Abstract
Human infections with influenza A(H5N1) virus in Cambodia increased sharply during 2013. Molecular characterization of viruses detected in clinical specimens from human cases revealed the presence of mutations associated with the alteration of receptor-binding specificity (K189R, Q222L) and respiratory droplet transmission in ferrets (N220K with Q222L). Discovery of quasispecies at position 222 (Q/L), in addition to the absence of the mutations in poultry/environmental samples, suggested that the mutations occurred during human infection and did not transmit further.
TEXT
Early detection of avian influenza A virus infection in humans and characterization of the viruses remain a global public health goal to better anticipate pandemic threats. Although there is little evidence of person-to-person transmission of these viruses to date, mutations in the hemagglutinin (HA) and internal protein genes associated with human adaptation have been reported in some groups of highly pathogenic avian influenza (HPAI) A(H5N1) viruses, as well as in A(H7N9) viruses in China (1–3). A glutamine-to-leucine substitution in the HA receptor-binding domain 220-loop (Q222L by H5 numbering) is recognized as one of the critical substitutions, altering the binding preference of the virus for host cells displaying sialic acid in α2,3 linkage to galactose on carbohydrate side chains (avian receptor) to sialic acid in α2,6 linkage to galactose (human receptor) (4, 5). Two recent studies identified additional HA (6, 7) and internal gene (7) mutations in H5N1 viruses with efficient respiratory droplet transmission in ferrets. While the exact contribution of each individual mutation described in either study has not been fully elucidated, the combination of as few as four HA mutations compared to parental strains (N154D, N220K, Q222L, T315I [6] and H103Y, T156A, Q222L, G224S [7]) was sufficient to facilitate aerosol droplet transmission. To date, only one of these four mutations, which results in loss of a glycosylation site at the 150 loop (either N154D or T156A), has been routinely identified in naturally occurring viruses detected in birds and humans. The other mutations remain extremely rare or undetected among circulating strains of HPAI H5N1 viruses (2).
During 2013, the number of reported H5N1-positive human cases detected in Cambodia (n = 26) exceeded the cumulative number identified from 2005 to 2012 (n = 21) (8) (Table 1). Twenty-six clinical specimens, one from each of the human cases positive for H5 HA by real-time reverse transcription (RT)-PCR, were inoculated into MDCK cells and/or embryonated chicken eggs, yielding 15 virus isolates (Table 1). The HA genes from 20 cases were sequenced directly from clinical specimens and/or virus isolates, and full-genome sequences were generated from the cases producing virus isolates. In addition to sequences obtained from human cases, sequences from 72 viruses obtained from poultry, environmental sources, and civet cats were determined.
TABLE 1.
Characteristics of 2,013 human cases of HPAI A(H5N1) virus infection detected in Cambodia
| WHO case no.a | Strain name | GenBank/GISAID accession number(s) | Isolate passage history and/or sequence availabilityb | Locationd | Agee | Sex | Illness onset date | Specimen collection date | Antiviral treatment start datef | Outcome | Detection methodg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD22 (1) | A/Cambodia/X0123311/2013 | PB2, KF001369; PB1, KF001370; PA, KF001371; HA, KF001372; NP, KF001373; NA, KF001374; M, KF001375; NS, KF001376 | C1 and C1/E1 | Phnom Penh | 8 mo | M | 8-Jan-13 | 9-Jan-13 | NA | Recovered | ILI surveillance |
| CD23 (2) | A/Cambodia/X0121311/2013 | No data | VNR (no data) | Takeo | 17 | F | 11-Jan-13 | 21-Jan-13 | NA | Fatal | Event based |
| CD24 (3) | A/Cambodia/X0123312/2013 | PB2, KF001377; PB1, KF001378; PA, KF001379; HA, KF001380; NP, KF001381; NA, KF001382; M, KF001383; NS, KF001384 | C1 and C1/E1 | Kampong Speu | 35 | M | 13-Jan-13 | 21-Jan-13 | NA | Fatal | SARI surveillance |
| CD25 (4) | A/Cambodia/X0125302/2013 | PB2, KF001385; PB1, KF001386; PA, KF001387; HA, KF001388; NP, KF001389; NA, KF001390; M, KF001391; NS, KF001392 | C1 and C1/E1 | Kampong Speu | 17 mo | F | 13-Jan-13 | 25-Jan-13 | 26-Jan-13 | Fatal | Event based |
| CD26 (5) | A/Cambodia/X0128304/2013 | No data | VNR (no data) | Kampot | 9 | F | 15-Jan-13 | 27-Jan-13 | 27-Jan-13 | Fatal | Event based |
