ABSTRACT
Advances in phage therapy and novel applications of phages in biotechnology encourage interest in phage impact on human and animal immunity. Here we present comparative studies of immunogenic properties of T4 phage head surface proteins gp23*, gp24*, Hoc, and Soc, both as elements of the phage capsid and as isolated agents. Studies comprise evaluation of specific antibodies in the human population, analysis of the proteins' impact on the primary and secondary responses in mice, and the effect of specific antibodies on phage antibacterial activity in vitro and in vivo in mice. In humans, natural antibodies specific to T4-like phages were abundant (81% of investigated sera). Among those, significantly elevated levels of IgG antibodies only against major head protein (gp23*) were found, which probably reflected cross-reactions of T4 with antibodies induced by other T4-like phages. Both IgM and IgG antibodies were induced mostly by gp23* and Hoc, while weak (gp24*) and very weak (Soc) reactivities of other head proteins were noticed. Thus, T4 head proteins that markedly contribute to immunological memory to the phage are highly antigenic outer capsid protein (Hoc) and major capsid protein (gp23*). Specific anti-gp23* and anti-Hoc antibodies substantially decreased T4 phage activity in vitro and to some extent in vivo. Cooperating with antibodies, the immune complement system also contributed to annihilating phages.
IMPORTANCE Current descriptions of phage immunogenicity and its biological consequences are still vague and incomplete; thus, the central problem of this work is timely and may have strong practical implications. Here is presented the very first description of the contribution of bacteriophage proteins to immunological memory of the phage. Understanding of interactions between phages and mammalian immunology may help in biotechnological adaptations of phages for therapeutic requirements as well as for better appreciation of phage ecology and their role in the biosphere.
INTRODUCTION
The main attribute of the bacterial viruses, their ability to kill bacteria, played a central role in phage biology almost from their discovery. Nowadays, phages are postulated to be an important alternative to the insufficient antibacterial drug arsenal (1–3). Phages as antibacterials offer a good way of treating or preventing many human diseases of bacterial etiology. Advances in phage therapy encourage scientific interest in interactions of phages with human and animal immunity. Since antibodies can decrease phage viability, dramatically resulting in the loss of antibacterial effects (3, 4), immunogenicity of phages is one of the important issues that may contribute to the success or failure of therapeutic use of bacterial viruses (5, 6).
The ability of bacteriophages to induce specific antibodies was one of the first useful properties of these viruses employed in microbiology and medicine: serological cross-reactions represent the earliest criteria for bacteriophage classification into related groups (7). Phage immunogenicity (ϕX174 phage) has been employed in medicine as a test for immune competence of immunodeficient patients, e.g., HIV-infected patients (8).
In the biosphere, one of the abundant phage groups is the T4-like phages (9). They are complex, multiantigenic objects. T4-like phages have been applied in early experimental phage therapies, as well as in recent phage safety tests (10). T4 has also been proposed as a platform for novel vaccines (11–13). T4 head offers a large, highly regular, three-dimensional structure. Its surface is formed by two essential proteins, gp23* and gp24*, and two dispensable ones, Hoc and Soc, which may effectively present foreign antigens (14). Extensive molecular studies of T4 resulted in a very good description of its structure, recently reviewed by Rao and Black (15, 16). However, which elements of the phage capsid shape phage immunological reactivity?
The first immunological studies of T4 were conducted almost 40 years ago by Mitsuhiro Yanagida, Tetsuro Ishii, and coworkers (17–19). The aim of those studies was in fact assignment of particular T4 genes to their functions and localization of the genes' products in the phage capsid. The approach was successful, contributing greatly to recognition of T4 phage capsid structure (20). Furthermore, Ishii and Yanagida provided some excellent examples of differences in the ability of phage capsid proteins to induce specific antibodies. One of these differences was reflected in a protein's name: highly antigenic outer capsid protein (Hoc) (17, 18). This name is often a reason for which Hoc is considered to be the most immunogenic element of the T4 phage capsid in general. Nevertheless, Hoc (at first provisionally named protein H) was studied together and compared only to Soc (small outer capsid protein, at first provisionally named protein O) (17, 18). Thus, confirmed differences in the proteins' immunogenicities as expressed by their names apply only to comparison of Hoc and Soc. Further, the goal of those early studies was to solve the T4 capsid structure, so proteins were isolated from capsids before application to animals. One should note that these proteins fixed on the T4 capsid may differ from isolated ones in exposure of their antigenic sites. Therefore, the contribution of particular capsid proteins to the antigenic reactivity of the phage particle remains unspecified.
A further problem is the ability of head-specific antibodies to affect phage viability. A decrease in phage activity that results from binding specific antibodies to proteins engaged in infecting bacteria was first postulated by Jerne and Avegno (21). This statement has further evolved into the general belief that immunization to phage head proteins must have little or no effect on phage viability, but in fact, this belief has never been verified.
MATERIALS AND METHODS
Phage protein isolation and purification.
