ABSTRACT
Natural dengue virus (DENV) infection in humans induces antibodies (Abs) that neutralize the serotype of infection in a potent and type-specific manner; however, most Abs generated in response to infection are serotype cross-reactive and poorly neutralizing. Such cross-reactive Abs may enhance disease during subsequent infection with a virus of a different DENV serotype. Previous screening assays for DENV-specific human B cells and antibodies, using viral and recombinant antigens, mainly led to the isolation of dominant nonneutralizing B cell clones. To improve upon our ability to recover and study rare but durable and potently neutralizing DENV-specific Abs, we isolated human DENV-specific B cells by using a primary screen of binding to live virus, followed by a secondary screen with a high-throughput, flow cytometry-based neutralization assay to identify DENV-specific B cell lines prior to generation of hybridomas. Using this strategy, we identified several new classes of serotype-specific and serotype-cross-neutralizing anti-DENV monoclonal Abs (MAbs), including ultrapotent inhibitory antibodies with neutralizing activity concentrations of <10 ng/ml. We isolated serotype-specific neutralizing Abs that target diverse regions of the E protein, including epitopes present only on the intact, fully assembled viral particle. We also isolated a number of serotype-cross-neutralizing MAbs, most of which recognized a region in E protein domain I/II containing the fusion loop. These data provide insights into targets of the protective Ab-mediated immune response to natural DENV infection, which will prove valuable in the design and testing of new experimental DENV vaccines.
IMPORTANCE Dengue virus infection is one of the most common mosquito-borne diseases and occurs in most countries of the world. Infection of humans with dengue virus induces a small number of antibodies that inhibit the infecting strain but also induces a large number of antibodies that can bind but do not inhibit dengue virus strains of other serotypes. We used a focused screening strategy to discover a large number of rare potently inhibiting antibodies, and we mapped the regions on the virus that were recognized by such antibodies. Our studies revealed that humans have the potential to generate very potent antibodies directed to diverse regions of the dengue virus surface protein. These studies provide important new information about protection from dengue virus infection that will be useful in the design and testing of new experimental dengue vaccines for humans.
INTRODUCTION
The range of dengue viruses (DENVs) has continued to expand, with DENVs causing an estimated 390 million infections in 2010, and the incidence of the most severe form of dengue disease is on a steep rise (1, 2). The immunopathogenic mechanisms underlying severe dengue disease are not completely understood, but there are a plethora of data consistent with a model of antibody (Ab)-mediated enhanced replication of DENV in cells bearing Ab Fc receptors. Serotype-cross-reactive Abs induced following primary DENV infection bind to DENVs of heterologous types, but they exhibit low potency for those serotypes in neutralization assays and do not protect against infection caused by the different serotypes. In fact, these Abs are thought to form nonneutralized antigen-Ab complexes that can allow the virus to enter cells expressing Fc receptors more efficiently, leading to increased viral replication and, ultimately, worse disease. This process, known as Ab-dependent enhancement (ADE) of infection, has been studied extensively using human immune sera and human monoclonal Abs (MAbs) in cell culture or animal models (3, 4).
DENVs are members of the Flaviviridae family that have pseudoicosahedral symmetry, displaying 180 copies of the envelope (E) glycoprotein and of the premembrane/membrane (prM/M) protein in the lipid bilayer membrane. There are three principal domains that make up the immunodominant E glycoprotein monomer, designated E domain I (EDI), EDII, and EDIII. Extensive mapping studies of epitopes recognized by potently neutralizing mouse MAbs have identified several hot spots on all three domains of the E protein. The most potent type-specific murine neutralizing Abs have been shown to bind a region on the lateral surface of the recombinant E protein DIII (5–9). As a result of these studies, considerable previous efforts focused on EDIII for possible use as a vaccine target. DENVs replicate poorly in mice, and recent studies suggest that the immunodominant regions recognized by experimentally inoculated mice and naturally infected humans may differ (10).
A comprehensive understanding of the locations of antigenic sites targeted by the protective human Ab response and the sites responsible for the development of potentially harmful infection-enhancing Abs is of critical importance. This topic has been an area of intense investigation, with recent results beginning to shed light on the nature of the human Ab response to DENV infection. Much of our knowledge of this response has come from studies using polyclonal sera from naturally infected patients. For instance, using Ab depletion experiments, we previously demonstrated that EDIII-binding Abs account for only a small fraction of the anti-DENV binding and neutralization activity of immune serum (11). Crill et al. confirmed that serotype-specific DIII Abs formed a very small proportion of the polyclonal response; however, these investigators detected a difference in DENV neutralization titers, albeit a small one, in the absence of these Abs (12). Studies of a recombinant DENV3 (rDENV3/4) strain containing a transplanted DENV4 E glycoprotein domain I/II (EDI/II) hinge revealed that hinge transplantation led to a gain of sensitivity to neutralization by DENV4 primary immune sera and a loss of neutralization by DENV3 primary immune sera (13). Also, we recently reported studies using human MAbs that support the importance of the hinge region as a critical determinant of type-specific neutralization activity directed to hinge regions from wild-type (14) or recombinant hinge-transplanted (13) viruses.
Understanding the fine specificity of the human B cell response to DENV requires the isolation of human MAbs, which have been difficult to generate in the past. In the last several years, however, we and others have isolated panels of naturally occurring human MAbs which suggest that the human response targets both the E and prM proteins and is made up largely of serotype-cross-reactive and weakly neutralizing Abs (14–18). Only a very small percentage of Abs isolated to date that are directed against surface-exposed epitopes are serotype specific, and the isolation of potent neutralizing human MAbs (those neutralizing DENV at concentrations of <0.5 μg/ml) is rare. The reasons for the difficulty in isolating potent neutralizing human MAbs are several but include the low frequency of such B cell clones in the human response and the complex structural nature of the epitopes recognized, which is difficult to recapitulate in laboratory protein reagents. DENV immune serum depletion studies have shown that the Abs responsible for serum neutralizing activity following primary DENV infection target complex epitopes that exist in a correct conformational state only on the virion particle, not on recombinant soluble forms of E protein (11, 14). The structure of a virion-only-binding Ab in complex with DENV1 was determined recently using cryo-electron microscopy, and this also revealed that the configuration of antigenic regions of E protein in particles differs from that of soluble E protein (19, 20). These observations raise the concern that studies using recombinant E protein to isolate human B cells for study may bias against the isolation of neutralizing Abs that recognize antigens that are presented best in the context of viral particles.
