Abstract
Haemophilus ducreyi causes the sexually transmitted disease chancroid and a chronic limb ulceration syndrome in children. In humans, H. ducreyi is found in an abscess and overcomes a hostile environment to establish infection. To sense and respond to membrane stress, bacteria utilize two-component systems (TCSs) and extracytoplasmic function (ECF) sigma factors. We previously showed that activation of CpxRA, the only intact TCS in H. ducreyi, does not regulate homologues of envelope protein folding factors but does downregulate genes encoding envelope-localized proteins, including many virulence determinants. H. ducreyi also harbors a homologue of RpoE, which is the only ECF sigma factor in the organism. To potentially understand how H. ducreyi responds to membrane stress, here we defined RpoE-dependent genes using transcriptome sequencing (RNA-Seq). We identified 180 RpoE-dependent genes, of which 98% were upregulated; a major set of these genes encodes homologues of envelope maintenance and repair factors. We also identified and validated a putative RpoE promoter consensus sequence, which was enriched in the majority of RpoE-dependent targets. Comparison of RpoE-dependent genes to those controlled by CpxR showed that each transcription factor regulated a distinct set of genes. Given that RpoE activated a large number of genes encoding envelope maintenance and repair factors and that CpxRA represses genes encoding envelope-localized proteins, these data suggest that RpoE and CpxRA appear to play distinct yet complementary roles in regulating envelope homeostasis in H. ducreyi.
INTRODUCTION
Haemophilus ducreyi is a Gram-negative, obligate human pathogen that causes chancroid. Chancroid is a sexually transmitted genital ulcer disease that presents as painful genital ulcers, often associated with regional lymphadenopathy. Chancroid is prevalent in the developing countries of Africa, Asia, and Latin America. Although the global prevalence of chancroid was estimated to be 4 to 6 million cases in the late 1990s, it is now undefined due to syndromic management of genital ulcer disease and the lack of surveillance programs (1, 2). In addition to causing its own morbidity, chancroid facilitates the acquisition and transmission of human immunodeficiency virus type 1 by providing a portal of viral entry, promoting viral shedding from the ulcer, and increasing viral replication due to immune activation (1, 2). Recent reports from the South Pacific islands implicate H. ducreyi as a predominant cause of a chronic limb ulceration syndrome in children, which is not sexually transmitted (3–6).
H. ducreyi is thought to enter the skin through abrasions that occur during intercourse. Clinical disease is often characterized initially by a papule at the site(s) of entry. The papules eventually develop into pustules, which finally erode into painful ulcers. To understand how H. ducreyi causes infection in its natural host, our laboratory developed a human challenge model of infection (7). In this model, healthy adult volunteers are inoculated with H. ducreyi on the skin of the upper arm via puncture wounds. Within 24 h of inoculation, neutrophils and macrophages traffic into the wound and coalesce into an abscess that eventually erodes the epidermis. During both experimental and natural infection, H. ducreyi is surrounded by neutrophils and macrophages, which fail to ingest the organism (8, 9). To successfully establish infection in its host, H. ducreyi must be able to sense and respond to the hostile environment of an abscess, including toxic products released by phagocytes and epithelial cells, the bactericidal activity of serum that transudates into the wound, hypoxia, and nutrient limitation.
Gram-negative bacteria frequently utilize two-component systems (TCSs) to sense and respond to extracellular stresses. The H. ducreyi genome encodes homologues of CpxRA, which is the only obvious intact TCS encoded in the genome. Activation of CpxR by deletion of cpxA downregulates the majority of its targets, including multiple virulence determinants, and attenuates the virulence of H. ducreyi in humans (10–12). However, deletion of cpxR does not affect the expression of virulence determinants or reduce the virulence of the organism in humans, suggesting that CpxRA is dispensable for H. ducreyi infection (13). Thus, H. ducreyi likely utilizes alternative mechanisms to sense and respond to extracellular stresses in vivo.
Gram-negative bacteria also utilize extracytoplasmic function (ECF) sigma factors to sense and respond to extracellular stresses; the Escherichia coli RpoE is one of the best-characterized ECF sigma factors. RpoE allows E. coli to sense and respond to stresses that perturb the cell envelope (14–17). In the absence of stress, RpoE is bound to the cytoplasmic domain of RseA, an anti-sigma factor, and to RseB, another negative regulator of RpoE (18, 19). RseC is a minor positive regulator of RpoE (19). RpoE is activated primarily by stresses that affect the folding of outer membrane proteins (OMPs), such as heat shock, oxidative stress, starvation, hyperosmotic stress, exposure to ethanol and detergents, and mutations in genes encoding chaperones for protein folding (20). In the presence of stress stimuli, the carboxyl ends of misfolded OMPs activate the protease activity of DegS, which in turn cleaves RseA in concert with RseP, ClpP, and other proteases, activating the RpoE regulon. Both OMP activation of DegS and lipopolysaccharide (LPS)-dependent relief of RseB inhibition are required for robust induction of RpoE (21, 22). One of the major subsets of genes regulated by RpoE encodes proteins involved in maintenance and repair of the cell envelope (23, 24). RpoE also regulates a number of cytoplasmic proteins involved in transcription, translation, and DNA synthesis and repair. RpoE is an essential sigma factor in E. coli; mutations in rpoE are either lethal or associated with suppressor mutations (25, 26).
The H. ducreyi genome (GenBank accession no. AE017143) contains homologues of RpoE, RseA, RseC, DegS, and RseP. RpoE is the only obvious ECF sigma factor in H. ducreyi. Our efforts to generate rpoE, rseA, and rseC mutants of H. ducreyi were unsuccessful. As an alternative approach to understand the role of the RpoE-mediated response in H. ducreyi, here we defined the genes regulated by RpoE using transcriptome sequencing (RNA-Seq). To this end, we compared the RNA-Seq-defined transcriptome of an H. ducreyi 35000HP wild-type strain containing an rpoE inducible plasmid to that of a wild-type strain containing a control plasmid. We show that RpoE differentially regulated ∼10% of the H. ducreyi genes, 98% of which were upregulated. Comparison of RpoE-dependent genes to those regulated by activated CpxR showed that the two transcription factors regulated unique sets of genes. While CpxRA represses the majority of its targets encoding envelope-localized proteins, RpoE activated a large number of genes involved in envelope maintenance and repair, suggesting that the two systems appear to play distinct yet complementary roles in regulating envelope homeostasis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. The H. ducreyi strains were grown on chocolate agar plates supplemented with 1% IsoVitaleX at 33°C with 5% CO2 or in Columbia broth supplemented with 5% tetracycline-free fetal bovine serum (Clontech), 1% IsoVitaleX, and 50 μg/ml of hemin (Aldrich Chemical Co.) at 33°C. E. coli strain DH5α and One Shot Top10 chemically competent cells (Invitrogen) were used for general cloning purposes. E. coli strains were grown in Luria-Bertani medium at 37°C, except for strain DY380, which was maintained in L-broth or L-agar and grown at 32°C or 42°C for induction of the λ red recombinase. When necessary, media were supplemented with spectinomycin (200 μg/ml for H. ducreyi and 50 μg/ml for E. coli), kanamycin (20 μg/ml for H. ducreyi and 50 μg/ml for E. coli), and/or streptomycin (100 μg/ml for H. ducreyi and 50 μg/ml for E. coli).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| H. ducreyi strain 35000HP | Human-passaged variant of strain 35000; parental strain | 41 |
| E. coli strains | ||
| DH5α and TOP10 | Strains used for general cloning procedures | Invitrogen |
| DY380 | DH10B derivative containing a defective λ prophage in which the red, bet, and gam genes are controlled by the temp-sensitive λcI857 repressor | 29 |
| Plasmids | ||
| pT | pLS88 derivative containing the tetracycline (tet) controlled expression system | 27 |
| pDG8 | pT derivative containing rpoE under control of the tet system | This study |
| pSPECR | Vector containing the spectinomycin resistance cassette | 28 |
| pDG9 | pT derivative containing the rpoE coding region and spectinomycin resistance cassette from pSPECR | This study |
| pDG10 | pT derivative containing the spectinomycin resistance cassette from pSPECR | This study |
| pDG11 | pT derivative containing a 3×-FLAG-tagged RpoE and a spectinomycin resistance cassette | This study |
| pRB157 | pLS88 derivative containing an ΩAmp cartridge followed by a BglII site for insertion of putative promoter sequences and a promoterless GFP cassette derived from pGreenTIR | 10 |
| pDG12 | pRB157 derivative containing the putative dsbA promoter region | This study |
| pDG13 | pRB157 derivative containing the putative degP promoter region | This study |
| pDG14 | pRB157 derivative containing the putative hfq promoter region | This study |
| pDG15 | pRB157 derivative containing the putative rpoE promoter region | This study |
| pDG16 | pRB157 derivative containing the putative rpoH promoter region | This study |
| pLS88 | H. ducreyi shuttle vector | 42 |
| pACYC177 | Low-copy-number H. ducreyi shuttle vector with a P15A origin of replication | New England Biolabs |
| pDG17 | pACYC177 derivative containing the tetracycline-regulated RpoE expression system | This study |
| pKF1 | pRB157 derivative containing the putative lspB promoter region | 10 |
| pDG18 | pDG16 derivative in which the first putative RpoE-dependent promoter is mutagenized from AAC to TTT at the −35 region | This study |
| pDG19 | pDG16 derivative in which the second putative RpoE-dependent promoter is mutagenized from AAC to TTT at the −35 region | This study |
Construction of a tetracycline-inducible RpoE expression vector.
