Abstract
Extending our previous analyses to the most recently described monoclonal broadly neutralizing antibodies (bNAbs), we confirmed a drift of HIV-1 clade B variants over 2 decades toward higher resistance to bNAbs targeting almost all the identified gp120-neutralizing epitopes. In contrast, the sensitivity to bNAbs targeting the gp41 membrane-proximal external region remained stable, suggesting a selective pressure on gp120 preferentially. Despite this evolution, selected combinations of bNAbs remain capable of neutralizing efficiently most of the circulating variants.
TEXT
Despite the ability of HIV-1 to evade the antibody response, some HIV-1-infected individuals develop high titers of broadly neutralizing antibodies (bNAbs) (1–11). Since 2009, thanks to the development of single-cell-based antibody cloning techniques, a large number of monoclonal bNAbs with outstanding potencies have been isolated from such individuals (12–21). Most of them target a few major sites of vulnerability on HIV-1 Env. Three of these sites are located within the exterior glycoprotein gp120: the CD4-binding site (13, 18, 21–23) and two glycan-dependent epitopes involving the V1/V2 and the V3 loops (16, 19, 20, 24, 25). A fourth site involves the membrane-proximal external region (MPER) of the transmembrane gp41 glycoprotein (15). A few additional sites involve recently defined epitopes at the gp41-gp120 interface (14, 17, 26). When passively transferred, these bNAbs can prevent either HIV-1 infection in humanized mice or simian-human immunodeficiency virus infection in macaques (27, 28). In addition, these bNAbs can also suppress established infection in humanized mice and macaques (29–32). The data strongly support testing the efficacy of bNAbs in human clinical trials. In this context, it is important to define the sensitivity to neutralization of the most representative HIV-1 variants that must be targeted. Due to the genetic bottleneck that occurs at transmission, transmitted variants may possess a selective advantage, and therefore preventive immunoprophylaxis should target early/transmitted variants. In addition, the rapid evolution of the HIV-1 species and its adaptation to its new host, the human population, suggests taking into account the most recently spreading variants, i.e., those that have been isolated at the time of primary infection during the recent years, as representative(s) of the contemporary epidemic.
Based on these hypotheses, we and others reported that HIV-1 appears to become more resistant to antibody neutralization over the course of the epidemic (33, 34). In our previous study, we compared the neutralization sensitivity of early/transmitted HIV-1 variants from patients infected by subtype B viruses at three periods of the epidemic (1987 to 1991, 1996 to 2000, and 2006 to 2010). A progressively increasing resistance to neutralization was observed over calendar time, for both human sera and the bNAbs b12, VRC01, VRC03, NIH45-46G54W, PG9, PG16, PGT121, PGT128, and PGT145 (33). Since then, bNAbs with increased potency or targeting different epitopes have been generated, including bNAbs improved by structure-based gene modifications (37–41). Therefore, we extended the analyses to a panel of new bNAbs described: PG9-iMab, PG16-iMab, 10E8, 3BNC117, NIH45-46m2, NIH45-46m7, 10-1074, JM4sdAb, 8ANC195, and PG9-16-RSH (Table 1). This new study provides an updated overview of the potency and breadth of these bNAbs against early/transmitted contemporary HIV-1 variants that could guide the selection of antibodies to be used in immunoprophylaxis strategies and of epitopes to be considered for vaccine design.
TABLE 1.
Characteristics of the bNAbs tested
| Antibody | Specificitya | Reference |
|---|---|---|
| b12 | gp120-CD4bs | 35 |
| VRC01 | gp120-CD4bs | 21 |
| VRC03 | gp120-CD4bs | 21 |
| NIH45-46G54W | gp120-CD4bs | 13 |
| NIH45-46m2 | gp120-CD4bs | 38 |
| NIH45-46m7 | gp120-CD4bs | 38 |
| 3BNC117 | gp120-CD4bs | 18 |
| PGT145 | gp120-V1V2 (glycan dependent) | 20 |
| PG9 | gp120-V1V2 (glycan dependent) | 19 |
| PG16 | gp120-V1V2 (glycan dependent) | 19 |
| PG9-16-RSH | gp120-V1V2 (glycan dependent) | 41 |
| PG9-iMab | Bispecific antibody | 40 |
| PG16-iMab | Bispecific antibody | 40 |
| PGT135 | gp120-V3 (glycan dependent) | 20 |
| PGT121 | gp120-V3 (glycan dependent) | 20 |
| PGT128 | gp120-V3 (glycan dependent) | 20 |
| 10-1074 | gp120-V3 (glycan dependent) | 16 |
| 2G12 | gp120-N332 dependent | 36 |
| 8ANC195 | g120/gp41 interface | 17 |
| JM4sdAb | gp120-CD4bs/CoRbs | 39 |
| 4E10 | gp41-MPER | 36 |
| 2F5 | gp41-MPER | 36 |
| 10E8 | gp41-MPER | 15 |
CD4bs, CD4 binding site; CoRbs, coreceptor binding site.
