Abstract
The Escherichia coli genome encodes approximately 30 two-component systems that are required for sensing and responding to a variety of environmental and physiological cues. Recent studies have revealed numerous regulatory connections between two-component systems and small noncoding RNAs (sRNAs), which posttranscriptionally regulate gene expression by base pairing with target mRNAs. In this study, we investigated the role of sRNAs in the CpxAR two-component system, which detects and mediates an adaptive response to potentially lethal protein misfolding in the Gram-negative bacterial envelope. Here, we showed for the first time that sRNAs are members of the Cpx regulon. We found that CpxR binds to the promoter regions and regulates expression of two sRNA genes, cyaR and rprA. We also investigated the roles that these sRNAs play in the Cpx response. Cpx repression of cyaR expression creates a feed-forward loop, in which CpxAR increases expression of the inner membrane protein YqaE both directly at the transcriptional level and indirectly at the translational level. Moreover, we found that RprA exerts negative feedback on the Cpx response, reducing Cpx activity in a manner that is dependent on the response regulator CpxR but independent of all of RprA's previously described targets. sRNAs therefore permit the fine-tuning of Cpx pathway activity and its regulation of target genes, which could assist bacterial survival in the face of envelope stress.
INTRODUCTION
Two-component systems (2CSs) are the primary means by which bacteria sense and respond to changes in their surroundings (1). Bacterial genomes frequently encode dozens of 2CSs, each of which detects a unique stimulus and performs a unique physiological role. Of the approximately 30 2CSs encoded in the Escherichia coli genome (2), the CpxAR 2CS is among the best characterized (reviewed in reference 3). The Cpx 2CS consists of the inner membrane (IM)-localized histidine kinase (HK) CpxA and the cytoplasmic response regulator (RR) CpxR. CpxA possesses two opposing enzymatic activities (4). In the presence of an inducing signal, CpxA acts as a kinase to phosphorylate CpxR at a conserved aspartate residue, thereby permitting CpxR to bind to DNA and modulate transcription. In the absence of an appropriate signal, CpxA acts as a CpxR∼P phosphatase, keeping CpxR dephosphorylated and therefore inactive.
The molecular nature of the signal sensed by CpxA remains unknown; however, several cues that induce the Cpx pathway have been identified. These include alkaline pH (5), alterations to the composition of the IM (6, 7), and ectopic expression of pilins such as PapE, PapG, and BfpA in the absence of their cognate chaperones (8, 9). All of these cues are expected to generate misfolded IM and/or periplasmic proteins; the Cpx system is therefore considered an envelope stress response (3). The Cpx pathway is also induced by overexpression of the outer membrane (OM) lipoprotein NlpE (10), which is believed to be an auxiliary regulator capable of sensing adhesion to hydrophobic surfaces (11). In accordance with the view of Cpx as an envelope stress response, many of the genes whose expression is most strongly increased by CpxR encode periplasmic protein folding and degrading factors, such as the protease/chaperone DegP (10), the disulfide bond oxidoreductase DsbA (12, 13), and CpxP, which functions as both a chaperone and a repressor of the Cpx response (14–16). CpxR also regulates a variety of other genes with envelope-related functions (3, 17); for example, expression of macromolecular complexes such as flagella and pili is repressed during the Cpx response, thereby reducing protein traffic to an already troubled periplasm.
2CSs can participate in regulatory networks by interacting with other types of regulators. Many such regulatory networks include small noncoding RNAs (sRNAs). sRNAs are regulatory molecules approximately 50 to 300 nucleotides in length (reviewed in references 18 and 19). The best-characterized type of sRNAs, trans-encoded sRNAs, act by base pairing with target mRNAs, using short regions of imperfect complementarity. This base pairing can have several different outcomes. sRNAs can negatively regulate expression of their target mRNAs by blocking ribosomal access to the mRNA's ribosome-binding site, thereby reducing translation, and/or by increasing degradation by RNases such as RNase E. Conversely, sRNAs can positively regulate mRNA expression by removing secondary structures in the mRNA that normally inhibit ribosome binding, thereby increasing translation, or by protecting the mRNA from degradation by RNases. Key to many of these activities is the RNA chaperone protein Hfq (reviewed in reference 20), which both stabilizes sRNAs and promotes annealing to their target mRNAs.
Interactions between 2CSs and sRNAs are numerous and can occur in both directions—2CSs can regulate the transcription of genes encoding sRNAs, while sRNAs can also regulate the translation and/or stability of mRNAs encoding 2CS components (reviewed in references 21 and 22). A prime example of a 2CS controlling the expression of sRNAs is EnvZ/OmpR, which activates the expression of three sRNA genes (micF, omrA, and omrB) and represses the expression of one sRNA gene (micC) (23–25). OmrA and OmrB, in turn, repress expression of the ompR mRNA, creating a negative feedback loop (26). Expression of mRNAs encoding 2CS proteins can also be regulated by sRNAs that are members of different regulons. Such is the case for the phoP mRNA, which encodes the RR of the PhoPQ 2CS. Expression of phoP is repressed by two sRNAs (MicA and GcvB), each of which is controlled by a different regulator (the alternative sigma factor σE and the transcription factors GcvA and GcvR, respectively), thereby allowing communication between these regulatory pathways (27, 28).
In this study, our aims were (i) to determine whether the Cpx regulon contains sRNAs and (ii) to determine whether any of these sRNAs regulate expression or activity of the Cpx 2CS. Preliminary evidence that CpxAR regulates the expression of sRNAs was obtained in a recent microarray examining changes in gene expression upon overexpression of NlpE (17). In this microarray, expression of several sRNA genes (micF, omrA, omrB, and rprA) was increased by NlpE overexpression, while expression of cyaR was repressed. Additional regulators and mRNA targets of these genes are already known and are summarized in Table S1 in the supplemental material. Interestingly, the majority of these sRNAs regulate the expression of mRNAs encoding envelope-localized proteins (indicated in bold in Table S1), which is in keeping with the role of the Cpx system as an envelope stress response. In the present work, we confirmed Cpx regulation of four of these sRNAs. We found that the Cpx response regulates the expression of cyaR and rprA. We show that CpxR binds to the promoters of these genes, consistent with a model in which it regulates their expression directly. We additionally found that these sRNAs endow the Cpx response with several regulatory network motifs, with CyaR participating in a feed-forward loop to regulate the IM protein YqaE and RprA participating in a novel feedback loop with CpxR.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table S2 in the supplemental material. Unless otherwise stated, strains were cultured in Luria-Bertani broth (LB) at 37°C with aeration at 225 rpm. Where indicated, isopropyl-β-d-thiogalactopyranoside (IPTG) (Invitrogen) was added to a concentration of 0.1 mM. Antibiotics (Sigma) were added where appropriate at the indicated concentrations: amikacin (Amk), 3 μg/ml; ampicillin (Amp), 100 μg/ml; chloramphenicol (Cam), 25 μg/ml; kanamycin (Kan), 30 μg/ml (E. coli K-12 strains) or 50 μg/ml (enteropathogenic E. coli [EPEC] strains); spectinomycin (Spc), 25 μg/ml; tetracycline (Tet), 10 μg/ml.
Strain and plasmid construction.
E. coli K-12 mutants and overexpression strains were constructed by standard techniques for P1 transduction and transformation (29). Donor strains harboring mutations in rcsC, mzrA, rpoS, csgD, ydaM, and ptsG were obtained from the Keio library (30). Where indicated, the kanamycin resistance cassette contained within these mutations was removed by Flp/Flp recombination target (FRT)-mediated recombination (31) to produce markerless deletions. EPEC strains were transformed by electroporation as previously described (32).
sRNA-lux transcriptional reporters were constructed as previously described (33). Briefly, promoters of sRNA genes were amplified by PCR, using the primer sequences listed in Table S3 in the supplemental material. Purified PCR products and the pJW15 lux reporter vector (34) were digested with BamHI and EcoRI, gel purified, and ligated together. Correct insertion of promoter sequences was verified by PCR and sequencing. In addition, sRNA-lux reporters were transformed into strains harboring mutations in known regulators of each gene (cyaA for cyaR-lux; ompR for micF-lux, omrA-lux, and omrB-lux; and rcsB for rprA-lux); regulator mutations affected expression of all lux reporters as expected based on published results (24, 25, 35–37; also data not shown).
