Abstract
The opportunistic pathogen Pseudomonas aeruginosa encodes a large and diverse complement of aerobic terminal oxidases, which is thought to contribute to its ability to thrive in settings with low oxygen availability. In this issue, Arai et al. (J. Bacteriol. 196:4206–4215, 2014, doi:http://dx.doi.org/10.1128/JB.02176-14) present a thorough characterization of these five complexes, enabling a more detailed understanding of aerobic respiration in this organism.
TEXT
Although the Earth's atmosphere contains approximately 21% oxygen, bacteria in environments that are ostensibly aerobic still encounter zones where the concentration is much lower, due to the effects of oxygen solubility, low diffusibility, and consumption by neighboring cells. Many bacteria cope with this in part through the use of branched respiratory chains that can be modulated in response to changing conditions (1). Aerobic terminal oxidases, which catalyze electron transfer from the respiratory apparatus to oxygen, vary in their affinities and efficiencies (2). Five such enzymes have been identified in the opportunistic pathogen Pseudomonas aeruginosa (Fig. 1), and these are thought to contribute to its ability to thrive under hypoxia (1, 3–8). A diverse collection of aerobic terminal oxidases may be especially critical for an organism that has a proficiency for persisting in biofilms, which are characterized by the formation of steep oxygen gradients (9–11). In addition to contributing to the growth of P. aeruginosa under microaerobic conditions, some of these complexes have been implicated in its ability to cope with various stresses (6, 12). In this issue of the Journal of Bacteriology, Arai et al. conduct a comprehensive study of these complexes, probing their biochemical properties and contributions to aerobic growth (13). They describe a systematic investigation of the attributes of each enzyme, which clarified many aspects of P. aeruginosa aerobic biology and revealed a new potential role for a particular high-affinity oxidase.
FIG 1.
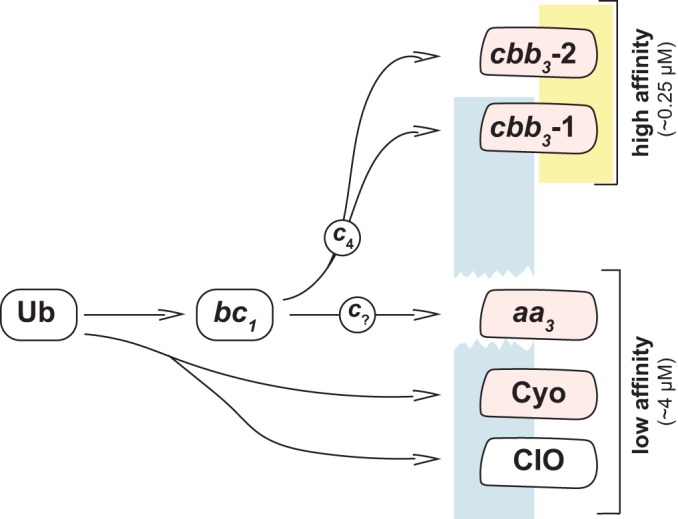
The P. aeruginosa membrane-bound electron transport chain can employ five different oxygen reductases. Ubiquinone (Ub) is reduced by a dehydrogenase (not shown) to ubiquinol, which acts as the electron donor for the cytochrome bc1 complex, Cyo, or CIO. The cytochrome bc1 complex reduces a c-type cytochrome, which then acts as the electron donor for aa3, cbb3-1, or cbb3-2. Heme-copper oxidases are represented by pink shapes. Oxidases that support growth better under microaerobic conditions are shaded yellow, while those that support growth better under typical conditions are shaded blue. These roles are influenced by intrinsic chemical properties and expression levels of the individual complexes. Approximate affinities for oxygen (Km values obtained amperometrically) are shown.
