Abstract
The Gram-negative soil bacterium Myxococcus xanthus utilizes its social (S) gliding motility to move on surfaces during its vegetative and developmental cycles. It is known that S motility requires the type IV pilus (T4P) and the exopolysaccharide (EPS) to function. The T4P is the S motility motor, and it powers cell movement by retraction. As the key regulator of the S motor, EPS is proposed to be the anchor and trigger for T4P retraction. The production of EPS is regulated in turn by the T4P in M. xanthus, and T4P− mutants are S− and EPS−. In this study, a ΔpilA strain (T4P− and EPS−) was mutagenized by a transposon and screened for EPS+ mutants. A pilA suppressor isolated as such harbored an insertion in the 3rd clustered regularly interspaced short palindromic repeat (CRISPR3) in M. xanthus. Evidence indicates that this transposon insertion, designated CRISPR3*, is a gain-of-function (GOF) mutation. Moreover, CRISPR3* eliminated developmental aggregation in both the wild-type and the pilA mutant backgrounds. Upstream of CRISPR3 are genes encoding the repeat-associated mysterious proteins (RAMPs). These RAMP genes are indispensable for CRISPR3* to affect development and EPS in M. xanthus. Analysis by reverse transcription (RT)-PCR suggested that CRISPR3* led to an increase in the processing of the RNA transcribed from CRISPR3. We propose that certain CRISPR3 transcripts, once expressed and processed, target genes critical for M. xanthus fruiting body development and EPS production in a RAMP-dependent manner.
INTRODUCTION
The soil bacterium Myxococcus xanthus is adapted to move and live on solid surfaces during both its vegetative and developmental cycles (1). In the vegetative cycle, this predatory organism spreads out or swarms over surfaces to consume other bacteria and organic matter in its environment as nutrients. Starvation conditions trigger the developmental cycle, which involves the coordinated movements of hundreds of thousands of cells to form a dome-shaped fruiting body on surfaces (2, 3). In the fruiting body, vegetative cells differentiate into metabolically dormant and environmentally resistant myxospores (3). M. xanthus relies on two genetically distinct forms of gliding motility to move. Adventurous (A) gliding enables the movement of well-isolated cells. Social (S) gliding, which is powered by the type IV pilus (T4P), requires cells to be in groups or in close physical proximity (4–6). The T4P is a polymeric protein filament composed of pilin monomers encoded by the pilA gene. It is the retraction of the assembled T4P filament that results in the movement of the cell on a surface.
Exopolysaccharide (EPS), another structural component on the cell surface, is required for S motility and M. xanthus development (2, 6). M. xanthus EPS may be associated with the outer cell surface and is thought to function as the anchor and trigger for T4P retraction (7). Many genes have been demonstrated to regulate EPS production in M. xanthus. In particular, the Dif chemotaxis-like proteins form a membrane-anchored signaling complex that positively regulates EPS production. Deletions of difA and difE, which encode methyl-accepting chemotaxis protein (MCP) and CheA kinase homologues, respectively, lead to a lack of EPS production. Elimination of SglK, an Hsp70/DnaK homologue, also results in an EPS− phenotype. Interestingly, the T4P, whose retraction is modulated by EPS, also regulates the production of EPS; T4P− mutants such as a pilA deletion mutant exhibit an EPS− phenotype. It has been demonstrated that the T4P functions upstream of Dif in the EPS regulatory pathway.
In an attempt to better understand the regulation of EPS, we carried out a genetic screen to identify transposon (Tn) mutations that could restore EPS production to a pilA deletion mutant. Here, we describe a transposon insertion in a CRISPR (clustered regularly interspaced short palindromic repeats) array that suppressed the EPS defect of a pilA mutant. CRISPRs are noncoding regions prevalent in the genomes of prokaryotes, and they have been demonstrated to function as an adaptive immune system against invading nucleic acids in certain organisms (8). A CRISPR typically consists of an array of identical repeats interspaced with spacers that are variable in length and sequence (9, 10). The repeats in some but not all of the CRISPRs harbor short palindrome sequences. Some of the spacers were found to be identical in sequence to various genetic elements such as phages and transposons (11–14). Most CRISPR loci have a set of CRISPR-associated (cas) genes that are adjacent to the CRISPR array (9, 10). Based on the current model, a CRISPR array may acquire a new spacer from an invading element as the first step toward resistance or immunity. To defend against an attack, a long transcript or pre-CRISPR RNA (pre-crRNA) is first produced from a CRISPR. The pre-crRNA is then processed into short and mature crRNAs, which may target corresponding mobile elements for destruction. The Cas proteins are proposed to function in the acquisition of new spacers and the processing of pre-crRNA, as well as targeting and destruction of foreign nucleic acids using crRNA as a guide.
In this study, we report our finding that a transposon insertion in a CRISPR suppressed the EPS defect of a pilA deletion in M. xanthus. This insertion occurred in CRISPR3, one of three arrays present in the organism. CRISPR3 consists of 53 repeats and 52 spacers in the wild-type (WT) strain. The transposon inserted into the 13th spacer of CRISPR3 (3SP13). Interestingly, this insertion, designated CRISPR3* here, is a gain-of-function (GOF) rather than a loss-of-function (LOF) mutation. While CRISPR3* restored EPS production to a pilA mutant, it led to no obvious EPS phenotype in a WT background. On the other hand, it adversely affected fruiting body development in both the ΔpilA and the WT backgrounds. The genes upstream of CRISPR3 encode the repeat-associated mysterious proteins (RAMPs). These RAMP genes classify CRISPR3 as a type IIIB CRISPR, which has been demonstrated to target RNA instead of DNA in vitro. Deletion analysis indicated that these RAMP genes are required for CRISPR3* to exert its function in both EPS production and fruiting body development. Based on analysis by reverse transcription (RT)-PCR, it appears that CRISPR3* altered the processing of the CRISPR3 pre-crRNA. We propose that one or more of the resulting crRNAs may target the RNA transcripts from certain M. xanthus chromosomal genes. As such, this novel type IIIB CRISPR influences cellular processes, such as EPS production and fruiting body development, that extend beyond the canonical function of prokaryotic immunity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The M. xanthus strains and plasmids used in this study are listed in Table 1. The medium for M. xanthus was Casitone-yeast extract (CYE) (2). The Escherichia coli strains used were XL1-Blue (Stratagene) and DH5α λpir (15), both grown in Luria-Bertani (LB) medium (16). The M. xanthus and E. coli strains were grown at 32°C and 37°C, respectively; 1.5% agar plates were used to grow M. xanthus and E. coli unless otherwise indicated. Media were supplemented with kanamycin at 100 μg/ml and/or oxytetracycline at 15 μg/ml when appropriate. Clone fruiting (CF) medium (17) was used to examine M. xanthus development.
