ABSTRACT
Relatively little is known about the extent of the polyclonal antibody (PAb) repertoire elicited by herpes simplex virus (HSV) glycoproteins during natural infection and how these antibodies affect virus neutralization. Here, we examined IgGs from 10 HSV-seropositive individuals originally classified as high or low virus shedders. All PAbs neutralized virus to various extents. We determined which HSV entry glycoproteins these PAbs were directed against: glycoproteins gB, gD, and gC were recognized by all sera, but fewer sera reacted against gH/gL. We previously characterized multiple mouse monoclonal antibodies (MAbs) and mapped those with high neutralizing activity to the crystal structures of gD, gB, and gH/gL. We used a biosensor competition assay to determine whether there were corresponding human antibodies to those epitopes. All 10 samples had neutralizing IgGs to gD epitopes, but there were variations in which epitopes were seen in individual samples. Surprisingly, only three samples contained neutralizing IgGs to gB epitopes. To further dissect the nature of these IgGs, we developed a method to select out gD- and gB-specific IgGs from four representative sera via affinity chromatography, allowing us to determine the contribution of antibodies against each glycoprotein to the overall neutralization capacity of the serum. In two cases, gD and gB accounted for all of the neutralizing activity against HSV-2, with a modest amount of HSV-1 neutralization directed against gC. In the other two samples, the dominant response was to gD.
IMPORTANCE Antibodies targeting functional epitopes on HSV entry glycoproteins mediate HSV neutralization. Virus-neutralizing epitopes have been defined and characterized using murine monoclonal antibodies. However, it is largely unknown whether these same epitopes are targeted by the humoral response to HSV infection in humans. We have shown that during natural infection, virus-neutralizing antibodies are principally directed against gD, gB, and, to a lesser extent, gC. While several key HSV-neutralizing epitopes within gD and gB are commonly targeted by human serum IgG, others fail to induce consistent responses. These data are particularly relevant to the design of future HSV vaccines.
INTRODUCTION
Herpes simplex virus (HSV) infects the mouth, skin, eye, and genital regions. The hallmark of herpes simplex viruses is their ability to establish a lifelong latent or persistent infection in sensory neurons, with reactivations that cause recurrent disease or viral shedding without evidence of clinical symptoms. Currently, 16% of the United States population is seropositive for HSV-2 and 54% for HSV-1 (1). Although HSV-2 is typically the causative agent of genital lesions, there is an increased incidence of genital HSV-1 infection. Control and clearing of the virus and genital lesions have been ascribed to the generation of cellular immunity (2). Recently, Schiffer et al. (3) suggested that mucosal HSV-2-specific CD8+ T cells represent containment of prior viral shedding rather than a correlate of future protection. The importance of the humoral response in humans with recurrent shedding is unknown; however, it has recently been shown that antibodies play a key role in HSV infection and protection (4–6). In particular, the essential HSV entry glycoproteins gD, gB, and gH/gL, which are the major stimuli of virus-neutralizing antibodies (7–12), are crucial to this host response. In addition, we showed that HSV gC is a major inhibitor of the complement cascade and therefore innate immunity (13–15).
In previous studies, we developed panels of polyclonal (PAbs) and monoclonal (MAbs) antibodies for all five glycoproteins, and these antibodies have been characterized for their ability to neutralize virus (8, 10, 16–18). For the gB MAbs, the epitopes have been further localized via electron microscopy (EM) imaging of gB-Fab complexes (19). Antibodies against entry glycoproteins use several different mechanisms to inhibit virus infection, namely (i) blocking the interaction between gD and receptor, (ii) blocking gD-gH/gL interaction, (iii) blocking gH/gL-gB interaction, and (iv) interfering with gB's ability to fuse membranes (12, 16, 20–22). Interestingly, the MAbs that show virus-neutralizing activity recognize both linear and conformation-dependent epitopes.
Specific antibodies that recognize important epitopes on HSV envelope glycoproteins are poorly defined in the human response to natural infection by virus. However, for either vaccine design or evaluation of sera from vaccines, it is important to identify those epitopes on the entry glycoproteins that stimulate virus-neutralizing antibodies. In addition, those antibodies are likely essential in clearing infectious HSV during reactivation events. The overall objective of this study is to determine the magnitude and repertoire of antibodies that recognize HSV viral glycoproteins in sera of patients that are naturally infected with HSV and then relate this information to the mechanisms of virus neutralization and virus shedding.
Here, we studied 10 sera of people who are naturally infected with HSV-2 and in whom the rate of genital shedding was previously assessed for frequency and magnitude (23). Six persons were infected with both HSV-1 and HSV-2, while four were infected only with HSV-2. We found that in every case, the patients developed type-common neutralizing sera to these viruses. In order to learn more about what constitutes the humoral response to HSV as it occurs in humans, we used four methods to further examine the IgGs isolated from serum: (i) testing the purified IgGs against Western blots of purified glycoproteins of both serotypes, (ii) epitope analyses of these 10 IgGs by biosensor competition assays with neutralizing mouse MAbs against specific epitopes of gD and gB, (iii) fractionation of four antibodies into gD-specific and gB-specific IgGs using tandem affinity chromatography, and (iv) determination of virus neutralization by these four anti-gD- and anti-gB-specific IgGs.
MATERIALS AND METHODS
Cells and soluble proteins.
African green monkey kidney (Vero) cells were grown in Dulbecco's modified Eagle medium (DMEM) with 5% fetal bovine serum (FBS). All soluble HSV glycoproteins were produced from baculovirus-infected insect (Sf9) cells. gD1(306t) and gD2(285t) were purified using a DL6 immunosorbent column (24–26). gC1(457t) and gC2(426t) were purified as previously described (27). gH1(803t)/gL1 and gH2(803t)/gL2 were purified via a C-terminal six-His tag by use of Ni-nitrilotriacetic acid resin and elution with imidazole (28). gB1(803t), gB2(720t)+His, and gB2(727t) were purified by use of a DL16 immunosorbent column (29–31).
Antibodies.
gD-specific MAbs 1D3, DL11, MC2, MC5, MC14, and MC23 (16, 17, 32) and gB-specific MAbs C226, DL16, H1817, H1695, SS10, SS55, SS63, and SS144 (8, 33–36) were characterized previously. PAbs used in this study were as follows: rabbit (R) serum R7 was raised against purified gD2 (and cross-reacts with gD1) (37), R176 was prepared against purified gH2(803t)/gL2 (38), R137 was raised against purified gH1(803t)/gL1 (39), R68 was raised against purified, denatured gB1 (8, 40), and R90 was raised against purified, native gB2 from HSV-2 strain 333-infected cell lysates; R47 and R64 were raised against native gC1 and gC2, respectively (41).
Human samples.
Serum samples were obtained from HSV-2-infected persons enrolled in natural history studies at the University of Washington Virology Research Clinic in Seattle, WA. From each participant, daily swabs of genital secretions were collected to characterize their viral shedding rate as described by Tronstein et al. (23). The samples were purposefully chosen for this study from 5 patients with high shedding rates, defined as detection of HSV on >10% of days, and 5 patients with low shedding rates, defined as detection of HSV on <10% of days. All laboratory assays were performed by investigators who were masked to the shedding phenotype. IgG was purified from human sera by protein G chromatography (HiTrap; GE Healthcare) according to the manufacturer's instructions. Following purification, IgGs were dialyzed against phosphate-buffered saline (PBS). IgG yields ranged from 2.6 mg/ml to 7.2 mg/ml. The IRB number is 815813 (human sera with HSV2 antibodies; approval date, April 2014).
Western blotting.
Purified glycoproteins were separated by electrophoresis on a 10% sodium dodecyl sulfate-polyacrylamide gel under denaturing or “native” (32) conditions. Separated proteins were transferred to nitrocellulose and probed with IgG purified from human sera. A control blot was cut into strips and probed with rabbit PAbs specific for each glycoprotein.
Biosensor/surface plasmon resonance (SPR) experiments.