| CD27 (6) | A/Cambodia/X0207301/2013 | PB2, KF001393; PB1, KF001394; PA, KF001395; HA, KF001396; NP, KF001397; NA, KF001398; M, KF001399; NS, KF001400 | C1 | Takeo | 5 | F | 25-Jan-13 | 6-Feb-13 | 6-Feb-13 | Fatal | Event based |
| CD28 (7) | A/Cambodia/X0212301/2013 | PB2, KF001401; PB1, KF001402; PA, KF001403; HA, KF001404; NP, KF001405; NA, KF001406; M, KF001407; NS, KF001408 | C1 | Kampot | 3 | F | 3-Feb-13 | 11-Feb-13 | 11-Feb-13 | Fatal | Event based |
| CD29 (8) | A/Cambodia/X0219301/2013 | PB2, KF001409; PB1, KF001410; PA, KF001411; HA, KF001412; NP, KF001413; NA, KF001414; M, KF001415; NS, KF001416 | C1 and C1/E1 | Kampot | 2 | M | 6-Feb-13 | 18-Feb-13 | NA | Fatal | Event based |
| CD30 (9) | A/Cambodia/X0215301/2013 | HA, KF001417; NA, KF001418 | VNR (HA, NA sequence)c | Kampong Cham | 35 | M | 13-Feb-13 | 13-Feb-13 | 13-Feb-13 | Fatal | Event based |
| CD31 (10) | A/Cambodia/X0401302/2013 | No data | VNR (no data) | Kampot | 5 | M | 27-Mar-13 | 1-Apr-13 | 1-Apr-13 | Recovered | Event based |
| CD32 (11) | A/Cambodia/X0502302/2013 | HA, KF369257 | VNR (HA sequence) | Kampong Speu | 5 | F | 2-May-13 | 2-May-13 | NA | Recovered | Fever surveillance |
| CD33 (12) | A/Cambodia/X0621333/2013 | HA, EPI538833 | VNR (HA sequence) | Phnom Penh | 58 | M | Unclear | 8-Jan-13 | NA | Recovered | SARI surveillance |
| CD34 (13) | A/Cambodia/X0628313/2013 | PB2, KF918453; PB1, KF918454; PA, KF918455; HA, KF918456; NP, KF918457; NA, KF918458; M, KF918459; NS, KF918460 | C1 and C1/E1 | Kampot | 6 | F | 24-Jun-13 | 28-Jun-13 | 28-Jun-13 | Fatal | Event based |
| CD35 (14) | A/Cambodia/X0709301/2013 | No data | VNR (no data) | Prey Veng | 3 | M | 4-Jul-13 | 9-Jul-13 | 9-Jul-13 | Recovered | Event based |
| CD36 (15) | A/Cambodia/X0808305/2013 | PB2, KF918461; PB1, KF918462; PA, KF918463; HA, KF918464; NP, KF918465; NA, KF918466; M, KF918467; NS, KF918468 | C1 | Battambang | 7 | M | 26-Jul-13 | 4-Aug-13 | 9-Aug-13 | Fatal | Event based |
| CD37 (16) | A/Cambodia/X0810301/2013 | PB2, EPI537652; PB1, EPI537653, PA, KF918469; HA, KF918470; NP, KF918471; NA, KF918472; M, KF918473; NS, KF918474 | E1 and E2 | Kandal | 5 | F | 1-Aug-13 | 9-Aug-13 | 10-Aug-13 | Recovered | Event based |
| CD38 (17) | A/Cambodia/X0817302/2013 | PB2, KF918487; PB1, KF918488; PA, KF918489; HA, KF918490; NP, KF918491; NA, KF918492; M, KF918493; NS, KF918494 | C1 and C1/E1 | Kandal | 6 | M | 21-Jul-13 | 23-Jul-13 | NA | Recovered | Fever surveillance |
| CD39 (18) | A/Cambodia/X0828324/2013 | PB2, KF918495; PB1, KF918496; PA, KF918497; HA, KF918498; NP, KF918499; NA, KF918500; M, KF918501; NS, KF918502 | C1 and C1/E1 | Phnom Penh | 15 mo | M | 16-Aug-13 | 27-Aug-13 | 27-Aug-13 | Recovered | Event based |
| CD40 (19) | A/Cambodia/X0913301/2013 | PB2, KF918503; PB1, KF918504; PA, KF918505; HA, KF918506; NP, KF918507; NA, KF918508; M, KF918509; NS, KF918510 | C1 | Takeo | 5 | F | 7-Sept-13 | 13-Sept-13 | 13-Sept-13 | Recovered | Event based |
| CD41 (20) | A/Cambodia/X0916322/2013 | PB2, KF918511; PB1, KF918512; PA, KF918513; HA, KF918514; NP, KF918515; NA, KF918516; M, KF918517; NS, KF918518 | C1 and C1/E1 | Kampot | 2 | F | 11-Sept-13 | 16-Sept-13 | 16-Sept-13 | Fatal | Event based |
| CD42 (21) | A/Cambodia/X1014305/2013 | No data | VNR (no data) | Kampong Thom | 8 | F | 8-Oct-13 | 11-Oct-13 | 14-Oct-13 | Recovered | Event based |
| CD43 (22) | A/Cambodia/X1024307/2013 | PB2, KF918527; PB1, KF918528; PA, KF918529; HA, KF918530; NP, KF918531; NA, KF918532; M, KF918533; NS, KF918534 | C1 | Battambang | 6 | F | 14-Oct-13 | 22-Oct-13 | 24-Oct-13 | Recovered | Event based |
| CD44 (23) | A/Cambodia/X1030304/2013 | PB2, EPI537641; PB1, EPI537642; PA, EPI537643; HA, EPI497961; NP, EPI537644; NA, EPI497962; M, EPI537645; NS, EPI537646 | C1 | Pursat | 3 | F | 17-Oct-13 | 25-Oct-13 | NA | Fatal | Event based |
| CD45 (24) | A/Cambodia/X1107305/2013 | HA, EPI537657 | VNR (HA sequence) | Kampot | 10 | M | 30-Oct-13 | 7-Nov-13 | 7-Nov-13 | Fatal | Event based |
| CD46 (25) | A/Cambodia/X1108306/2013 | HA, EPI537675 | VNR (HA sequence) | Phnom Penh | 3 | M | 5-Nov-13 | 8-Nov-13 | 8-Nov-13 | Recovered | Event based |
| CD47 (26) | A/Cambodia/X1108305/2013 | HA, EPI538834 | VNR (HA sequence) | Pailin | 29 | M | 26-Oct-13 | 7-Nov-13 | 7-Nov-13 | Fatal | Event based |
Numbers in parentheses were the case numbers during 2013.