Increasing resistance to bNAbs targeting gp120.
The HIV-1 population that we studied was described previously (33). It was derived from 40 Caucasian men having sex with men (MSM) with primary infection, infected by clade B viruses at three periods of the French epidemic: between 1987 and 1991 (historical patients [HP]), 1996 and 2000 (intermediate patients [IP]), and 2006 and 2010 (contemporary patients [CP]). For each patient, blood samples were collected shortly after infection (before 3 months postinfection, except for a few cases). Pseudotyped viruses expressing envelope glycoprotein (Env) variants representative of the viral quasispecies infecting each patient were generated from the entire env gene amplified by reverse transcription-PCR from plasma-extracted viral RNA. Phylogenetic analysis of the env sequences of these viruses with a large series of env sequences issued from clade B variants isolated at the time of primary infection from patients of various geographic origins indicated that our viral population did not belong to a genetically restricted subset of viruses but could be considered representative of the entire clade B HIV-1 population worldwide (33). In addition, the genetic diversity among the env sequences of our viral population increased gradually from HP to CP, suggesting that it mirrors the global genetic evolution of HIV-1 over the course of the epidemic (33).
The neutralizing activities of bNAbs described in Table 1 were evaluated by using a luciferase reporter gene assay in TZM-bl cells based on single-round infection of Env-pseudotyped viruses (33). Although the recent bNAbs that target the CD4-binding site, NIH45-46m2, NIH45-46m7 (both derived from NIH45-46G54W), and 3BNC117 were more potent than the previously tested b12, VRC01, VRC03, and NIH45-46G54W, we observed still a significant decrease in sensitivity to neutralization over the three periods of the epidemic for all these bNAbs (Fig. 1A). The median 50% inhibitory concentration (IC50) of NIH45-46m2 increased progressively and significantly from 0.029 μg/ml for HP to 0.098 μg/ml for CP (P = 0.012). For NIH45-46m7 and 3BNC117, the median IC50 increased, respectively, from 0.035 and 0.020 μg/ml for HP to 0.098 and 0.270 μg/ml for IP and remained stable for CP (0.087 μg/ml and 0.175 μg/ml, respectively; P = 0.013 and 0.017, respectively).
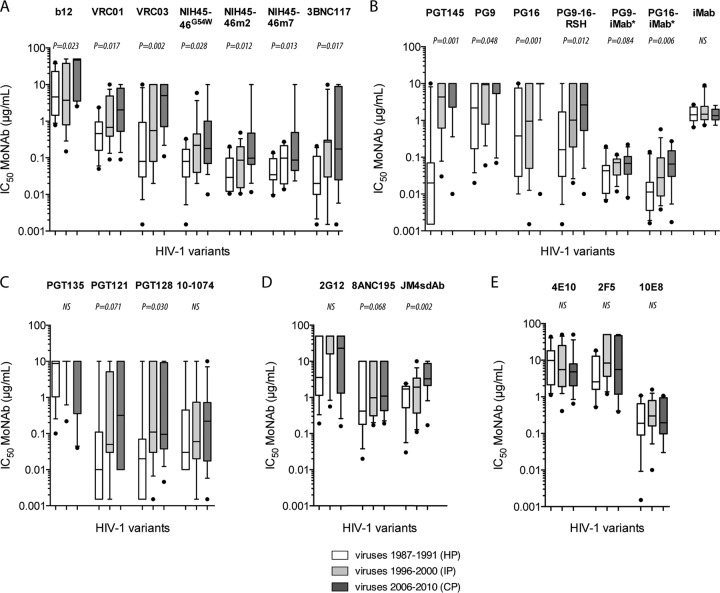
FIG 1.