To construct a tetracycline-responsive RpoE expression vector, the rpoE coding region was amplified using primers P1/P2 (see Table S1 in the supplemental material), which generated an NdeI site at the 5′ end and a BamHI site at the 3′ end of rpoE. The amplified fragment was ligated into NdeI- and BamHI-digested pT, which contains a tetracycline-inducible regulatory system (27); the resulting construct was designated pDG8. Preliminary experiments showed that 35000HP transformed with pDG8 had severe growth defects in broth, raising the possibility that RpoE was overexpressed in the absence of induction. To reduce the basal level of expression of RpoE, a 1.2-kb spectinomycin cassette from pSPECR was digested with BamHI and ligated in the orientation opposite to that of rpoE into BamHI-digested pDG8, generating pDG9 (28). The growth of 35000HP transformed with pDG9 was identical to that of 35000HP (data not shown). As a control, a plasmid that contained all features of pDG9 but lacked rpoE, designated pDG10, was also constructed. All constructs were confirmed by sequencing. pDG9 and pDG10 were transformed into H. ducreyi 35000HP, and the resulting strains were designated 35000HP(pDG9) and 35000HP(pDG10), respectively.
A FLAG-tagged RpoE expression vector was constructed by engineering a 3×-FLAG tag sequence into rpoE immediately after its translation start codon in pDG9 using λ red recombinase. Briefly, pDG9 was transformed into the E. coli strain DY380, which contains the temperature-sensitive λ red recombinase (29). A 150-bp cassette was amplified by PCR using primers P3/P4 (see Table S1 in the supplemental material) with P3 containing the engineered 3×-FLAG tag sequence. The amplified fragment was electroporated into DY380. Following induction of λ red recombinase, the 3×-FLAG tag sequence was inserted immediately downstream of the rpoE translation start codon. The resulting construct was designated pDG11 and was confirmed by sequencing using primers P5/P6. pDG11 was transformed into H. ducreyi 35000HP, and the resulting strain was designated 35000HP(pDG11).
RT-PCR and qRT-PCR.
Total RNA was extracted from bacterial cells using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. To remove DNA, the RNA was treated twice with the Turbo DNA-free DNase (Ambion). The integrity and the concentration of the RNA were determined using the Agilent 2100 Bioanalyzer (Agilent Technologies) and the NanoDrop ND-1000 spectrophotometer (Thermo Scientific), respectively. cDNA was synthesized from total RNA using the Super Smart cDNA synthesis kit (Clontech); reverse transcriptase PCR (RT-PCR) analysis of rpoE-rseA, rseA-rseC, and rseC-fadD was performed using primers P7/P8, P9/P10, and P11/P12, respectively (see Table S1 in the supplemental material).
Quantitative RT-PCR (qRT-PCR) was performed using the QuantiTect SYBR green RT-PCR kit (Qiagen) in an ABI Prism 7000 sequence detection system (Applied Biosystems). qRT-PCR was performed to amplify internal gene-specific fragments ranging from 70 to 200 bp of HD0518, rluA, dsbA, degP, and HD0192 using primers P13/P14, P15/P16, P17/P18, P19/P20, and P21/P22, respectively (see Table S1 in the supplemental material); qRT-PCR analysis of HD0430, HD0930, hfq, and ompP2B was performed using primers described previously (11). The amplification efficiency was determined for each primer pair; all primer pairs had greater than 95% efficiency. The expression levels of target genes were normalized to that of dnaE using primers described previously (11). The fold change in expression was calculated as (Etarget)ΔCTtarget [35000HP(pDG9) − 35000HP(pDG10)]/(Ereference)ΔCTreference [35000HP(pDG9) − 35000HP(pDG10)], where E is the amplification efficiency (equal to 10−1/slope) and ΔCT is the change in cycle threshold (10).
RpoE induction.
35000HP(pDG9) and 35000HP(pDG10) were grown to mid-log phase (optical density at 660 nm [OD660] = 0.2) and induced for RpoE expression by adding 200 ng/ml of anhydrotetracycline (ATc) (Clontech). To determine if RpoE induction affected H. ducreyi growth and viability, OD660 and CFU were measured at different time points after induction. rpoE transcripts were quantified from 35000HP(pDG9) and 35000HP(pDG10) cells harvested before and 5, 10, and 20 min after induction by qRT-PCR.
For induction of FLAG-tagged RpoE expression, 35000HP(pDG11) and 35000HP(pDG10) were grown to mid-log phase (OD660 = 0.2) and then treated with 200 ng/ml of ATc, harvested, and analyzed as described above. Western blotting was performed on whole-cell lysates of 35000HP(pDG11) and 35000HP(pDG10) before and 5, 10, and 20 min after adding ATc, using the monoclonal anti-FLAG M2 antibody (Sigma) (11).
RNA-Seq analysis and identification of RpoE-dependent genes.
RNA samples were isolated from 35000HP(pDG9) and 35000HP(pDG10) before and 5 and 10 min after induction of rpoE. RNA quality and quantity assessment, mRNA enrichment, preparation of RNA-Seq libraries, sequence mapping, quantification of transcript levels, and identification of differentially expressed genes were performed as described previously (11). Sequence was obtained as 50-bp single-end reads on an Illumina HiSeq 2500 instrument. Since rpoE transcription was induced during the experiments, rpoE transcript levels were excluded from the analysis. Functional classification and clustering analysis of the differentially expressed genes were performed using DAVID bioinformatics resources (30).
Identification of putative RpoE promoter motifs upstream of induced transcriptional units.
Putative RpoE promoter motifs were identified de novo using the Multiple EM for Motif Elicitation (MEME) algorithm (31). Briefly, the differentially regulated genes were organized into transcriptional units (TUs) using the predicted operon structures from the DOOR database (32). Transcription start sites (TSSs) were predicted for the differentially regulated TUs using the RNA-Seq data. The 100-bp upstream regions from the predicted TSSs were used for de novo motif identification by the MEME algorithm, restricting the motif length to 15 to 50 bp. The identified motif was then represented as a position-specific scoring matrix (PSSM). The significance of the discovered motif was tested by calculating the matching scores based on the putative promoter sequence and the motif feature characterized by the PSSM, using a published strategy (33). The score cutoff was determined by maximizing the enrichment of identified motifs in the putative promoter regions of differentially regulated TUs compared to those of the unaffected genes.
Reporter assays.
Reporter assays were performed as previously described (11). Approximately 450-bp upstream regions from the translation start codon containing the putative promoter regions of dsbA, degP, hfq, rpoE, and rpoH were amplified by PCR using primers P23/P24, P25/P26, P27/P28, P29/P30, and P31/P32, respectively (see Table S1 in the supplemental material). The fragments were ligated to pRB157 using the BglII restriction site preceding a promoterless green fluorescent protein (GFP) gene cassette (34). The orientation of the insert with respect to the GFP gene cassette was confirmed by PCR using a promoter-specific forward primer and the reverse primer that hybridizes to a region of the GFP gene cassette downstream of the BglII site described previously (11). The final constructs, designated pDG12, pDG13, pDG14, pDG15, and pDG16, containing the respective putative promoter regions from dsbA, degP, hfq, rpoE, and rpoH were confirmed by sequencing.
Given that the reporter constructs and the rpoE inducible expression system (pDG9) both contained a pLS88 origin of replication, we replaced the pLS88 origin of replication in pDG9 with a p15A origin of replication from pACYC177. Briefly, the regions containing the origin of replication and the ampicillin resistance cassette in pACYC177 and the rpoE inducible expression system, including the terminators on either side in pDG9, were amplified using primers P33/P34 and P35/P36, respectively (see Table S1 in the supplemental material). The two fragments were assembled together using the Gibson Assembly cloning kit (New England BioLabs). After confirmation by sequencing, the final construct, designated pDG17, was electroporated into H. ducreyi 35000HP, resulting in 35000HP(pDG17). The growth of 35000HP(pDG17) in broth was identical to that of 35000HP (data not shown). The reporter constructs were then electroporated into 35000HP(pDG17). As a control, a reporter containing the putative lspB promoter region was also electroporated into 35000HP(pDG17) (10). Whole-cell lysates were prepared from each transformant harvested at 0, 30, 60, and 120 min after RpoE induction and analyzed by Western blotting using monoclonal antibodies specific to GFP (Clontech) and the peptidoglycan-associated protein (PAL) (10). For each strain, the level of expression of GFP protein normalized to PAL was determined by densitometry using ImageJ software (35).
Site-directed mutagenesis of putative RpoE-dependent rpoH promoter elements.
Mutations were introduced into the putative RpoE-dependent rpoH promoter region in pDG16 using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies) following the manufacturer's instructions. The upstream region of rpoH in pDG16 contains two putative RpoE-dependent promoters. The third, fourth, and fifth conserved nucleotides in the −35 conserved region of each putative promoter were mutated from A to T, A/C to T, and C to T, respectively (see Fig. 7A). Mutagenic primers were designed using the QuikChange Primer Design program (Agilent Technologies). The primers P37/P38 and P39/P40 were used to mutagenize putative promoter 1 and 2 regions, respectively; the mutagenized constructs were designated pDG18 and pDG19 (see Table S1 in the supplemental material). The mutations in pDG18 and pDG19 were confirmed by sequencing. The mutagenized constructs were used to transform 35000HP(pDG17); the transformants were grown to mid-log phase and induced for rpoE expression, and the expression ratio of GFP/PAL was measured as described above.
FIG 7.