The P. aeruginosa aerobic terminal oxidases include enzymes that can use ubiquinol or cytochromes as electron donors, ones that have low or high affinities for O2, and a cyanide-insensitive oxidase (CIO) that functions in the presence of this endogenously produced virulence factor (12). Aside from CIO, the four other aerobic terminal oxidases encoded by the P. aeruginosa genome are the bo3 oxidase (Cyo), the aa3 oxidase (aa3), cbb3 oxidase 1 (cbb3-1), and cbb3 oxidase 2 (cbb3-2). Earlier studies predicted that Cyo and aa3 (which is most similar to the mitochondrial terminal oxidase) would have low affinities for oxygen and that CIO and the cbb3 enzymes would have high oxygen affinities (4–6, 14, 15). In batch cultures grown under typical conditions (i.e., in nutrient-rich medium and atmospheric oxygen, with vigorous shaking), the low-affinity enzymes Cyo and/or aa3 would thus be expected to play major roles during exponential growth, when oxygen is relatively abundant. The high-affinity enzymes cbb3-1 and cbb3-2 would be expected to function primarily in stationary phase, when oxygen is relatively scarce, and/or during growth under microaerobic conditions. Hypothetically, CIO would be particularly important in stationary phase, as it is the only terminal oxidase that functions in the presence of cyanide, which P. aeruginosa produces at high cell density (7, 12, 16).
Using gene expression and mutant analyses, previous studies have revised and clarified this working model of P. aeruginosa aerobic respiration. They have shown that the aa3 oxidase, which plays a major role in aerobic respiration in other bacteria (14, 15), is expressed primarily under nutrient-limited conditions and is otherwise a minor player under typical conditions (6). Rather, the cbb3-1, cbb3-2, and CIO oxidases play major roles, and their importance is exaggerated when oxygen becomes limiting and during growth in biofilms (4–6, 17). The two cbb3 oxidases are very similar at the amino acid level (with a similarity of 87% for the catalytic subunit in strain PAO1), but they are differentially regulated: the cbb3-1 complex is expressed constitutively, while the cbb3-2 complex is induced by oxygen limitation (4, 5). Critically, these studies also showed that oxidase gene expression can be compensatory, such that loss of one or more oxidases leads to induction of others (6).
To study the physiological roles of the P. aeruginosa aerobic terminal oxidases in isolation, Arai et al. (13) took the important step of generating combinatorial mutants, each containing only one of these complexes, in strain PAO1. Growth experiments confirmed the unique primary physiological roles of the cbb3 complexes in this bacterium (4–6). Under typical conditions, the strain containing the cbb3-1 oxidase grew like the wild type, while the strain containing the cbb3-2 oxidase showed a defect earlier in the growth experiment before “catching up” to the final wild-type density. This growth profile is consistent with the prior observation that cbb3-2 is induced specifically by oxygen limitation (4); basal levels of expression could support initial growth before increased oxygen consumption by the denser culture brings the oxygen level down and thereby enhances expression. The growth phenotypes of the other individual single-oxidase strains also reflected what was known about regulation of the P. aeruginosa terminal oxidases (4, 6).
Prior work looking at expression of genes encoding putative c-type cytochromes had provided clues as to which of these proteins might carry electrons from the cytochrome bc1 complex to the cbb3-type oxidases (6). Arai et al. (13) used a genetic approach to determine that cytochrome c4, encoded by open reading frame (ORF) PA5490 in strain PAO1, is the predominant mediator functioning at this step in the electron transport pathway under typical and microaerobic conditions. This finding fills in an important gap that was remaining in our model of the P. aeruginosa aerobic respiratory chain.
To assess their positions in the electron transport chain and oxygen affinities, Arai et al. (13) conducted in vitro studies with membranes prepared from strains producing each of the individual oxidases. Oxygen consumption assays confirmed the electron donor (ubiquinol or cytochrome c) predictions for each complex (4), as well as the cyanide sensitivity of all of the oxidases but CIO. A battery of affinity assays also confirmed predictions for the relative affinities of the oxidases, with one exception: CIO, a cytochrome bd-type enzyme, was found to differ from the canonical Escherichia coli cytochrome bd (18) in that it showed a low affinity for oxygen. The physiological consequences of the low oxygen affinity of CIO were examined by expressing the P. aeruginosa cioAB genes in an E. coli mutant lacking native terminal oxidases. The growth of this strain was compared to a version of the same mutant expressing the E. coli cytochrome bd under the same promoter. Growth rates and yields for the two strains were similar under typical conditions, but the CIO-expressing strain showed a lower growth rate under microaerobic conditions, further supporting the conclusion that CIO functions optimally when oxygen availability is relatively high.