Phage proteins gp23*, gp24*, Hoc, and Soc were purified to immunological purity grade as described by Miernikiewicz et al. (22). Briefly, genes coding for the proteins were cloned using Gateway technology into pDEST15 and pDEST24 vectors, allowing expression of recombinant products with glutathione S-transferase (GST) affinity tags, and expressed in the Escherichia coli expression system. Bacteria of strain B834(DE3) F− ompT hsdSB(rB− mB−) gal dcm met (DE3) (EMD) were grown in LB high-salt (10 g/liter of NaCl) culture medium (Sigma-Aldrich or AppliChem). gp24* was expressed without additional chaperones, Soc was produced in coexpression with a TF chaperone of E. coli (from pTf16 vector; TaKaRa Bio Inc.), Hoc was produced in coexpression with groES plus groEL of E. coli (from pGRO7 vector; TaKaRa Bio Inc.), and gp23* was produced in coexpression with a gp31 chaperone of T4 phage. gp31 is a specific cochaperonin functionally replacing GroES in the groES/groEL chaperonin complex. It is required for gp23 folding in the course of the phage infection of E. coli. This chaperone was expressed from pG31t. Expression of the recombinant constructions was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) (0.2 mM). TF and groES/groEL were induced with l-arabinose (3 mM) at 25°C. Harvested bacteria were lysed by freeze-thawing with lysozyme in phosphate buffer with phenylmethylsulfonyl fluoride (PMSF) (50 mM Na2HPO4, 300 mM NaCl, 1 mM PMSF; pH 7.5). The soluble fraction was incubated with glutathione sorbent slurry (glutathione Sepharose 4B; GE Healthcare Life Sciences) and washed with phosphate buffer, and proteins were released by proteolysis with AcTev protease (5 U/ml) (Invitrogen, Life Technologies Corporation) at 10°C; GST tags remained bound in the resin. Then the first step of lipopolysaccharide (LPS) removal was done with EndoTrap blue (Hyglos GmbH, Germany). Control bovine albumin (Sigma) was also processed at this stage. Phage proteins and control albumin were separated by gel filtration fast protein liquid chromatography) (FPLC) on a Superdex 75 10/300 GL column (GE Healthcare Life Sciences). The final step of LPS removal was performed with EndoTrap blue (Hyglos GmbH). The sample was dialyzed against phosphate-buffered saline (PBS) and filtered through 0.22-μm polyvinylidene difluoride (PVDF) filters (Millipore). Protein concentrations were determined by the Lowry chromogenic method (Fermentas International Inc.). These preparations were used for mouse immunizations or for enzyme-linked immunosorbent assays (ELISAs).
Phage cultures and purification.
T4 phage was purchased from American Type Culture Collection (ATCC) (Rockville, MD). HAP1 (a T4 mutant without Hoc on its capsid) was selected at our institute, the Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław (IIET) (23). The bacteriophages were cultured on Escherichia coli B host obtained from the Collection of Microorganisms at the IIET. The bacteriophages were purified by filtration through polysulfone membranes and by three subsequent steps of chromatography: gel filtration on Sepharose 4B (Sigma-Aldrich, Poland) followed by cellulofine sulfate (Millipore, Billerica, MA) chromatography (24) and then removal of residual LPS by LPS affinity chromatography with EndoTrap blue according to the manufacturer's instructions (Hyglos GmbH), carried out by three successive incubations of the preparations with the slurry followed by centrifugations. The samples were dialyzed against PBS and filtered with 0.22-μm PVDF filters (Millipore). The phage concentrations were measured by the double-layer method of Adams (25).
LPS content determination.
The endotoxin level of the purified phages was assessed using EndoLISA (ELISA-based endotoxin detection assay; Hyglos GmbH) according to the manufacturer's instructions. Diluted samples or standard dilutions with binding buffer were incubated overnight at room temperature with shaking. Subsequently, the plate was washed and assay reagent was added. Fluorescent signal was detected immediately in a fluorescence reader (Synergy H4 H4MLFPTAD; BioTek Instruments, USA). Protein preparations and phages were used for ELISA or mouse injection only if the LPS content was less than 1 unit per ml or per mouse.
Immunization of mice.
All animal experiments were performed according to EU directive 2010/63/EU for animal experiments and were approved by the 1st Local Committee for Experiments with the Use of Laboratory Animals, Wrocław, Poland. Six- to twelve-week-old C57BL6/J female or male mice were bred in the Animal Breeding Centre of the IIET and kept under specific-pathogen-free (SPF) conditions.
For studies of gp23*, gp24*, Hoc, and Soc immunogenicity, mice were inoculated intraperitoneally (i.p.) with 0.2 ml of phage. IgM production was measured after a single injection with the phage at 6 × 109 PFU/mouse. IgG production was measured after triple injections: 5 × 109 PFU/mouse on day 0, 5 × 109 PFU/mouse on day 20, and 2 × 109 PFU/mouse on day 40.