Our goal here was to develop a screening strategy to identify and isolate very potent DENV-neutralizing human MAbs directed to conformation-dependent E protein antigenic sites. We sought to overcome the obstacle of the low frequency of circulating human B cells specifying high-potency Abs and the problem of conformational complexity of DENV neutralizing determinants. We used a high-efficiency B cell transformation protocol to generate thousands of human B cell lines from immune donors, screened secreted Abs for binding to live DENV, and then used a secondary screen to identify functional Abs by using a high-throughput, flow cytometry-based neutralization assay with live DENV. Cell lines secreting Abs that bound to live virus in immunoassay and neutralized live virus in vitro were then fused to make hybridomas, resulting in MAb-secreting clones. Using this new screening strategy, we identified a large panel of human MAbs that were enriched for potent neutralizing human MAbs and then performed detailed characterization of these MAbs. Analysis of binding patterns of the resulting DENV-neutralizing MAbs allowed us to define novel recognition groups of epitopes that were associated with unique patterns of fine specificity and neutralization and enhancing activities.
MATERIALS AND METHODS
Human subjects and peripheral blood cell isolation.
We identified a panel of subjects in North Carolina and Tennessee who had acquired DENV infection naturally by screening volunteers with suspected exposure during past travel to regions where DENV is endemic. Subjects were confirmed to have had DENV infection by testing their sera for the presence of Abs that neutralized one or more of the DENV serotypes. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient separation on Ficoll. The cells were cryopreserved immediately and stored in liquid nitrogen until study. The protocol for recruiting and collecting blood samples from subjects was approved by the Institutional Review Boards of the University of North Carolina at Chapel Hill and the Vanderbilt University Medical Center.
Viruses and recombinant proteins.
DENV1 (American genotype) WestPac-74, DENV1 (Asian genotype) Philippines 2004, DENV2 (Asian genotype) S-16803, DENV3 (American genotype) Nicaragua 1998, DENV3 (Asian genotype) CH-53489, DENV3 (Asian genotype) Thailand 1974, and DENV4 (American genotype) TVP-376, provided by Robert Putnak (Walter Reed Army Institute of Research, Silver Spring, MD), were used in the present study for both binding enzyme-linked immunosorbent assays (ELISAs) and neutralization assays. Infected cell culture monolayer supernatants containing live DENV for use in virus-capture ELISA were prepared in C6/36 mosquito cell monolayers grown in complete minimal essential medium (MEM) (Gibco).
Recombinant proteins representing fragments of the E or prM protein, prepared as previously described (21), were used to determine antigens and domains recognized by human MAbs. Fragments included the full ectodomain of the E protein (which represents ∼80% of the E protein; commonly referred to as E80 and here designated “rE”), a recombinant fragment containing domains I and II (rEDI/II), and a recombinant fragment containing domain III (rEDIII). cDNAs encoding recombinant DENV proteins were constructed using the sequences of the above-described strains. Sequence optimization, gene synthesis, and molecular cloning of all recombinant DENV protein constructs for expression in insect cells by use of a baculovirus vector were performed by GenScript USA Inc. Protein production and purification were described previously (21).
Generation of human hybridomas.
Previously cryopreserved samples were thawed rapidly in a 37°C water bath and washed prior to transformation with Epstein-Barr virus (EBV) in the presence of CpG, a Chk2 inhibitor drug, and cyclosporine, as described previously (15). Cultures were incubated at 37°C with 5% CO2 for 10 days prior to screening for virus-specific cell lines by ELISA. Cells from wells with supernatants reacting in an ELISA with captured live DENV were then expanded prior to screening by flow cytometric neutralization assay (see below) and cytofusion with HMMA2.5 nonsecreting myeloma cells, as previously described (15). Following cytofusion, hybridomas were selected by growth in HAT medium containing ouabain and biologically cloned.
MAb production and purification.
Wells containing hybridoma cells producing DENV-specific Abs were cloned biologically by three rounds of limiting dilution plating or by use of a ClonePix device (Molecular Devices) per the manufacturer's recommendations. Once clonality was achieved, each hybridoma was expanded until 50% confluent in 75-cm2 flasks. For Ab expression, the cells in 75-cm2 flasks were collected with a cell scraper; the hybridomas were washed in serum-free medium (Gibco Hybridoma-SFM from Invitrogen) and split equally among four 225-cm2 flasks (Corning) containing 250 ml serum-free medium. Flasks were incubated for 21 days before the medium was clarified by centrifugation and sterile filtered (0.2 μm). Abs were purified from clarified medium by protein G chromatography (protein G HP columns; GE Life Sciences).
Virus-capture and recombinant protein-capture ELISAs.
For virus-capture ELISA, purified mouse MAb 4G2, prepared in carbonate binding buffer, was used to coat ELISA plates (Nunc) and incubated at 4°C overnight. After blocking for 1 h, plates were washed 5 times with phosphate-buffered saline (PBS), and 50 μl of live-DENV-containing culture supernatant from infected C6/36 cell culture monolayers was added. Plates then were washed 10 times with PBS, and 10 μl of B cell culture supernatant (during screening) or 5 μl of purified human MAb at 1 μg/μl (during characterization of clones) was added to 25 μl/well of blocking solution. Plates were incubated at room temperature for 1 h prior to washing 5 times with PBS. A secondary Ab conjugated to alkaline phosphatase (goat anti-human Fc; Meridian Life Science) was applied at a 1:5,000 dilution in blocking solution (25 μl/well), and plates were again incubated at room temperature for 1 h. Following repeat PBS washing 5 times, phosphatase substrate solution (1 mg/ml phosphatase substrate in 1 M Tris aminomethane; Sigma) was added at 25 μl/well, and plates were incubated at room temperature for 2 h before reading of the optical density at 405 nm on a Biotek plate reader.
To define the binding domains of purified MAbs, we used ELISA binding to DENV protein fragments. Recombinant E or prM protein constructs fused to the Strep-tag II epitope were expressed using a baculovirus vector and insect cells. Mouse anti-Strep-tag II MAb (StrepMAB-Immo; IBA 2-1517-001) prepared in carbonate binding buffer was used to coat ELISA plates (Nunc) and incubated at 4°C overnight. After blocking for 1 h, plates were washed 5 times with PBS, and 50 μl of culture supernatant from insect cells was inoculated with a baculovirus expressing a prM or E recombinant protein fragment construct. Plates were then washed 10 times with PBS, and 5 μl of purified human monoclonal Ab (1 μg/μl) was added to 25 μl/well of blocking solution. All other steps were performed as described above for the virus-capture ELISA.
Competition assays.
