Abstract
The concentrations and relative ratios of various aroma compounds produced by fermenting yeast cells are essential for the sensory quality of many fermented foods, including beer, bread, wine, and sake. Since the production of these aroma-active compounds varies highly among different yeast strains, careful selection of variants with optimal aromatic profiles is of crucial importance for a high-quality end product. This study evaluates the production of different aroma-active compounds in 301 different Saccharomyces cerevisiae, Saccharomyces paradoxus, and Saccharomyces pastorianus yeast strains. Our results show that the production of key aroma compounds like isoamyl acetate and ethyl acetate varies by an order of magnitude between natural yeasts, with the concentrations of some compounds showing significant positive correlation, whereas others vary independently. Targeted hybridization of some of the best aroma-producing strains yielded 46 intraspecific hybrids, of which some show a distinct heterosis (hybrid vigor) effect and produce up to 45% more isoamyl acetate than the best parental strains while retaining their overall fermentation performance. Together, our results demonstrate the potential of large-scale outbreeding to obtain superior industrial yeasts that are directly applicable for commercial use.
INTRODUCTION
During industrial fermentations, yeasts convert simple carbohydrates into ethanol and CO2. However, in addition to these primary metabolites, they also produce smaller quantities of several other metabolites that have a marked effect on the product's sensory quality. These secondary metabolites include higher alcohols, aldehydes, sulfur-containing compounds, esters, phenols, carbonyl compounds, and organic acids, all of which contribute to the product aroma. Volatile acetate esters, such as isoamyl acetate (IA) and ethyl acetate (EA), are considered one of the most important groups of aroma-active yeast metabolites. While EA is often perceived negatively in excessively high concentrations, IA is the main determinant of the often desired fruity characteristics of fermented beverages (1). Volatile acetate esters are the product of an enzyme-catalyzed condensation reaction between acyl-coenzyme A (CoA) and a higher alcohol (2). Being lipid soluble, acetate esters diffuse through the cellular membrane of the fermenting yeast cell into the medium. Although the exact genetic mechanisms underlying the regulation of acetate ester production are yet to be elucidated, it has been shown that several different enzymes are involved, most notably the alcohol acyltransferases 1 and 2, encoded by the genes ATF1 and ATF2, respectively (3–6). The production of acetate esters during the fermentation process is influenced by many parameters, including medium composition (e.g., sugar profile and density, nitrogen content, and lipid concentration), oxygen availability, fermentation temperature, initial yeast concentration, and fermentor design (1). Altering these factors therefore allows some adjustment of flavor formation although the changes are often only minor. Moreover, adjusting general fermentation parameters like aeration and medium composition may be prohibitively expensive and may also have unwanted side effects. Therefore, direct tuning of the yeast's inherent flavor production is a much better strategy.
There are several ways to tune the aroma production of industrial yeast strains. Genetic engineering of genes coding for key enzymes, such as ATF1 and ATF2, has been demonstrated to yield remarkable changes in the production of acetate esters (5, 7, 8). However, these strategies all entail the use of recombinant DNA techniques and/or genetic transformation, which implies that the resulting strains are classified as genetically modified organisms (GMOs). Because of the complex legislation and (most importantly) negative consumer perception, these strains are only marginally used for commercial food and beverage production. Therefore, non-GMO strategies, based on random mutagenesis and/or directed evolution, are often more appropriate to improve complex industrially relevant yeast traits (reviewed in reference 9). These approaches were already successfully applied to improve several stress-related phenotypes of industrial yeast strains, including ethanol tolerance (10), acetic acid tolerance (11), and copper resistance (12). Perhaps the most important factor determining the success of these strategies is the availability of an easy way to identify the few superior cells among a large pool of inferior variants. While this can be readily achieved for stress tolerance by simply applying the stress condition for which improvement is desired, other phenotypes, like aroma production, are much more difficult to select for. In these cases, sexual hybridization strategies using phenotypically characterized haploid segregants of carefully selected parental strains (cell-to-cell mating) is an appealing strategy (9). However, the phenotypic characterization of the haploid segregants to identify the ones that outperform the parental strains for the phenotype of interest is often an important bottleneck that can limit the success of this approach.
In this study, we measured the production of 15 different aroma compounds for a set of 301 genetically diverse industrial and natural (wild) Saccharomyces strains. The obtained data set provides a comprehensive overview of the aromatic potential of Saccharomyces yeasts. Our results show that the production of aroma-active acetate esters shows extraordinary differences between different yeast strains and that industrial strains have clearly been selected for high aroma production. Next, we selected three genetically diverse heterothallic strains (designated Y141, Y354, and Y397) with high IA production to serve as parental strains for the production of 46 different intraspecific hybrids (both inbred and outbred strains). Aroma production analysis of these hybrids in lab-scale ale fermentations indicates that while inbreeding yields only very minor improvements in IA production, outbreeding with carefully selected parental strains can serve as a valuable strategy to obtain superior hybrids, even beyond the phenotypic borders of both parental strains (a phenomenon called best-parent heterosis or hybrid vigor).
The aims of this study were (i) to obtain a detailed overview of the phenotypic diversity in aroma production of a genetically diverse collection of yeast strains, (ii) to use this data set as a tool to select strains for further phenotypic improvement using sexual hybridization, a non-GMO technique, (iii) to develop a breeding scheme to improve complex, polygenic industrially relevant traits, such as aroma production, and (iv) to test the industrial potential of these resulting hybrids for production of fruity ales or flavorful low-alcohol beers.
MATERIALS AND METHODS
Yeast strains and storage conditions.
For this research paper, 301 industrial and wild Saccharomyces strains were analyzed (Table 1). Part of this collection is a set of 60 representative (homozygous diploid) Saccharomyces cerevisiae and Saccharomyces paradoxus strains described previously (13). Due to their limited industrial relevance, the lab/reference strains and clinical isolates of this set were not included. Industrial strains were ordered from various culture collections or directly provided by producers of fermented products. Wild strains were selected in order to cover a broad geographical diversity (more details on the origin of the wild strains are provided in Table S1 in the supplemental material).
TABLE 1.
Overview of the yeast collection used in this study
| Yeast source(s) | No. of strainsa |
|---|---|
| Ale beer | 104 |
| Lager beer | 29 (S. pastorianus) |
| Wine | 63 |
| Sake | 13 |
| Spirit | 11 |
| Bakery | 10 |
| Bioethanol | 7 |
| Wild (nondomesticated) isolates | 36 (S. paradoxus), 28 (S. cerevisiae) |
All strains belong to S. cerevisiae unless otherwise noted.
All strains were stored long term at −80°C using a glycerol-based standard storage medium (2% [wt/vol] Bacto peptone, 1% [wt/vol] yeast extract, 2% [wt/vol] glucose, 25% [vol/vol] glycerol).
Sporulation, tetrad dissection, and mating type characterization.