The model of infection in preimmunized mice as well as in vitro studies of phage inactivation by specific sera required animals with the same serum levels of antibodies specific to each investigated protein. Since the proteins differed in their abilities to induce IgG production (data not shown), the immunization schema was experimentally developed for obtaining the same antibody levels; after experimental determination, it was established as follows: gp23* was injected at 50 μg/mouse subcutaneously (s.c.) on day 0, 70 μg/mouse i.p. on day 30, and 100 μg/mouse s.c. on day 55; gp24* was injected at 50 μg/mouse s.c. on day 0, 200 μg/mouse i.p. on day 30, and 200 μg/mouse s.c. on day 55; Hoc was injected at 50 μg/mouse s.c. on day 0, 70 μg/mouse i.p. on day 30, and 100 μg/mouse s.c. on day 55; and Soc was injected at 50 μg/mouse s.c. on day 0, 100 μg/mouse i.p. on day 30, and 300 μg/mouse s.c. on day 55. Sera of the mice were tested for specific antibody levels on days 60 to 62, and when the levels were equal, the mice were used for the infection model.
Murine blood was collected into heparinized tubes, under anesthesia, from the tail vein (IgM days 1, 5, and 10, and in the case of IgG, before infection model) or from the orbital plexus vein (IgM day 15 and IgG day 60). Then animals were sacrificed by cervical dislocation. Serum was separated from the blood by double centrifugation (2,250 rpm and 10,000 rpm) and, if necessary, also filtered by 0.22-μm syringe filter and used for ELISA. One sample experiment of each type is presented.
Experimental E. coli infection treated with T4 phage in mice.
Six- to twelve-week-old C57BL6/J male mice of SPF standard were preimmunized with purified T4 head proteins up to equal levels (according to the developed schedule of immunization; see above), as confirmed by ELISA. Then mice were infected with E. coli at 109 CFU/mouse and 1 h later treated with T4 phage at 109 PFU/mouse. Mice were kept in microbiologically separated breeding boxes with filtered air supply and offtake. Their condition was monitored during the whole experiment. The model of infection was developed experimentally as follows: 50% of phage-treated mice survived 5 days of the infection, and untreated mice were all dead within 4 days. The experiment was terminated on day 5 due to ethical reasons.
Human sera.
Human IgG was evaluated in sera of 50 healthy volunteers who had never been subjected to phage therapy or involved in phage work (age range, 18 to 40 years). Blood was collected into heparinized tubes, and serum was separated from the blood by double centrifugation at 2,250 rpm and 10,000 rpm and used for ELISA. Experiments were approved by the local Commission of Bioethics, Wrocław Medical University.
Specific antibody level measurement by ELISA.
A MaxiSorp flat-bottom 96-well plate (Nunc; Thermo Scientific) was sterilely coated overnight at 4°C with phage proteins and albumin (100 μl per well; 10 μg/ml). Subsequently, wells were washed 5 times with PBS and blocked for 1 h with 1% albumin at 100 μl per well at room temperature. Albumin was removed and the plate was washed 5 times with PBS. Diluted serum (from 1/20 to 1/1,000 in PBS) was applied to the wells coated with relevant proteins and control albumin (100 μl of diluted serum per well). Each sample was investigated in duplicate. The plate was incubated at 37°C for 2 h. Serum was removed and the plate was washed with PBS with 0.05% Tween 20 (Serva) 5 times. Diluted detection antibody was applied (100 μl per well): peroxidase-conjugated AffiniPure goat anti-mouse IgM (Jackson ImmunoResearch Laboratories) or peroxidase-conjugated AffiniPure goat anti-mouse IgG (Jackson ImmunoResearch Laboratories). It was incubated with the plate for 1 h at room temperature in the dark. Antibody solution was removed and the plate was washed with PBS with 0.05% Tween 20 (BD Biosciences) 5 times. The DuoSet of substrate reagents for peroxidase was used (50 μl) according to the manufacturer's instructions (DY999; R&D Systems) and incubated for 20 min. Twenty-five microliters of 2 N H2SO4 was added, and absorbance was measured at 450 nm (main reading) and 570 nm (background). The background values were subtracted from the main readings (as recommended by the manufacturer).
Highly responsive sera from animals were used to establish a reference standard serum according to references 26 and 27. Briefly, to calculate ELISA units (EU), 10 points of standard serum dilutions were determined on each plate. For IgG antibody level, standard serum was diluted in PBS in 2-fold steps from 100 to 51,200, and for IgM antibody level, standard serum was diluted in PBS in 2-fold steps from 10 to 5,120. The dilution giving an optical density at 450 nm (OD450) of ≤1 was assigned as 1,000 EU and then 500 EU, 250 EU, 125 EU, etc. The obtained standard curve was fitted to a function that converted OD values to EU. Each EU was normalized, and the average value of each duplicate was calculated. These values were presented as final results in groups and statistically analyzed by one-way analysis of variance (ANOVA; Tukey) or the Kruskal-Wallis test with the Statistica 8.0 software package.
Serum samples from healthy volunteers (n = 50) were tested at a 1:100 dilution by ELISA. Reverse cumulative distribution plots for each head protein (gp23*, gp24*, Hoc, and Soc) were assigned from direct OD values according to references 28 and 29.
All experiments were repeated 2 to 4 times and were not summarized; sample experiments with their individual n values and statistical significance are presented.