Enhanced resistance of early/transmitted HIV-1 clade B variants to neutralization by monoclonal NAbs (MoNAbs) over the course of the epidemic. Comparisons are shown for the neutralization sensitivity of Env-pseudotyped viruses derived from historical patients (HP; n = 11), intermediate patients (IP; n = 15), and contemporary patients (CP; n = 14) by the following groups of antibodies: b12, VRC01, VRC03, and NIH45-46G54W (adapted from reference 33) and NIH45-46m2, NIH45-46m7, and 3BNC117 (this study) (A); PGT145, PG9, and PG16 (adapted from reference 33) and PG9-16-RSH, PG9-iMab, PG16-iMab, and iMab (this study) (B); PGT135, PGT121, and PGT128 (adapted from reference 33) and 10-1074 (this study) (C); 2G12 (adapted from reference 33) and 8ANC195 and JM4sdAb (this study) (D); 4E10 and 2F5 (adapted from reference 33) and 10E8 (this study) (D). Box plots show the distributions of antibody titers (IC50s) of each bNAb toward pseudotyped viruses of each period; the horizontal lines represent the 10th, median, and 90th percentiles. Each datum point represents the mean value of the assay performed in duplicate. Differences of neutralization sensitivity between viruses over calendar time were evaluated using a Jonckheere-Terpstra test.
The modified bNAbs that target the N160-glycan V1V2 epitopes PG9-16-RSH, PG9-iMab, and PG16-iMab were also more potent than the previously tested PG9, PG16, and PGT145 to neutralize the early/transmitted viruses (Fig. 1B). However, we still observed a significant decrease in sensitivity to neutralization over the three periods of the epidemic. The median IC50s of PG9-16-RSH, PG9-iMab, and PG16-iMab increased progressively from 0.160, 0.043, and 0.011 μg/ml for HP to 2.495, 0.068, and 0.065 μg/ml for CP, respectively. This trend was significant for PG9-16-RSH (P = 0.012) and PG16-iMab (P = 0.006) and just above the limit of significance for PG9-iMAb (P = 0.084). PG9-iMab and PG16-iMab are bispecific antibodies that were constructed using the humanized anti-CD4 antibody ibalizumab (iMab) as the scaffold, onto which the antigen-binding domains of PG9 and PG16 were engrafted (40). Interestingly, the sensitivity to the parental iMab was similar for HP, IP, and CP (median IC50s of 1.42, 1.47, and 1.35 μg/ml, respectively; P = 0.564) (Fig. 1B), suggesting that the increasing resistance to PG9-iMAb and PG16-iMAb over the course of the epidemic was specifically due to the paratope domain of PG9 and PG16.
The potency of the bNAb 10-1074, isolated from the B-cell lineage encoding PGT121 targeting an N332-glycan V3 epitope (16), was somewhat similar to that of the previous bNAbs PGT121 and PGT128. Although we also observed a trend for a decreasing sensitivity to neutralization by these reagents over the three periods, the phenomenon reached significance only for PGT128 (Fig. 1C). These slight differences could be attributed to modalities of glycan recognition by these antibodies (16, 20).
As previously reported (33), the sensitivity to neutralization by 2G12 was low, even for viruses from HP, and no trend of increasing resistance to this bNAb was observed (Fig. 1D). The exceptional nature of this antibody (42) associated with its low potency might explain the absence of drift for its targeted epitope. HIV-1 variants from CP were more resistant to the llama-derived antibody JM4sdAb than the variants that were transmitted 2 decades earlier (median IC50s of 1.720, 1.930, and 3.065 μg/ml for HP, IP, and CP, respectively; P = 0.002). This bNAb reacts with a CD4-induced epitope, and this reaction involves elements of both the coreceptor- and CD4-binding sites (37, 39). Although not significant, a similar trend was observed for the human bNAb 8ANC195, which targets a newly defined glycan-dependent epitope adjacent to the CD4-binding site and spanning gp120-gp41 interface (17). The median IC50 increased progressively from HP to CP (median IC50s, 0.420, 0.980, and 3.020 μg/ml for HP, IP, and CP, respectively; P = 0.068) (Fig. 1D). Future works with other bNAbs belonging to this class should provide additional information on the drift of this antigenic site which involves both envelope subunits, gp120 and gp41, and on the selective pressure that it undergoes.
The more recent bNAb 10E8 that targets the MPER was more potent than the previously tested MPER bNAbs 2F5 and 4E10. However, in agreement with our previous observations obtained with 2F5 and 4E10, no trend of increased resistance to 10E8 over time was observed (median IC50s, 0.195, 0.300, and 0.190 μg/ml for HP, IP, and CP, respectively; P = 0.416) (Fig. 1E).