Experiments were performed using a Biacore 3000 optical biosensor (GE Healthcare, Biacore Life Sciences) at 25°C. Filtered and degassed HBS-EP buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20) was used in all experiments. An anti-His antibody (Qiagen, Inc.) was covalently coupled to a CM5 sensor chip (Biacore) following our previous protocol (42). Next, 200 resonance units (RU) of purified gD2(285t) or gB2(720t) were captured by the anti-His antibody via their C-terminal His tags. Purified human IgGs were then injected for 240 s, followed by IgGs from mouse MAbs (75 to 150 μg/ml), also for 240 s. After each experiment, the chip surface was treated with brief pulses of 0.2 M Na2CO3 (pH 11) until the RU signal returned to baseline, and then a new cycle was started. All injections were performed at a flow rate of 5 μl/min.
Virus neutralization assays.
Serial dilutions of IgG were mixed with HSV-1 (strain KOS) or HSV-2 (strain 333), and the mixture was incubated at 37°C for 1 h. Monolayers of Vero cells grown in 48-well plates were then incubated with the IgG-virus mixture for 24 h. Cells were fixed with methanol-acetone (2:1), and plaques were visualized by the black plaque assay (27).
Selection of gD- and gB-specific IgGs with glycoprotein antigen-sorbent columns.
Briefly, 1.1 g of activated CNBr Sepharose 4B (Pharmacia) was swollen in 0.001 N HCl at room temperature (RT) for 1 h. The gel was washed with 100 ml of 0.001 N HCl on a glass filter. Soluble, purified gB2(727t) or gD2(285t) (10 mg) in 2.8 ml PBS plus 2.8 ml of freshly made 0.1 M carbonate buffer (0.2 M sodium bicarbonate, 1 M NaCl, pH 8.3) was mixed with the gel overnight at 4°C. Next, the mixtures were washed with 1 M ethanolamine (pH 8.0), then with 0.1 M sodium acetate 1 M NaCl (pH 4.0), and finally with 0.1 M sodium borate 1 M NaCl (pH 8.0), all at RT. The protein-gel mixtures were then equilibrated with TS washing buffer (10 mM Tris-HCl [pH 7.2], 0.5 M NaCl) at 4°C and loaded into individual columns. Both columns were washed extensively with TS buffer before loading IgGs from human sera. The flowthrough was collected and recycled through each column 5 times, followed by washing with TS buffer. The gD- or gB-specific IgGs were eluted with 3 ml of 0.1 M ethanolamine (pH 11.5). The selection of gD IgG was done first, followed by passing the gD-depleted IgG sample through the gB column. The eluted samples were further dialyzed with PBS and concentrated with centrifugal filter units (Millipore). For sample 6, we passed the gD- and gB-depleted IgG over a gC1(346t) column (43) using the same procedure.
RESULTS
We obtained 10 blinded serum samples from a study of people naturally infected with either HSV-2 alone (samples 2, 4, 5, 8) or both HSV-1 and HSV-2 (samples 1, 3, 6, 7, 9, 10) (Table 1) (23). Serum samples 1 to 5 were from people who shed virus infrequently (low shedders), while samples 6 to 10 were from people who shed virus frequently (high shedders). Purified IgG was used in all analyses.
TABLE 1.
Characteristics of IgG from each human serum sample
| Sample | HSV status | Sheddinga | Neutralization (μg/ml IgG)b |
Reaction with glycoproteinc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSV-1 | HSV-2 | gB-1 | gB-2 | gC-1 | gC-2 | gD-1 | gD-2 | gHgL-1 | gHgL-2 | |||
| 1 | 1, 2 | Low | 23 | 90 | +++ | +++ | +/− | + | + | +++ | + | − |
| 2 | 2 | Low | 125 | 90 | +++ | +++ | − | +++ | + | +++ | +/− | − |
| 3 | 1, 2 | Low | 125 | 190 | +++ | +++ | − | + | + | +++ | + | − |
| 4 | 2 | Low | 23 | 18 | +++ | +++ | +/− | +++ | +++ | +++ | + | − |
| 5 | 2 | Low | 30 | 12 | ++ | +++ | − | +++ | + | +++ | − | + |
| 6 | 1, 2 | High | 8 | 5 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | − |
| 7 | 1, 2 | High | 7 | 12 | +++ | +++ | − | +++ | + | +++ | − | − |
| 8 | 2 | High | 55 | 38 | +++ | +++ | − | +++ | ++ | +++ | − | − |
| 9 | 1, 2 | High | 3 | 10 | +++ | +++ | +/− | + | ++ | +++ | + | + |
| 10 | 1, 2 | High | 9 | 7 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | + |
As determined by Tronstein et al. (23). “High” shedding rates are defined as detection of HSV on >10% of days, while “low” shedding rates are defined as detection of HSV on <10% of days.
Values represent a 50% reduction in plaques as compared to the control (no IgG).
Determined by IgG reactivity to purified proteins via Western blotting (Fig. 2). +++, very strong; ++, strong; +, moderate; +/−, weakly positive; −, negative.
Virus neutralization.
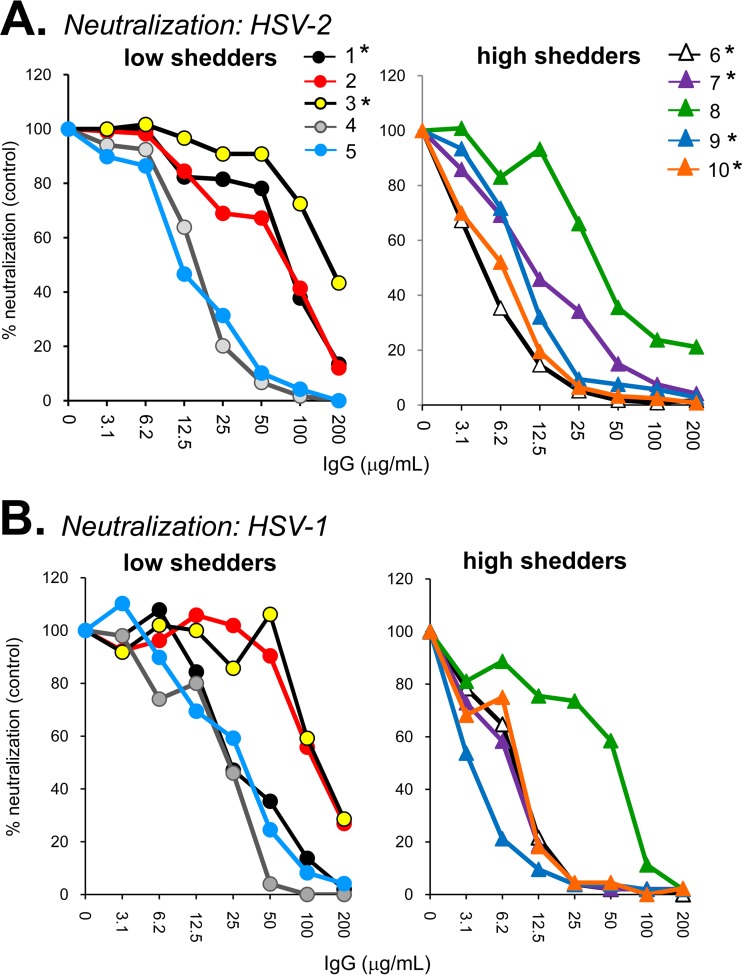
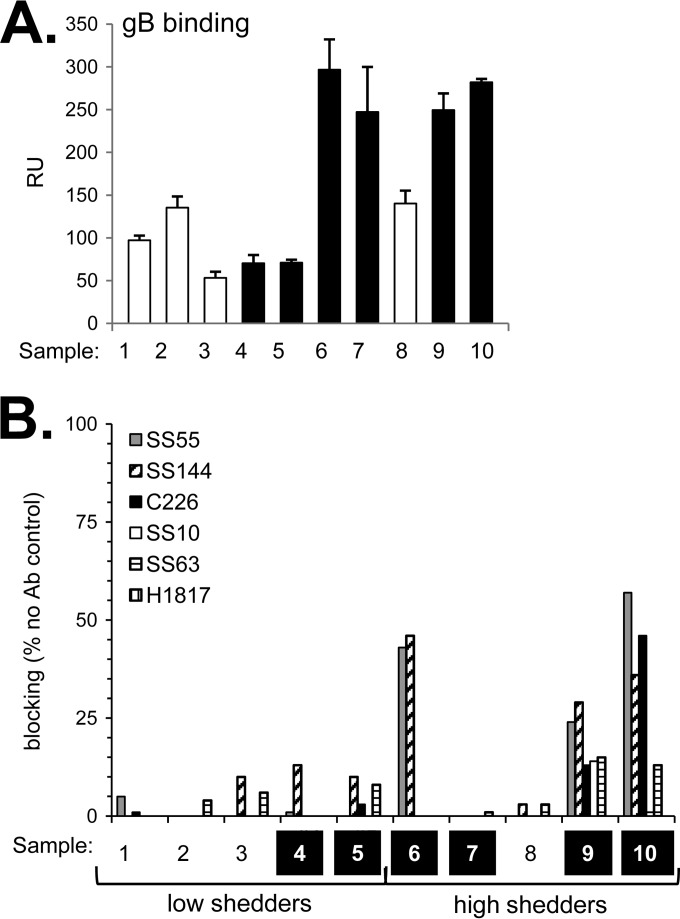
The human antibodies were screened for virus-neutralizing activity against either HSV-2 (333) (Fig. 1A) or HSV-1 (KOS) (Fig. 1B) via a standard plaque reduction assay. Virus neutralization activity was classified as weak if it required >30 μg/ml IgG to elicit 50% plaque reduction (Table 1). Of the five IgGs from low shedders, three (samples 1 to 3) neutralized HSV-2 poorly, while the remaining two (samples 4 and 5) neutralized HSV-2 well (Fig. 1A). For the high shedders, four of five samples (samples 6, 7, 9, and 10) showed strong neutralizing activity against both HSV serotypes. While the numbers are small, it does not appear that high neutralizing activity effectively controls shedding.