The cyaR::Kan mutation in strain SV514 was constructed by λ Red recombination (38). The FRT-flanked kanamycin resistance cassette was amplified from the Keio library using primers cyaRKOFor and cyaRKORev (see Table S3 in the supplemental material). The purified PCR product was electroporated into strain DY378, which encodes λ Red recombinase functions. cyaR::Kan mutations in kanamycin-resistant transformants were verified by PCR. The cyaR::Kan cassette was then transduced into yqaE′-lacZ reporter strain NRD397.
The sRNA overexpression plasmids were obtained from the Gottesman lab's library. All plasmids were shown by Northern blotting to cause accumulation of the encoded sRNA, as expected (39).
Luminescence assays.
Activity of lux reporters was measured as previously described (40). Strains were cultured overnight in LB at 30°C with aeration and then subcultured 1:100 into fresh LB and grown at 37°C with aeration for 4 h (with IPTG induction after 2 h if necessary). Normalized luminescence was determined by dividing raw luminescence (in counts per second [cps]) by the optical density at 600 nm (OD600) of the same culture. Luminescence assays were performed two or three times, with five replicate cultures each time.
Electrophoretic mobility shift assays (EMSAs).
Maltose binding protein (MBP)-CpxR was purified from JM109(pMCR) as described previously (4) with a few modifications. First, cells were disrupted by passage though a French pressure cell once at 20,000 lb/in2. Second, the crude extract was incubated with the amylose resin overnight with gentle agitation for batch protein purification. This mixture was then poured into a column for subsequent washing and elution steps. MBP-CpxR at the indicated concentration was incubated in the presence of acetyl phosphate (20 mM) at 37°C for 30 min in 15 μl of binding buffer (10 mM Tris [pH 7.4], 50 mM KCl, 1 mM EDTA, 5% glycerol, 50 μg/ml bovine serum albumin [BSA], 1 mM dithiothreitol [DTT], 20 mM potassium glutamate, 10 mM MgSO4). Next, 1.5 pmol of purified, PCR-amplified promoter DNA was added and the mixture was incubated for another 30 min at 37°C. Reactions were stopped by the addition of 6× DNA loading dye (0.25% bromophenol blue, 0.25% xylene cyanol FF, 30% glycerol in water). Reactions were electrophoresed on a 5% nondenaturing TBE polyacrylamide gel (Bio-Rad) in 1× TBE running buffer (89 mM Tris, 89 mM boric acid, 1 mM EDTA [pH 8.0]). DNA was visualized with an ethidium bromide stain.
β-Galactosidase assays.
Strains to be assayed were cultured overnight in LB containing appropriate antibiotics at 37°C with aeration. For the PBAD::yqaE′-lacZ reporter experiments, strains were subcultured 1:200 in fresh LB with antibiotics and grown at 37°C with shaking for 6 h; arabinose was added to a final concentration of 0.01% 4 h postsubculture to induce reporter expression. For all other experiments, strains were subcultured 1:50 into fresh LB with antibiotics and grown at 37°C with shaking to early stationary phase (5 h). Strains harboring sRNA overexpression plasmids were induced with IPTG after 3 h. β-Galactosidase activity was measured as previously described (41), with 5 μl of cell culture being added to 195 μl of 1× Z buffer for strains carrying the cpxP-lacZ reporter gene due to high reporter activity. Each strain was assayed in triplicate.
Western blot analysis.
Subcultures for whole-cell lysates of bacterial strains used for Western blot analysis were prepared by diluting overnight cultures 1:50 into 5 ml fresh LB containing appropriate concentrations of antibiotics. Cultures were grown to early stationary phase (5 h) at 37°C with shaking, with induction of expression plasmids after 3 h of growth by addition of IPTG. One-milliliter samples, standardized to the same optical density at 600 nm, were pelleted, and cell pellets were lysed in 50 μl of 2× SDS-PAGE loading dye (125 mM Tris [pH 6.8], 20% glycerol, 10% β-mercaptoethanol, 6% sodium dodecyl sulfate, 0.2% bromophenol blue). Electrophoresis and blotting were performed as previously described (15) with rabbit anti-MBP-CpxR (1:10,000 dilution) or anti-MBP-CpxA (1:50,000 dilution) primary antibodies and alkaline phosphatase–anti-rabbit secondary antibodies (Sigma; 1:25,000 dilution). Proteins were detected by chemiluminescence using a Bio-Rad ChemiDoc MP imaging system and an Immun-Star alkaline phosphatase chemiluminescence kit (Bio-Rad). Western blots were exposed using a cumulative signal acquisition mode, and the longest exposure in which no pixels were saturated was selected for band intensity analysis.
RESULTS
In order to confirm the preliminary microarray results indicating that the sRNA genes cyaR, micF, omrA, omrB, and rprA are members of the Cpx regulon (17), we examined the effect of Cpx pathway activation and inactivation upon expression of these genes using lux transcriptional reporters. We were able to confirm Cpx regulation, either direct or indirect, of four of the five genes: omrA, omrB, cyaR, and rprA; expression of the micF-lux reporter was not consistently altered by activation or inactivation of the Cpx response (data not shown). We also confirmed that expression of both omrA-lux and omrB-lux was activated by the Cpx response indirectly via the Cpx-regulated connector protein MzrA and the EnvZ/OmpR 2CS as previously reported (reference 42 and data not shown). We therefore chose to focus on cyaR and rprA, as they represented potentially novel Cpx regulon members. Unless otherwise specified, we use the term “regulation” to include both direct (binding of CpxR to a gene's promoter) and indirect (changes in gene expression mediated through any means other than direct binding of CpxR to a gene's promoter) mechanisms of regulation throughout the remainder of this paper.
Cpx regulation of cyaR.
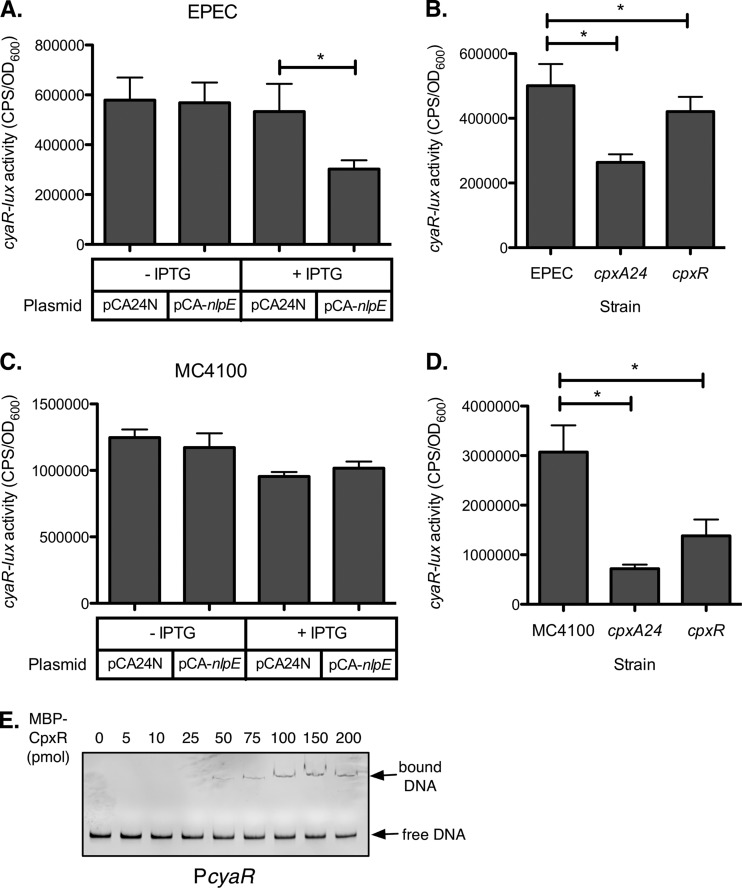
In the microarray, Cpx activation via nlpE overexpression decreased cyaR expression to one-third of the uninduced control in enteropathogenic E. coli (EPEC), while no significant change in cyaR expression was observed upon nlpE overexpression in E. coli strain MC4100 (17). Using a cyaR-lux transcriptional reporter, we confirmed that nlpE overexpression represses cyaR expression in EPEC (Fig. 1A) but has an effect indistinguishable from that of the vector control in MC4100 (Fig. 1C). Activation of the Cpx response by a different means (using the gain-of-function allele cpxA24) repressed cyaR-lux activity in both EPEC and MC4100 (Fig. 1B and D, respectively). Interestingly, inactivation of the Cpx response through mutation of cpxR also decreased cyaR-lux expression (Fig. 1B and D), although the effect of the cpxR mutation was consistently smaller than the effect of the cpxA24 mutation.