Altogether, these results fully confirmed our first observations that indicated an increasing resistance of HIV-1 clade B to bNAbs targeting the major gp120 epitopes over 2 decades. Interestingly, drift was observed—not for a single major neutralization region of gp120, but for almost all the identified neutralization targets evaluated. This suggests a continuous selective pressure involving all epitopes, and therefore a drift of the entire gp120. In contrast, we observed a constant stable sensitivity to bNAbs targeting the gp41 MPER, suggesting either a lack of selective pressure, a weak tolerance to mutations in this region, or a combination of both. The first possibility, lack of selective pressure, is supported by the low reported frequency of NAbs elicited to this region (4). Although anti-MPER antibodies have been detected in HIV-1-infected individuals, they are associated with the neutralizing activity of sera only in rare cases (4, 5, 43, 44). The second possibility is supported by the fact that it is difficult to select resistant strains in vitro without impairing infectivity (45). However, exceptional 4E10-resistant HIV-1 isolates with rare MPER polymorphisms have been described (46). The fact that mutations within the MPER did not occur during passive immunization of HIV-1-infected individuals with 2F5 and 4E10 could be a result of both mechanisms (45).
Neutralization potency and breadth of bNAbs against early/transmitted HIV-1 variants.
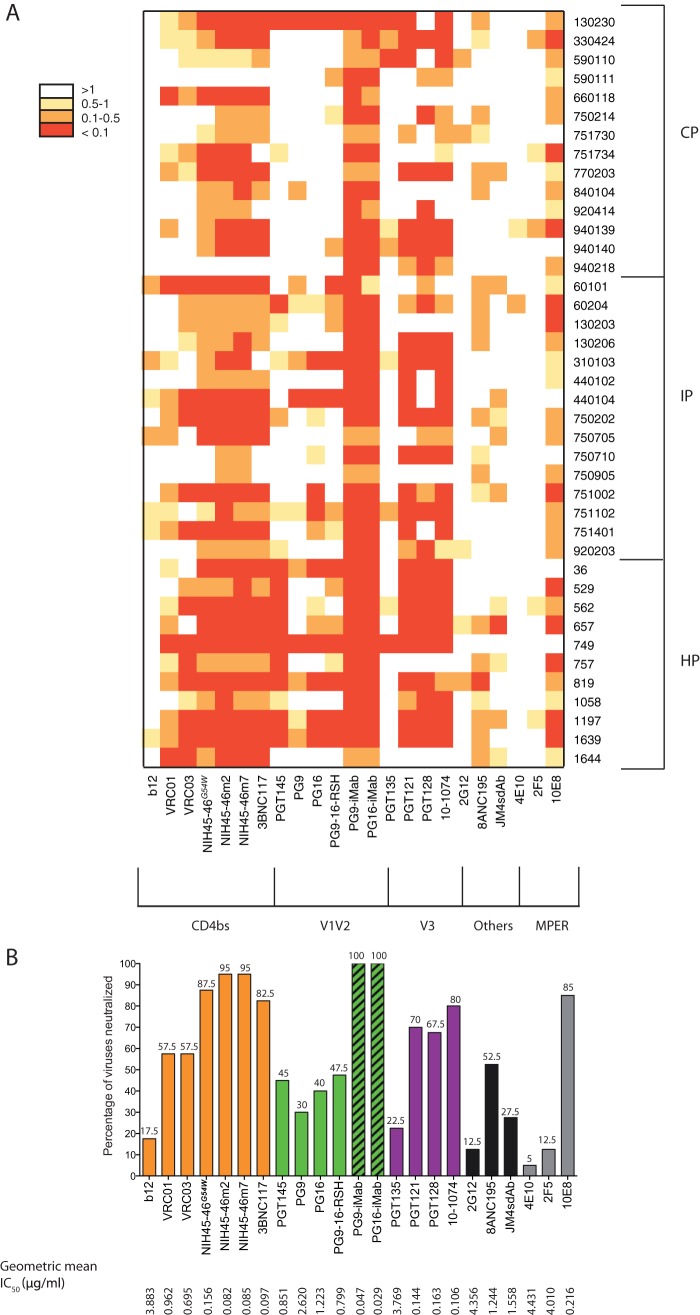
The neutralizing activities of bNAbs against our panel of pseudotyped viruses were compared by using IC50 heat map analysis (Fig. 2A). Only the bispecific antibodies PG9-iMab and PG16-iMab were able to neutralize all viruses at less than 1 μg/ml. Their neutralization breadth was exceptional compared to that of the parental PG9 and PG16, or to PG9-16-RSH (a chimeric derivate of PG9 and PG16). PG9-iMab and PG16-iMab were also the most potent of all bNAbs tested, with geometric mean IC50s of 0.047 μg/ml and 0.029 μg/ml, respectively, which indicate 32-fold and 52-fold higher potency than iMab (1.51 μg/ml) and 56-fold and 42-fold higher potency than PG9 (2.62 μg/ml) and PG16 (1.22 μg/ml), respectively (Fig. 2B). The geometric mean IC50s of PG9-iMab, PG16-iMab, iMab, PG9, and PG16 observed with our panel of early/transmitted subtype B viruses were approximately 10-fold higher than those previously reported with a panel of viral isolates from all major circulating genetic subtypes derived from patients at various stages of infection (40). These differences might be due to the fact that the virus populations were very different. Among all the other bNAbs, NIH45-46m2 and NIH45-46m7, which target the CD4-binding site, were the broadest and most potent. They neutralized 95% of viruses at less than 1 μg/ml, with a geometric mean IC50s of 0.082 μg/ml for NIH45-46m2 and 0.085 μg/ml for NIH45-46-m7. The breadth and potency of 3BNC117 was only slightly lower, neutralizing 82.5% of viruses at less than 1 μg/ml with a geometric mean IC50 of 0.097 μg/ml. The bNAb 10-1074 was the broadest and most potent antibody among those targeting the N332-glycan V3 region. It neutralized 80% of the viruses at less than 1 μg/ml, with a geometric mean IC50 of 0.106 μg/ml, whereas PGT121 and PGT128 neutralized 70% and 67.5% of the viruses with geometric mean IC50s of 0.144 μg/ml and 0.163 μg/ml, respectively. Among antibodies that do not target the three major epitopes on gp120, 8ANC195 was the broadest and most potent. It neutralized 52.5% of viruses with a geometric mean IC50 of 1.244 μg/ml. (Fig. 2B). The bNAb 10E8 was the most potent of the anti-MPER reagents, neutralizing 85% of the viruses at less than 1 μg/ml with a geometric mean IC50 of 0.216 μg/ml (Fig. 2B).