RESULTS
Natural anti-T4 phage antibodies in humans.
Antiphage antibodies in humans who have never been subjected to phage therapy may occur due to natural contact with bacteriophages: after infections of relevant bacteria (30, 31) or resulting from constant exposure to phages propagating on symbiotic bacterial flora. It is, however, not easy to draw a general conclusion on the rate of antiphage antibody occurrence in the human population, since both negative (10) and positive (32–36) observations have been reported.
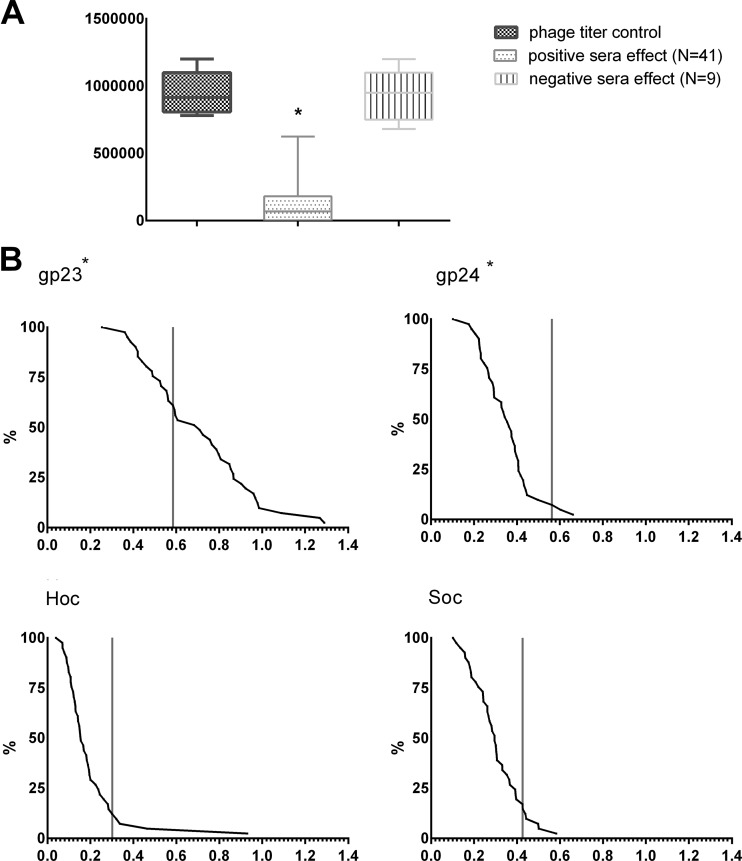
Studies of immunogenic properties of the T4 phage head surface proteins were therefore started with evaluation of specific antibodies in humans. Fifty healthy volunteers who had never been subjected to phage therapy or involved in phage work participated in the tests. Eighty-two percent (41 out of 50) of investigated sera significantly decreased phage activity (positive sera), and only 18% (9 out of 50) of the sera did not induce an antiphage effect (negative sera). Loss of the phage activity after incubation with positive sera reached 95% of the control (Fig. 1A). Then, natural IgG antibodies specific to the phage proteins gp23*, gp24*, Hoc, and Soc were identified in this population. Significant contributions of specific antibodies were observed only in the case of gp23* (P < 0.00001). As much as 61% of positive human sera reacted with this protein more strongly than the control cutoff value; no other protein was found to react with the sera to a noticeable extent, including Hoc (Fig. 1B). These results show that anti-T4 phage antibodies are frequent in the human population, but it is not the highly antigenic outer capsid protein that induced most of the humoral response. Antibodies specific to major capsid protein (gp23*) of T4 prevail.
FIG 1.
Occurrence of T4 phage neutralizing antibodies in human population. (A) Phage inactivation by human sera. Bacteriophage T4 (106 PFU/ml) was incubated for 2 h with human sera (50 healthy volunteers); after that, phage viability was tested on bacteria by the double-layer method. Results were compared to the phage titer control and classified into two groups. Negative sera were those not inactivating the phage (phage titer after incubation was within the range of the mean value of the control ± 2 SDs); nine samples could be classified into this group. Positive sera were those inactivating the phage (phage titer after incubation was lower than the range of the negative samples); 41 samples were classified into this group. Control was calculated from titers of the same phage preparation incubated with sera of nonimmunized SPF standard mice (n = 8, mean = 0.955 × 106 PFU/ml, SD = 0.159 × 106 PFU/ml). *, phage titer after incubation with phage-inactivating (positive) sera was significantly decreased in comparison to phage titer after incubation with the noninactivating (negative) sera (P = 0.00002) and in comparison to the titer control (P = 0.00001; Kruskal-Wallis ANOVA). Whiskers represent minimal and maximum readouts, boxes represent SD, and medians are marked by bars. (B) Natural IgG antibodies specific to T4 head surface proteins in human population. Reverse cumulative distribution plots for each head protein (gp23*, gp24*, Hoc, and Soc) were assigned to the human sera, according to the work of Miura et al. (28). Cutoff values for nonimmunized individuals are represented by vertical lines in each plot. Comparison of the plots and statistical analysis show that immunization to gp23* markedly prevails in the human population (P < 0.00001; Kruskal-Wallis ANOVA).