Sporulation was induced on acetate medium (1% [wt/vol] potassium acetate, 0.05% [wt/vol] amino acid mix, 2% [wt/vol] agar) after 5 to 10 days at 25°C. The ascus wall was digested with 4 mg ml−1 Zymolyase (Seikagaku, Tokyo, Japan) suspension (dissolved in 2 M sorbitol) and incubated for 3 min at room temperature. Tetrads were dissected using a micromanipulator (MSM manual micromanipulator; Singer, Somerset, United Kingdom) on YPD-agar (2% [wt/vol] Bacto peptone, 1% [wt/vol] yeast extract, 2% [wt/vol] glucose, 2% [wt/vol] agar). The hetero- or homothallic nature of the parental strain was determined by mating type testing of all viable spores originating from four different tetrads. Mating type was determined by a PCR approach using MAT-A (5′-ACTCCACTTCAAGTAAGAGTT-3′), MAT-α (5′-GCACGGAATATGGGACTACTTCG-3′), and MAT-R (5′-AGTCACATCAAGATCGTTTATGG-3′) as primers and a temperature profile consisting of an initial denaturation step (98°C for 2 min), followed by 30 cycles of 98°C for 30s, 55°C for 30s, and 72°C for 40s, with a final extension of 72°C for 5 min.
Lab-scale fermentations in rich growth medium.
Yeast precultures were shaken overnight at 30°C in test tubes containing 5 ml of yeast extract (1%, wt/vol), peptone (2%, wt/vol), and glucose (4%, wt/vol) medium (4% YPGlu medium). After 16 h of growth, 0.5 ml of the preculture was used to inoculate 50 ml of 4% YPGlu medium in 250-ml Erlenmeyer flasks, and this second preculture was shaken at 30°C for 16 h. This preculture was used for inoculation of the fermentation medium (YPGlu containing 10% glucose [10% YPGlu]) at an initial optical density at 600 nm (OD600) of 0.5, roughly equivalent to 107 cells ml−1. The fermentations, performed in 250-ml Schott bottles with a water lock placed on each bottle, were incubated statically for 7 days at 20°C. Weight loss was measured daily to estimate fermentation progress. After 7 days, the fermentation bottles were removed from the incubator, the fermentation medium was filtered (0.15-mm-pore-size paper filter; Macherey-Nagel, Düren, Germany), and samples for chromatographic analysis and density and ethanol measurements were taken.
Screening of segregants for aroma production.
A single colony of the segregants to be analyzed was picked and inoculated in Erlenmeyer flasks (250 ml) containing 50 ml of 10% YPGlu. The bottles were sealed with a cotton plug and Parafilm and incubated at 30°C in a shaking incubator at 200 rpm. After 144 h of growth, the fermentations were analyzed sensorially and scored for fruitiness. To ensure correct identification of fruity segregants and validate experimental robustness, all segregants were tested in duplicate, and fruity (strains Y141, Y354, and Y397) and nonfruity (strains Y137, Y325, and Y349) control strains were included in the experiment. The most fruity segregants were subsequently used for the hybridization experiments.
Lab-scale ale fermentations.
Hybrid strains were tested in mimicked ale fermentations, performed in industrial wort of 21.5° Plato (21.5°P) supplied by a Belgian brewery. The wort was sampled aseptically from the brewery and sparged with air for 1 h to saturation prior to the fermentation experiments. The yeasts were propagated as follows: a single colony was used as inoculant for the preculture of each strain in 5 ml of YPGlu with 2% [wt/vol] glucose (2% YPGlu) medium. This preculture was grown for 16 h (shaking) at 30°C. Next, a second propagation step consisting of the inoculation of 0.5 ml of the first preculture in 50 ml of 2% YPGlu and incubation at 30°C for 16 h was executed. This culture was used for inoculation of the industrial wort at an OD600 of 0.5, roughly equivalent to 107 cells ml−1. Blank fermentations (containing no yeast inoculum) were included to detect potential contaminations. Fermentations were carried out statically at 20°C for 7 days in 250-ml Schott bottles, which were equipped with an Ankom gas production system (Ankom, NY, USA) for online measurement of gas production. The headspace of the fermentation was flushed with N2 prior to the experiment, and the overpressure on the bottles was held constant at 7.38 lb/in2. After 7 days, the fermentations were cooled on ice to prevent evaporation of volatiles, and samples for chromatographic analysis and density and ethanol measurements were taken. Lastly, descriptive sensory analysis of the leftover fermented medium was performed. All fermentations were performed in biological duplicates.
Analytical analysis of fermentation samples.
Headspace gas chromatography coupled with flame ionization detection (HS-GC-FID), calibrated for 15 important aroma compounds, including esters, higher alcohols, and acetaldehyde, was used for the quantification of yeast aroma production. The GC was equipped with a headspace autosampler (PAL system; CTC Analytics, Zwingen, Switzerland) and contained a DB-WAXeter column (length, 30 m; internal diameter, 0.25 mm; layer thickness, 0.5 μm [Agilent Technologies, Santa Clara, CA]), and N2 was used as the carrier gas. Samples were heated for 25 min at 70°C in the autosampler. The injector block and FID temperatures were both kept constant at 250°C. Samples of 5 ml were collected in 15-ml glass tubes containing 1.75 g of sodium chloride each. These tubes were immediately closed and cooled to minimize evaporation of volatile compounds. The oven temperature was held at 50°C for 5 min, after which it was increased to 80°C at 4°C min−1. Next, the temperature was increased to 200°C at 5°C min−1 and held at 200°C for 3 min. Results were analyzed with Agilent Chemstation software (Agilent, Santa Clara, CA).
Cell-to-cell mating.
The screened haploid segregants of both parental strains were streaked to single colonies on a YPD-agar plate. One colony of each segregant was picked, and both were mixed on a second YPD-agar plate. Ten microliters of distilled water was added to the mixed cell cultures to increase mixing efficiency. The plate was dried and incubated at room temperature for 24 h. A small fraction of the spot was picked with a toothpick and streaked to single colonies on a new YPD-agar plate. After 48 h of incubation, the diploid status of the resulting colonies was verified by mating type PCR.
Genetic fingerprinting.
Genomic DNA was extracted in a 96-well format, executed in a TissueLyser II instrument (Qiagen, Venlo, Netherlands) according to the manufacturer's instructions (ether extraction). Two types of PCR-based genetic fingerprinting were performed: interdelta analysis and R3 randomly amplified polymorphic DNA (RAPD-R3) analysis. The PCRs were executed in a C1000 Thermal Cycler (Bio-Rad, Hercules, CA). For the interdelta analysis, primers delta12 (5′-TCAACAATGGAATCCCAAC-3′) and delta21 (5′-CATCTTAACACCGTATATGA-3′) and the temperature profile described in Legras and Karst (14) were used. For RAPD-R3, primer R3 (5′-ATGCAGCCAC-3′) was used, with the temperature profile described in Corte et al. (15). The PCR products were visualized using QIAxcel Advanced Systems (Qiagen, Venlo, Netherlands).
Flow cytometry.
The DNA content of yeast was measured by staining of the DNA with propidium iodide (PI) combined with fluorescence-activated cell sorting (FACS). As a reference, S288c haploid and diploid strains were used.
Genetic stability test.
