ABSTRACT
DDX3 is a member of the DEAD-box RNA helicase family, involved in mRNA metabolism, including transcription, splicing, and translation. We previously identified DDX3 as a hepatitis B virus (HBV) polymerase (Pol) binding protein, and by using a transient transfection, we found that DDX3 inhibits HBV replication at the posttranscriptional level, perhaps following encapsidation. To determine the exact mechanism of the inhibition, we here employed a diverse HBV experimental system. Inconsistently, we found that DDX3-mediated inhibition occurs at the level of transcription. By using tetracycline-inducible HBV-producing cells, we observed that lentivirus-mediated DDX3 expression led to a reduced level of HBV RNAs. Importantly, knockdown of DDX3 by short hairpin RNA resulted in augmentation of HBV RNAs in two distinct HBV replication systems: (i) tetracycline-inducible HBV-producing cells and (ii) constitutive HBV-producing HepG2.2.15 cells. Moreover, DDX3 knockdown in HBV-susceptible HepG2-NTCP cells, where covalently closed circular DNA (cccDNA) serves as the template for viral transcription, resulted in increased HBV RNAs, validating that transcription regulation by DDX3 occurs on a physiological template. Overall, our results demonstrate that DDX3 represents an intrinsic host antiviral factor that restricts HBV transcription.
IMPORTANCE Upon entry into host cells, viruses encounter host factors that restrict viral infection. During evolution, viruses have acquired the ability to subvert cellular factors that adversely affect their replication. Such host factors include TRIM5α and APOBEC3G, which were discovered in retroviruses. The discovery of host restriction factors provided deeper insight into the innate immune response and viral pathogenesis, leading to better understanding of host-virus interactions. In contrast to the case with retroviruses, little is known about host factors that restrict hepatitis B virus (HBV), a virus distantly related to retroviruses. DDX3 DEAD box RNA helicase is best characterized as an RNA helicase involved in RNA metabolism, such as RNA processing and translation. Here, we show that DDX3 inhibits HBV infection at the level of viral transcription.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection represents a major public health burden, affecting more than 300 million individuals worldwide, and carries a high risk for developing cirrhosis and hepatocellular carcinoma (HCC) (1). HBV virions contain a small, partially double-stranded circular DNA genome of 3.2 kb in length. Although it is a DNA virus, HBV replicates its DNA genome via reverse transcription. Upon infection, the virion DNA is converted into covalently closed circular DNA (cccDNA), which then serves as the template for viral transcription (2). Among the viral transcripts, only pregenomic RNA (pgRNA), 3.5 kb in length, is selectively packaged into nucleocapsids along with HBV polymerase (Pol). Inside the nucleocapsid, the pgRNA is reverse transcribed by HBV Pol to yield relaxed circular (RC) DNA. These mature RC DNA-containing nucleocapsids are enveloped at the endoplasmic reticulum and released extracellularly via budding.
Upon entry to host cells, viruses encounter cellular factors that frequently determine the fate of viral infection. Some cellular factors contribute to the establishment of viral infection, whereas others adversely affect viral infection. Viruses have acquired the ability to co-opt or subvert these cellular factors to benefit establishment of infection. In addition to the host immune system, host cells possess cellular factors that restrict viral infection. Such factors were first documented in lentiviruses, including human immunodeficiency virus (HIV), where a host restriction factor, TRIM5α, was identified as the long-sought host factor responsible for determining host tropism of primate lentiviruses (3). Other host restriction factors have been documented, including APOBEC3G, BST-2/tetherin, and SAMHD1 (4). In contrast, little is known about host factors that restrict HBV infection. Accumulating evidence has indicated that members of the APOBEC3 family exhibit antiviral activity against HBV infection (5–10). Importantly, some members of the APOBEC3 family were shown to be involved in cytokine-induced decay of cccDNA (11).
DDX3, which is a member of the highly conserved DEAD-box RNA helicases, plays a role in a multitude of RNA metabolic states, such as transcription regulation, pre-mRNA splicing, RNA export, and mRNA translation (12–18). Evidence suggests that DDX3 is a host factor coopted by multiple viruses, including HIV and hepatitis C virus (HCV) (19–23). One report showed that DDX3 facilitates the Rev/Rev-responsive element (RRE)-mediated nuclear export of HIV genomic RNA (21); others then showed that DDX3 promotes HIV-1 genomic RNA translation (23). In addition, DDX3 supports HCV genome replication, although the molecular mechanism remains uncertain (20, 22). In contrast to these supportive roles, we previously reported that DDX3 inhibits genome replication of HBV, perhaps following pgRNA encapsidation (24). However, the exact mechanism of the inhibition remains to be elucidated.
Here, we sought to determine the step in the HBV life cycle at which DDX3 inhibits HBV genome replication by employing diverse HBV replication systems. We found that DDX3 inhibits viral genome replication at the level of viral transcription by showing that the inhibition by DDX3 occurs independent of HBV Pol-DDX3 interaction. The downregulation of DDX3 led to enhanced viral RNA levels, suggesting that DDX3 represents a host factor that restricts HBV infection. By using HBV-susceptible cells, we demonstrated that DDX3 affects transcription from cccDNA, the natural template for viral transcription.
MATERIALS AND METHODS
Plasmids.
HBV 1.3-mer replicon constructs (i.e., 1.3-mer and its P-null version) and all DDX3 expression vectors were previously described (24). An X-null counterpart of the 1.3-mer replicon was generated by introducing two stop codons into the X open reading frame (ORF) without altering the P-ORF, as similarly described (25). pHBV-Luc, an HBV core promoter/enhancer reporter construct, was previously described (25). Specifically, it contains HBV enhancer I and enhancer II, as well as the basal core promoter upstream of the luciferase ORF of the pGL3-Basic plasmid (Promega). pHBV-S1-Luc and pHBV-S2-Luc were constructed by insertion of fragments containing the HBV S1 promoter region (nucleotides [nt] 2718 to 2798; HBV ayw) and S2 promoter region (nt 2958 to 3158; HBV ayw) into XhoI/HindIII sites of the pGL3-Basic plasmid (Promega), respectively. The pCDH-DDX3-Flag plasmid encoding DDX3 in a lentiviral vector was generated by inserting the DDX3-Flag cDNA into the NheI/EcoRI sites of pCDH-CMV-MCS-EF1-Puro (System Biosciences). Five short hairpin RNA (shRNA) constructs targeting multiple regions of the DDX3 gene (shDDX3) were purchased from Sigma. A shDDX3 construct showing the best knockdown efficiency was selected and used to produce lentiviral vectors. The sources of the remaining plasmids are as follows: pTet-off and pTREHBVDE, Ju-Tao Guo, Drexel University College of Medicine, USA; pHIV-gag/pol/rev, pVSV-G, and pLKO.1-puro, Sigma; pLKO.1-puro GFP shRNA, Sigma; pMD2.G, Addgene (plasmid 12,259); psPAX2, Addgene (plasmid 12,260); and hemagglutinin (HA)-DDX5, Yasuo Ariumi, Kumamoto University, Japan.
Cell culture.
Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C. The HBV-Tof cell line, which supports the HBV genome replication in a tetracycline-inducible manner, was established as described elsewhere (26). Briefly, HepG2 cells were cotransfected with pTet-off and pTREHBVDE plasmids and selected with G418 in the presence of doxycycline (1 μg/ml) for 2 weeks. Selected clones were picked, propagated in doxycycline-free medium, and subjected to Southern blot analysis to determine the levels of HBV replication. A clone that supports the highest level of HBV replication was chosen for this study. The HBV-Tof-DDX3 cell line, which stably expresses DDX3 in the HBV-Tof cell, was established by transducing a lentiviral vector encoding DDX3. HBV-Tof cells stably expressing shDDX3 or shRNA targeting green fluorescent protein (GFP) (shGFP) were established by puromycin selection after transducing lentiviral vectors encoding either shDDX3 or shGFP. Three days after selection, surviving cells were pooled and used for a further experiment. HepG2-NTCP cells, stably expressing human NTCP, were maintained in DMEM supplemented with 10% FBS–2 mM l-glutamine (27). HepAD38 cells (kindly provided by Christopher Seeger, Fox Chase Cancer Center) were cultured to produce HBV inoculum, as described previously (28).
Transfection.
