Abstract
Genetic characterization was performed for 10 group I Clostridium botulinum strains isolated from botulism cases in Japan between 2006 and 2011. Of these, 1 was type A, 2 were type B, and 7 were type A(B) {carrying a silent bont/B [bont/(B)] gene} serotype strains, based on botulinum neurotoxin (BoNT) production. The type A strain harbored the subtype A1 BoNT gene (bont/A1), which is associated with the ha gene cluster. The type B strains carried bont/B5 or bont/B6 subtype genes. The type A(B) strains carried bont/A1 identical to that of type A(B) strain NCTC2916. However, bont/(B) genes in these strains showed single-nucleotide polymorphisms (SNPs) among strains. SNPs at 2 nucleotide positions of bont/(B) enabled classification of the type A(B) strains into 3 groups. Pulsed-field gel electrophoresis (PFGE) and multiple-locus variable-number tandem-repeat analysis (MLVA) also provided consistent separation results. In addition, the type A(B) strains were separated into 2 lineages based on their plasmid profiles. One lineage carried a small plasmid (5.9 kb), and another harbored 21-kb plasmids. To obtain more detailed genetic information about the 10 strains, we sequenced their genomes and compared them with 13 group I C. botulinum genomes in a database using whole-genome SNP analysis. This analysis provided high-resolution strain discrimination and enabled us to generate a refined phylogenetic tree that provides effective traceability of botulism cases, as well as bioterrorism materials. In the phylogenetic tree, the subtype B6 strains, Okayama2011 and Osaka05, were distantly separated from the other strains, indicating genomic divergence of subtype B6 strains among group I strains.
INTRODUCTION
Clostridium botulinum bacteria are Gram-positive, spore-forming, anaerobic bacteria that produce extremely potent proteinaceous neurotoxins known as botulinum neurotoxins (BoNTs). These BoNTs cause a neuroparalytic illness, botulism, in humans and animals (1, 2). Because of their high toxicities, BoNTs and C. botulinum are considered potential weapon materials for bioterrorism and are managed under strict biosafety regulations (3, 4). BoNTs are classified into 7 distinct serotypes (BoNT/A to BoNT/G) based on their immunological specificity (5). A novel serotype, BoNT/H, has also been reported recently (6, 7). The nucleotide sequences of the BoNT gene (bont) vary within each serotype, allowing further classification of BoNTs into subtypes or genetic variants (8, 9).
Most C. botulinum strains produce a single serotype of BoNT; however, several strains, such as type Ab, Af, Ba, and Bf bivalent strains, express more than 1 toxin serotype. The uppercase letter denotes the dominant toxin type. Additionally, some type A strains carry a type B bont gene (bont/B) that does not produce active toxin proteins due to mutations or truncations. Type A strains carrying the silent bont/B [bont/(B)] gene are designated type A(B) strains (10, 11). The bont genes exist in several specific locations of the C. botulinum genome (on a chromosome or plasmid), depending on the strain type. In type A(B) strains, the bont/A and bont/(B) genes are located in the arsC and oppA/brnQ operon, respectively, on the chromosome (12). In these genomic locations, these genes are associated with toxin accessory genes and form toxin gene clusters. Two main toxin gene clusters, ha and orfX, are known (13–16).
C. botulinum strains are also classified into 4 groups (I to IV) based on their biochemical and physiological properties. Strains in groups I and II primarily cause human botulism (17, 18). Group I strains are proteolytic and produce A, B, or F toxins, whereas group II strains are nonproteolytic and produce B, E, or F toxins. There are 4 naturally occurring forms of botulism in humans (19, 20). Food-borne botulism and infant botulism are common forms of botulism and are reported relatively often in many areas of the world. On the other hand, adult intestinal colonization (or adult intestinal toxemia) and wound botulism are rare forms of botulism. There are also reports of botulism caused by accidental overdose of BoNTs during medical and cosmetic use (21, 22).
Several large outbreaks of food-borne botulism were reported in Japan between 1951 and 1985. Most of these outbreaks occurred in the northern area of Japan and were type E botulism caused by the traditional fermented fish food izushi (23, 24). Food-borne botulism became sporadic after this period, although a large outbreak of type B botulism caused by imported preserved green olives occurred in 1998 (18 patients, no deaths) (25). Since 2000, only 2 food-borne cases (3 patients, no deaths) have been reported to date (26). The first case of infant botulism in Japan was reported in 1986 (27); thereafter, 31 additional infant botulism cases have been reported to date (28). These cases included 21 type A (68%), 5 type B (16%), and 5 other or unknown types (16%). No deaths from infant botulism have been reported in Japan. Although no cases of wound or adult intestinal colonization botulism have been officially reported in Japan, 3 cases of unclassified adult botulism were reported between 2008 and 2013 (3 patients, 1 death). Botulism is a rare disease in Japan, and the isolation of C. botulinum is infrequently reported. Therefore, detailed genetic characterization of recently isolated clinical strains in Japan is of significance both for genotype comparison with strains from other areas and for better understanding of botulism's epidemiology. In this study, we performed detailed characterizations of 10 group I C. botulinum strains isolated in Japan between 2006 and 2011.
MATERIALS AND METHODS
Bacterial strains.
The C. botulinum strains analyzed in this study are listed in Table 1. These strains were isolated from 9 independent botulism cases in Japan. The isolation of C. botulinum strains from clinical and environmental specimens was performed with a conventional culture method using cooked meat (CM) medium (Becton Dickinson, Sparks, MD) and Gifu anaerobic medium (GAM) and Clostridium welchii egg yolk agar plates (Nissui Pharmaceutical, Tokyo, Japan). The BoNT serotype of each strain was determined with a mouse protection assay (19) using standard anti-BoNT antiserum (National Institute of Infectious Diseases [NIID], Tokyo Japan). All animal experiments were conducted with the approval of the NIID Institutional Animal Care and Use Committee. The presence of corresponding bont genes in these strains was confirmed using PCR methods (29, 30).
TABLE 1.
C. botulinum strains isolated in Japan between 2006 and 2011
| Strain | BoNT serotypea | bont subtypeb | Type of botulismc (source of isolate) |
|---|---|---|---|
| Miyagi2006 | A(B) | A1(B) | IB (feces) |
| Miyagi2006-01 | A(B) | A1(B) | IB (well water at patient's house) |
| Iwate2007 | A(B) | A1(B) | IB (feces) |
| Ibaraki2007 | A(B) | A1(B) | IB (feces) |
| Tochigi2008 | A(B) | A1(B) | Adult botulism of unknown cause (feces) |
| Iwate2008 | A(B) | A1(B) | IB (feces) |
| Fukuoka2010 | A(B) | A1(B) | IB (feces) |
| Okayama2011 | B | B6 | IB (feces) |
| Ehime2011 | B | B5 | IB (feces) |
| Aichi2011 | A | A1 | IB (feces) |
Examined with mouse protection assay and PCR.
Examined by sequencing bont genes.
IB, infant botulism.
DNA extraction.
Before DNA preparation, CM medium cultures of the C. botulinum strains were spread on Clostridium welchii egg yolk agar plates and incubated at 37°C under anaerobic condition to form colonies. A single colony of each strain was selected, inoculated into CM medium, and cultured at 37°C for 24 to 48 h. After growth in CM medium, strains were inoculated into brain heart infusion (BHI) medium (Becton Dickinson) and cultured anaerobically at 37°C until the mid-log phase. Genomic DNAs were extracted from 1 ml of BHI culture using a DNeasy blood and tissue kit (Qiagen, Hilden, Germany) with pretreatment for Gram-positive bacteria according to the manufacturer's instructions. Small plasmid DNAs were extracted from the same BHI culture using a QIAprep spin miniprep kit (Qiagen) according to the manufacturer's instructions, with slight modifications. Briefly, 20 mg/ml lysozyme in buffer P1 (bacterial pellet resuspension buffer) was added and the mixture was incubated at 37°C for 15 min before the addition of buffer P2 (lysis buffer). Plasmid DNA fractions were concentrated via ethanol precipitation depending on yield.