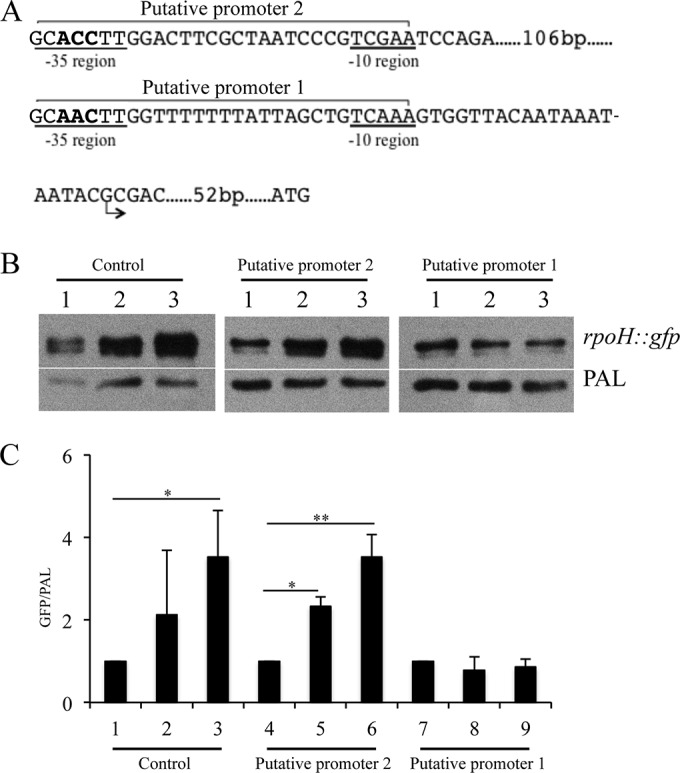
The predicted RpoE promoter is required for RpoE-dependent regulation of rpoH. (A) The predicted −10 and −35 regions of the two tandem RpoE-dependent promoters as well as the transcriptional and translational start sites are shown. In order to demonstrate that these promoter regions were functional, we mutagenized nucleotides within each −35 region. The mutagenized nucleotides are indicated in bold. (B) Effect of site-directed mutagenesis on promoter activity measured in the GFP reporter assay. Plasmids containing the wild-type and mutagenized putative rpoH promoter regions were electroporated into 35000HP(pDG17). Whole-cell lysates were prepared at 0 (lane 1), 60 (lane 2), and 120 (lane 3) minutes after RpoE induction and probed with an anti-GFP monoclonal antibody. PAL detected with the monoclonal antibody 3B9 served as a loading control. Putative promoter 1 and putative promoter 2, reporters with nucleotide substitutions in each putative promoter; control, reporter containing the wild-type putative rpoH promoter region. (C) Densitometry analysis of the Western blots from panel B. The data represent the means ± SD from three independent experiments. *, P < 0.05; **, P < 0.01.
Statistical analyses.
Densitometry data were analyzed using one-way analysis of variance (ANOVA) with Tukey's honestly significant difference post hoc test; a P value of <0.05 was considered statistically significant. The overlap of RpoE-dependent genes with those regulated by CpxRA, Hfq, and stationary phase was analyzed using Fisher's exact test; the overlap was considered significant if the odds ratio was >1 and the P value was <0.05. Throughout, the data are expressed as means ± standard deviations (SD).
RESULTS
Identification and characterization of the rpoE locus in H. ducreyi.
The H. ducreyi genome contains putative homologues of rpoE (HD1172), rseA (HD1173), rseC (HD1174), degS (HD1350), rseP (HD1192), and clpP (HD0221). However, H. ducreyi lacks an obvious homologue of rseB. By BLAST analysis, the H. ducreyi RpoE, RseA, RseC, DegS, RseP, and ClpP had 69%, 32%, 35%, 51%, 43%, and 73% amino acid identity to their respective homologues in E. coli. In E. coli, rpoE, rseA, rseB, and rseC are organized together in an operon (Fig. 1A) (19). The H. ducreyi rpoE, rseA, and rseC genes are also organized in a putative operon with gene order rpoE→rseA→rseC→fadD (Fig. 1B); fadD is a pseudogene in H. ducreyi. RT-PCR analysis suggested that rpoE is cotranscribed with its downstream genes (Fig. 1B).
FIG 1.
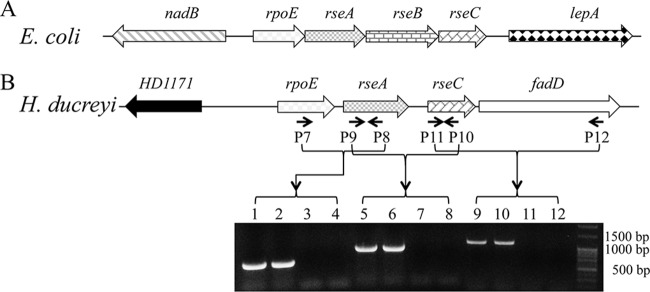
The rpoE loci in E. coli and H. ducreyi. (A) Genomic organization of the rpoE locus in E. coli K-12. (B) Genomic organization and RT-PCR analysis of the rpoE locus in H. ducreyi. Arrows indicate the binding sites for primers used for RT-PCR analysis. Lanes 1, 2, 3, and 4, RT-PCR with primers P7 and P8; lanes 5, 6, 7, and 8, RT-PCR with primers P9 and P10; lanes 9, 10, 11, and 12, RT-PCR with primers P11 and P12. Lanes 1, 5, and 9, positive control using genomic DNA; lanes 2, 6, and 10, RT-PCR; lanes 3, 7, and 11, control without reverse transcriptase; lanes 4, 8, and 12, control without template. The RT-PCR data are representative of cDNA made from three independent RNA samples.
Induction of RpoE expression.
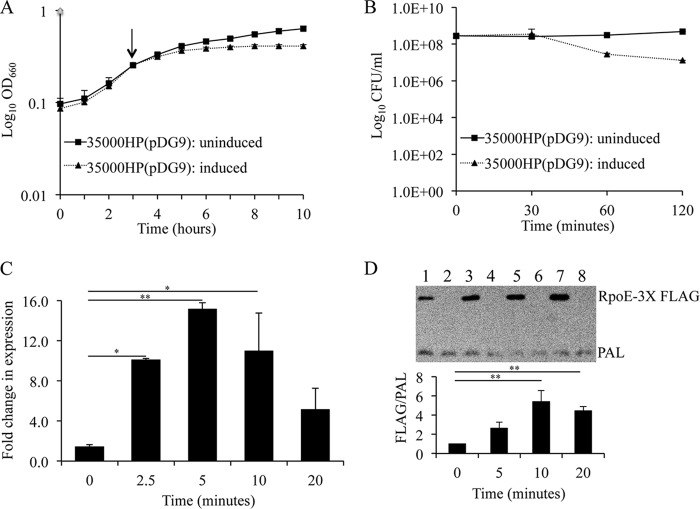
In E. coli, RpoE overexpression has been used to define RpoE-dependent genes (23). H. ducreyi RpoE was overexpressed under the control of a tetracycline (tet) regulatory system. Compared to uninduced 35000HP(pDG9), 35000HP(pDG9) induced with ATc lagged in growth at 1 h after RpoE induction (Fig. 2A). By quantitative culture, the viability of 35000HP(pDG9) induced for RpoE expression was identical to that of uninduced 35000HP(pDG9) at 30 min but declined by 1.1 ± 0.16 log and 1.4 ± 0.2 log units at 1 and 2 h after induction, respectively (Fig. 2B). These data suggested that RpoE induction needed to be confined to less than 30 min to avoid confounding effects on cell viability. Next, we compared rpoE transcripts in 35000HP(pDG9) and 35000HP(pDG10) before and at 2.5, 5, 10, and 20 min after addition of ATc. qRT-PCR analysis showed that rpoE was significantly induced at 2.5 (10.1-fold ± 0.1-fold), 5 (15.1-fold ± 0.7-fold), and 10 (11.0-fold ± 3.8-fold) minutes after addition of ATc, with maximal induction at 5 min (Fig. 2C).
FIG 2.
RpoE overexpression using a tetracycline-responsive expression system. (A) Effect of RpoE induction on the growth of 35000HP(pDG9) as assessed by OD measurements at 660 nm. An arrow indicates the point at which ATc was added to induce RpoE expression. (B) Effect of RpoE induction on the viability of 35000HP(pDG9) as assessed by quantitative culture. (C) RpoE induction as assessed by qRT-PCR analysis of rpoE transcripts following addition of ATc. (D) RpoE induction as assessed by Western blotting of RpoE expression following addition of ATc. Whole-cell lysates were prepared from 35000HP(pDG11) (lanes 1, 3, 5, and 7) and 35000HP(pDG10) (lanes 2, 4, 6, and 8) at 0, 5, 10, and 20 min after RpoE induction. RpoE expression was determined using a monoclonal antibody specific to the 3×-FLAG tag fused to the N-terminal end of RpoE immediately after the start codon. PAL, detected using the 3B9 antibody, served as a loading control. The data represent the means ± SD from four independent experiments. *, P < 0.05 and **P < 0.01.
In order to confirm that induction of rpoE led to increased expression of RpoE, we constructed a FLAG-tagged version of rpoE in the plasmid pDG11. In terms of rpoE transcription, growth, and viability, 35000HP(pDG11) induced for RpoE expression behaved identically to 35000HP(pDG9) (data not shown). Compared to its vector control [35000HP(pDG10)], Western blot analysis of 35000HP(pDG11) using an anti-FLAG antibody showed that RpoE expression was significantly induced at 10 (5.4-fold ± 1.1-fold) and 20 (4.4-fold ± 0.4-fold) minutes after addition of ATc (Fig. 2D).
Transcriptome analysis.
To define RpoE-dependent genes, we compared the transcriptome of the RpoE-overexpressing strain [35000HP(pDG9)] to that of its vector control [35000HP(pDG10)]. We grew the strains to mid-log phase, induced their expression systems with ATc, isolated RNA, and determined their transcriptomes by RNA-Seq.
To reduce indirect transcriptional changes caused by RpoE overexpression, we harvested cells before and at 5 and 10 min after RpoE induction. Within each strain, fold changes in gene expression levels at 5 and 10 min were determined by comparison to preinduction levels. As described previously, a false-discovery rate (FDR) of ≤0.1 and a fold change of ≥2 were used as criteria for differential transcript expression (11). For each strain and time point, four biological replicates were included, totaling 24 samples (see Table S2 in the supplemental material). The percentage of total reads aligned to the reference genome, the percentage of reads aligned to the coding regions, and the average coverage per nucleotide from all strains and time points ranged from 90.7 to 96.7%, 81.3 to 91.4%, and 5.9 to 10.5, respectively (see Table S2 in the supplemental material). The coefficients of determination (R2) of gene expression levels between the samples within each time point and strain ranged from 0.98 to 0.99, indicating that the RNA-Seq data were highly reproducible.