FIG 1.
Virus neutralization. Sample IgGs were tested for their ability to neutralize HSV-2 (A) or HSV-1 (B). Plaque numbers were determined for each sample and plotted as a percentage of plaques obtained in the absence of human IgG (y axis). IgG concentration is indicated on the x axis. Sample numbers are indicated at the top of panel A; those from dually infected (HSV-1/2) individuals are indicated with an asterisk (*).
IgG binding to purified HSV glycoproteins.
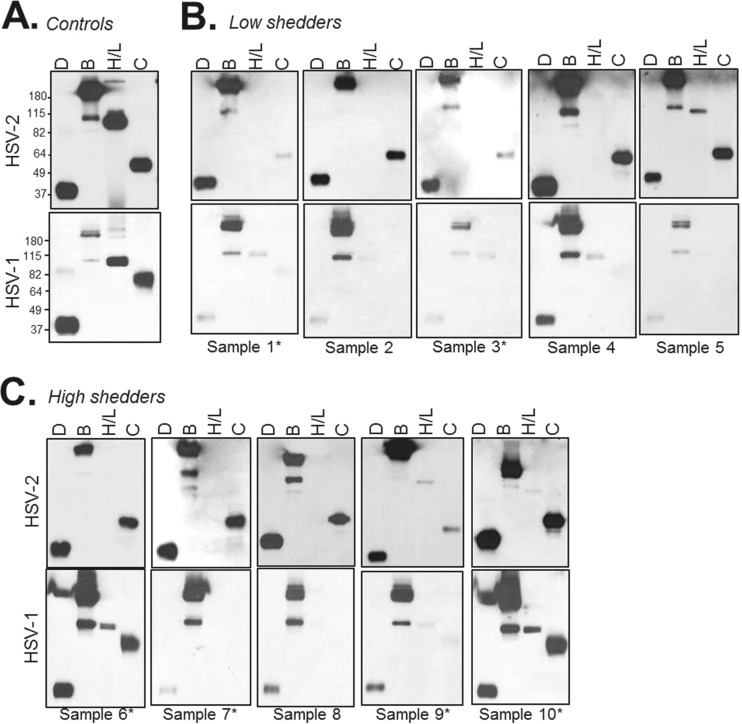
Purified gD, gB, gH/gL, and gC (for both HSV-1 and HSV-2) were run on SDS-PAGE gels under “native” (conformationally correct) (32) conditions. Western blots were then probed with each of the 10 sample IgGs. As a control, we probed another blot with a rabbit PAb generated specifically to each purified glycoprotein (Fig. 2A). Each of the 10 human IgGs reacted against gD and gB of both HSV serotypes (Fig. 2B and C). All sample IgGs recognized HSV-2 gC. However, only two samples (numbers 6 and 10) reacted strongly against HSV-1 gC or gH/gL of either serotype. It is possible that the lack of detection of gH/gL may be due to improper conformation of the heterodimer, as even “native” Western blots contain a small amount of detergent (32).
FIG 2.
Western blots. Purified glycoprotein D, gB, gH/gL, and gC were blotted onto nitrocellulose via nondenaturing Western blotting. (A) Control blots were probed with a mixture of rabbit polyclonal IgG against each protein. For HSV-2 proteins (top), rabbit PAbs R90 (anti-gB), R176 (anti-gH/gL), and R64 (anti-gC) were used. For HSV-1 proteins (bottom), PAbs R68 (anti-gB), R47 (anti-gC), and R137 (anti-gH/gL) were used. Anti-gD PAb R7 is type common and was used for both sets of blots. Molecular mass markers are shown to the left in kilodaltons. For the human samples, blots probed with IgG from individuals classified as low virus shedders are shown in panel B, and high shedders are shown in panel C. Samples from dually infected (HSV-1/2) individuals are indicated with an asterisk (*).
Thus, the Western blot data indicate that all 10 sera had a significant response to type 2 gD, gB, and gC, as well as type 1 gD and gB. It was surprising that so few samples recognized gH/gL of either serotype, either under native (Fig. 2) or denaturing (data not shown) conditions. As the most consistent responses were directed at gD and gB, we focused our next series of experiments on them.
Competition with murine anti-gD MAbs.
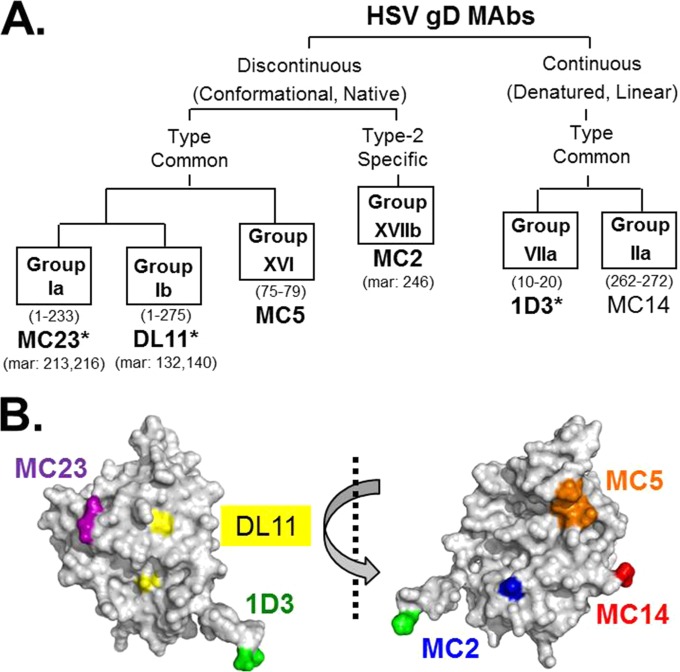
Antibodies against entry glycoproteins use several different mechanisms to inhibit virus infection (12, 16, 20–22). The six anti-gD MAbs used in this study (Fig. 3A) all affect gD function, but in different ways. Three MAbs block gD-receptor binding (1D3, MC23, Dl11), and two block the gD-gH interaction (MC2 and MC5) (16). We also included the nonneutralizing MAb MC14, which significantly enhances neutralization by MAb MC2 (16). The locations of the epitopes of these MAbs are shown mapped to the gD crystal structure (Fig. 3B).
FIG 3.