To confirm genetic stability, hybrids were grown in 250-ml Schott bottles containing 20 ml of industrial wort (21.5°P). Initially, a single colony of each of the hybrids was picked and inoculated in 5 ml of industrial wort and incubated overnight at 23°C with shaking. Next, this preculture was used to inoculate a first Schott bottle containing 20 ml of industrial wort at approximately an OD600 of 0.5 (∼107 cells ml−1) and grown at 23°C for 7 days, reaching a cell density of approximately 108 cells ml−1. Next, these cultures were resuspended in the medium, and 0.5 ml of each culture was transferred to the next batch of 20-ml industrial wort. This procedure was repeated six times to yield approximately 36 yeast generations in total. Next, genetic fingerprinting was used to compare the genotypes of the initial and stabilized hybrids. To check the homogeneity of the population, six isolates of the same population were genotyped.
Data analysis and data visualization.
Analysis of the genetic fingerprint data was executed using BioNumerics (Applied Maths, Sint-Martens-Latem, Belgium). Preprocessing of the GC data consisted of a log transformation and subsequent conversion to Z-scores to correct for noise, calculated as follows: Z-score = (X − μ)/σ, where X is the log-transformed concentration measurement, μ is the mean log-transformed value of all strains, and σ is the standard deviation of log-transformed values of all strains.
The BioNumerics software was used to analyze and cluster the strains based on their genotypes by using the Dice correlation coefficient to build a similarity matrix and an unweighted pair group method with arithmetic mean (UPGMA) algorithm for clustering.
Prior to data analysis of the aroma production in lab-scale fermentation experiments, interstrain differences in fermentation capacities were corrected for by equating total ethanol production to 5% (vol/vol) and normalizing all data accordingly. Cluster analysis of the aroma compounds was performed using the Pearson correlation coefficient for building the similarity matrix and UPGMA algorithm for clustering. Statistical analysis tests that were performed when the aroma data were analyzed were the Pearson's r test (correlation test), Shapiro-Wilk test (normality test), Mann-Whitney U test (test of stochastic equality), and Levene's test (test for equality of variances). Scatter, box, and dot plots were developed in R (16).
RESULTS
In this section, we present and analyze the diversity of aroma production in Saccharomyces yeasts, the correlation between the production of different compounds, and differences in aroma production between domesticated and nondomesticated strains and demonstrate the applicability of this data set to select parental strains for hybridization experiments.
Aroma production is a highly diverse trait and a domestication phenotype.
To develop new hybrid strains with superior aroma profiles, careful selection of the parental strains is of utmost importance. We therefore measured the production of different aroma-active acetate esters, ethyl esters, higher alcohols, and acetaldehyde in a collection of 301 different Saccharomyces cerevisiae, Saccharomyces pastorianus, and Saccharomyces paradoxus strains from various (industrial and natural) niches. The fermentation experiments were performed in rich growth medium (10% YPGlu) to avoid origin-specific biases in the experiment. An overview of the flavor profile of all strains is depicted in Fig. 1 and in Table S1 in the supplemental material.
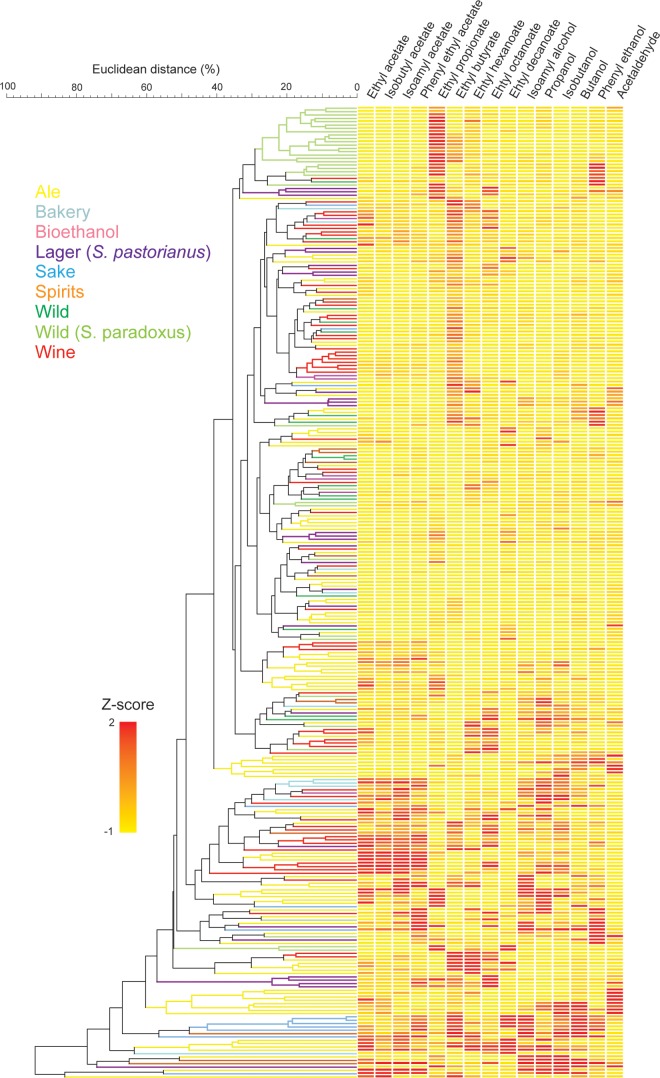
FIG 1.
Visual representation of the aroma production of the Saccharomyces yeasts analyzed in this study. All strains are S. cerevisiae strains unless noted differently. Measurements were converted into Z-scores, pairwise similarities were calculated by Euclidean distance, and a UPGMA clustering algorithm was applied to cluster the data.
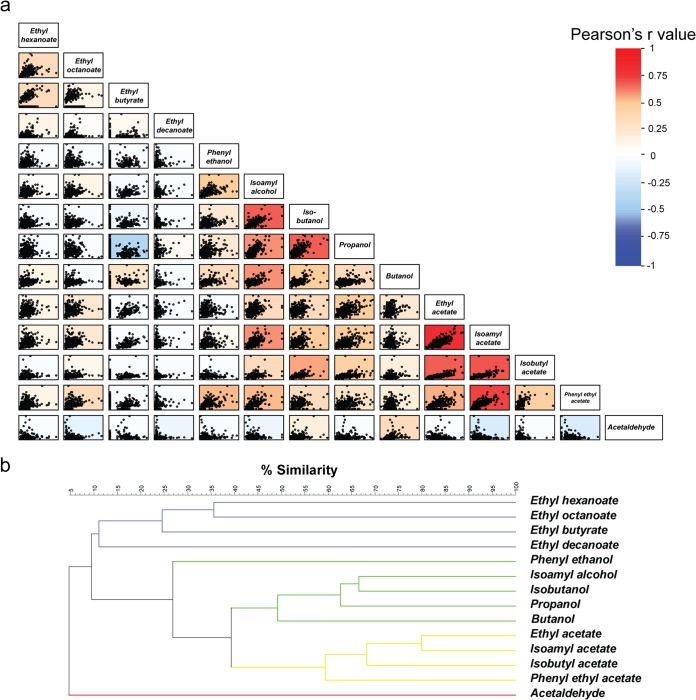
A first observation is that the production of aroma compounds belonging to the same group of molecules (ethyl esters, acetate esters, or higher alcohols) is correlated (Fig. 2). The highest correlation values were measured for different acetate esters. Moreover, the production of specific acetate esters also correlates with the production of the respective higher alcohol that serves as a substrate to produce the ester (e.g., r = 0.77 for IA and isoamyl alcohol production; P < 0.001). Interestingly, acetate esters formed from different precursors often show very high correlations (e.g., for IA and EA, r = 0.88 and P < 0.001). These data confirm the presence of a shared, but not identical, metabolic pathway of different acetate esters (e.g., IA and EA) and suggests that enzyme activity (Atf1 and/or Atf2), more than precursor concentration, is the main limiting factor for acetate ester production, as suggested earlier (5). Moreover, these correlations also indicate that it may often be difficult to change the production of one aroma compound without changing other compounds that show a high positive correlation.
