Abstract
It is still unclear whether expanded and activated regulatory T cells (Tregs) in chronic viral infections can influence primary immune responses against superinfections with unrelated viruses. Expanded Tregs found in the spleens of chronically Friend virus (FV)-infected mice decreased murine cytomegalovirus (mCMV)-specific CD8+ T cell responses during acute mCMV superinfection. This suppression of mCMV-specific T cell immunity was found only in organs with FV-induced Treg expansion. Surprisingly, acute mCMV infection itself did not expand or activate Tregs.
TEXT
Previous infections can influence the immune response to infections with unrelated pathogens, which is called heterologous immunity (1, 2). This phenomenon has been found in closely related and completely unrelated viral infections in humans and in mouse models (3). To date, limited information is available as to whether virus-expanded regulatory T cells (Tregs) in a chronically infected host play a role in heterologous immunity. In this study, we investigated the influence of Friend virus (FV)-expanded Tregs on the primary murine cytomegalovirus (mCMV)-specific CD8+ T cell response during an acute mCMV superinfection.
We first confirmed the expansion of Tregs during FV infection by determining the number of Tregs in chronically FV-infected mice in different organs (Fig. 1a) (4, 5). C57BL/6 mice (males, 8 to 9 weeks old) were infected intravenously with 40,000 spleen focus-forming units (SFFU) of FV, and CD4+ Foxp3+ Tregs were measured at day 42 postinfection (6, 7). Significantly enhanced percentages (not shown) and absolute numbers of Tregs were found in the spleens but not in the livers and peritoneal exudate cells (PECs) of chronically FV-infected mice compared to results for naive, age-matched controls (Fig. 1a). FV-expanded Tregs had an activated and differentiated phenotype (CD43+ CD69+ and KLRG-1+), in contrast to Tregs from uninfected mice at day 10 and day 42 after FV infection (Fig. 1c and reference 8, respectively).
FIG 1.
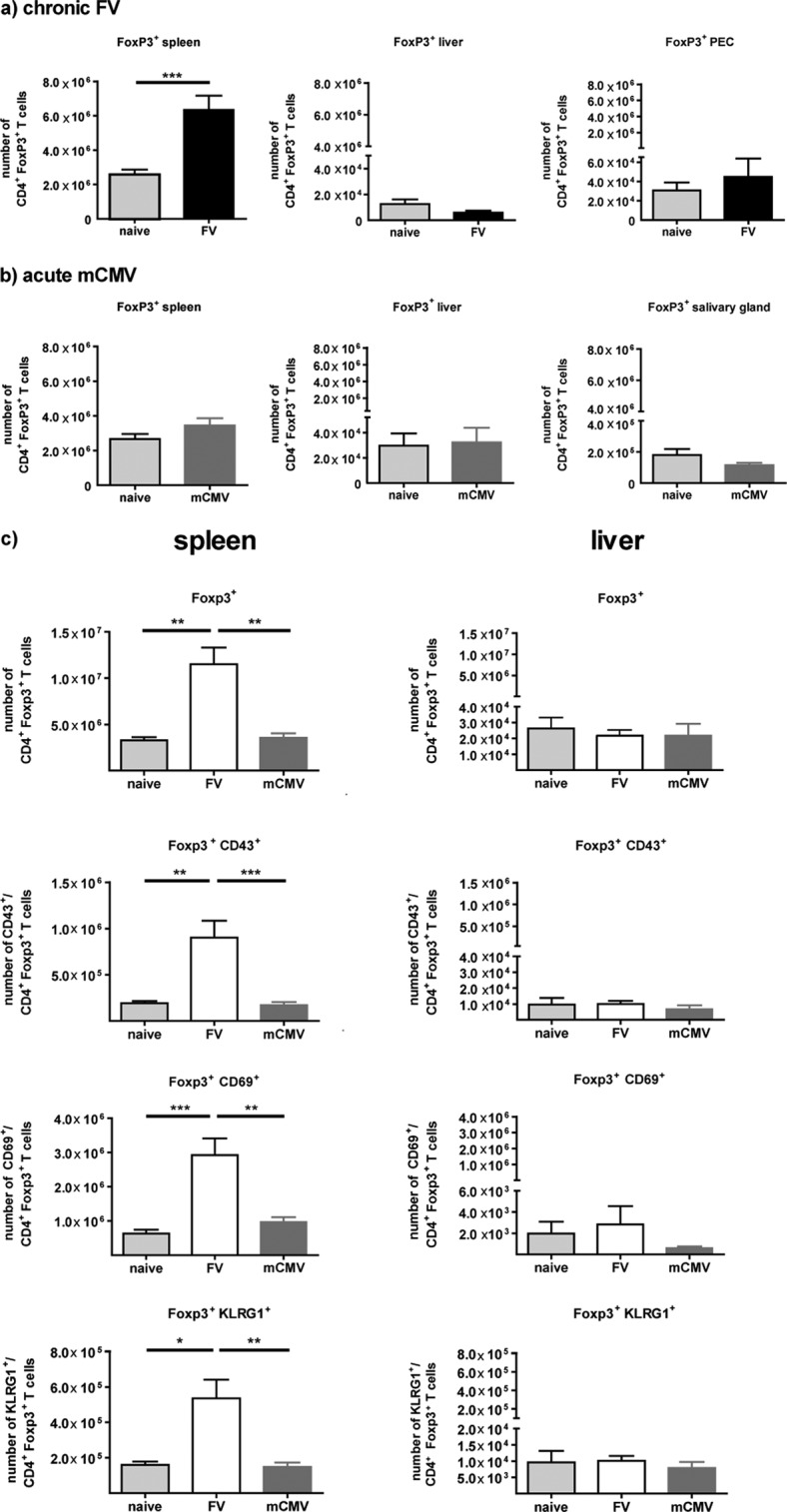