PCR analysis and DNA sequencing.
PCR amplification of the orfX and ha cluster regions was performed using the primer pairs listed in Table 2. PrimeStar HS DNA polymerase (TaKaRa Bio, Tokyo, Japan) was used for PCR under the following conditions: 30 cycles at 98°C for 10 s, 55°C for 15 s, and 72°C for 4 min. PrimeStar Max DNA polymerase (TaKaRa Bio) was also used for PCR under the following conditions: 30 cycles at 98°C for 10 s, 55°C for 15 s, and 72°C for 25 s. Amplified DNA fragments that contained bont genes (X4 and H3 fragments) were sequenced with a primer-walking strategy using an ABI 3130xl genetic analyzer (Applied Biosystems, Foster City, CA). The sequencing primers are listed in Table S1 in the supplemental material. The detection of 21-kb plasmids (pMI06, pMI06-01, and pTO08) in type A(B) strains was also performed with PCR using PrimeStar Max DNA polymerase and the primer pairs listed in Table 2. The conditions were 30 cycles at 98°C for 10 s, 55°C for 15 s, and 72°C for 30 s.
TABLE 2.
Oligonucleotide DNA primers used in this study
| PCR targeta | Primer |
|---|---|
| orfX cluster | |
| X1F | GTTCCTCCTAATTGAAGAGG |
| X1R | AATTCCACACTAAATGGTAC |
| X2F | TTAGTTTGAATTCTAGATTT |
| X2R | GCTGTATAATAATGTTGATT |
| X3F | AGGAAATGCAAATTAAAGAA |
| X3R | CCATTTACAGGATCTTTATA |
| X4F | GTGAAGAACGATTAGAATAC |
| X4R | TTCTTGACAGACTCATGTAG |
| ha cluster | |
| H1F | AAGCTGGTGGACTCTTATTT |
| H1R | TATACTTAAGTTGTCATTTA |
| H2F | ATGATAAGAATATACTGAAA |
| H2R | TTATTTTAAGTTTATATCCC |
| H3F | GGTGCTAAGTTTAAATTAAT |
| H3R | TTATTCAGTCCACCCTTCAT |
| 21-kb plasmids of type A(B) strains | |
| 1F | TCTATGACTAGATTCTTTTC |
| 1R | CTTGCTGAGTAAGGTCTATG |
| 2F | GCGATACCAGTTGCCCACGC |
| 2R | GCTTTTCCCTTTATCTCTTC |
| 3F | AAGATCTTGAGAAATTAGTA |
| 3R | CAATAATTGAACTTGATAAG |
| 4F | AAGACGTTATAATATTAAGA |
| 4R | TAGCTTTGCCGCTATATATT |
| 5F | CTTTATTTATGGAATAGTTA |
| 5R | ATTGTTATTAAAACTATTTT |
| 6F | AATAGGAAGTAGAAGTTTTA |
| 6R | TAATGACTACACTAAATATC |
Forward (F) and reverse (R) primers for each amplicon are shown.
PFGE and MLVA of type A(B) strains.
Pulsed-field gel electrophoresis (PFGE) was performed based on a protocol reported by Johnson et al. (31). The restriction endonuclease SmaI was used to digest genomic DNA. Fingerprinting II software (Bio-Rad, Hercules, CA) was used to compare PFGE patterns and draw the dendrogram. Multiple-locus variable-number tandem repeat analysis (MLVA) was performed based on a protocol reported by Macdonald et al. (32). Ten genomic regions containing variable-number tandem repeat (VNTR) markers were amplified by the reported primers (32) and sequenced. The lengths of these amplified fragments were compared between strains. A dendrogram was created with the Ward method using R software.
Whole-genome sequencing and single-nucleotide polymorphism (SNP) analysis.
DNA libraries of the ∼600-bp-long inserts of the C. botulinum strains were prepared using a Nextera XT sample preparation kit (Illumina, San Diego, CA). The sequencing run for paired-end reads was performed using an Illumina Genome Analyzer IIx (GA IIx) and TruSeq SBS kit version 5 or an Illumina MiSeq and MiSeq reagent kit version 2 (Illumina) according to the manufacturer's instructions. To construct simulated paired-end reads from the available genomic sequences of other C. botulinum strains, we used Maq software (version 0.7.1) (33) with the “Maq simulate” command and the following modifications of the default parameters: number of pairs of reads, N 10,000,000; mutation rate, r 0; fraction of 1-bp indels, R 0; and paired-end read length of 80-mer, 1 80 2 80. These parameters generated 20 million 80-mer hypothetical reads with neither mutations nor indels from the genomic sequences used for single-nucleotide variation (SNV) identification. To generate short-read mapping data of all C. botulinum strains compared to the reference chromosome sequence of C. botulinum ATCC 3502 (NC_009495), we used BWA-SW (34) and SAMtools (35) software as the default parameters. All SNVs were extracted using VarScan version 2.3.4 (36) with the default parameters. All SNVs were concatenated to generate a pseudosequence for phylogenetic analysis. The maximum-likelihood-based DNA analysis program (RAxML version 7.25) (37) was used for phylogenetic analysis with 1,000-times bootstrapping. FigTree version 1.2.3 software was used to display the tree that was generated. Before de novo assembly, the short reads obtained were assembled using A5 assembler (38) or CLC Genomics Workbench 5.0 (CLC Bio, Katrinebjerg, Denmark) with the default parameters.
Nucleotide sequence accession numbers.
The nucleotide sequences of the representative bont genes analyzed in this study were deposited into the DDBJ, ENA, and GenBank databases under the following accession numbers: bont/A1 and bont/(B) of Miyagi2006, AB665554 and AB665555; bont/(B) of Iwate2007, AB665556; bont/(B) of Ibaraki2007, AB665557; bont/B5 of Ehime2011, AB665553; and bont/B6 of Okayama2011, AB665558. The nucleotide sequences of plasmids pIB07, pMI06, pMI06-01, and pTO08 were deposited into the databases under accession numbers AB859731, AB910391, AB910519, and AB910520, respectively. The whole-genome-sequencing short reads have been deposited in the DDBJ Sequence Read Archive under accession number DRA002253.
RESULTS
C. botulinum strains isolated from botulism cases in Japan.
Ten botulism cases were reported in Japan between September 2006 and June 2011. Eight of these cases were infant botulism, and 2 were adult cases (annual surveillance data of notifiable diseases, NIID Infectious Diseases Surveillance Center [http://www.niid.go.jp/niid/ja/allarticles/surveillance/2085-idwr/ydata/3329-report-ea2011.html]). One of the adult cases was reported in 2007 as type E food-borne botulism caused by homemade fermented fish food. The other adult case (reported in 2008) was type A botulism of unknown cause. With the exception of the type E adult food-borne case, C. botulinum strains were isolated in all cases. These strains are listed in Table 1. Nine C. botulinum strains were isolated from 8 infant botulism cases. Of these strains, 6 were type A(B) serotype (strains Miyagi2006, Miyagi2006-01, Iwate2007, Ibaraki2007, Iwate2008, and Fukuoka2010), 1 was type A (strain Aichi2011), and 2 were type B (strains Ehime2011 and Okayama2011). Two of these strains, Miyagi2006 and Miyagi2006-01, were obtained from the same infant botulism case (isolated from patient feces and well water at the patient's house) (Table 1). In the adult type A botulism case, a type A(B) strain, Tochigi2008, was isolated. This adult case was recorded as an unclassified botulism case; however, a retrospective review has suggested the possibility of adult intestinal colonization botulism, based on the following characteristics: (i) the presence of abundant C. botulinum bacteria in the patient's feces, (ii) detection of C. botulinum bacteria in feces more than 1 month after disease onset, (iii) no detection of BoNTs or C. botulinum in remaining foods or patient environment, and (iv) advanced age of the patient (83 years).