FIG 2.
Correlation study of aroma compound production. (a) Pair plots of all aroma compounds. The correlation coefficient r is indicated by the color of each graph (red, positive correlation; blue, negative correlation; white, no correlation). (b) UPGMA clustering of the production of all aroma compounds. Colors indicate the type of aroma compound (red, aldehydes; yellow, acetate esters; green, higher alcohols; purple, ethyl esters).
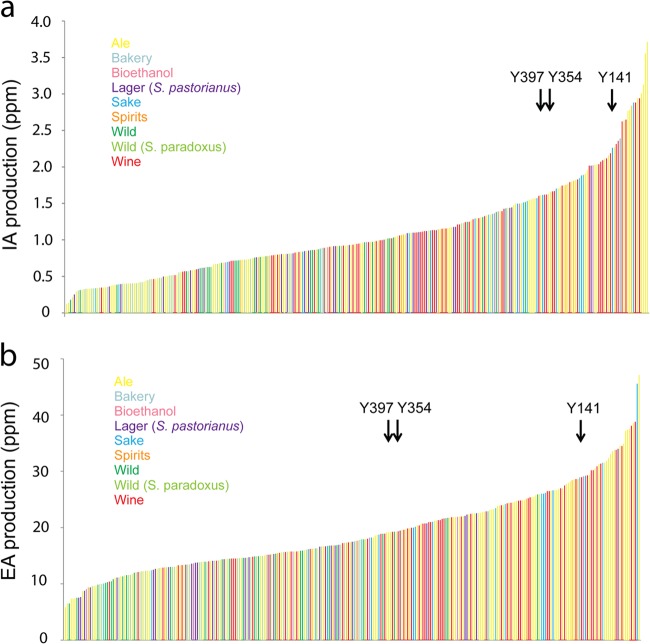
Two of the most important aroma compounds are IA (a fruity, banana-like aroma) and EA (an alcoholic, fruity, but in high concentrations also solvent-like aroma). Interestingly, the production of these aroma compounds is extremely variable among different Saccharomyces strains, with the production of IA and EA differing by 31- and 7-fold between the highest and lowest producing strains, respectively (Fig. 3).
FIG 3.
Different Saccharomyces strains show a wide range of isoamyl acetate (a) and ethyl acetate (b) production in 10% YPGlu fermentations. Strains are color-coded based on their origins. Arrows indicate the selected parental strains (cf. “Selection of parental strains to generate hybrids with high aroma production” below).
Figure 4 and Table S1 in the supplemental material show the difference in IA and EA production levels between all 301 tested Saccharomyces strains, revealing interesting trends. For example, S. paradoxus (genetically the most closely related species to S. cerevisiae) strains show, on average, only very low acetate ester production compared to S. cerevisiae. To assess this further, the IA and EA production profiles of 60 representative S. cerevisiae and S. paradoxus strains (13) were compared. It became apparent that S. cerevisiae generally produces significantly more IA and EA (P values for IA and EA production [PIA and PEA, respectively] of 0.0002 and 0.0007, respectively) than S. paradoxus (see Fig. S1 in the supplemental material). This trend remains when only nondomesticated S. cerevisiae strains were compared to S. paradoxus strains (data not shown). Furthermore, despite the low genetic diversity between S. cerevisiae yeasts compared to S. paradoxus strains (as determined by Liti et al. [13]), the aroma profile of S. cerevisiae yeasts is significantly more variable (i.e., shows higher trait variability) than that of S. paradoxus (PIA = 0.0062 and PEA = 0.0059).
FIG 4.
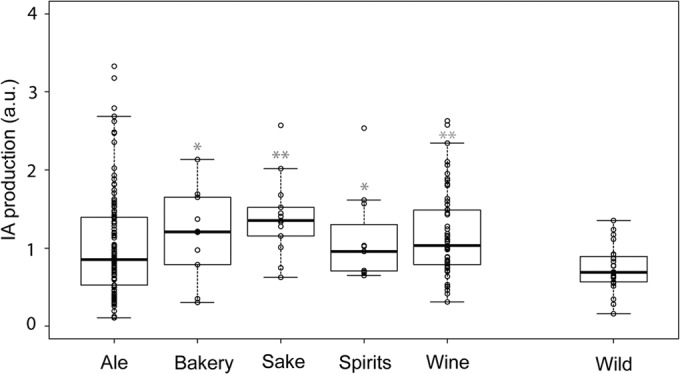
Comparison of isoamyl acetate production of Saccharomyces cerevisiae strains from different origins. Values are normalized to the average of all 301 yeasts in the data set. Statistical significance of differences between pairwise comparisons of domesticated strains (ale, bakery, sake, spirits, and wine) and nondomesticated strains (wild) is indicated. The asterisks indicate the level of significance (*, P < 0.05; **, P < 0.01).
Our data also provide an interesting basis to study whether industrial yeasts show signs of domestication and/or selection for increased aroma production. Analysis of the IA production of the S. cerevisiae strain collection (237 strains, of which 209 were industrial strains and 28 were wild strains) revealed a significant difference in production of this acetate ester between wild isolates and strains used in different industrial fermentation processes. Strains used in industrial food and beverage fermentations (the domesticated strains) generally produced more of this compound than wild (nondomesticated) strains (Fig. 4). For example, when the strains are grouped according to their origins, we found that strains used in the sake industry generally showed the highest IA production (PIA < 0.001 compared to wild isolates). Looking at individual strains, we found that the strains with the highest IA production, however, originate from the ale industry. These data suggest that production of certain desirable aroma compounds, such as IA, might have been selected for in industrial yeasts and is therefore a domestication phenotype. Additionally, the variability in IA production levels was higher in domesticated than in the nondomesticated S. cerevisiae strains, with the highest variability in strains used in the ale industry (coefficient of variation for IA production in ale [CVIA(Ale)] = 0.686, which is 77% more variable than for wild isolates, with PIA = 0.0022). This might indicate a selection for sensorial extremes in these ale yeasts, which may contribute to obtaining diverse ale types.
Interestingly, the production of acetaldehyde, a mostly undesirable flavor compound, did not show strong signs of selection except in wine strains, which show a significantly reduced production of acetaldehyde compared to wild strains (PAcetaldehyde = 0.0041), suggesting counterselection for the production of this compound.