FIG 1.
CpxR regulates expression of cyaR. (A and C) Luminescence assays comparing cyaR-lux reporter expression in wild-type EPEC (A) or MC4100 (C) carrying vector control pCA24N or the overexpression plasmid pCA-nlpE. (B and D) Luminescence assays comparing cyaR-lux reporter expression in wild-type, cpxA24 (Cpx-activating mutation), and cpxR (Cpx-inactivating mutation) strains of EPEC (B) or MC4100 (D). Luminescence was normalized to the OD600 of the culture. Data for luminescence assays represent the means and standard deviations of five replicate cultures. Asterisks denote a statistically significant difference from the relevant wild-type or vector control (P < 0.05, one-way ANOVA with Bonferroni's multiple-comparison test). (E) EMSA. A PCR product containing the cyaR promoter region was incubated alone or with increasing concentrations of MBP-CpxR∼P protein and then subjected to 5% native polyacrylamide electrophoresis. DNA was detected with ethidium bromide staining.
To explain the observation that cyaR expression is decreased both when the Cpx response is activated and when Cpx is inactivated, we hypothesized that Cpx might exert both direct and indirect regulatory effects on cyaR. cyaR is also known to be regulated by catabolite repression (35, 36). We therefore examined the effects of cpx mutations upon cyaR-lux expression under high-glucose conditions where cyclic AMP (cAMP) levels would be expected to be low, and therefore cAMP receptor protein (CRP)/cAMP would have little effect on cyaR expression. Figure S2A in the supplemental material shows that, under high-glucose conditions, the cpxA24 mutation still represses cyaR-lux expression, while the cpxR mutation no longer has an effect, suggesting that the cpxR mutation affects cyaR expression via cAMP/CRP. We then searched the microarray results to find any Cpx-regulated genes that might affect levels of cAMP. Indeed, the PtsG and UhpT glucose transporters are negatively regulated by the Cpx response (17). In support of the hypothesis that the cpxR mutation affects cyaR expression via effects on cAMP levels, we found that deletion of cpxR did not affect cyaR expression in a ptsG mutant background (see Fig. S2B). We therefore believe that the reduced cyaR expression in the cpxR mutant is secondary to altered expression of glucose transporters in this strain.
We hypothesized that the repression of cyaR expression in the cpxA24 mutant could be the result of direct binding of phosphorylated CpxR to the cyaR promoter. In order to examine this possibility, we performed an electrophoretic mobility shift assay (EMSA) to assess the ability of a purified, phosphorylated MBP-CpxR (MBP-CpxR∼P) fusion protein to bind to the cyaR promoter in vitro. This MBP-CpxR fusion has previously been shown to complement a cpxR mutation (4). We found that addition of 50 pmol or more of MBP-CpxR∼P to the cyaR promoter DNA caused the appearance of a distinct CpxR-DNA complex (Fig. 1E). The cyaR promoter had lower affinity for MBP-CpxR∼P than the positive control, cpxP, which gave a shifted band with 25 pmol of protein (see Fig. S1A in the supplemental material), but higher affinity than the negative control, rpoD, which did not bind to MBP-CpxR∼P unless at least 100 pmol of protein was added (see Fig. S1B). We therefore hypothesize that CpxR weakly binds the cyaR promoter region, which is consistent with the relatively small change in expression of cyaR when the Cpx response was activated (Fig. 1A to D).
Cpx regulation of both cyaR and yqaE creates a feed-forward loop.
We next turned to the question of the role of CyaR in the Cpx response. Many sRNAs exert feedback regulation on their own regulator (43); we examined whether CyaR might affect Cpx pathway activity by transforming a cyaR overexpression plasmid into a cpxP-lacZ reporter strain. No change in activity of the cpxP-lacZ reporter was observed when CyaR was overexpressed relative to the empty vector control (data not shown). sRNAs also participate in feed-forward loops, in which a regulator controls expression of a target gene both directly (by binding to its promoter) and indirectly, by regulating expression of a third gene, which is itself a regulator of the target gene (43). We identified a potential feedforward loop consisting of CpxR, CyaR, and the IM protein YqaE. Transcription of the yqaE gene was previously shown to be activated by the Cpx response (17), while translation of yqaE is known to be repressed by CyaR (35). Combined with our results from Fig. 1, these data suggested that CpxR could increase expression of yqaE both directly, by binding to its promoter, and indirectly, by decreasing expression of cyaR.
In order to address whether CpxR directly regulates transcription of yqaE, we performed an EMSA. We found that MBP-CpxR∼P binds to the yqaE promoter with similar affinity as to the cpxP positive control (Fig. 2A; see also Fig. S1A in the supplemental material), suggesting that the previously reported transcriptional regulation of yqaE is direct. To assess whether the Cpx response regulates translation of yqaE, we used a previously described PBAD::yqaE′-lacZ translational reporter (35). Transcription of this construct is driven by the arabinose-inducible PBAD promoter and is therefore not subject to regulation by CpxR. When the Cpx response was activated by the plasmid pCA-nlpE, expression of the yqaE translational reporter increased approximately 2-fold relative to the vector control (Fig. 2B). The majority of the Cpx enhancement of yqaE translation was CyaR dependent, since pCA-nlpE increased reporter expression only ∼1.2-fold in a cyaR::Kan mutant strain (Fig. 2B). Our data suggest that Cpx repression of cyaR expression gives rise to a coherent feed-forward loop, in which CpxR both directly and indirectly activates yqaE expression.
FIG 2.
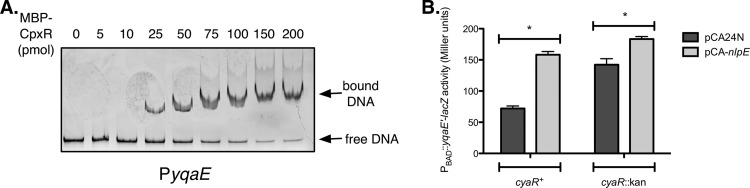
CyaR participates in a feed-forward loop regulating expression of yqaE. (A) EMSA. A PCR product containing the yqaE promoter region was incubated alone or with increasing concentrations of MBP-CpxR∼P protein and then subjected to 5% native polyacrylamide electrophoresis. DNA was detected with ethidium bromide staining. (B) β-Galactosidase assay comparing expression of a PBAD::yqaE′-lacZ translational reporter in the presence of the vector control pCA24N or the nlpE overexpression plasmid pCA-nlpE, in strains harboring a wild-type copy of cyaR or a cyaR::Kan mutation. Data represent the means and standard deviations of three replicate cultures. Asterisks denote a statistically significant difference from the relevant vector control (P < 0.05, one-way ANOVA with Bonferroni's multiple-comparison test).
Cpx regulation of rprA.