FIG 2.
Potency and breadth comparison of bNAbs against early/transmitted HIV-1 variants. (A) A heat map of the neutralizing activities (IC50s) of the bNAbs against a panel of 40 pseudotyped viruses from patients with primary infection (HP, IP, and CP), with increasingly darker colors indicating increasing neutralization sensitivity, as indicated by the key. (B) Neutralization breadth of bNAbs against the panel of pseudotyped viruses. Percentages of viruses neutralized for each bNAb at less than 1 μg/ml are indicated above the graph. IC50s greater than the highest bNAb concentration tested (10 μg/ml) were assigned a value of 10 in the geometric mean IC50 calculations.
Neutralization coverage of the most recently transmitted HIV-1 variants.
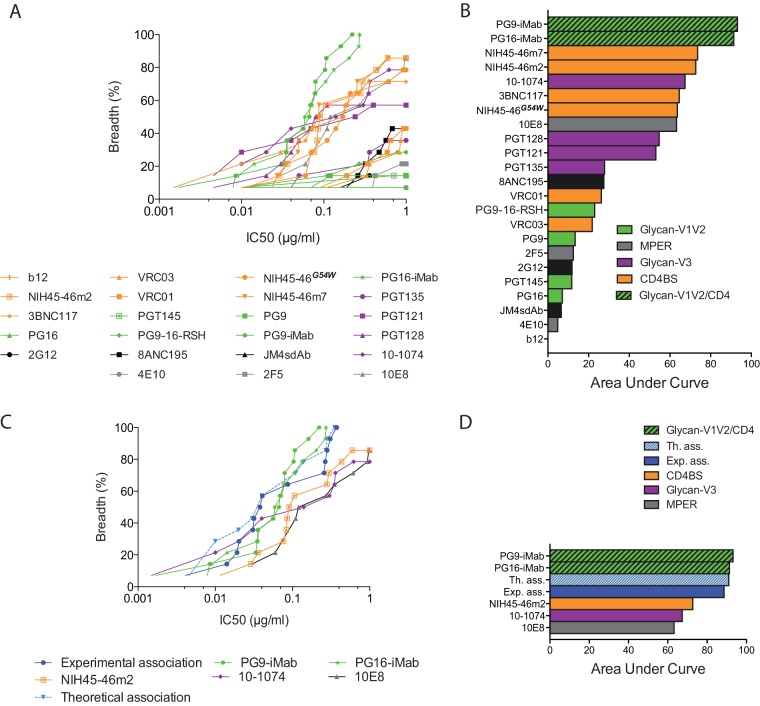
The perspective of using some bNAbs in human clinical trials and as tools for vaccine design and the evolution of the HIV-1 species that we have described necessitate focusing our attention on early/transmitted viruses collected during the most recent years of the epidemic. We therefore analyzed the cumulative frequency of neutralization of the viruses from the CP according to IC50s of up to 1 μg/ml, considering the area under the curve for each bNAb (Fig. 3A and B). Besides the bispecific antibodies PG9-iMab and PG16-iMab, which showed exceptional neutralization coverage, the bNAbs NIH45-46m7, NIH45-46m2, 10-1074, 3BNC117, NIH45-46G54W, and 10E8 were the most potent (Fig. 3B). They neutralized between 71% and 86% of viruses at less than 1 μg/ml (Fig. 3A). It would be useful to identify a combination of bNAbs that would neutralize 100% of HIV-1 variants at a low concentration, in order to prevent escape of any variant resistant to 1 or 2 of the bNAbs. We therefore selected three of the most potent bNAbs identified above, each targeting a different epitope, and investigated the sensitivity of CP variants to neutralization by a 1:1:1 combination of NIH45-46m2, 10-1074, and 10E8. The observed neutralization coverage by various concentrations of this combination was compared to the theoretical coverage that would be obtained if the neutralizing activities were fully additive (Fig. 3C and D). The NIH45-46m2/10-1074/10E8 combination was able to neutralize all the contemporary HIV-1 variants with an IC50 of ≤0.37 μg/ml, reaching approximately the theoretical curve and the potency of the bispecific antibodies PG9-iMab (IC50, ≤0.22 μg/ml) and PG16-iMab (IC50, ≤0.27 μg/ml). The effect of this combination was nearly additive but neither synergistic nor antagonistic. Compared to the NIH45-46G54W/PGT128 combination tested in our previous study, this new combination was more potent, reaching similar neutralization coverage at approximately 3-fold-lower bNAb concentrations (33). These results suggest that optimal neutralization coverage of recently transmitted variants may be achieved by combining three bNAbs targeting different epitopes, i.e., the CD4-binding site, the N332-glycan-dependent V3 epitope, and the MPER.
FIG 3.