Comparison of the transcriptomes of 35000HP(pDG9) and 35000HP(pDG10) yielded 180 and 312 differentially expressed genes at 5 and 10 min after RpoE induction, respectively; of these, 98.3% and 98% were upregulated (Fig. 3 and Tables 2 and 3; see Table S3 in the supplemental material). Of the 180 and 312 differentially expressed genes, 175 were differentially expressed at both time points (Fig. 3). Since it was possible that some of the transcriptional effects seen after 10 min of induction were indirect, we focused primarily on transcriptional changes seen after 5 min of induction for the remainder of the study.
FIG 3.
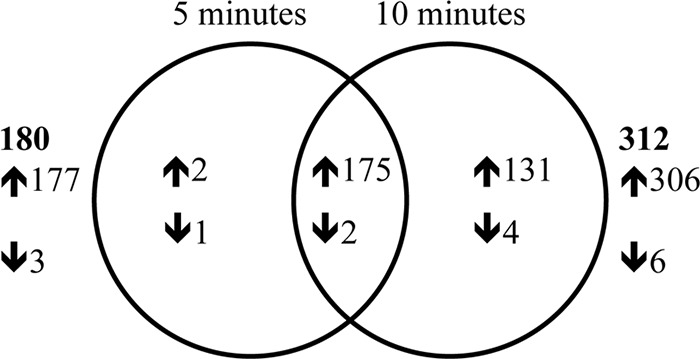
Venn diagram showing the overlap in genes differentially expressed at 5 and 10 min after induction of RpoE expression. The up- and downregulated genes are indicated by ↑ and ↓, respectively. The total number of differentially expressed genes at each time point is indicated in bold. 5 min and 10 min, genes differentially expressed in 35000HP(pDG9) relative to 35000HP(pDG10) at 5 and 10 min after RpoE induction, respectively.
TABLE 2.
Functional classification of genes/open reading frames differentially expressed by overexpression of RpoE in H. ducreyi
| Regulation and function | No. of genesa |
|
|---|---|---|
| 5 min | 10 min | |
| Total differentially expressed genes | 180 (98% upregulated, 2% downregulated) | 312 (98% upregulated, 2% downregulated) |
| Upregulated genes | ||
| Envelope | ||
| Lipooligosaccharide biosynthesis and export | 13 | 21 |
| Outer membrane protein assembly and insertion | 4 | 4 |
| Peptidoglycan metabolism | 10 | 13 |
| Periplasmic chaperones and folding catalysts | 5 | 5 |
| Pilus | —b | 4 |
| Other envelope components | 9 | 10 |
| Cytoplasm | ||
| DNA mismatch repair | 8 | 17 |
| RNA modification | 8 | 10 |
| Regulation of gene expression | 12 | 13 |
| Cofactor biosynthesis | 7 | 14 |
| Amino acid biosynthesis | 1 | 3 |
| Monosaccharide metabolism | 1 | 5 |
| Stress adaptation | 3 | 3 |
| Miscellaneous | 1 | 2 |
| Hypothetical proteins | 95 | 182 |
| Downregulated genes | ||
| Envelope | 1 | 4 |
| Cytoplasm | 1 | 1 |
| Hypothetical proteins | 1 | 1 |
Number of genes differentially expressed in 35000HP(pDG9) compared to 35000HP(pDG10) at 5 or 10 min after induction of RpoE.
—, no genes were found to be differentially expressed.
TABLE 3.
Genes differentially expressed by overexpression of RpoE in H. ducreyi
| Function and gene | Description of product | 5 min |
10 min |
||
|---|---|---|---|---|---|
| Fold changea | P value | Fold changea | P value | ||
| Upregulated genes | |||||
| Envelope | |||||
| Lipopolysaccharide biogenesis and exportb | |||||
| neuAc | CMP N-acetylneuraminic acid synthetase | 2.0 | 5.5E−21 | ||
| lst | Lipooligosaccharide sialyltransferase | 2.3 | 3.3E−22 | ||
| lsgAc | Putative lipopolysaccharide biosynthesis protein | 3.0 | 9.3E−40 | 4.3 | 2.4E−86 |
| lsgD | Possible lipooligosaccharide n-acetylglucosamine glycosyltransferase | 2.8 | 5.3E−44 | 4.7 | 1.4E−107 |
| lsgE | Putative lipooligosaccharide galactosyltransferase | 2.0 | 1.2E−21 | 3.8 | 9.0E−81 |
| lsgF | Putative lipooligosaccharide galactosyltransferase | 2.0 | 5.4E−19 | 3.9 | 6.4E−76 |
| lgtB | Lipooligosaccharide galactosyltransferase II | 3.8 | 2.9E−24 | 4.9 | 3.7E−50 |
| waaF | ADP-heptose-lipooligosaccharide heptosyltransferase II | 3.9 | 5.0E−61 | 6.7 | 3.6E−142 |
| lpxL | Lipid A acyltransferase | 2.3 | 1.9E−32 | ||
| lpxH | UDP-2,3-diacylglucosamine hydrolase | 2.2 | 3.5E−23 | ||
| lpxD | UDP-3-O-(3-hydroxymyristoyl) glucosamine N-acyltransferase | 2.6 | 4.6E−36 | ||
| lpxA | UDP-N-acetylglucosamine O-acyltransferase | 2.1 | 5.6E−24 | ||
| gcp | Putative sialylglycoprotease | 2.5 | 4.8E−28 | 3.3 | 1.8E−56 |
| fabZ | (3R)-Hydroxymyristoyl-acyl carrier protein dehydratase | 2.5 | 2.6E−33 | ||
| galE | UDP-glucose-4-epimerase | 2.2 | 5.0E−21 | 3.3 | 1.1E−56 |
| rmlBc | dTDP-d-glucose 4,6-dehydratase | 2.1 | 2.9E−23 | ||
| glmMc | Phosphoglucosamine mutase | 3.2 | 1.0E−50 | 5.2 | 4.4E−115 |
| ftsH | Cell division protein FtsH | 2.5 | 5.5E−31 | 3.8 | 2.7E−75 |
| HD0552 | Lipopolysaccharide export system permease protein | 3.1 | 2.1E−43 | 4.8 | 5.2E−99 |
| HD0553 | Lipopolysaccharide export system permease protein | 2.6 | 1.9E−34 | 3.7 | 3.5E−77 |
| HD0586 | Lipopolysaccharide export system ATP-binding protein | 2.7 | 3.1E−30 | 3.8 | 1.5E−69 |
| Outer membrane protein assembly and insertion | |||||
| D15/bamA | Outer membrane protein D-15 | 2.5 | 1.4E−28 | 4.3 | 1.2E−82 |
| smpA/bamE | Small protein A | 8.4 | 1.0E−130 | 15.0 | 8.0E−252 |
| lppc | 15-kDa outer membrane lipoprotein | 7.0 | 1.9E−117 | 10.8 | 2.4E−212 |
| lolA | Outer membrane lipoprotein carrier protein | 6.3 | 6.2E−132 | 11.6 | 1.0E−257 |
| Peptidoglycan metabolism | |||||
| murA | UDP-N-acetylglucosamine-1-carboxyvinyltransferase | 3.5 | 2.1E−52 | 5.7 | 8.7E−120 |
| ampG | Permease and possible signal transducer AmpG | 2.4 | 4.1E−24 | 3.8 | 2.2E−67 |
| ampD | Anhydro-N-acetylmuramyl-tripeptide amidase | 7.7 | 4.1E−130 | 13.9 | 1.6E−252 |
| uppSc | Undecaprenyl pyrophosphate synthetase | 8.1 | 2.1E−133 | 11.8 | 8.4E−227 |
| nlpD | Lipoprotein | 2.5 | 3.5E−28 | 4.8 | 4.6E−96 |
| prc | Tail-specific protease | 3.8 | 1.0E−66 | 5.5 | 4.1E−130 |
| mepA | Penicillin-insensitive murein endopeptidase A | 2.5 | 2.9E−29 | ||
| dacA | d-Alanyl-d-alanine carboxypeptidase fraction A | 2.0 | 7.6E−26 | 2.8 | 3.4E−60 |
| lysA | Diaminopimelate decarboxylase | 3.0 | 2.6E−61 | ||
| HD0112 | N-Acetylmuramoyl-l-alanine amidase | 2.4 | 1.1E−04 | 2.7 | 1.2E−06 |
| HD0501 | N-Acetylmuramoyl-acetylmuramoyl-l-alanine amidase | 2.6 | 2.9E−32 | ||
| HD0922 | Hypothetical protein | 2.6 | 9.3E−35 | 5.4 | 1.7E−108 |
| HD1339 | Hypothetical protein | 3.5 | 6.6E−63 | 5.1 | 3.0E−124 |
| Periplasmic chaperones and folding catalysts | |||||
| degP | Periplasmic serine protease do | 7.8 | 1.2E−135 | 13.0 | 5.2E−249 |
| ecfE | Protease EcfE | 3.7 | 3.9E−55 | 5.3 | 1.1E−108 |
| dsbA | Probable thiol:disulfide interchange protein | 3.9 | 8.1E−56 | 6.3 | 1.7E−126 |
| surAc | Peptidyl-prolyl cis-trans isomerase | 3.5 | 5.7E−50 | 4.3 | 1.7E−87 |
| dsbC | Thiol:disulfide interchange protein | 3.4 | 7.1E−55 | 5.3 | 1.4E−119 |
| Pilus | |||||
| fimAc | Possible fimbrial major pilin protein | 2.4 | 1.7E−26 | ||
| fimB | Possible fimbrial structural subunit | 2.2 | 2.4E−17 | ||
| fimC | Probable fimbrial outer membrane usher protein | 2.1 | 1.1E−19 | ||