An Octet Red instrument (ForteBio) for biolayer interferometry was used for competition binding studies to determine which Abs bound to common major antigenic sites. For DENV1, we used live virus as antigen; for DENV2, we used recombinant E protein. For competition assays using whole DENV1 virions, purified biotinylated mouse anti-DENV prM MAb 2H2 was loaded onto streptavidin tips (ForteBio). A suspension of live DENV1 WestPac-74 was prepared by pelleting virus from 250 ml of sterile-filtered supernatant from infected C6/36 cell culture monolayers by ultracentrifugation for 12 h. The pellet containing crude virion particles was then suspended in 5 ml PBS and used for capture on biosensor tips coated with MAb 2H2. After a wash step, the first anti-DENV MAb was added to saturation, followed immediately by application of the second MAb to assess whether binding of the first MAb interfered with binding of the second. For DENV2 recombinant E protein competition assays, purified mouse anti-Strep-tag II MAb (StrepMAB-Immo; IBA 2-1517-001) was loaded onto anti-mouse IgG Fc capture tips (ForteBio) in order to capture the epitope-tagged recombinant protein. We added a soluble form of recombinant E protein from DENV2, designated rE (containing the extracellular domain, which is about 80% of the E protein sequence), that was generated previously (18). After a wash step, the first anti-dengue virus MAb was added. Without washing, the second Ab was then added and binding assessed. We defined competition for binding to the same antigenic site as a reduction of maximum binding of the second Ab to <25% of its binding in the absence of the first Ab. When maximum binding of the second Ab was >75% of its binding in the absence of the first Ab, we interpreted this finding as indicating a lack of competition. All Ab competition experiments resulted in maximum binding values for the second Ab that were <25% or >75% compared to maximum binding without competition (i.e., there were no ambiguous results due to intermediate levels of competition).
DENV Western blotting.
Live DENV in infected cell culture supernatants was concentrated by ultracentrifugation and then loaded into a 4 to 12% SDS-PAGE gel run under denaturing, nonreducing conditions. After transfer, the nitrocellulose membrane was probed with the purified human MAb in question (diluted 1:1,000) for 1 h at 37°C. The membrane was washed with PBS plus Tween 20 (PBST) 3 times and then incubated with alkaline phosphatase-conjugated goat anti-human Fc secondary Ab (Meridian Life Science) for 1 h at 37°C prior to washing and development using 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP/NBT) chromogenic substrate (Invitrogen).
Neutralization assay.
The neutralizing potency of MAbs was measured using a flow cytometry-based 96-well neutralization assay with either Vero cells or a U937 human monocytic cell line stably transfected with DC-SIGN, as previously described (22, 23).
ADE assay.
The ability of Abs to enhance DENV infection was measured using U937 cells that had not been engineered to express DC-SIGN. In the absence of the virus attachment factor, these Fc receptor-bearing cells are susceptible to infection only in the presence of DENV-specific Abs. The assay was performed as previously described in detail (15). ADE activity was expressed as the fold enhancement of infected cells in the DENV-specific Ab-treated sample compared to the sample treated with a control Ab.
RESULTS
Frequencies of DENV-reactive B cells in subjects following DENV infection.
We selected three DENV-immune subjects for our study: one following primary DENV2 infection, one following primary DENV3 infection, and one following secondary infection. Serum neutralization titers specific to the four DENV serotypes were used to define whether the subjects had a primary or secondary infection (Table 1). PBMCs were obtained and studied 1 to 9 years following resolution of the infection. PBMC samples were transformed, and transformed B cell culture supernatants were first screened for binding to live DENV in virus-capture ELISA. Based on the number of positive wells and the number of lymphocytes tested (determined by average colony counts in transformed wells), the frequency of DENV-specific B cells in circulation was estimated for each subject. The frequencies of DENV-specific B cells were determined to be similar between the three DENV-immune samples: between 1.4 and 1.8 per thousand transformable B cells (Table 1). In a separate experiment, additional screening was performed on EBV-transformed B cell cultures from donor 1 (previously exposed to secondary DENV infection), using recombinant E protein from each DENV serotype (Fig. 1). We detected the presence of B cell lines secreting serotype-specific Abs to DENV1 or DENV3 but not to DENV2 or DENV4, which is suggestive of previous infections with the former two serotypes. This strategy for determining the infecting serotypes following secondary infection has not been described previously.
TABLE 1.
Subject demographics, serologies, and hybridoma yields from DENV-immune subjectsa
| Type of infection | Donor no. | Infecting serotype(s) | Location acquired | Yr of infection | Time since infection (yr) | Reciprocal serum Ab 50% neutralization titer to DENV serotype: |
No. of wells with DENV-reactive supernatants/total no. of wells tested | Estimated frequency of DENV-specific B cells in circulation (10−4) | No. of hybridomas obtained | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||||
| Secondary | 1 | 1 and 3 | Mexico | 2006 | 4 | 282 | 209 | 166 | 76 | 178/3,840 | 14 | 27 |
| Primary | 2 | 3 | Nicaragua | 2009 | 1 | 60 | 32 | 980 | 76 | 90/1,536 | 18 | 14 |
| 3 | 2 | Sri Lanka | 1996 | 9 | < | 271 | < | 42 | 117/2,304 | 16 | 9 | |
<, titer of <1:20. Live-DENV-reactive B cell frequencies were estimated using the number of wells with DENV-reactive supernatants divided by the total number of lymphoblastoid B cell colony counts in the transformation plate (i.e., the total number of B cells investigated).
FIG 1.