Cells were transfected with polyethylenimine (PEI) (linear,25 kDa; Polysciences, Inc.) as previously described (29). For lentiviral production, HEK293T cells were seeded in 100-mm plates and transfected as described below. For the luciferase reporter assay, HepG2 cells were seeded in 12-well plates and harvested a day posttransfection.
Lentiviruses.
Lentiviruses were produced from HEK293T cells by a transfection procedure. For the generation of DDX3-expressing lentiviral vectors, cells were cotransfected with 11 μg of pCDH-CMV-MCS-EF1-puro-DDX3 construct, 11 μg of pHIV-gag/pol/rev, and 2 μg of pVSV-G. For the production shDDX3-expressing lentiviral vectors, cells were cotransfected with 12 μg of shDDX3 construct, 6 μg of psPAX2, and 3 μg of pMD2.G. Medium was replaced 8 h after transfection, and the supernatant was collected 2 days posttransfection. Lentiviral transduction was performed by dispensing appropriate amount of supernatants (ca. 2 ml per 100-mm plate) derived from the above transfected cells with Polybrene (Sigma) at a final concentration of 4 μg/ml. One day after transduction, cells were extensively washed and fed with fresh medium containing puromycin for the selection. Routinely, more than 90% of cells were transduced.
Southern blot analysis.
Southern blot analysis was performed using viral DNAs isolated from cytoplasmic capsids, exactly as previously described (29). For the detection of cccDNA, a modified Hirt method was used to extract protein-free vial DNAs, as described previously (26, 30, 31). Briefly, protein-free vial DNAs were isolated from infected HepG2-NTCP cells in 35-mm plates (30). The extracted DNAs were treated with Plasmid-Safe ATP-dependent DNase according to the manufacturer's instructions (Epicentre). The viral DNAs were separated on an agarose gel, transferred to a nylon membrane, and hybridized with an HBV-specific probe, as described previously (26, 31).
Western blot analysis.
Western blot analysis was performed, as described previously (24). Primary antibodies used include anti-DDX3 (diluted 1:1,000; Abcam) and polyclonal rabbit antiserum against HBcAg (diluted 1:3,000; kindly provided by Kyongmin Kim, Ajou University, South Korea). After being incubated with appropriate secondary antibodies, the proteins were visualized by chemiluminescence and quantified by using a LAS-4000 instrument (Fujifilm).
Native agarose gel analysis of capsid particles.
Native agarose gel analysis of capsid particles was performed as previously described (32), with some modifications. Cytoplasmic cell lysates containing viral capsid were directly resolved in a 1.2% agarose gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Immuno-Blot PVDF; Bio-Rad) through capillary action in 10× SSC (1.5 M NaCl and 0.15 M sodium citrate) buffer. The membrane blot was blocked with 1% nonfat dry milk and probed with anti-HBcAg antibody (diluted 1:5,000; Dako). The HBV capsids were then visualized as described for Western blot analysis.
Northern blot analysis.
Total RNA was extracted using acid guanidinium thiocyanate-phenol-chloroform extraction (33). Northern blot analysis was essentially carried out as described previously (34).
RNase protection assay.
The RNase protection assay (RPA) was performed as described previously (32). Briefly, one-third of the RNA isolated from a 35-mm plate was subjected to an RNase protection assay as recommended by the manufacturer (Ambion). The pgRNA-specific riboprobe was generated as described previously (29). A riboprobe for the detection of gylceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA was also generated by in vitro transcription of the GAPDH gene fragment.
Luciferase assay.
Cells were harvested 1 day posttransfection and assayed using the Dual-Luciferase assay system (Promega) according to the manufacturer's instructions. Firefly luciferase activities were normalized by Renilla luciferase activities.
Real time RT-PCR.
Total RNA was reverse transcribed into cDNA using oligo(dT) primers and Moloney murine leukemia virus (M-MuLV) reverse transcriptase (New England Biolabs). cDNA derived from 20 ng of total RNA was subjected to real-time reverse transcription-PCR (RT-PCR) using SYBR green Supermix on a CFX96 real-time PCR detection system (Bio-Rad). Two different sets of primers were used to amplify either pgRNA (3.5-kb transcript) or all four major HBV RNAs (3.5-, 2.4-, 2.1-, and 0.7-kb transcripts) (30). The rRNA (18S) was used as a reference gene.
HBV infection.
An HBV inoculum was prepared from the culture supernatant of HepAD38 cells as described previously (28). The viral DNA in the inoculum was quantitated by Southern blotting. The titer of the HBV inoculum was approximately 1 × 1010 genome equivalents (GEq) per ml. For HBV infection, HepG2-NTCP cells were seeded in 12-well plates coated with collagen type I (BD Biosciences) and infected with HBV at 103 GEq per cell in the medium containing 4% polyethylene glycol (PEG 8000; Sigma). One day after infection, cell were washed with phosphate-buffered saline (PBS) 3 times to remove residual viral particles and maintained in the same medium containing 2.5% dimethyl sulfoxide (DMSO), as described previously (35). The medium was refreshed every other day.
RESULTS
DDX3 inhibits both HBV replication and core protein expression.
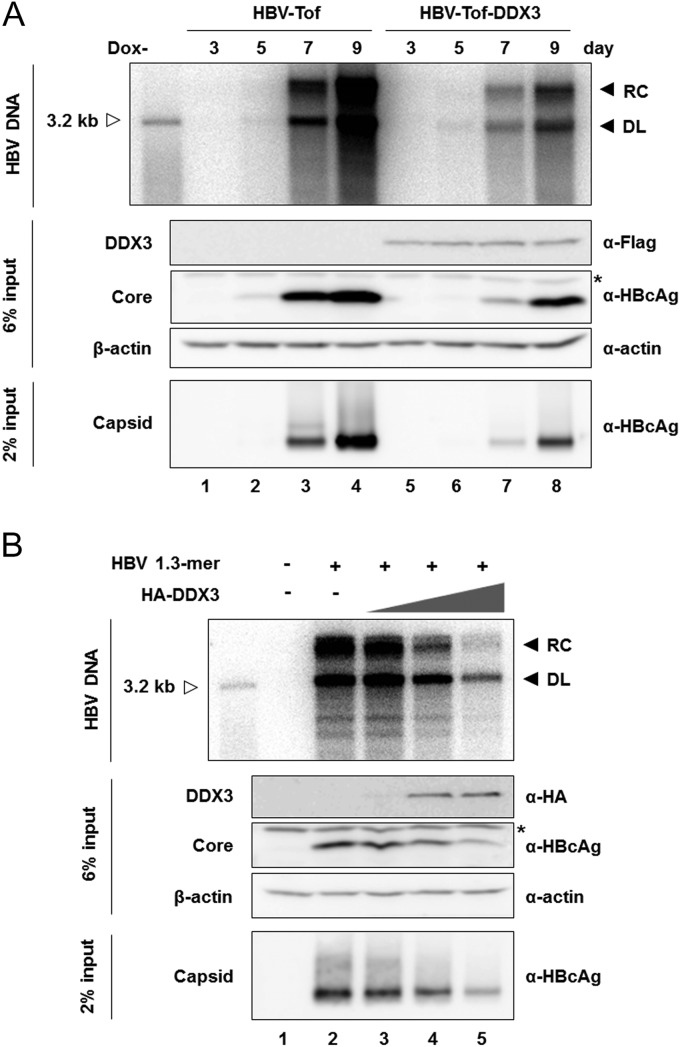
We previously reported that ectopic expression of DDX3 suppresses HBV replication in HepG2 cells (24). However, the underlying mechanism of inhibition has remained elusive. To this end, we first established a HepG2-derived stable cell line, designated HBV-Tof, in which HBV pgRNA is expressed under the control of a tetracycline-responsive minimal cytomegalovirus (CMV) promoter. Subsequently, HBV-Tof cells stably expressing DDX3, HBV-Tof-DDX3, were generated in parallel by transduction with lentiviral vectors expressing Flag-DDX3, as describe in Materials and Methods. Stable expression of DDX3 was confirmed by Western blotting (Fig. 1A, middle). Cells were cultured in doxycycline-free medium to induce HBV pgRNA transcription and harvested at the indicated time points for the detection of HBV replication intermediates within intracellular capsids by Southern blotting (Fig. 1A, top). Following induction, HBV replication intermediates became detectable at day 5 and markedly increased over time (Fig. 1A, top, lanes 1 to 4), in agreement with a previous report (26). In HBV-Tof-DDX3 cells, HBV replication intermediates became detectable at day 5 upon withdrawal of doxycycline. However, a significantly reduced amount of viral DNAs was detected in the HBV-Tof-DDX3 cells at days 7 and 9 (Fig. 1A, top; compare lanes 3 and 4 to 7 and 8), suggesting that DDX3 inhibits viral DNA synthesis. In contrast, the lack of reduction in viral DNAs in HBV-Tof-ctrl cells, in which the control lentiviral vector was stably transduced, corroborated that the reduction in viral DNAs in HBV-Tof-DDX3 cells is attributable to DDX3 (data not shown). In addition, core protein expression and core particle formation were determined by Western blotting and native agarose gel analysis followed by Western blotting, respectively. Stable expression of DDX3 reduced the levels of core particles and of core protein (Fig. 1A, bottom and middle). The observation that the extent of core protein reduction was comparable to that of viral DNA reduction implied that the inhibition of viral DNA replication by DDX3 might occur at the RNA level. A similar observation was made in a transient-transfection system (Fig. 1B), confirming the results found using the HBV-Tof cell line. HepG2 cells were transfected with the HBV 1.3-mer replicon containing an overlength HBV genome, which can initiate viral replication by an endogenous viral promoter, along with increasing amounts of HA-DDX3 expression plasmid. As shown in Fig. 1B, dose-related reduction in the viral replication intermediates was observed upon ectopic expression of DDX3. In parallel, the levels of core proteins and capsid particles were decreased to an extent similar to that for the viral DNAs. Similar results were obtained in Huh7 cells (data not shown). These data suggested that DDX3 inhibits HBV replication, a step prior to the synthesis of viral proteins and viral DNAs. The above findings led us to examine the level of HBV RNAs.