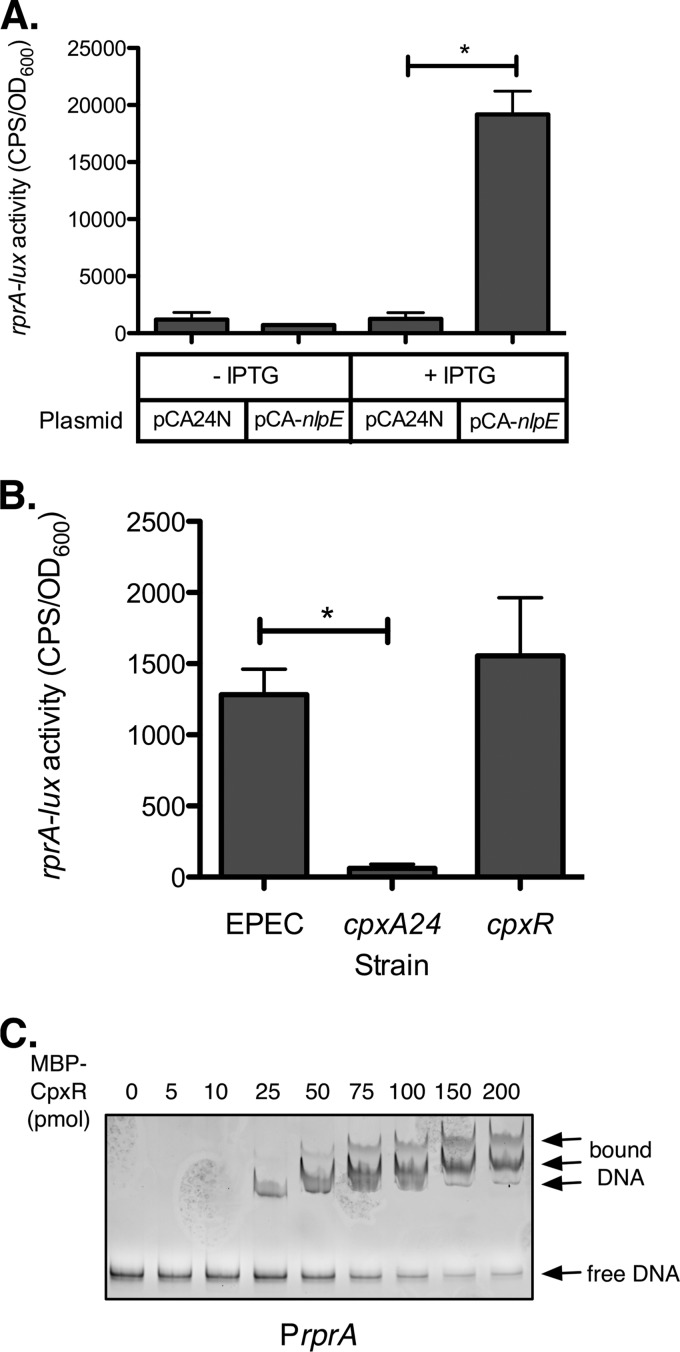
Microarray analysis showed that rprA expression was increased almost 8-fold when the Cpx pathway was activated by nlpE overexpression in EPEC, while the rprA transcript was not detected in the MC4100 microarrays (17). Therefore, we initially characterized the effect of activating and inactivating the Cpx response on rprA-lux activity in EPEC. In concordance with the microarray results, overexpression of nlpE activated expression of the rprA-lux reporter more than 15-fold relative to the vector control (Fig. 3A). Unexpectedly, activation of the Cpx response using the cpxA24 mutation had the opposite effect on the rprA-lux reporter, strongly repressing its expression (Fig. 3B). Inactivation of the Cpx response by mutation of cpxR had little effect on the rprA-lux reporter (Fig. 3B). EMSA showed that MBP-CpxR∼P bound to the rprA promoter region (Fig. 3C), with similar affinity as for the cpxP promoter positive control (see Fig. S1A in the supplemental material). Three distinct shifted bands were observed when MBP-CpxR∼P was added to the rprA promoter DNA (Fig. 3C), suggesting the presence of multiple CpxR binding sites in the promoter region.
FIG 3.
CpxR regulates expression of rprA in EPEC. (A) Luminescence assays comparing rprA-lux reporter expression in wild-type EPEC carrying vector control pCA24N or the overexpression plasmid pCA-nlpE. (B) Luminescence assays comparing rprA-lux reporter expression in wild-type EPEC and cpxA24 (Cpx-activating mutation) and cpxR (Cpx-inactivating mutation) strains of EPEC. Luminescence was normalized to the OD600 of the culture. Data for luminescence assays represent the means and standard deviations of five replicate cultures. Asterisks denote a statistically significant difference from the relevant wild-type or vector control (P < 0.05, one-way ANOVA with Bonferroni's multiple-comparison test). (C) EMSA. A PCR product containing the rprA promoter region was incubated alone or with increasing concentrations of MBP-CpxR∼P protein and then subjected to 5% native polyacrylamide electrophoresis. DNA was detected with ethidium bromide staining.
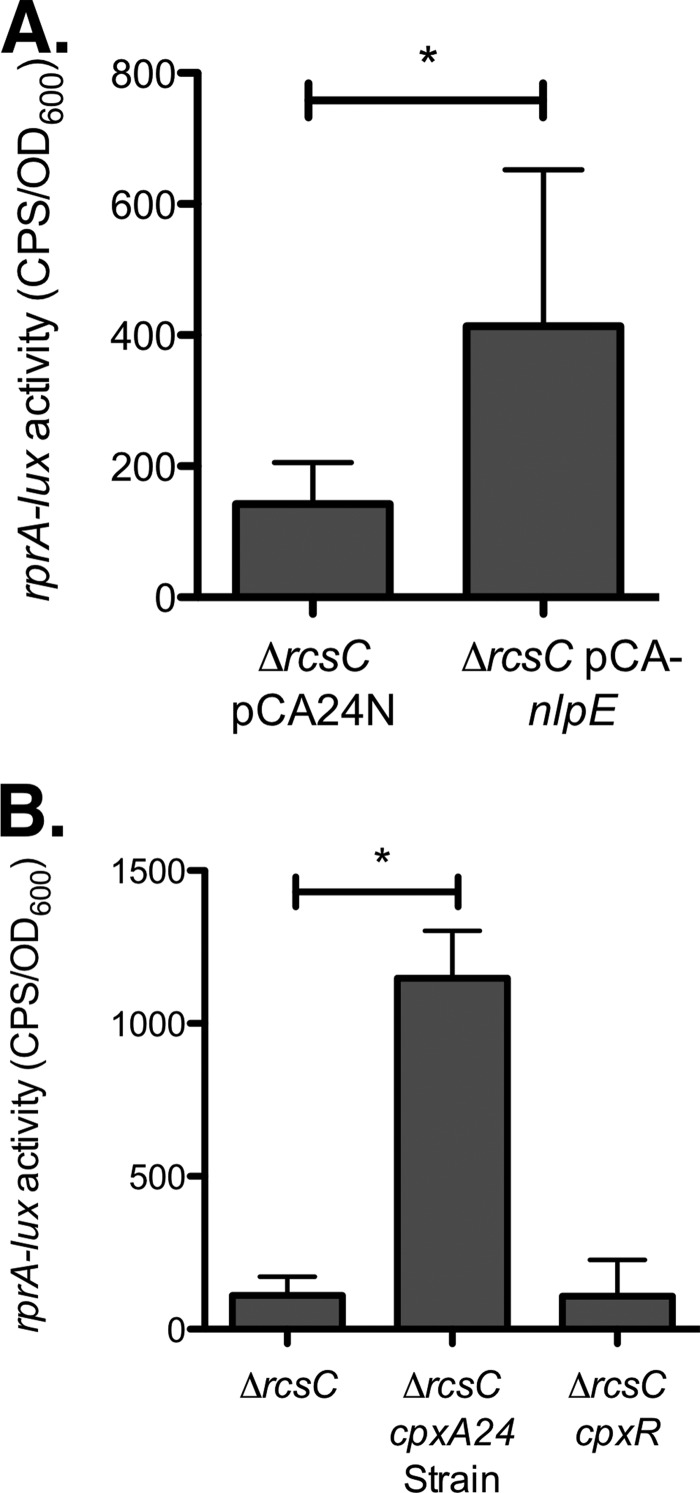
To gain more insight into Cpx regulation of rprA, we repeated the rprA-lux experiments in MC4100 wild-type and cpx mutant strains; however, no reporter activity was observed in any of the strains (data not shown), consistent with previous findings by microarray (17). In an attempt to boost reporter activity to detectable levels, we introduced a ΔrcsC mutation. This HK mutation increases activity of the Rcs pathway and was previously shown to elevate rprA expression in the E. coli MG1655 background (37). Mutation of rcsC has the additional benefit of eliminating any indirect effects of Cpx activation on Rcs signaling. In the MC4100 ΔrcsC background, we were able to detect a low level of activity of the rprA-lux reporter (Fig. 4). Critically, in the absence of a functional Rcs phosphorelay, both overexpression of nlpE and the cpxA24 mutation increased rprA-lux activity (Fig. 4A and B, respectively). We therefore concluded that activation of the Cpx response enhances rprA expression, possibly as a result of direct binding of CpxR to the rprA promoter. Although we currently do not understand why the cpxA24 allele leads to inhibition of rprA transcription in EPEC, we believe that unidentified connections between the Cpx and Rcs pathways, complex regulation involving multiple regulators, and/or strain differences may be involved (see Discussion).