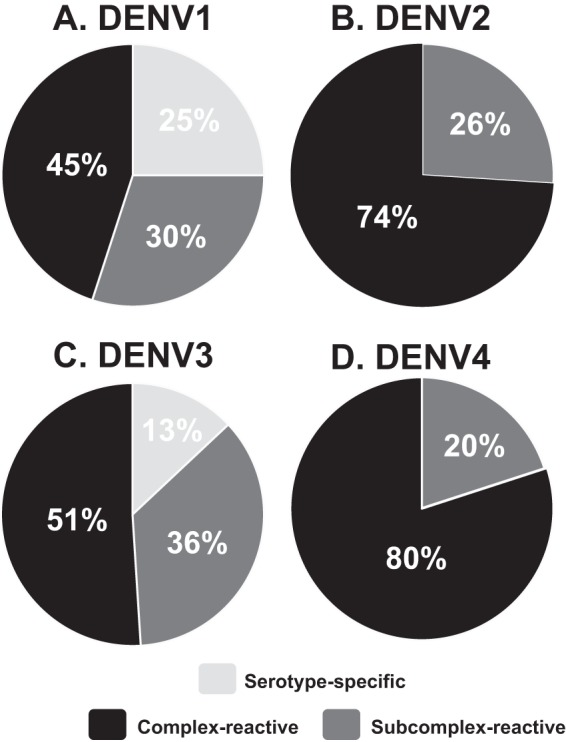
DENV rE protein-reactive Ab responses reveal that the two infecting serotypes for donor 1 (with secondary infection) were DENV1 and DENV3. Supernatants from EBV-transformed B cell cultures from donor 1 were screened for binding to rE protein from each DENV serotype. A total of 249 cultures were determined to react with one or more DENV rE proteins in ELISA. Serotype cross-reactivity profiles are shown for 81/249 (33%) cultures that bound DENV1 rE protein (A), 50/249 (20%) that bound DENV2 rE protein (B), 72/249 (29%) that bound DENV3 rE protein (C), and 46/249 (18%) that bound DENV4 rE protein (D). Serotype-specific, binds only one serotype; subcomplex-reactive, binds more than one but fewer than four serotypes; complex-reactive, binds all four serotypes.
Secondary screening of live-DENV-reactive B cell cultures by use of a flow cytometric neutralization assay to enrich for B cell lines secreting neutralizing Abs.
Previous studies have demonstrated that most DENV-specific memory B cells secrete serotype-cross-reactive, poorly neutralizing Abs and that only a minor fraction (<5% of DENV-specific B cells) secrete strongly neutralizing Abs associated with long-term protection (14, 15, 18). We used a flow cytometry-based neutralization assay (22, 23) as a secondary screen to identify B cells producing rare strongly neutralizing Abs (Fig. 2). In each case, the cultures exhibiting the highest neutralization capacity were directed against the serotype(s) responsible for the donor's infection according to serology (Fig. 2). As expected, a very large percentage of cultures from each donor mediated <50% neutralization in the assay. In fact, many lines had no detectable neutralizing capacity or caused an apparent enhancement of DENV infection in the assay. We selected for cytofusion the top 30 to 40% of DENV-reactive B cell lines, based on percent neutralizing activity against the serotype(s) of the infecting strain(s) in the donor, as indicated in the boxed areas in Fig. 2.
FIG 2.
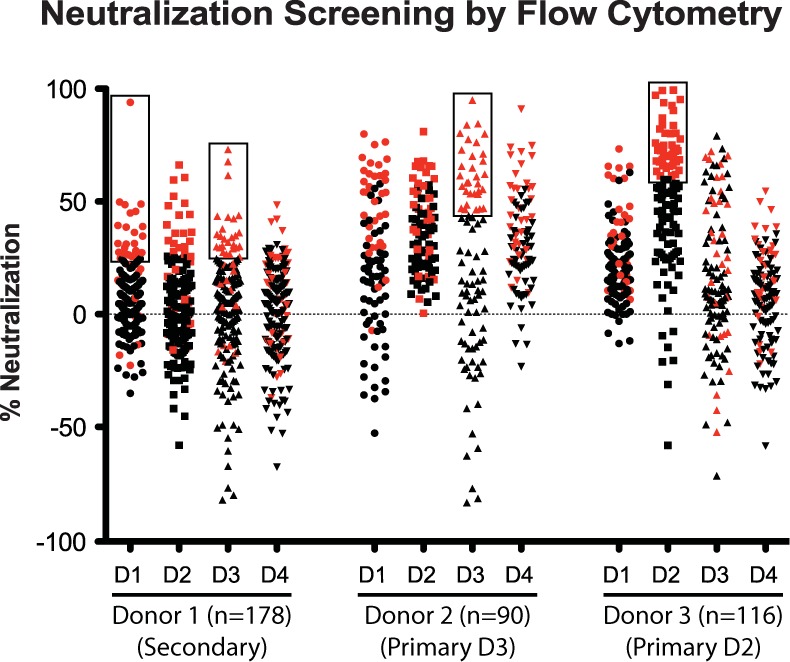
Secondary screening of live-DENV-reactive B cell cultures in a flow cytometric neutralization assay using U937 cells expressing DC-SIGN. The percent neutralization mediated by Abs in supernatants of EBV-transformed B cell cultures from three donors is shown for viruses of the four DENV serotypes. We tested 178 supernatants from donor 1 (previous secondary infection with DENV1 and DENV3), 90 supernatants from donor 2 (primary DENV3 infection), and 116 supernatants from donor 3 (primary DENV2 infection). Individual B cell lines chosen for cytofusion and generation of hybridomas are indicated by symbols within black boxes and are also highlighted in red to show their distribution of neutralizing activity for all serotypes. For donor 1, we selected lines with highest activity toward DENV1 or DENV3; for donor 2, we selected those with highest activity toward DENV3; and for donor 3, we chose those with highest activity toward DENV2. The concentrations of individual MAbs in this screening assay of cultures containing multiple B cell clones were unknown; however, in some cases, a high level of activity in this screen predicted a high potency of the identified clone. For example, the highest point of neutralization in donor 1 clones neutralizing DENV1, which neutralized DENV1 at a 95% level, was later isolated as the potent MAb 1F4.
Distinct groups of neutralizing MAbs exhibiting diverse binding and functional properties.
DENV-reactive B cell cultures selected based on live virus binding plus the virus neutralization screen were processed by cytofusion with a myeloma cell partner to generate human hybridoma cells stably secreting fully human anti-DENV MAbs. A total of 50 human MAbs were generated from 130 cytofusions attempted with cells of the three donors, with a significant number of clones having strong neutralizing potency (see Table S1 in the supplemental material). Detailed characterization revealed that many strongly neutralizing and serotype-specific MAbs were identified in this panel. Still, a significant proportion of the MAbs generated were DENV complex reactive, displayed weak or no neutralizing potency, and exhibited significant ADE capacity in cell culture.