Analysis of bont gene subtypes and organization.
Before undertaking large-scale genomic analysis of the C. botulinum strains in Table 1, we characterized the bont gene subtypes and organization of these strains. We designed PCR primers to amplify bont gene clusters (orfX and ha) based on the draft genome sequence of the type A(B) strain NCTC2916 (GenBank accession number ABDO02000001). The primer pairs listed in Table 2 amplify the orfX cluster with 4 amplicons (X1 to X4) and the ha cluster with 3 amplicons (H1 to H3) (Fig. 1A). The results of this PCR analysis are shown in Fig. 1B. We obtained all 7 PCR products (X1 to X4 and H1 to H3) in the 7 type A(B) strains, suggesting that these strains have a bont gene organization similar to that of NCTC2916. Three PCR products (H1 to H3) were obtained in the 2 type B strains (Fig. 1B). In the type A strain Aichi2011, only X4 and H1 segments were amplified (Fig. 1B), suggesting that bont/A of this strain is present in the ha cluster. The absence of PCR products of the X3 or H2 amplicon (containing the ntnh gene) in Aichi2011 is probably attributable to the hybrid nature (ha/orfX) of the ntnh gene region in this strain (12).
FIG 1.
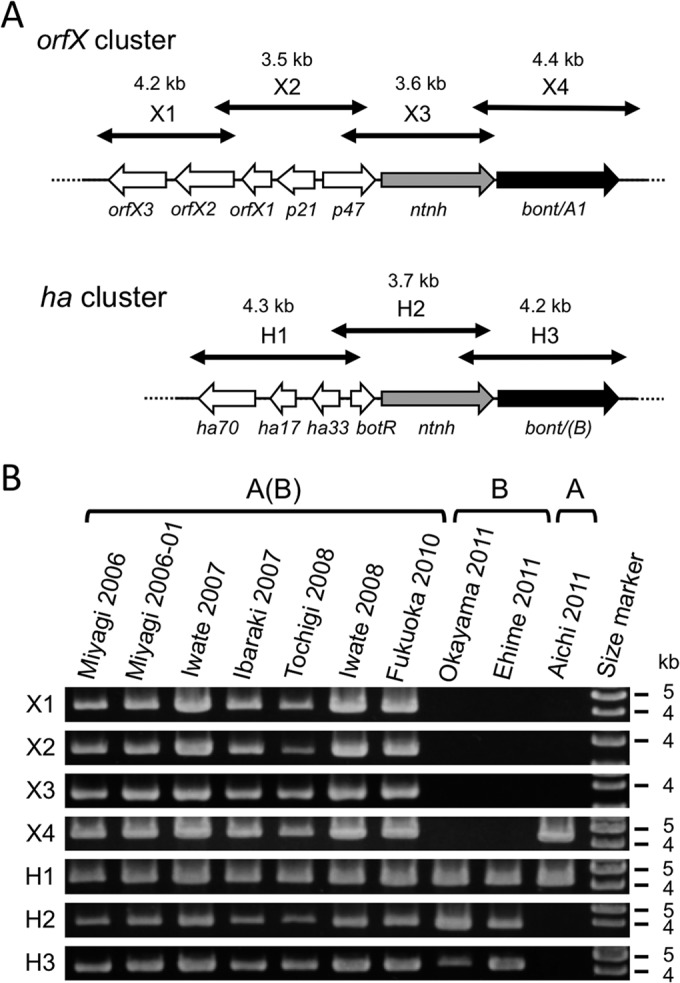
PCR scanning analysis of bont gene cluster organization. (A) Schematic illustration of two bont gene clusters (orfX and ha) of type A(B) strain NCTC2916. Solid and gray arrows indicate bont and ntnh (nontoxin nonhemagglutinin) genes, respectively. Open arrows indicate genes in the orfX and ha clusters. Double-headed arrows indicate the approximate regions of amplicons in the PCR scanning analysis (X1 to X4 and H1 to H3 segments). Approximate sizes of amplicons are shown above the arrows. The PCR primer pairs used for the analysis are listed in Table 2. (B) Results of PCR scanning of 10 C. botulinum strains. Strain names and serotypes are shown at the top. The positions of the molecular size markers are shown at the right.
We then sequenced the bont genes in the X4 and H3 fragments, which showed that the bont/A genes from the 7 type A(B) strains were identical to bont/A1 of the NCTC2916 strain. The bont/(B) genes of type A(B) strains were also almost identical to that of NCTC2916 but exhibited SNPs among strains. Two SNPs at nucleotide (nt) positions 53 and 565 of bont/(B) were identified. In the bont/(B) genes of Ibaraki2007 and Iwate2008 strains, nt 53 and 565 are A and G, respectively. This pattern was identical to that of the NCTC2916 strain (Table 3, SNP pattern 3). However, Miyagi2006, Miyagi2006-01, and Tochigi2008 have T at nt 53, whereas Iwate2007 and Fukuoka2010 have T at nt 53 and A at nt 565. The bont/(B) genes of Miyagi2006, Miyagi2006-01, and Tochigi2008 (Table 3, SNP pattern 1) are identical to those of the CDC3517 (GenBank accession number FJ981691) and CDC5178 (GenBank accession number FJ981693) strains (15). The bont/(B) genes of Iwate2007 and Fukuoka2010 (Table 3, SNP pattern 2) are identical to those of the CDC4893 (GenBank accession number FJ981692) and CDC5277 (GenBank accession number FJ981694) strains (15).
TABLE 3.
SNPs in bont/(B) genes of type A(B) strains
| Strain | Nucleotide at indicated position of bont/(B) gene |
SNP pattern | |
|---|---|---|---|
| 53 | 565 | ||
| Miyagi2006 | T | G | 1 |
| Miyagi2006–01 | T | G | 1 |
| Tochigi2008 | T | G | 1 |
| Iwate2007 | T | A | 2 |
| Fukuoka2010 | T | A | 2 |
| Ibaraki2007 | A | G | 3 |
| Iwate2008 | A | G | 3 |
| NCTC2916 | A | G | 3 |
The bont/A gene of Aichi2011 was subtype A1 and was identical to those of the ATCC 3502 (GenBank accession number NC_009495) and Hall (GenBank accession number NC_009698) strains (39). The bont/B gene of Ehime2011 was bont/B5 and was identical to that of the type Bf strain CDC3281 (GenBank accession number GQ244313) (40) and plasmid pCLJ of the type Ba strain 657 (GenBank accession number CP001081) (41). The bont/B gene of Okayama2011 was similar to that of the Osaka05 strain (GenBank accession number AB302852) (42) and was established as subtype B6 (8). Four SNPs were found in a comparison of the bont/B6 genes of Osaka05 and Okayama2011. One of these SNPs (A2562C, with C instead of A at position 2562) affects the amino acid sequence (K854N).
PFGE and MLVA analyses of type A(B) strains.