Numbers of Tregs in the spleens, livers, PECs, and salivary glands of acutely mCMV- as well as chronically FV-infected mice. (a) Naive C57BL/6 mice were infected intravenously with 40,000 SFFU FV; control mice received phosphate-buffered saline (PBS). In the chronic phase, 6 weeks after FV infection, the numbers of CD4+ Foxp3+ T cells were determined in the spleens, livers, and PECs. (b) Naive C57BL/6 mice were infected intraperitoneally with 5 × 104 PFU of mCMV; control mice received PBS. At day 10 after mCMV infection, the number of CD4+ Foxp3+ T cells was measured in the spleens, livers, and salivary glands. (c) Mice were infected with FV or mCMV, and control (naive) mice received PBS. At 10 days postinfection, the absolute numbers of CD4+ Foxp3+ Tregs as well as activated Tregs were determined in the spleens and livers of acutely FV- or mCMV-infected or naive mice. (a, b) Data were pooled from 2 to 6 independent experiments with 4 to 24 mice per group. (c) Statistical analysis was performed with an unpaired Student t test, and the data were pooled from 1 to 4 independent experiments with 6 to 19 mice per group. Statistical analysis was performed with the Bonferroni multiple-comparison or Kruskal-Wallis test. Statistically significant differences between the groups are indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
To investigate whether mCMV infection also induces an expansion of Tregs, naive mice were infected intraperitoneally with 5 × 104 PFU of mCMV (Smith strain [7]) and CD4+ Foxp3+ Tregs were analyzed at day 10 postinfection in the spleen, liver, and salivary gland, sites where mCMV replicates (9). Surprisingly, no expansion or activation of Tregs was found in the spleens of acutely mCMV-infected mice, in contrast to what is described for FV or lymphocytic choriomeningitis virus (LCMV) infection (Fig. 1b and c) (10–12). Additionally, no expansion of the Vβ5+ CD4+ Foxp3+ Tregs, which recognize self-superantigens expressed by endogenous mouse mammary tumor virus, were detectable in mCMV-infected mice (8, 10, 11; data not shown). Furthermore, Tregs from mCMV-infected mice displayed the same phenotype as Tregs from naive animals (Fig. 1c). This was also the case for later time points after mCMV infection (>10 days postinfection) (data not shown), indicating that mCMV does not induce Treg responses in C57BL/6 mice.