By generating P. aeruginosa mutants that each expressed only one terminal oxidase, Arai et al. (13) were able to address outstanding questions regarding oxygen respiration and provide a more complete picture of the aerobic electron transport chain in this pathogen. A particularly intriguing feature of the P. aeruginosa system is that a high-affinity oxidase, cbb3-1, functions as the major oxygen reductase in well-aerated cultures (Fig. 1). The authors point out that this is a unique trait, as most other bacteria regulate high-affinity oxidases such that they are expressed specifically under low-oxygen conditions, with low-affinity enzymes similar to aa3 and/or Cyo acting as the major oxidase(s) under high-oxygen conditions (14, 15, 19). Even the closely related Pseudomonas putida, which also possesses two cbb3-type enzymes, employs Cyo as its major oxidase under typical conditions (19). Though the measured proton translocation efficiency (protons translocated across the membrane per oxygen atom consumed) values of the cbb3-type oxidases were lower than that of the aa3 oxidase, they were higher than the efficiency value of CIO and comparable to that of the Cyo oxidase.
The critical role of cbb3-1 during exponential growth with relatively high oxygen availability raises the question of whether this enzyme contributes capabilities beyond oxygen reduction in typical P. aeruginosa cultures. The cbb3 oxidases differ from other heme-copper oxidases in their subunit and binuclear active site composition, exhibiting structural similarities to nitric oxide reductases (NORs) (20, 21). cbb3 oxidase purified from Pseudomonas stutzeri has been shown to have NOR activity (22), and the cbb3 oxidases of P. aeruginosa have been implicated in anaerobic denitrification and NO-mediated effects on biofilm formation (17). In diverse organisms, effects of the cbb3 oxidases on the expression of other metabolic genes and on biomineralization have suggested that they may be involved in sensing and responding to changes in redox homeostasis (4, 23, 24). Finally, results from the current study and others suggest that modulation of the P. aeruginosa respiratory chain is strongly influenced by environmental stresses, and the different oxidases provide activity under distinct, specialized conditions (6, 7, 16). aa3 is expressed during nutrient starvation, Cyo is expressed under iron starvation and in the presence of a NO-generating reagent, and CIO is induced by copper starvation and cyanide (6). As Arai et al. note (13), utilization of cbb3-1 during exponential growth may provide protection from reactive oxygen species or another unknown source of stress. Further investigation into the ecophysiology of P. aeruginosa biofilm formation and infection may uncover the significance of cbb3 activity for this bacterium, which utilizes oxygen preferentially and yet dominates in environments where it is in short supply.
ACKNOWLEDGMENTS
Research done in our laboratory is supported by NIH/NIAID R01-AI103369 to L.E.P.D.
We apologize to authors whose work could not be cited due to space limitations.
Footnotes
Published ahead of print 29 September 2014
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Poole RK, Cook GM. 2000. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 43:165–224. 10.1016/S0065-2911(00)43005-5. [DOI] [PubMed] [Google Scholar]
- 2.Morris RL, Schmidt TM. 2013. Shallow breathing: bacterial life at low O2. Nat. Rev. Microbiol. 11:205–212. 10.1038/nrmicro2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita K, Yamada M, Shinagawa E, Adachi O, Ameyama M. 1980. Membrane-bound respiratory chain of Pseudomonas aeruginosa grown aerobically. J. Bacteriol. 141:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comolli JC, Donohue TJ. 2004. Differences in two Pseudomonas aeruginosa cbb3 cytochrome oxidases. Mol. Microbiol. 51:1193–1203. 10.1046/j.1365-2958.2003.03904.x. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Ortega C, Harwood CS. 2007. Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol. Microbiol. 65:153–165. 10.1111/j.1365-2958.2007.05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami T, Kuroki M, Ishii M, Igarashi Y, Arai H. 2010. Differential expression of multiple terminal oxidases for aerobic respiration in Pseudomonas aeruginosa. Environ. Microbiol. 12:1399–1412. 10.1111/j.1462-2920.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams HD, Zlosnik JE, Ryall B. 2007. Oxygen, cyanide and energy generation in the cystic fibrosis pathogen Pseudomonas aeruginosa. Adv. Microb. Physiol. 52:1–71. 10.1016/S0065-2911(06)52001-6. [DOI] [PubMed] [Google Scholar]