Selection of parental strains to generate hybrids with high aroma production.
To generate hybrids with improved aroma production, three S. cerevisiae parental strains that showed high IA production (without excessively high EA production) and limited genetic relatedness (thereby maximizing the chance for a different genetic basis for their high aroma production) were selected (Fig. 5. Moreover, this selection was also based on the strain's ability to produce stable haploid segregants that are needed to generate hybrids using the cell-to-cell mating approach (T. Snoek, unpublished data). In total, 53 strains of the collection were shown to possess this ability. Based on these criteria, we selected three genetically diverse S. cerevisiae strains (Y141 originating from the sake industry and the ale beer strains Y354 and Y397) that produce high concentrations of IA (2.02×, 1.46×, and 1.43× the population average, respectively) as parental strains for the hybridization experiments (Fig. 3).
FIG 5.
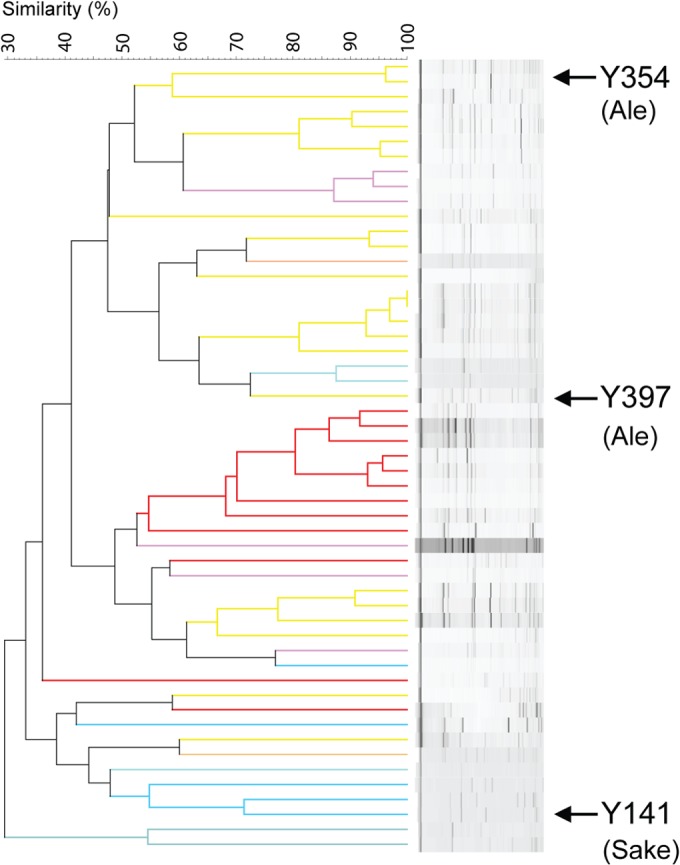
Genetic relatedness (based on interdelta analysis) of the 53 strains in the collection able to produce stable haploid segregants. The three strains selected for the breeding experiment are indicated. Color codes are identical to those on Fig. 1.
Generation of hybrid strains by cell-to-cell mating.
Complex, polygenic traits can vary significantly among meiotic segregants. Therefore, after sporulation and tetrad dissection, 142 segregants (93 of strain Y141, 29 of Y354, and 20 of Y397) were screened to identify the ones that showed high production of fruity aroma compounds. Seventeen haploid segregants (nine from Y141, six from Y354, and two from Y397) were subsequently selected for hybridization. Thirty different hybrids were constructed using the cell-to-cell mating procedure. Next, sporulation and subsequent screening of the segregants of two of these hybrids (H3 and H16) yielded eight more high-IA-producing haploids (six from H3 and two from H16), which were used to develop 16 more hybrids, bringing the total number of hybrids to 46 (see Table S2 in the supplemental material). For each of these 46 strains, we used interdelta fingerprinting to confirm the hybrid nature of the genomes. Furthermore, we used propidium iodide staining and flow cytometry to measure the DNA content of parental and hybrid strains. While most hybrids showed a DNA content corresponding to a diploid genome, aberrant genotypes indicative of haploid, diploid, or aneuploidy genomes were also observed. For example, while H31 (a hybrid of Y397 segregant 1 [Y397-S1] and Y141-S8) is a diploid strain, H32 (a hybrid of S397-S1 and Y141-S6) was identified to be triploid. A subset of these hybrids (H12, H20, H41, and H46) was subsequently checked for genetic stability by performing six consecutive fermentations (where the yeast strain was serially reinoculated into the next fermentation batch) in wort medium. No differences in the fingerprinting profiles were observed before and after the stabilization procedure, and a homogeneous yeast population was observed (examined using two different fingerprinting methods, interdelta analysis and RAPD-R3) (see Fig. S2 in the supplemental material). This suggests that the hybrids are genetically stable.
Outbreeding yields hybrid ale yeasts with superior aroma production.
To test the newly developed hybrids for their potential to produce aroma-rich ale beer and compare their performance to that of their respective parental strains, all hybrids and the parental strains were tested in duplicate in lab-scale wort fermentations mimicking industrial-scale ale fermentations. Three additional ale strains, designated Y137, Y325, and Y349, were included in the experiments and were used together with the ale parental strains Y354 and Y397 as reference strains for the fermentation performance (ale reference strains). The aroma profile was measured analytically using HS-GC-FID as well as by sensory analysis. Moreover, we also monitored the kinetics of the fermentations to evaluate the hybrid's fermentation efficiency in ale fermentations (Fig. 6). The results show that the hybrids differ greatly in their fermentation efficiencies, with most hybrids performing at levels similar to those of the parental strains but others showing significantly worse or better fermentation efficiencies.
FIG 6.
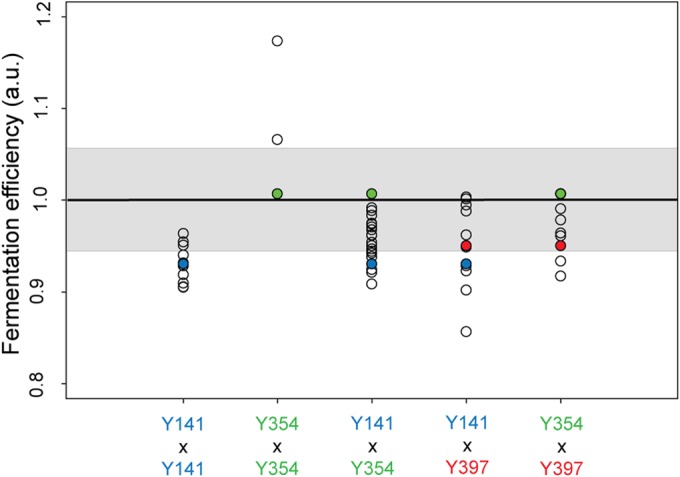
Fermentation characteristics of the 46 developed hybrids (open circles) and the three respective parental strains (colored circles). The total volume of gas produced (a proxy for the conversion of sugars into CO2 and ethanol) during the fermentations was measured using an automated online system (see Materials and Methods for details). All values were normalized to the average value of five ale reference strains (Y137, Y325, Y349, Y354, and Y397). Gray shading indicates 2 standard deviations of values for the five reference ale strains. a.u., arbitrary units.