Individual immunogenic properties of T4 head surface proteins: gp23*, gp24*, Hoc, and Soc.
The primary response (IgM) and secondary response (IgG) to T4 phage proteins in mice were studied. Two phage strains were used: the wild-type T4 phage and the HAP1 mutant, which was deprived of Hoc. This mutant was used to assess whether Hoc protein modifies the immunogenicity of other T4 head proteins. HAP1 immunization also served as an internal control (no anti-Hoc antibodies were expected in mice treated with HAP1 phage). The control group of mice was injected with PBS.
One day after immunization with phages, anti-head protein antibodies were not detectable (data not shown). Maximum IgM production was observed 5 days after the challenge, accordingly to typical schema of the humoral response. A marked increase (in comparison to the control mice) was observed in anti-Hoc IgM (approximately a 100-fold increase) and in anti-gp23* (approximately a 10-fold increase) (Fig. 2). The secondary response (IgG) was also induced mostly by Hoc (approximately a 10,000-fold increase) and gp23* (approximately a 100-fold increase), but also by gp24* (less than a 10-fold increase). Protein Soc was the least effective in stimulation of the immunological response (Fig. 2). These observations show that there are two T4 head proteins that markedly contribute to immunological memory to the phage: highly antigenic outer capsid protein (Hoc) but also major capsid protein (gp23*).
FIG 2.
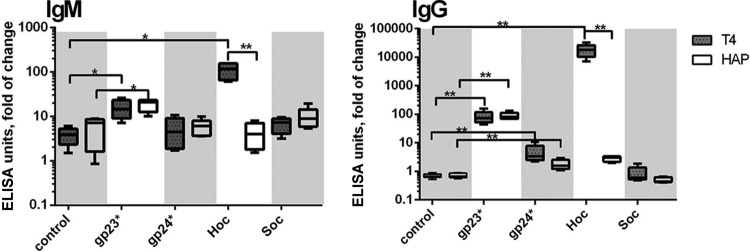
Humoral response to individual T4 head proteins in animal model. Mice (n = 8) were injected i.p. with T4 phage or HAP1 phage (for dose schema, see Materials and Methods). Control mice were injected with PBS. Antibody levels were tested in murine sera on day 5 (IgM) or day 40 (IgG) by ELISA with a high-titer standard serum as a reference, and ELISA units were calculated. Fold increase in comparison to the control is presented. Whiskers represent minimal and maximum values, boxes represent SD, and medians are marked by bars. Results are presented in logarithmic scale. **, difference statistically significant (P < 0.001; Kruskal-Wallis ANOVA); *, difference statistically significant (P < 0.02; Kruskal-Wallis ANOVA).
Effect of anti-T4 head immunization on phage antibacterial activity.
Antibodies that bind to phage proteins engaged in infection may impede it and thus decrease phage viability, which was first postulated by Jerne and Avegno (21). This statement has been often repeated in discussions on particular phage proteins' immunogenicity and phage neutralization. It has further evolved into the general belief that immunization to phage head proteins has little or no effect on phage viability, but in fact this belief has never been verified. Therefore, in this work the effect of specific preimmunization to T4 head surface proteins on T4 phage antibacterial activity was tested in vitro and in vivo. A murine model was applied.
Mice were immunized with purified gp23* or gp24* or Hoc or Soc or with albumin (control) up to the same antibody level. After in vitro serum incubation with the phage, phage antibacterial activity was determined by phage titer. As a result, a dramatic decrease of phage activity was observed in the samples incubated with anti-gp23* sera (P < 0.005 in comparison to all other groups). A decrease in phage activity was also induced by anti-Hoc sera (P < 0.006 in comparison to all other groups except gp23*). This decrease was significantly stronger in noninactivated sera, which suggests engagement of serum complement in the antiphage effect of immunization (Fig. 3).
FIG 3.
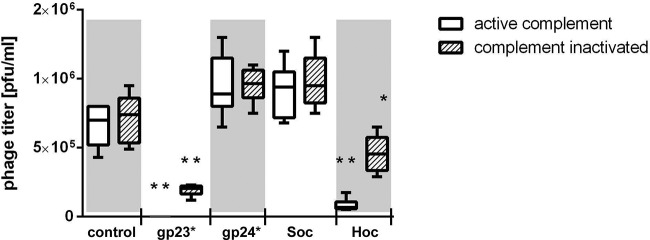
Effect of antihead antibodies on T4 phage activity in vitro. T4 bacteriophage (106 PFU/ml) was incubated for 1.5 h with inactivated sera of mice preimmunized to gp23* or gp24* or Hoc or Soc or albumin (control). Mice were preimmunized up to the same antibody level in each group, as confirmed by ELISA. After incubation, phage viability was tested on bacteria by the double-layer method. Phage titer in each group (n = 6) is presented. Whiskers represent minimal and maximum readouts, boxes represent SD, and medians are marked by bars. *, decrease of phage activity in the samples exposed to inactivated anti-Hoc sera was statistically significant in comparison to all other groups except inactivated gp23* (P < 0.006); **, decrease of the phage activity was statistically significant in comparison to all control groups, gp24*, and Soc (P < 0.005).
