, Berlin, Germany). Another valve port was directly connected to a 0.5-liter-volume aluminum bag having one syringe port (GL Sciences, Tokyo, Japan) to collect gas evolved during the cultivation. A portion of the collected gas was taken through the syringe port by using a 500-μl gas-tight syringe (Hamilton, Reno, NV), and the concentrations of H2 and CO2 were determined by a gas chromatograph (GC-2014; Shimadzu, Kyoto, Japan) equipped with a stainless packed column (3.0 mm by 4 m; Shincarbon ST; Shinwa Chemical Industries, Kyoto, Japan) (carrier gas, Ar) and a thermal conductivity detector (current value, 50 mA). After that, the collected gas phase in the aluminum bag was transferred to a 1,011-ml vacuum bottle (GL Science), and the gas volume was calculated from the pressure change in the bottle measured by using a digital pressure meter.
The S0-dependent growth properties of T. kodakarensis strains were determined as follows. The cells were precultured in 100 ml of ASW-YT-S0 at 85°C for 14 h, except for the KUW1ΔΔhyh_vor strain, which was precultured in ASW-YT-Pyr due to the growth defect in S0-dependent condition, and then the cells were inoculated into 8 ml of ASW-YT-S0 (5%, vol/vol) in a tightly capped test tube. The optical density at 600 nm of the culture was directly measured every 2 h with the S1200 spectrophotometer.
Analyses of substrates and products in culture medium.
After 12 to 14 h of cultivation of T. kodakarensis strains in ASW-YT-Pyr, the culture broth was centrifuged (5,000 × g, 20 min, 4°C), and the supernatant was ultrafiltrated with a Vivaspin 500 (3,000-molecular-weight cutoff; Sartorius, Göttingen, Germany). The concentration of each carboxylic acid in the supernatant was determined by ion chromatography (ICS-2100; Dionex, CA) equipped with a series of two ion exchange columns (IonPac AS11-HC and IonPac AS-19; both 4.0 mm by 250 mm; both from Dionex) and a conductivity detector (current value, 140 mA). The mobile phase was a gradient of KOH from 0.5 mM to 60 mM. The concentration of each amino acid in the supernatant was determined by high-performance liquid chromatography (HPLC) after acid hydrolysis and derivatization. The acid hydrolysis of the supernatant was done in the presence of 12 N HCl (Fe-free) (Nacalai Tesque, Kyoto, Japan) under reduced pressure in a sealed glass tube at 110°C for 24 h. The hydrolysate was reacted with phenylisothiocyanate according to the procedure described previously (43), and the derivatives were analyzed using a Prominence HPLC apparatus (Shimadzu) equipped with a reverse-phase column (3.0 mm by 75 mm; Shim-pack XR-ODS; Shimadzu). The mobile phase was a linear gradient of acetonitrile in the range of 5% to 36% in 5 mM potassium phosphate buffer (pH 6.9), and the absorbance at 254 nm was monitored. The concentration of acetate was enzymatically determined by using F-kit acetate (Roche Diagnostics, Basel, Switzerland), and that of NH3 was measured by using an ammonia test (Wako Pure Chemicals, Osaka, Japan). The amount of biomass was calculated based on the estimation that the organic fraction of the cell is C5H7NO2, and this comprises 90% of cell dry weight (11).
RESULTS
Construction of T. kodakarensis mutants lacking genes for POR, VOR, or PFL potentially involved in pyruvate oxidation.
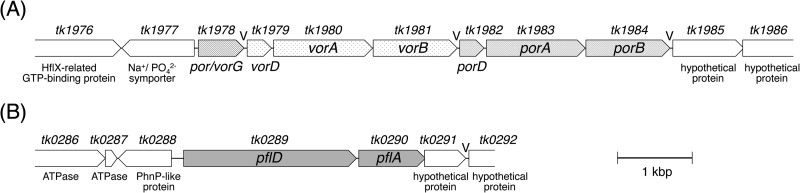
It has been reported that POR and VOR from P. furiosus were heterotetramers, wherein the γ-subunit was shared with the two enzymes, and the genes were clustered as porG (vorG)-vorDAB-porDAB (6). T. kodakarensis also possesses a gene cluster with the same organization (tk1978-tk1984) as that shown in Fig. 1A, of which deduced amino acid sequences were highly homologous to the counterparts from P. furiosus and T. litoralis (>80% identities). PFL and its activating enzyme, encoded by pflDA (tk0289-tk0290) (Fig. 1B), shared 33% and 30% identities with the corresponding homologs from E. coli, respectively. The genes of POR, VOR, or PFL were individually deleted in T. kodakarensis by homologous recombination to evaluate the contribution of the enzymes to pyruvate/amino acid oxidation in this hyperthermophile. We used an Hyh-deficient strain, the KUW1Δhyh mutant, which was constructed by deletion of hyhBGSL (tk2072-tk2069) in T. kodakarensis KUW1 (ΔpyrF ΔtrpE) as a host strain in order to simplify the H2-evolving metabolisms by blocking the uptake of the evolved H2 (26, 27). Considering the presence of a common γ-subunit for POR and VOR, the knockout mutants of POR and VOR were constructed by deletion of the genes encoding δ, α, and β subunits of POR (porDAB) and those of VOR (vorDAB), respectively. The strain lacking PFL was constructed by deletion of almost the entire region of pflDA except an 8-bp region of the 3′ end of pflA overlapped with the downstream gene tk0291, encoding a hypothetical protein. The genotype of the resulting KUW1Δhyh, KUW1ΔΔhyh_por, KUW1ΔΔhyh_vor, and KUW1ΔΔhyh_pfl strains were confirmed by PCR analyses as shown in Fig. S1 in the supplemental material.