C1, first passage in cell culture; E1, first passage in embryonated eggs; VNR, virus not recovered.
Sequence generated directly from clinical specimen when the virus was not recovered after inoculation in MDCK cells or embryonated chicken eggs.
Province where case was identified.
Ages are in years except where otherwise noted.
NA, antiviral treatment not received.
ILI, influenza-like illness; SARI, severe acute respiratory infection.
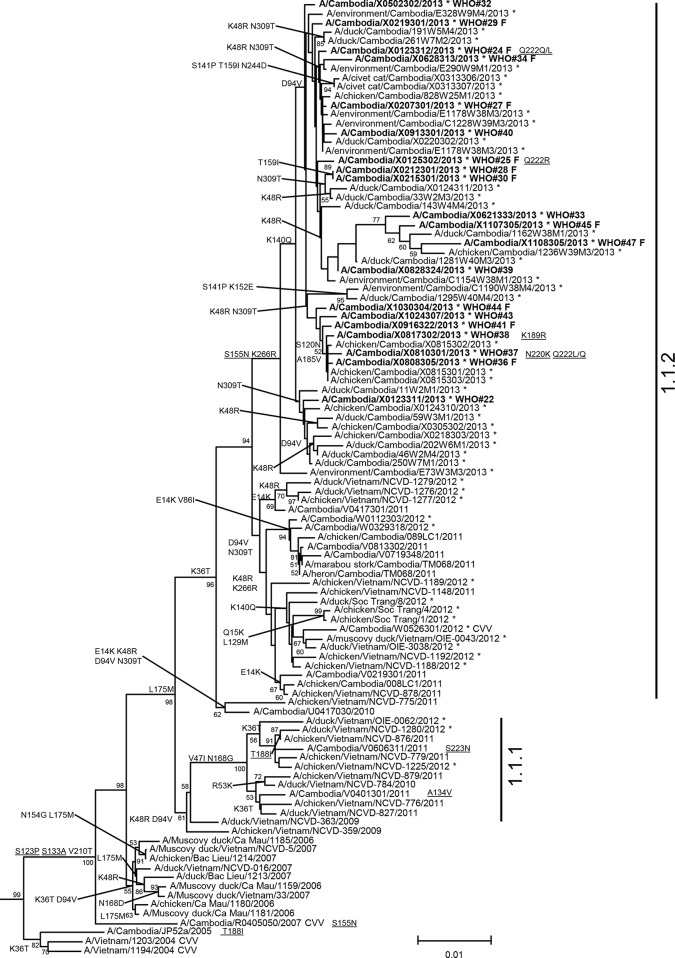
Nucleotide sequence analysis of the HA genes revealed that all viruses belong to the clade 1.1.2 phylogenetic group (9, 10). While all 2013 data from neighboring countries may not be available, the Cambodian viruses clustered in a discrete group made up of samples collected only in Cambodia during 2013 (Fig. 1). Amino acid sequence comparisons to clade 1 progenitor strains (e.g., A/Vietnam/1203/2004) revealed that all viruses in the 2013 group shared mutations at four positions (HA S123P, S133A, S155N, and K266R). Three of these four HA substitutions were previously recognized as contributing to increased binding of H5 viruses to mammalian host cell sialic acid receptors in α2,6 linkage either alone (S133A, S155N) or in combination with other mutations (S123P) (11–13). While the S155N mutation resides within the 150-loop glycosylation motif, the mutation of serine to asparagine does not change the predicted N-linked glycosylation motif of these viruses (NST to NNT; underlining indicates the mutation within the motif). In addition, several individual viruses from human cases had other HA mutations previously linked to increased binding to α2,6 host cell receptors (K189R, Q222L) and enhanced respiratory droplet transmission in a ferret model (N220K with Q222L) (5, 6, 11). Virus sequenced from the clinical specimen of case 24 had a mixed base population of Q222Q/L. Another virus isolated from Cambodian case 25 had a mixed base population of Q222R/Q. A clinical specimen collected from case 37 (A/Cambodia/X0810301/2013) had both the N220K and Q222L/Q mutations, and the K189R mutation was identified in the virus infecting case 38 (Fig. 1). Although all clade 1.1.2 viruses detected to date, including 2013 Cambodian viruses from poultry, civet cats, and environmental samples, share three previously recognized HA markers (S123P, S133A, S155N), other mutations associated with mammalian adaptation were not found in the nonhuman samples, but viruses from the recently identified human cases in Cambodia had four to five amino acid residues in the HA associated with alteration of receptor-binding specificity.
FIG 1.
Neighbor-joining phylogenetic tree of the HA genes of clade 1 highly pathogenic avian influenza A(H5N1) viruses constructed in MEGA5. The nearest reassortant WHO candidate vaccine viruses (CVV) for each group of clade 1 are denoted by CVV at the end of the strain name. Viruses collected in 2012-2013 are denoted with an asterisk. Sequences were aligned using MUSCLE, and amino acid differences at branch nodes indicate shared HA1 substitutions relative to the reference strain, A/Vietnam/1203/2004. Mutations to the right of a strain name indicate amino acid changes found only in that individual virus. Underlined amino acid substitutions indicate previously recognized molecular markers and/or markers of note as listed in the H5N1 Genetic Changes Inventory (24). Branches on the tree with HA sequences from human cases are in bold. Bootstraps greater than 50 generated from 1,000 replicates are shown at branch nodes. The scale bar represents the number of nucleotide substitutions per site.