FIG 4.
The Cpx response regulates rprA expression independently of the Rcs pathway in E. coli MC4100. (A) Luminescence assays comparing rprA-lux reporter expression in MC4100 ΔrcsC carrying vector control pCA24N or the overexpression plasmid pCA-nlpE. (B) Luminescence assays comparing rprA-lux reporter expression in ΔrcsC, ΔrcsC cpxA24 (Cpx-activating mutation), and ΔrcsC cpxR (Cpx-inactivating mutation) strains of MC4100. Luminescence was normalized to the OD600 of the culture. Data for luminescence assays represent the means and standard deviations of five replicate cultures. Asterisks denote a statistically significant difference from the relevant wild-type or vector control (P < 0.05, one-way ANOVA with Bonferroni's multiple-comparison test).
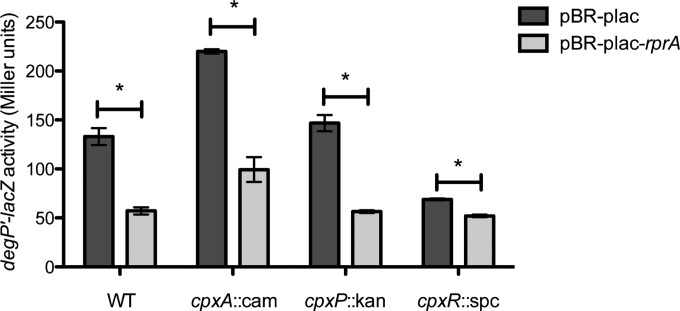
RprA overexpression inhibits the Cpx response.
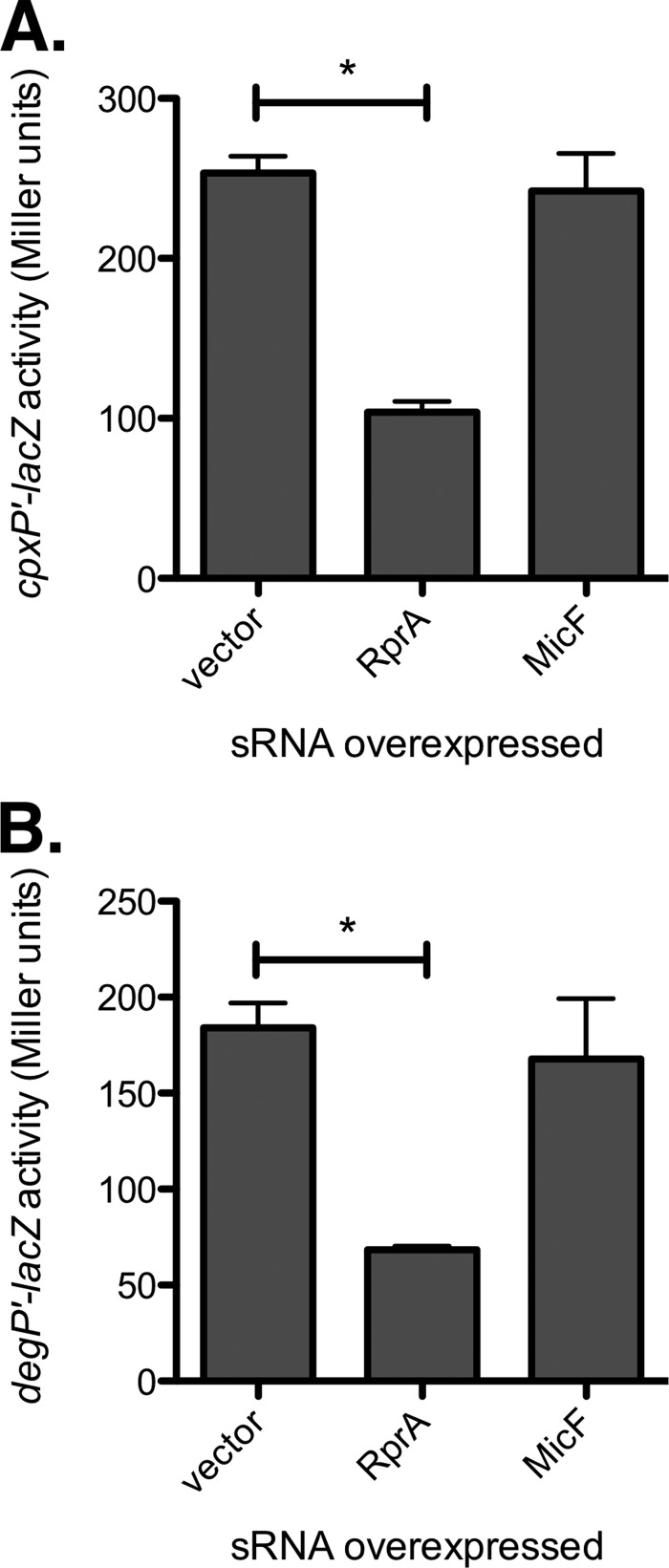
To address the function of RprA in the Cpx response, we examined whether RprA can exert feedback regulation on the Cpx pathway. The effect of overexpression of RprA on Cpx pathway activity was measured using cpxP-lacZ and degP-lacZ reporters. The sRNA MicF, although not a member of the Cpx regulon (data not shown), was included in these experiments as a control because it has previously been shown to repress cpxR translation (44). Overexpression of RprA significantly repressed activity of both the cpxP-lacZ and the degP-lacZ reporter (Fig. 5) (P < 0.05, one-way analysis of variance [ANOVA] with Bonferroni's multiple-comparison test). Since degP is transcriptionally activated by both the Cpx 2CS and the σE stress response (10, 45), we also assayed expression of a uniquely σE-controlled transcriptional reporter, rpoHP3-lacZ, and found that it was not substantially inhibited by RprA overexpression (see Fig. S3 in the supplemental material). Surprisingly, MicF overexpression did not repress expression of any of the reporters (Fig. 5; see also Fig. S3).
FIG 5.
Overexpression of rprA inhibits Cpx pathway activity. Effect of micF and rprA overexpression on activity of the cpxP-lacZ (A) and degP-lacZ (B) transcriptional reporters. Asterisks denote a statistically significant difference from the vector control (P < 0.05, one-way ANOVA with Bonferroni's multiple-comparison test). Data represent the means and standard deviations of three replicate cultures.
We used Western blotting to examine the effects of the sRNAs on CpxA and CpxR protein abundance. The blots showed that overexpression of RprA did not affect the intracellular abundance of either of these proteins (Fig. 6). This result implies that repression of the Cpx pathway by RprA does not result from direct translational inhibition of either cpxA or cpxR. In agreement with previous results showing that MicF overexpression reduces translation of cpxR (44), we observed a significant decrease in CpxR protein levels upon overexpression of MicF (Fig. 6). We additionally found a previously unreported decrease in CpxA protein levels upon MicF overexpression (Fig. 6).
FIG 6.

Overexpression of rprA does not decrease CpxA or CpxR protein levels. The effect of overexpressing MicF and RprA on CpxA and CpxR protein levels was measured by Western blotting with primary antibodies against either CpxA (A) or CpxR (B), with a nonspecific band (NSB) as a loading control. Quantification of CpxA and CpxR bands was performed using a ChemiDoc MP imager (Bio-Rad) with Image Lab software and is shown beneath the images.
Cpx pathway inhibition by RprA is independent of RprA's known targets and dependent on CpxR.
In order to determine whether inhibition of the Cpx pathway by RprA occurred through one of RprA's known targets, rpoS, ydaM, and csgD (46–48), we deleted each of these individually and assayed for a disappearance of Cpx repression upon rprA overexpression. Overexpression of RprA in E. coli W3110 strains harboring deletions of rpoS, ydaM, or csgD still resulted in a decrease in cpxP-lacZ reporter activity (Fig. 7), suggesting that inhibition of the Cpx pathway by RprA does not occur via its regulation of these target genes.