While we were characterizing these MAbs, a number of interesting different neutralizing classes of Abs emerged (which we designated groups A through F), exhibiting diverse binding and functional properties (Table 2). Groups of MAbs that target prM or different regions of the E protein could be distinguished. We used the following definitions to describe the breadth of Abs for the four serotypes: serotype specific, binds only one serotype; DENV subcomplex reactive, binds more than one but fewer than four serotypes; and DENV complex reactive, binds all four serotypes. The diverse characteristics displayed by these groups of MAbs represent binding to various epitopes, and possibly different mechanisms of neutralization. For example, in one case, for the group C MAb 1F4, which possesses potent serotype 1-specific neutralizing activity, the Ab was found to target an epitope present only on the intact viral particle (14, 20). As can be seen in Fig. 3A, MAb 1F4 exhibited strong DENV1-specific neutralization potency (50% effective concentration [EC50] of 0.1 μg/ml), without the ability to bind or enhance infectivity of other serotypes. Another group of potently neutralizing serotype-specific Abs is exemplified by 3F9 (group B) (Fig. 3B). MAb 3F9 is serotype specific in binding and neutralization (in this case, to DENV2; EC50 = 0.03 μg/ml), but unlike 1F4, which binds only to virions, 3F9 binds to recombinant E protein and targets EDIII. Another group of strongly neutralizing MAbs, designated group D, are DENV complex reactive in binding but serotype specific in neutralization. MAb 4L5, isolated from donor 1, who had a history of secondary infection, bound viruses of all four DENV serotypes equally in ELISA, but it neutralized only DENV1. One interesting DENV complex-reactive Ab (MAb 1I12) bound the prM protein and strongly neutralized DENV3, which is an unexpected property for a prM-specific Ab.
TABLE 2.
Patterns of binding to recombinant proteins and functional activities of DENV-reactive neutralizing MAbsa
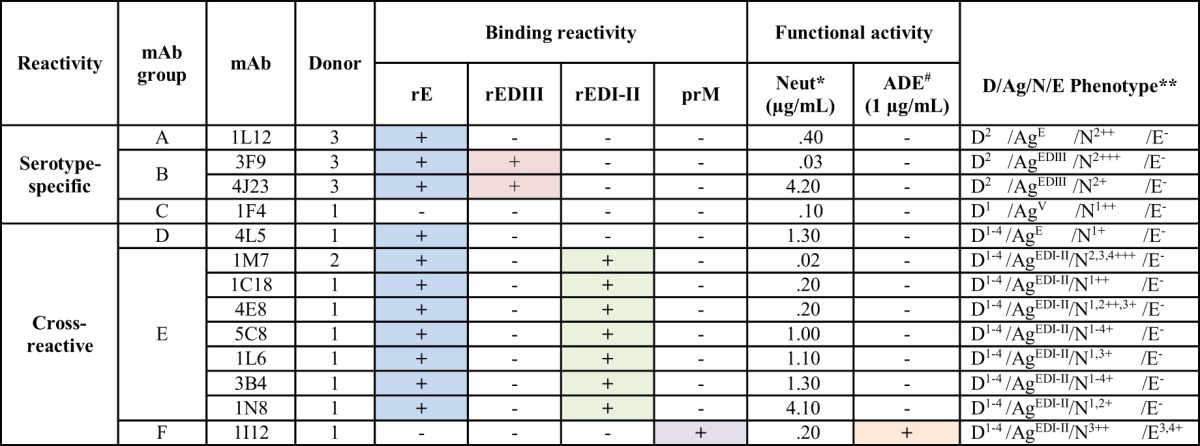
*, lowest concentration at which 50% neutralization (Neut) occurred in flow cytometry-based neutralization assay against any of 4 serotypes. #, presence of >25-fold Ab-dependent enhancement for any of the 4 serotypes tested at 1 μg/ml. **, the key to phenotypes is as follows: D, dengue virus binding [the superscript indicates the serotype(s) of virus bound in whole-virus-capture ELISA]; Ag, antigen binding pattern, i.e., which recombinant E protein domain antigen, if any, was bound in ELISA (E = envelope, V = virion-only binding, DI-II = domain I-II fusion protein, and DIII = domain III); N, neutralization (the superscript indicates which of the 4 serotypes of virus were neutralized in the flow cytometric neutralization assay) (+, ++, and +++ indicate concentrations where 50% of virus was neutralized, in ranges of 1 to 5, 0.1 to 0.9, and <0.1 μg/ml, respectively); and E, enhancement, i.e., the serotype(s) of virus enhanced in a flow cytometric ADE assay at 1.0 μg/ml (−, <25-fold enhancement; +, 25- to 49-fold enhancement).
FIG 3.
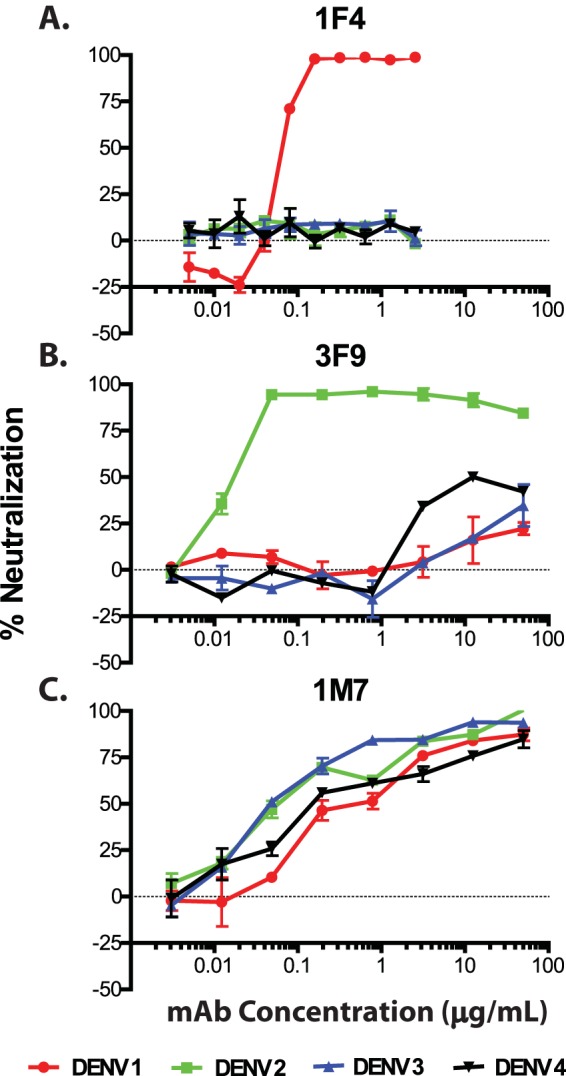
Neutralization profiles of potent serotype-specific and complex-reactive human MAbs in U937 cells expressing DC-SIGN. Neutralization curves are shown for the following representative potent, serotype-specific human MAbs: DENV1-specific MAb 1F4 (A), DENV2-specific MAb 3F9 (B), and serotype-cross-neutralizing human MAb 1M7 (C). Neutralization is shown across a series of concentrations (in μg/ml). EC50s were determined for MAb 1F4 (DENV1 EC50 = 0.1 μg/ml), MAb 3F9 (DENV2 EC50 = 0.03 μg/ml), and MAb 1M7 (DENV1 EC50 = 0.55 μg/ml, DENV2 EC50 = 0.14 μg/ml, DENV3 EC50 = 0.06 μg/ml, and DENV4 EC50 = 0.60 μg/ml).