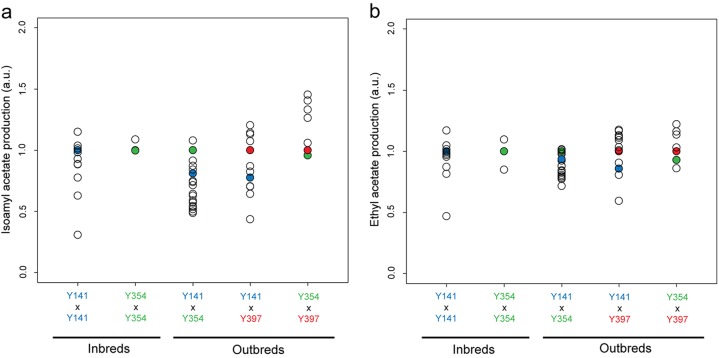
Analysis of the aroma production of the 46 different hybrid strains revealed that inbreeding yielded only minor changes in aroma production (even when multiple rounds of inbreeding were performed) (see Table S2 in the supplemental material). In contrast, outbreeding, especially when Y397 was used as one of the parental strains, resulted in large differences in aroma production, with some hybrids showing IA concentrations exceeding the levels of the parental strains (Fig. 7). Production of other acetate esters, such as EA, was also increased, although to a lesser extent. A summary of the 10 strains with the most pronounced fruity aroma profile, their respective parental strains, and reference strains (ale strains with average acetate ester production) is given in Table 2.
FIG 7.
Production levels of isoamyl acetate (a) and ethyl acetate (b) of the 46 different hybrids. The concentrations are normalized to the value of the parent with the highest production of this compound. Thus, hybrids scoring more than 1 show best-parent heterosis. a.u., arbitrary units.
TABLE 2.
Characteristics of the 10 hybrids with highest production of isoamyl acetate as measured in the industrial wort fermentationsa
| Strain group and name | EA production (ppm) | IA production (ppm) | Fermentation efficiency (au)b |
|---|---|---|---|
| Parental strains (source) | |||
| Y141 (sake) | 12.21 ± 2.88 | 1.47 ± 0.45 | 0.94 ± 0.05 |
| Y354 (ale) | 13.24 ± 4.91 | 1.81 ± 0.32 | 1.02 ± 0.00 |
| Y397 (ale) | 14.25 ± 2.25 | 1.89 ± 0.32 | 0.96 ± 0.00 |
| Additional ale reference strains | |||
| Y137 | 6.80 ± 1.59 | 0.32 ± 0.09 | 1.03 ± 0.06 |
| Y325 | 6.01 ± 1.19 | 0.27 ± 0.03 | 1.03 ± 0.00 |
| Y349 | 10.19 ± 0.78 | 0.39 ± 0.07 | 1.02 ± 0.04 |
| Y354 × Y397 strains | |||
| H41 | 16.55 ± 2.64 | 2.65 ± 0.44 | 0.97 ± 0.03 |
| H42 | 12.27 ± 4.66 | 2.00 ± 0.66 | 0.99 ± 0.00 |
| H43 | 16.20 ± 1.33 | 2.39 ± 0.39 | 0.95 ± 0.01 |
| H44 | 17.39 ± 1.13 | 2.75 ± 0.12 | 0.93 ± 0.02 |
| H45 | 14.69 ± 3.32 | 2.52 ± 0.09 | 0.98 ± 0.01 |
| H46 | 16.08 ± 1.73 | 2.16 ± 0.37 | 1.00 ± 0.01 |
| Y141 × Y397 strains | |||
| H34 | 15.82 ± 1.81 | 2.13 ± 0.42 | 0.87 ± 0.12 |
| H35 | 16.64 ± 1.07 | 2.28 ± 0.33 | 0.97 ± 0.01 |
| H37 | 14.38 ± 1.60 | 2.03 ± 0.32 | 0.96 ± 0.00 |
| H39 | 15.54 ± 3.42 | 2.03 ± 0.38 | 0.94 ± 0.02 |
Values for the parental strains and three additional reference ale yeasts are also included.
Fermentation efficiency is represented as the total gas production at the end of the fermentation, relative to the average of the five ale reference strains. au, arbitrary units.
Fourteen hybrids (∼30%) showed best-parent heterosis for IA production. Hybrid H44, a hybrid strain developed from Y354-S5 and Y397-S2, showed the greatest increase in the production of IA (2.745 ppm) compared to both parental strains (152% and 145% higher IA production than that of the parental strains Y354 and Y397, respectively). Similarly, H45, a hybrid strain developed from Y354-S5 and Y397-S1, showed 39% and 33% increases in IA production compared to its parents Y354 and Y397, respectively. Since this strain showed normal fermentation kinetics, it may be an interesting candidate for the efficient production of ale beers with distinct fruity notes.
DISCUSSION
The beer market has become global and increasingly competitive, with a high demand for new, distinctive products (17). New yeast variants, capable of producing distinct flavor profiles, enable brewers to tailor the sensory characteristics of their products and develop novel, highly aromatic specialty beers. This study describes the development of 46 new hybrids using a large-scale selective breeding approach, starting from three parental strains selected from 301 candidate Saccharomyces strains on the basis of their aroma production, genetic relatedness, and sexual life cycle. Some of the resulting hybrids showed increased IA production and appear directly applicable for commercial beer fermentations.
The quantitative analysis of the aroma production of a large collection of different wild and industrial Saccharomyces strains also yielded a deeper understanding of aroma production in yeast. First, the production of IA was revealed to be a so-called domestication phenotype, a phenotype that has been selected for by humans. The production of certain aromatic compounds was previously suggested to be a domestication phenotype for wine strains (18), but here it was shown that S. cerevisiae strains used in the production of ale, bread, sake, and spirits also generally produce more IA than their wild counterparts. While the high production of fruity aromas of yeast strains used to produce fermented beverages is perhaps not surprising given the importance of these compounds for the organoleptic quality of beverages, it was surprising to discover the same trend for bread yeasts. Despite the recent report on the (slight) influence of baker's yeast on bread's ester profile (19), these volatile esters are generally quickly evaporated during the baking process and are therefore present only in a concentration well below the detection limit. The high production of flavor compounds by baker's yeasts is therefore more likely a consequence of their close link with beer yeasts. Indeed, baker's yeasts have been proposed to originate from a relatively recent allotetraploidization event between domesticated ale and wine strains (20).
Domestication phenotypes in industrial yeasts have been proposed to be artifacts of population bottlenecks, allowing many loss-of-function mutations to get fixed in the population by genetic drift (21, 22). Many previously described domestication phenotypes might, therefore, merely reflect the confounding influence of a shared population history, and thus a bias in the strains analyzed, and are not caused by a selection for this phenotype in the fermentation environment. To estimate the potential effect of a bias in our strain collection due to shared population histories, we repeated our analysis with strains showing <80% similarity of their genetic fingerprint (calculated using the Dice correlation) to diminish the number of genetically identical or very similar strains in the collection. Whereas this correction reduced the number of strains analyzed by 43%, the average IA production of strains from different origins did not show important changes. This suggests that IA production may be a true domestication phenotype that has been selected for in several industrial processes.