| fimD | Probable periplasmic fimbrial chaperone | 2.1 | 4.2E−20 | ||
| Other envelope components | |||||
| hlp | Lipoprotein Hlp | 3.0 | 7.2E−39 | 3.8 | 5.3E−72 |
| lspA | Lipoprotein signal peptidase | 2.7 | 1.0E−33 | 3.9 | 2.2E−77 |
| yfeB | Iron (chelated) transporter, ATP-binding protein | 3.4 | 8.1E−49 | 4.4 | 1.3E−87 |
| cdsA | CDP-diglyceride pyrophosphorylase | 6.3 | 2.7E−108 | 8.7 | 8.3E−184 |
| comAc | Possible competence protein A-like protein | 2.1 | 1.8E−10 | 2.8 | 1.0E−23 |
| HD1126 | Leader peptidase HopD | 2.1 | 3.3E−10 | ||
| HD1820 | ABC transporter ATP binding protein | 2.5 | 2.8E−23 | 3.8 | 3.6E−62 |
| HD1821 | ABC transporter | 2.4 | 2.0E−19 | 3.6 | 1.8E−52 |
| oapA | Opacity associated protein A | 6.0 | 4.6E−102 | 9.9 | 6.7E−200 |
| oapB | Opacity associated protein B | 4.5 | 4.2E−72 | 7.4 | 5.6E−153 |
| Cytoplasm | |||||
| DNA mismatch repair | |||||
| topB1 | DNA topoisomerase III | 2.5 | 1.1E−34 | ||
| ssb2 | Single-stranded DNA-binding protein | 2.8 | 9.3E−27 | ||
| topB2 | DNA topoisomerase III | 2.3 | 7.6E−28 | ||
| sbcBc | Exodeoxyribonuclease I | 2.9 | 1.7E−36 | 3.9 | 2.2E−74 |
| trpH | TrpH-like protein | 2.3 | 8.4E−31 | ||
| dnaX | DNA polymerase III subunits gamma and tau | 2.8 | 3.2E−31 | 4.1 | 2.8E−71 |
| holB | DNA polymerase III delta′ subunit | 3.1 | 5.3E−35 | 4.8 | 6.5E−88 |
| mutYc | A/G-specific adenine glycosylase | 2.5 | 2.3E−44 | ||
| recJ | Single-stranded-DNA-specific exonuclease RecJ | 3.0 | 2.0E−47 | 5.2 | 7.2E−120 |
| gam | Putative mu phage host nuclease inhibitor protein | 2.1 | 2.9E−06 | ||
| recQ | ATP-dependent DNA helicase | 2.0 | 4.3E−21 | ||
| uvrD | DNA helicase II | 2.2 | 2.4E−19 | 3.8 | 2.5E−66 |
| uvrB | Excinuclease ABC subunit B | 2.3 | 5.4E−29 | ||
| mutT | 7,8-Dihydro-8-oxoguanine-triphosphatase | 4.6 | 1.2E−79 | 6.9 | 6.0E−153 |
| aptc | Adenine phosphoribosyltransferase | 3.7 | 1.2E−44 | 5.0 | 6.3E−89 |
| tmk | Thymidylate kinase | 3.4 | 4.3E−43 | 5.3 | 3.6E−102 |
| upp | Uracil phosphoribosyltransferase | 3.2 | 1.2E−59 | ||
| RNA modification | |||||
| trmAc | tRNA (uracil-5-)-methyltransferase | 2.2 | 2.1E−31 | ||
| miaA | tRNA delta-2-isopentylpyrophosphate transferase | 4.1 | 4.2E−62 | 5.8 | 6.0E−120 |
| rsuA | Ribosomal small subunit pseudouridine synthase A | 3.3 | 8.7E−57 | 4.3 | 3.7E−102 |
| ksgA | Dimethyladenosine transferase | 3.0 | 2.7E−36 | 3.7 | 1.8E−67 |
| tgt | tRNA-guanine transglycosylase | 2.3 | 2.0E−27 | ||
| rluA | Pseudouridylate synthase | 4.5 | 8.9E−71 | 6.8 | 1.1E−139 |
| rumB | 23S rRNA (uracil-5-)-methyltransferase | 3.7 | 2.0E−42 | 4.6 | 8.5E−73 |
| rimK | Ribosomal protein S6 modification protein | 6.4 | 4.7E−116 | 11.5 | 2.2E−232 |
| HD1138 | tRNA pseudouridine synthase C | 2.4 | 3.6E−28 | 3.7 | 3.4E−74 |
| HD1770 | Nitrogen regulatory protein | 2.0 | 1.2E−20 | 2.5 | 4.9E−41 |
| Regulation of gene expression | |||||
| rpoH | RNA polymerase sigma 32 factor | 4.5 | 8.9E−73 | 6.4 | 4.4E−135 |
| rseA | Possible sigma E factor negative regulatory protein | 4.9 | 1.5E−79 | 6.6 | 5.2E−139 |
| rseC | Possible sigma E factor regulatory protein | 2.8 | 9.0E−40 | 3.5 | 3.1E−71 |
| cpxR | Transcriptional regulatory protein CpxR | 3.0 | 1.9E−42 | 4.3 | 1.6E−90 |
| cpxA | Sensor kinase CpxA | 2.4 | 1.1E−27 | 3.2 | 5.0E−58 |
| ptsN | Phosphotransferase system, nitrogen regulatory IIA-like protein | 2.5 | 4.2E−28 | 3.8 | 2.2E−69 |
| hfq | Putative host factor I protein | 3.0 | 1.2E−35 | 4.1 | 3.9E−73 |
| glnB | Putative nitrogen regulatory protein P-II | 2.6 | 5.3E−35 | 3.9 | 8.0E−83 |
| argRc | Arginine repressor | 2.3 | 4.5E−29 | ||
| asnC | Transcription regulatory protein, AsnC | 2.0 | 4.0E−19 | 2.6 | 8.3E−42 |
| cysB | Cys regulon transcriptional activator | 4.3 | 5.7E−75 | 7.0 | 3.2E−156 |
| fabR | DNA-binding transcriptional repressor FabR | 4.2 | 2.0E−74 | 6.6 | 8.3E−151 |
| Cofactor biosynthesis | |||||
| mogAc | Molybdopterin biosynthesis protein | 2.7 | 3.2E−38 | 4.1 | 2.9E−90 |
| ispH | Hydroxymethylbutenyl pyrophosphate reductase | 2.2 | 5.5E−22 | 3.4 | 3.0E−62 |
| visC | Probable monooxygenase | 2.7 | 3.0E−40 | ||
| hemY | HemY protein | 2.3 | 4.8E−40 | ||
| hemX | Putative uroporphyrinogen III C-methyltransferase | 2.2 | 2.3E−39 | ||
| modA | Molybdate-binding periplasmic protein | 3.1 | 4.7E−48 | ||
| modB | Molybdenum ABC transporter, permease protein | 2.8 | 6.2E−41 | ||
| modC | Molybdenum ABC transporter, ATP-binding protein | 2.7 | 4.7E−39 | ||
| lipA | Lipoic acid synthetase | 3.0 | 2.3E−58 | ||
| lipB | Lipoate biosynthesis protein B | 2.1 | 2.3E−27 | 3.3 | 8.3E−74 |
| rpiA | Ribose 5-phosphate isomerase A | 2.7 | 1.6E−39 | 4.9 | 1.2E−111 |
| mdh | Malate dehydrogenase | 3.2 | 1.0E−41 | 4.4 | 2.7E−84 |
| HD0261 | Hypothetical protein | 3.7 | 4.6E−53 | 5.6 | 4.5E−115 |
| HD1410 | Hypothetical protein | 2.9 | 1.1E−35 | 4.0 | 1.1E−74 |
| Amino acid biosynthesis | |||||
| argH | Arginosuccinate lyase | 2.9 | 4.8E−41 | ||
| aroC | Chorismate synthase | 2.4 | 2.8E−31 | ||
| cysZ | Cysteine synthetase | 2.8 | 1.3E−35 | 4.6 | 1.1E−89 |
| Monosaccharide metabolism | |||||
| pflB | Formate acetyltransferase | 2.4 | 3.7E−34 | 3.8 | 4.5E−89 |
| citC | Citrate lyase synthetase | 3.6 | 5.0E−72 | ||
| citDc | Citrate lyase gamma chain | 3.1 | 3.5E−51 | ||
| citE | Citryl coenzyme A lyase subunit | 2.9 | 6.4E−54 | ||
| citF | Citrate coenzyme A transferase subunit | 2.1 | 8.2E−26 | ||
| Stress adaptation | |||||
| surEc | Acid phosphatase stationary-phase survival protein | 5.0 | 5.0E−90 | 8.9 | 2.2E−191 |
| proQ | Possible ProP effector | 4.5 | 3.1E−79 | 6.1 | 3.8E−141 |
| mazGc | MazG protein | 3.1 | 5.9E−46 | 4.2 | 5.6E−88 |
| Miscellaneous | |||||
| vacB | RNase R, virulence associated VacB-like protein | 2.9 | 1.2E−33 | 5.1 | 4.1E−98 |
| hflX | GTP-binding protein HflX | 2.4 | 4.5E−25 | ||
| Downregulated genes | |||||
| Envelope | |||||
| ccmD | Cytochrome c maturation protein D | −2.1 | 2.2E−04 | ||
| ompA2c | Major outer membrane protein-like protein OmpA2 | −2.6 | 2.3E−45 | ||
| ompP2A | Outer membrane protein P2-like protein | −2.3 | 1.7E−30 | ||
| ompP2B | Outer membrane protein P2-like protein | −2.2 | 8.1E−19 | −2.7 | 5.3E−36 |
| Cytoplasm | |||||
| purM | Phosphoribosylformylglycinamidine cyclo-ligase | −2.7 | 7.0E−10 | ||
| pyrE | Orotate phosphoribosyltransferase | −2.1 | 7.9E−28 | ||
Mean fold change in expression of genes in 35000HP(pDG9) compared to 35000HP(pDG10) at 5 or 10 min after induction of RpoE; the fold change was normalized by dividing the fold change at 5 or 10 min with that of 0 min.