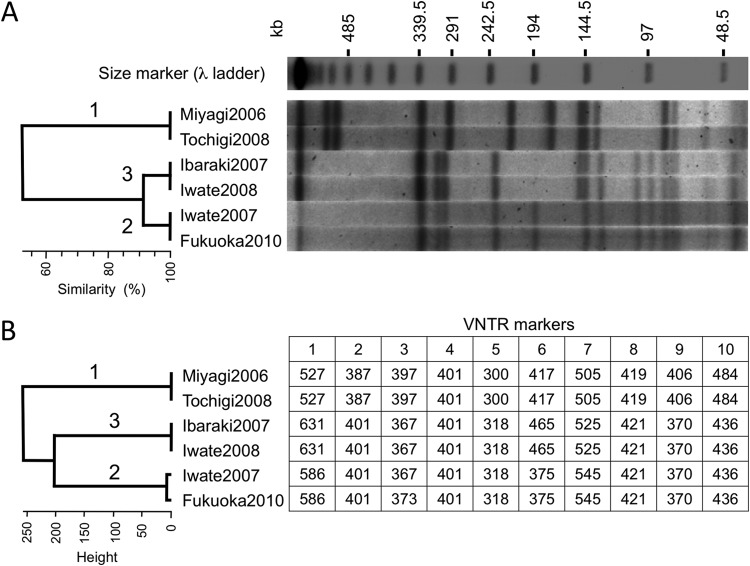
The type A(B) strains analyzed in this study were separated into 3 groups according to the SNP patterns of bont/(B) (Table 3). To test whether other typing methods could discriminate type A(B) strains, we performed PFGE and MLVA. Figure 2A shows the results of PFGE using the SmaI-digested genomic DNA of 6 type A(B) strains. We observed 3 distinct PFGE patterns. The patterns of the Miyagi2006 and Tochigi2008 strains [bont/(B) SNP pattern 1] were identical. Similarly, the patterns of Iwate2007 and Fukuoka2010 [bont/(B) SNP pattern 2] were indistinguishable, as were those of Ibaraki2007 and Iwate2008 [bont/(B) SNP pattern 3] (Fig. 2A). Three genetic background groups among type A(B) strains predicted by bont/(B) SNPs were also discriminated using PFGE.
FIG 2.
PFGE and MLVA analyses of C. botulinum type A(B) strains. (A) PFGE patterns of 6 type A(B) strains digested with restriction enzyme SmaI. The dendrogram of the PFGE patterns was generated with Fingerprinting II software and is shown at the left. (B) MLVA of 6 type A(B) strains. Sizes (bp) of PCR products of 10 VNTR marker regions are shown. The dendrogram generated from VNTR fragment sizes is shown at the left. Numbers 1 to 3 for the dendrogram clades correspond to the type of bont/(B) gene SNP pattern (Table 3). The Miyagi2006-01 strain had PFGE and MLVA patterns identical to those of the Miyagi2006 and Tochigi2008 strains (data not shown).
Figure 2B shows the results of MLVA typing according to 10 VNTR markers (32). MLVA was also able to separate the 6 type A(B) strains into 3 groups consistent with those determined with bont/(B) SNP patterns and PFGE. However, MLVA identified a difference between Iwate2007 and Fukuoka2010 in a single VNTR marker (VNTR3). MLVA provided better resolution than PFGE in this study.
Small plasmids in type A(B) strains.
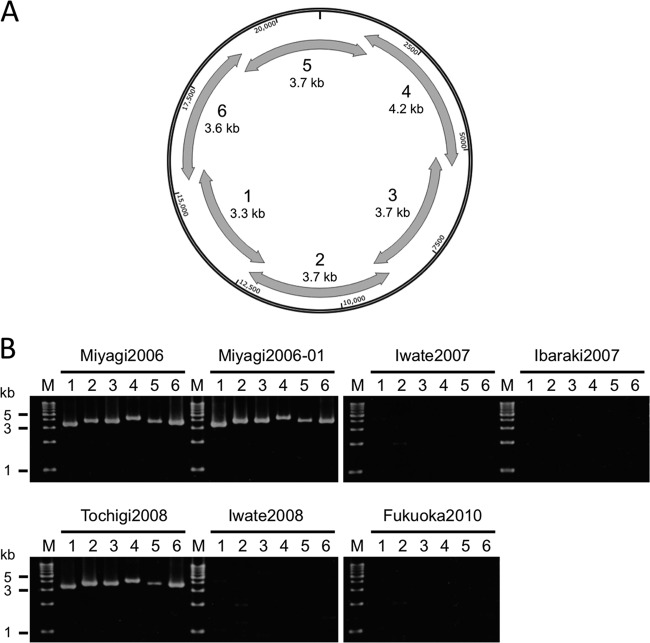
While preparing genomic DNA, we noticed that 4 of the type A(B) strains (Iwate2007, Ibaraki2007, Iwate2008, and Fukuoka2010) harbored small plasmidlike DNAs of approximately 6 kb in size (Fig. 3A, lanes 3, 4, 6, and 7). We characterized the DNA from the Ibaraki2007 strain (designated pIB07). The digestion of pIB07 DNA with HindIII yielded 2 DNA fragments of approximately 3.2 and 2.8 kb (Fig. 3B). We cloned these 2 fragments into a pUC19 plasmid and sequenced them using a primer-walking strategy, which showed that the pIB07 sequence was identical to one of the contigs of the NCTC2916 genome sequence (GenBank accession number NZ_ABDO02000015). The confirmed size of pIB07 is 5,926 bp, and it carries 5 genes larger than 150 bp (Fig. 3C). These genes are for a replication initiation protein, a plasmid recombination enzyme, a putative lipoprotein, and two hypothetical proteins. The electrophoresis patterns shown in Fig. 3A also indicate that 3 type A(B) strains (Miyagi2006, Miyagi2006-1, and Tochigi2008) might lack the pIB07-like plasmid, but these strains were assumed to have larger plasmids (Fig. 3A, lanes 1, 2, and 5).
FIG 3.
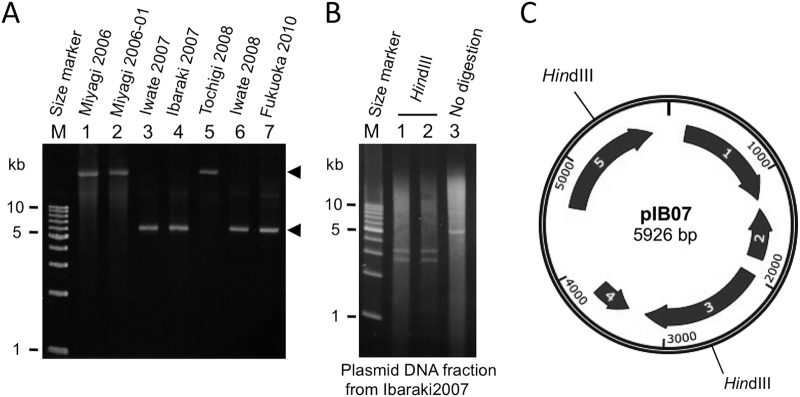
Analysis of plasmids of C. botulinum type A(B) isolates. (A) Plasmid DNA fractions extracted from 7 type A(B) strains were analyzed with 0.8% agarose gel electrophoresis. Arrowheads indicate positions of plasmidlike DNAs. (B) HindIII digestion pattern of plasmidlike DNA from Ibaraki2007 (pIB07). (C) Structure of the pIB07 plasmid. The positions of the 2 HindIII sites are indicated. Arrows indicate 5 genes larger than 150 bp. These genes were annotated as follows: 1, replication initiation protein; 2, putative lipoprotein; 3, hypothetical protein; 4, hypothetical protein; and 5, plasmid recombination enzyme.
Genome sequencing analysis.