The production of acetate esters such as IA and EA was found to be both significantly higher and significantly more diverse in S. cerevisiae than in S. paradoxus. These results are in line with previous studies (13, 21), where S. paradoxus strains, despite their greater single-nucleotide polymorphism (SNP) diversity, generally show less phenotypic variability than S. cerevisiae. However, S. paradoxus generally produces more ethyl esters (especially ethyl propionate). Whereas most genetic studies on aroma production focus on S. cerevisiae, our data highlight that a quantitative trait locus (QTL) analysis of ethyl ester production in S. paradoxus could lead to the identification of superior alleles for this industrially relevant phenotype.
Our results also show that targeted cell-to-cell mating is a promising approach to obtain industrial yeasts with superior aroma production. The single biggest advantage of this approach is the phenotyping of the haploid segregants of the selected parents prior to the breeding experiment (23). Since parental traits can be transferred to the F1 generation unequally due to allele segregation during meiosis, this additional screening step increases the chances of obtaining superior hybrids. Indeed, after sporulation, only ∼10% (Y397) or ∼20% (Y141 and Y354) of the segregants showed a sensory profile with a level of fruitiness similar to or higher than that of the parental strain. This indicates that production of IA is a polygenic trait and underscores the importance of haploid segregant screening. Additionally, haploid-haploid mating (especially between segregants from the same species) yields hybrids which are genetically more stable than hybrids developed with other hybridization techniques, such as rare mating or protoplast fusion of diploid cells (24). As a consequence, the risk of losing phenotypes of interest by genome rearrangements in the hybrids is reduced. Indeed, the proliferation phase on agar plates directly after the mating procedure and subsequent separation in single-cell-derived colonies, equivalent to approximately 40 yeast generations, is probably sufficient to establish a stable hybrid genome (at least in this experimental setup), and further stabilization in liquid wort medium is not necessary.
Interestingly, the ploidy level of the developed hybrids, even between hybrids originating from the same parental strains, was variable. In most cases the obtained hybrids were diploid, but in some cases, triploid hybrids were identified. This might be explained by the surprising and interesting observation that some haploid segregants (especially those originating from Y397) have the ability to relatively quickly duplicate their genome and form diploids with only one mating type (a/a or α/α) (data not shown), a phenomenon often encountered in experimental evolution experiments (25). Therefore, some hybrids might be the result of a hybridization event between two haploid segregants, while others might result from hybridization between a haploid and a diploid cell, homozygous at the mating type locus.
When hybrids originating from the same parents (inbreds) and hybrids from two different parents (outbreds) are compared, it becomes apparent that inbreeding generally does not yield strongly increased IA production (at least not for the selected parental strains in this study), with a maximum of a 15% IA increase for H2 (a Y141 inbred). Further inbreeding of these strains (e.g., H9, a hybrid of selected segregants derived from H3) (see Table S2 in the supplemental material) did not yield significant further improvements. It has to be noted that we could not obtain inbreds of Y397 since only haploid segregants with mating type a could be isolated from this strain. On the other hand, when Y397 segregants were crossed with Y141 or Y354 segregants, several hybrids showing a significant best-parent heterosis effect for IA production were identified (e.g., H44 showed a 45% increase of IA). Such best-parent heterosis has already been observed for other phenotypes in S. cerevisiae hybridization experiments (26, 27), but it does not seem to occur for all traits (28). However, it was recently shown that best-parent heterosis occurs relatively frequently when genetically distant domesticated strains are crossed, while the incidence is lower in wild strains (29). Interestingly, this extreme effect could not be detected in hybrids of Y141 and Y354, possibly because these strains share the same genetic polymorphisms that are driving their aroma production or because of complex epistasis between their polymorphisms.
It is important that there are several potential side effects of hybridization. First, selective breeding contains the risk of yielding so-called crippled strains, i.e., strains that show improvement for the selected trait but perform worse for other industrially important phenotypes that were not selected for. This was encountered, for example, in the study of Bellon et al. (30), where three out of five developed hybrids showed inferior fermentation performance. Therefore, the fermentation kinetics of the newly developed hybrids was monitored during the ale fermentations. These measurements indicate that although some hybrids show defects in their fermentation efficiencies, most hybrids show fermentation profiles that are comparable to the established ale production strains used as a reference in this study.
A second potential risk of selecting for a specific phenotype is the hitchhiking of undesired traits. In our case, selection for IA is often accompanied by an increase in other acetate esters, especially EA (Pearson r of 0.88; P < 0.001) (Fig. 2) since these compounds share part of their metabolic pathway. Despite the fact that EA often contributes positively to the flavor of fermented products, it can sometimes be perceived negatively because it can impart a solvent-like aroma when it is present in high concentrations. However, in our study, EA production of the hybrids did not reach critically high levels; the maximal concentration measured was 17.39 ppm in H44, while the odor threshold was measured to be approximately 30 ppm in beer (31). Indeed, solvent-like off-flavors were not detected during the sensory analyses of the fermentation products, while distinct notes of banana and pineapple (characteristic for high IA levels) were clearly perceived (data not shown). Therefore, in these lower concentrations, EA does not induce any detectable off-flavor but, rather, contributes to the overall flavor balance and is perceived as sweet or fruity.
In conclusion, our results provide an overview of the natural diversity in yeast aroma production and put this knowledge into practice by demonstrating how targeted breeding, a non-GMO approach, yields ale yeasts with superior IA production without increasing EA production to undesired levels. These new strains are readily usable in industrial applications, where they could be used to brew new, fruity types of ale beer or to help obtain more flavorful low-alcohol beers.
Supplementary Material
ACKNOWLEDGMENTS
We thank all Verstrepen lab members for their help and suggestions.
Research in the lab of K.J.V. is supported by Barry Callebaut, ERC Starting Grant 241426, VIB, EMBO YIP program, FWO, and Human Frontier Science.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 5 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02235-14.