FIG 7.
Inhibition of the Cpx pathway by RprA is not dependent on any of RprA's known target genes. β-Galactosidase assay showing the effect of rprA overexpression on activity of a cpxP-lacZ reporter in wild-type (WT) and rpoS, ydaM, and csgD mutant strains. Data represent the means and standard deviations of three replicate cultures. Asterisks indicate P < 0.05 (one-way ANOVA with Bonferroni's multiple-comparison test).
Since RprA does not appear to inhibit the Cpx response by acting directly on the transcripts of cpxA or cpxR, or through any of its published targets, we sought to determine whether inhibition of Cpx activity by RprA involves sensing of an envelope-localized inducing cue by CpxA by assessing whether mutations to cpxA, cpxP, or cpxR in E. coli W3110 abolished inhibition of the Cpx pathway by RprA. Experiments with a degP-lacZ reporter revealed that RprA overexpression still obviously repressed the Cpx pathway in cpxA::Cam and cpxP::Kan strains (Fig. 8). In contrast, although RprA overexpression in the absence of cpxR still led to a statistically significant decrease in degP-lacZ expression, this decrease was greatly diminished (Fig. 8). In fact, inhibition disappeared almost entirely in a cpxR::Spc strain (Fig. 8). These data suggested that inhibition by RprA occurs not through its regulation of some envelope-localized component sensed by CpxA but, rather, predominantly by signaling through CpxR.
FIG 8.
Inhibition of the Cpx pathway by RprA is CpxR dependent but CpxA independent. β-Galactosidase assay showing the effect of rprA overexpression on activity of a degP-lacZ reporter in wild-type (WT), cpxA::Cam, cpxP::Kan, and cpxR::Spc mutant strains. Data represent the means and standard deviations of three replicate cultures. Asterisks indicate P < 0.05 (one-way ANOVA with Bonferroni's multiple-comparison test).
It has previously been shown that phosphorylation of CpxR can occur in a CpxA-independent manner by the low-molecular-weight phosphodonor acetyl phosphate, a product of the Pta-AckA pathway (49). Thus, we investigated the possibility that Cpx inhibition by RprA occurs through the Pta-AckA pathway by deleting both pta and ackA and measuring inhibition of the cpxP-lacZ reporter by RprA. No difference between the wild-type strain and the pta-ackA::Tn10 strain was observed upon RprA overexpression (see Fig. S4 in the supplemental material). Therefore, RprA inhibits the Cpx pathway via CpxR in a Pta-AckA-independent manner.
DISCUSSION
Two-component systems and sRNAs are both widely used by bacteria to regulate gene expression in response to environmental changes. In recent years, many connections between these two types of regulators have been revealed, with at least six of E. coli's 30 2CSs shown to regulate the expression of one or more sRNAs, and numerous sRNAs demonstrated to directly or indirectly regulate 2CS activity (21). In this work, we showed that the Cpx 2CS regulates the expression of at least four sRNA genes: cyaR, rprA, omrA, and omrB (Fig. 9). We found that CpxR regulates expression of cyaR and rprA in a manner consistent with direct binding to their promoters. These Cpx-regulated sRNAs create new regulatory motifs not previously identified within the Cpx response, including both feed-forward and negative feedback loops (Fig. 9).
FIG 9.
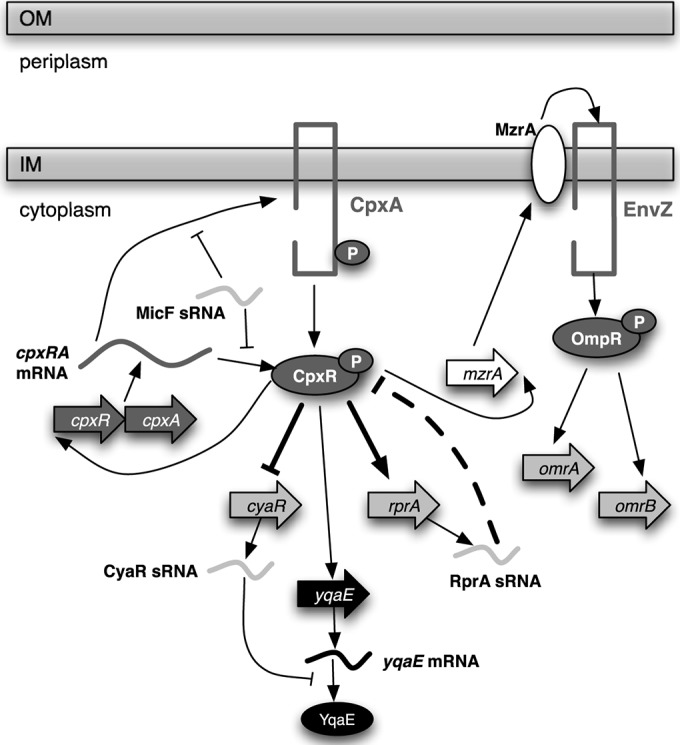
Model of regulatory connections between the Cpx two-component system and sRNAs. In the presence of envelope stress, the CpxA histidine kinase autophosphorylates and then phosphorylates the response regulator CpxR. CpxR directly regulates the transcription of two sRNA genes: cyaR transcription is repressed, while rprA transcription is either activated or repressed, depending on the growth phase. CpxR indirectly regulates the transcription of the sRNAs OmrA and OmrB by activating transcription of the inner membrane protein MzrA; MzrA physically interacts with EnvZ, increasing the activity of the EnvZ/OmpR two-component system, which activates omrA and omrB transcription. CpxR regulates the expression of the inner membrane protein YqaE in two ways: directly at the transcriptional level and indirectly at the translational level through repression of CyaR, creating a feed-forward loop. Two sRNAs affect expression or activity of the Cpx response: RprA indirectly represses activity of CpxR by an unknown mechanism (indicated by a dashed line), while MicF represses translation of the cpxRA mRNA. Boldface arrows and lines indicate regulatory connections described for the first time in this work. OM, outer membrane; IM, inner membrane; P, phosphate.
Cpx regulation of sRNA expression.
Regulation of both cyaR and rprA by the Cpx response is complex. In the case of cyaR, both activation and inactivation of the Cpx response decrease cyaR expression (Fig. 1). We believe that the decreased cyaR expression in the cpxR mutant results from secondary effects on glucose transporters, since mutation of cpxR does not affect cyaR expression either in high-glucose medium or in the absence of the glucose transporter ptsG (see Fig. S2 in the supplemental material). Microarray results suggest that when the Cpx response is inactivated in the cpxR mutant, expression of PtsG and UhpT increases (17), increasing the rate of glucose transport into the cell. The increased cellular glucose concentration would decrease cAMP production and thereby decrease cyaR expression. The role of the Cpx response in sugar transport is further supported by the recent finding that CpxR represses expression of the glucose 6-phosphate transporter UhpT in enterohemorrhagic E. coli (50). In contrast, activation of the Cpx response does not appear to repress cyaR expression via effects on catabolite repression, since the cpxA24 mutation still reduces cyaR-lux activity under high-glucose conditions (see Fig. S2A). The simplest explanation for these results is that phosphorylated CpxR represses cyaR expression through direct binding to its promoter, since CpxR can bind the cyaR promoter in vitro (Fig. 1E). However, we cannot formally rule out indirect effects of Cpx pathway activation on cyaR expression.
Our initial experiments with rprA in EPEC showed that activating the Cpx response using two different methods produced opposite effects on rprA expression—nlpE overexpression increased rprA-lux activity, while the cpxA24 mutation repressed rprA expression (Fig. 3). When these experiments were repeated in the MC4100 ΔrcsC mutant background, we found that both nlpE overexpression and the cpxA24 mutation increased rprA-lux expression (Fig. 4). Since CpxR also binds to the rprA promoter in vitro (Fig. 3C), we favor the explanation that binding of CpxR to the rprA promoter enhances rprA expression (although, again, we cannot exclude the possibility of indirect regulation at this time). In this case, the rprA repression observed in the EPEC cpxA24 mutant could result from indirect effects on Rcs activity. In support of this idea, Evans and colleagues (51) have recently shown that the Cpx response affects Rcs activity in response to peptidoglycan alterations. Thus, it seems likely that the Cpx response can interact with the Rcs pathway through an unknown mechanism. In addition, in silico analysis indicates that other regulators in addition to RcsB and CpxR may bind to the rprA promoter region; we are currently investigating whether any of these other regulators may play a role in the complex, strain-dependent regulation of rprA.