FIG 1.
Structures of T. kodakarensis vor-por and pfl gene clusters. The oligo(T) sequences that potentially acted as archaeal transcription terminators are indicated with a “V” (46).
Growth properties of the T. kodakarensis mutants.
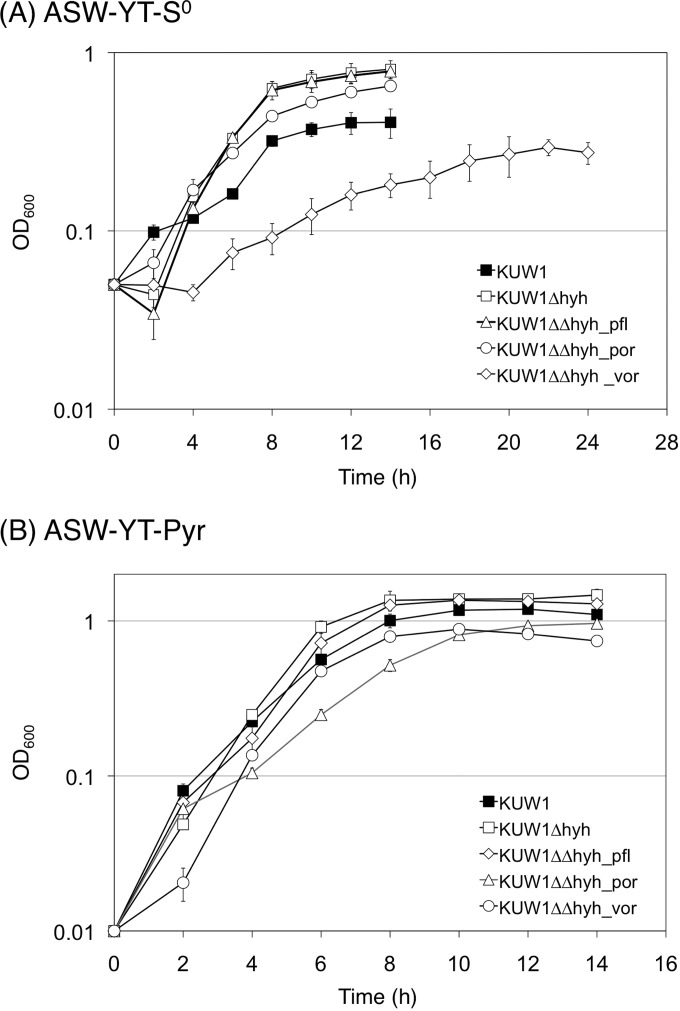
The growth properties of the constructed strains were evaluated in nutrient-rich media supplemented with S0 (ASW-YT-S0; H2S-evolving conditions) or sodium pyruvate (ASW-YT-Pyr; H2-evolving conditions). Compared to those of the parent strain KUW1, the KUW1Δhyh strain showed a similar growth rate and slightly higher final cell density in both media (Fig. 2). Among the modified strains derived from the KUW1Δhyh strain, the most drastic change was observed for the KUW1ΔΔhyh_vor strain; growth was severely impaired in ASW-YT-S0. The growth rate and final cell density of the KUW1ΔΔhyh_vor strain in ASW-YT-Pyr also were decreased, although the change was less significant than that in ASW-YT-S0. The KUW1ΔΔhyh_por strain showed slightly lower growth ability than the KUW1Δhyh control strain. Almost no change in growth properties was observed for the KUW1ΔΔhyh_pfl strain.
FIG 2.
Growth property of T. kodakarensis strains in ASW-YT medium containing either S0 or sodium pyruvate. The cells were cultivated at 85°C, and optical densities at 600 nm were measured.
Mass balance of pyruvate/amino acid oxidation in T. kodakarensis and the effects of hyh deletion.