HSV gD MAb tree. (A) Diagram showing gD antigenic sites and associated MAbs used in this study. The defining properties of MAbs within each branch of the tree are indicated. Antigenic sites are defined by “groups” of MAbs that compete with each other for gD binding. MAb group designations are shown in boxes. gD residues implicated in MAb binding are in parentheses below group names. Representative MAbs are shown in large font below group names, with known residues of mar (MAb-resistant) mutants indicated below. Virus-neutralizing MAbs are shown in bold type. Receptor-blocking MAbs are indicated by an asterisk. (B) Two views of the gD structure rotated 180 degrees. The locations of the epitopes of MAbs listed above are indicated.
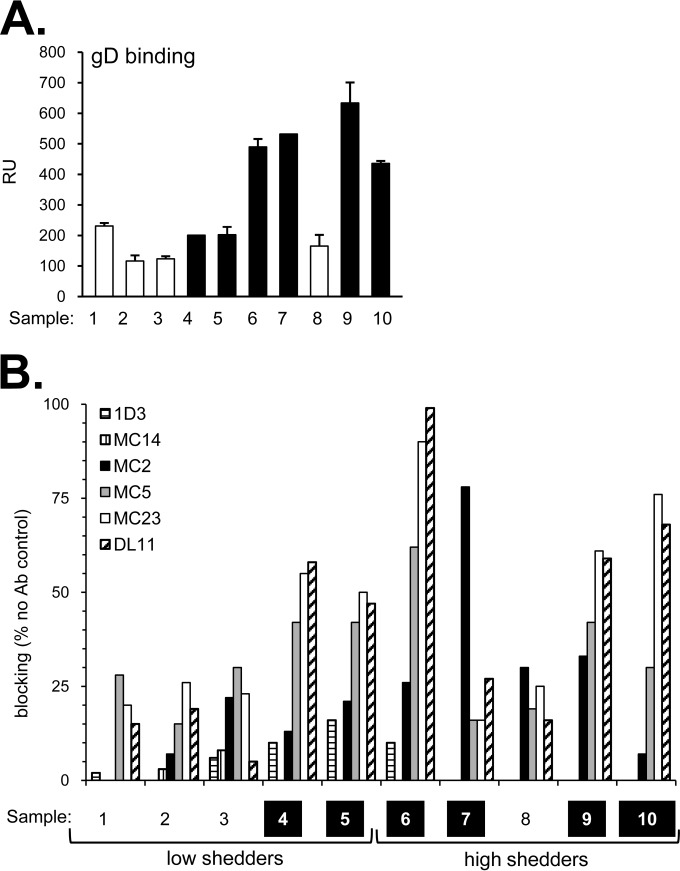
To characterize the responses to these six gD epitopes in naturally infected individuals, we used a protein-binding competition assay using surface plasmon resonance/biosensor (8, 44, 45). The goal was to determine whether and to what extent the binding of these MAbs to gD is diminished by preincubation of gD with human IgG. Reduced MAb binding would indicate the presence of human antibodies to an epitope that binds to or overlaps that of the neutralizing MAb. We first captured soluble gD2(285t) (to accommodate the type 2 specificity of MAb MC2) (16) on the surface of a biosensor chip via its 6-His tag. Each human IgG was then flowed across the chip surface to allow binding of gD-specific antibodies (Fig. 4A). A separate chip surface containing gD was exposed, in parallel, to buffer alone. Individual mouse MAb IgGs were flowed across the test and control surfaces. The difference in MAb binding to the two surfaces upon completion of the injection was used to calculate the MAb blocking activity of each human IgG. For example, if the human IgG contained antibodies that bound the same (or nearby) epitope as that of the mouse MAb, the mouse MAb would be unable to bind gD. However, if these antibodies were absent from the human IgG, the mouse MAb should have free access to the epitope and be able to bind to gD. Curves for all samples are shown in Fig. S1 in the supplemental material, and blocking of each MAb is summarized in bar graphs (Fig. 4B). We used an arbitrary cutoff point of 20% in scoring samples positive for blocking (45).
FIG 4.
(A) Binding of subject IgG to gD2 via biosensor. A fixed amount of each IgG was injected across a chip surface coated with purified protein. The levels of IgG binding (response units [RU]) for human sample IgG are shown. The samples with the strongest HSV-2 neutralization activity are represented by black bars and the weaker neutralizers by white bars. Error bars indicate standard errors for at least two experiments. (B) Biosensor analysis: blocking of neutralizing gD MAbs via human subject IgG. Each bar indicates the percent blocking activity (compared to the no-antibody control) against the anti-gD MAbs tested (1D3, MC14, MC2, MC5, MC23, DL11). Samples classified as strong HSV-2 neutralizers are highlighted in black along the bottom row. A representative experiment is shown.
Surprisingly, none of the 10 IgGs blocked MAbs 1D3 or MC14 (Fig. 4B, Table 2), both of which bind to linear epitopes (Fig. 3A). However, all 10 IgGs blocked at least one of the six MAbs, and the overall level of binding correlated with the extent of neutralization. Thus, four of the weak virus-neutralizing IgGs (samples 1, 2, 3, and 8) blocked MC2, MC5, and MC23 at or below 30% (Fig. 4). Five IgGs with high virus-neutralizing activity (samples 4, 5, 6, 9, 10) significantly blocked MC5, MC23, and DL11 from binding to gD but had less effect on MC2. Consequently, these IgGs targeted both receptor binding and a postreceptor binding step. In contrast, sample 7 was unique. It blocked predominantly MC2 (78% blocking) and only weakly blocked DL11 (27%) (Fig. 4B, Table 2), suggesting that IgG from sample 7 may neutralize primarily by blocking a post-receptor binding step (based on the mechanism of action of MAb MC2). Thus, for gD, there were a variety of responses among the 10 human sera, and both receptor binding and post-receptor binding functions were targets.
TABLE 2.
Percent blocking of mouse MAbs from human IgG samples
| Sample | % blockinga |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| αgD MAbs |
αgB MAbs |
|||||||||||
| 1D3 | MC14 | MC2 | MC5 | MC23 | DL11 | SS55 | SS144 | C226 | SS10 | SS63 | H1817 | |
| 1 | 2 | − | 0 | 28 | 20 | 15 | 5 | − | 1 | − | − | − |
| 2 | 0 | 3 | 7 | 15 | 26 | 19 | − | − | − | − | 4 | − |
| 3 | 6 | 8 | 22 | 30 | 23 | 5 | − | 10 | − | − | 6 | − |
| 4 | 10 | − | 13 | 42 | 55 | 58 | 1 | 13 | 0 | − | − | − |
| 5 | 16 | − | 21 | 42 | 50 | 47 | − | 10 | 3 | − | 8 | − |
| 6 | 10 | − | 26 | 62 | 90 | 99 | 43 | 46 | − | − | − | − |
| 7 | − | − | 78 | 16 | 16 | 27 | − | − | − | − | 1 | − |
| 8 | 0 | 0 | 30 | 19 | 25 | 16 | − | 3 | − | − | 1 | − |
| 9 | − | − | 33 | 42 | 61 | 59 | 24 | 29 | 13 | 14 | 15 | − |
| 10 | − | − | 7 | 30 | 76 | 68 | 57 | 36 | 46 | 1 | 13 | − |
Percent blocking activity (as compared to the no-antibody control). A dash represents numbers below 0% (no blocking).
Competition with murine anti-gB MAbs.