The group of neutralizing Abs that contained the most members (group E) was DENV complex reactive in binding, and these Abs neutralized viruses of more than one DENV serotype. MAb 1M7, for example, bound and strongly neutralized viruses of DENV serotypes 1 through 4 in cells, with EC50s of 0.55, 0.14, 0.06, and 0.60 μg/ml, respectively (Fig. 3C). Abs in two of the three cross-reactive groups, groups D and E, targeted the EDI/II region. Abs in these two major groups were quite variable in their neutralization potency. For example, MAb 1M7 was strongly neutralizing (EC50 of 0.02 μg/ml), while 1N8 was only weakly neutralizing (EC50 of 4.1 μg/ml), representing at least a 200-fold range of activity. Potent MAbs (EC50s of <0.5 μg/ml) were isolated from both primary and secondary cases, at similar frequencies (3/23 clones from primary cases and 4/27 clones from secondary cases).
Binding and functional properties of groups of nonneutralizing and enhancing MAbs.
Despite our strategy to enrich the number of neutralizing MAbs generated, characterization of the resulting MAbs also revealed several different classes of nonneutralizing MAbs (designated groups G through K), which were grouped based on binding and functional properties (Table 3). Many Abs identified in this study (groups H to K) were DENV complex reactive and had little or no neutralizing or enhancement capacity. These groups of MAbs exhibited similar functional properties, but they targeted different sites on the virus. The group of nonneutralizing Abs that contained the most members (group I) was DENV complex reactive in binding and targeted EDI/II. Group K MAbs were complex reactive in binding but bound to prM. Several of these Abs exhibited remarkable ADE activity. For example, MAb 3I18 from group I and MAb 5E15 from group K were potent at enhancing infection in vitro, increasing infectivity by 66- and 61-fold, respectively.
TABLE 3.
Pattern of binding to recombinant proteins and functional activities of DENV-reactive nonneutralizing MAbsa
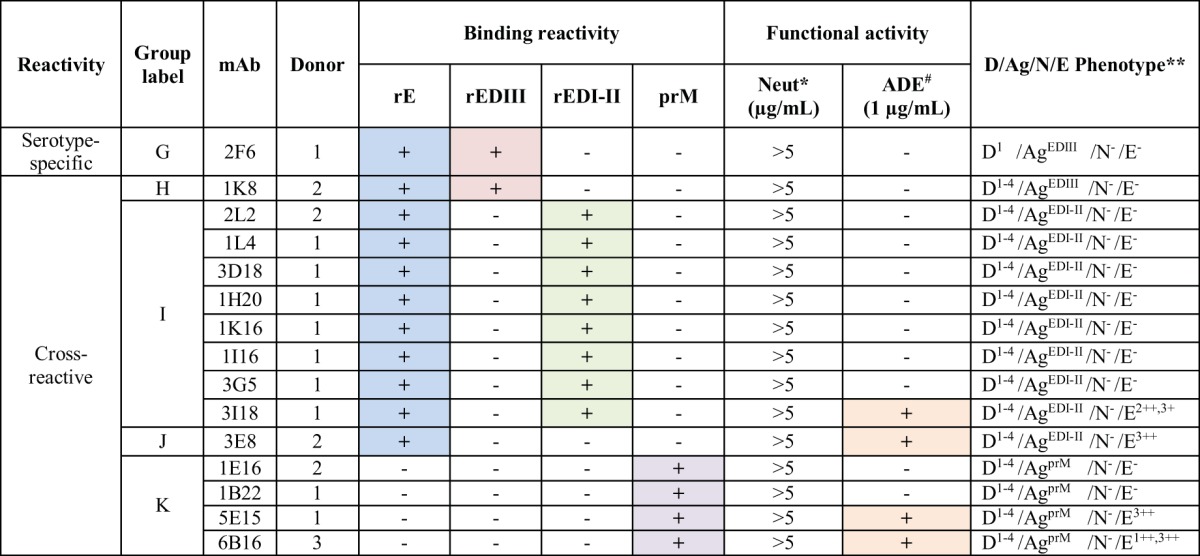
*, 50% neutralization was not detected at any concentration up to 5 μg/ml in flow cytometry-based neutralization assays against any of the 4 serotypes. #, presence of >25-fold Ab-dependent enhancement for any of the 4 serotypes tested at 1 μg/ml. **, the key to phenotypes is as follows: D, dengue virus binding [the superscript indicates the serotype(s) of virus bound in whole-virus-capture ELISA]; Ag, antigen binding pattern, i.e., which recombinant E protein domain antigen, if any, was bound in ELISA (E = envelope protein, prM = premembrane protein, DI-II = domain I-II fusion protein, and DIII = domain III); N, neutralization; and E, enhancement, i.e., the serotype(s) of virus enhanced in a flow cytometric ADE assay at 1.0 μg/ml (−, <25-fold enhancement; +, 25- to 49-fold enhancement; ++, 50- to 100-fold enhancement).
Potent serotype-specific MAbs display little enhancement potential.
Many of the most potent neutralizing Abs identified here were serotype specific in their binding and neutralization (groups A to C) (Table 2; see Table S1 in the supplemental material). When these were tested for the ability to enhance DENV infection in cell culture at 1.0 μg/ml, little activity was detected. We also tested representative antibodies from different groups in a broad range of dilutions in the ADE assay (clones indicated with yellow highlighting in Table S1; data not shown). We found that one of the cross-neutralizing antibodies (1M7) of group E that was not identified previously as possessing ADE activity did show enhancement capability at a very low concentration (0.008 μg/ml). The neutralization potency of MAbs in groups A through D was tested in several cell lines, since activity is known to vary in differing lines (Table 4). We also tested the neutralization activity against viruses of Asian versus American genotypes for representative clones, since two of the donors were infected in the Americas (data not shown). In most cases, the activities were comparable, although the activity appeared to be higher for the 1M7 MAb against the DENV3 strain from Nicaragua, the country where the relevant donor was infected.
TABLE 4.