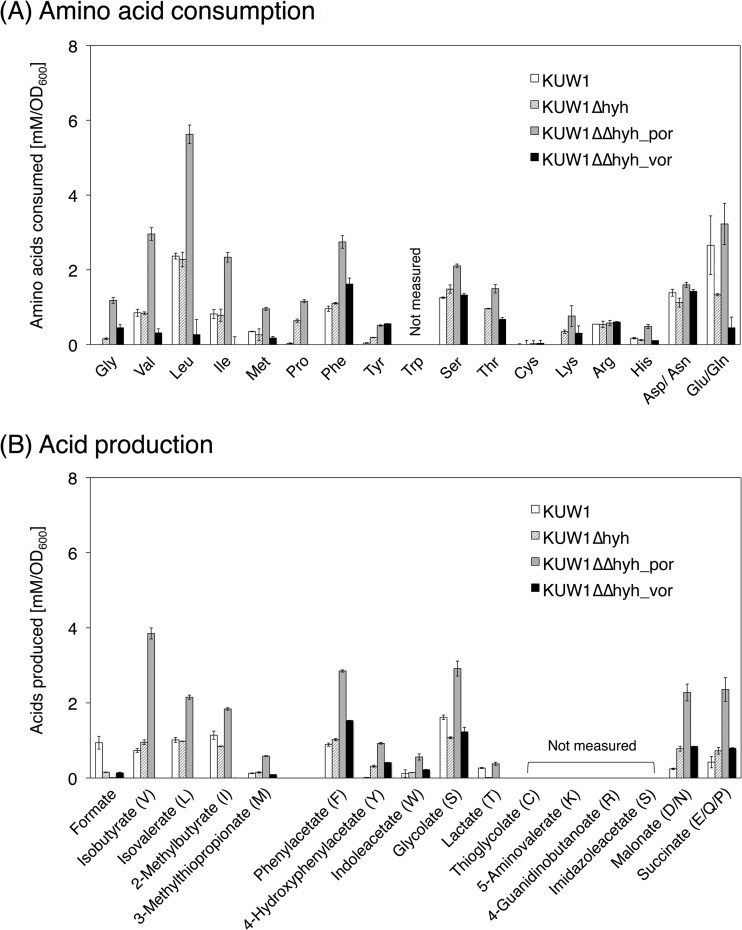
Although previous reports described an increase of H2 evolution by gene disruption of Hyh acting on H2 uptake in T. kodakarensis (26, 27), the alterations in pyruvate/amino acid oxidation have not been investigated in detail. Hence, the KUW1 and KUW1Δhyh strains were cultivated in ASW-YT-Pyr, and the changes in concentrations of substrates and end products in the culture broth and those of H2 and CO2 in the evolved gas phase were compared (Table 2). Among the amino acids and the corresponding carboxylic acids to be analyzed, the concentration of Trp could not be determined due to degradation during the acid hydrolysis of proteins/peptides, and thioglycolate (derived from Cys), 5-aminovalerate (Lys), 4-guanidinobutanoate (Arg), and imidazoleacetate (His) could not be detected by ion chromatography. Therefore, the consumption of Trp and production of the four acids were estimated from the production of indoleacetate (derived from Trp) and consumption of the corresponding amino acids, respectively. In Table 2 and the data below, the terms “amino acids” and “acids” indicated the sum of 19 amino acids besides alanine and that of 15 carboxylic acids except for acetate and formate, respectively. The consumption of each amino acid and the production of each acid are shown in Fig. 3.
TABLE 2.
Consumption of substrates and production of fermentation products during the batch cultivation in ASW-YT-Pyr
| Strain | OD600 | Consumption (mmol/liter of culture) |
Production (mmol/liter of culture) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyruvate | Amino acidsa | NH3 | Acetate | Alanine | Acidsb | Formate | CO2 | H2 | NH3 | Biomassc | ||
| KUW1 | 1.12 ± 0.08 | 37.0 ± 0.3 | 10.2 ± 1.0 | 4.50 ± 0.07 | 24.8 ± 1.1 | 20.4 ± 0.4 | 7.5 ± 0.2 | 0.97 ± 0.13 | 21.5 ± 1.8 | 14.7 ± 1.0 | 3.14 ± 0.22 | |
| KUW1Δhyh | 1.51 ± 0.09 | 32.9 ± 0.01 | 17.0 ± 0.1 | 31.7 ± 1.8 | 14.4 ± 1.6 | 11.5 ± 0.3 | 0.18 ± 0.02 | 33.4 ± 1.9 | 33.6 ± 1.9 | 8.01 ± 0.40 | 4.24 ± 0.26 | |
| KUW1ΔΔhyh_pfl | 1.54 ± 0.05 | 32.8 ± 0.4 | 16.0 ± 1.9 | 32.5 ± 1.8 | 15.4 ± 0.4 | 12.7 ± 0.01 | 0.22 ± 0.03 | 34.1 ± 4.0 | 33.9 ± 2.7 | 8.24 ± 0.68 | 4.31 ± 0.13 | |
| KUW1ΔΔhyh_por | 0.73 ± 0.02 | 22.9 ± 0.2 | 21.4 ± 0.6 | 8.3 ± 0.1 | 23.4 ± 0.7 | 16.7 ± 0.6 | 0.01 ± 0.00 | 17.8 ± 1.4 | 18.3 ± 0.6 | 5.84 ± 0.29 | 2.05 ± 0.07 | |
| KUW1ΔΔhyh_vor | 1.19 ± 0.08 | 32.3 ± 0.1 | 9.4 ± 0.3 | 36.6 ± 0.2 | 8.9 ± 0.1 | 7.2 ± 0.1 | 0.15 ± 0.02 | 33.9 ± 2.2 | 34.0 ± 2.5 | 7.69 ± 0.04 | 3.34 ± 0.23 | |
Trp consumption is estimated from indoleacetate (derived from Trp) production. Ala is not included in the sum.
The amount includes carboxylic acids corresponding to amino acids, except for formate and acetate. Thioglycolate (derived from Cys), 5-aminovalerate (Lys), 4-guanidinobutanoate (Arg), and imidazoleacetate (His) production was estimated from the corresponding amino acid consumption.
Based on the assumption that the organic fraction of cells is C5H7NO2, and this comprises 90% of dry cell weight (11).
FIG 3.
Consumption of amino acids (A) and production of the corresponding acids (B) during the cultivation of T. kodakarensis strains in an ASW-YT-Pyr medium. Each strain was cultivated for 12 h, except the KUW1ΔΔhyh_por strain, which was cultivated for 14 h. The letters in parentheses in panel B indicate the corresponding amino acids. Pro was estimated to be degraded after the conversion to Glu and formed succinate as the corresponding acid.