FIG 1.
DDX3 inhibits HBV replication and gene expression. (A) HBV-Tof and HBV-Tof-DDX3 cells were seed in 35-mm plates and maintained in the presence of doxycycline (1 μg/ml). At confluence, the culture medium was replaced by medium without doxycycline to induce viral RNA synthesis. Cells were harvested at the indicated time points, and intracellular core DNAs were isolated and analyzed by Southern blotting. The replication intermediates (relaxed-circular [RC] and duplex-linear [DL] DNAs) are indicated as leftward filled arrows. A 3.2-kb size marker was coelectrophoresed and is denoted by a rightward open arrow. An aliquot of cell lysate was subjected to Western blot analysis with anti-Flag antibody for the detection of DDX3 and with anti-HBcAg antibody for the detection of core protein. A nonspecific band reproducibly detectable by HBcAg antibody is denoted by an asterisk. β-Actin served as a loading control. HBV core particles were resolved by native agarose gel electrophoresis, as described in Materials and Methods. (B) HepG2 cells were transfected with the HBV 1.3-mer replicon construct (1 μg) along with increasing amounts (0.5 μg, 2 μg, and 6 μg) of HA-DDX3 construct. Cells were harvested 4 days posttransfection. The readouts were measured as shown in panel A.
DDX3 downregulates HBV RNA levels.
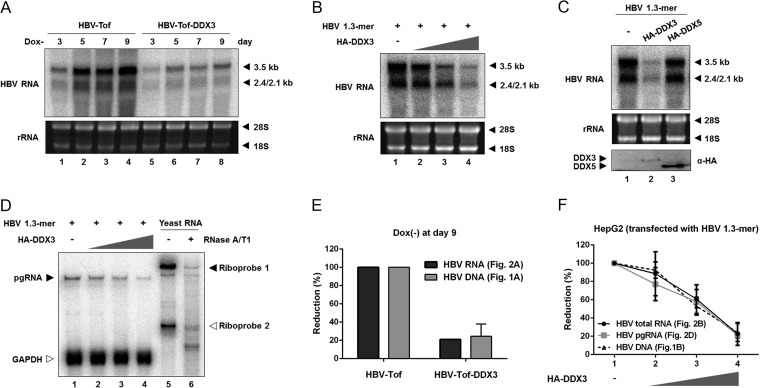
DDX3-mediated inhibition of both HBV replication and core protein synthesis implied that DDX3 has an antiviral function affecting the viral RNA. To test this hypothesis, HBV-Tof and HBV-Tof-DDX3 cells were cultured in the absence of doxycycline and harvested at the indicated time points, as described for Fig. 1A. Total RNA was extracted and analyzed by Northern blotting using an HBV-specific probe (Fig. 2A). In HBV-Tof cells, viral RNAs (i.e., 3.5-kb, 2.4-kb, and 2.1-kb transcripts) were detectable (Fig. 2A, lanes 1 to 4), consistent with a previous report (36). In contrast, stable expression of DDX3 reduced accumulation of viral RNAs (Fig. 2A, compare lanes 1 to 4 to lanes 5 to 8), suggesting that DDX3 negatively modulates viral RNA synthesis. Likewise, Northern blot analysis shown in Fig. 2B demonstrated that DDX3 diminished the amount of viral RNAs in a dose-related manner when HepG2 cells were transiently transfected with the HBV 1.3-mer replicon and increasing amounts of HA-DDX3 expression plasmid.
FIG 2.
Inhibition by DDX3 occurs at the HBV RNA level. (A) HBV-Tof and HBV-Tof-DDX3 cells were cultured for the indicated time periods without doxycycline. Viral RNAs were isolated and analyzed by Northern blotting. The position of 3.5-kb and 2.4/2.1-kb RNAs is denoted by leftward filled arrows. Ribosomal RNAs (28S and 18S rRNA) are included as a loading control. (B) HepG2 cells were transfected with a constant amount of HBV 1.3-mer replicon in combination with incremental amounts of the HA-DDX3 construct, as described in the legend to Fig. 1B. After 48 h, cells were harvested and the levels of viral RNAs were examined by Northern blotting. (C) HepG2 cells transfected with HBV 1.3-mer replicon (1 μg) along with either HA-DDX3 (6 μg) or HA-DDX5 (6 μg). Isolated RNAs were subjected to Northern blot analysis. DDX3 and DDX5 expression was measured by Western blotting using anti-HA antibody. (D) RNase protection assay (RPA). Cells were transfected as described for panel B. Total RNAs were extracted and analyzed by RPA to examine the pgRNA following treatment by ribonucleases (i.e., RNase A and T1). Riboprobes for the detection of HBV pgRNA (Riboprobe 1) and GAPDH mRNA (Riboprobe 2) are denoted by filled and open arrows, respectively. Yeast RNA served as a negative control. (E) Quantitation of viral RNAs and viral DNAs. Relative amount of viral RNAs (see panel A) and viral DNAs (see Fig. 1A) extracted from both HBV-Tof cells and HBV-Tof-DDX3 cells at day 9 upon withdrawal of doxycycline were quantified. (F) Quantitation of the viral RNAs and viral DNAs. Relative amounts of viral RNAs detected by Northern blotting (see panel B), pgRNA detected by RPA (see panel D), and viral DNAs detected by Southern blotting (see Fig. 1B) were quantified.
In addition to DDX3, DDX5, another member of the DEAD box RNA helicase family, is strongly implicated in transcriptional regulation (37). To determine if the aforementioned inhibitory effect was specific to DDX3, viral RNA synthesis from the HBV 1.3-mer replicon was examined following the ectopic expression of either HA-DDX3 or HA-DDX5. Northern blot analysis showed that overexpression of DDX5 did not alter HBV RNA levels (Fig. 2C, top), even though DDX5 expression was significantly higher than DDX3 expression (Fig. 2C, bottom), indicating that the HBV antiviral activity is DDX3 specific.
In addition, an RNase protection assay (RPA) performed in parallel showed that DDX3 exhibited a similar inhibitory effect on HBV pgRNA accumulation (Fig. 2D). As shown in Fig. 2D, the protected fragment of pgRNA was decreased whereas the protected fragment of GAPDH mRNA remained unaltered in the presence of incremental amounts of DDX3. These data reinforce the finding that DDX3 is involved in the reduction of HBV transcripts.