Tregs in influenza A virus (IAV)-immune mice control IAV-specific memory CD8+ T cells during sequential infection with a different IAV strain by IAV-specific memory Tregs in the lung (13). Additionally, we could show that IAV-expanded Tregs negatively influenced the strength of non-cross-reactive LCMV-specific CD8+ T cell responses in mice sequentially infected with IAV and LCMV (14). Since nothing is known about the influence of virus-expanded Tregs in chronic viral infections on a primary unrelated infection, chronically FV-infected mice were superinfected with mCMV (FV/mCMV). mCMV-specific CD8+ T cell responses were determined in the spleen, liver, and PECs at day 7 after mCMV superinfection (Fig. 2). Therefore, lymphocytes were simulated with the mCMV-specific major histocompatibility complex (MHC) class I peptides M45985–993 (HGIRNASFI), M57816–824 (SCLEFWQRV), M102446–455 (SIVDLRFAVL), m139419–426 (TVYGFCLL), or m14115–23 (VIDAFSRL), and functional mCMV-specific CD8+ T cells were determined by gamma interferon (IFN-γ) production (7, 15). Significant 2- to 3-fold reductions in mCMV-specific IFN-γ+ CD44+ CD8+ T cell numbers were detectable in the spleens of FV/mCMV-superinfected mice compared to numbers in mice infected only with mCMV (Fig. 2a). Importantly, this reduction was detectable in those spleens where FV induced a sustained Treg expansion and activation but not in PECs (Fig. 2b) or in livers (data not shown). In these PECs and livers, the mCMV-specific CD8+ T cell responses were similar between FV/mCMV- and mCMV-only-infected mice (Fig. 2b). This is in line with our previous findings showing that FV-induced Treg responses are locally defined to organs in which viral replication occurs and in which effector CD4+ and CD8+ T cell responses are induced (16). These effector T cells play a major role in initiating the activation and expansion of different subsets of Tregs (8, 10, 17).
FIG 2.
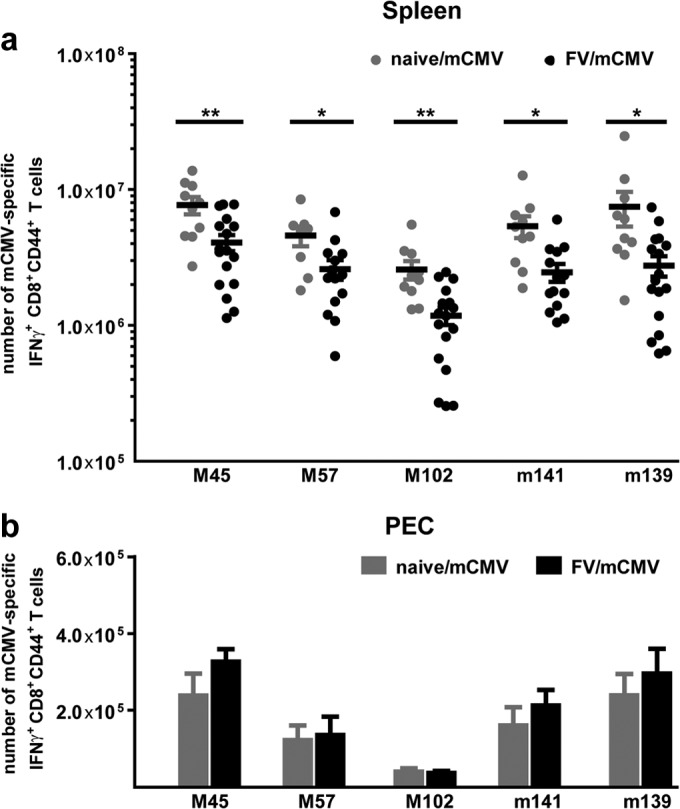
Primary mCMV-specific CD8+ T cell responses in the spleens and PECs of naive mCMV-infected and FV/mCMV-superinfected mice. Naive C57BL/6 mice were infected intravenously with 40,000 SFFU FV; control mice received PBS. In the chronic phase, 6 weeks after FV infection, mice were infected intraperitoneally with 5 × 104 PFU mCMV. As a control, age-matched naive mice were infected with mCMV. mCMV-specific CD8+ T cell responses in the spleens (a) and PECs (b) of naive and chronically FV-infected mice were obtained at day 7 after mCMV infection. Data from the spleens were pooled from 4 independent experiments with 10 to 17 mice and from PECs from 2 independent experiments with 3 to 9 mice per group. Statistical analysis was performed with an unpaired Student t test. Statistically significant differences between the groups are indicated (*, P < 0.05; **, P < 0.01).