The strain KUW1, expressing hyhBGSL, evolved 14.7 mmol/liter of culture of H2 on pyruvate, which was much lower than the 21.5 mmol/liter of culture of CO2 (Table 2). The amount of the produced acids corresponded to 74% of the consumed amino acids, and the tendency of less formation of acids always was observed for T. kodakarensis strains in this study. The alanine formation was about two times greater than amino acid consumption, and consumption of NH3 and production of formate during the cultivation were detected. Val, Leu, Ile, Phe, Ser, Asp/Asn, and Glu/Gln were preferred amino acids for KUW1 (Fig. 3), which agreed with the results in a previous continuous cultivation of T. kodakarensis KOD1 (11). The results also were consistent with substrate specificities of KORs and acyl-CoA synthetases, except for Asp/Asn (18–20, 30–34). The consumption of Val, Ile, and Phe coincided well with the formation of the corresponding acids, whereas Leu, Ser, Asp/Asn, and Glu/Gln were consumed more than the formation of the corresponding acids. This discrepancy was especially significant for Leu, as about only half of isovalerate was produced compared to the consumption of Leu.
The deletion of hyhBGSL increased evolution of both H2 and CO2, and the increase of H2 was larger than that of CO2, leading to equal production of H2 and CO2 (∼33 mmol/liter of culture) by the KUW1Δhyh strain (Table 2). This strain showed less consumption of pyruvate and less formation of alanine and formate than the parent strain KUW1. Notably, unlike KUW1, the KUW1Δhyh strain produced NH3 during S0-independent growth on pyruvate. The equimolar formation of H2 and CO2 and production of NH3 also were seen for all of the Hyh-deficient strains examined in this study. As shown in Fig. 3, the KUW1Δhyh strain showed tendencies for the amino acid consumption and acid formation that were similar to those of KUW1, except it showed significantly more consumption of Thr.
Based on the two major fates of pyruvate, conversion to alanine and acetate, it can be expected that the sum of the produced acetate and alanine corresponded to the consumption of pyruvate. Nevertheless, in the determined mass balances for KUW1 and the KUW1Δhyh mutant, the actual sums of acetate and alanine (45.2 and 46.1 mmol/liter of culture, respectively) were remarkably larger than those of pyruvate consumption (37.0 and 32.9 mmol/liter of culture, respectively).
Effects of PFL or POR knockout on metabolism in T. kodakarensis.
Almost no change in mass balances was observed between the pflDA deletion strain KUW1ΔΔhyh_pfl and the KUW1Δhyh parent strain (Table 2). Although formate formation was detected for both strains, the levels were low (0.18 to 0.22 mmol/liter of culture) and not altered by the disruption of pflDA. The results strongly suggested that the small amount of formate was not produced by Pfl-mediated cleavage of pyruvate.
In contrast, quite drastic changes were observed in the stoichiometry of pyruvate/amino acid oxidation by the porDAB deletion strain KUW1ΔΔhyh_por; more consumption of amino acids and less consumption of pyruvate were observed compared to those by the KUW1Δhyh strain (Table 2), and alanine production and pyruvate consumption almost matched each other (∼23 mmol/liter of culture). As shown in Fig. 3, this strain showed more consumption for most of the detectable amino acids than the KUW1Δhyh strain, particularly for Gly, Val, Leu, Ile, Met, Phe, and Glu/Gln. The consumption of Leu and Thr was significantly higher than the formation of the corresponding acids, as seen for the KUW1Δhyh strain.
Effects of VOR knockout on metabolism in T. kodakarensis.
Deletion of vorDAB resulted in a drastic increase and decrease in the formation of acetate and alanine, respectively, leading to a much larger ratio of acetate to alanine (4.1) than that by the KUW1Δhyh control strain (2.2) (Table 2). The amino acid consumption by the KUW1ΔΔhyh_vor strain remarkably decreased compared to that by the KUW1Δhyh strain, while it was well balanced to alanine formation, as seen for the other Hyh-deficient strains. Despite the great impact of the deletion of vorDAB on catabolism, H2 and CO2 were evolved in equal amounts to levels as high as those by the KUW1Δhyh strain.
The KUW1ΔΔhyh_vor strain was scarcely able to consume Val, Leu, and Ile and completely lost the ability to produce the corresponding acids (Fig. 3), whereas the consumption of Phe and Tyr and the formation of the corresponding acids were rather increased compared to the levels for the KUW1Δhyh strain.