Neutralization coverage of the most recently transmitted HIV-1 variants. (A) Coverage graph comparing the neutralization breadth and potencies of bNAbs against pseudotyped viruses from contemporary patients. The y axis shows the cumulative frequency of IC50s up to the concentration shown on the x axis. (B) Bar graph showing values for the area under the curve for the bNAbs shown in the coverage graph. (C) The neutralization coverage of viruses from contemporary patients was tested against a 1:1:1 combination of NIH45-46m2, 10-1074, and 10E8. Solid lines show the coverage of each bNAb used alone or in combination (experimental association). Each datum point represents the mean value of the assay performed in duplicate. The dashed line shows the theoretical coverage that would be obtained if the neutralizing activities of combined antibodies were fully additive. (D) Bar graph showing values for the area under the curve for the bNAbs used alone or in combination, as shown in the coverage graph.
In conclusion, the data confirm the ongoing adaptation of the HIV-1 species to the humoral immunity of the human population over the course of the epidemic, even using the most potent and broadly neutralizing monoclonal antibodies described to date. The drift in resistance to neutralization concerns only the external glycoprotein gp120, but not the MPER. It could suggest a high selective pressure on gp120, in agreement with the higher frequency of NAbs targeting the gp120 epitopes compared to the low frequency of NAbs to MPER in sera from elite neutralizers (4). Interestingly enough, almost all the identified major neutralization epitopes of gp120 are affected by this antigenic drift, suggesting that gp120 as a whole has progressively evolved in less than 3 decades. What mechanism has been responsible for this evolution? Has gp120 been shaped progressively by the neutralizing responses of the transmitters? It is difficult to privilege this hypothesis if we consider that most transmissions occur during acute infection, when bNAbs are not yet present in the transmitters, and that ancestral viruses are preferentially transmitted when transmissions occur during long-lasting infections (47, 48). However, if it were the case, this would suggest that the evolution of sensitivity to neutralization has been driven by rare events of late transmission of isolates, selected under NAb pressure, that might have an advantage over ancestral viruses. In any case, it remains to be determined whether the antigenic drift has been associated with other functional modifications of gp120 properties, such as interactions with the receptor and coreceptors, or cell entry efficacy more globally. Such a functional drift could be associated with an increase or, alternatively, a decrease of HIV-1 virulence, a major question that must be addressed. Despite this evolution, the good news is that the most recently identified bNAbs, either modified as bispecific antibodies or associated in combinations selected to target different epitopes, still remain capable of efficiently neutralizing the most recently transmitted HIV-1 clade B variants. These bispecific antibodies or these documented combinations should be favored for human trials.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale de Recherches sur le SIDA et les hépatites (ANRS, Paris, France) and in part by the intramural program of the Vaccine Research Center, NIAID, NIH. Mélanie Bouvin-Pley was supported by doctoral fellowships from the Région Centre and Sidaction (France). Marion Morgand was supported by a doctoral fellowship from the ANRS.
