Abstract
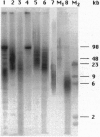
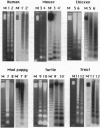
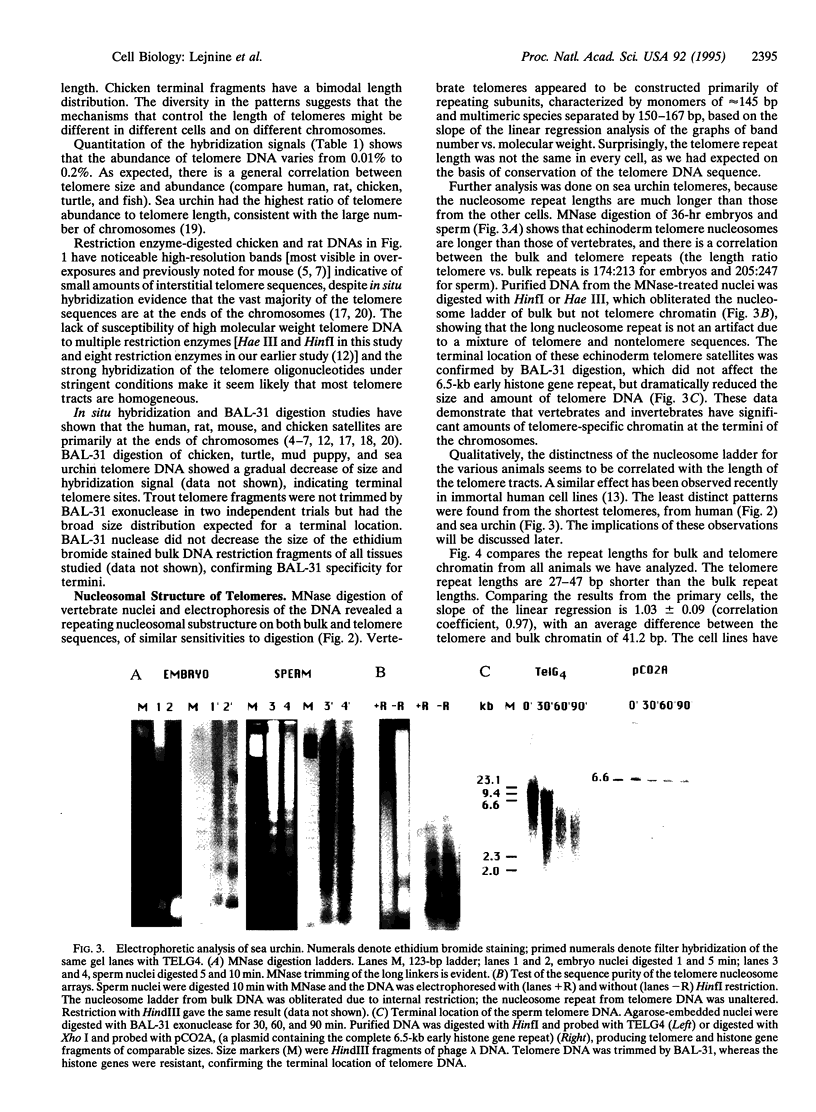
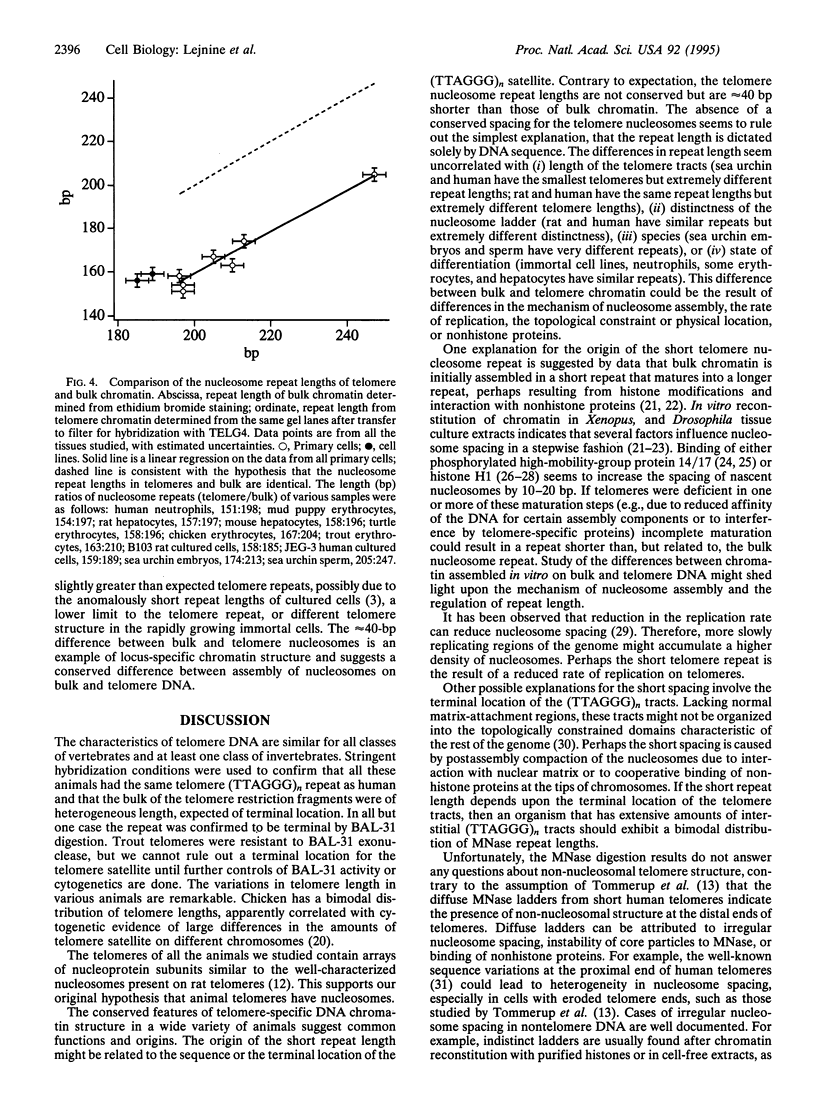
Eukaryotic chromosomes terminate with telomeres, nucleoprotein structures that are essential for chromosome stability. Vertebrate telomeres consist of terminal DNA tracts of sequence (TTAGGG)n, which in rat are predominantly organized into nucleosomes regularly spaced by 157 bp. To test the hypothesis that telomeres of other animals have nucleosomes, we compared telomeres from eight vertebrate tissues and cell cultures, as well as two tissues from an invertebrate. All telomeres have substantial tracts of (TTAGGG)n comprising 0.01-0.2% of the genome. All telomeres are long (20-100 kb), except for those of sea urchin, human, and some chicken chromosomes, which are 3-10 kb in length. All of the animal telomeres contained nucleosome arrays, consistent with the original hypothesis. The telomere repeat lengths vary from 151 to 205 bp, seemingly uncorrelated with telomere size, regularity of nucleosome spacing, species, or state of differentiation but surprisingly correlated with the repeat of bulk chromatin within the same cells. The telomere nucleosomes were consistently approximately 40 bp smaller than bulk nucleosomes. Thus, animal telomeres have highly conserved sequences and unusually short nucleosomes with cell-specific structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Gosden J. R., Cross S. H., Cranston G., Rout D., Sugawara N., Szostak J. W., Fantes P. A., Hastie N. D. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988 Apr 14;332(6165):656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- Almouzni G., Wolffe A. P. Nuclear assembly, structure, and function: the use of Xenopus in vitro systems. Exp Cell Res. 1993 Mar;205(1):1–15. doi: 10.1006/excr.1993.1051. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Blasquez V., Stein A., Ambrose C., Bina M. Simian virus 40 protein VP1 is involved in spacing nucleosomes in minichromosomes. J Mol Biol. 1986 Sep 5;191(1):97–106. doi: 10.1016/0022-2836(86)90425-0. [DOI] [PubMed] [Google Scholar]