TABLE 1.
M. xanthus strains and plasmids
| Strain or plasmid | Genotype/description | Reference |
|---|---|---|
| Strains | ||
| BY802 | ΔpilA::tet CRISPR3* | This study |
| BY850 | CRISPR3* | This study |
| DK1622 | Wild type | 2 |
| DK10407 | ΔpilA::tet | 18 |
| DK10416 | ΔpilB | 44 |
| YZ601 | ΔdifA | 45 |
| YZ603 | ΔdifE | 46 |
| YZ811 | ΔsglK | 47 |
| YZ1200 | ΔpilA::tet ΔCRISPR3 | This study |
| YZ1201 | ΔpilA::tet ΔRAMP | This study |
| YZ1202 | ΔCRISPR3 | This study |
| YZ1203 | ΔRAMP | This study |
| YZ1261 | ΔpilA::tet ΔRAMP CRISPR3* | This study |
| YZ1262 | ΔRAMP CRISPR3* | This study |
| YZ1263 | ΔMXAN | This study |
| YZ1267 | ΔpilA::tet ΔMXAN | This study |
| YZ1270 | ΔpilA::tet ΔCRISPR3 att::pXY105 | This study |
| YZ1273 | ΔpilA::tet ΔMXAN CRISPR3* | This study |
| YZ1279 | ΔpilB CRISPR3* | This study |
| YZ1280 | ΔsglK CRISPR3* | This study |
| YZ1281 | ΔdifA CRISPR3* | This study |
| YZ1282 | ΔdifE CRISPR3* | This study |
| Plasmids | ||
| pMY7 | Cloning vector; Kanr; E. coli galK | Unpublished data |
| pMycoMar | magellan4 mutagenesis vector | 19 |
| pRW100 | CRISPR3 deletion in pMY7 | This study |
| pRW107 | MXAN deletion in pMY7 | This study |
| pRW112 | RAMP deletion in pMY7 | This study |
| pWB425 | M. xanthus expression vector | 20 |
| pXY105 | CRISPR3 in pWB425 | This study |
| pZErO-2 | Cloning vector; Kanr | Invitrogen |
Isolation of a pilA suppressor mutant.
DK10407 (ΔpilA) (18) was mutagenized with the mariner transposon magellan4 (19, 20). This transposon contains the E. coli R6Kγ origin of replication and nptII, which confers kanamycin resistance (Kanr) (19). pMycoMar, a plasmid carrying the transposon, was transformed into DK10407 by electroporation (21). Transformants were plated on CYE plates with kanamycin and Congo red (CR) at 30 μg/ml (22). Red colonies, potentially EPS+ mutants, were further examined using CYE plates with calcofluor white (CW) at 50 μg/ml (23).
To clone the transposon insertions, genomic DNA of a transposon mutant was isolated and digested with SacII, which does not cut within the magellan4 transposon (20). The digestion mixture was then used for ligation and subsequent transformation of the E. coli strain DH5α λpir by selection on kanamycin. Plasmids from the transformants were sequenced using MarR1 and MarL1 (24), and the sequence flanking the transposon was compared with the genome sequence of M. xanthus DK1622 (25) to identify the insertion site.
Construction of plasmids.
Two plasmids were constructed to delete two M. xanthus gene clusters, the RAMP and MXAN_7275 to MXAN_7270 (MXAN_7275-7270) genes. One additional plasmid was constructed to delete CRISPR3. PCR primers were designed to amplify regions upstream and downstream of the target for deletion. The fragments were then joined by overlapping PCR to generate the deletion allele, which was cloned into pMY7 using HindIII and XbaI. pMY7 contains nptII and the E. coli galK gene (unpublished data). The primer pairs to delete the CRISPR3 array were ΔCRISPR3_F1 (GCATAAGCTTGCGCTGTTCACCGGAGGT) and ΔCRISPR3_R1 (TTCGCTCATGGAGGCCCTGTAGCCCATCTGAATCTCCCAG) for the upstream fragment and ΔCRISPR3_F2 (ACAGGGCCTCCATGAGCGAA) and ΔCRISPR3_R2 (TAGCTCTAGAAGGCACGGAGCAACTCGGA) for the downstream fragment. The primers to delete MXAN_7275-7270 were ΔMXAN_F1 (GACCAAGCTTTCAACATATCGCCGTCGA) and ΔMXAN_R1 (GTATTCGTTCCAGAACCGGG) for the upstream and ΔMXAN_F2 (CCCGGTTCTGGAACGAATACGAGAAGCCTACGGCGAGTTC) and ΔMXAN_R2 (GCTCTAGATGTTGGTTGCGTGCATGG) for the downstream. The primers to delete the RAMP genes were ΔRAMP_F1 (GATTACAAGCTTCTCGCTCCTGGTGCGGAT) and ΔRAMP_R1 (CGGAGCGAGTGGTTGCCGAA) for the upstream and ΔRAMP_F2 (ACAGGGCCTCCATGAGCGAA) and ΔRAMP_R2 (CGTCCTCTCTAGATTCCAAACCCCATGGAA) for the downstream. The resulting plasmids with ΔCRISPR3, ΔMXAN_7275-7270, and ΔRAMP alleles were pRW100, pRW107, and pRW112, respectively.
pXY105 was constructed to express CRISPR3 from PnptII, the promoter of the Kanr gene. CRISPR3 was amplified using CRISPR3_F1 and CRISPR3_R (AGGTCTAGATGGCCTCGCAGCTTCCAGAT). It was digested with HindIII and XbaI and cloned into pWB425 (20) at the same restriction sites to produce pXY105.
Construction of M. xanthus mutants.
The deletion plasmids mentioned above were used in a two-step procedure (26) to replace their targets on the chromosome (Table 1). pRW100 was used to delete CRISPR3 from DK1622 (WT) and DK10407 (ΔpilA) to construct YZ1202 (ΔCRISPR3) and YZ1200 (ΔpilA ΔCRISPR3), respectively. pRW107 was used to delete ΔMXAN_7275-7270 from DK1622 and DK10407 to construct YZ1263 (ΔMXAN) and YZ1267 (ΔpilA ΔMXAN), respectively. pRW112 was used to delete the RAMP genes from DK1622 and DK10407 to construct YZ1203 (ΔRAMP) and YZ1201 (ΔpilA ΔRAMP), respectively.