CyaR-dependent feed-forward loop.
Interactions between regulators can produce regulatory network motifs that have unique properties. One of these motifs is the feed-forward loop, in which a regulator controls the expression of a target gene both directly (by binding to its promoter) and indirectly (by regulating another regulator that also affects expression of the target) (43, 52). Feedforward loops can be classified as either coherent, if the direct and indirect routes of regulation are both positive or both negative, or incoherent, if one route is positive and the other negative (52). sRNAs have been shown to participate in both coherent and incoherent feed-forward loops (53). We have identified a coherent feed-forward loop, in which CpxR activates expression of yqaE both directly, by binding to its promoter and increasing yqaE transcription (Fig. 2A) (17), and indirectly, by repressing expression of cyaR and thereby increasing translation of yqaE (Fig. 2B) (35). A similar feed-forward loop involving the sRNA Spot42 was recently shown to reduce leaky expression of its target genes in the absence of an activating cue (54). Thus, CyaR could help to reduce leaky expression of yqaE under Cpx-inactive conditions. Although the function of YqaE is currently unknown, it was shown that a yqaE mutant was more resistant to several envelope-damaging compounds in a Biolog phenotype microarray (17), suggesting that yqaE expression makes cells more sensitive to some toxic agents. Therefore, CyaR repression of yqaE could help to ensure that yqaE is expressed only when the Cpx response is fully activated.
sRNAs affecting Cpx pathway activity.
Recent studies have identified numerous sRNAs that affect the expression or activity of 2CSs (21). Our data, combined with those of Holmqvist et al. (44), indicate that several sRNAs affect the Cpx pathway (Fig. 9), with RprA mediating feedback regulation and MicF possibly permitting other regulators to communicate with the Cpx response.
Our data demonstrate that RprA exerts negative feedback on the Cpx response without directly affecting expression of CpxR or CpxA. Overexpression of RprA decreased expression of two Cpx-regulated genes (Fig. 5) but did not affect the abundance of CpxR and CpxA proteins (Fig. 6). Furthermore, RprA overexpression did not affect activity of a cpxR′-′gfp translational fusion containing the 5′ untranslated region (UTR) and first 20 codons of cpxR (data not shown); however, the ability of RprA to interact with other regions of the cpxR coding sequence remains to be thoroughly tested. These results suggest that RprA modulates the activity of the Cpx pathway rather than its expression. Since signals can enter the Cpx pathway through numerous signaling components (3), we overexpressed RprA in mutants lacking the Cpx system components CpxP, CpxA, and CpxR in order to gain more information about the mechanism of RprA feedback regulation. This experiment showed that RprA's effects on the Cpx pathway were dependent on CpxR but not on CpxA (Fig. 8). Surprisingly, RprA-mediated repression was not dependent on the Pta-AckA pathway (see Fig. S4 in the supplemental material), which was previously shown to be the major CpxA-independent source of CpxR phosphorylation when cells are grown in the presence of excess glucose (5). Furthermore, RprA repression of Cpx activity was not dependent on any of the known RprA targets rpoS, ydaM, and csgD (Fig. 7), suggesting that additional targets of RprA regulation remain to be identified.
There are several mechanisms by which RprA could conceivably influence the activity of CpxR without altering its expression. One possibility is that RprA regulates a noncognate HK (i.e., not CpxA) that is capable of cross-phosphorylating CpxR. We do not favor this possibility, however, since cross talk between 2CSs has been shown to be unlikely to occur in vivo (55, 56). Another exciting possibility is that RprA regulates the expression of a novel auxiliary regulator that influences CpxR activity. Such auxiliary regulators can affect the activity of an RR by modulating its rate of phosphorylation or dephosphorylation or its ability to bind to DNA (57). Identification of the mechanism by which RprA influences Cpx pathway activity could therefore shed light on both the cellular role of this sRNA and on the regulation of 2CS activity.
Holmqvist and colleagues previously reported that MicF decreases expression of a cpxR-gfp translational fusion (44), in agreement with our finding that overexpression of MicF decreases the abundance of both CpxR and CpxA (Fig. 6). Curiously, despite this decrease in Cpx protein levels, overexpression of micF did not significantly decrease expression of the Cpx-regulated genes cpxP and degP (Fig. 5). We considered the possibility that MicF overexpression affects Cpx activity only under pathway-activating conditions, since gene regulation by CpxR depends not only on its abundance but also on its phosphorylation status. However, when we repeated the β-galactosidase assay from Fig. 5A in LB at pH 8, which is an inducing condition for the Cpx pathway (5), we still found no effect of MicF overexpression on cpxP-lacZ activity (data not shown). Thus, the significance of MicF regulation of cpxRA is currently unclear. One possibility that we are currently investigating is that MicF overexpression has differential effects on Cpx regulon members—decreased levels of CpxR could have a larger effect on its ability to bind to low-affinity promoters than its ability to bind to high-affinity promoters like cpxP. If this hypothesis is correct, MicF could provide a mechanism for its regulators, including EnvZ/OmpR and Lrp, to restrict the size of the Cpx regulon to include only select targets.
In summary, we have demonstrated that sRNAs comprise a previously unrecognized part of the Cpx regulon, with roles in both feed-forward and feedback regulation. In addition, multiple sRNAs affect the expression or activity of CpxR. These results demonstrate that sRNAs link the Cpx response to a variety of other cellular regulators. Such regulatory connections may play an important role in E. coli's ability to withstand envelope stress.
Supplementary Material
ACKNOWLEDGMENTS
We thank Susan Gottesman for providing strain NRD397 and the pBR-plac sRNA overexpression plasmids.
This work was supported by the Canadian Institute of Health Research operating grant 97819. S.L.V. was supported by a Natural Sciences and Engineering Research Council Post-Graduate Scholarship, an Alberta Ingenuity Graduate Scholarship, and an Izaak Walton Killam Memorial Scholarship. T.L.R. was supported by a Senior Scholar Award from Alberta Innovates Health Solutions.
Footnotes
Published ahead of print 22 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02138-14.