In the in vivo model of E. coli infection, mice were preimmunized with purified phage proteins or with albumin (control) also to the same antibody level and then infected with E. coli and treated with T4 phage. Numbers of mice that survived in each group in subsequent days were counted. The experiment was terminated on day 5 due to ethical reasons. Surprisingly, a significantly higher death rate was observed only in the group of mice that was preimmunized with Hoc; all animals were dead in this group on day 4 (Fig. 4). Immunization specific to gp23*, which was previously found to have a devastating effect on T4 in vitro, did not result in a significant decrease in length of animal survival in vivo (Fig. 4).
FIG 4.
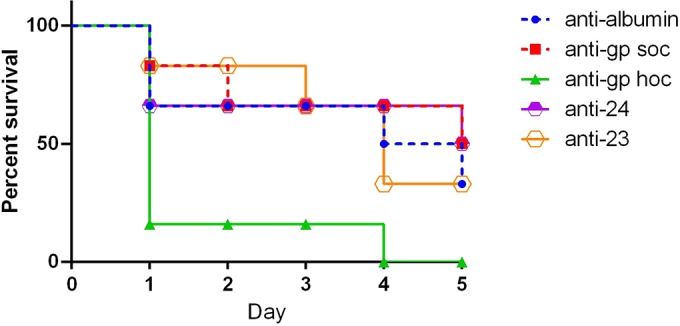
Effect of preimmunization of mice with T4 head proteins on phage activity against bacterial infection. Mice (n = 6) were preimmunized with purified T4 head proteins up to equal levels (according to the developed schedule of immunization; see Materials and Methods), as confirmed by ELISA. Then mice were infected with E. coli at 109 CFU/mouse and 1 h later treated with T4 phage at 109 PFU/mouse. Numbers of living animals in particular groups on days 0 to 5 are presented. Animal condition was monitored during the whole experiment. The model of infection was developed experimentally as follows: 50% of phage-treated mice survived 5 days of the infection, and untreated mice were all dead within 4 days. The experiment was terminated on day 5 due to ethical reasons.
These results show the biological effects of specific immunization to T4 head proteins: contrary to common expectations, it can decrease phage activity and counteract the antibacterial effect of phages in vitro and in vivo. However, in vivo interactions between phages and the immunological system cannot be simply transferred from in vitro antiphage activity of specific sera. In our studies, only preimmunization to Hoc protein was able to decrease the therapeutic effect of the phage in the bacterial infection.
DISCUSSION
One of the important issues that contribute to the success or failure of medical use of bacterial viruses is the immunogenicity of phages. In these studies, anti-T4 phage antibodies were frequent in the human population (81% of individuals). Since investigated individuals had not been subjected to phage therapy, T4-specific antibodies resulted from natural contact with bacteriophages. Significantly elevated levels of IgG antibodies only against gp23* were found (Fig. 1), with no significant representation of individuals immunized to Hoc, which seems to be in opposition to the relatively high immunogenicity of Hoc (Fig. 2). People are naturally exposed to an extremely large spectrum of phages that are present almost everywhere in the environment. Thus, in humans, antibodies that bind the phage must be cross-reacting ones. Importantly, not all T4-like phages have the nonessential protein Hoc on their capsids (19). Very weak immunization against Hoc in humans could be explained with the hypothesis that T4-like phages without Hoc are more frequent in the environment than those with this domain. It is difficult to determine how frequent are natural Hoc-lacking phages, but probably in fact there are fewer T4-like phages that lack Hoc and those with this protein prevail (19). In that case, the almost complete lack of anti-Hoc antibodies in the human population is surprising. This may be explained by the fact that Hoc proteins, as nonessential ones, are much more differentiated among the T4-like group than essential and thus relatively conservative major capsid proteins (gp23*). Antibodies' cross-reactions strongly depend on the level of similarity, so they are weaker for highly differentiated capsid elements.
The practical importance of specific immunization to the T4 head derives from its effect on antibacterial activity of the phage. In our studies, sera containing antihead antibodies decreased T4 phage activity and counteracted its antibacterial effect in vitro and in vivo (Fig. 3 and 4). Preimmunization to Hoc protein decreased the therapeutic effect of T4 phage in experimental bacterial infection, while in vitro, both anti-Hoc and anti-gp23* sera neutralized the phage. Thus, antibodies specific to T4 head proteins decreased antibacterial effectiveness of the phage, but in vitro antiphage activity of specific sera cannot be simply transferred to in vivo interactions between phages and the immunological response. The effect may depend on such conditions as route of immunization, dose, additives, etc. (35). Further, the immunological response to an antigen in a living organism engages many other (not only humoral) components of the immunological system. Here we present the role of the immune complement system, which increased phage neutralization (Fig. 3).