Genomic DNA from the ΔpilA suppressor strain BY802 was transformed into DK1622, YZ1267, YZ1201, YZ1203, DK10416 (ΔpilB), YZ601 (ΔdifA), YZ603 (ΔdifE), and YZ811 (ΔsglK) to construct the following strains: BY850 (CRISPR3*), YZ1273 (ΔpilA ΔMXAN CRISPR3*), YZ1261 (ΔpilA ΔRAMP), YZ1262 (ΔRAMP CRISPR3*), YZ1279 (ΔpilB CRISPR3*), YZ1280 (ΔsglK CRISPR3*), YZ1281 (ΔdifA CRISPR3*), and YZ1282 (ΔdifE CRISPR3*), respectively. pXY105 was transformed into YZ1200 (ΔpilA ΔCRISPR3) to construct YZ1270 (ΔpilA ΔCRISPR3 att::pXY105).
Examination of EPS production and fruiting body development.
EPS production and fruiting body development were examined on CYE-plus-CW and CF plates, respectively. Briefly, cells were resuspended at 5 × 109 cells/ml in CYE, and 5 μl was spotted on CYE-plus-CW plates for EPS assays. For the examination of development, cells were resuspended at 2.5 × 109 cells/ml in MOPS buffer (10 mM morpholinepropanesulfonic acid [pH 7.6], 2 mM MgSO4), and 5 μl was spotted on CF plates. Both sets of plates were incubated at 32°C for 5 days before documentation.
Examination of CRISPR3 expression by RT-PCR.
To perform RT-PCR, overnight cultures in CYE were used to inoculate a culture to an optical density of 0.15 at 600 nm. Cells were harvested after 20 h of growth and resuspended at 5 × 108 cells/ml in CYE. A CYE plate with 1.0% agar was inoculated with 100 μl of the cell suspension by spreading and incubated for 4 days at 32°C. The cells were then scraped off and resuspended in MOPS buffer. Samples from five plates for each strain were pooled for RNA isolation using a TriSure RNA isolation kit (Bioline). These RNA preparations were treated with DNase I (Promega), repurified using the TriSure RNA isolation kit, and resuspended in RNase-free water. RT-PCR was performed using Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega) for reverse transcription and Taq DNA polymerase (New England BioLabs) for PCR. For RT-PCR two pairs of primers targeting different regions of CRISPR3 relative to the CRISPR3* insertion were used. The primers upstream of the insertion were CRISPR3_UF (TGGGAGATTCAGATGGGCT) and CRISPR3_UR (TGCTCGTCGTCACGATGCTGGA). Those downstream were CRISPR3_DF (CGTCTGGCCTTCGCCGTCGT) and CRISPR3_DR (TGGACGGGAGAAGACGTTCA). Only the reverse primers CRISPR3_UR and CRISPR3_DR, respectively, were used in the two RT reactions. The PCR products were resolved on a 1.4% agarose gel, and ImageJ (27) was used for quantification. To determine the sequences of the RT-PCR products, the bands of interest were excised from the agarose gel, cloned into pZErO-2 (Invitrogen), and sequenced.
RESULTS
Isolation of a pilA suppressor in EPS production.
M. xanthus pilA mutants are EPS− because they do not assemble the T4P due to a lack of pilin. To better understand EPS regulation in M. xanthus, a genetic screen was carried out to isolate suppressors of pilA in EPS production. Briefly, a pilA deletion (ΔpilA) mutant (DK10407) was mutagenized with a mariner Tn, and mutants were selected on plates supplemented with the dye CR. EPS− colonies appear yellowish orange, while EPS+ colonies are red due to the binding of CR to M. xanthus EPS. Here, we report studies of BY802, one of two pilA suppressor mutants isolated from screening ∼20,000 colonies using this method. The other suppressor will be reported elsewhere.
The EPS+ phenotype of BY802 was confirmed by binding of the fluorescent dye CW as an alternative EPS assay. As shown in Fig. 1A, the WT (DK1622) and BY802 fluoresced under UV illumination while the pilA mutant did not. The genomic DNA of BY802 was transformed into the parental ΔpilA mutant to verify that the EPS+ phenotype was linked to one locus with a Tn insertion(s). All resulting transformants examined were found to be EPS+ by dye binding assays (data not shown), demonstrating that BY802 harbors a single Tn insertion responsible for the restoration of EPS to the pilA mutant. This insertion is designated CRISPR3* here (see below). It should be noted that the suppressor strain, despite being EPS+, is still S− due to a lack of the T4P. Consequently, the colony morphology of BY802 differs from that of the WT, which is EPS+ and S+.
FIG 1.
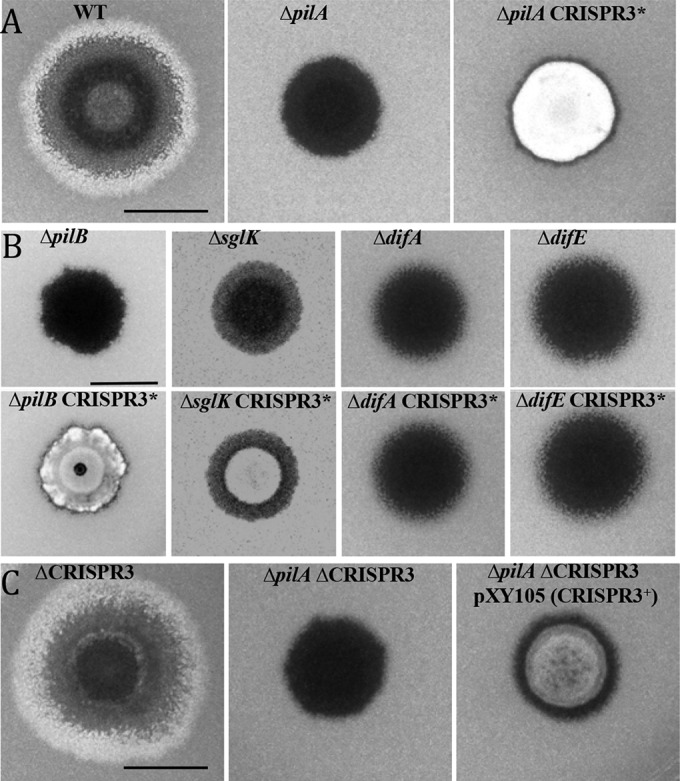
CRISPR3* is a gain-of-function mutation that suppresses ΔpilA, ΔpilB, and ΔsglK in EPS production. Five microliter samples of cell suspensions of various strains at 5 × 109 cells/ml were spotted on CYE-plus-CW plates and incubated for 5 days at 32°C. The photographs were taken under UV illumination. The fluorescence intensity approximates the level of EPS production. (A) CRISPR3* suppresses a pilA deletion. Strains: WT (DK1622), ΔpilA (DK10407), and ΔpilA CRISPR3* (BY802). (B) CRISPR3* suppresses pilB and sglK but not difA or difE mutations. Strains: ΔpilB (DK10416), ΔpilB CRISPR3* (YZ1279), ΔsglK (YZ811), ΔsglK CRISPR3* (YZ1280), ΔdifA (YZ601), ΔdifA CRISPR3* (YZ1281), ΔdifE (YZ603), and ΔdifE CRISPR3* (YZ1282). (C) Expression of CRISPR3 suppresses ΔpilA. Strains: ΔCRISPR3 (YZ1202), ΔpilA ΔCRISPR3 (YZ1200), and ΔpilA ΔCRISPR3 pXY105 (YZ1270). The scale bars in all three panels represent 1 cm.