Neutralization potencies of serotype-specific human MAbs in two different cell lines
| Serotype specificity | MAb | EC50 (μg/ml)a in: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| U937 cells expressing DC-SIGN |
Vero-81 cells |
||||||||
| DENV1 | DENV2 | DENV3 | DENV4 | DENV1 | DENV2 | DENV3 | DENV4 | ||
| DENV1 | 1F4 | 0.1 | > | > | > | 0.02 | > | > | > |
| 4L5 | 1.3 | > | > | > | 0.05 | > | > | > | |
| DENV2 | 1L12 | > | 0.4 | > | > | > | 0.05 | > | > |
| 3F9 | > | 0.03 | > | > | > | 0.003 | > | > | |
| 4J23 | > | 4.0 | > | > | > | 0.08 | > | > | |
EC50s of 1.0 to 10.0 μg/ml are shown. EC50s of <1.0 μg/ml are shown in bold, and EC50s of <0.1 μg/ml are shown in bold italics. >, neutralization was not detected when the MAb was tested at concentrations as high as 10 μg/ml.
Epitope mapping using competition assays.
We further characterized our panel of MAbs by determining competition binding groups. Using a real-time biosensor, we performed competitive binding studies with representative full-length MAbs to intact DENV1 particles or recombinant soluble DENV2 rE protein. As can be seen in Fig. 4, top panel, representative MAbs from the panel segregated into three principal competition groups when binding to the intact DENV1 particles. Inclusion of MAbs with known binding domains in the assay allowed us to attribute these groups to prM, fusion loop, and DI/II hinge region specificity. In testing for competition using DENV2 recombinant E protein, two main competition groups emerged (Fig. 4, bottom panel), including a large group recognizing the fusion loop region. Representative MAbs from the complex-reactive group E (neutralizing) or group I (nonneutralizing) MAbs competed for the same major antigenic site. MAb 5E15, which is representative of group K, bound to prM protein and was found to compete with the previously isolated human prM-binding Ab 4F8, which was included for comparative purposes.
FIG 4.
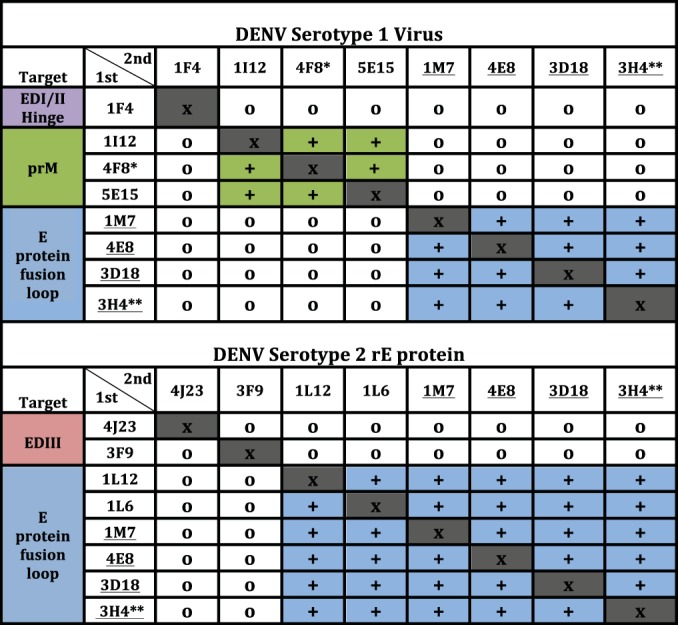
Antibody competition groups for ELISA binding to DENV1 or DENV2 rE protein antigen. Underlined clone names indicate MAbs that bound to both DENV1 and DENV2 and thus were tested in both assays. x, completion with the same antibody (competition was detected in all cases); +, binding of the first antibody applied (listed in vertical column) reduced binding of the second antibody applied (indicated in top row); o, competition was not detected. *, MAb 4F8 was previously isolated and was included here as a control, since it was shown previously to bind to the prM protein; **, MAb 3H4 was isolated previously and was included here as a control, since it was previously shown to bind to the fusion loop of E protein DI-II.
DENV complex-reactive MAbs target a region of EDII around the fusion loop.
The largest groups of neutralizing and nonneutralizing Abs identified in our studies were groups E and I. Group E MAbs are DENV complex reactive and exhibit neutralizing activity toward viruses of more than one DENV serotype. We performed competitive binding studies, using either intact DENV1 particles or rE protein, with the previously isolated fusion loop-binding human MAb 3H4. Representative DENV complex-reactive MAbs from groups D, E, and I, each of which binds to rE protein, competed for binding with the human fusion loop-binding MAb 3H4. Competition of these MAbs was observed using either DENV1 particles (Fig. 4, top panel) or rE protein (Fig. 4, bottom panel), suggesting that MAbs in groups D, E, and I target similar antigenic sites focused on or near the fusion loop.
DISCUSSION
This study describes the isolation and characterization of 50 human MAbs to DENV by employment of a unique screening strategy to enrich MAbs possessing neutralizing activity. The rationale for the strategy was to focus the MAb isolation on Abs that recognized epitopes present in live virus particles, using a two-step screening strategy based on live virus binding and neutralization of wild-type virus in vitro. Supernatants from a total of 384 different live-DENV-reactive B cell cultures from three donors were assayed for neutralization potency. Cultures with Abs that bound to live virus and that possessed the highest degree of neutralizing potency were then selected for electrical cytofusion to generate human hybridomas, resulting in 50 new human MAbs. Characterization of the final purified MAbs resulted in the identification of a large number of diverse groups of Abs exhibiting different unique neutralization potencies, binding locations, and ADE activities.
In the process of generating human MAbs, we expanded DENV-reactive B cell cultures to allow for screening in neutralization assays. During this stage, we were able to estimate the frequency of circulating B cells that were reactive to DENV in the donor blood samples. Each of the donors tested had a remote history of DENV infection, ranging from 1 to 9 years prior. Despite the differences in serotype, location, and number of years since infection, the estimated frequencies of DENV-reactive circulating B cells were remarkably similar, at about 1 to 2 per thousand. This finding suggests that there may be a typical long-term set point frequency of B cells in the circulating peripheral blood memory B cell pool following DENV infection.
By testing our B cell culture supernatants for binding to all four DENV serotypes, we were able to determine the frequencies of complex-reactive or serotype-specific B cells. This information allowed us to determine the virus serotypes likely responsible for remote secondary infection in a donor with more than one prior infection, a method not previously described. Donor 1 demonstrated the typical serum titers seen in an individual following secondary DENV infection, with elevated serum titers to all four serotypes. Using the B cell transformation and culture technique, we were able to determine that among the many clones detected, this donor possessed some that were derived from DENV1 or DENV3 (but not DENV2 or DENV4) serotype-specific B cells, suggesting prior exposure to viruses of these two serotypes.