REFERENCES
- 1.Wolanin PM, Thomason PA, Stock JB. 2002. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol. 3:REVIEWS3013. 10.1186/gb-2002-3-10-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima T, Aiba H, Masuda Y, Kanaya S, Sugiura M, Wanner BL, Mori H, Mizuno T. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281–291. 10.1046/j.1365-2958.2002.03170.x. [DOI] [PubMed] [Google Scholar]
- 3.Vogt SL, Raivio TL. 2012. Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol. Lett. 326:2–11. 10.1111/j.1574-6968.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 4.Raivio TL, Silhavy TJ. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese PN, Silhavy TJ. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danese PN, Oliver GR, Barr K, Bowman GD, Rick PD, Silhavy TJ. 1998. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J. Bacteriol. 180:5875–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mileykovskaya E, Dowhan W. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol. 179:1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones CH, Danese PN, Pinkner JS, Silhavy TJ, Hultgren SJ. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394–6406. 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nevesinjac AZ, Raivio TL. 2005. The Cpx envelope stress response affects expression of the type IV bundle-forming pili of enteropathogenic Escherichia coli. J. Bacteriol. 187:672–686. 10.1128/JB.187.2.672-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese PN, Snyder WB, Cosma CL, Davis LJ, Silhavy TJ. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387–398. 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 11.Otto K, Silhavy TJ. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 99:2287–2292. 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danese PN, Silhavy TJ. 1997. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183–1193. 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 13.Pogliano J, Lynch AS, Belin D, Lin EC, Beckwith J. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169–1182. 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 14.Quan S, Koldewey P, Tapley T, Kirsch N, Ruane KM, Pfizenmaier J, Shi R, Hofmann S, Foit L, Ren G, Jakob U, Xu Z, Cygler M, Bardwell JCA. 2011. Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nat. Struct. Mol. Biol. 18:262–269. 10.1038/nsmb.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raivio TL, Popkin DL, Silhavy TJ. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Keller R, Volkmer R, Krauss N, Scheerer P, Hunke S. 2011. Structural basis for two-component system inhibition and pilus sensing by the auxiliary CpxP protein. J. Biol. Chem. 286:9805–9814. 10.1074/jbc.M110.194092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raivio TL, Leblanc SKD, Price NL. 2013. The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J. Bacteriol. 195:2755–2767. 10.1128/JB.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Lay N, Schu DJ, Gottesman S. 2013. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J. Biol. Chem. 288:7996–8003. 10.1074/jbc.R112.441386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell 43:880–891. 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 9:578–589. 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Göpel Y, Görke B. 2012. Rewiring two-component signal transduction with small RNAs. Curr. Opin. Microbiol. 15:132–139. 10.1016/j.mib.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Mandin P, Guillier M. 2013. Expanding control in bacteria: interplay between small RNAs and transcriptional regulators to control gene expression. Curr. Opin. Microbiol. 16:125–132. 10.1016/j.mib.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Zhang A, Blyn LB, Storz G. 2004. MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J. Bacteriol. 186:6689–6697. 10.1128/JB.186.20.6689-6697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coyer J, Andersen J, Forst SA, Inouye M, Delihas N. 1990. micF RNA in ompB mutants of Escherichia coli: different pathways regulate micF RNA levels in response to osmolarity and temperature change. J. Bacteriol. 172:4143–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillier M, Gottesman S. 2006. Remodelling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol. Microbiol. 59:231–247. 10.1111/j.1365-2958.2005.04929.x. [DOI] [PubMed] [Google Scholar]
- 26.Guillier M, Gottesman S. 2008. The 5′ end of two redundant sRNAs is involved in the regulation of multiple targets, including their own regulator. Nucleic Acids. Res. 36:6781–6794. 10.1093/nar/gkn742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coornaert A, Lu A, Mandin P, Springer M, Gottesman S, Guillier M. 2010. MicA sRNA links the PhoP regulon to cell envelope stress. Mol. Microbiol. 76:467–479. 10.1111/j.1365-2958.2010.07115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coornaert A, Chiaruttini C, Springer M, Guillier M. 2013. Post-transcriptional control of the Escherichia coli PhoQ-PhoP two-component system by multiple sRNAs involves a novel pairing region of GcvB. PLoS Genet. 9:e1003156. 10.1371/journal.pgen.1003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silhavy TJ, Berman ML. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 30.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 32.Hobson N, Price NL, Ward JD, Raivio TL. 2008. Generation of a restriction minus enteropathogenic Escherichia coli E2348/69 strain that is efficiently transformed with large, low copy plasmids. BMC Microbiol. 8:134. 10.1186/1471-2180-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong JL, Vogt SL, Raivio TL. 2013. Using reporter genes and the Escherichia coli ASKA overexpression library in screens for regulators of the Gram negative envelope stress response. Methods Mol. Biol. 966:337–357. 10.1007/978-1-62703-245-2_21. [DOI] [PubMed] [Google Scholar]
- 34.MacRitchie DM, Ward JD, Nevesinjac AZ, Raivio TL. 2008. Activation of the Cpx envelope stress response down-regulates expression of several locus of enterocyte effacement-encoded genes in enteropathogenic Escherichia coli. Infect. Immun. 76:1465–1475. 10.1128/IAI.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Lay N, Gottesman S. 2009. The Crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J. Bacteriol. 191:461–476. 10.1128/JB.01157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansen J, Eriksen M, Kallipolitis B, Valentin-Hansen P. 2008. Down-regulation of outer membrane proteins by noncoding RNAs: unraveling the cAMP-CRP- and σE-dependent CyaR-ompX regulatory case. J. Mol. Biol. 383:1–9. 10.1016/j.jmb.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 37.Majdalani N, Hernandez D, Gottesman S. 2002. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 46:813–826. 10.1046/j.1365-2958.2002.03203.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomason L, Court DL, Bubunenko M, Costantino N, Wilson H, Datta S, Oppenheim A. 2007. Recombineering: genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. Chapter 1:Unit 1.16. 10.1002/0471142727.mb0116s78. [DOI] [PubMed] [Google Scholar]
- 39.Mandin P, Gottesman S. 2010. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 29:3094–3107. 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191:1798–1815. 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buelow DR, Raivio TL. 2005. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J. Bacteriol. 187:6622–6630. 10.1128/JB.187.19.6622-6630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerken H, Charlson ES, Cicirelli EM, Kenney LJ, Misra R. 2009. MzrA: a novel modulator of the EnvZ/OmpR two-component regulon. Mol. Microbiol. 72:1408–1422. 10.1111/j.1365-2958.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beisel CL, Storz G. 2010. Base pairing small RNAs and their roles in global regulatory networks. FEMS Microbiol. Rev. 34:866–882. 10.1111/j.1574-6976.2010.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmqvist E, Unoson C, Reimegård J, Wagner EGH. 2012. A mixed double negative feedback loop between the sRNA MicF and the global regulator Lrp. Mol. Microbiol. 84:414–427. 10.1111/j.1365-2958.2012.07994.x. [DOI] [PubMed] [Google Scholar]
- 45.Erickson JW, Gross CA. 1989. Identification of the σE subunit of Escherichia coli RNA polymerase: a second alternate σ factor involved in high-temperature gene expression. Genes Dev. 3:1462–1471. 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 46.Jørgensen MG, Nielsen JS, Boysen A, Franch T, Møller-Jensen J, Valentin-Hansen P. 2012. Small regulatory RNAs control the multi-cellular adhesive lifestyle of Escherichia coli. Mol. Microbiol. 84:36–50. 10.1111/j.1365-2958.2012.07976.x. [DOI] [PubMed] [Google Scholar]
- 47.Majdalani N, Chen S, Murrow J, St. John K, Gottesman S. 2001. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol. Microbiol. 39:1382–1394. 10.1111/j.1365-2958.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- 48.Mika F, Busse S, Possling A, Berkholz J, Tschowri N, Sommerfeldt N, Pruteanu M, Hengge R. 2012. Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli. Mol. Microbiol. 84:51–65. 10.1111/j.1365-2958.2012.08002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolfe AJ, Parikh N, Lima BP, Zemaitaitis B. 2008. Signal integration by the two-component signal transduction response regulator CpxR. J. Bacteriol. 190:2314–2322. 10.1128/JB.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurabayashi K, Hirakawa Y, Tanimoto K, Tomita H, Hirakawa H. 2014. The role of the CpxAR two-component signal transduction system in control of fosfomycin resistance and carbon substrate uptake. J. Bacteriol. 196:248–256. 10.1128/JB.01151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans KL, Kannan S, Li G, de Pedro M, Young KD. 2013. Eliminating a set of four penicillin binding proteins triggers the Rcs phosphorelay and Cpx stress responses in Escherichia coli. J. Bacteriol. 195:4415–4424. 10.1128/JB.00596-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alon U. 2007. Network motifs: theory and experimental approaches. Nat. Rev. Genet. 8:450–461. 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- 53.Mank NN, Berghoff BA, Klug G. 2013. A mixed incoherent feed-forward loop contributes to the regulation of bacterial photosynthesis genes. RNA Biol. 10:347–352. 10.4161/rna.23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beisel CL, Storz G. 2011. The base-pairing RNA Spot 42 participates in a multioutput feedforward loop to help enact catabolite repression in Escherichia coli. Mol. Cell 41:286–297. 10.1016/j.molcel.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA. 2009. Kinetic buffering of cross talk between bacterial two-component sensors. J. Mol. Biol. 390:380–393. 10.1016/j.jmb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siryaporn A, Goulian M. 2008. Cross-talk suppression between the CpxA-CpxR and EnvZ-OmpR two-component systems in E. coli. Mol. Microbiol. 70:494–506. 10.1111/j.1365-2958.2008.06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitrophanov AY, Groisman EA. 2008. Signal integration in bacterial two-component regulatory systems. Genes Dev. 22:2601–2611. 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.