CRISPR3* suppressed ΔpilB and ΔsglK but not ΔdifA or ΔdifE.
Many genes are known to play roles in EPS regulation in M. xanthus. They include the sglK and dif genes. To examine the genetic relationship of CRISPR3* with these genes, genomic DNA of BY802 was used to transform ΔsglK, ΔdifA, and ΔdifE mutant strains. A ΔpilB mutant was also transformed to determine the specificity of the suppression of T4P− mutations by CRISPR3*. The resulting double mutants were examined on CW plates (Fig. 1B). The ΔpilB CRISPR3* and ΔsglK CRISPR3* strains fluoresced, while ΔdifA CRISPR3* and ΔdifE CRISPR3* did not. The results described here demonstrate that in EPS regulation, CRISPR3* is epistatic to ΔpilA, ΔpilB, and ΔsglK but not to ΔdifA and ΔdifE mutations in M. xanthus.
CRISPR3* is a gain-of-function mutation.
The site of the Tn insertion in BY802 was identified by cloning and sequencing. The insertion occurred in CRISPR3, the 3rd of three CRISPR regions on the M. xanthus chromosome (Fig. 2). CRISPR3 contains 53 nearly identical repeats, each 36 bp long. Between these repeats are 52 spacers ranging from 33 to 40 bp in length. The Tn inserted after a TA dinucleotide at the 2nd and 3rd positions of the 13th spacer of CRISPR3 (3SP13). At the 5′ end of the CRISPR3 array is a typical A/T-rich leader sequence (L), which is where the promoter is anticipated to be for this CRISPR array. This insertion in CRISPR3 was surprising, as we had expected mutations in or near protein-coding sequences.
FIG 2.
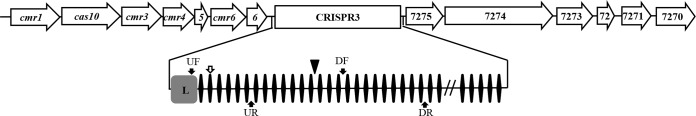
M. xanthus CRISPR3 locus. A region of 20.1 kb at the CRISPR3 locus is shown at the top, with genetic elements drawn to scale. The arrows represent ORFs, and the rectangle represents the CRISPR3 array. Upstream of CRISPR3 are seven RAMP genes: cmr1, cas10, cmr3, cmr4, cmr5 (indicated by 5), cmr6, and cas6 (indicated by 6). Downstream are six ORFs: MXAN_7275-7270 (MXAN_7272 is indicated by 72). Below is a closeup of CRISPR3. It contains 53 repeats (diamonds), 52 spacers (horizontal lines), and a leader sequence (L). The double slash indicates the omission of repeats 28 through 49. The arrowhead indicates the CRISPR3* Tn insertion in the 13th spacer. The solid arrows indicate the positions of the primers used for RT-PCR. These primers are CRISPR3_UF (UF), CRISPR3_UR (UR), CRISPR3_DF (DF), and CRISPR3_DR (DR). The open arrow indicates where CRISPR3_UR (UR) annealed in RT-PCR to produce a smaller product (shown in Fig. 5). pXY105 contains the entire CRISPR3 region, including 746 bp upstream of the first repeat and 1,542 bp downstream of the last repeat.
We sought to determine the genetic nature of the 3SP13 insertion in BY802. Tn insertions tend to result in LOF mutations more often than GOF mutations. In principle, however, a Tn insertion may result in either type. We first deleted the entire CRISPR3 array to examine if a CRISPR3-null or LOF mutation could suppress a pilA deletion. The ΔCRISPR3 allele, which deleted all 53 repeats and 52 spacers, was constructed in a ΔpilA mutant background as well as in a WT background. In the WT background, the ΔCRISPR3 allele did not affect EPS production appreciably in qualitative (Fig. 1C) and quantitative (not shown) assays under our experimental conditions. Unexpectedly, the ΔpilA ΔCRISPR3 double mutant lacked EPS production, as indicated by its lack of fluorescence on CW plates. Since the deletion of CRISPR3 is unable to suppress ΔpilA, the original 3SP13 insertion in BY802 is unlikely to be a LOF mutation of CRISPR3.
We considered the alternative that the 3SP13 insertion could be a GOF mutation next. One possibility is that the insertion activates the function of CRISPR3 by increasing the level of CRISPR3 crRNA. We sought to express CRISPR3 from PnptII, the promoter for the kanamycin resistance gene in the Tn used for mutagenesis. PnptII in the CRISPR3* mutant is oriented in the same direction as the predicted promoter for CRISPR3 in the L sequence (Fig. 2). As such, it could lead to the expression of the CRISPR3 region downstream of the insertion. CRISPR3 was cloned in its entirety into an expression vector with PnptII and the Mx8 phage attachment site (28) (Fig. 2). The resulting expression plasmid (pXY105) was transformed into a ΔpilA mutant with the chromosomal CRISPR3 deleted to circumvent homologous recombination. As shown in Fig. 1C, the resulting strain (YZ1270) fluoresced on the CW plate, indicating that the artificial expression of CRISPR3 is sufficient to suppress ΔpilA. It was noted that this suppression is not as strong as the 3SP13 insertion in BY802. As a control, the same CRISPR3 fragment cloned in the inverted orientation in the same vector did not result in suppression (data not shown). These results with the deletion and expression of CRISPR3 (Fig. 1C) led to the conclusion that the CRISPR3 insertion in BY802 is a GOF mutation. This mutation is therefore designated CRISPR3* to differentiate it from a LOF mutation.
CRISPR3* led to defects in fruiting body development.