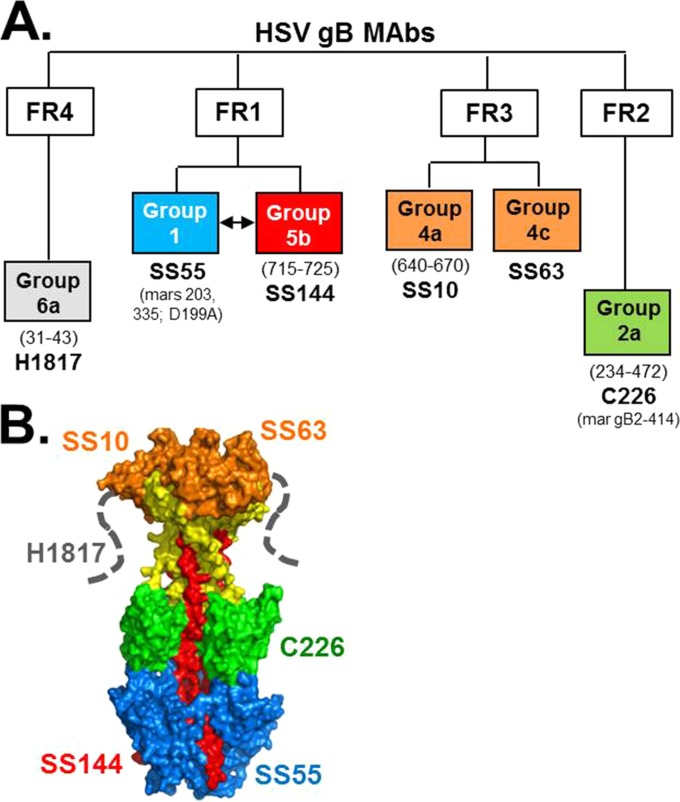
A subset of our panel of anti-gB MAbs (Fig. 5A) (8) were chosen for this study. We grouped the antibodies into four functional regions (FR; as defined by Bender et al. [8]) involved in virus neutralization (36, 46) and mapped them to the crystal structure of postfusion gB (Fig. 5B). FR1 is located at the base of gB and contains the fusion loops; it is the site of gB-lipid association (28, 46, 47). FR1 is made up of two distinct structural domains, and different MAbs bind to each of these (SS55 to domain I, blue; SS144 to domain V, red) (Fig. 5B). FR2 is in the middle lobe of gB and is postulated to associate with gH/gL during the fusion process (20, 48); it is represented by MAb C226. FR3 is within the gB crown, which is a site for gB-cell interaction (29, 33); MAbs SS10 and SS63 bind here. Lastly, FR4 is located within the gB N terminus (8), and its crystal structure is unknown (dashed lines, Fig. 5B). MAb H1817 binds within FR4 (residues 31 to 43) (8, 35).
FIG 5.
HSV gB MAb tree. (A) Diagram showing gB antigenic sites and associated MAbs used in this study. Antigenic sites are defined first within gB functional regions (FR) as defined by Bender et al. (8) and secondly by “groups” of MAbs that compete with each other for gB binding. MAb group designations are shown in boxes, color-coded to their epitope binding region as shown on the gB crystal structure in panel B. gB residues implicated in MAb binding are in parentheses below group names. Representative MAbs are shown in large font below group names, with known residues of mar mutants indicated below.
We next screened within the 10 human IgGs, whose binding to gB is shown in Fig. 6A, for the ability to block the binding of these six gB-specific antibodies using the biosensor competition assay (see Fig. S2 in the supplemental material). We used gB2(720t) in this assay, as all of the anti-gB MAbs bind to both type 1 and type 2 gB. Remarkably, seven out of the 10 IgGs (samples 1, 2, 3, 4, 5, 7, and 8) did not block any of the MAbs from binding to gB (Fig. 6B, Table 2), suggesting that none of these individuals raised antibody to these important neutralizing sites. This much more limited response is in contrast to what we saw for gD. Of note, all 10 samples reacted strongly with gB via native Western blotting (Fig. 2B and C), suggesting that the majority of people raised antibody to nonneutralizing epitopes of gB.
FIG 6.
(A) Binding of subject IgG to gB2 via biosensor. (B) Biosensor analysis: blocking of neutralizing gB MAbs via human subject IgG. Data for both panels A and B are presented as described for Fig. 4.
Three IgG samples competed with at least one neutralizing gB MAb. IgG from these samples blocked the binding of the SS55 and SS144 MAbs (Fig. 6B), two MAbs to FR1. Sample 10 also blocked the binding of FR2 MAb C226 (Fig. 6B). It is noteworthy that none of the IgGs competed with MAbs with epitopes in the gB crown/FR3 (SS10, SS63) or the gB N terminus/FR4 (H1817) (Fig. 6B, Table 2). We suggest that these IgGs neutralize virus by binding to FR1, thereby inhibiting fusion loop insertion. For sample 10, gB-specific IgG likely neutralized virus by inhibiting the gB-gH/gL interaction as well (20, 49).
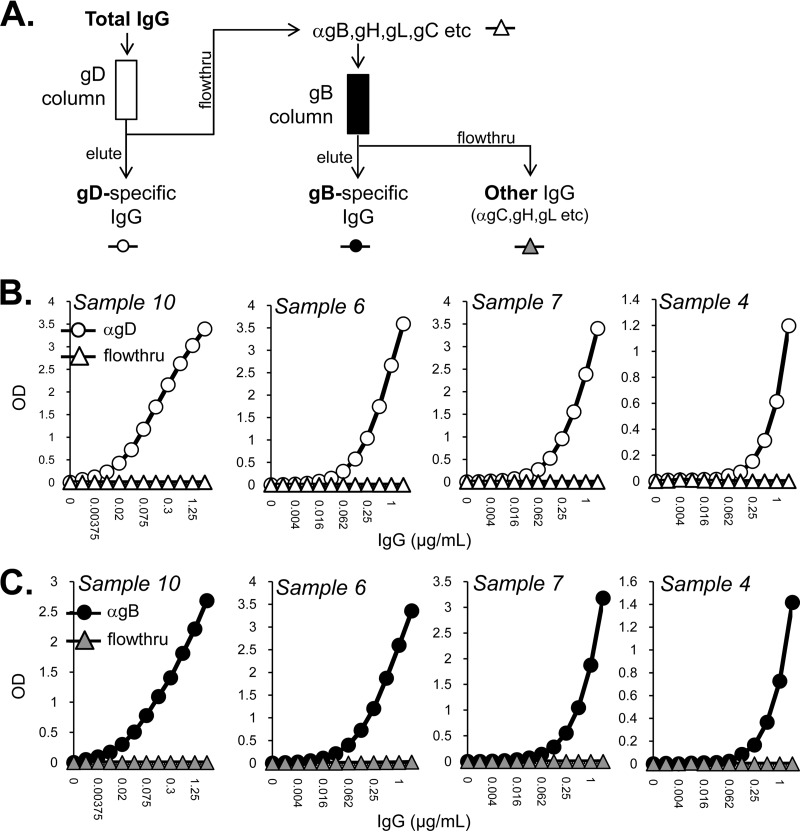
Purification of gD- and gB-specific IgGs.
The following experiments were designed to dissect within the human IgGs for glycoprotein-specific neutralization activity. We developed antigen-sorbent columns to capture gD-specific and gB-specific IgGs (Fig. 7A). We selected four highly neutralizing human IgGs (samples 4, 6, 7, and 10) that were predicted by our biosensor competition assay to have different mixes of antibodies directed against gD- and gB-neutralizing epitopes (Fig. 4B and 6B, Table 2).
FIG 7.
Purification of gD- and gB-specific IgGs. (A) Total human IgGs were passed over a gD2(306t) column, where soluble gD is covalently linked to Sepharose 4B. The elute (white circles) and flowthrough (white triangles) for each sample were tested by ELISA for binding to soluble gD (B); optical density at 405 nm (OD405) is shown on the y axis, and IgG concentration is on the x axis of each ELISA graph. The flowthrough was next passed over a gB2(724t) antigen-sorbent column, separating out the gB-specific IgGs (eluate, black circles) from the flowthrough which contained anti-gC, anti-gH/gL, and other anti-HSV IgGs (gray triangles). Once again, the elute and flowthrough for each sample were tested by ELISA, this time for binding to soluble gB (C).
Total IgGs were passed over a gD2(306t) column to which the soluble gD was covalently linked to Sepharose 4B. The flowthrough for each sample was collected and saved for the next step; IgGs bound to the gD column were eluted under acidic conditions (Fig. 7A). The eluate and flowthrough were then tested by enzyme-linked immunosorbent assay (ELISA) against soluble gD2. The flowthrough was found to be devoid of anti-gD activity (Fig. 7B). This flowthrough fraction was then passed over a gB2(727t) antigen-sorbent column (Fig. 7A). The eluate contained anti-gB activity, and none remained in the column flowthrough (Fig. 7C). The gD- and gB-specific pools of IgGs represented approximately 2% each of the total starting IgG. The final flowthrough contained all of the remaining anti-HSV IgGs (“other,” e.g., anti-gC, anti-gH/gL). Since three of the IgGs subjected were from people dually infected with HSV-1 and HSV-2 (samples 6, 7, and 10) (Table 1), we tested each IgG pool for its ability to neutralize both HSV-1 and HSV-2 (Fig. 8). For clarity, we report our analysis of each sample separately.