We propose three parallel mechanisms as inactivating effects of antihead immunization. The first is aggregation of phage particles, which makes phage proteins mediating infection less accessible; this is a complement-independent mechanism. Specific antibodies may interact with immune complement complexes, which are large (in comparison to phage capsids), highly active structures that are potentially able to destabilize phage capsids (second mechanism) or constitute steric hindrance for proper functions of phage proteins engaged in infection (third mechanism). Understanding of interactions between phages and mammalian immunology may help in biotechnological adaptations of phages for therapeutic requirements as well as for better appreciation of phage ecology and the role of phages in the biosphere.
Conclusions.
In humans, natural antibodies specific to T4-like phages are abundant (81% of investigated sera). Among those, significantly elevated levels of IgG antibodies only against major head protein (gp23*) were found, which probably reflects cross-reactions of T4 with antibodies induced by other T4-like phages. Specific anti-gp23* and anti-Hoc antibodies can substantially decrease T4 phage activity in vitro and to some extent in vivo. Cooperating with antibodies, the immune complement system also contributes to annihilation of phages.
ACKNOWLEDGMENTS
We are most grateful to Mitsuhiro Yanagida for his kind help and advice and for making his archives accessible to us for recognition of the Hoc story.
This work was supported by EU Structural Funds (Operational Programme Innovative Economy) and the National Science Centre in Poland (UMO-2012/05/E/NZ6/03314).
Footnotes
Published ahead of print 20 August 2014
REFERENCES
- 1.Borysowski J, Międzybrodzki R, Górski A. 2014. Phage therapy current research and applications. Caister Academic Press, Norfolk, England. [Google Scholar]
- 2.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. 2010. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11:69–86. 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 3.Międzybrodzki R, Borysowski J, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Szufnarowski K, Pawełczyk Z, Rogóż P, Kłak M, Wojtasik E, Górski A. 2012. Clinical aspects of phage therapy. Adv. Virus Res. 83:73–121. 10.1016/B978-0-12-394438-2.00003-7. [DOI] [PubMed] [Google Scholar]
- 4.Smith HW, Huggins MB, Shaw KM. 1987. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J. Gen. Microbiol. 133:1127–1135. [DOI] [PubMed] [Google Scholar]
- 5.Górski A, Międzybrodzki R, Borysowski J, Dąbrowska K, Wierzbicki P, Ohams M, Korczak-Kowalska G, Olszowska-Zaremba N, Łusiak-Szelachowska M, Kłak M, Jończyk E, Kaniuga E, Gołaś A, Purchla S, Weber-Dąbrowska B, Letkiewicz S, Fortuna W, Szufnarowski K, Pawełczyk Z, Rogóż P, Kłosowska D. 2012. Phage as a modulator of immune responses: practical implications for phage therapy. Adv. Virus Res. 83:41–71. 10.1016/B978-0-12-394438-2.00002-5. [DOI] [PubMed] [Google Scholar]
- 6.Sulakvelidze A, Alavidze Z, Morris JG., Jr 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649–659. 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stent GS. 1963. Molecular biology of bacterial viruses. W.H. Freeman and Company, San Francisco, CA. [Google Scholar]
- 8.Fogelman I, Davey V, Ochs HD, Elashoff M, Feinberg MB, Mican J, Siegel JP, Sneller M, Lane HC. 2000. Evaluation of CD4+ T cell function in vivo in HIV-infected patients as measured by bacteriophage phiX174 immunization. J. Infect. Dis. 182:435–441. 10.1086/315739. [DOI] [PubMed] [Google Scholar]
- 9.Krisch HM, Comeau AM. 2008. The immense journey of bacteriophage T4-from d'Hérelle to Delbrück and then to Darwin and beyond. Res. Microbiol. 159:314–324. 10.1016/j.resmic.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Bruttin A, Brüssow H. 2005. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49:2874–2878. 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J, Abu-Shilbayeh L, Rao VB. 1997. Display of a PorA peptide from Neisseria meningitidis on the bacteriophage T4 capsid surface. Infect. Immun. 65:4770–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren S, Fengyu Zuo S, Zhao M, Wang X, Wang X, Chen Y, Wu Z, Ren Z. 2011. Inhibition of tumor angiogenesis in lung cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine 29:5802–5811. 10.1016/j.vaccine.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Sathaliyawala T, Rao M, Maclean DM, Birx DL, Alving CR, Rao VB. 2006. Assembly of human immunodeficiency virus (HIV) antigens on bacteriophage T4: a novel in vitro approach to construct multicomponent HIV vaccines. J. Virol. 80:7688–7698. 10.1128/JVI.00235-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Z, Black LW. 1998. Phage T4 SOC and HOC display of biologically active, full-length proteins on the viral capsid. Gene 215:439–444. 10.1016/S0378-1119(98)00298-4. [DOI] [PubMed] [Google Scholar]