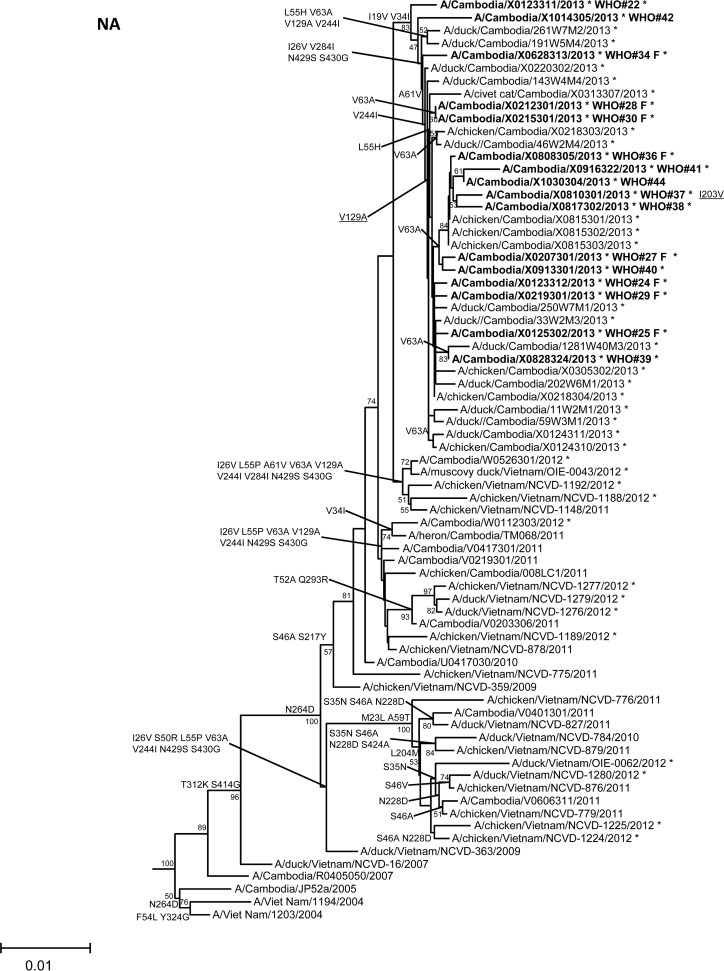
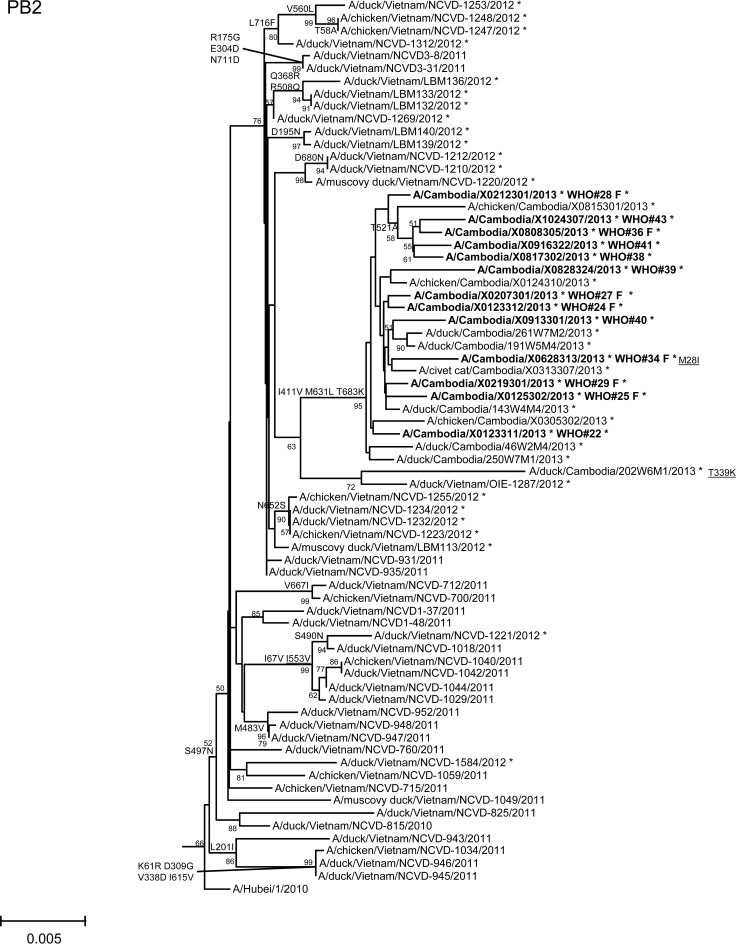
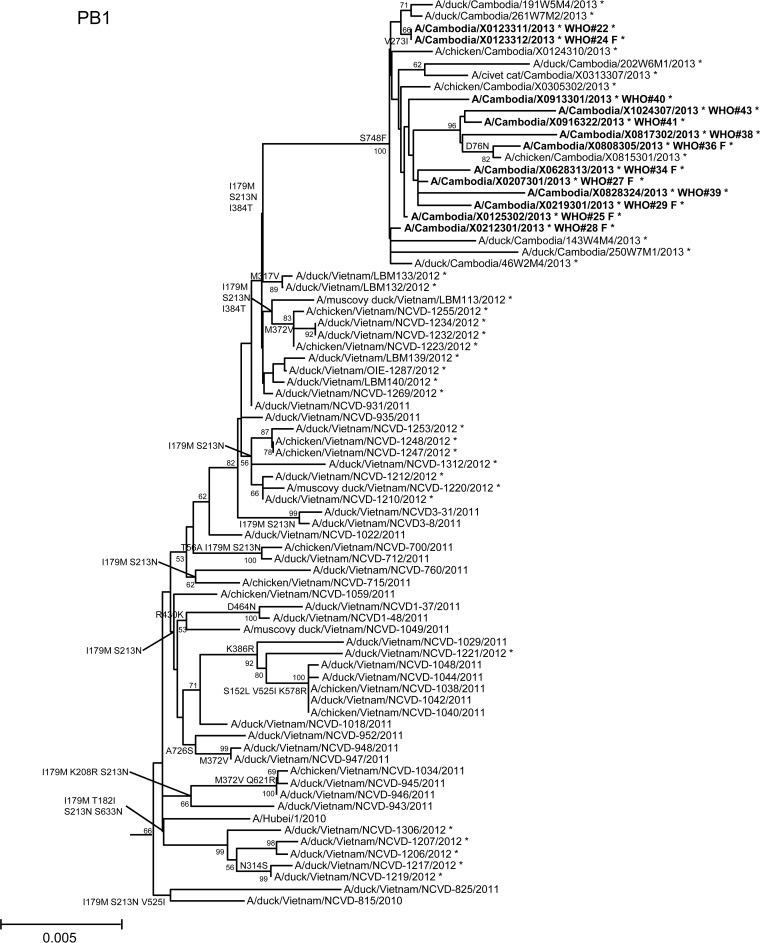
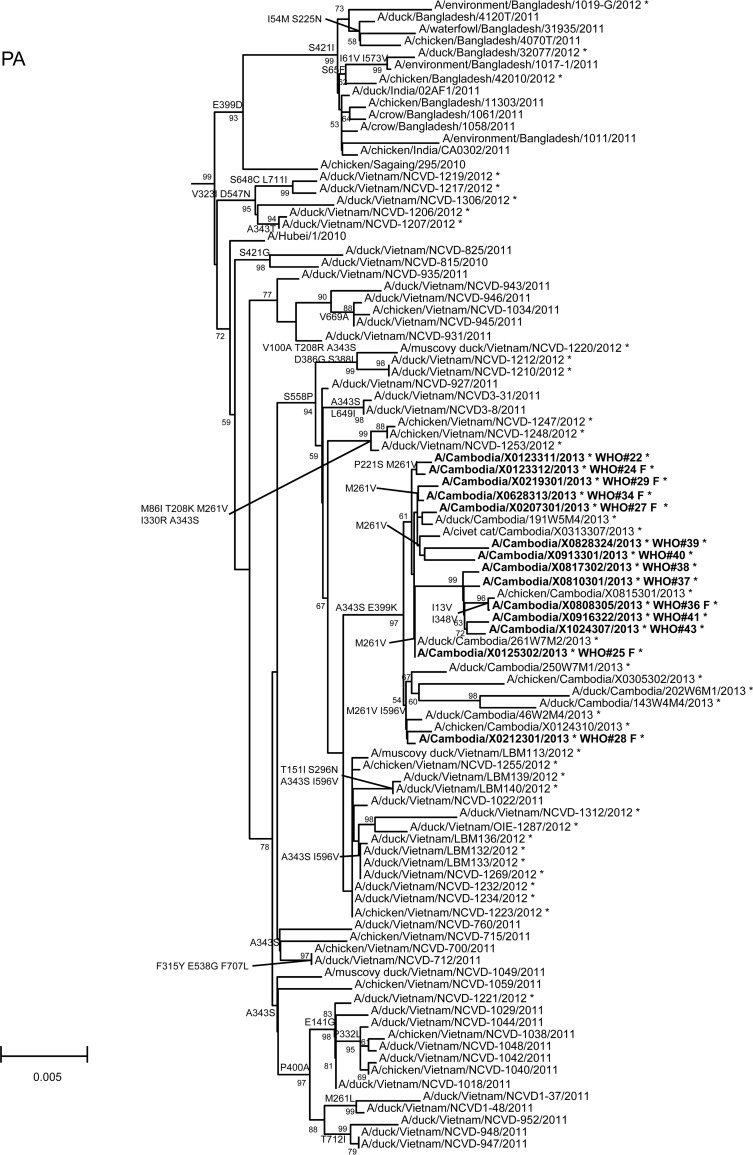
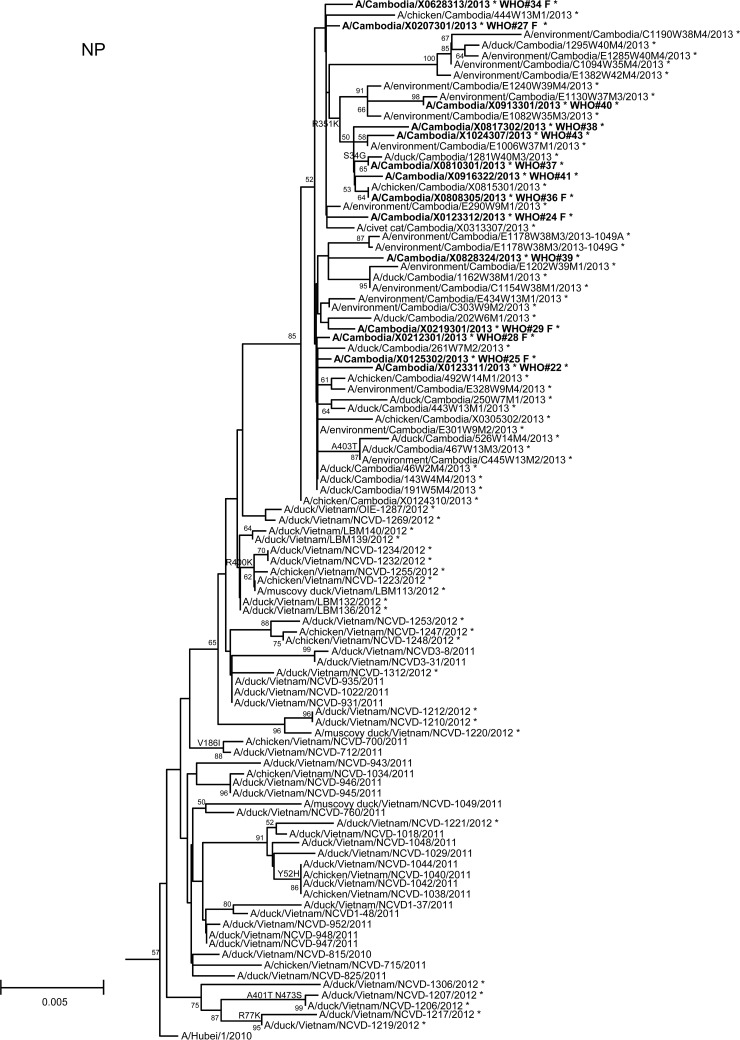
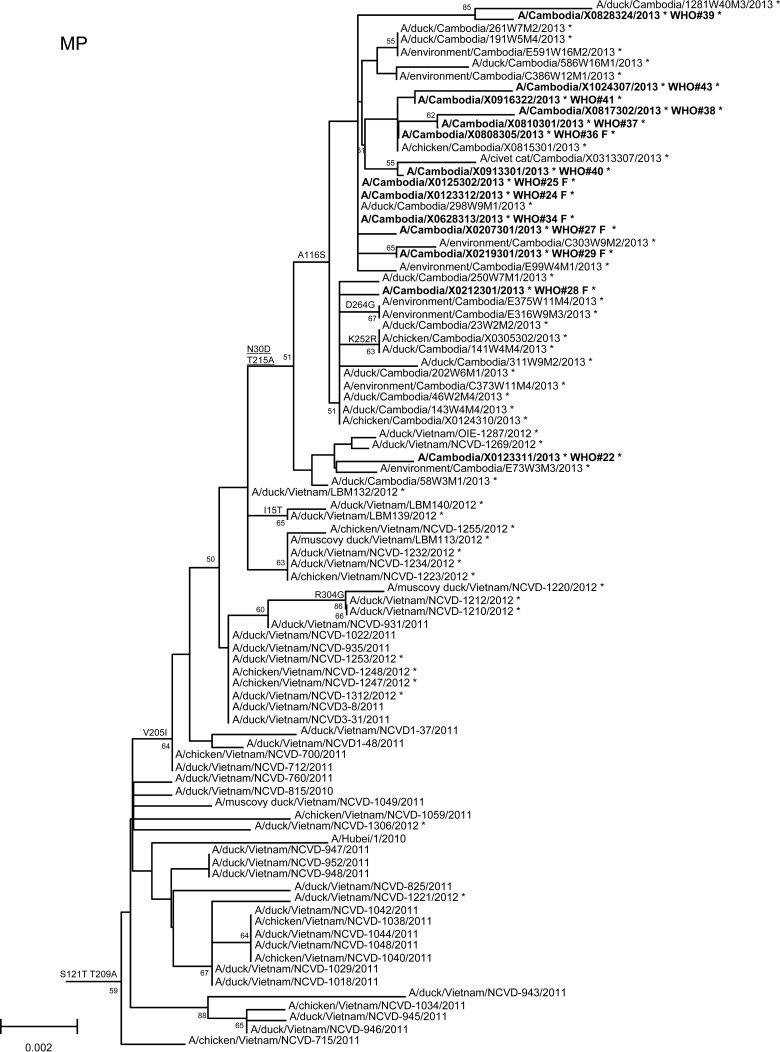
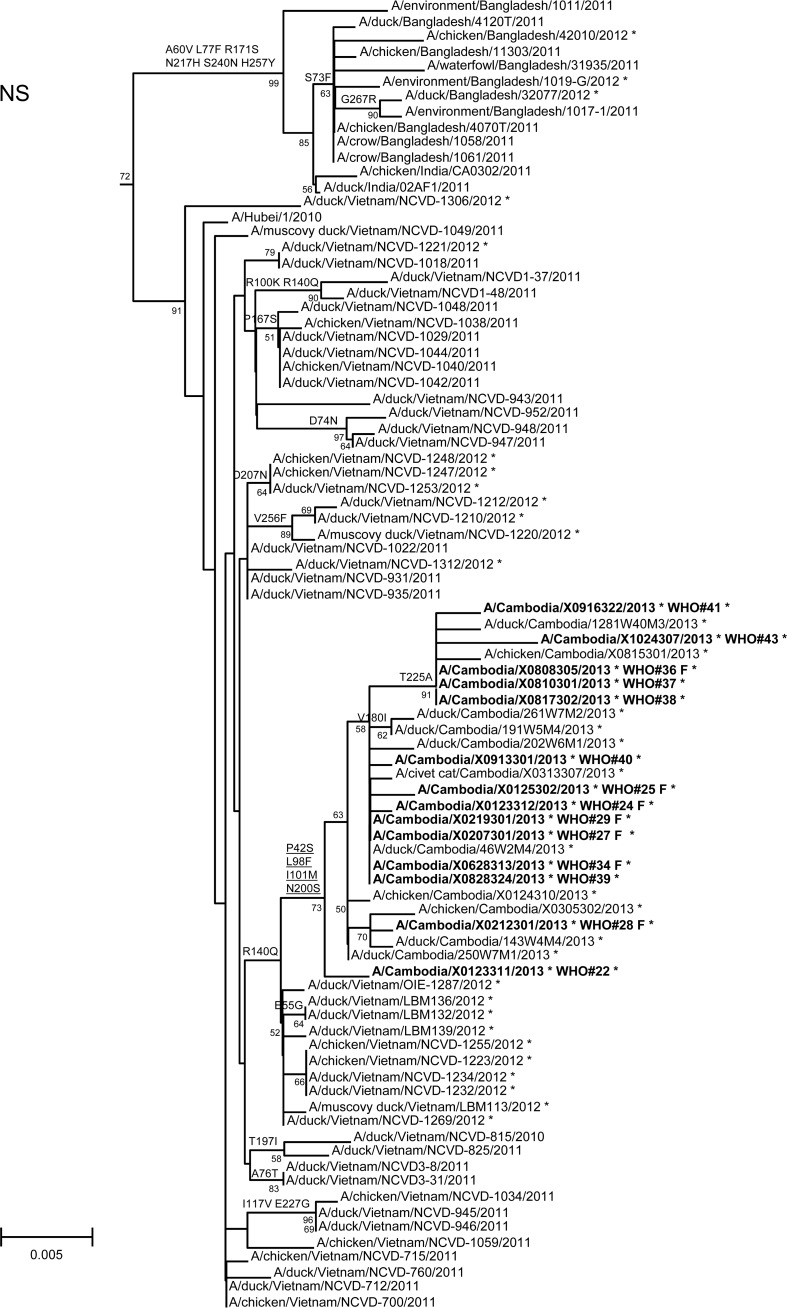
In order to assess additional potential markers of mammalian adaptation, we analyzed the other influenza virus protein sequences and annotated amino acids of note on phylogenetic trees (Fig. 2). The neuraminidase (NA) of all 2013 viruses had a V129A mutation, which has been shown to reduce susceptibility to zanamivir (14). Virus isolated from case 37 had a mutation associated with reduced susceptibility to oseltamivir (NA I203V) (15). Unlike the HA and NA genes of the 2013 viruses, which were phylogenetically related to those of previous clade 1.1 viruses belonging to genotype Z, matrix (M) and internal protein genes analyzed from all animal, human, and environmental samples clustered with clade 2.3.2.1a viruses circulating in Vietnam in 2012. This finding indicated that all of these viruses represent a novel, previously undescribed genotype resulting from