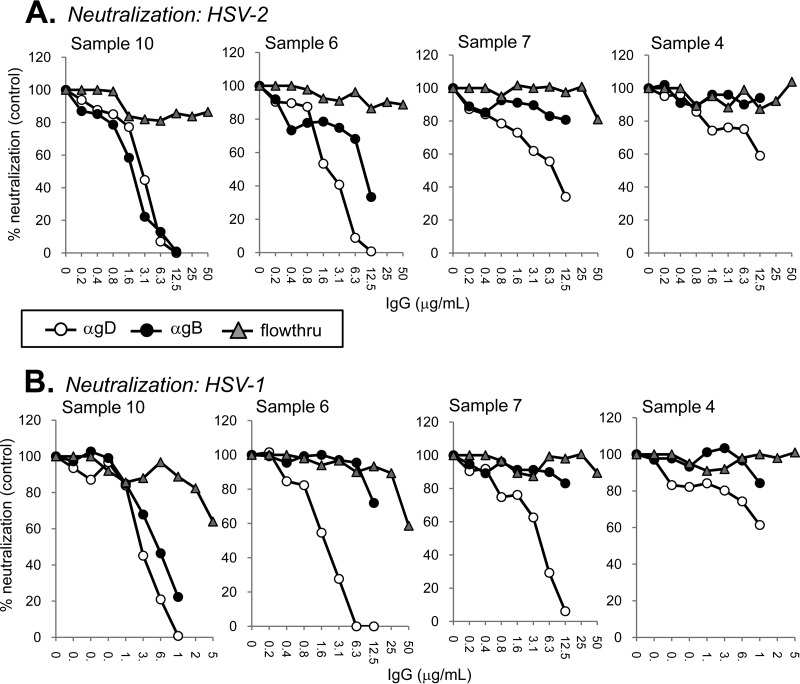
FIG 8.
Virus neutralization of antigen-sorbent column-selected IgGs. Anti-gD, anti-gB, and flowthrough (“other”) IgGs were obtained as outlined in the legend to Fig. 7. IgGs were tested for their ability to neutralize HSV-2 (A) or HSV-1 (B). Samples 6, 7, and 10 were from HSV-1 and HSV-2 dual-seropositive individuals, while sample 4 was from a person infected with HSV-2 only. Plaque numbers were determined for each sample and plotted as a percentage of plaques obtained in the absence of human IgG (y axis). IgG concentration is indicated on the x axis.
Sample 10.
Both anti-gD-specific IgG (white circles) and anti-gB-specific IgG (black circles) neutralized HSV-2 (Fig. 8A) and HSV-1 (Fig. 8B) equally well. Interestingly, the remaining IgG (gray triangles), devoid of anti-gD- and anti-gB-specific antibodies, failed to neutralize HSV-2 (Fig. 8A) yet neutralized 36% of HSV-1 at the highest concentration tested (50 μg/ml) (Fig. 8B). The data suggest that for sample 10, all of its neutralizing activity against HSV-2 comes from anti-gD and anti-gB IgGs, but other antibodies (such as anti-gC or anti-gH/gL) play some role in neutralizing HSV-1.
Sample 6.
gD- and gB-specific IgGs prepared from sample 6 neutralized HSV-2, although the gD-specific IgG was more potent (Fig. 8A). Against HSV-1, the difference in neutralizing activity was quite striking. Whereas the gD-specific IgG was just as potent against HSV-1 as against HSV-2, the gB-specific IgG failed to reach 50% neutralization at the highest concentration tested (12.5 μg/ml) (Fig. 8B). Sample 6 did not compete with a key anti-gB neutralizing MAb (C226 in FR2) (Fig. 8A), and this may be the reason why its gB-specific neutralization activity is lower than that of sample 10. Similar to sample 10, the remaining IgG (“other”) for sample 6 exhibited anti-HSV-1- but not anti-HSV-2-neutralizing activity (Fig. 8). To determine what might be in the “other” fraction, we passed the flowthrough of sample 6 (after passage over the gD and gB columns) over a column that contained gC1. The IgG in the eluate had virus-neutralizing activity against HSV-1 but not HSV-2 (data not shown). There was no detectable virus neutralization activity remaining in the flowthrough (data not shown). Thus, sample 6 contains modest neutralizing activity targeting gC1, but antibodies against other glycoproteins, including gH/gL, were insufficient to neutralize virus.
Sample 7.
This sample competed well against the anti-gD MAb MC2 but to a much lesser extent against the other anti-gD MAbs (Fig. 4). Interestingly, sample 7 failed to compete with any gB-specific MAbs (Fig. 6). After separation, gD-specific IgG neutralized HSV-2 and HSV-1. However, as expected, the gB-specific IgG and the remaining flowthrough IgG did not (Fig. 8). We conclude that for sample 7, all of its virus-neutralizing activity comes from gD-specific IgG and, within that IgG, an “MC2-like” antibody accounts for the majority of the response to gD.
Sample 4.
IgG from sample 4 blocked several anti-gD MAbs with a pattern similar to that of sample 10 (Fig. 4). Additionally, sample 4 exhibited poor blocking of gB-specific MAbs (Fig. 6). All neutralizing activity against both HSV-1 and HSV-2 was contained within the gD-specific IgG fraction (Fig. 8). We saw no difference in neutralizing activity between HSV-1 and HSV-2, even though sample 4 IgG came from an individual infected with HSV-2 only (Table 1). The anti-gD-neutralizing activity of sample 4 was weaker than that of samples 6 and 10, which is also what we observed for virus neutralization by the samples' total IgG (Table 1). In addition, gD binding activity of the total IgG was much lower for sample 4 than for sample 6 or 10 (see Fig. S1A in the supplemental material).
For the samples fractionated using our tandem-capture system, most of the virus-neutralizing activity was traced to either anti-gD IgGs (samples 4 and 7) or a combination of anti-gD and anti-gB antibodies (samples 6 and 10). For samples 6 and 10, there appeared to be some HSV-1-specific neutralization activity within the “other” IgG fraction at the highest concentration tested (50 μg/ml) (Fig. 8B). In the case of sample 6, this is due largely to antibody directed at gC1 (data not shown). Both of these samples were obtained from patients infected with both HSV-1 and HSV-2 (Table 1).
DISCUSSION
Little is known about the antibody repertoire in humans elicited by HSV glycoproteins during infection, although neutralizing activity against gD and gB has been documented in human sera (50, 51). We examined the sera from 10 HSV-seropositive individuals who had been previously tested and classified according to their frequency of viral shedding (23). When we began this study, we started with what could be regarded as two relatively simple questions: (i) what comprises a neutralizing antibody response to HSV in naturally infected humans and (ii) is there any relationship between the extent of virus shedding and neutralization? Our results highlight the complexity of the answers, even in this small set of samples. While all 10 samples contained neutralizing IgG, six were identified as containing strong neutralizing antibodies (Table 1). However, we could find no association between IgG neutralization activity and the rate of viral shedding. Furthermore, all 10 samples contained IgGs strongly reactive against HSV gB, gD, and gC. We were surprised by how few samples contained antibodies that react against purified gH/gL of either serotype.
Competition with neutralizing MAbs.
Here, we showed that we could delve more deeply into specific epitopes of gD and gB that were recognized by the 10 samples using competition analysis. However, it should be noted that blocking of the neutralizing mouse MAb by the human PAb does not mean the epitopes are identical; rather, we can say a similar neutralizing antibody is likely to be in the polyclonal mix. What did all of the IgGs have in common? All 10 contained IgG that blocked MAbs which interfere with gD-receptor binding (MC23, DL11). Thus, this interaction is a prime target of an effective immune response. We plotted the percent MAb blocking of these 10 samples against their neutralization scores and calculated R2 values for each plot (data not shown). The level of competition against DL11 and MC23 correlated quite well with total IgG neutralization rank (DL11, R2 = 0.83; MC23, R2 = 0.73). MAb MC5 blocking activity was observed in all samples but was not as consistently predictive of neutralizing activity (R2 = 0.36).