Screening of a large number of live-DENV-reactive B cell culture supernatants for neutralization potency clearly demonstrated the consistency of the pattern of induction of serotype-cross-reactive and weakly or nonneutralizing Abs that has been observed previously following DENV infection. For the donor with prior secondary infection studied here, more than 98% of B cell line supernatants with DENV-specific Abs showed <70% neutralization activity. Interestingly, a large percentage of live-DENV-reactive unpurified supernatants tested exhibited significantly negative values in the neutralization assay (meaning a higher percentage of inoculated cells were infected than the case in the absence of Ab). It should be noted that at the screening stage, as shown in Fig. 2, the concentration of a particular live-DENV-reactive MAb could not be determined in the culture supernatant from EBV-transformed B cell lines containing multiple transformed colonies (i.e., there were multiple transformed B cell clones in each well, only one of which was likely to be DENV specific). Therefore, the exact neutralization or enhancing potency could not be established for the screening supernatants, in contrast to the data with purified IgG from cloned hybridoma cells shown in later experiments.
To further study the neutralizing Ab response to DENV infection, B cell cultures possessing the highest potencies in our neutralization screening assay were selected for the generation of human hybridomas. Ultimately, 50 human hybridomas were generated, with isolation of a relatively large number of those secreting MAbs with strong neutralizing potency. Since the initial screen was performed with unpurified supernatants from EBV-transformed B cell lines, it was not surprising that some of the resulting clones did not possess neutralizing activity when tested as purified immunoglobulins. Analysis of this large Ab panel demonstrated that both neutralizing and nonneutralizing MAbs segregated into distinct phenotypic groups based on functional characteristics. Groups of serotype-specific and complex-reactive MAbs were characterized further. Interestingly, the most potent serotype-specific MAbs bound either to EDIII or to complex epitopes found only on the intact viral particle. Competition studies between groups of serotype-specific neutralizing MAbs revealed the presence of Abs to prM, the fusion loop, the DI/II hinge, and DIII. The most potent Ab that we isolated here, MAb 3F9, targeted EDIII, with a neutralizing activity of 3 ng/ml in Vero-81 cells. This finding was interesting because most human neutralizing Abs in polyclonal sera do not recognize DIII, and many neutralizing Abs in mice do recognize DIII.
Determining the epitopes recognized by potently neutralizing Abs induced in humans by natural infection may be one of the best tools that we have for understanding the determinants of human protection. Sites that induce ultrapotent neutralizing activity should be the focus of future rational DENV vaccine design. Based on the information provided here, we propose two distinct but compatible strategies for rational design of an effective DENV vaccine. One strategy would make use of the low potential for disease enhancement intrinsic to the induction of neutralizing Abs that recognize viruses of only one serotype (serotype-specific Abs). In this case, antigenic preparations incorporating epitopes with type-specific determinants could be developed to induce potent protection, without the generation of unwanted, potentially dangerous cross-reactive and nonneutralizing Abs. We designated this scheme a “four-by-one” vaccination plan (a mix of four vaccine candidates, each targeting one serotype). A second strategy would be to focus on synthesis of immunogens that possess cross-reactive antigenic determinants, but only incorporating those finely mapped features of epitopes that induce ultrapotent Abs that neutralize viruses of all four DENV serotypes. This scheme we designate a “one-by-four” vaccination strategy (one immunogen targeting all four serotypes). In this case, the response would be across serotypes, but the Abs elicited should narrowly target potent neutralizing epitopes, reducing the chance of enhancement at physiologic Ab concentrations. Of course, one might consider a five-valent strategy in which the “four-by-one” and “one-by-four” immunogens are combined, with the goal of inducing both type-specific and cross-reactive neutralizing Abs without induction of cross-reactive nonneutralizing responses.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant U54 AI057157 (to the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense) and NIH grant K08 AI103038 (to S.A.S.).
We thank Frances Smith-House for excellent laboratory management support.
Footnotes
Published ahead of print 6 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00247-14.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ. 2002. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 10:100–103. 10.1016/S0966-842X(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 3.Halstead SB, O'Rourke EJ. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741. 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 4.Zellweger RM, Prestwood TR, Shresta S. 2010. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7:128–139. 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crill WD, Roehrig JT. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769–7773. 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromowski GD, Barrett AD. 2007. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349–360. 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Gromowski GD, Barrett ND, Barrett AD. 2008. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J. Virol. 82:8828–8837. 10.1128/JVI.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin B, Parrish CR, Murray JM, Wright PJ. 1994. Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology 202:885–890. 10.1006/viro.1994.1410. [DOI] [PubMed] [Google Scholar]
- 9.Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. 2007. Type and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J. Virol. 81:12816–12826. 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahala WM, de Silva AM. 2011. The human antibody response to dengue virus infection. Viruses 3:2374–2395. 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. 2009. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103–113. 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crill WD, Hughes HR, Delorey MJ, Chang GJ. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:e4991. 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messer WB, de Alwis R, Yount B, Royal S, Huynh J, Smith SA, Crowe JE, Doranz B, White LJ, Sariol CA, de Silva AM, Baric RS. 2014. Dengue virus envelope protein domain I/II hinge determines long-lived serotype-specific dengue immunity. Proc. Natl. Acad. Sci. U. S. A. 111:1939–1944. 10.1073/pnas.1317350111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE, Jr, de Silva AM. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. U. S. A. 109:7439–7444. 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE., Jr 2012. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J. Virol. 86:2665–2675. 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. 2011. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl. Trop. Dis. 5:e1188. 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748. 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283. 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci. Transl. Med. 4:139–183. 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 20.Fibriansah G, Tan J, Smith SA, de Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Harris E, de Silva A, Crowe JE, Jr, Lok SM. 2014. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol. Med. 6:358–371. 10.1002/emmm.201303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SA, de Alwis R, Kose N, Durbin AP, Whitehead SS, de Silva AM, Crowe JE., Jr 2013. Human monoclonal antibodies derived from memory B cells following live attenuated dengue virus vaccination or natural infection exhibit similar characteristics. J. Infect. Dis. 207:1898–1908. 10.1093/infdis/jit119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraus AA, Messer W, Haymore LB, de Silva AM. 2007. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J. Clin. Microbiol. 45:3777–3780. 10.1128/JCM.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambeth CR, White LJ, Johnston RE, de Silva AM. 2005. Flow cytometry-based assay for titrating dengue virus. J. Clin. Microbiol. 43:3267–3272. 10.1128/JCM.43.7.3267-3272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