DISCUSSION
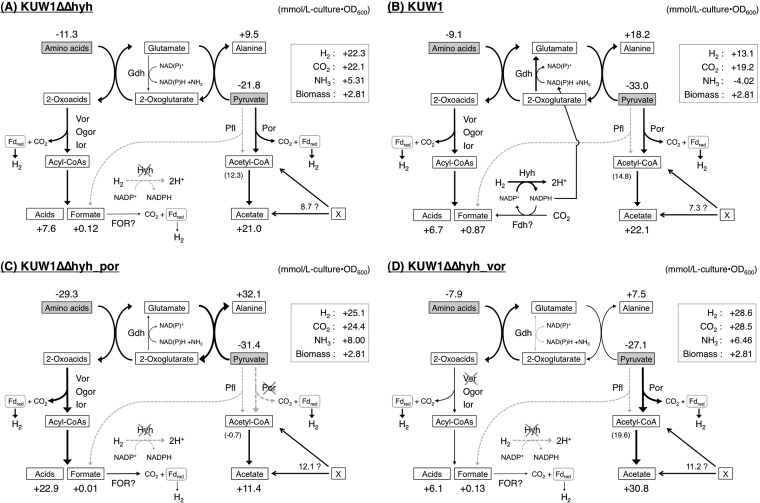
The present study determined the mass balance of the simultaneous oxidation of pyruvate and amino acids concomitant with H2 evolution by T. kodakarensis grown in the absence of S0, and the cellular consumption of substrates and production of end products in the estimated catabolic pathways are presented in Fig. 4. Pyruvate played two roles as an acceptor for the amino acid-derived amino group via glutamate, forming alanine, and as an energy source to be oxidized to acetate. As shown in Fig. 4A, the determined mass balance for the hyhBGSL deletion strain KUW1Δhyh rather agreed with the estimated metabolisms except for a few points. The H2/CO2 ratio was 1.0, and the sum of the cellular production of acids/formate and that of acetyl-CoA estimated by subtraction of alanine formation from pyruvate consumption (20 mmol/liter of culture · OD600) was comparable to the formation of H2 and CO2 (22 mmol/liter of culture · OD600). The alanine formation also was comparable to the amino acid consumption, suggesting most amino groups in the consumed amino acids were transferred to pyruvate. The production of acids was slightly lower than the consumption of amino acids, which was probably due to the anabolic metabolism of amino acids. One severe discrepancy in the stoichiometry, not only for the KUW1Δhyh strain but also for other strains examined in this study, was excess production of acetate, which is discussed below.
FIG 4.
Mass balances of pyruvate/amino acid oxidation in T. kodakarensis KUW1Δhyh (A), KUW1 (B), KUW1ΔΔhyh_por (C), and KUW1ΔΔhyh_vor (D) strains. The cellular consumption and production were calculated as mmol/liter of culture · OD600. The formation of acetyl-CoA shown in parentheses was estimated by subtraction of alanine formation from pyruvate consumption. An unidentified source for the excess formation of acetate or acetyl-CoA is shown by an “X.”
Although the actual amount of CO2 evolved by the strain KUW1 was less than that by the KUW1Δhyh strain, this was due mainly to the lower cell growth of KUW1 (Table 2), as the cellular formation of CO2 by KUW1 (Fig. 4B) was similar to that of the KUW1Δhyh strain (Fig. 4A). On the other hand, the H2/CO2 ratio for KUW1 was 0.68, which indicated the uptake of H2 by HYH as reported previously (26, 27). It was further clarified that KUW1 showed the formation of less H2 and more alanine than the KUW1Δhyh strain, and NH3 was consumed during the cultivation, in contrast to the significant production of NH3 by the KUW1Δhyh strain. These facts strongly suggested that NADPH formed by the Hyh-driven reoxidation of H2 was used in glutamate formation through GDH-catalyzed reductive amination of 2-oxoglutarate, and the amino group in the resulting glutamate consequently was transferred to pyruvate to form alanine (Fig. 4B). Indeed, the increase of alanine formation attributed to the function of Hyh (8.7 mmol/liter of culture · OD600) nearly corresponded to the change of NH3 concentration (−9.3 mmol/liter of culture · OD600) observed for KUW1. This suggests that the reducing equivalent recovered from H2 was consumed mostly by GDH; thus, it hardly contributed to anabolic metabolisms in T. kodakarensis, at least under the cultivation conditions examined. The results also demonstrated an advantage of the deletion of hyhBGSL in the improvement of the yield of H2 to the consumed substrates (amino acids and pyruvate) from 31% to 67%.
Although PFL-mediated cleavage of pyruvate was one possible pathway for formate formation in T. kodakarensis, the concentration of formate was not changed by the deletion of pflDA (Table 2). Moreover, the KUW1ΔΔhyh_pfl strain showed almost no differences in growth property and mass balance compared to the KUW1Δhyh parent strain, indicating that the PFL homolog did not participate in pyruvate/amino acid oxidation in T. kodakarensis. Interestingly, a higher level of formation of formate was observed for KUW1 than for the KUW1Δhyh strain (Table 2), suggesting some of the reducing equivalent recovered from H2 by HYH was used to reduce CO2 to formate by an unknown formate oxidoreductase. We assumed that the unknown formate dehydrogenase was related to a gene, tk2076, showing 40% identity to the formate dehydrogenase α-subunit FdhF from E. coli, and this was supported by the observation that the deletion of tk2076 from KUW1 decreased formate production to a level similar to that by the KUW1Δhyh strain (data not shown).