The development of CRISPR3* mutants was examined because defects in EPS have been directly tied to M. xanthus fruiting (29). As shown in Fig. 3, the WT strain formed regular fruiting bodies. When the CRISPR3* mutation was introduced into the WT, the resulting strain showed no obvious aggregation under the same conditions. Consistent with previous reports, the pilA single mutant formed developmental aggregates. The pilA suppressor strain, however, showed no obvious sign of aggregation, like the CRISPR3* single mutant. Similarly, when pXY105 was introduced into the ΔpilA ΔCRISPR3 double-mutant background, it resulted in significantly diminished aggregation under developmental conditions (Fig. 3). In contrast, the ΔCRISPR3 null allele had no obvious effect on development in either the WT or the pilA mutant background. Thus, CRISPR3* is a GOF mutation in development, which argues that CRISPR3 can function to deter fruiting body formation. Because CRISPR3* results in developmental defects with no effect on EPS in the WT background, CRISPR3* may influence development and EPS production through distinct mechanisms.
FIG 3.
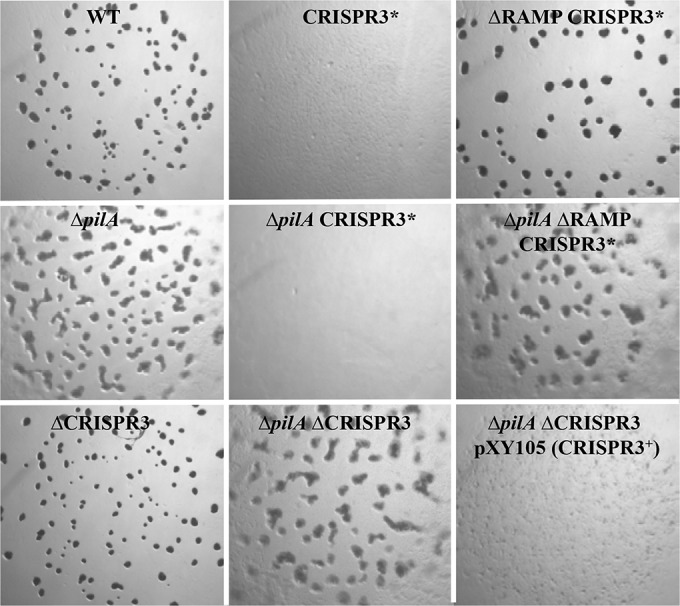
CRISPR3* adversely affects development in a RAMP-dependent manner. Five microliter samples of cell suspensions at 2.5 × 109 cells/ml of the indicated strains were spotted on CF plates. The photographs were taken after 5 days. Strains: WT (DK1622), CRISPR3* (BY850), ΔRAMP CRISPR3* (YZ1262), ΔpilA (DK10407), ΔpilA CRISPR3* (BY802), ΔpilA ΔRAMP CRISPR3* (YZ1261), ΔCRISPR3 (YZ1202), ΔpilA ΔCRISPR3 (YZ1200), and ΔpilA ΔCRISPR3 pXY105 (YZ1270). ΔRAMP (YZ1203) is wild type in development (not shown).
RAMP genes are required for CRISPR3* to exert its function.
Further upstream of the CRISPR3 array are seven cas genes, cmr1, cas10, cmr3, cmr4, cmr5, cmr6, and cas6 (Fig. 2). Their products are also known as RAMPs. The presence of cas10 classifies the M. xanthus CRISPR3 system as type III, and cmr5 further defines it as type IIIB. Downstream of CRISPR3 are six open reading frames (ORFs), MXAN_7275 to MXAN_7270. These ORFs read in the same direction as the RAMP genes and CRISPR3. We examined whether the ORFs were related to CRISPR3 function by constructing a deletion allele of the MXAN_7275-7270 gene cluster (ΔMXAN) in the WT, the ΔpilA, and the ΔpilA CRISPR3* backgrounds. As shown in Fig. 3, ΔMXAN did not alter the fluorescence of any of these strains on CW plates. These results indicate that the MXAN_7275-7270 genes are not required for CRISPR3* to suppress ΔpilA and are unlikely to be related to CRISPR3 function or EPS production.
The seven RAMP genes were also examined for their roles in the function of CRISPR3*. A deletion allele of all seven RAMP genes (ΔRAMP) was constructed in the ΔpilA CRISPR3* background. As shown in Fig. 4, the resulting mutant (YZ1261) failed to fluoresce on CW plates. The EPS− phenotype of this mutant indicates that the RAMP genes are required for the suppression of pilA by CRISPR3*. As controls, the deletion of RAMP genes did not affect EPS production in the WT and the ΔpilA backgrounds as analyzed on CW plates (Fig. 4). The same strains were also examined for development to determine if CRISPR3* required the RAMP genes to adversely affect fruiting body formation. As shown in Fig. 3, the deletion of the RAMP genes alleviated the detrimental effect of CRISPR3* on development in both the WT and the ΔpilA backgrounds. These results demonstrate that these RAMP genes are required for CRISPR3* to exert its effect on both fruiting body development and EPS production.
FIG 4.
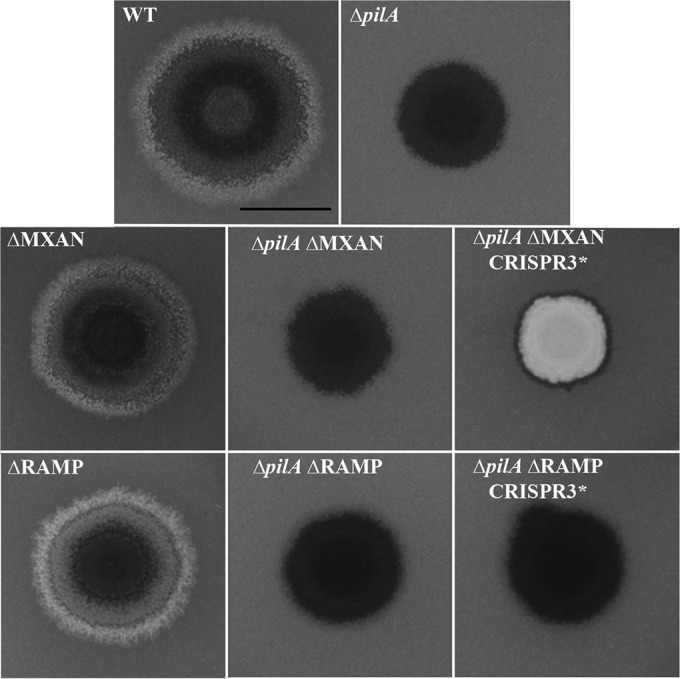
RAMP genes, but not MXAN_7275-7270, are required for CRISPR3* to suppress ΔpilA. Experiments were performed as for Fig. 1. Strains: WT (DK1622), ΔpilA (DK10407), ΔMXAN (YZ1263), ΔpilA ΔMXAN (YZ1267), ΔpilA ΔMXAN CRISPR3* (YZ1273), ΔRAMP (YZ1203), ΔpilA ΔRAMP (YZ1201), and ΔpilA ΔRAMP CRISPR3* (YZ1261). The scale bar represents 1 cm. The suppressor strain BY802 (Fig. 1) is indistinguishable from YZ1273.