REFERENCES
- 1.Verstrepen KJ, Derdelinckx G, Dufour JP, Winderickx J, Thevelein JM, Pretorius IS, Delvaux FR. 2003. Flavor-active esters: adding fruitiness to beer. J. Biosci. Bioeng. 96:110–118. 10.1016/S1389-1723(03)90112-5. [DOI] [PubMed] [Google Scholar]
- 2.Nordström K. 1964. Formation of esters from alcohols by brewer's yeast. J. Inst. Brew. 70:328–336. 10.1002/j.2050-0416.1964.tb01999.x. [DOI] [Google Scholar]
- 3.Fujii T, Nagasawa N, Iwamatsu A, Bogaki T, Tamai Y, Hamachi M. 1994. Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl. Environ. Microbiol. 60:2786–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilly M, Bauer FF, Lambrechts MG, Swiegers JH, Cozzolino D, Pretorius IS. 2006. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 23:641–659. 10.1002/yea.1382. [DOI] [PubMed] [Google Scholar]
- 5.Verstrepen KJ, Van Laere SD, Vanderhaegen BM, Derdelinckx G, Dufour JP, Pretorius IS, Winderickx J, Thevelein JM, Delvaux FR. 2003. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. 69:5228–5237. 10.1128/AEM.69.9.5228-5237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossouw D, Bauer FF. 2009. Comparing the transcriptomes of wine yeast strains: toward understanding the interaction between environment and transcriptome during fermentation. Appl. Microbiol. Biotechnol. 84:937–954. 10.1007/s00253-009-2204-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang CY, Liu YL, Qi YN, Zhang JW, Dai LH, Lin X, Xiao DG. 2013. Increased esters and decreased higher alcohols production by engineered brewer's yeast strains. Eur. Food Res. Technol. 236:1009–1014. 10.1007/s00217-013-1966-1. [DOI] [Google Scholar]
- 8.Hirosawa I, Aritomi K, Hoshida H, Kashiwagi S, Nishizawa Y, Akada R. 2004. Construction of a self-cloning sake yeast that overexpresses alcohol acetyltransferase gene by a two-step gene replacement protocol. Appl. Microbiol. Biotechnol. 65:68–73. 10.1007/s00253-004-1563-0. [DOI] [PubMed] [Google Scholar]
- 9.Steensels J, Snoek T, Meersman E, Nicolino MP, Voordeckers K, Verstrepen KJ. 11 April 2014. Improving industrial yeast strains: exploiting natural and artificial diversity. FEMS Microbiol. Rev. 10.1111/1574-6976.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley D, Fraser S, Chambers PJ, Rogers P, Stanley GA. 2010. Generation and characterisation of stable ethanol-tolerant mutants of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 37:139–149. 10.1007/s10295-009-0655-3. [DOI] [PubMed] [Google Scholar]
- 11.Aarnio T, Suikho M, Kauppinen V. 1991. Isolation of acetic acid tolerant baker's yeast variants in a turbidostat. Appl. Biochem. Biotechnol. 27:55–63. 10.1007/BF02921515. [DOI] [Google Scholar]
- 12.Adamo GM, Brocca S, Passolunghi S, Salvato B, Lotti M. 2012. Laboratory evolution of copper tolerant yeast strains. Microb. Cell Fact. 11:1. 10.1186/1475-2859-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liti G, Carter DM, Moses AM, Warringer J, Parts L, James SA, Davey RP, Roberts IN, Burt A, Koufopanou V, Tsai IJ, Bergman CM, Bensasson D, O'Kelly MJ, van Oudenaarden A, Barton DB, Bailes E, Nguyen AN, Jones M, Quail MA, Goodhead I, Sims S, Smith F, Blomberg A, Durbin R, Louis EJ. 2009. Population genomics of domestic and wild yeasts. Nature 458:337–341. 10.1038/nature07743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legras JL, Karst F. 2003. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol. Lett. 221:249–255. 10.1016/S0378-1097(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 15.Corte L, Lattanzi M, Buzzini P, Bolano A, Fatichenti F, Cardinali G. 2005. Use of RAPD and killer toxin sensitivity in Saccharomyces cerevisiae strain typing. J. Appl. Microbiol. 99:609–617. 10.1111/j.1365-2672.2005.02631.x. [DOI] [PubMed] [Google Scholar]
- 16.R Development Core Team. 2004. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 17.Tremblay CH, Tremblay VJ. 2011. Recent economic developments in the import and craft segment of the US brewing industry, p 141–160 In Swinnen J. (ed), The economics of beer. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 18.Hyma KE, Saerens SM, Verstrepen KJ, Fay JC. 2011. Divergence in wine characteristics produced by wild and domesticated strains of Saccharomyces cerevisiae. FEMS Yeast Res. 11:540–551. 10.1111/j.1567-1364.2011.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birch AN, Petersen MA, Arneborg N, Hansen ÅS. 2013. Influence of commercial baker's yeasts on bread aroma profiles. Food Res. Int. 52:160–166. 10.1016/j.foodres.2013.03.011. [DOI] [Google Scholar]
- 20.Randez-Gil F, Cocoles-Saz I, Prieto JA. 2013. Genetic and phenotypic characteristics of baker's yeast: relevance to baking. Annu. Rev. Food Sci. Technol. 4:191–214. 10.1146/annurev-food-030212-182609. [DOI] [PubMed] [Google Scholar]
- 21.Warringer J, Zorgo E, Cubillos FA, Zia A, Gjuvsland A, Simpson JT, Forsmark A, Durbin R, Omholt SW, Louis EJ, Liti G, Moses A, Blomberg A. 2011. Trait variation in yeast is defined by population history. PLoS Genet. 7:e1002111. 10.1371/journal.pgen.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zörgö E, Gjuvsland A, Cubillos FA, Louis EJ, Liti G, Blomberg A, Omholt SW, Warringer J. 2012. Life history shapes trait heredity by accumulation of loss-of-function alleles in yeast. Mol. Biol. Evol. 29:1781–1789. 10.1093/molbev/mss019. [DOI] [PubMed] [Google Scholar]
- 23.Lindegren CC, Lindegren G. 1943. A new method for hybridizing yeast. Proc. Natl. Acad. Sci. U. S. A. 29:306–308. 10.1073/pnas.29.10.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Traves L, Lopes CA, Barrio E, Querol A. 2012. Evaluation of different genetic procedures for the generation of artificial hybrids in Saccharomyces genus for winemaking. Int. J. Food Microbiol. 156:102–111. 10.1016/j.ijfoodmicro.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Gerstein AC, Chun H-JE, Grant A, Otto SP. 2006. Genomic convergence toward diploidy in Saccharomyces cerevisiae. PLoS Genet. 2:e145. 10.1371/journal.pgen.0020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marullo P, Bely M, Masneuf-Pomarede I, Pons M, Aigle M, Dubourdieu D. 2006. Breeding strategies for combining fermentative qualities and reducing off-flavor production in a wine yeast model. FEMS Yeast Res. 6:268–279. 10.1111/j.1567-1364.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- 27.Timberlake WE, Frizzell MA, Richards KD, Gardner RC. 2011. A new yeast genetic resource for analysis and breeding. Yeast 28:63–80. 10.1002/yea.1821. [DOI] [PubMed] [Google Scholar]
- 28.Zörgö E, Gjuvsland A, Cubillos FA, Louis EJ, Liti G, Blomberg A, Omholt SW, Warringer J. 2012. Life history shapes trait heredity by accumulation of loss-of-function alleles in yeast. Mol. Biol. Evol. 29:1781–1789. 10.1093/molbev/mss019. [DOI] [PubMed] [Google Scholar]
- 29.Plech M, de Visser JAG, Korona R. 2013. Heterosis is prevalent among domesticated but not wild strains of Saccharomyces cerevisiae. G3 (Bethesda) 4:315–323. 10.1534/g3.113.009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellon JR, Schmid F, Capone DL, Dunn BL, Chambers PJ. 2013. Introducing a new breed of wine yeast: interspecific hybridisation between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS One 8:e62053. 10.1371/journal.pone.0062053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meilgaard MC. 1975. Flavor chemistry of beer. Part II: flavor and threshold of 239 aroma volatiles. MBAA Tech. Q. 12:151–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.