Differentially expressed genes were categorized into different functional categories using DAVID bioinformatics resources.
First gene of a known or putative operon predicted by the DOOR database.
qRT-PCR confirms the fold changes determined by RNA-Seq.
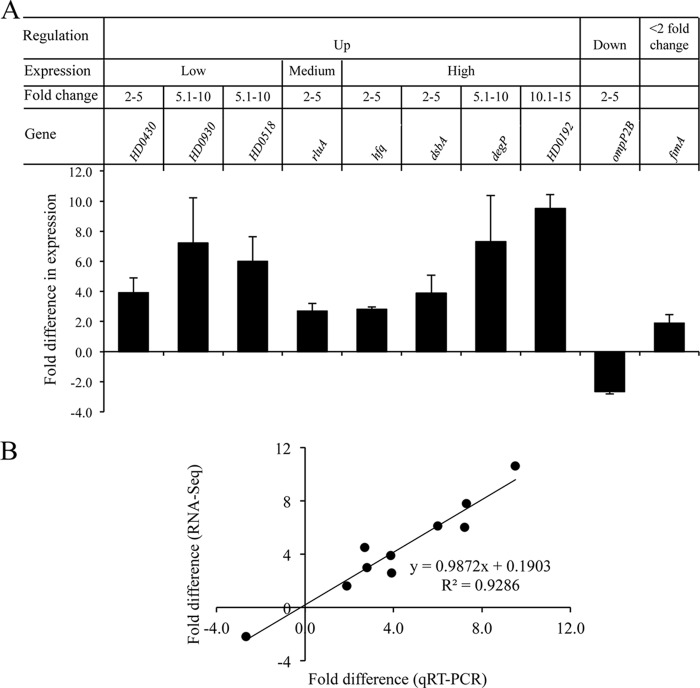
We validated selected differentially regulated targets using qRT-PCR (11). The targets were first grouped into up- and downregulated genes (Fig. 4A). The upregulated genes were then subgrouped based on their expression levels (high, medium, and low) (Fig. 4A). Genes in each expression level were further stratified based on their fold change (2.0 to 5.0, 5.1 to 10.0, and 10.1 to 15.0) (Fig. 4A). Representative genes were selected arbitrarily from each category; a total of 9 genes were evaluated by qRT-PCR. qRT-PCR analysis confirmed the differential expression of 9/9 targets identified by RNA-Seq (Fig. 4A). Fold changes derived from RNA-Seq were in good agreement with those derived from qRT-PCR (Fig. 4B).
FIG 4.
qRT-PCR validation of the RNA-Seq data. (A) Fold change in the expression of target genes in 35000HP(pDG9) relative to 35000HP(pDG10) at 5 min after induction. The criteria used for selecting the targets for qRT-PCR validation are outlined in the figure. The expression levels of target genes were normalized to that of dnaE. The data represent the means ± SD from four independent experiments. (B) Correlation between the fold changes derived from qRT-PCR and RNA-Seq.
De novo identification of putative RpoE promoter motifs.
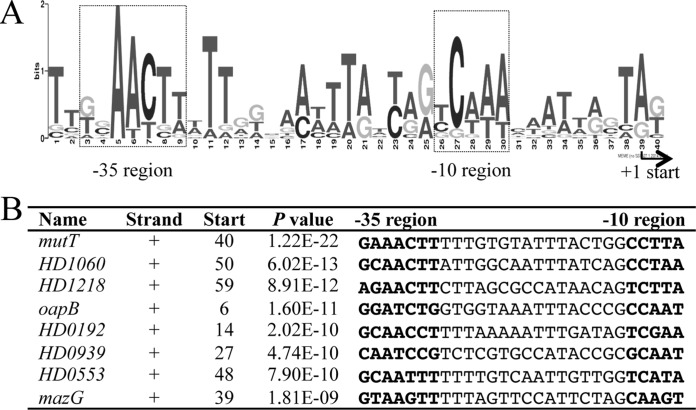
The differentially regulated genes belonged to 73 putative transcriptional units (TUs); transcription start sites (TSSs) were predicted for 44 of these TUs using RNA-Seq data (see Table S4 in the supplemental material). A de novo motif analysis of the 100-bp upstream putative promoter regions from the predicted TSSs of the 44 RpoE-dependent TUs identified an RpoE consensus sequence with an expected value of 3.5 × 10−2 (Fig. 5A and B). The identified sequence logo consisted of a −35 region, a 15- to 17-nucleotide spacer, and a −10 region. The H. ducreyi logo was similar to the E. coli RpoE consensus sequence, which contains the −35 motif GGAACTT, a 15- to 19-nucleotide spacer, and the −10 motif TCAAA (23).
FIG 5.
Sequence logo of the H. ducreyi RpoE promoter motifs. (A) Sequence logo of the H. ducreyi RpoE promoter motifs. The logo was generated by the MEME algorithm using the 100-bp upstream sequences from the TSSs of 44 of 73 RpoE-dependent TUs for which the TSSs could be predicted based on the RNA-Seq data. The putative TSS is indicated, and the putative −10 and −35 regions are boxed. (B) Multiple-sequence alignment of the RpoE promoter region in the eight putative RpoE-dependent promoters that contained the most significant matches. Name, gene in which the motif was identified; Strand, strand in which the gene is located (+, sense strand; −, antisense strand); Start, start position of the binding site relative to the TSS; P value, P value for the predicted promoter motifs; −35 and −10 regions of the predicted promoter are highlighted in bold.
A genome-wide search using a position-specific scoring matrix (PSSM) derived from the H. ducreyi −35 and −10 motifs in the 450-bp upstream and 100-bp downstream regions from the translational start codon showed that this logo was present in the upstream putative promoter sequences of 51 out of 73 (70%) RpoE-dependent TUs and in 326 out of 616 (53%) TUs whose expression levels were not differentially regulated (odds ratio = 2.06; P < 0.006) (see Table S5 in the supplemental material). Given that a relatively higher percentage of nondifferentially regulated TUs contained the RpoE promoter motifs, we calculated the random occurrence of the motifs in the H. ducreyi genome using Fisher's exact test; the results from this analysis showed that the motif was randomly found in 337 out of 616 (55%) nondifferentially regulated TUs. The percentage of nondifferentially regulated TUs that contained the RpoE motifs by chance (55%) is close to the percentage of nondifferentially regulated TUs that contained the RpoE motifs in our enrichment analysis (53%). Using random occurrence as 55%, the expected occurrence of the predicted motifs in differentially regulated TUs is 40 out of 73. However, our enrichment analysis showed that 51 out of 73 differentially regulated TUs contained the RpoE motifs, indicating that the promoter motifs were 27.5% enriched in the differentially regulated TUs compared to those whose expression levels were not differentially regulated. These data suggest that the high occurrence of the RpoE motifs in nondifferentially regulated TUs is likely due to random occurrence of the RpoE promoter motifs in the H. ducreyi genome. Our data also suggest that, despite the high random occurrence of the RpoE promoter motifs in the H. ducreyi genome, the identified promoter motifs were enriched in RpoE-dependent TUs.
Reporter assays confirm RpoE regulation of putative target promoters.
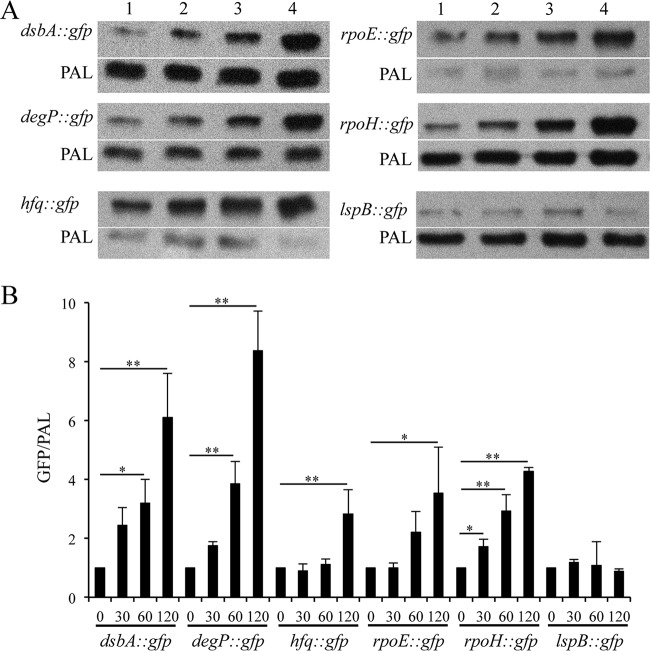
We sought to determine the RpoE dependence of putative target promoters in 35000HP. We constructed reporter plasmids containing the promoter regions of the RpoE targets dsbA, degP, hfq, rpoE, and rpoH. As a negative control, we used an lspB reporter, which lacks the putative RpoE-dependent promoter. The reporters were transformed into 35000HP(pDG17), which contains the rpoE inducible system. Overexpression of RpoE from the low-copy-number plasmid pDG17 did not affect the viability of H. ducreyi after induction for up to 2 h (data not shown). Therefore, we induced RpoE expression and measured the ratio of GFP protein levels relative to PAL at 0, 30, 60, and 120 min after RpoE induction. Compared to at 0 min, the reporter activity of dsbA (60 min, 3.2-fold ± 0.8-fold; 120 min, 6.1-fold ± 1.4-fold), degP (60 min, 3.8-fold ± 0.7-fold; 120 min, 8.3-fold ± 1.3-fold), hfq (120 min, 2.8-fold ± 0.8-fold), and rpoH (30 min, 1.7-fold ± 0.3-fold; 60 min, 2.9-fold ± 0.5-fold; 120 min, 4.3-fold ± 0.4-fold) significantly increased after induction, confirming RpoE regulation of these targets (Fig. 6A and B). As expected, the activity of an lspB reporter did not increase after induction (Fig. 6A and B).