reassortment of clade 1.1.2 viruses with clade 2.3.2.1a viruses. The M1 protein sequences of all viruses possessed mutations associated with increased virulence in mice (N30D and T215A), which are common to the majority of circulating strains of H5N1 viruses (16). Interestingly, replacement of the clade 1.1 (genotype Z) M gene, which had two highly conserved markers of adamantane drug resistance (M2 L26I and S31N), with the clade 2.3.2.1a gene that possesses neither mutation indicates a noteworthy change for possible antiviral treatment options (17). None of the 2013 Cambodian viruses had mutations in polymerase genes associated with mammalian host adaptation. One polymerase basic 2 protein (PB2) mutation (R368Q) identified in all 2013 viruses and two other mutations (M28I, T339R) found in individual viruses were shown to alter polymerase activity and enhance virulence in mice when found in combination with other mutations (18–20). Finally, the NS1 protein of all viruses had four molecular markers (P42S, L98F, I101M, N200S) of enhanced virulence in mice and decreased the antiviral response commonly found with circulating H5N1 viruses (21–23).
FIG 2.
Neighbor-joining phylogenetic tree of the neuraminidase (NA) and internal genes of highly pathogenic avian influenza A(H5N1) viruses constructed in MEGA5. Viruses collected in 2012-2013 are denoted with an asterisk. Sequences were aligned using MUSCLE; amino acid differences at branch nodes indicate substitutions relative to reference strain A/Vietnam/1203/2004 for the NA gene and A/Hubei/1/2010 for the internal genes. Mutations to the right of each strain name indicate amino acid changes found only in that individual virus. Underlined amino acid substitutions indicate previously recognized molecular markers and/or markers of note as listed in the H5N1 Genetic Changes Inventory (24). Branches on the tree with sequences from human cases are in bold. Bootstraps greater than 50 generated from 1,000 replicates are shown at branch nodes. The scale bar represents the number of nucleotide substitutions per site.
This study provides the first identification of two HA mutations (220K and 222L) in a clinical sample collected from a human infection following exposure to poultry. These are two of the four mutations described in the ferret-transmissible H5 mutant (6). There was no evidence that either of these cases resulted from or led to human-to-human transmission, as indicated by follow-up investigations with close contacts of the human cases after these and other cases were detected.
ACKNOWLEDGMENTS
We gratefully acknowledge the authors and the originating and submitting laboratories for the sequences from GISAID's EpiFlu Database which were used in this analysis.
This work was funded by the World Health Organization (WHO) in Cambodia, the Food and Agriculture Organization (FAO) in Cambodia, and the Office of the Assistant Secretary for Preparedness and Response within the U.S. Department of Health and Human Services. We thank the Centers for Disease Control and Prevention's Division of Global Disease Detection and Emergency Response for their support.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry. The opinions expressed herein are those of the authors and do not represent those of the U.S. Navy, the U.S. Department of Defense, or the U.S. government.
Some of the authors are employed by the U.S. government, and this work was done as part of their official duties.