CRISPR3* may lead to increased processing of pre-crRNA.
How does CRISPR3* influence both fruiting body development and EPS production? One scenario was that the CRISPR3* mutation might activate the transcription of CRISPR3 downstream of the insertion. This would lead to increased or artificial production of CRISPR3 pre-crRNA and crRNA. The CRISPR3 crRNA could affect both EPS production and development by targeting the transcripts of certain genes that function in these processes. This scenario was consistent with the orientation of the PnptII promoter in the Tn and the suppression of ΔpilA by the CRISPR3 expression construct (pXY105) (Fig. 1C).
Two pairs of primers were used in RT-PCR to examine the transcripts from CRISPR3 both down- and upstream of the CRISPR3* mutation. The first pair, CRISPR3_UF and CRISPR3_UR, target a region from the leader sequence to the 6th spacer upstream of CRISPR3* (Fig. 2). The second pair, CRISPR3_DF and CRISPR3_DR, are complementary to spacers 16 and 25, respectively, downstream of the mutation (Fig. 2). No obvious differences between CRISPR3 and CRISPR3* strains were observed when the downstream pair was used in RT-PCR (data not shown). In contrast, there were reproducible differences when the upstream pair was used (Fig. 5). This pair was expected to amplify a fragment of 441 bp. A band of similar size was present in all but the ΔCRISPR3 strain, as anticipated. However, there was a smaller band around 350 bp long in the two strains with the CRISPR3* allele. Estimations from multiple experiments with technical replications indicated that the upper band is consistently less intense in CRISPR3* mutants than in strains with the WT CRISPR3 allele. These results suggested that the CRISPR3* mutation may have led to an increase in processing of pre-crRNA upstream of its insertion site.
FIG 5.
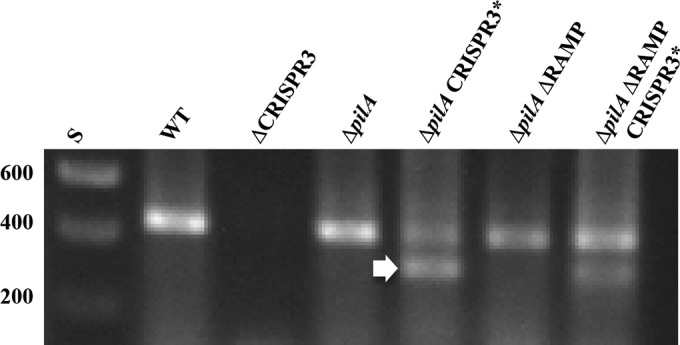
CRISPR3* mutation may affect processing of CRISPR3 RNA transcripts upstream of the insertion. RT-PCR was performed using the primers CRISPR3_UF and CRISPR3_UR (Fig. 2) as described in Materials and Methods. RT-PCR products were resolved on a 1.4% agarose gel and visualized by ethidium bromide staining and UV illumination. The sizes of DNA standards (S) in base pairs are indicated on the left. The arrow indicates the extra band in CRISPR3* mutants. Strains: WT (DK1622), ΔCRISPR3 (YZ1202) ΔpilA (DK10407), ΔpilA ΔRAMP (YZ1201), ΔpilA CRISPR3* (BY802), and ΔpilA ΔRAMP CRISPR3* (YZ1261).
The DNA fragments from RT-PCR were cloned and sequenced to investigate their identities and origins. The upper band (Fig. 5) from the suppressor strain (ΔpilA CRISPR3*) was identical to the one from the WT. Both were 441 bp long, with the anticipated sequence flanked by the two upstream primers (Fig. 2). The lower band from the suppressor strain was 351 bp long, and both its 5′ and 3′ ends matched CRISPR3_UR (Fig. 2), the primer used in both the RT and the PCRs (Fig. 6). This primer, which is complementary to the 6th spacer, serendipitously matched 15 out of 22 positions in the CRISPR3 repeat (Fig. 6). As a consequence, it also annealed with the second repeat, in addition to the 6th spacer, to give rise to the 351-bp fragment in the suppressor strain. The smaller RT-PCR fragment from YZ1261 (ΔpilA CRISPR3* ΔRAMP) was also examined, and it was identical to the 351-bp fragment from the suppressor strain (ΔpilA CRISPR3*). The observations with RT-PCR indicate that the CRISPR3* mutation resulted in the production of RNA species that are not present in the WT CRISPR3 strains. We suggest that CRISPR3* led to an increase or change in the processing of CRISPR3 pre-crRNA, which is possibly responsible for the effect of CRISPR3* on fruiting and EPS in M. xanthus.
FIG 6.

Alignments of the repeats and two Cas6 proteins from M. xanthus CRISPR2 and CRISPR3. (A) Comparison of the CRISPR2 repeat (IIR) and the CRISPR3 repeat (IIIR). They have two mismatches, indicated in boldface. The last line is the sequence of the CRISPR3_UR primer (3UR) aligned with the CRISPR3 repeat. The underlined nucleotides match the sequence of the repeat. (B) Alignment of CRISPR2 Cas6 (Cas6′) and CRISPR3 Cas6 over 195 amino acids. Identical and conserved residues are indicated by colons and periods, respectively.
DISCUSSION
CRISPRs have been demonstrated to function as adaptive immune systems in prokaryotes in three steps (30): adaptation, crRNA biogenesis, and targeting or interference. During adaptation, a new spacer is acquired from exogenous nucleic acids, along with the addition of a new repeat adjacent to the leader sequence. crRNA biogenesis begins with the transcription of the CRISPR array from a promoter in the leader sequence. The resulting pre-crRNA is then processed to generate the small and mature interfering crRNA. In targeting or interference, a crRNA-Cas nucleoprotein complex with nuclease activity targets and cleaves DNA or RNA homologous to the crRNA (10). There are three major types of CRISPRs, defined mainly by their associated Cas proteins. Type I has six subtypes, IA through IF. Types II and III each have two subtypes, IIA and IIB, and IIIA and IIIB. All subtypes of types I and II cleave DNA, as does type IIIA. Type IIIB has been demonstrated to target RNA rather than DNA. Regardless of their targets, all CRISPRs are proposed to go through these three steps to confer on prokaryotes immunity or resistance to invading nucleic acids. It is noteworthy that Cas9 and its associated type II CRISPR systems have been developed for genome editing (31); it has generated considerable excitement, as it has been successfully applied to organisms from bacteria to mammals and from worms to plants (31).