This study also elucidated that POR and VOR were specifically involved in the oxidation of pyruvate and branched-chain amino acids in vivo, respectively. In particular, the pyruvate consumption by the KUW1ΔΔhyh_por strain was well matched with alanine formation, and the evolution of CO2 and H2 was comparable to that of acid production. These results most likely were because the pyruvate oxidation was nearly completely blocked by the deletion of porDAB; thus, the energy conservation was shifted from the oxidation of pyruvate/amino acids to the oxidation of amino acids only (Fig. 4C). VOR appeared not to participate in the pyruvate oxidation, although it has been reported that VOR from T. litoralis showed weak activity to pyruvate (30). The deletion of vorDAB diminished the oxidation of branched-chain amino acids (Fig. 3 and 4D), which was consistent with the substrate specificity (30), as well as suggesting that VOR has a unique role in the oxidation of branched-chain amino acids in T. kodakarensis. The remaining oxidation ability for amino acids in the KUW1ΔΔhyh_vor strain was supposed to be mediated by OGOR oxidizing 2-oxoglutarate derived from Glu/Gln and IOR preferring 2-oxoacids derived from aromatic amino acids. The related metabolic changes by the vorDAB deletion were the decrease in alanine formation and increase in pyruvate consumption. The KUW1ΔΔhyh_vor strain showed a much lower growth rate and final cell density than the KUW1Δhyh strain in ASW-YT-S0, whereas such growth impairment was not significant in ASW-YT-Pyr (Fig. 2). It was estimated that the VOR-mediated oxidation of branched-chain amino acids was an important energy-conserving pathway in T. kodakarensis grown in the presence of S0, and the weakened amino acid oxidation in the KUW1ΔΔhyh_vor strain was compensated for by the enhancement of pyruvate oxidation when pyruvate was available. From the viewpoint of microbial H2 production, the deletion of vorDAB was a beneficial modification, because the H2 yield from the consumed substrates was increased up to 82% from 67% by the KUW1Δhyh parent strain. We also attempted the double deletion of vorDAB and porDAB in T. kodakarensis KUW1 and KUW1Δhyh strains, but this was unsuccessful even after several trials using selection medium supplemented with S0, starch, and an increased concentration of aromatic amino acids. This result suggested that the action of VOR or POR is essential for the growth of T. kodakarensis.
The most severe discrepancy in the mass balances determined in this study was the excess formation of acetate or alanine toward pyruvate consumption. Considering that alanine formation was well balanced with amino acid consumption, as described above, this discrepancy probably was attributed to the excess formation of acetate in T. kodakarensis. The levels of excess acetate (or acetyl-CoA) reached 7.3 to 12.1 mmol/liter · OD600 (Fig. 4), and a similar discrepancy in acetate formation by T. kodakarensis has been reported by Yokooji et al. (17). Although we initially considered that the excess acetate was formed through the hydrolysis of acetylated compounds in rich medium during cell growth, acetate was not detected after acid hydrolysis of the fresh rich medium (data not shown). Another possible source of the excess acetate was assumed to be some sugars in the rich medium. However, this was unlikely, because the degradation of sugars to acetate led to the formation of more H2 than CO2 (2:1 with hexoses), which did not agree with the equimolar formation of H2 and CO2 for the hyhBGSL deletion strains examined. These results suggested the presence of an acetate-generating pathway from an unidentified source (indicated with an “X” in Fig. 4) in T. kodakarensis. Interestingly, such an excess formation of acetate was not observed in continuous cultivation of T. kodakarensis with a rich medium and batch cultivation of P. furiosus with a semisynthetic medium (11, 16). We also observed the formation of NH3 in the Hyh-deficient strains of T. kodakarensis. As the present results exhibited almost stoichiometric transfer of an amino group in amino acids to pyruvate, such release of free NH3 during the oxidation of amino acids was unexpected. Another disagreement was seen between consumption of some amino acids and formation of the corresponding acids, particularly for Leu and Thr, as shown in Fig. 3. It was supposed that some Leu residues were consumed by anabolic reactions in T. kodakarensis lacking the Leu biosynthesis pathway (35). The higher level of consumption of Thr may be caused by threonine dehydrogenase activity in T. kodakarensis (17, 44). Given the formation of acetyl-CoA by the threonine dehydrogenase-initiated degradation of Thr, this side pathway may contribute to the excess formation of acetate, although this would not be sufficient to explain the large amount of excess acetate. The results of mass balance analyses in this study suggested the presence of unknown metabolisms of some compounds in rich medium during batch cultivation of T. kodakarensis. Further analysis will be needed to elucidate these phenomena in the hyperthermophilic archaeon.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by a research grant from the Institute of Fermentations, Osaka, Japan.
Footnotes
Published ahead of print 25 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02021-14.
REFERENCES
- 1.Huber R, Stetter KO. 2001. Discovery of hyperthermophilic microorganisms. Methods Enzymol. 330:11–24. 10.1016/S0076-6879(01)30367-1. [DOI] [PubMed] [Google Scholar]
- 2.Itoh T. 2003. Taxonomy of nonmethanogenic hyperthermophilic and related thermophilic archaea. J. Biosci. Bioeng. 96:203–212. 10.1016/S1389-1723(03)80183-4. [DOI] [PubMed] [Google Scholar]