Although most IgGs blocked MC2, which targets a post-receptor binding function of gD (16, 49), by less than 33%, sample 7 blocked this MAb by 78%. Fractionation of antigen-specific IgG showed that this MC2-like IgG component likely accounts for the majority of this sample's HSV-neutralizing activity. Thus, antibodies that target activation of gH/gL by gD can be induced in naturally infected humans and provide potent virus-neutralizing activity.
None of the human IgGs competed with the anti-gD MAbs 1D3 and MC14, both of which bind to linear epitopes. In a study just completed using samples from the recent GlaxoSmithKline vaccine trial with gD as the immunogen, we also noted a lack of competition between antibodies raised against gD and anti-gD MAbs to linear epitopes (45). Thus, these regions of gD are poorly immunogenic in the natural host regardless of whether the protein is presented to the human immune system as a subunit vaccine or as one of many antigens produced during infection. One wonders if future HSV vaccines (52, 53) should be designed to induce PAbs in humans against these important linear gD epitopes.
In contrast to the rich response to neutralizing epitopes of gD, we found only three individuals with IgG that blocked anti-gB neutralizing MAbs. FR1 epitopes were targeted by all three of these samples, while an FR2 epitope was targeted by only one sample. FR1 is located at the base of gB and contains the fusion loops (FL) (8, 46, 47). One mechanism of neutralization by FR1 MAbs is by blocking FL insertion into target membranes. A previous study of the humoral response to gB in HSV-infected humans found that antibodies targeting FR1 epitopes were well represented (50). This same study found that sera from HSV-1-infected individuals exhibited more effective blocking of FR1 epitopes than sera from HSV-2-infected patients. Consistent with this, the 3 samples from the current study that strongly blocked FR1 epitopes also came from patients infected with both HSV-2 and HSV-1. FR2 MAbs block infection by blocking an essential interaction between gH/gL and gB. Although several other gB epitopes contained within FR3 and FR4 were screened, none were strongly blocked by the serum IgG from infected individuals. This was particularly surprising in light of the strong reactivity of all samples to gB by Western blotting (Fig. 2). Thus, it appears that these individuals targeted epitopes (perhaps nonneutralizing) that were not represented within our panel of MAbs.
Human anti-HSV-neutralizing IgG is predominantly against gD and gB.
Using affinity chromatography, we isolated gD- and gB-specific IgGs from four representative sera with strong or very strong neutralizing activity. Consistent with the well-documented role of gD as a dominant immunogen and inducer of neutralizing antibodies (51), virus-neutralizing activity was observed in the gD-specific IgG fraction from each sample. In the two samples shown to possess gB MAb blocking antibodies, the gB-specific IgG fraction also neutralized. Interestingly, the gB-specific IgG from sample 6 exhibited greater potency against HSV-2 than HSV-1. This was somewhat unexpected, since the ectodomains of gB1 and gB2 (except for the extreme N termini) exhibit high sequence identity (54, 55) and very few neutralizing gB MAbs are type specific. In particular, the gB MAbs blocked by sample 6 IgG (SS55 and SS144) are type common.
For samples 6 and 10, there was a small amount of HSV-1-specific neutralizing activity in the IgG depleted of gD- and gB-specific antibodies. Further analysis of sample 6 showed that this activity was directed against HSV-1 gC. Despite this, the relatively weak neutralizing activity directed against this protein was somewhat surprising. In addition to its role in immune evasion (13–15), gC1 has been shown to generate neutralizing antibodies in animal models (56–59). We found no additional neutralizing activity in the flowthrough after removal of gD2-, gB2-, and gC1-specific fractions from sample 6 IgG. This was unexpected, since sample 6 IgG was one of only a few that recognized gH/gL by Western blotting. gH/gL is highly immunogenic in rabbits and mice (39) and generates high-titer neutralizing antibodies directed against gH (10, 11, 60–62). Whether the poor response to gH/gL of either serotype is limited to the samples tested here or is more generally true is worth examining on additional samples.
We believe we have demonstrated that we have the tools to dissect the human immune response to HSV glycoproteins. The methods we applied to this small set of samples represent a template for examining samples taken from other cohorts of infected individuals and from subjects who are vaccinated against HSV using protein-based vaccines.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NIH grants AI-076231 and AI-056045 (to R.J.E) and AI-18289 (to G.H.C.). C.K. is supported by Public Health Service grants AI-097171 from the National Institute of Allergy and Infectious Diseases and DE-022137 from the National Institute of Dental and Craniofacial Research.
We thank Harvey Friedman, Debbie Long, Doina Atanasiu, and Reagan Greene Cox for critical readings of the manuscript.
Footnotes
Published ahead of print 20 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01930-14.
REFERENCES
- 1.Bradley H, Markowitz LE, Gibson T, McQuillan GM. 2014. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J. Infect. Dis. 209:325–333. 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 2.Kinchington PR, Leger AJ, Guedon JM, Hendricks RL. 2012. Herpes simplex virus and varicella zoster virus, the house guests who never leave. Herpesviridae 3:5. 10.1186/2042-4280-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffer JT, Swan D, Al Sallaq R, Magaret A, Johnston C, Mark KE, Selke S, Ocbamichael N, Kuntz S, Zhu J, Robinson B, Huang ML, Jerome KR, Wald A, Corey L. 2013. Rapid localized spread and immunologic containment define herpes simplex virus-2 reactivation in the human genital tract. eLife 2:e00288. 10.7554/eLife.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, Distefano DJ, Cai M, Brown M, Smith JF, Kowalski R, Swoyer R, Galli J, Copeland V, Rios S, Davidson RC, Salnikova M, Kingsley S, Bryan J, Casimiro DR, Friedman HM. 2014. Protection provided by an HSV-2 glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1 seropositive guinea pigs. J. Virol. 88:2000–2010. 10.1128/JVI.03163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awasthi S, Friedman HM. 2014. A paradigm shift: vaccine-induced antibodies as an immune correlate of protection against herpes simplex virus type 1 genital herpes. J. Infect. Dis. 209:813–815. 10.1093/infdis/jit658. [DOI] [PubMed] [Google Scholar]
- 6.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. 2014. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J. Infect. Dis. 209:828–836. 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender FC, Samanta M, Heldwein EE, de Leon MP, Bilman E, Lou H, Whitbeck JC, Eisenberg RJ, Cohen GH. 2007. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J. Virol. 81:3827–3841. 10.1128/JVI.02710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitbeck JC, Muggeridge MI, Rux AH, Hou W, Krummenacher C, Lou H, van Geelen A, Eisenberg RJ, Cohen GH. 1999. The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain. J. Virol. 73:9879–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns TM, Shaner MS, Zuo Y, Ponce-de-Leon M, Baribaud I, Eisenberg RJ, Cohen GH, Whitbeck JC. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J. Virol. 80:2596–2608. 10.1128/JVI.80.6.2596-2608.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski JM, Eisenberg RJ, Cohen GH. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 72:3595–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fries LF, Friedman HM, Cohen GH, Eisenberg RJ, Hammer CH, Frank MM. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 137:1636–1641. [PubMed] [Google Scholar]