Besides its ability to function in immunity, a CRISPR system may also regulate other cellular functions in bacteria. Of the organisms possessing CRISPRs, 18% were found to have spacers originating from their genomes (14, 32), suggesting that CRISPR systems could affect chromosomal genes not involved in immunity. There are indeed a few such examples in the literature. DevR and DevS, which are essential for M. xanthus development (33, 34), are now known as CRISPR-associated proteins Cas7 and Cas5. They have been found to be affiliated with most of the type I CRISPR systems (35, 36). In M. xanthus, they are associated with CRISPR2, a type IC CRISPR. devR and devS are regulated by developmental progression, along with other genes at the same locus in M. xanthus (33, 34). While the functions of Cas5 and Cas7 have yet to be elucidated, they contain RNA binding domains (35) and may potentially influence gene expression or directly perform functions crucial for M. xanthus development. Early studies of a Pseudomonas aeruginosa lysogen provided additional and more direct evidence that CRISPR systems may regulate chromosomal genes (37, 38). A WT P. aeruginosa strain produces biofilm and displays swarming motility. However, its lysogen with the DMS3 prophage loses both biofilm formation and swarming motility. Disruption of a type IF CRISPR or its associated cas genes restored biofilm formation and swarming to the lysogen. These results suggest that a type IF CRISPR system may regulate the functions of genes that are not directly related to bacterial immunity.
A more recent example came from a type II CRISPR in Francisella novicida, an intracellular bacterial pathogen of animals. In this case, Cas9, as well as its associated transactivating crRNA (tracrRNA) and small CRISPR/Cas-associated RNA (scaRNA), was found to repress the expression of a bacterial lipoprotein (BLP). Such BLPs are the ligands for the Toll-like receptor TLR2 for the activation of innate immunity of the animal host. It appears that Cas9 and its associated RNAs destabilize the mRNA for BLP to subvert detection of the bacterium by its host. The abrogation of this regulatory mechanism of BLP attenuates the pathogenesis of F. novicida. Cas9 had been engineered previously to regulate gene expression in an artificial setting (39–41). The new discovery indicated that Cas9 can directly influence the level of transcripts and proteins in a natural and biologically relevant context. These observations provide evidence that type II CRISPR systems can regulate genes involved in critical cellular processes distinct from prokaryotic immunity.
This study provides strong evidence that a type IIIB CRISPR system can regulate EPS production and fruiting body development in M. xanthus. A CRISPR3 Tn insertion, CRISPR3*, restored EPS production to a pilA mutant in M. xanthus. It was also found to suppress pilB and sglK in EPS production. CRISPR3* is a GOF mutation because the artificial expression of CRISPR3, but not CRISPR3 deletion, resulted in the suppression of the ΔpilA EPS defect. Moreover, the CRISPR3* mutation itself was also found to have a detrimental effect on fruiting body development in both the pilA mutant and the WT backgrounds. Our findings here clearly indicate that CRISPR3 is involved in the regulation of fruiting body development and EPS production in M. xanthus, neither of which is directly related to the canonical function of CRISPR in immunity.
We propose a molecular model to explain how CRISPR3 may regulate EPS production and fruiting body development in M. xanthus. We propose that CRISPR3, like other CRISPR arrays, can be transcribed as a long pre-crRNA. crRNAs are then produced by pre-crRNA processing. One or more CRISPR3 crRNAs can target mRNAs from genes involved in the regulation of EPS production and fruiting body development, possibly by cleavage or degradation. Because EPS and fruiting are not typical responses to phage infection, the target genes here can be inferred to be M. xanthus chromosomal genes critical for these normal cellular processes. Because the deletion of CRISPR3 led to no obvious phenotype in M. xanthus (Fig. 1), CRISPR3 pre-crRNA is likely not produced and/or processed at sufficient levels to affect fruiting or EPS production in a WT background. In contrast, the expression of CRISPR3 from a heterologous promoter affected both fruiting body development and EPS production, similarly to CRISPR3* (Fig. 1 and 3). Because the deletion of RAMP genes abrogated the effect of CRISPR3* in both processes, it is proposed that one or more of the RAMP proteins are indispensable for regulation of the targeted genes to affect EPS production and development.
It was surprising that the deletion of RAMP genes, including cas6, did not eliminate the new CRISPR3 RNA species detected by RT-PCR in the CRISPR3* strains (Fig. 5). In Pyrococcus furiosus, the riboendonuclease Cas6 appears to be the first enzyme involved in the processing of pre-crRNA (42, 43). It cleaves RNA in the repeat 8 nucleotides upstream of a spacer. This produces an RNA intermediate with a spacer flanked by part of the repeat at both ends (43). The 3′ end of this intermediate is believed to be further processed by other RAMP proteins to generate crRNA, yet in the M. xanthus RAMP deletion, CRISPR3 pre-crRNA appeared to be processed, at least to some degree, in the CRISPR3* mutants (Fig. 5). It is possible that CRISPR3 Cas6 (MXAN_7276) does not catalyze the first processing step or that the later steps do not require priming by Cas6, at least in M. xanthus CRISPR3* mutants. Alternatively, the Cas6 associated with CRISPR2 in M. xanthus may partially process CRISPR3 pre-crRNA. CRISPR2 is about 21 kb away from CRISPR3. CRISPR2 Cas6 (MXAN_7265) is 65% identical and 86% similar to CRISPR3 Cas6 (Fig. 6). The repeats of these two CRISPRs are both 36 bp long, and they differ at only two positions. Future studies may determine if there are biochemical and functional overlaps between these two CRISPR systems in M. xanthus.
ACKNOWLEDGMENTS
This work was partially supported by National Science Foundation grants MCB-1239889 and MCB-1417726, as well as by National Institutes of Health grant GM071601 and by the Fralin Life Science Institute. R.A.W. was partially supported by the Post Baccalaureate Research and Education Program (PREP) and the Multicultural Academic Opportunities Program (MAOP) at Virginia Tech.