Next, we looked at whether DDX3-mediated reduction of HBV RNA is sufficient for the inhibition of HBV replication, so the extent of viral DNA and RNA reduction in the presence of DDX3 was quantified (Fig. 2E and F). The reduction in viral DNA levels in HBV-Tof-DDX3 cells was similar to that in viral RNA levels compared to results for parental HBV-Tof cells (Fig. 2E). Likewise, the reduction in viral DNAs by ectopically expressed DDX3 was comparable to that in viral RNAs (Fig. 2F). This quantitative analysis led us to conclude that the reduction in the RNA levels fully accounts for the reduction seen in the DNA levels.
DDX3-mediated reduction of HBV RNA is dependent of ATPase activity.
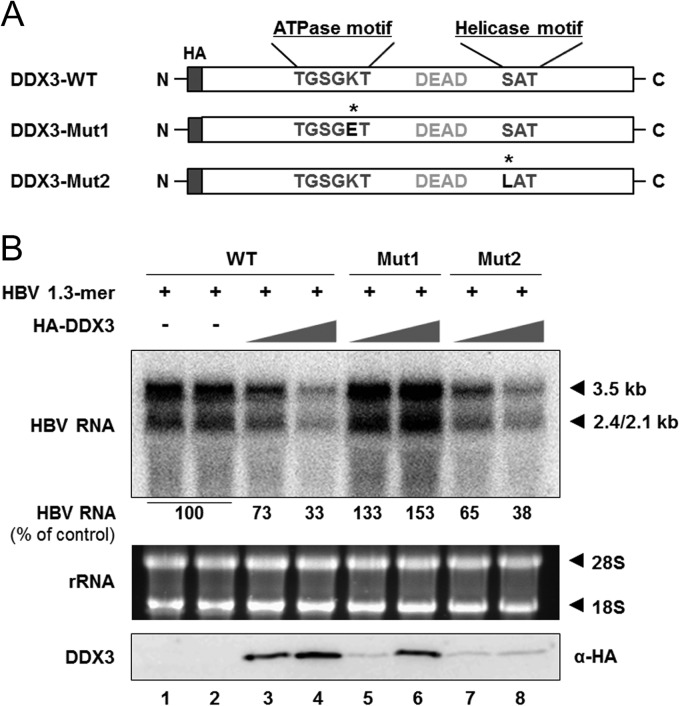
The above findings suggested that the reduction in viral DNA is primarily attributable to the downregulation of viral RNA (Fig. 2E and F). We have previously shown that DDX3 ATPase activity is required for the inhibition of viral DNA synthesis (24). Therefore, ATPase activity could also be required for HBV RNA synthesis. To address this issue, DDX3 mutants harboring a point mutation in either the conserved ATPase motif or the helicase motif were utilized (21, 24). The K230E mutation in the ATPase motif of DDX3-Mut1 abrogates not only ATPase activity but also helicase activity, while the S382L mutation in the helicase motif of DDX3-Mut2 abrogates helicase activity (Fig. 3A). The HBV 1.3-mer replicon was transfected into HepG2 cells with increasing amounts of DDX3 construct. As expected, Northern blot analysis showed that overexpression of DDX3-Mut1 did not reduce viral RNA levels but rather resulted in increased viral RNA levels (Fig. 3B, compare lanes 1 and 2 to lanes 5 and 6), similar to the result we obtained from previous Southern blot analysis (24). The upregulation of HBV RNA implies that DDX3-Mut1 may exhibit a dominant negative effect on endogenous DDX3. On the other hand, overexpression of DDX3-Mut2 showed inhibitory activity for HBV RNA levels comparable to that of the wild type (WT) (Fig. 3B, compare lane 3 and 4 to 7 and 8), even though the expression of DDX3-Mut2 was markedly lower (Fig. 3B, bottom). This result indicates that the helicase activity is dispensable for DDX3-mediated viral RNA reduction. Overall, we conclude that DDX3 reduces the steady-state level of HBV RNAs in an ATPase activity-dependent manner.
FIG 3.
ATPase activity is required for inhibition of HBV RNA synthesis by DDX3. (A) A schematic representation of WT DDX3 (DDX3-WT) and its mutants (Mut1 and Mut2). Amino acids both in the ATPase motif and the helicase motif are denoted, along with the DEAD box. Asterisks indicate the replaced amino acid in the ATPase motif of DDX3-Mut1 (Lys to Glu) and in the helicase motif of DDX3-Mut2 (Ser to Leu). All constructs have a HA tag at the N terminus. (B) HepG2 cells were cotransfected with an HBV 1.3-mer replicon (1 μg) and increasing amounts (2 μg or 6 μg) of DDX3-WT or the DDX3 mutants in duplicate. At 48 h posttransfection, cells were harvested and viral RNAs were analyzed by Northern blotting. The average intensity of viral RNA bands obtained from cells transfected with the HBV 1.3-mer replicon (lanes 1 and 2) was set to 100%. Ribosomal RNAs were detected to confirm that equivalent amounts of RNAs were loaded. DDX3 expression was measured by Western blotting using anti-HA antibody.
DDX3 downregulates HBV RNA via transcriptional regulation.
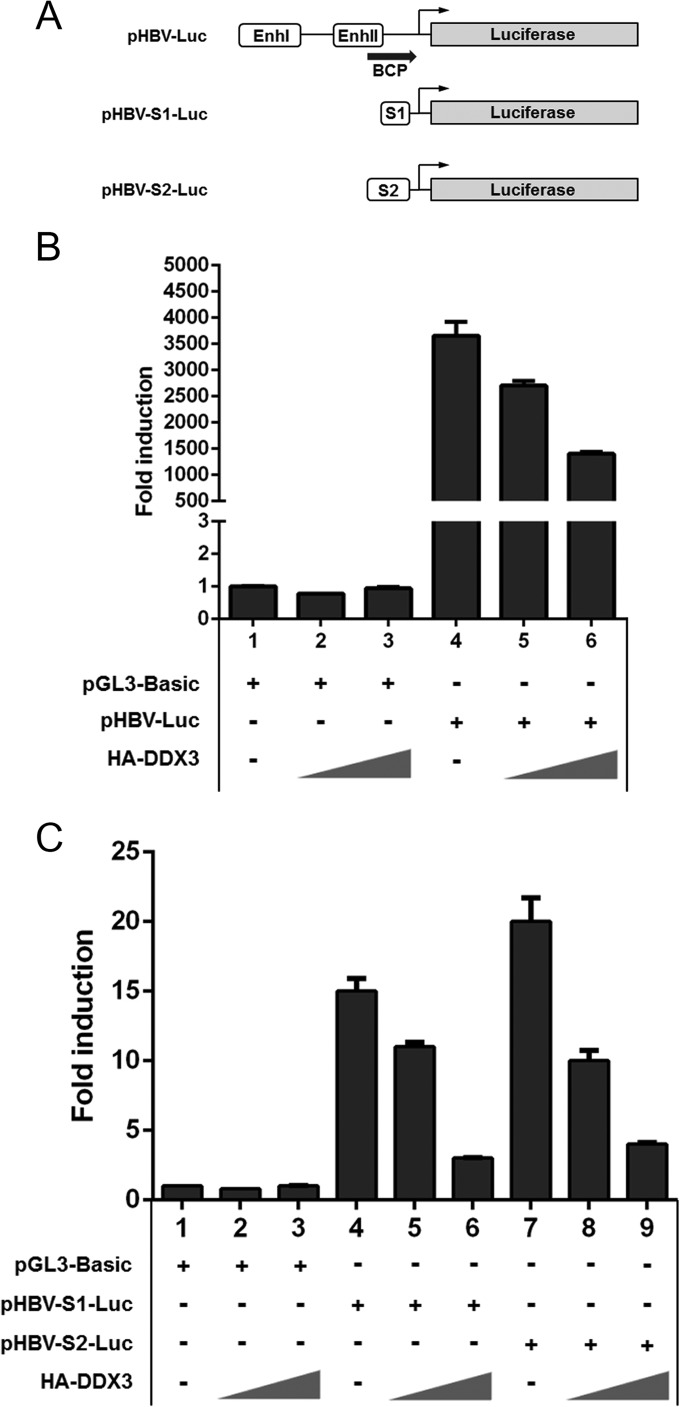
DDX3 has been implicated in the transcriptional regulation of several promoters (14–16). Thus, it is possible that DDX3 inhibits viral RNA synthesis by suppressing transcription. To address this issue, an HBV promoter-driven reporter assay was performed as previously described (25). Each HBV reporter construct was transfected into HepG2 cells with an increasing amount of the HA-DDX3 construct, and then luciferase activities were measured (Fig. 4A). Results revealed that overexpression of DDX3 inhibited core promoter activity in a dose-dependent manner (Fig. 4B). Similarly, both S1 and S2 promoter activities were inhibited by DDX3 expression (Fig. 4C). In contrast, the inhibition was not observed when luciferase activities from cells transfected with either pRL-TK or pRL-CMV, where Renilla luciferase expression is driven from either the HSV-1 TK promoter or the CMV promoter, respectively, were measured (data not shown). Because transcriptional repression is a nuclear event, we examined the subcellular localization of DDX3. Consistent with previous reports (21, 38), cell fractionation experiments showed that DDX3 is somewhat equally distributed both in the nucleus and in the cytosol (data not shown).