FIG 6.
Promoter-reporter analysis of the RpoE-dependent targets identified by RNA-Seq. (A) In vivo transcriptional activity of the putative RpoE-dependent promoters at 0 (lane 1), 30 (lane 2), 60 (lane 3), and 120 (lane 4) minutes after RpoE induction. For assessing the promoter activity, whole-cell lysates were prepared from H. ducreyi strains containing pDG17 and the reporter constructs at 0, 30, 60, and 120 min after RpoE induction and probed with an anti-GFP monoclonal antibody and anti-PAL monoclonal antibody 3B9, which served as a loading control. (B) Densitometry analysis of the Western blots from panel A. The data represent the means ± SD from three independent experiments. *, P < 0.05; **, P < 0.01.
RpoE overexpression increased the transcript levels of rseA and rseC (Table 2). RT-PCR analysis suggested that rpoE, rseA, and rseC are in an operon (Fig. 1B). Compared to at 0 min, the reporter activity of the putative promoter preceding the rpoE TU significantly increased at 120 min after RpoE induction (P < 0.05), suggesting that RpoE is autoregulated in H. ducreyi (Fig. 6A and B).
Site-directed mutagenesis confirms the predicted RpoE-dependent promoter.
The majority of the RpoE-dependent targets contained one or more putative RpoE promoter motifs (see Table S5 in the supplemental material). To validate whether RpoE utilizes the predicted RpoE-dependent promoter motifs to control its targets, we selected the reporter strain containing the putative rpoH promoter region. Bioinformatics analysis showed the presence of two putative RpoE-dependent promoter motifs in the putative rpoH promoter region (Fig. 7A). To identify which of these motifs was required for RpoE-dependent regulation of rpoH, we mutagenized the third, fourth, and fifth nucleotides in the −35 regions of the 2 putative promoters (Fig. 7A). Compared to at 0 min, the reporter activity of 35000HP(pDG16)(pDG17), which contains the wild-type putative rpoH promoter, significantly increased after RpoE induction (120 min, 3.5-fold ± 1.3-fold) (Fig. 7B and C). Compared to at 0 min, the reporter activity of 35000HP(pDG19)(pDG17), which harbors mutations in the putative promoter 2 region, significantly increased after RpoE induction (60 min, 2.3-fold ± 0.2-fold; 120 min, 3.5-fold ± 0.5-fold) (Fig. 7B and C). However, compared to at 0 min, the reporter activity of 35000HP(pDG18)(pDG17), which harbors mutations in the putative promoter 1 region, was unaltered after RpoE induction (Fig. 7B and C). These data suggest that the putative promoter 1 region is required for RpoE-dependent regulation of rpoH and that RpoE likely utilizes the predicted promoter sequence to control its targets (Fig. 5A).
Functional analysis of genes regulated by RpoE.
We analyzed the genes that were differentially regulated after 5 and 10 min of induction (Tables 2 and 3). The RpoE-dependent genes belonged to several functional categories; DAVID bioinformatics analyses showed that several pathways or functional clusters were statistically enriched (30). Clusters involved in envelope homeostasis such as those encoding factors involved in LPS biosynthesis and export (lsgA, lsgD, lsgE, lsgF, lgtB, waaF, glmM, galE, ftsH, HD0552, HD0553, and HD0586), OMP assembly and insertion (D15/bamA, smpA/bamE, lpp, and lolA), peptidoglycan metabolism (murA, ampD, uppS, ampG, nlpD, prc, dacA, HD0112, HD0922, and HD1339), and periplasmic chaperones and folding catalysts (degP, ecfE, dsbA, surA, and dsbC) constituted a major part of the enriched clusters (Table 3). A second major enriched cluster encoded homologues of factors involved in DNA mismatch repair (recJ, sbcB, mutT, uvrB, apt, tmk, and dnaX) and RNA modification (ksgA, rsuA, rluA, miaA, rumB, HD1138, and HD1770) (Table 3). A third major cluster of enriched genes encoded homologues of factors involved in regulation of gene expression (glnB, cysB, cpxR, cpxA, hfq, rpoH, rseA, rseC, ptsN, and asnC) (Table 3).
Comparison of RpoE-dependent genes to the CpxR and Hfq regulons and genes differentially regulated during stationary phase.
We recently defined the genes differentially regulated by CpxR, Hfq, and growth in stationary phase relative to mid-log phase in H. ducreyi; we found that Hfq regulates stationary-phase gene expression in H. ducreyi (11, 36). Since RpoE overexpression increased the transcription of cpxRA and hfq, we compared the RpoE-dependent genes to those differentially regulated by CpxR, Hfq, and stationary phase relative to mid-log phase. For this analysis, overlap was defined as differentially regulated genes whose expression was positively correlated (i.e., both upregulated or both downregulated). Compared to a cpxR deletion mutant, a cpxR-activating mutant differentially regulates approximately 140 genes in H. ducreyi harvested from stationary phase. There was no overlap between the 140 genes differentially regulated by CpxR and the 180 genes differentially regulated by RpoE overexpression. There are approximately 282 Hfq-dependent genes in H. ducreyi. There was less overlap of the genes differentially regulated by RpoE overexpression and those regulated by Hfq than was expected by chance (12/180 genes) (odds ratio = 0.35; P = 0.00015). Compared to organisms grown to mid-log phase, H. ducreyi harvested from stationary phase differentially regulated approximately 288 genes. Considering these 288 genes, there was a significant overlap of 180 genes differentially regulated by RpoE overexpression (46/180 genes) (odds ratio = 1.93; P = 0.0006). Taken together, these data suggest that under the conditions tested, the RpoE, CpxRA, and Hfq regulons do not appear to significantly overlap and that RpoE may play a role in stationary-phase gene regulation in H. ducreyi.
DISCUSSION
The H. ducreyi genome encodes homologues of two systems that generally respond to stresses affecting the cell envelope: RpoE and CpxRA. We previously showed that activation of CpxRA in H. ducreyi represses the majority of its targets encoding envelope-localized proteins, including known virulence determinants, but does not affect the transcription of genes involved in envelope protein folding and chaperoning (11, 12). To potentially understand how H. ducreyi regulates envelope stress responses, here we defined RpoE-dependent genes in H. ducreyi. We compared the transcriptome of a strain containing a plasmid that can be induced to expresses RpoE to that of a strain containing a vector control. The rationale for this approach is discussed in detail by Rhodius et al., who employed the same strategy to identify RpoE-dependent genes in E. coli (23). RpoE overexpression upregulated cpxRA and hfq, which are also involved in gene regulation in H. ducreyi (11, 12, 36). This raised the possibility that some of the transcriptional effects caused by overexpression of RpoE were indirect. Comparison of RpoE-dependent genes to those regulated by CpxR and Hfq showed no or little overlap. All together, these data suggest that the transcriptional changes resulting from RpoE overexpression are more likely due to direct regulation by RpoE and less likely due to secondary activation of other regulatory systems.
We showed that H. ducreyi RpoE upregulates 98% of its targets; a large number of these encode factors involved in envelope maintenance and repair. Unlike RpoE, H. ducreyi CpxRA downregulates the majority of its targets; most of these encode envelope-localized proteins (11, 12). Thus, RpoE functions primarily to maintain and repair the envelope, while CpxRA functions primarily to reduce protein traffic across the envelope, which otherwise may exacerbate preexisting envelope stress. These data suggest that RpoE and CpxRA appear to play distinct yet complementary roles in regulating envelope homeostasis in H. ducreyi.
H. ducreyi lacks a homologue of RpoS, which regulates stationary-phase gene expression in other organisms (37). Compared to mid-log phase, entry into stationary phase is associated with differential regulation of 288 H. ducreyi genes (36). Comparison of the genes altered by overexpression of RpoE to those differentially regulated in stationary phase relative to mid-log phase showed that approximately 26% of the genes altered by overexpression of RpoE overlapped and were positively correlated with those differentially regulated in stationary phase relative to mid-log phase (36). Hfq is a major regulator of stationary-phase gene expression in H. ducreyi (36). Although RpoE overexpression increased hfq transcription, comparison of RpoE-dependent genes to those regulated by Hfq showed little overlap, suggesting that the overlap between RpoE-dependent genes and those upregulated in stationary phase is not due to RpoE-dependent upregulation of hfq expression (36). Although not experimentally addressed in this study, the data suggest that RpoE may also play a role in stationary-phase gene regulation in H. ducreyi.
One limitation of our study is that we did not define physiological signals that activate RpoE in H. ducreyi. We considered using the reporter constructs containing putative RpoE-dependent promoters to study such signals. However, due to our inability to recover an rpoE mutant, we did not have an appropriate negative control for these studies. In E. coli, RpoE is activated by a variety of envelope-perturbing stresses that lead to misfolded proteins in the outer membrane, upregulates several genes involved in envelope maintenance and repair, and coordinates with other stress response regulators (16, 17, 20, 23). Similarly, H. ducreyi RpoE upregulated a large number of genes encoding factors involved in envelope maintenance and repair as well as those encoding several other stress response regulators. Despite the lack of information about the signals that activate RpoE in H. ducreyi, our results suggest that RpoE likely controls the response to envelope stress in this organism.