- 3.Amend JP, Shock EL. 2001. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25:175–243. 10.1111/j.1574-6976.2001.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 4.Bräsen C, Esser D, Rauch B, Siebers B. 2014. Carbohydrate metabolism in Archaea: current insights into unusual enzymes and pathways and their regulation. Microbiol. Mol. Biol. Rev. 78:89–175. 10.1128/MMBR.00041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blamey JM, Adams MW. 1993. Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim. Biophys. Acta 1161:19–27. 10.1016/0167-4838(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 6.Kletzin A, Adams MW. 1996. Molecular and phylogenetic characterization of pyruvate and 2-ketoisovalerate ferredoxin oxidoreductases from Pyrococcus furiosus and pyruvate ferredoxin oxidoreductase from Thermotoga maritima. J. Bacteriol. 178:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma K, Hutchins A, Sung SJ, Adams MW. 1997. Pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus, functions as a CoA-dependent pyruvate decarboxylase. Proc. Natl. Acad. Sci. U. S. A. 94:9608–9613. 10.1073/pnas.94.18.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon AL, Hendrix H, Hutchins A, Verhagen MF, Adams MW. 1998. The δ-subunit of pyruvate ferredoxin oxidoreductase from Pyrococcus furiosus is a redox-active, iron-sulfur protein: evidence for an ancestral relationship with 8Fe-type ferredoxins. Biochemistry 37:12838–12846. 10.1021/bi980979p. [DOI] [PubMed] [Google Scholar]
- 9.Mai X, Adams MW. 1996. Purification and characterization of two reversible and ADP-dependent acetyl coenzyme A synthetases from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 178:5897–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glasemacher J, Bock AK, Schmid R, Schönheit P. 1997. Purification and properties of acetyl-CoA synthetase (ADP-forming), an archaeal enzyme of acetate formation and ATP synthesis, from the hyperthermophile Pyrococcus furiosus. Eur. J. Biochem. 244:561–567. 10.1111/j.1432-1033.1997.00561.x. [DOI] [PubMed] [Google Scholar]
- 11.Kanai T, Imanaka H, Nakajima A, Uwamori K, Omori Y, Fukui T, Atomi H, Imanaka T. 2005. Continuous hydrogen production by the hyperthermophilic archaeon, Thermococcus kodakaraensis KOD1. J. Biotechnol. 116:271–282. 10.1016/j.jbiotec.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Ward DE, de Vos WM, van der Oost J. 2002. Molecular analysis of the role of two aromatic aminotransferases and a broad-specificity aspartate aminotransferase in the aromatic amino acid metabolism of Pyrococcus furiosus. Archaea 1:133–141. 10.1155/2002/959031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreotti G, Cubellis MV, Nitti G, Sannia G, Mai X, Marino G, Adams MW. 1994. Characterization of aromatic aminotransferases from the hyperthermophilic archaeon Thermococcus litoralis. Eur. J. Biochem. 220:543–549. 10.1111/j.1432-1033.1994.tb18654.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsui I, Matsui E, Sakai Y, Kikuchi H, Kawarabayasi Y, Ura H, Kawaguchi S, Kuramitsu S, Harata K. 2000. The molecular structure of hyperthermostable aromatic aminotransferase with novel substrate specificity from Pyrococcus horikoshii. J. Biol. Chem. 275:4871–4879. 10.1074/jbc.275.7.4871. [DOI] [PubMed] [Google Scholar]
- 15.Ward DE, Kengen SWM, van der Oost J, de Vos WM. 2000. Purification and characterization of the alanine aminotransferase from the hyperthermophilic archaeon Pyrococcus furiosus and its role in alanine production. J. Bacteriol. 182:2559–2566. 10.1128/JB.182.9.2559-2566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kengen SWM, Stams AJM. 1994. Formation of l-alanine as a reduced end product in carbohydrate fermentation by the hyperthermophilic archaeon Pyrococcus furiosus. Arch. Microbiol. 161:168–175. 10.1007/BF00276479. [DOI] [Google Scholar]
- 17.Yokooji Y, Sato T, Fujiwara S, Imanaka T, Atomi H. 2013. Genetic examination of initial amino acid oxidation and glutamate catabolism in the hyperthermophilic archaeon Thermococcus kodakarensis. J. Bacteriol. 195:1940–1948. 10.1128/JB.01979-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shikata K, Fukui T, Atomi H, Imanaka T. 2007. A novel ADP-forming succinyl-CoA synthetase in Thermococcus kodakaraensis structurally related to the archaeal NDP-forming acetyl-CoA synthetases. J. Biol. Chem. 282:26963–26970. 10.1074/jbc.M702694200. [DOI] [PubMed] [Google Scholar]
- 19.Awano T, Wilming A, Tomita H, Fukui T, Imanaka T, Atomi H. 2014. Characterization of two members among the five ADP-forming acyl coenzyme A (acyl-CoA) synthetases reveals the presence of a 2-(imidazole-4-yl) acetyl-CoA synthetase in Thermococcus kodakarensis. J. Bacteriol. 196:140–147. 10.1128/JB.00877-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JW, Poole FL, Adams MW. 2014. Characterization of ten heterotetrameric NDP-dependent acyl-CoA synthetases of the hyperthermophilic archaeon Pyrococcus furiosus. Archaea 10.1155/2014/176863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva PJ, van den Ban ECD, Wassink H, Haaker H, de Castro B, Robb FT, Hagen WR. 2000. Enzymes of hydrogen metabolism in Pyrococcus furiosus. Eur. J. Biochem. 267:6541–6551. 10.1046/j.1432-1327.2000.01745.x. [DOI] [PubMed] [Google Scholar]
- 22.Sapra R, Bagramyan K, Adams MW. 2003. A simple energy-conserving system: proton reduction coupled to proton translocation. Proc. Natl. Acad. Sci. U. S. A. 100:7545–7550. 10.1073/pnas.1331436100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schut GJ, Boyd ES, Peters JW, Adams MW. 2013. The modular respiratory complexes involved in hydrogen and sulfur metabolism by heterotrophic hyperthermophilic archaea and their evolutionary implications. FEMS Microbiol. Rev. 37:182–203. 10.1111/j.1574-6976.2012.00346.x. [DOI] [PubMed] [Google Scholar]