FIG 4.
DDX3 inhibits HBV promoter activities. (A) A schematic representation showing promoter regions and enhancers of HBV reporter constructs. pHBV-Luc contains HBV enhancer I (EnhI), enhancer II (EnhII), and basal core promoter (BCP) regions. pHBV-S1-Luc and pHBV-S2-Luc constructs contain the S1 promoter region and S2 promoter region, respectively. Transcription start sites are denoted by black arrows. (B and C) HepG2 cells were transfected with the pGL3-Basic construct (1 μg) or each HBV reporter construct (1 μg), together with increasing amounts (0.5 μg or 1 μg) of the DDX3 expression plasmid. For each transfection, the pRL-TK plasmid (50 ng) was cotransfected to normalize the transfection efficiency. Luciferase activity was measured at 30 h posttransfection. Representative data from three independent experiments performed in duplicate are shown.
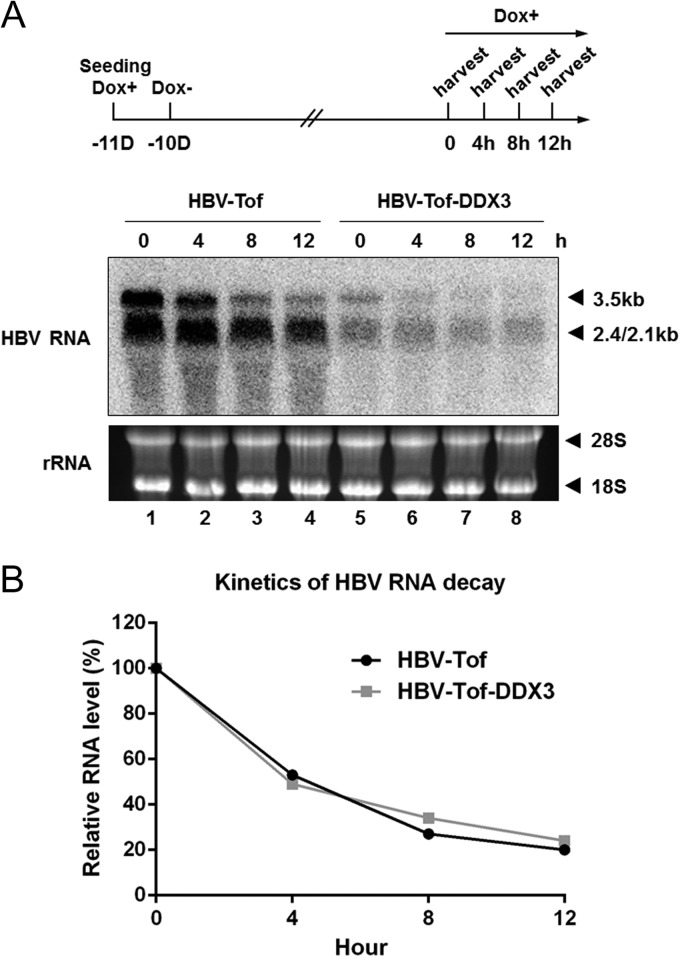
To rule out the possibility that the reduction in viral RNAs can be partially attributed to accelerated decay of viral RNAs caused by DDX3, we examined whether DDX3 promotes HBV RNA decay, as previously described (39, 40). Our data showed that regardless of DDX3 expression, the half-life of pgRNA was determined to be approximately 5 h (Fig. 5A and B), indicating that DDX3 did not alter decay kinetics of HBV RNAs. Taken together, we concluded that DDX3 suppresses HBV RNA expression through transcriptional control.
FIG 5.
DDX3 does not affect viral RNA decay. (A) The experimental scheme is shown on the top. HBV-Tof cells were cultured in a medium containing doxycycline (1 μg/ml) (Dox+) for 24 h, and then cells were maintained in doxycycline-free medium (Dox−) for 10 days. Cells were harvested immediately after adding doxycycline back to the culture medium (0) and 4, 8, and 12 h thereafter. Total RNA was isolated and analyzed by Northern blotting. (B) The relative RNA levels at each time point were plotted to estimate the HBV RNA decay kinetics.
The effect of endogenous DDX3 on HBV RNA synthesis.
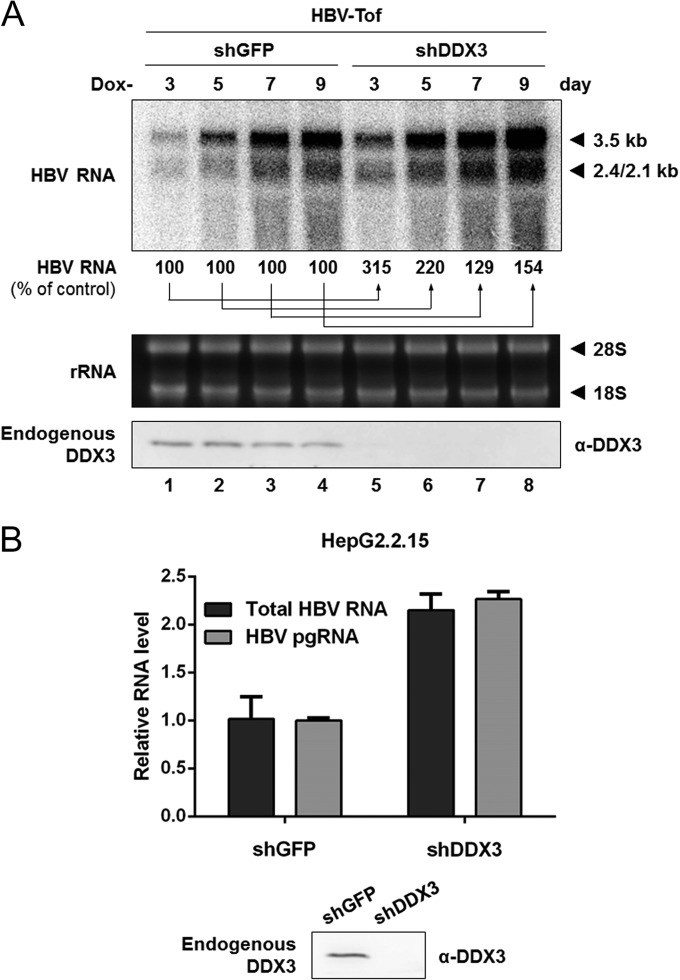
Our data have demonstrated DDX3-mediated inhibition of viral transcription only by ectopically expressed DDX3, so it remained to be elucidated whether endogenous DDX3 exerts a similar antiviral phenotype. We attempted to address this question by silencing endogenous DDX3. We predicted that viral RNAs would increase under conditions where endogenous DDX3 was reduced. As shown in Fig. 6A, cells expressing DDX3-targeting shRNA (shDDX3) showed an increase in HBV RNAs (Fig. 6A, compare lanes 1 to 4 to lanes 5 to 8) compared to results for control cells expressing GFP-targeting shRNA (shGFP). Efficient DDX3 knockdown (>90%) was achieved in HBV-Tof cells, as shown by Western blotting (Fig. 6A, bottom).
FIG 6.