To obtain more detailed genomic information, we sequenced the genomes of the 10 C. botulinum strains using a next-generation DNA sequencing platform. The sequencing and assembly results are summarized in Table S2 in the supplemental material. The estimated genome coverage of the sequences obtained ranged from 66- to 309-fold among strains, although the assembly results were less favorable in 3 strains (Miyagi2006, Miyagi2006-01, and Ehime2011).
In these sequencing data, we found sequences that were identical to that of the pIB07 plasmid in 4 type A(B) strains (Iwate2007, Ibaraki2007, Iwate2008, and Fukuoka2010), whereas the other 3 type A(B) strains (Miyagi2006, Miyagi2006-01, and Tochigi2008) did not carry this sequence (data not shown). This finding was consistent with the results of electrophoresis shown in Fig. 3A. Interestingly, however, we found plasmidlike sequences of approximately 21 kb in the Miyagi2006, Miyagi2006-01, and Tochigi2008 strains instead of the pIB07 sequence. These 21-kb sequences showed partial homology to previously reported C. botulinum plasmids pBOT3502 (GenBank accession number AM412318) of the type A strain ATCC 3502 (39), pCLI (GenBank accession number CP000729) of the type F strain Langeland, and pCBF (GenBank accession number CP002012) of the type F strain 230613 (43). We hypothesized that these 21-kb sequences might be the larger plasmids observed in the electrophoresis results shown in Fig. 3A (lanes 1, 2, and 5).
To confirm this hypothesis, we undertook PCR scanning analysis to detect the plasmids from 3 strains. The genomic DNA fractions of the type A(B) strains were analyzed using PCR with primer pairs that covered the 21-kb plasmid with 6 amplicons (Fig. 4). As expected, these PCR scans were all positive in the Miyagi2006, Miyagi2006-01, and Tochigi2008 strains but negative in the type A(B) strains that carry the pIB07 plasmid. These results indicated that 3 strains carry 21-kb plasmids, as predicted by electrophoresis (Fig. 3A) and genome sequencing. The plasmids from these strains were 21,001 bp in size and contained 17 coding sequences (CDSs), including a bacteriocin gene that was identical to the boticin B gene (btcB) from the type B C. botulinum strain 213B (44). The sequences of plasmids pMI06 (from Miyagi2006) and pMI06-01 (from Miyagi2006-01) were identical; however, compared with the other 2 plasmids, plasmid pTO08 from Tochigi2008 had 3 SNPs.
FIG 4.
Detection of 21-kb plasmid predicted by genome sequencing. (A) Strategy of PCR scanning for plasmid detection. Six double-headed arrows indicate amplicons that cover the whole plasmid sequence. Sizes of amplicons are shown. PCR primer pairs used for the analysis are listed in Table 2. (B) Results of PCR scanning. Genomic DNAs from type A(B) C. botulinum strains were used for the template. PCR products of the six amplicons were analyzed by 0.8% agarose gel electrophoresis. Names of strains analyzed are shown above the gels. Lane numbers correspond to amplicon numbers. Lanes M, 1-kb-ladder DNA size markers.
Comparison of these plasmids with pBOT3502 and pCLI (pCBF) revealed their chimeric nature. Half of the pMI06, pMI06-01, and pTO08 regions (the region containing dnaE and bacteriocin biosynthesis genes) had homology with the pBOT3502 plasmid, and the other half (containing a putative replication protein gene) was similar to the pCLI (pCBF) plasmid, suggesting genetic exchange events among plasmids between different C. botulinum serotype lineages (Fig. 5).
FIG 5.
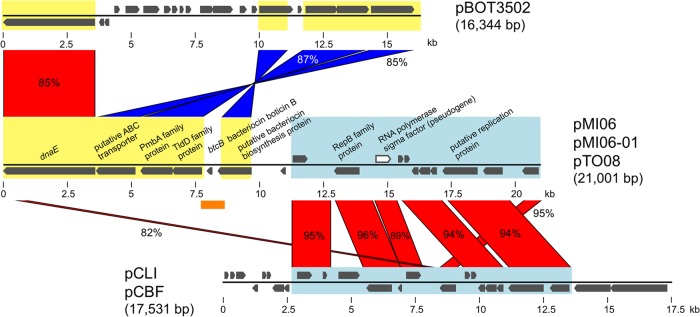
Comparison of plasmids from C. botulinum strains. The plasmids from type A(B) strains Miyagi2006, Miyagi2006-01, and Tochigi2008 (pMI06, pMI06-01, and pTO08, respectively) were compared with plasmids pBOT3502, pCLI, and pCBF from C. botulinum strains ATCC 3502 (type A), Langeland (type F), and 230613 (type F), respectively. Plasmid regions with sequence homology are indicated in red and blue (inverted regions). The first halves of the pMI06, pMI06-01, and pTO08 plasmids show homology with the pBOT3502 plasmid (yellow). The other halves have homology with the pCLI and pCBF plasmids (light blue). A region of about 1 kb containing the bacteriocin boticin B gene (orange bar) is highly homologous to the sequence of the 18.8-kb plasmid of C. botulinum strain 213B (GenBank accession number AF278540) (44). Three SNPs were observed in a comparison of the pMI06, pMI06-01, and pTO08 plasmids (see the text).
Whole-genome SNP analysis.
Using genome sequence data, we then performed whole-genome SNP analysis. In addition to the 10 genomes sequenced in this study, 13 previously reported group I C. botulinum genomes, from the ATCC 3502, Hall, ATCC 19397, Kyoto, Loch Maree, H04402 065, NCTC2916, Okra, 657, Bf, Osaka05, 230613, and Langeland strains, were analyzed (Table 4). Comparative genome analysis revealed a 1,284,104-bp sequence as a core genome (33.04% of the genome sequence of the ATCC 3502 strain), and 86,140 core genome SNP sites were extracted (see Table S3 in the supplemental material). The phylogenetic tree based on these SNP markers is shown in Fig. 6 and is well matched with the trees based on bont gene sequences or amplified fragment length polymorphism (AFLP) analysis (5), suggesting that it reflects genomic features and variations among strains. In this tree, the subtype B6 strains Okayama2011 and Osaka05 were distantly separated from the other strains (Fig. 6). Approximately 50% of the total core genome SNP markers of these strains did not match those of the other subtype strains (see Table S4 in the supplemental material). The subtype B5 strain Ehime2011 was clustered with bivalent strains 657 and Bf. The subtype A1 strain Aichi2011 was clustered with type A strains ATCC 3502, ATCC 19397, and Hall. The type A(B) strains and subtype A2 strain Kyoto formed a large cluster within which type A(B) strains were separated into two clades (Fig. 6).
TABLE 4.
Genome sequences included in whole-genome SNP analysis
| C. botulinum strain | bont subtype | GenBank accession no. |
|---|---|---|
| ATCC 3502 | A1 | NC_009495 |
| Hall | A1 | NC_009698 |
| ATCC 19397 | A1 | NC_009697 |
| Kyoto | A2 | NC_012563 |
| Loch Maree | A3 | NC_010520 |
| H04402 065 | A5(B3′) | NC_017299 |
| NCTC2916 | A1(B) | ABDO02000000 |
| Okra | B1 | NC_010516 |
| 657 | B5 a4 | NC_012658 |
| Bf | B5 f2 | ABDP00000000 |
| Osaka05 | B6 | BAUF00000000 |
| 230613 | F1 | NC_017297 |
| Langeland | F1 | NC_009699 |
FIG 6.