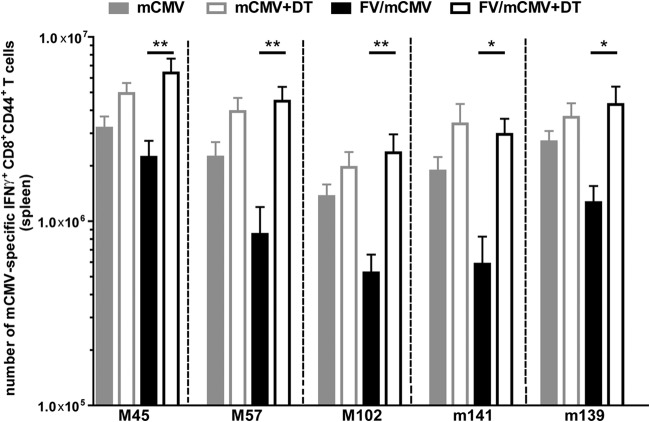
To prove that FV-expanded Tregs are important mediators of the reduction in mCMV-specific CD8+ T cell responses, we made use of the DEREG mouse model. DEREG mice express the human diphtheria toxin receptor (DTR) under the control of the Foxp3 promoter (18). Treatment of DEREG mice with diphtheria toxin (DT) results in a selective depletion of Foxp3+ CD4+ Tregs. For Treg depletion, 0.5 μg DT/mouse/time point was injected intraperitoneally on days −4, −1, 2, and 5 of mCMV superinfection of chronically FV-infected mice (19). Depletion of Tregs during mCMV superinfection resulted in significant 3- to 5-fold increases in the total numbers of mCMV-specific CD8+ T cells that produced IFN-γ in chronically FV-infected mice (Fig. 3). This significant increase was detectable for all analyzed mCMV epitope-specific CD8+ T cell responses in the spleen. Previously, it had been shown that the activation and expansion of Tregs during FV infection were not dependent on FV antigens but rather were initiated by self-antigens and cytokines, like interleukin 2 (IL-2) and tumor necrosis factor alpha (TNF-α) (8, 10). Hence, no FV-specific Tregs were found during FV infection (10). Our current findings show that not only is the induction of Tregs independent of the FV antigen, but also the suppression of effector T cell responses is not restricted to FV-specific T cells. FV-expanded Tregs inhibit T cell responses against unrelated viral antigens (mCMV), indicating that their activity is not antigen but rather organ specific.
FIG 3.
mCMV-specific CD8+ T cell responses after the depletion of Tregs in naive mCMV-infected and FV/mCMV-superinfected mice. Tregs were depleted by intraperitoneal injection of 0.5 μg DT at days −4, −1, 2, and 5 of mCMV infection in naive and chronically FV-infected DEREG mice. Control mice received PBS. mCMV-specific CD8+ T cell responses were determined at day 7 after mCMV infection. Data were pooled from 3 independent experiments with 5 to 15 mice per group, and statistical analysis was performed with a one-way analysis of variance (ANOVA) multiple-comparison test (*, P < 0.05; **, P < 0.01).