Footnotes
Published ahead of print 10 September 2014
REFERENCES
- 1. Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368:1888–1897. 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2. Russell CA, Fonville JM, Brown AEX, Burke DF, Smith DL, James SL, Herfst S, van Boheemen S, Linster M, Schrauwen EJ, Katzelnick L, Mosterín A, Kuiken T, Maher E, Neumann G, Osterhaus AD, Kawaoka Y, Fouchier RA, Smith DJ. 2012. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science 336:1541–1547. 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stevens J, Blixt O, Chen L-M, Donis RO, Paulson JC, Wilson IA. 2008. Recent avian H5N1 viruses exhibit increased propensity for acquiring human receptor specificity. J. Mol. Biol. 381:1382–1394. 10.1016/j.jmb.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. 2006. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 312:404–410. 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 5. Chutinimitkul S, van Riel D, Munster VJ, van den Brand JMA, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA, de Wit E. 2010. In vitro assessment of attachment pattern and replication efficiency of H5N1 influenza A viruses with altered receptor specificity. J. Virol. 84:6825–6833. 10.1128/JVI.02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herfst S, Schrauwen EJA, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. 2013. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html Accessed 13 February 2014.
- 9.World Health Organization/World Organisation for Animal Health/Food and Agriculture Organization (WHO/OIE/FAO) H5N1 Evolution Working Group. 2014. Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses. Influenza Other Respir. Viruses 8:384–388. 10.1111/irv.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sorn S, Sok T, Ly S, Rith S, Tung N, Viari A, Gavotte L, Holl D, Seng H, Asgari N, Richner B, Laurent D, Chea N, Duong V, Toyoda T, Yasuda CY, Kitsutani P, Zhou P, Bing S, Deubel V, Donis R, Frutos R, Buchy P. 2013. Dynamic of H5N1 virus in Cambodia and emergence of a novel endemic sub-clade. Infect. Genet. Evol. 15:87–94. 10.1016/j.meegid.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Lu B, Zhou H, Suguitan AL, Cheng X, Subbarao K, Kemble G, Jin H. 2010. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J. Virol. 84:6570–6577. 10.1128/JVI.00221-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamada S, Suzuki Y, Suzuki T, Le MQ, Nidom CA, Sakai-Tagawa Y, Muramoto Y, Ito M, Kiso M, Horimoto T, Shinya K, Sawada T, Kiso M, Usui T, Murata T, Lin Y, Hay A, Haire LF, Stevens DJ, Russell RJ, Gamblin SJ, Skehel JJ, Kawaoka Y. 2006. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 444:378–382. 10.1038/nature05264. [DOI] [PubMed] [Google Scholar]
- 13. Yang Z-Y, Wei C-J, Kong W-P, Wu L, Xu L, Smith DF, Nabel GJ. 2007. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 317:825–828. 10.1126/science.1135165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naughtin M, Dyason JC, Mardy S, Sorn S, von Itzstein M, Buchy P. 2011. Neuraminidase inhibitor sensitivity and receptor-binding specificity of Cambodian clade 1 highly pathogenic H5N1 influenza virus. Antimicrob. Agents Chemother. 55:2004–2010. 10.1128/AAC.01773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hurt AC, Holien JK, Barr IG. 2009. In vitro generation of neuraminidase inhibitor resistance in A(H5N1) influenza viruses. Antimicrob. Agents Chemother. 53:4433–4440. 10.1128/AAC.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan S, Deng G, Song J, Tian G, Suo Y, Jiang Y, Guan Y, Bu Z, Kawaoka Y, Chen H. 2009. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 384:28–32. 10.1016/j.virol.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 17. Lan Y, Zhang Y, Dong L, Wang D, Huang W, Xin L, Yang L, Zhao X, Li Z, Wang W, Li X, Xu C, Yang L, Guo J, Wang M, Peng Y, Gao Y, Guo Y, Wen L, Jiang T, Shu Y. 2010. A comprehensive surveillance of adamantane resistance among human influenza A virus isolated from mainland China between 1956 and 2009. Antivir. Ther. 15:853–859. 10.3851/IMP1656. [DOI] [PubMed] [Google Scholar]
- 18. Leung BW, Chen H, Brownlee GG. 2010. Correlation between polymerase activity and pathogenicity in two duck H5N1 influenza viruses suggests that the polymerase contributes to pathogenicity. Virology 401:96–106. 10.1016/j.virol.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 19. Li J, Ishaq M, Prudence M, Xi X, Hu T, Liu Q, Guo D. 2009. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 144:123–129. 10.1016/j.virusres.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 20. Salomon R. 2006. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 203:689–697. 10.1084/jem.20051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiao P, Tian G, Li Y, Deng G, Jiang Y, Liu C, Liu W, Bu Z, Kawaoka Y, Chen H. 2008. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 82:1146–1154. 10.1128/JVI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spesock A, Malur M, Hossain MJ, Chen L-M, Njaa BL, Davis CT, Lipatov AS, York IA, Krug RM, Donis RO. 2011. The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol. 85:7048–7058. 10.1128/JVI.00417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imai H, Shinya K, Takano R, Kiso M, Muramoto Y, Sakabe S, Murakami S, Ito M, Yamada S, Le MT, Nidom CA, Sakai-Tagawa Y, Takahashi K, Omori Y, Noda T, Shimojima M, Kakugawa S, Goto H, Iwatsuki-Horimoto K, Horimoto T, Kawaoka Y. 2010. The HA and NS genes of human H5N1 influenza A virus contribute to high virulence in ferrets. PLoS Pathog. 6:e1001106. 10.1371/journal.ppat.1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. 2014. H5N1 genetic changes inventory: a tool for influenza surveillance and preparedness. http://www.cdc.gov/flu/pdf/avianflu/h5n1-inventory.pdf Accessed 20 August 2014.