Phylogenetic dendrogram based on whole-genome SNP analysis. Genome sequences of the 10 C. botulinum strains analyzed in this study (Table 1) and 13 group I C. botulinum genomes reported in GenBank (Table 4) were compared. The dendrogram was generated using a maximum-likelihood estimation method based on 86,140 SNP markers identified in a comparison of the core genomes of 23 C. botulinum strains (see Table S3 in the supplemental material). The serotypes/subtypes of the C. botulinum strains in the clusters are shown at the right. The bootstrap values are indicated at the branches.
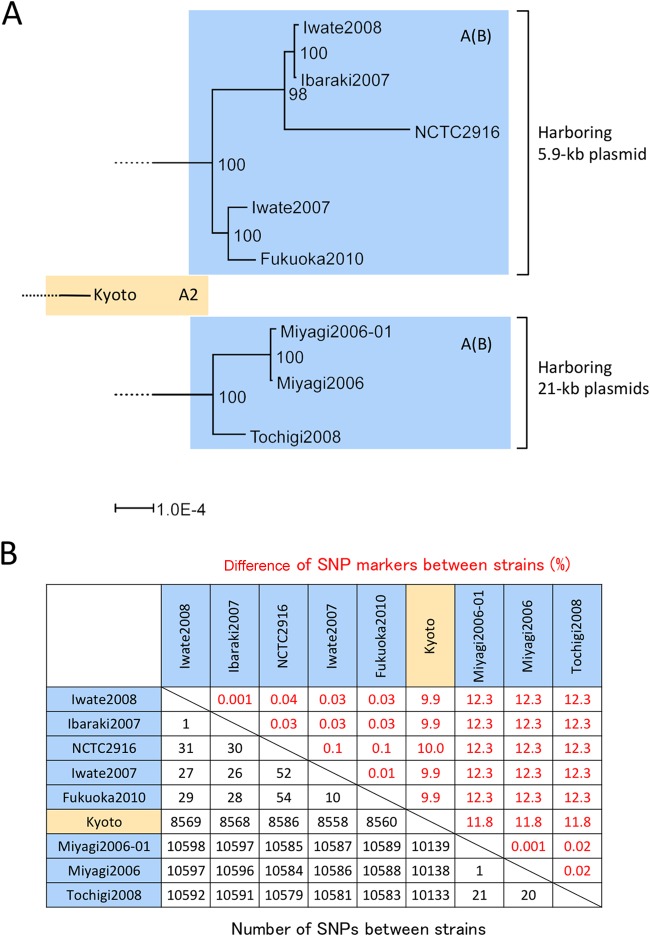
One of these type A(B) clades contained strains harboring a 5.9-kb plasmid (the pIB07 plasmid), and the other contained strains carrying 21-kb plasmids (pMI06, pMI06-01, and pTO08). The phylogenetic tree of these A(B) strain clades is magnified in Fig. 7A. In the clade of pIB07-harboring strains, Ibaraki2007 and Iwate2008 are not discriminated from each other by bont/(B) gene sequencing (Table 2), PFGE (Fig. 2A), or MLVA (Fig. 2B); however, 1 SNP was detected in a comparison of these strains (Fig. 7B). Conversely, the Iwate2007 and Fukuoka2010 strains, in which a single VNTR marker difference was observed in the MLVA results (Fig. 2B), had 10 SNP marker mismatches (Fig. 7B). A single SNP marker mismatch was also found in a comparison of the Miyagi2006 and Miyagi2006-01 strains, which were derived from the same infant botulism case (Fig. 7B).
FIG 7.
Genetic relationship of C. botulinum type A(B) strains isolated in Japan. (A) Magnified portion of the phylogenetic dendrogram in Fig. 6 showing 2 A(B) strain clades and A2 strain Kyoto. The bootstrap values are indicated at the branches. The brackets indicate clades of type A(B) strains harboring 5.9- and 21-kb plasmids. (B) Summary of the differences in core genome SNP markers between type A(B) and A2 strains.
DISCUSSION
We performed genetic characterization of 10 C. botulinum strains isolated from 9 botulism cases in Japan. These cases occurred across a wide area of Japan; therefore, we were particularly interested in determining whether these strains have genetic relationships or regional differences. The analysis showed that most of the bont genes sequenced in this study were found in the database with complete identity; only the bont/B6 gene of Okayama2011 was a novel sequence, with 4 SNPs compared with bont/B6 of the Osaka05 strain (42). Osaka05 is the first subtype B6 strain isolated in Japan, from a 2005 infant botulism case. To our knowledge, only 2 subtype B6 strains have been reported to date. Both strains were isolated in Japan; however, whether these strains are indigenous to the country or distributed worldwide is unknown. In the whole-genome SNP analysis, Okayama2011 and Osaka05 formed a distinct clade and separated distantly from the other group I strains (Fig. 6). However, 14,123 SNPs were identified between the Okayama2011 and Osaka05 strains (16.4% of total core genome SNP markers), suggesting considerable genetic variations in these strains (see Table S4 in the supplemental material). Previous studies of Osaka05 have reported that the bont/B6 gene of this strain is located on a large plasmid of approximately 280 kb (42, 45). In the present study, we found that bont/B6 of Okayama2011 was present on a large contig that has homology to the plasmids pCLD (GenBank accession number CP000940) of strain Okra, pCLJ (GenBank accession number CP001081) of strain 657, pCLK (GenBank accession number CP000963) of strain Loch Maree (41), and pCLQ (GenBank accession number AOSX01000021) of strain Af84 (46) (data not shown), suggesting that bont/B6 of Okayama2011 is also located on a large plasmid, similar to the bont gene-carrying plasmids reported to date. Further genomic analysis of the Okayama2011 and Osaka05 strains will clarify the structure of bont/B6-carrying plasmids and genetic variations between these strains.
Another type B strain characterized in this study was Ehime2011, which carries bont/B5 as the only bont gene in the genome. Subtype B5 is known as a bivalent B subtype (bvB or Bbv) and is often found in bivalent strains like 657 and Bf (Table 4). However, there are also reports of strains that carry bont/B5 as the single toxin gene in the genome (47). Ehime2011 is thought to be genetically related to bivalent strains, as demonstrated by whole-genome SNP phylogenetic analysis (Fig. 6).
Type A C. botulinum strains carrying bont/A1 genes associated with the ha cluster are frequently identified in botulism cases worldwide (12, 13). ATCC 3502, ATCC 19397, and Hall are the type strains of this lineage. Aichi2011 in this study also belongs to this lineage. However, whole-genome SNP analysis identified approximately 900 to 1,300 SNPs in comparisons of Aichi2011 and the ATCC 3502, ATCC 19397, and Hall strains (see Tables S3 and S4 in the supplemental material). These core genome SNP markers may be useful for epidemiological and forensic studies of this lineage that require high resolution for strain discrimination.
Type A(B) strains have been isolated most frequently in recent botulism cases in Japan. Among the 7 type A(B) strains in this study, Miyagi2006 and Miyagi2006-01were derived from the same infant botulism case. At the time this case was investigated, these strains were considered identical because no differences were found in their PFGE patterns (48). The Miyagi2006-01 strain (isolated from well water) was thought to be an infection source or a contaminating bacterium originating in the patient's feces. However, the results of whole-genome SNP analysis showed a difference in one SNP marker between the Miyagi2006 and Miyagi2006-01 strains (Fig. 7B). We interpreted this difference as the variation of a single C. botulinum strain and consider these strains identical, although the frequency of SNPs in C. botulinum has not been thoroughly investigated. Similarly, we also regarded the Ibaraki2007 and Iwate2008 strains as identical (Fig. 7B). However, Ibaraki2007 and Iwate2008 were isolated from independent infant botulism cases that occurred in distantly separated areas at different times, raising the question of whether these type A(B) strains are distributed widely in the natural environment, including soil, in Japan or are present in a common source of infection, such as commercial food products (49, 50), although honey was not ingested in either of the cases. This question should be addressed in further studies.