- Conconi A., Widmer R. M., Koller T., Sogo J. M. Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell. 1989 Jun 2;57(5):753–761. doi: 10.1016/0092-8674(89)90790-3. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M., Dingwall C. Chromatin assembly in vitro and in vivo. Bioessays. 1988 Aug-Sep;9(2-3):44–49. doi: 10.1002/bies.950090203. [DOI] [PubMed] [Google Scholar]
- Drew H. R. Reconstitution of short-spaced chromatin from the histone octamer and either HMG-14,17 or histone H1. J Mol Biol. 1993 Apr 5;230(3):824–836. doi: 10.1006/jmbi.1993.1204. [DOI] [PubMed] [Google Scholar]
- German J. The chromosomal complement of blastomeres in Arbacia punctulata. Chromosoma. 1966;20(2):195–201. doi: 10.1007/BF00335207. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Kipling D., Cooke H. J. Hypervariable ultra-long telomeres in mice. Nature. 1990 Sep 27;347(6291):400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Leffak I. M. Chromatin assembled in the presence of cytosine arabinoside has a short nucleosome repeat. Nucleic Acids Res. 1983 Aug 25;11(16):5451–5466. doi: 10.1093/nar/11.16.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov V. L., Lejnine S., Bedoyan J., Langmore J. P. Nucleosomal organization of telomere-specific chromatin in rat. Cell. 1993 May 21;73(4):775–787. doi: 10.1016/0092-8674(93)90256-p. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda I., Schmid M. Localization of the telomeric (TTAGGG)n sequence in chicken (Gallus domesticus) chromosomes. Cytogenet Cell Genet. 1994;65(3):190–193. doi: 10.1159/000133630. [DOI] [PubMed] [Google Scholar]