We thank the patients and clinicians who participated in the ANRS SEROCO CO2 and PRIMO CO6 cohorts. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pNL4.3.LUC.R-E- from Nathaniel Landau; TZM-bl cells from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.; HIV-1 anti-gp41 MAb (10E8) from Mark Connors.
Footnotes
Published ahead of print 17 September 2014
REFERENCES
- 1. Haynes BF, Moody MA, Liao H-X, Verkoczy L, Tomaras GD. 2011. B cell responses to HIV-1 infection and vaccination: pathways to preventing infection. Trends Mol. Med. 17:108–116. 10.1016/j.molmed.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, Gao H, Greene KM, McCutchan F, Williamson C, Kim JH, Tovanabutra S, Hahn BH, Swanstrom R, Thomson MM, Gao F, Harris L, Giorgi E, Hengartner N, Bhattacharya T, Mascola JR, Montefiori DC. 20 August 2014. Impact of clade, geography and age of the epidemic on HIV-1 neutralization by antibodies. J. Virol. 10.1128/JVI.01705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mascola JR, Montefiori DC. 2010. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 28:413–444. 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 4. Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 6:e1001028. 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braibant M, Brunet S, Costagliola D, Rouzioux C, Agut H, Katinger H, Autran B, Barin F. 2006. Antibodies to conserved epitopes of the HIV-1 envelope in sera from long-term non-progressors: prevalence and association with neutralizing activity. AIDS 20:1923–1930. 10.1097/01.aids.0000247113.43714.5e. [DOI] [PubMed] [Google Scholar]
- 6. Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, the CAPRISA002 Study Team 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85:4828–4840. 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, Mascola JR. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 13:1032–1034. 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7:e1001251. 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sather DN, Carbonetti S, Kehayia J, Kraft Z, Mikell I, Scheid JF, Klein F, Stamatatos L. 2012. Broadly neutralizing antibodies developed by an HIV-positive elite neutralizer exact a replication fitness cost on the contemporaneous virus. J. Virol. 86:12676–12685. 10.1128/JVI.01893-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, DeHovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337–7348. 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. 2014. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS 28:163–169. 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407. 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diskin R, Scheid JF, Marcovecchio PM, West AP, Klein F, Gao H, Gnanapragasam PNP, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293. 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, Ramirez A, Derking R, van Gils MJ, Liang CH, Mcbride R, von Bredow B, Shivatare SS, Wu CY, Chan-Hui PY, Liu Y, Feizi T, Zwick MB, Koff WC, Seaman MS, Swiderek K, Moore JP, Evans D, Paulson JC, Wong CH, Ward AB, Wilson IA, Sanders RW, Poignard P, Burton DR. 2014. Broadly neutralizing HIV antibodies define a glycan-dependant epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity 40:657–668. 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PNP, Spencer DIR, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U. S. A. 109:E3268–E3277. 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scharf L, Scheid JF, Lee JH, West AP, Jr, Chen C, Gao H, Gnanapragasam PN, Mares R, Seaman MS, Ward AB, Nussenzweig MC, Bjorkman PJ. 2014. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Rep. 7:785–795. 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G, Principal Investigators. Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent Neutralizing Antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G, Principal Investigators. Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, Scheid JF, Kraft Z, Liu Y, Pietzsch J, Hurley A, Poignard P, Feizi T, Morris L, Walker BD, Fatkenheuer G, Seaman MS, Stamatatos L, Nussenzweig MC. 2012. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J. Exp. Med. 209:1469–1479. 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103. 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Peña AT, Cupo A, Julien JP, van Gils M, Lee PS, Peng W, Paulson JC, Poignard P, Burton DR, Moore JP, Sanders RW, Wilson IA, Ward AB. 2014. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 40:669–680. 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. U. S. A. 109:18921–18925. 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. 25 August 2014. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang H-W, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Büning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. 2013. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc. Natl. Acad. Sci. U. S. A. 110:16538–16543. 