DDX3 knockdown leads to increased HBV RNA levels. (A) The effect of DDX3 knockdown in HBV-Tof cells. HBV-Tof cells stably expressing shDDX3 or shGFP were cultured in doxycycline-free medium and harvested for total RNA extraction at the indicated time points. HBV transcripts were analyzed by Northern blotting, with ribosomal RNAs serving as the loading control. The intensity of viral RNAs from HBV-Tof cells (lanes 1, 2, 3, and 4) was set to 100%. Endogenous DDX3 expression was evaluated by Western blotting using anti-DDX3 antibody. (B) The effect of DDX3 knockdown in HepG2.2.15 cells. Total RNAs were extracted from HepG2.2.15 cells stably expressing shDDX3 or shGFP and analyzed by real-time RT-PCR. Western blot analysis was performed to confirm efficient DDX3 knockdown in HepG2.2.15 cells.
To substantiate the above finding with HBV-producing cells, HepG2.2.15 cells, a well-characterized cell line that stably produces HBV from an integrated HBV genome (41), were transduced with lentiviral vectors encoding shDDX3 or shGFP, as described above. Viral RNA synthesis was measured by real-time RT-PCR analysis using two different primer sets: (i) a core region primer set for the detection of pgRNA (a 3.5-kb transcript) and (ii) an X region primer set for the detection of all four HBV RNAs (30). As shown in Fig. 6B, a 2-fold increase in viral transcripts was observed in HepG2.2.15 cells stably expressing shDDX3 compared to results for cells expressing the shGFP control. Similar results were obtained when the extracted viral RNAs from HepG2.2.15 cells expressing either shDDX3 or shGFP were analyzed by Northern blotting (data not shown). We conclude that endogenous DDX3, as well as ectopically expressed DDX3, represses HBV transcription.
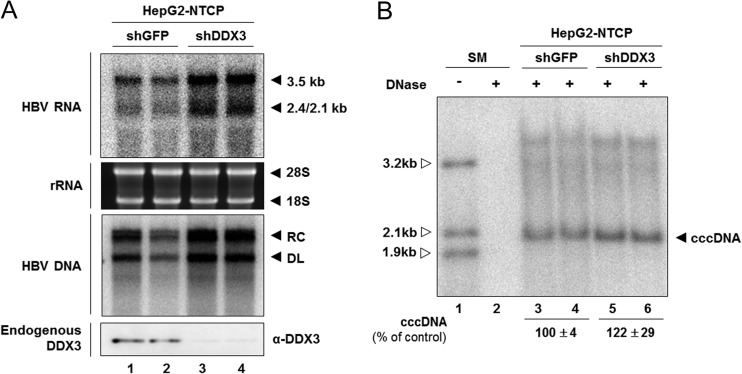
The above findings indicate that endogenous DDX3 inhibits HBV transcription. However, in our system, we could not determine whether DDX3 is recruited to cccDNA to negatively regulate viral promoter activities because most viral transcripts are derived from transfected plasmids in a transient-transfection system or the integrated viral transgene in HBV-Tof cell lines. Therefore, we confirmed the antiviral role of DDX3 in HBV infection by using HepG2-NTCP cells that are susceptible to HBV infection (30, 35). Prior to HBV infection, HepG2-NTCP cells were transduced with lentivirus containing shDDX3. At day 9 postinfection, cells were harvested for the detection of viral transcripts and viral replication intermediates (Fig. 7A). As expected, both viral transcripts and viral replication intermediates were increased when endogenous DDX3 was depleted (Fig. 7A, compare lanes 1 and 2 to lanes 3 and 4). Next, we sought to examine the level of cccDNA by using a modified Hirt extraction method (Fig. 7B) (26, 42). Because Hirt extraction involves direct phenol extraction of DNA from cell lysates without protease digestion, the HBV DNA species in Hirt preparations should be free of the viral DNA covalently linked to HBV Pol. Southern blot analysis of Hirt extracts revealed viral DNA species that comigrated with the 2.1-kb size marker. This DNA species was interpreted as cccDNA because (i) it was resistant to linear DNA-specific DNase treatment (Fig. 7B, lanes 3 to 6), (ii) the size was very similar to that of previously reported cccDNA (11, 26, 30, 42), and (iii) it was converted to a 3.2-kb-unit-length double-stranded DNA following EcoRI digestion (data not shown) (26, 42). Intriguingly, the cccDNA level appeared slightly increased upon silencing of endogenous DDX3, although the increase was statistically insignificant (P = 0.06) (Fig. 7B, compare lanes 3 and 4 to lanes 5 and 6). The increase in the cccDNA level, albeit the magnitude of increase was only modest, is consistent with a notion that intracellular amplification is executed to replenish cccDNA pools in HepG2-NTCP cells. The observation that DDX3 represses the transcription of HBV cccDNA, the genuine template for viral RNA transcription, further validated our conclusion.
FIG 7.
The effect of DDX3 knockdown in a HBV-susceptible HepG2-NTCP cell line. (A) Viral RNAs and viral DNAs. HepG2-NTCP cells were transduced with either shDDX3- or shGFP-expressing lentiviral vectors. Cells were infected with HBV in duplicate and then cultured for an additional 9 days. Intracellular viral RNAs and DNAs were analyzed by Northern blotting (top) and Southern blotting (middle), respectively. Silencing of DDX3 in HepG2-NTCP cells is shown by Western blotting (bottom). (B) cccDNA. HepG2-NTCP cells were similarly transduced with lentiviral vectors, infected with HBV, and maintained as shown in panel A. Viral DNAs were extracted using a modified Hirt method and treated with Plasmid-Safe ATP-dependent DNase prior to gel electrophoresis, as described in Materials and Methods. The extracted DNAs were subjected to Southern blot analysis. The cccDNA is denoted by leftward filled arrows. Results are shown as means ± SD from three independent experiments.
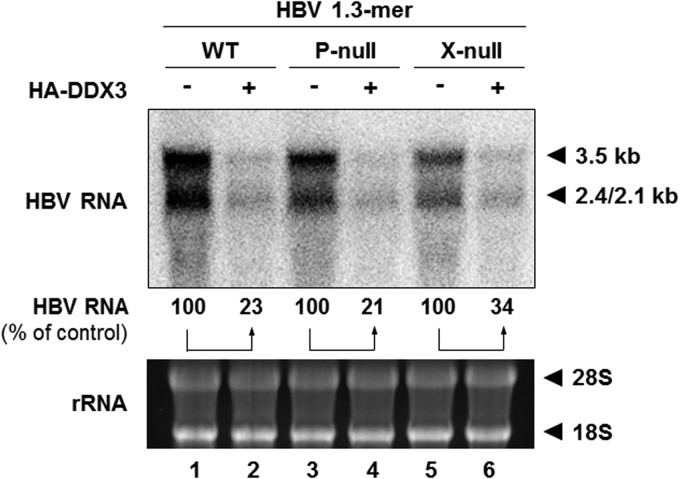
HBV Pol and HBx are not involved in DDX3-mediated reduction of HBV RNA.
Because DDX3 has been identified as an HBV Pol-interacting protein (24, 43), it is plausible that its regulatory function is exerted via interaction with HBV Pol. In addition, HBx acts as a transcriptional transactivator and is recruited to the HBV cccDNA (44, 45). Thus, it is possible that HBx could contribute to DDX3-mediated HBV transcriptional suppression. We tested this hypothesis by using different HBV 1.3-mer replicons in which expression of each viral gene was abrogated: P-null and X-null. HepG2 cells were transfected with each HBV 1.3-mer replicon, along with either the control plasmid or the DDX3 expression plasmid. Two days following transfection, viral RNAs were extracted and analyzed by Northern blotting (Fig. 8). In contrast to our speculation, overexpression of DDX3 downregulated HBV RNA levels in both the 1.3-mer P-null and 1.3-mer X-null replicon-transfected cells, to a degree similar to that found in HBV 1.3-mer WT replicon-transfected cells. Thus, we conclude that neither HBV Pol nor HBx is involved in DDX3-mediated transcription inhibition.
FIG 8.
The inhibition of viral RNA synthesis by DDX3 is independent of HBV Pol or HBx. HepG2 cells were transfected with 1 μg of each indicated HBV 1.3-mer replicon (WT, P-null, or X-null), together with 6 μg of HA-DDX3 construct for 48 h. Levels of viral RNAs were determined by Northern blotting. Ribosomal RNAs are shown as a loading control. The amount of viral RNAs from each replicon-transfected sample (lanes 1, 3, and 5) was set to 100%.