- 14.Friedman HM, Wang L, Fishman NO, Lambris JD, Eisenberg RJ, Cohen GH, Lubinski J. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 70:4253–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubinski JM, Wang L, Soulika AM, Burger R, Wetsel RA, Colten H, Cohen GH, Eisenberg RJ, Lambris JD, Friedman HM. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 72:8257–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J. Virol. 86:1563–1576. 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muggeridge MI, Isola VJ, Byrn RA, Tucker TJ, Minson AC, Glorioso JC, Cohen GH, Eisenberg RJ. 1988. Antigenic analysis of a major neutralization site of herpes simpelx virus glycoprotein D using deletion mutants and monoclonal antibody-resistant mutants. J. Virol. 62:3274–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muggeridge MI, Wu T-T, Johnson DC, Glorioso JC, Eisenberg RJ, Cohen GH. 1990. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology 174:375–387. 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 19.Cairns TM, Fontana J, Huang ZY, Whitbeck JC, Atanasiu D, Rao S, Shelly SS, Lou H, Ponce de Leon M, Steven AC, Eisenberg RJ, Cohen GH. 2014. Mechanism of neutralization of herpes simplex virus by antibodies directed at the fusion domain of glycoprotein B. J. Virol. 88:2677–2689. 10.1128/JVI.03200-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ. 2010. Bimolecular complementation defines functional regions of herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J. Virol. 84:3825–3834. 10.1128/JVI.02687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 17:882–888. 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sisk WP, Bradley JD, Leipold RJ, Stoltzfus AM, Ponce de Leon M, Hilf M, Peng C, Cohen GH, Eisenberg RJ. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicola AV, Willis SH, Naidoo NN, Eisenberg RJ, Cohen GH. 1996. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J. Virol. 70:3815–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krummenacher C, Rux AH, Whitbeck JC, Ponce-de-Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty RJ, Spear PG, Eisenberg RJ, Cohen GH. 1999. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J. Virol. 73:8127–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannah BP, Cairns TM, Bender FC, Whitbeck JC, Lou H, Eisenberg RJ, Cohen GH. 2009. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J. Virol. 83:6825–6836. 10.1128/JVI.00301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender FC, Whitbeck JC, Ponce de Leon M, Lou H, Eisenberg RJ, Cohen GH. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542–9552. 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelly SS, Cairns TM, Whitbeck JC, Lou H, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. The membrane-proximal region (MPR) of herpes simplex virus gB regulates association of the fusion loops with lipid membranes. mBio 3(6):e00429-12. 10.1128/mBio.00429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. 2011. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J. Virol. 85:6175–6184. 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen GH, Isola VJ, Kuhns J, Berman PW, Eisenberg RJ. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bender FC, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588–11597. 10.1128/JVI.79.18.11588-11597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro D, Paz P, Pereira L. 1992. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology 186:99–112. 10.1016/0042-6822(92)90064-V. [DOI] [PubMed] [Google Scholar]
- 35.Pereira L, Ali M, Kousoulas K, Huo B, Banks T. 1989. Domain structure of herpes simplex virus 1 glycoprotein B: neutralizing epitopes map in regions of continuous and discontinuous residues. Virology 172:11–24. 10.1016/0042-6822(89)90102-5. [DOI] [PubMed] [Google Scholar]
- 36.Cairns TM, Fontana J, Huang ZY, Whitbeck JC, Atanasiu D, Rao S, Shelly SS, Lou H, de Leon MP, Steven AC, Eisenberg RJ, Cohen GH. 2014. Mechanism of neutralization of HSV by antibodies directed at the fusion domain of glycoprotein B. J. Virol. 88:2677–2689. 10.1128/JVI.03200-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isola VJ, Eisenberg RJ, Siebert GR, Heilman CJ, Wilcox WC, Cohen GH. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns TM, Landsburg DJ, Whitbeck JC, Eisenberg RJ, Cohen GH. 2005. Contribution of cysteine residues to the structure and function of herpes simplex virus gH/gL. Virology 332:550–562. 10.1016/j.virol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Peng T, Ponce de Leon M, Novotny MJ, Jiang H, Lambris JD, Dubin G, Spear PG, Cohen GH, Eisenberg RJ. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 72:6092–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenberg RJ, Ponce de Leon M, Friedman HM, Fries LF, Frank MM, Hastings JC, Cohen GH. 1987. Complement component c3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423–435. 10.1016/0882-4010(87)90012-X. [DOI] [PubMed] [Google Scholar]
- 41.Hung SL, Peng C, Kostavasili I, Friedman HM, Lambris JD, Eisenberg RJ, Cohen GH. 1994. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology 203:299–312. 10.1006/viro.1994.1488. [DOI] [PubMed] [Google Scholar]
- 42.Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J. Virol. 74:10863–10872. 10.1128/JVI.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rux AH, Lou H, Lambris JD, Friedman HM, Eisenberg RJ, Cohen GH. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component c3b. Virology 294:324–332. 10.1006/viro.2001.1326. [DOI] [PubMed] [Google Scholar]
- 44.Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Hirao L, Isaacs SN, Moss B, Eisenberg RJ, Cohen GH. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein b5r. J. Virol. 79:6260–6271. 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitbeck JC, Huang ZY, Cairns TM, Gallagher JR, Lou H, Ponce-de-Leon M, Belshe RB, Eisenberg RJ, Cohen GH. 2014. Repertoire of epitopes recognized by serum IgG from humans vaccinated with herpes simplex virus type 2 glycoprotein D. J. Virol. 88:7786–7795. 10.1128/JVI.00544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220. 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 47.Hannah BP, Heldwein EE, Bender FC, Cohen GH, Eisenberg RJ. 2007. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J. Virol. 81:4858–4865. 10.1128/JVI.02755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. U. S. A. 104:18718–18723. 10.1073/pnas.0707452104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 84:12292–12299. 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Pescador L, Pereira L, Charlebois ED, Kohl S. 1993. Antibodies to epitopes of herpes simplex virus type 1 glycoprotein B (gB) in human sera: analysis of functional gB epitopes defined by inhibition of murine monoclonal antibodies. J. Infect. Dis. 168:844–853. 10.1093/infdis/168.4.844. [DOI] [PubMed] [Google Scholar]
- 51.Eing BR, Kuhn JE, Braun RW. 1989. Neutralizing activity of antibodies against the major herpes simplex virus type 1 glycoproteins. J. Med. Virol. 27:59–65. [DOI] [PubMed] [Google Scholar]
- 52.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43. 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston C, Koelle DM, Wald A. 2014. Current status and prospects for development of an HSV vaccine. Vaccine 20:1553–1560. 10.1016/j.vaccine.2013.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goade DE, Bell R, Yamada T, Mertz GJ, Jenison S. 1996. Locations of herpes simplex virus type 2 glycoprotein B epitopes recognized by human serum immunoglobulin G antibodies. J. Virol. 70:2950–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wittekindt C, Fleckenstein B, Wiesmuller K, Eing BR, Kuhn JE. 2000. Detection of human serum antibodies against type-specifically reactive peptides from the N terminus of glycoprotein B of herpes simplex virus type 1 and type 2 by surface plasmon resonance. J. Virol. Methods 87:133–144. 10.1016/S0166-0934(00)00160-9. [DOI] [PubMed] [Google Scholar]
- 56.Adamiak B, Trybala E, Mardberg K, Johansson M, Liljeqvist JA, Olofsson S, Grabowska A, Bienkowska-Szewczyk K, Szewczyk B, Bergstrom T. 2010. Human antibodies to herpes simplex virus type 1 glycoprotein C are neutralizing and target the heparan sulfate-binding domain. Virology 400:197–206. 10.1016/j.virol.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 57.Svennerholm B, Jeansson S, Vahlne A, Lycke E. 1991. Involvement of glycoprotein C (gC) in adsorption of herpes simplex virus type 1 (HSV-1) to the cell. Arch. Virol. 120:273–279. [DOI] [PubMed] [Google Scholar]
- 58.Weir JP, Bennett M, Allen EM, Elkins KL, Martin S, Rouse BT. 1989. Recombinant vaccinia virus expressing the herpes simplex virus type 1 glycoprotein C protects mice against herpes simplex virus challenge. J. Gen. Virol. 70(Part 10):2587–2594. [DOI] [PubMed] [Google Scholar]
- 59.Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2011. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J. Virol. 85:10472–10486. 10.1128/JVI.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Showalter SD, Zweig M, Hampar B. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckmaster EA, Gompels U, Minson A. 1984. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 × 10(3) molecular weight. Virology 139:408–413. [DOI] [PubMed] [Google Scholar]
- 62.Gompels U, Minson A. 1986. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology 153:230–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.