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Lifson JD, Dimitrov D, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bouvin-Pley M, Morgand M, Moreau A, Jestin P, Simonnet C, Tran L, Goujard C, Meyer L, Barin F, Braibant M. 2013. Evidence for a continuous drift of the HIV-1 species towards higher resistance to neutralizing antibodies over the course of the epidemic. PLoS Pathog. 9:e1003477. 10.1371/journal.ppat.1003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bunnik EM, Euler Z, Welkers MRA, Boeser-Nunnink BDM, Grijsen ML, Prins JM, Schuitemaker H. 2010. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nat. Med. 16:995–997. 10.1038/nm.2203. [DOI] [PubMed] [Google Scholar]
- 35. Burton DR, Barbas CF, III, Persson MA, Koenig S, Chanock RM, Lerner RA. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. U. S. A. 88:10134–10137. 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, Katinger H. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses 10:359–369. 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 37. Acharya P, Luongo TS, Georgiev IS, Matz J, Schmidt SD, Louder MK, Kessler P, Yang Y, McKee K, O'Dell S, Chen L, Baty D, Chames P, Martin L, Mascola JR, Kwong PD. 2013. Heavy chain-only IgG2b llama antibody effects near-pan HIV-1 neutralization by recognizing a CD4-induced epitope that includes elements of coreceptor- and CD4-binding sites. J. Virol. 87:10173–10181. 10.1128/JVI.01332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diskin R, Klein F, Horwitz JA, Halper-Stromberg A, Sather DN, Marcovecchio PM, Lee T, West AP, Gao H, Seaman MS, Stamatatos L, Nussenzweig MC, Bjorkman PJ. 2013. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J. Exp. Med. 210:1235–1249. 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matz J, Kessler P, Bouchet J, Combes O, Ramos OHP, Barin F, Baty D, Martin L, Benichou S, Chames P. 2013. Straightforward selection of broadly neutralizing single-domain antibodies targeting the conserved CD4 and coreceptor binding sites of HIV-1 gp120. J. Virol. 87:1137–1149. 10.1128/JVI.00461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pace CS, Song R, Ochsenbauer C, Andrews CD, Franco D, Yu J, Oren DA, Seaman MS, Ho DD. 2013. Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1. Proc. Natl. Acad. Sci. U. S. A. 110:13540–13545. 10.1073/pnas.1304985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pancera M, Shahzad-ul Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, Zhang B, Parks R, Eudailey J, Lloyd KE, Blinn J, Alam SM, Haynes BF, Amin MN, Wang L-X, Burton DR, Koff WC, Nabel GJ, Mascola JR, Bewley CA, Kwong PD. 2013. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat. Struct. Mol. Biol. 20:804–813. 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 43. Shen X, Parks RJ, Montefiori DC, Kirchherr JL, Keele BF, Decker JM, Blattner WA, Gao F, Weinhold KJ, Hicks CB, Greenberg ML, Hahn BH, Shaw GM, Haynes BF, Tomaras GD. 2009. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J. Virol. 83:3617–3625. 10.1128/JVI.02631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Molinos-Albert LM, Carrillo J, Curriu M, Rodriguez de la Concepción ML, Marfil S, García E, Clotet B, Blanco J. 2014. Anti-MPER antibodies with heterogeneous neutralization capacity are detectable in most untreated HIV-1 infected individuals. Retrovirology 11:44. 10.1186/1742-4690-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manrique A, Rusert P, Joos B, Fischer M, Kuster H, Leemann C, Niederöst B, Weber R, Stiegler G, Katinger H, Günthard HF, Trkola A. 2007. In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J. Virol. 81:8793–8808. 10.1128/JVI.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakamura KJ, Gach JS, Jones L, Semrau K, Walter J, Bibollet-Ruche F, Decker JM, Heath L, Decker WD, Sinkala M, Kankasa C, Thea D, Mullins J, Kuhn L, Zwick MB, Aldrovandi GM. 2010. 4E10-resistant HIV-1 isolated from four subjects with rare membrane-proximal external region polymorphisms. PLoS One 5:e9786. 10.1371/journal.pone.0009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sagar M, Laeyendecker O, Lee S, Gamiel J, Wawer MJ, Gray RH, Serwadda D, Sewankambo NK, Shepherd JC, Toma J, Huang W, Quinn TC. 2009. Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J. Infect. Dis. 199:580–589. 10.1086/596557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Redd AD, Collinson-Streng AN, Chatziandreou N, Mullis CE, Laeyendecker O, Martens C, Ricklefs S, Kiwanuka N, Nyein PH, Lutalo T, Grabowski MK, Kong X, Manucci J, Sewankambo N, Wawer MJ, Gray RH, Porcella SF, Fauci AS, Sagar M, Serwadda D, Quinn TC. 2012. Previously transmitted HIV-1 strains are preferentially selected during subsequent sexual transmissions. J. Infect. Dis. 206:1433–1442. 10.1093/infdis/jis503. [DOI] [PMC free article] [PubMed] [Google Scholar]