DISCUSSION
We have previously shown that ectopic expression of DDX3 inhibits HBV genome replication (24). Here, we investigated the mechanism of inhibition and demonstrated that DDX3 inhibits viral replication at the level of viral transcription. In contrast, HIV and HCV co-opt DDX3, where it enhances viral replication at viral RNA export and/or translation and viral RNA replication, respectively (20–23). The regulatory role of DDX3 in transcription is not totally unexpected, because DDX3 has been shown to regulate transcription as well as RNA metabolism. For example, studies have shown that DDX3 upregulates the p21WAF promoter by interacting with transcription factor Sp1 (14), while it downregulates the E-cadherin promoter (15).
Our conclusion is supported by three independent experiments. First, by using tetracycline-inducible HBV-producing cell lines, we showed that cells stably expressing DDX3 significantly decreased viral RNA levels (Fig. 2A), whereas cells stably expressing shDDX3 increased viral RNA levels (Fig. 6A). Second, ectopic expression of DDX3 also reduced not only viral DNAs but also viral RNAs (Fig. 1B and 2B). Third, by using HBV-susceptible cells, we corroborated the above findings that the inhibitory effect of DDX3 occurs at the level of viral RNA (Fig. 7A).
The results described above were carried out either by transient transfection of HBV replicon constructs or by using an inducible HBV-producing cell line, where the templates for viral transcription were transfected plasmid DNAs or chromosomally integrated copies of viral DNAs, respectively. Because these templates are surrogates for episomal cccDNA, the natural template for viral transcription, we studied the effects of DDX3 on viral transcription of cccDNA (Fig. 7A and B). These results corroborated the initial conclusions obtained by using surrogate templates. Nonetheless, how DDX3 inhibits viral transcription remains elusive, and whether DDX3 inhibits HBV transcription through binding to cccDNA merits further investigation.
A question arises as to how DDX3 suppresses transcription from an HBV promoter. It was noted that transcription from HBV gene promoters (i.e., core, S1, and S2 promoters) as well as an HBV-Tof promoter (i.e., CMV promoter) was reduced by DDX3 (Fig. 2 and 4). In contrast, transcription of pCMV-Renilla and pTK-Renilla was unaffected by DDX3 (data not shown). We speculated that a common transcription factor that regulates both the HBV-Tof promoter and HBV gene promoters but not a CMV or TK promoter is targeted by DDX3-mediated transcription suppression. Alternatively, a transcription factor that binds to HBV enhancers could be regulated by DDX3 because the HBV enhancers linked to both HBV-Tof promoter and HBV gene promoters.
Regarding the step at which DDX3 acts, we have previously reported the inhibition occurring at the posttranscription level, most likely following pgRNA encapsidation (24). In contrast, our data documented here pointed out the inhibition occurring at the transcription level. The discrepancy can be partly ascribed to the relatively smaller amount of DDX3 plasmids used in our earlier work. More important, our data eliminate the possibility that DDX3 inhibits viral genome replication following incorporation into nucleocapsids, since the extent of inhibition by DDX3 was fully accounted for by the inhibition of viral RNA synthesis (Fig. 2E and F). In line with this argument, the impact of DDX3 on in vitro protein priming by the HBV Pol, as recently established by Jones et al. (43), was not evident, since the in vitro protein priming was unaffected by DDX3 overexpression (data not shown), excluding the possibility that DDX3 affects viral genome replication. In addition, it should be noted that the inhibitory effect of DDX3 on HBV transcription is mediated via a mechanism not involving HBV Pol interaction, because inhibition was observed even in the absence of HBV Pol expression (Fig. 6). It remains uncertain whether DDX3 incorporated into the nucleocapsid has a role, if any, in the HBV life cycle. It could be that the incorporation of DDX3 into the nucleocapsid is simply a consequence of the HBV Pol-DDX3 interaction in the context of innate immunity (24).
Given that DDX3 contains ATPase-dependent RNA helicase activity, we determined whether the helicase activity was also required for its transcriptional regulation; we showed that the effect of DDX3 on viral transcription is independent of its helicase activity but dependent on its ATPase activity (Fig. 3B). Our result is consistent with the previous finding that DDX3 exerts its stimulatory function on the p21WAF promoter in an ATPase-dependent but helicase-independent manner (14), suggesting that RNA helicase activity is dispensable for transcriptional regulation.
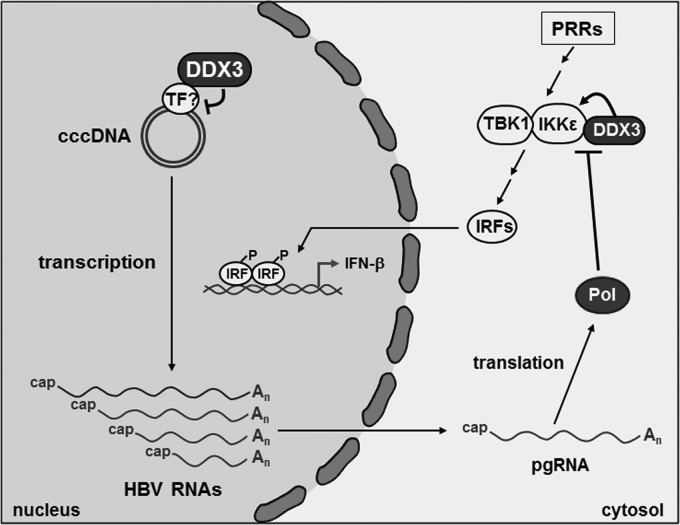
In conclusion, our work clearly demonstrated that DDX3 restricts HBV infection by suppressing transcription of the viral episome. In addition to the transcriptional regulation, DDX3 was shown to promote interferon induction by the activation of interferon regulatory factor (IRF) signaling via interaction with TBK1/IKKε (16, 17). Common attributes of host restriction factors are that their actions are counteracted by viral proteins, and they are often interferon inducible (4). In line with this notion, we and others showed that DDX3-promoted IRF signaling is counteracted by HBV Pol (46, 47). On the other hand, DDX3 was not induced by interferon treatment in HepG2 cells (data not shown). Overall, our work showed that DDX3 represents a host factor that restricts HBV infection via two distinct mechanisms: by suppressing viral transcription of cccDNA template and by promoting IRF signaling (Fig. 9).
FIG 9.
A schematic model illustrating the antiviral action of DDX3 for the HBV infection. In the nucleus, DDX3 suppresses the viral transcription of cccDNA, perhaps via interaction with a transcription factor. In the cytoplasm, upon the activation of PRRs (i.e., Toll-like receptors or RIG-I), TBK1/IKKε become activated and phosphorylate IRFs. Then, the phosphorylated IRFs translocate to the nucleus and bind to the interferon promoter, thereby inducing expression of interferon-stimulated genes. DDX3 restricts viral infection by promoting IRF signaling via interaction with TBK1/IKKε. DDX3-mediated restriction is counteracted by HBV Pol. PRRs, pattern recognition receptors; TF, transcription factor.
ACKNOWLEDGMENTS
This work was supported in part by a National Research Foundation of Korea (NRF) grant, funded by the South Korean government (MSIP), no. 2012-007811. C.K. was supported by training grant NRF-2014: Fostering Core Leaders of the Future Basic Science Program.