Before this study, 7 type A(B) strains had been regarded as similar strains. However, the results of whole-genome SNP analysis clearly delineated 2 distinct lineages of type A(B) strains (Fig. 6 and 7A). Approximately 10,000 SNP markers (12% of the total SNP markers analyzed in this study) differed between these 2 lineages (Fig. 7B). Subtype A2 strain Kyoto is located between these 2 lineages (Fig. 6 and 7A). The presence of these 2 lineages was also determined through analysis of the plasmid profiles of type A(B) strains (Fig. 3A and 7A). C. botulinum strains reportedly carry plasmids of a variety of sizes (41, 47, 51, 52); however, small and medium plasmids without bont genes have not been extensively characterized. The analysis of small plasmids in C. botulinum may be useful for strain characterization and discrimination.
In this study, we characterized C. botulinum strains isolated recently in Japan and showed that small-plasmid profiling and whole-genome SNP analyses are promising tools for epidemiological studies and discrimination of C. botulinum strains. Further analysis of C. botulinum strains using these strategies will provide better understanding of the epidemiological aspects and genetic features of this bacterium.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by a Grant for Research on Emerging and Reemerging Infectious Diseases (grant H23 Shinko-Ippan-007) from the Ministry of Health, Labor and Welfare of Japan and a Grant-in Aid for Scientific Research on Innovative Areas (grant 25117530) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
We thank Yumiko Ogasawara of the NIID Pathogen Genomics Center for experimental assistance.
Footnotes
Published ahead of print 5 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02134-14.
REFERENCES
- 1.Peck MW. 2009. Biology and genomic analysis of Clostridium botulinum. Adv. Microb. Physiol. 55:183–265, 320. 10.1016/S0065-2911(09)05503-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang JC, Sun L, Nie QH. 2010. Botulism, where are we now? Clin. Toxicol. (Phila.) 48:867–879. 10.3109/15563650.2010.535003. [DOI] [PubMed] [Google Scholar]
- 3.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059–1070. 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 4.Rainey GJ, Young JA. 2004. Antitoxins: novel strategies to target agents of bioterrorism. Nat. Rev. Microbiol. 2:721–726. 10.1038/nrmicro977. [DOI] [PubMed] [Google Scholar]
- 5.Hill KK, Smith TJ, Helma CH, Ticknor LO, Foley BT, Svensson RT, Brown JL, Johnson EA, Smith LA, Okinaka RT, Jackson PJ, Marks JD. 2007. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J. Bacteriol. 189:818–832. 10.1128/JB.01180-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barash JR, Arnon SS. 2014. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins. J. Infect. Dis. 209:183–191. 10.1093/infdis/jit449. [DOI] [PubMed] [Google Scholar]
- 7.Dover N, Barash JR, Hill KK, Xie G, Arnon SS. 2014. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 209:192–202. 10.1093/infdis/jit450. [DOI] [PubMed] [Google Scholar]
- 8.Hill KK, Smith TJ. 2013. Genetic diversity within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Curr. Top. Microbiol. Immunol. 364:1–20. 10.1007/978-3-642-33570-9_1. [DOI] [PubMed] [Google Scholar]
- 9.Whitemarsh RC, Tepp WH, Bradshaw M, Lin G, Pier CL, Scherf JM, Johnson EA, Pellett S. 2013. Characterization of botulinum neurotoxin A subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect. Immun. 81:3894–3902. 10.1128/IAI.00536-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franciosa G, Ferreira JL, Hatheway CL. 1994. Detection of type A, B, and E botulism neurotoxin genes in Clostridium botulinum and other Clostridium species by PCR: evidence of unexpressed type B toxin genes in type A toxigenic organisms. J. Clin. Microbiol. 32:1911–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutson RA, Zhou Y, Collins MD, Johnson EA, Hatheway CL, Sugiyama H. 1996. Genetic characterization of Clostridium botulinum type A containing silent type B neurotoxin gene sequences. J. Biol. Chem. 271:10786–10792. 10.1074/jbc.271.18.10786. [DOI] [PubMed] [Google Scholar]
- 12.Hill KK, Xie G, Foley BT, Smith TJ, Munk AC, Bruce D, Smith LA, Brettin TS, Detter JC. 2009. Recombination and insertion events involving the botulinum neurotoxin complex genes in Clostridium botulinum types A, B, E and F and Clostridium butyricum type E strains. BMC Biol. 7:66. 10.1186/1741-7007-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson MJ, Lin G, Raphael B, Andreadis J, Johnson EA. 2008. Analysis of neurotoxin cluster genes in Clostridium botulinum strains producing botulinum neurotoxin serotype A subtypes. Appl. Environ. Microbiol. 74:2778–2786. 10.1128/AEM.02828-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luquez C, Raphael BH, Joseph LA, Meno SR, Fernandez RA, Maslanka SE. 2012. Genetic diversity among Clostridium botulinum strains harboring bont/A2 and bont/A3 genes. Appl. Environ. Microbiol. 78:8712–8718. 10.1128/AEM.02428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luquez C, Raphael BH, Maslanka SE. 2009. Neurotoxin gene clusters in Clostridium botulinum type Ab strains. Appl. Environ. Microbiol. 75:6094–6101. 10.1128/AEM.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raphael BH, Luquez C, McCroskey LM, Joseph LA, Jacobson MJ, Johnson EA, Maslanka SE, Andreadis JD. 2008. Genetic homogeneity of Clostridium botulinum type A1 strains with unique toxin gene clusters. Appl. Environ. Microbiol. 74:4390–4397. 10.1128/AEM.00260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutson RA, Thompson DE, Collins MD. 1993. Genetic interrelationships of saccharolytic Clostridium botulinum types B, E and F and related clostridia as revealed by small-subunit rRNA gene sequences. FEMS Microbiol. Lett. 108:103–110. 10.1111/j.1574-6968.1993.tb06081.x. [DOI] [PubMed] [Google Scholar]
- 18.Hutson RA, Thompson DE, Lawson PA, Schocken-Itturino RP, Bottger EC, Collins MD. 1993. Genetic interrelationships of proteolytic Clostridium botulinum types A, B, and F and other members of the Clostridium botulinum complex as revealed by small-subunit rRNA gene sequences. Antonie Van Leeuwenhoek 64:273–283. [DOI] [PubMed] [Google Scholar]
- 19.Lindstrom M, Korkeala H. 2006. Laboratory diagnostics of botulism. Clin. Microbiol. Rev. 19:298–314. 10.1128/CMR.19.2.298-314.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobel J. 2005. Botulism. Clin. Infect. Dis. 41:1167–1173. 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 21.Chertow DS, Tan ET, Maslanka SE, Schulte J, Bresnitz EA, Weisman RS, Bernstein J, Marcus SM, Kumar S, Malecki J, Sobel J, Braden CR. 2006. Botulism in 4 adults following cosmetic injections with an unlicensed, highly concentrated botulinum preparation. JAMA 296:2476–2479. 10.1001/jama.296.20.2476. [DOI] [PubMed] [Google Scholar]
- 22.Ghasemi M, Norouzi R, Salari M, Asadi B. 2012. Iatrogenic botulism after the therapeutic use of botulinum toxin-A: a case report and review of the literature. Clin. Neuropharmacol. 35:254–257. 10.1097/WNF.0b013e31826248b8. [DOI] [PubMed] [Google Scholar]
- 23.Horowitz BZ. 2010. Type E botulism. Clin. Toxicol. (Phila.) 48:880–895. 10.3109/15563650.2010.526943. [DOI] [PubMed] [Google Scholar]