- 24.Pisa KY, Huber H, Thomm M, Müller V. 2007. A sodium ion-dependent A1AO ATP synthase from the hyperthermophilic archaeon Pyrococcus furiosus. FEBS J. 274:3928–3938. 10.1111/j.1742-4658.2007.05925.x. [DOI] [PubMed] [Google Scholar]
- 25.Kanai T, Ito S, Imanaka T. 2003. Characterization of a cytosolic NiFe-hydrogenase from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 185:1705–1711. 10.1128/JB.185.5.1705-1711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai T, Matsuoka R, Beppu H, Nakajima A, Okada Y, Atomi H, Imanaka T. 2011. Distinct physiological roles of the three [NiFe]-hydrogenase orthologs in the hyperthermophilic archaeon Thermococcus kodakarensis. J. Bacteriol. 193:3109–3016. 10.1128/JB.01072-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santangelo TJ, Cuboňová L, Reeve JN. 2011. Deletion of alternative pathways for reductant recycling in Thermococcus kodakarensis increases hydrogen production. Mol. Microbiol. 81:897–911. 10.1111/j.1365-2958.2011.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schut GJ, Nixon WJ, Lipscomb GL, Scott RA, Adams MW. 2012. Mutational analyses of the enzymes involved in the metabolism of hydrogen by the hyperthermophilic archaeon Pyrococcus furiosus. Front. Microbiol. 3:163–168. 10.3389/fmicb.2012.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schut GJ, Menon AL, Adams MW. 2001. 2-Keto acid oxidoreductases from Pyrococcus furiosus and Thermococcus litoralis. Methods Enzymol. 331:144–158. 10.1016/S0076-6879(01)31053-4. [DOI] [PubMed] [Google Scholar]
- 30.Heider J, Mai X, Adams MW. 1996. Characterization of 2-ketoisovalerate ferredoxin oxidoreductase, a new and reversible coenzyme A-dependent enzyme involved in peptide fermentation by hyperthermophilic archaea. J. Bacteriol. 178:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozawa Y, Nakamura T, Kamata N, Yasujima D, Urushiyama A, Yamakura F, Ohmori D, Imai T. 2005. Thermococcus profundus 2-ketoisovalerate ferredoxin oxidoreductase, a key enzyme in the archaeal energy-producing amino acid metabolic pathway. J. Biochem. 137:101–107. 10.1093/jb/mvi012. [DOI] [PubMed] [Google Scholar]
- 32.Mai X, Adams MW. 1994. Indolepyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. A new enzyme involved in peptide fermentation. J. Biol. Chem. 269:16726–16732. [PubMed] [Google Scholar]
- 33.Ozawa Y, Siddiqui MA, Takahashi Y, Urushiyama A, Ohmori D, Yamakura F, Arisaka F, Imai T. 2012. Indolepyruvate ferredoxin oxidoreductase: an oxygen-sensitive iron-sulfur enzyme from the hyperthermophilic archaeon Thermococcus profundus. J. Biosci. Bioeng. 114:23–27. 10.1016/j.jbiosc.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Mai X, Adams MW. 1996. Characterization of a fourth type of 2-keto acid-oxidizing enzyme from a hyperthermophilic archaeon: 2-ketoglutarate ferredoxin oxidoreductase from Thermococcus litoralis. J. Bacteriol. 178:5890–5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukui T, Atomi H, Kanai T, Matsumi R, Fujiwara S, Imanaka T. 2005. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 15:352–363. 10.1101/gr.3003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knappe J, Blaschkowski HP, Grobner P, Schmitt T. 1974. Pyruvate formate-lyase of Escherichia coli: the acetyl-enzyme intermediate. Eur. J. Biochem. 263:253–263. [DOI] [PubMed] [Google Scholar]
- 37.Buis JM, Broderick JB. 2005. Pyruvate formate-lyase activating enzyme: elucidation of a novel mechanism for glycyl radical formation. Arch. Biochem. Biophys. 433:288–296. 10.1016/j.abb.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Bagramyan K, Trchounian A. 2003. Structural and functional features of formate hydrogen lyase, an enzyme of mixed-acid fermentation from Escherichia coli. Biochemistry 68:1159–1170. [DOI] [PubMed] [Google Scholar]
- 39.Leonhartsberger S, Korsa I, Böck A. 2002. The molecular biology of formate metabolism in Enterobacteria. J. Mol. Microbiol. Biotechnol. 4:269–276. [PubMed] [Google Scholar]
- 40.Sato T, Fukui T, Atomi H, Imanaka T. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 185:210–220. 10.1128/JB.185.1.210-220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobori H, Ogino M, Orita I, Nakamura S, Imanaka T, Fukui T. 2010. Characterization of NADH oxidase/NADPH polysulfide oxidoreductase and its unexpected participation in oxygen sensitivity in an anaerobic hyperthermophilic archaeon. J. Bacteriol. 192:5192–5202. 10.1128/JB.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato T, Fukui T, Atomi H, Imanaka T. 2005. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl. Environ. Microbiol. 71:3889–3899. 10.1128/AEM.71.7.3889-3899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koop DR, Morgan ET, Tarr GE, Coon MJ. 1982. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J. Biol. Chem. 257:8472–8480. [PubMed] [Google Scholar]
- 44.Bashir Q, Rashid N, Jamil F, Imanaka T, Akhtar M. 2009. Highly thermostable L-threonine dehydrogenase from the hyperthermophilic archaeon Thermococcus kodakaraensis. J. Biochem. 146:95–120. 10.1093/jb/mvp051. [DOI] [PubMed] [Google Scholar]
- 45.Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263–267. 10.1155/2004/204953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santangelo TJ, Cubonová L, Skinner KM, Reeve JN. 2009. Archaeal intrinsic transcription termination in vivo. J. Bacteriol. 191:7102–7108. 10.1128/JB.00982-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.