To determine the influence of non-virus-induced, natural Tregs on mCMV-specific CD8+ T cell responses, Tregs were depleted in age-matched mice infected only with mCMV (Fig. 3, gray bars). No significant differences in the magnitudes of mCMV-specific CD8+ T cell responses were found between Treg-depleted and nondepleted mice during acute mCMV infection. This was in line with the finding that mCMV infection did not result in Treg expansion or activation (Fig. 1c). In contrast to our findings, a stronger effect on mCMV-specific T cell responses in Treg-depleted mice was described by Arens et al. (20). This difference might be explained by the different technical approaches which were used to deplete Tregs during acute mCMV infection. In our study, Foxp3-expressing cells were selectively depleted, whereas in the study of Arens et al., Tregs were depleted with anti-CD25 antibodies. Approximately 80% of all NK cells express CD25 at days 2 and 3 after mCMV infection (21). Thus, treatment with the anti-CD25 antibody may have also depleted NK cells, whereas in our approach, only Foxp3+ Tregs were ablated. Depletion of NK cells has been shown to enhance mCMV titers and mCMV-specific T cell responses during acute mCMV infection (22–26), which might be the reason for the strong effect reported by Arens et al. (20). Unlike with mCMV, the expansion of Tregs has been found in mice infected with other herpesviruses, like herpes simplex virus (HSV) (12, 27). It is currently not known why mCMV induces such weak Treg responses, which is clearly different from what occurs in many other viral infections.
To investigate whether the mCMV clearance in chronically FV-infected mice was negatively influenced by the fact that FV-expanded Tregs reduced mCMV-specific CD8+ T cell responses, we determined mCMV titers by plaque assay at day 7 after mCMV superinfection (Fig. 4) (7). No differences in mCMV titers in the spleen (the organ where reduced mCMV-specific CD8+ T cell responses were found) were detected between mCMV-infected and FV/mCMV-superinfected mice (Fig. 4). This suggests that the enhanced number of Tregs in chronically FV-infected mice did not alter mCMV clearance. Similar results were obtained in the models of acute LCMV infection of IAV-immune mice and chronic LCMV infection (14, 28). As mentioned above, NK cells play a very important role in viral clearance in early mCMV infection of C57BL/6 mice, and even the strength of the mCMV-specific T cell response is partly controlled by NK cells (22–26). Binding of the mCMV protein m157 to Ly49H+ on NK cells is responsible for the strong NK cell effect in C57BL/6 mice (22, 29–31). Additionally, the depletion of CD8+ T cells during mCMV infection does not change the kinetics of mCMV clearance (32). This shows that mCMV clearance is driven mainly by NK cells during acute mCMV infection of C57BL/6 mice, which explains why CD8+ T cell responses suppressed by FV-expanded Tregs did not significantly affect viral loads.
FIG 4.