- 24.Nishiura H. 2007. Incubation period as a clinical predictor of botulism: analysis of previous izushi-borne outbreaks in Hokkaido, Japan, from 1951 to 1965. Epidemiol. Infect. 135:126–130. 10.1017/S0950268806006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cawthorne A, Celentano LP, D'Ancona F, Bella A, Massari M, Anniballi F, Fenicia L, Aureli P, Salmaso S. 2005. Botulism and preserved green olives. Emerg. Infect. Dis. 11:781–782. 10.3201/eid1105.041088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momose Y, Asakura H, Kitamura M, Okada Y, Ueda Y, Hanabara Y, Sakamoto T, Matsumura T, Iwaki M, Kato H, Shibayama K, Igimi S. 2014. Food-borne botulism in Japan in March 2012. Int. J. Infect. Dis. 24:20–22. 10.1016/j.ijid.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, Shimizu T, Ooi K, Noda H, Nasu T, Sakaguchi G. 1988. Quantification of Clostridium botulinum type A toxin and organisms in the feces of a case of infant botulism and examination of other related specimens. Jpn. J. Med. Sci. Biol. 41:21–26. [DOI] [PubMed] [Google Scholar]
- 28.Koepke R, Sobel J, Arnon SS. 2008. Global occurrence of infant botulism, 1976–2006. Pediatrics 122:e73–e82. 10.1542/peds.2007-1827. [DOI] [PubMed] [Google Scholar]
- 29.Lindstrom M, Keto R, Markkula A, Nevas M, Hielm S, Korkeala H. 2001. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 67:5694–5699. 10.1128/AEM.67.12.5694-5699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshi K, Fujinaga Y, Inoue K, Nakajima H, Oguma K, Ueno T, Sunagawa H, Ohyama T. 1996. Simple method for detection of Clostridium botulinum type A to F neurotoxin genes by ploymerase chain reaction. Microbiol. Immunol. 40:5–11. 10.1111/j.1348-0421.1996.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson EA, Tepp WH, Bradshaw M, Gilbert RJ, Cook PE, McIntosh ED. 2005. Characterization of Clostridium botulinum strains associated with an infant botulism case in the United Kingdom. J. Clin. Microbiol. 43:2602–2607. 10.1128/JCM.43.6.2602-2607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macdonald TE, Helma CH, Ticknor LO, Jackson PJ, Okinaka RT, Smith LA, Smith TJ, Hill KK. 2008. Differentiation of Clostridium botulinum serotype A strains by multiple-locus variable-number tandem-repeat analysis. Appl. Environ. Microbiol. 74:875–882. 10.1128/AEM.01539-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Ruan J, Durbin R. 2008. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 18:1851–1858. 10.1101/gr.078212.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H. 2011. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27:2987–2993. 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koboldt DC, Chen K, Wylie T, Larson DE, McLellan MD, Mardis ER, Weinstock GM, Wilson RK, Ding L. 2009. VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 25:2283–2285. 10.1093/bioinformatics/btp373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 38.Tritt A, Eisen JA, Facciotti MT, Darling AE. 2012. An integrated pipeline for de novo assembly of microbial genomes. PLoS One 7:e42304. 10.1371/journal.pone.0042304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sebaihia M, Peck MW, Minton NP, Thomson NR, Holden MT, Mitchell WJ, Carter AT, Bentley SD, Mason DR, Crossman L, Paul CJ, Ivens A, Wells-Bennik MH, Davis IJ, Cerdeno-Tarraga AM, Churcher C, Quail MA, Chillingworth T, Feltwell T, Fraser A, Goodhead I, Hance Z, Jagels K, Larke N, Maddison M, Moule S, Mungall K, Norbertczak H, Rabbinowitsch E, Sanders M, Simmonds M, White B, Whithead S, Parkhill J. 2007. Genome sequence of a proteolytic (group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res. 17:1082–1092. 10.1101/gr.6282807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raphael BH, Choudoir MJ, Luquez C, Fernandez R, Maslanka SE. 2010. Sequence diversity of genes encoding botulinum neurotoxin type F. Appl. Environ. Microbiol. 76:4805-4812. 10.1128/AEM.03109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith TJ, Hill KK, Foley BT, Detter JC, Munk AC, Bruce DC, Doggett NA, Smith LA, Marks JD, Xie G, Brettin TS. 2007. Analysis of the neurotoxin complex genes in Clostridium botulinum A1-A4 and B1 strains: BoNT/A3, /Ba4 and /B1 clusters are located within plasmids. PLoS One 2:e1271. 10.1371/journal.pone.0001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umeda K, Seto Y, Kohda T, Mukamoto M, Kozaki S. 2009. Genetic characterization of Clostridium botulinum associated with type B infant botulism in Japan. J. Clin. Microbiol. 47:2720–2728. 10.1128/JCM.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian RM, Li T, Hou XJ, Wang Q, Cai K, Liu YN, Gao X, Liu H, Xiao L, Tu W, Shi J, Cao WC, Wang H. 2011. The complete genome sequence of Clostridium botulinum F str. 230613, insertion sites, and recombination of BoNT gene clusters. Genome 54:546–554. 10.1139/g11-019. [DOI] [PubMed] [Google Scholar]
- 44.Dineen SS, Bradshaw M, Johnson EA. 2000. Cloning, nucleotide sequence, and expression of the gene encoding the bacteriocin boticin B from Clostridium botulinum strain 213B. Appl. Environ. Microbiol. 66:5480–5483. 10.1128/AEM.66.12.5480-5483.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi Y, Hosomi K, Uchiyama J, Ogura Y, Umeda K, Sakaguchi M, Kohda T, Mukamoto M, Misawa N, Matsuzaki S, Hayashi T, Kozaki S. 2014. Draft genome sequence of Clostridium botulinum type B strain Osaka05, isolated from an infant patient with botulism in Japan. Genome Announc. 2(1):e01010-13. 10.1128/genomeA.01010-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dover N, Barash JR, Hill KK, Davenport KW, Teshima H, Xie G, Arnon SS. 2013. Clostridium botulinum strain Af84 contains three neurotoxin gene clusters: bont/A2, bont/F4 and bont/F5. PLoS One 8:e61205. 10.1371/journal.pone.0061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franciosa G, Maugliani A, Scalfaro C, Aureli P. 2009. Evidence that plasmid-borne botulinum neurotoxin type B genes are widespread among Clostridium botulinum serotype B strains. PLoS One 4:e4829. 10.1371/journal.pone.0004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi T, Haginoya K, Morimoto T, Hatakeyama T, Tsuchiya S. 2014. A case of infant botulism infection due to consumption of untreated well-water. J. Pediatr. 164:931–933. 10.1016/j.jpeds.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 49.Barash JR, Hsia JK, Arnon SS. 2010. Presence of soil-dwelling clostridia in commercial powdered infant formulas. J. Pediatr. 156:402–408. 10.1016/j.jpeds.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 50.Smith JK, Burns S, Cunningham S, Freeman J, McLellan A, McWilliam K. 2010. The hazards of honey: infantile botulism. BMJ Case Rep. 2010:bcr0520103038. 10.1136/bcr.05.2010.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall KM, Bradshaw M, Pellett S, Johnson EA. 2007. Plasmid encoded neurotoxin genes in Clostridium botulinum serotype A subtypes. Biochem. Biophys. Res. Commun. 361:49–54. 10.1016/j.bbrc.2007.06.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strom MS, Eklund MW, Poysky FT. 1984. Plasmids in Clostridium botulinum and related Clostridium species. Appl. Environ. Microbiol. 48:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.