- 8.Arai H. 2011. Regulation and function of versatile aerobic and anaerobic respiratory metabolism in Pseudomonas aeruginosa. Front. Microbiol. 2:103. 10.3389/fmicb.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu KD, Stewart PS, Xia F, Huang CT, McFeters GA. 1998. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 64:4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich LE, Okegbe C, Price-Whelan A, Sakhtah H, Hunter RC, Newman DK. 2013. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol. 195:1371–1380. 10.1128/JB.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wessel AK, Arshad TA, Fitzpatrick M, Connell JL, Bonnecaze RT, Shear JB, Whiteley M. 2014. Oxygen limitation within a bacterial aggregate. mBio 5(2):e00992. 10.1128/mBio.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham L, Williams HD. 1995. Isolation and characterization of mutants defective in the cyanide-insensitive respiratory pathway of Pseudomonas aeruginosa. J. Bacteriol. 177:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arai H, Kawakami T, Osamura T, Hirai T, Sakai Y, Ishii M. 2014. Enzymatic characterization and in vivo function of five terminal oxidases in Pseudomonas aeruginosa. J. Bacteriol. 196:4206–4215. 10.1128/JB.02176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosma G, Braster M, Stouthamer AH, van Verseveld HW. 1987. Isolation and characterization of ubiquinol oxidase complexes from Paracoccus denitrificans cells cultured under various limiting growth conditions in the chemostat. Eur. J. Biochem. 165:657–663. 10.1111/j.1432-1033.1987.tb11491.x. [DOI] [PubMed] [Google Scholar]
- 15.Winstedt L, von Wachenfeldt C. 2000. Terminal oxidases of Bacillus subtilis strain 168: one quinol oxidase, cytochrome aa3 or cytochrome bd, is required for aerobic growth. J. Bacteriol. 182:6557–6564. 10.1128/JB.182.23.6557-6564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham L, Pitt M, Williams HD. 1997. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol. Microbiol. 24:579–591. 10.1046/j.1365-2958.1997.3561728.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamada M, Toyofuku M, Miyano T, Nomura N. 2 September 2014. Aerobic respiratory enzymes, Cbb3-type cytochrome c oxidases, impact the anaerobic life of Pseudomonas aeruginosa PAO1. J. Bacteriol. 10.1128/JB.01978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Mello R, Hill S, Poole RK. 1996. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology 142:755–763. 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 19.Ugidos A, Morales G, Rial E, Williams HD, Rojo F. 2008. The coordinate regulation of multiple terminal oxidases by the Pseudomonas putida ANR global regulator. Environ. Microbiol. 10:1690–1702. 10.1111/j.1462-2920.2008.01586.x. [DOI] [PubMed] [Google Scholar]
- 20.Buschmann S, Warkentin E, Xie H, Langer JD, Ermler U, Michel H. 2010. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science 329:327–330. 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 21.Pitcher RS, Watmough NJ. 2004. The bacterial cytochrome cbb3 oxidases. Biochim. Biophys. Acta 1655:388–399. 10.1016/j.bbabio.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Forte E, Urbani A, Saraste M, Sarti P, Brunori M, Giuffre A. 2001. The cytochrome cbb3 from Pseudomonas stutzeri displays nitric oxide reductase activity. Eur. J. Biochem. 268:6486–6491. 10.1046/j.0014-2956.2001.02597.x. [DOI] [PubMed] [Google Scholar]
- 23.Oh JI, Kaplan S. 2000. Redox signaling: globalization of gene expression. EMBO J. 19:4237–4247. 10.1093/emboj/19.16.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Raschdorf O, Silva KT, Schuler D. 2014. The terminal oxidase cbb3 functions in redox control of magnetite biomineralization in Magnetospirillum gryphiswaldense. J. Bacteriol. 196:2552–2562. 10.1128/JB.01652-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