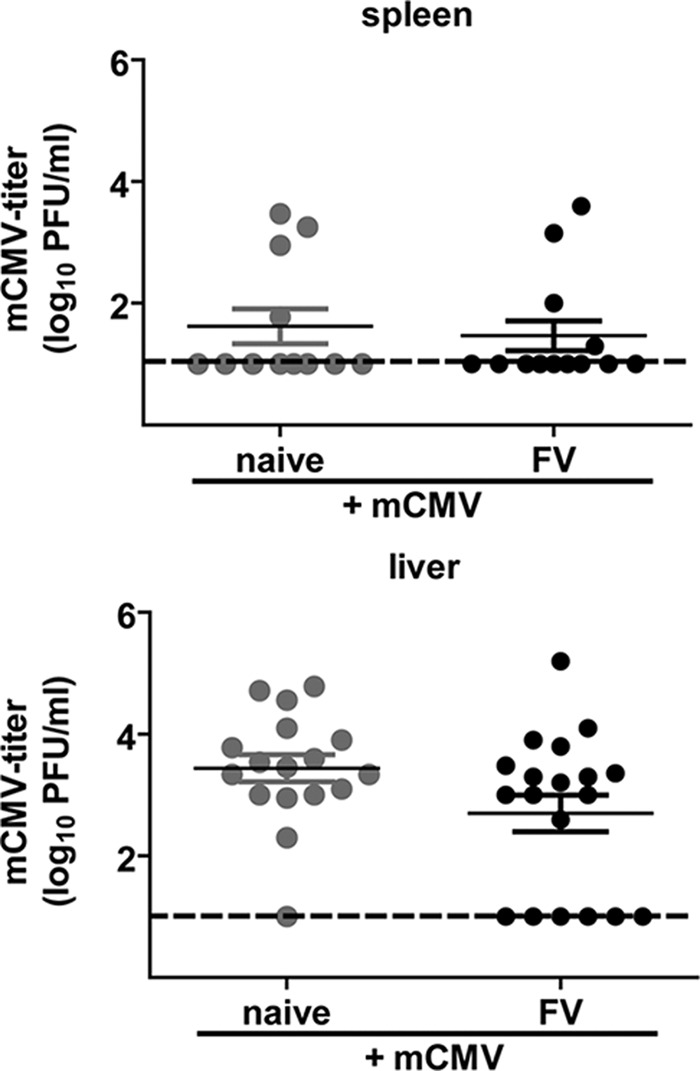
Detection of mCMV titers in naive mCMV-infected and FV/mCMV-superinfected mice. Naive C57BL/6 mice were infected with FV; control mice received PBS. In the chronic phase, 6 weeks after FV infection, mice were superinfected with mCMV. mCMV titers were determined in the spleens and livers at day 7 after mCMV infection. Data were pooled from 3 to 4 independent experiments. Dashed lines display the detection limits.
Taken together, our data show that Tregs expanded by a chronic retroviral infection can dampen primary immune responses to mCMV. This effect might impair the immunity of individuals to acute infections with chronic infectious diseases.
ACKNOWLEDGMENTS
This work was supported by the German Research Association (DFG) via Transregio 60.
We thank Tim Sparwasser (Institute for Infection Immunology, TWINCORE, Hannover, Germany) for providing the DEREG mice.
Footnotes
Published ahead of print 17 September 2014
REFERENCES
- 1. Selin LK, Varga SM, Wong IC, Welsh RM. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 188:1705–1715. 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welsh RM, Selin LK. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417–426. 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 3. Welsh RM, Che JW, Brehm MA, Selin LK. 2010. Heterologous immunity between viruses. Immunol. Rev. 235:244–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zelinskyy G, Kraft AR, Schimmer S, Arndt T, Dittmer U. 2006. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur. J. Immunol. 36:2658–2670. 10.1002/eji.200636059. [DOI] [PubMed] [Google Scholar]
- 5. Myers L, Messer RJ, Carmody AB, Hasenkrug KJ. 2009. Tissue-specific abundance of regulatory T cells correlates with CD8+ T cell dysfunction and chronic retrovirus loads. J. Immunol. 183:1636–1643. 10.4049/jimmunol.0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kabat D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1–42. [DOI] [PubMed] [Google Scholar]
- 7. Francois S, Peng J, Schwarz T, Duppach J, Gibbert K, Dittmer U, Kraft AR. 2013. NK cells improve control of friend virus infection in mice persistently infected with murine cytomegalovirus. Retrovirology 10:58. 10.1186/1742-4690-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joedicke JJ, Dietze KK, Zelinskyy G, Dittmer U. 2014. The phenotype and activation status of regulatory T cells during Friend retrovirus infection. Virol. Sin. 29:48–60. 10.1007/s12250-014-3396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell AE, Cavanaugh VJ, Slater JS. 2008. The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence. Med. Microbiol. Immunol. 197:205–213. 10.1007/s00430-008-0077-2. [DOI] [PubMed] [Google Scholar]
- 10. Myers L, Joedicke JJ, Carmody AB, Messer RJ, Kassiotis G, Dudley JP, Dittmer U, Hasenkrug KJ. 2013. IL-2-independent and TNF-alpha-dependent expansion of Vbeta5+ natural regulatory T cells during retrovirus infection. J. Immunol. 190:5485–5495. 10.4049/jimmunol.1202951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Punkosdy GA, Blain M, Glass DD, Lozano MM, O'Mara L, Dudley JP, Ahmed R, Shevach EM. 2011. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc. Natl. Acad. Sci. U. S. A. 108:3677–3682. 10.1073/pnas.1100213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889–901. 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brincks EL, Roberts AD, Cookenham T, Sell S, Kohlmeier JE, Blackman MA, Woodland DL. 2013. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J. Immunol. 190:3438–3446. 