- 15.Rao VB, Black LW. 2010. Structure and assembly of bacteriophage T4 head. Virol. J. 7:356. 10.1186/1743-422X-7-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black LW, Rao VB. 2012. Structure, assembly, and DNA packaging of the bacteriophage T4 head. Adv. Virus Res. 82:119–153. 10.1016/B978-0-12-394621-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii T, Yanagida M. 1974. Morphogenesis of the head-part of the T4 phage. Abstr. Jpn. Biophys. Soc. 29-E:82 (In Japanese.) [Google Scholar]
- 18.Ishii T, Yanagida M. 1975. Molecular organization of the shell of the Teven bacteriophage head. J. Mol. Biol. 97:655–660. 10.1016/S0022-2836(75)80065-9. [DOI] [PubMed] [Google Scholar]
- 19.Yanagida M, Suzuki Y, Toda T. 1984. Molecular organization of the head of bacteriophage Teven: underlying design principles. Adv. Biophys. 17:97–146. 10.1016/0065-227X(84)90026-1. [DOI] [PubMed] [Google Scholar]
- 20.Karam JD. 1994. Molecular biology of bacteriophage T4, p 213–317 American Society for Microbiology, Washington, DC. [Google Scholar]
- 21.Jerne NK, Avegno P. 1956. The development of the phage-inactivating properties of serum during the course of specific immunization of an animal: reversible and irreversible inactivation. J. Immunol. 76:200–208. [PubMed] [Google Scholar]
- 22.Miernikiewicz P, Owczarek B, Piotrowicz A, Boczkowska B, Rzewucka K, Figura G, Letarov A, Kulikov E, Kopciuch A, Świtała-Jeleń K, Oślizło A, Hodyra K, Gubernator J, Dąbrowska K. 2012. Recombinant expression and purification of T4 phage gpHoc, gpSoc, gp23, gp24 proteins in native conformations with stability studies. PLoS One 7:e38902. 10.1371/journal.pone.0038902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dąbrowska K, Zembala M, Boratynski J, Kujawa M, Świtala-Jelen K, Wietrzyk J, Opolski A, Szczaurska K, Godlewska J, Gorski A. 2007. Hoc protein regulates the biological effects of T4 phage in mammals. Arch. Microbiol. 187:489–498. 10.1007/s00203-007-0216-y. [DOI] [PubMed] [Google Scholar]
- 24.Boratyński J, Syper D, Weber-Dabrowska B, Łusiak-Szelachowska M, Poźniak G, Górski A. 2004. Preparation of endotoxin-free bacteriophages. Cell. Mol. Biol. Lett. 9:253–259. [PubMed] [Google Scholar]
- 25.Adams MH. 1959. Bacteriophages. Interscience Inc, New York, NY. [Google Scholar]
- 26.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. 2008. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 26:193–200. 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becherelli M, Prachi P, Viciani E, Biagini M, Fiaschi L, Chiarot E, Nosari S, Brettoni C, Marchi S, Biancucci M, Fontana MR, Montagnani F, Bagnoli F, Barocchi MA, Manetti AG. 2013. Protective activity of the CnaBE3 domain conserved among Staphylococcus aureus Sdr proteins. PLoS One 8(9):e74718. 10.1371/journal.pone.0074718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, Nikolaeva D, Diakite M, Fairhurst RM, Fay MP, Long CA, Tsuboi T. 2013. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect. Immun. 81:4377–4382. 10.1128/IAI.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed GF, Meade BD, Steinhoff MC. 1995. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 96(3 Part 2):600–603. [PubMed] [Google Scholar]
- 30.Hedström SA, Kamme C. 1973. Antibodies against staphylococcal bacteriophages in human sera. II. Assay of antibodies in exacerbation and regression of chronic staphylococcal osteomyelitis. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 81:749–752. [PubMed] [Google Scholar]
- 31.Kamme C. 1973. Antibodies against staphylococcal bacteriophages in human sera. I. Assay of antibodies in healthy individuals and in patients with staphylococcal infections. Acta Pathol. Microbiol. Scand. B. Microbiol. Immunol. 81:741–748. [PubMed] [Google Scholar]
- 32.Kucharewicz-Krukowska A, Slopek S. 1987. Immunogenic effect of bacteriophage in patients subjected to phage therapy. Arch. Immunol. Ther. Exp. 35:553–561. [PubMed] [Google Scholar]
- 33.Weber-Dabrowska B, Dabrowski M, Slopek S. 1987. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch. Immunol. Ther. Exp. 35:563–568. [PubMed] [Google Scholar]
- 34.Keller R, Engley FB. 1958. Fate of bacteriophage particles introduced into mice by various routes. Proc. Soc. Exp. Biol. Med. 98:577–579. 10.3181/00379727-98-24112. [DOI] [PubMed] [Google Scholar]
- 35.Łusiak-Szelachowska M, Zaczek M, Weber-Dąbrowska B, Międzybrodzki R, Kłak M, Fortuna W, Letkiewicz S, Rogóż P, Szufnarowski K, Jończyk-Matysiak E, Owczarek B, Górski A. 2014. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 27:295–304. 10.1089/vim.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merril CR, Biswas B, Carlton RM, Jensen NC, Creed GJ, Zullo S, Adhya S. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. U. S. A. 93:3188–3192. 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]