Footnotes
Published ahead of print 17 September 2014
REFERENCES
- 1. El-Serag HB. 2012. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142:1264–1273 e1. 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. 2009. Control of cccDNA function in hepatitis B virus infection. J. Hepatol. 51:581–592. 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 3. Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853. 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 4. Harris RS, Hultquist JF, Evans DT. 2012. The restriction factors of human immunodeficiency virus. J. Biol. Chem. 287:40875–40883. 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turelli P, Mangeat B, Jost S, Vianin S, Trono D. 2004. Inhibition of hepatitis B virus replication by APOBEC3G. Science 303:1829. 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- 6. Baumert TF, Rosler C, Malim MH, von Weizsacker F. 2007. Hepatitis B virus DNA is subject to extensive editing by the human deaminase APOBEC3C. Hepatology 46:682–689. 10.1002/hep.21733. [DOI] [PubMed] [Google Scholar]
- 7. Rosler C, Kock J, Kann M, Malim MH, Blum HE, Baumert TF, von Weizsacker F. 2005. APOBEC-mediated interference with hepadnavirus production. Hepatology 42:301–309. 10.1002/hep.20801. [DOI] [PubMed] [Google Scholar]
- 8. Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S, Vartanian JP, Greeve J. 2006. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology 43:1364–1374. 10.1002/hep.21187. [DOI] [PubMed] [Google Scholar]
- 9. Suspene R, Guetard D, Henry M, Sommer P, Wain-Hobson S, Vartanian JP. 2005. Extensive editing of both hepatitis B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:8321–8326. 10.1073/pnas.0408223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen DH, Gummuluru S, Hu J. 2007. Deamination-independent inhibition of hepatitis B virus reverse transcription by APOBEC3G. J. Virol. 81:4465–4472. 10.1128/JVI.02510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, Remouchamps C, Chou WM, Thasler WE, Huser N, Durantel D, Liang TJ, Munk C, Heim MH, Browning JL, Dejardin E, Dandri M, Schindler M, Heikenwalder M, Protzer U. 2014. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 343:1221–1228. 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rocak S, Linder P. 2004. DEAD-box proteins: the driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 5:232–241. 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- 13. Schroder M. 2010. Human DEAD-box protein 3 has multiple functions in gene regulation and cell cycle control and is a prime target for viral manipulation. Biochem. Pharmacol. 79:297–306. 10.1016/j.bcp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 14. Chao CH, Chen CM, Cheng PL, Shih JW, Tsou AP, Lee YH. 2006. DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res. 66:6579–6588. 10.1158/0008-5472.CAN-05-2415. [DOI] [PubMed] [Google Scholar]
- 15. Botlagunta M, Vesuna F, Mironchik Y, Raman A, Lisok A, Winnard P, Jr, Mukadam S, Van Diest P, Chen JH, Farabaugh P, Patel AH, Raman V. 2008. Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 27:3912–3922. 10.1038/onc.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soulat D, Burckstummer T, Westermayer S, Goncalves A, Bauch A, Stefanovic A, Hantschel O, Bennett KL, Decker T, Superti-Furga G. 2008. The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J. 27:2135–2146. 10.1038/emboj.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schroder M, Baran M, Bowie AG. 2008. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 27:2147–2157. 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gu L, Fullam A, Brennan R, Schroder M. 2013. Human DEAD box helicase 3 couples IkappaB kinase epsilon to interferon regulatory factor 3 activation. Mol. Cell. Biol. 33:2004–2015. 10.1128/MCB.01603-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Ge LL, Li PP, Wang Y, Dai JJ, Sun MX, Huang L, Shen ZQ, Hu XC, Ishag H, Mao X. 2014. Cellular DDX3 regulates Japanese encephalitis virus replication by interacting with viral un-translated regions. Virology 449:70–81. 10.1016/j.virol.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ariumi Y, Kuroki M, Abe K, Dansako H, Ikeda M, Wakita T, Kato N. 2007. DDX3 DEAD-box RNA helicase is required for hepatitis C virus RNA replication. J. Virol. 81:13922–13926. 10.1128/JVI.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. 2004. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119:381–392. 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 22. Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. 2007. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. U. S. A. 104:12884–12889. 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soto-Rifo R, Rubilar PS, Ohlmann T. 2013. The DEAD-box helicase DDX3 substitutes for the cap-binding protein eIF4E to promote compartmentalized translation initiation of the HIV-1 genomic RNA. Nucleic Acids Res. 41:6286–6299. 10.1093/nar/gkt306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Kim S, Ryu WS. 2009. DDX3 DEAD-box RNA helicase inhibits hepatitis B virus reverse transcription by incorporation into nucleocapsids. J. Virol. 83:5815–5824. 10.1128/JVI.00011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cha MY, Ryu DK, Jung HS, Chang HE, Ryu WS. 2009. Stimulation of hepatitis B virus genome replication by HBx is linked to both nuclear and cytoplasmic HBx expression. J. Gen. Virol. 90:978–986. 10.1099/vir.0.009928-0. [DOI] [PubMed] [Google Scholar]
- 26. Guo H, Jiang D, Zhou T, Cuconati A, Block TM, Guo JT. 2007. Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J. Virol. 81:12472–12484. 10.1128/JVI.01123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nkongolo S, Ni Y, Lempp FA, Kaufman C, Lindner T, Esser-Nobis K, Lohmann V, Mier W, Mehrle S, Urban S. 2014. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J. Hepatol. 60:723–731. 10.1016/j.jhep.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 28. Watashi K, Liang G, Iwamoto M, Marusawa H, Uchida N, Daito T, Kitamura K, Muramatsu M, Ohashi H, Kiyohara T, Suzuki R, Li J, Tong S, Tanaka Y, Murata K, Aizaki H, Wakita T. 2013. Interleukin-1 and tumor necrosis factor-alpha trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID). J. Biol. Chem. 288:31715–31727. 10.1074/jbc.M113.501122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ko C, Shin YC, Park WJ, Kim S, Kim J, Ryu WS. 2014. Residues Arg703, Asp777, and Arg781 of the RNase H domain of hepatitis B virus polymerase are critical for viral DNA synthesis. J. Virol. 88:154–163. 10.1128/JVI.01916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1:e00049. 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai D, Nie H, Yan R, Guo JT, Block TM, Guo H. 2013. A southern blot assay for detection of hepatitis B virus covalently closed circular DNA from cell cultures. Methods Mol. Biol. 1030:151–161. 10.1007/978-1-62703-484-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryu DK, Kim S, Ryu WS. 2008. Hepatitis B virus polymerase suppresses translation of pregenomic RNA via a mechanism involving its interaction with 5′ stem-loop structure. Virology 373:112–123. 10.1016/j.virol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 33. Chomczynski P, Sacchi N. 2006. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat. Protoc. 1:581–585. 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 34. Guo H, Jiang D, Ma D, Chang J, Dougherty AM, Cuconati A, Block TM, Guo JT. 2009. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J. Virol. 83:847–858. 10.1128/JVI.02008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, Stindt J, Koniger C, Nassal M, Kubitz R, Sultmann H, Urban S. 2014. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146:1070–1083. 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 36. Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM, Cuconati A, Guo H. 2012. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob. Agents Chemother. 56:4277–4288. 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fuller-Pace FV. 2013. The DEAD box proteins DDX5 (p68) and DDX17 (p72): multi-tasking transcriptional regulators. Biochim. Biophys. Acta 1829:756–763. 10.1016/j.bbagrm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 38. Owsianka AM, Patel AH. 1999. Hepatitis C virus core protein interacts with a human DEAD box protein DDX3. Virology 257:330–340. 10.1006/viro.1999.9659. [DOI] [PubMed] [Google Scholar]
- 39. Li J, Lin S, Chen Q, Peng L, Zhai J, Liu Y, Yuan Z. 2010. Inhibition of hepatitis B virus replication by MyD88 involves accelerated degradation of pregenomic RNA and nuclear retention of pre-S/S RNAs. J. Virol. 84:6387–6399. 10.1128/JVI.00236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mao R, Nie H, Cai D, Zhang J, Liu H, Yan R, Cuconati A, Block TM, Guo JT, Guo H. 2013. Inhibition of hepatitis B virus replication by the host zinc finger antiviral protein. PLoS Pathog. 9:e1003494. 10.1371/journal.ppat.1003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sells MA, Chen ML, Acs G. 1987. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. U. S. A. 84:1005–1009. 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gao W, Hu J. 2007. Formation of hepatitis B virus covalently closed circular DNA: removal of genome-linked protein. J. Virol. 81:6164–6174. 10.1128/JVI.02721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones SA, Boregowda R, Spratt TE, Hu J. 2012. In vitro epsilon RNA-dependent protein priming activity of human hepatitis B virus polymerase. J. Virol. 86:5134–5150. 10.1128/JVI.07137-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bouchard MJ, Schneider RJ. 2004. The enigmatic X gene of hepatitis B virus. J. Virol. 78:12725–12734. 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Belloni L, Pollicino T, De Nicola F, Guerrieri F, Raffa G, Fanciulli M, Raimondo G, Levrero M. 2009. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc. Natl. Acad. Sci. U. S. A. 106:19975–19979. 10.1073/pnas.0908365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang H, Ryu WS. 2010. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog. 6:e1000986. 10.1371/journal.ppat.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu S, Chen J, Wu M, Chen H, Kato N, Yuan Z. 2010. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKK and DDX3. J. Gen. Virol. 91(8):2080–2090. 10.1099/vir.0.020552-0. [DOI] [PubMed] [Google Scholar]