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraft AR, Wlodarczyk MF, Kenney LL, Selin LK. 2013. PC61 (anti-CD25) treatment inhibits influenza A virus-expanded regulatory T cells and severe lung pathology during a subsequent heterologous lymphocytic choriomeningitis virus infection. J. Virol. 87:12636–12647. 10.1128/JVI.00936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. 2006. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 176:3760–3766. 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 16. Zelinskyy G, Dietze KK, Husecken YP, Schimmer S, Nair S, Werner T, Gibbert K, Kershaw O, Gruber AD, Sparwasser T, Dittmer U. 2009. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood 114:3199–3207. 10.1182/blood-2009-03-208736. [DOI] [PubMed] [Google Scholar]
- 17. Joedicke JJ, Myers L, Carmody AB, Messer RJ, Wajant H, Lang KS, Lang PA, Mak TW, Hasenkrug KJ, Dittmer U. 2014. Activated CD8+ T cells induce expansion of Vbeta5+ regulatory T cells via TNFR2 signaling. J. Immunol. 193:2952–2960. 10.4049/jimmunol.1400649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63. 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dietze KK, Zelinskyy G, Liu J, Kretzmer F, Schimmer S, Dittmer U. 2013. Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads. PLoS Pathog. 9:e1003798. 10.1371/journal.ppat.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arens R, Loewendorf A, Redeker A, Sierro S, Boon L, Klenerman P, Benedict CA, Schoenberger SP. 2011. Differential B7-CD28 costimulatory requirements for stable and inflationary mouse cytomegalovirus-specific memory CD8 T cell populations. J. Immunol. 186:3874–3881. 10.4049/jimmunol.1003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee SH, Fragoso MF, Biron CA. 2012. Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J. Immunol. 189:2712–2716. 10.4049/jimmunol.1201528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bukowski JF, Woda BA, Welsh RM. 1984. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 52:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lanier LL. 2008. Evolutionary struggles between NK cells and viruses. Nat. Rev. Immunol. 8:259–268. 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49. 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scalzo AA, Corbett AJ, Rawlinson WD, Scott GM, Degli-Esposti MA. 2007. The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol. Cell Biol. 85:46–54. 10.1038/sj.icb.7100013. [DOI] [PubMed] [Google Scholar]
- 26. Mitrovic M, Arapovic J, Jordan S, Fodil-Cornu N, Ebert S, Vidal SM, Krmpotic A, Reddehase SM, Jonjic S. 2012. The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8(+) T-cell response. J. Virol. 86:2165–2175. 10.1128/JVI.06042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Veiga-Parga T, Suryawanshi A, Mulik S, Gimenez F, Sharma S, Sparwasser T, Rouse BT. 2012. On the role of regulatory T cells during viral-induced inflammatory lesions. J. Immunol. 189:5924–5933. 10.4049/jimmunol.1202322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O'Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R. 2014. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 211:1905–1918. 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. 2003. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 5:1263–1277. 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 30. Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. U. S. A. 99:8826–8831. 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323–1326. 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 32. Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski UH. 1990. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 64:5457–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]