Footnotes
Published ahead of print 8 September 2014
REFERENCES
- 1.Dworkin M, Kaiser D. 1993. Myxobacteria II. ASM Press, Washington, DC. [Google Scholar]
- 2.Kaiser D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. U. S. A. 76:5952–5956. 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuner JM, Kaiser D. 1982. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J. Bacteriol. 151:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodgkin J, Kaiser D. 1979. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): genes controlling movements of single cells. Mol. Gen. Genet. 171:167–176. 10.1007/BF00270003. [DOI] [Google Scholar]
- 5.Hodgkin J, Kaiser D. 1979. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol. Gen. Genet. 171:177–191. 10.1007/BF00270004. [DOI] [Google Scholar]
- 6.Shimkets LJ. 1986. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J. Bacteriol. 166:837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Sun H, Ma X, Lu A, Lux R, Zusman D, Shi W. 2003. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl. Acad. Sci. U. S. A. 100:5443–5448. 10.1073/pnas.0836639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 9.Jansen R, Embden JD, Gaastra W, Schouls LM. 2002. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 43:1565–1575. 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 10.Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV. 2006. A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct 1:7. 10.1186/1745-6150-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. 2005. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151:2551–2561. 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- 12.Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. 2005. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60:174–182. 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- 13.Pourcel C, Salvignol G, Vergnaud G. 2005. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151:653–663. 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Li Y, Gorgé O, Platonov ME, Yan Y, Guo Z, Pourcel C, Dentovskaya SV, Balakhonov SV, Wang X, Song Y, Anisimov AP, Vergnaud G, Yang R. 2008. Insight into microevolution of Yersinia pestis by clustered regularly interspaced short palindromic repeats. PLoS One 3:e2652. 10.1371/journal.pone.0002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolter R, Helinski DR. 1978. Activity of the replication terminus of plasmid R6K in hybrid replicons in Escherichia coli. J. Mol. Biol. 124:425–441. 10.1016/0022-2836(78)90180-8. [DOI] [PubMed] [Google Scholar]
- 16.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 17.Hagen DC, Bretscher AP, Kaiser D. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284–296. 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- 18.Wall D, Wu SS, Kaiser D. 1998. Contact stimulation of Tgl and type IV pili in Myxococcus xanthus. J. Bacteriol. 180:759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin EJ, Akerley BJ, Novik VN, Lampe DJ, Husson RN, Mekalanos JJ. 1999. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc. Natl. Acad. Sci. U. S. A. 96:1645–1650. 10.1073/pnas.96.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black WP, Xu Q, Cadieux CL, Suh S-J, Shi W, Yang Z. 2009. Isolation and characterization of a suppressor mutation that restores Myxococcus xanthus exopolysaccharide production. Microbiology 155:3599–3610. 10.1099/mic.0.031070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashefi K, Hartzell PL. 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF− defect. Mol. Microbiol. 15:483–494. 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 22.Dana JR, Shimkets LJ. 1993. Regulation of cohesion-dependent cell interactions in Myxococcus xanthus. J. Bacteriol. 175:3636–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaswamy S, Dworkin M, Downard J. 1997. Identification and characterization of Myxococcus xanthus mutants deficient in calcofluor white binding. J. Bacteriol. 179:2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youderian P, Burke N, White DJ, Hartzell PL. 2003. Identification of genes required for adventurous gliding motility in Myxococcus xanthus with the transposable element mariner. Mol. Microbiol. 49:555–570. 10.1046/j.1365-2958.2003.03582.x. [DOI] [PubMed] [Google Scholar]
- 25.Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS, Eisen JA, Ronning CM, Barbazuk WB, Blanchard M, Field C, Halling C, Hinkle G, Iartchuk O, Kim HS, Mackenzie C, Madupu R, Miller N, Shvartsbeyn A, Sullivan SA, Vaudin M, Wiegand R, Kaplan HB. 2006. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. U. S. A. 103:15200–15205. 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueki T, Inouye S, Inouye M. 1996. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene 183:153–157. 10.1016/S0378-1119(96)00546-X. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck E, Ludwig G, Auerswald EA, Reiss B, Schaller H. 1982. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene 19:327–336. 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- 29.Bonner PJ, Black WP, Yang Z, Shimkets LJ. 2006. FibA and PilA act cooperatively during fruiting body formation of Myxococcus xanthus. Mol. Microbiol. 61:1283–1293. 10.1111/j.1365-2958.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- 30.Bhaya D, Davison M, Barrangou R. 2011. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 45:273–297. 10.1146/annurev-genet-110410-132430. [DOI] [PubMed] [Google Scholar]
- 31.Pennisi E. 2013. The CRISPR craze. Science 341:833–836. 10.1126/science.341.6148.833. [DOI] [PubMed] [Google Scholar]
- 32.Stern A, Keren L, Wurtzel O, Amitai G, Sorek R. 2010. Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet. 26:335–340. 10.1016/j.tig.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thony-Meyer L, Kaiser D. 1993. devRS, an autoregulated and essential genetic locus for fruiting body development in Myxococcus xanthus. J. Bacteriol. 175:7450–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viswanathan P, Murphy K, Julien B, Garza AG, Kroos L. 2007. Regulation of dev, an operon that includes genes essential for Myxococcus xanthus development and CRISPR-associated genes and repeats. J. Bacteriol. 189:3738–3750. 10.1128/JB.00187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makarova KS, Aravind L, Wolf YI, Koonin EV. 2011. Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. Biol. Direct 6:38. 10.1186/1745-6150-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, van der Oost J, Koonin EV. 2011. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 9:467–477. 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zegans ME, Wagner JC, Cady KC, Murphy DM, Hammond JH, O'Toole GA. 2009. Interaction between bacteriophage DMS3 and host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa. J. Bacteriol. 191:210–219. 10.1128/JB.00797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cady KC, O'Toole GA. 2011. Non-identity-mediated CRISPR-bacteriophage interaction mediated via the Csy and Cas3 proteins. J. Bacteriol. 193:3433–3445. 10.1128/JB.01411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. 2013. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154:442–451. 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fineran PC, Dy RL. 2014. Gene regulation by engineered CRISPR-Cas systems. Curr. Opin. Microbiol. 18:83–89. 10.1016/j.mib.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, Terns RM, Terns MP. 2009. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139:945–956. 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carte J, Wang R, Li H, Terns RM, Terns MP. 2008. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 22:3489–3496. 10.1101/gad.1742908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu SS, Wu J, Kaiser D. 1997. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 23:109–121. 10.1046/j.1365-2958.1997.1791550.x. [DOI] [PubMed] [Google Scholar]
- 45.Xu Q, Black WP, Ward SM, Yang Z. 2005. Nitrate-dependent activation of the Dif signaling pathway of Myxococcus xanthus mediated by a NarX-DifA interspecies chimera. J. Bacteriol. 187:6410–6418. 10.1128/JB.187.18.6410-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Black WP, Yang Z. 2004. Myxococcus xanthus chemotaxis homologs DifD and DifG negatively regulate fibril polysaccharide production. J. Bacteriol. 186:1001–1008. 10.1128/JB.186.4.1001-1008.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z. 2007. Demonstration of interactions among Dif proteins and the identification of KapB as a regulator of exopolysaccharide in Myxococcus xanthus. Virginia Polytechnic Institute and State University, Blacksburg, VA. [Google Scholar]