- Poccia D. L., Simpson M. V., Green G. R. Transitions in histone variants during sea urchin spermatogenesis. Dev Biol. 1987 Jun;121(2):445–453. doi: 10.1016/0012-1606(87)90181-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Campos A., Shimamura A., Worcel A. Assembly and properties of chromatin containing histone H1. J Mol Biol. 1989 Sep 5;209(1):135–150. doi: 10.1016/0022-2836(89)90177-0. [DOI] [PubMed] [Google Scholar]
- Starling J. A., Maule J., Hastie N. D., Allshire R. C. Extensive telomere repeat arrays in mouse are hypervariable. Nucleic Acids Res. 1990 Dec 11;18(23):6881–6888. doi: 10.1093/nar/18.23.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Bina M. A model chromatin assembly system. Factors affecting nucleosome spacing. J Mol Biol. 1984 Sep 15;178(2):341–363. doi: 10.1016/0022-2836(84)90148-7. [DOI] [PubMed] [Google Scholar]
- Tolley J. O., Omann G. M., Jesaitis A. J. A high-yield, high-purity elutriation method for preparing human granulocytes demonstrating enhanced experimental lifetimes. J Leukoc Biol. 1987 Jul;42(1):43–50. doi: 10.1002/jlb.42.1.43. [DOI] [PubMed] [Google Scholar]
- Tommerup H., Dousmanis A., de Lange T. Unusual chromatin in human telomeres. Mol Cell Biol. 1994 Sep;14(9):5777–5785. doi: 10.1128/mcb.14.9.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremethick D. J., Drew H. R. High mobility group proteins 14 and 17 can space nucleosomes in vitro. J Biol Chem. 1993 May 25;268(15):11389–11393. [PubMed] [Google Scholar]
- Tremethick D. J., Frommer M. Partial purification, from Xenopus laevis oocytes, of an ATP-dependent activity required for nucleosome spacing in vitro. J Biol Chem. 1992 Jul 25;267(21):15041–15048. [PubMed] [Google Scholar]
- Vincenz C., Fronk J., Tank G. A., Langmore J. P. Nucleoprotein hybridization: a method for isolating active and inactive genes as chromatin. Nucleic Acids Res. 1991 Mar 25;19(6):1325–1336. doi: 10.1093/nar/19.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]