Our inability to obtain an rpoE mutant suggests that this ECF sigma factor is likely essential in H. ducreyi. A large set of RpoE-dependent genes encodes homologues of factors involved in maintenance or repair of the cell envelope, suggesting that loss of RpoE may lead to loss of envelope integrity. On the other hand, RpoE overexpression was also toxic to H. ducreyi. This is perhaps due to redirection of cellular resources away from essential functions toward the synthesis of envelope components. Alternatively, as most RpoE-dependent genes encode homologues of proteins that localize to the periplasm/outer membrane, overexpression of RpoE may lead to saturation of membrane protein translocation machinery, resulting in cellular toxicity. These data underscore the fact that RpoE expression needs to be tightly regulated in order to ensure viability.
In E. coli, the CpxRA and RpoE systems are interconnected. For example, both RpoE and CpxRA positively regulate a large number of genes encoding protein folding and chaperoning factors and jointly regulate degP (17, 20, 23, 38–40). In H. ducreyi, RpoE regulates homologues of these genes, including degP, while CpxRA does not. In E. coli, activation of CpxRA negatively regulates rpoE transcription, and RpoE does not regulate cpxRA transcription (20, 23, 38). In contrast, activation of CpxRA does not regulate rpoE transcription in H. ducreyi, but RpoE positively regulates cpxRA transcription (11). Thus, the interconnections between the CpxRA and RpoE systems in H. ducreyi differ from those in E. coli.
We conclude that H. ducreyi RpoE positively regulates a large number of genes involved in envelope maintenance and repair. In addition to its role in envelope homeostasis, our findings also suggest a possible role for RpoE in stationary-phase gene regulation in H. ducreyi. Future studies will focus on characterizing the contribution of RpoE-dependent genes to the ability of H. ducreyi to survive in humans.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant AI27863 to S.M.S. from the National Institute of Allergy and Infectious Diseases (NIAID).
We have no relevant financial relationships to disclose.
We thank Margaret Bauer, Julia Williams, and Concerta Holley for their thoughtful criticism of the manuscript.
Footnotes
Published ahead of print 8 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02034-14.
REFERENCES
- 1.Spinola SM, Ballard RC. 2010. Chancroid, p 141–156 In Morse SA, Holmes KK, Ballard RC. (ed), Atlas of sexually transmitted diseases and AIDS, 4th ed. Saunders, Philadelphia, PA. [Google Scholar]
- 2.Spinola SM. 2008. Chancroid and Haemophilus ducreyi, p 689–699 In Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH. (ed), Sexually transmitted diseases, 4th ed. McGraw-Hill, New York, NY. [Google Scholar]
- 3.Ussher JE, Wilson E, Campanella S, Taylor SL, Roberts SA. 2007. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin. Infect. Dis. 44:e85–e87. 10.1086/515404. [DOI] [PubMed] [Google Scholar]
- 4.McBride WJ, Hannah RC, Le Cornec GM, Bletchly C. 2008. Cutaneous chancroid in a visitor from Vanuatu. Australas. J. Dermatol. 49:98–99. 10.1111/j.1440-0960.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 5.Peel TN, Bhatti D, De Boer JC, Stratov I, Spelman DW. 2010. Chronic cutaneous ulcers secondary to Haemophilus ducreyi infection. Med. J. Aust. 192:348–350. [DOI] [PubMed] [Google Scholar]
- 6.Mitjà O, Lukehart SA, Pokowas G, Moses P, Kapa A, Godornes C, Robson J, Cherian S, Houinei W, Kazadi W, Siba P, de Lazzari E, Bassat Q. 2014. Haemophilus ducreyi as a cause of skin ulcers in children from a yaws-endemic area of Papua New Guinea: a prospective cohort study. Lancet Global Health 2:e235–e241. 10.1016/S2214-109X(14)70019-1. [DOI] [PubMed] [Google Scholar]
- 7.Janowicz DM, Ofner S, Katz BP, Spinola SM. 2009. Experimental infection of human volunteers with Haemophilus ducreyi: fifteen years of clinical data and experience. J. Infect. Dis. 199:1671–1679. 10.1086/598966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer ME, Goheen MP, Townsend CA, Spinola SM. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549–2557. 10.1128/IAI.69.4.2549-2557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer ME, Townsend CA, Ronald AR, Spinola SM. 2006. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microbe Infect. 8:2465–2468. 10.1016/j.micinf.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Spinola SM, Fortney KR, Baker B, Janowicz DM, Zwickl B, Katz BP, Blick RJ, Munson RS., Jr 2010. Activation of the CpxRA system by deletion of cpxA impairs the ability of Haemophilus ducreyi to infect humans. Infect. Immun. 78:3898–3904. 10.1128/IAI.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangaiah D, Zhang X, Fortney KR, Baker B, Liu Y, Munson RS, Jr, Spinola SM. 2013. Activation of CpxRA in Haemophilus ducreyi primarily inhibits the expression of its targets, including major virulence determinants. J. Bacteriol. 195:3486–3502. 10.1128/JB.00372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labandeira-Rey M, Brautigam CA, Hansen EJ. 2010. Characterization of the CpxRA regulon in Haemophilus ducreyi. Infect. Immun. 78:4779–4791. 10.1128/IAI.00678-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labandeira-Rey M, Dodd D, Fortney KR, Zwickl B, Katz BP, Janowicz DM, Spinola SM, Hansen EJ. 2011. A Haemophilus ducreyi cpxR deletion mutant is virulent in human volunteers. J. Infect. Dis. 203:1859–1865. 10.1093/infdis/jir190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ades SE. 2008. Regulation by destruction: design of the sigmaE envelope stress response. Curr. Opin. Microbiol. 11:535–540. 10.1016/j.mib.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz N, Silhavy TJ. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122–126. 10.1016/j.mib.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Alba BM, Gross CA. 2004. Regulation of the Escherichia coli sigmaE-dependent envelope stress response. Mol. Microbiol. 52:613–619. 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- 17.Raivio TL, Silhavy TJ. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591–624. 10.1146/annurev.micro.55.1.591. [DOI] [PubMed] [Google Scholar]
- 18.De Las Penas A, Connolly L, Gross CA. 1997. The sigmaE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigmaE. Mol. Microbiol. 24:373–385. 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 19.Missiakas D, Mayer MP, Lemaire M, Georgopoulos C, Raina S. 1997. Modulation of the Escherichia coli sigmaE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol. Microbiol. 24:355–371. 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 20.Rowley G, Spector M, Kormanec J, Roberts M. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4:383–394. 10.1038/nrmicro1394. [DOI] [PubMed] [Google Scholar]
- 21.Chaba R, Alba BM, Guo MS, Sohn J, Ahuja N, Sauer RT, Gross CA. 2011. Signal integration by DegS and RseB governs the sigmaE-mediated envelope stress response in Escherichia coli. Proc. Natl. Acad. Sci., U. S. A. 108:2106–2111. 10.1073/pnas.1019277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima S, Guo MS, Chaba R, Gross CA, Sauer RT. 2013. Dual molecular signals mediate the bacterial response to outer-membrane stress. Science 340:837–841. 10.1126/science.1235358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. 2006. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 4:e2. 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bury-Mone S, Nomane Y, Reymond N, Barbet R, Jacquet E, Imbeaud S, Jacq A, Bouloc P. 2009. Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 5:e1000651. 10.1371/journal.pgen.1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Las Penas A, Connolly L, Gross CA. 1997. SigmaE is an essential sigma factor in Escherichia coli. J. Bacteriol. 179:6862–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayden JD, Ades SE. 2008. The extracytoplasmic stress factor, sigmaE, is required to maintain cell envelope integrity in Escherichia coli. PLoS One 3:e1573. 10.1371/journal.pone.0001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison A, Santana EA, Szelestey BR, Newsom DE, White P, Mason KM. 2013. Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae. Infect. Immun. 81:1221–1233. 10.1128/IAI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitby PW, Morton DJ, Stull TL. 1998. Construction of antibiotic resistance cassettes with multiple paired restriction sites for insertional mutagenesis of Haemophilus influenzae. FEMS Microbiol. Lett. 158:57–60. 10.1111/j.1574-6968.1998.tb12800.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56–65. 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 30.Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4:44–57. 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 31.Bailey TL, Elkan C. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2:28–36. [PubMed] [Google Scholar]
- 32.Mao F, Dam P, Chou J, Olman V, Xu Y. 2009. DOOR: a database for prokaryotic operons. Nucleic Acids Res. 37:D459–D463. 10.1093/nar/gkn757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Taylor MW, Edenberg HJ. 2006. Model-based identification of cis-acting elements from microarray data. Genomics 88:452–461. 10.1016/j.ygeno.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Cormack BP, Valdivia RH, Falkow S. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38. 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 35.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangaiah D, Labandeira-Rey M, Zhang X, Fortney KR, Ellinger S, Zwickl B, Baker B, Liu Y, Janowicz DM, Katz BP, Brautigam CA, Munson RS, Jr, Hansen EJ, Spinola SM. 2014. Haemophilus ducreyi Hfq contributes to virulence gene regulation as cells enter stationary phase. mBio 5(1):e01028-13. 10.1128/mBio.01081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65:189–213. 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191:1798–1815. 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connolly L, De Las Penas A, Alba BM, Gross CA. 1997. The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways. Genes Dev. 11:2012–2021. 10.1101/gad.11.15.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raivio TL, Leblanc SK, Price NL. 2013. The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J. Bacteriol. 195:2755–2767. 10.1128/JB.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Tawfiq JA, Thornton AC, Katz BP, Fortney KR, Todd KD, Hood AF, Spinola SM. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684–1687. 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 42.Dixon LG, Albritton WL, Willson PJ. 1994. An analysis of the complete nucleotide sequence of the Haemophilus ducreyi broad-host-range plasmid pLS88. Plasmid 32:228–232. 10.1006/plas.1994.1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.