Abstract
Yeast has long been considered the microorganism of choice for second-generation bioethanol production due to its fermentative capacity and ethanol tolerance. However, tolerance toward inhibitors derived from lignocellulosic materials is still an issue. Flocculating yeast strains often perform relatively well in inhibitory media, but inhibitor tolerance has never been clearly linked to the actual flocculation ability per se. In this study, variants of the flocculation gene FLO1 were transformed into the genome of the nonflocculating laboratory yeast strain Saccharomyces cerevisiae CEN.PK 113-7D. Three mutants with distinct differences in flocculation properties were isolated and characterized. The degree of flocculation and hydrophobicity of the cells were correlated to the length of the gene variant. The effect of different strength of flocculation on the fermentation performance of the strains was studied in defined medium with or without fermentation inhibitors, as well as in media based on dilute acid spruce hydrolysate. Strong flocculation aided against the readily convertible inhibitor furfural but not against less convertible inhibitors such as carboxylic acids. During fermentation of dilute acid spruce hydrolysate, the most strongly flocculating mutant with dense cell flocs showed significantly faster sugar consumption. The modified strain with the weakest flocculation showed a hexose consumption profile similar to the untransformed strain. These findings may explain why flocculation has evolved as a stress response and can find application in fermentation-based biorefinery processes on lignocellulosic raw materials.
INTRODUCTION
Despite the fact that the first large-scale lignocellulosic ethanol plants are under development or have recently become operational (1, 2), there are still major hurdles to overcome before this second-generation biofuel can become economically competitive. First, lignocellulosic feedstocks contain significant amounts of pentose sugars originating from hemicelluloses, and second, the harsh pretreatment methods that are often necessary lead to the creation of inhibitors that slow down or even stop the subsequent fermentation (3). There are a number of different recombinant yeast strains that can utilize the pentoses xylose and/or arabinose, as reviewed previously (4). However, most of these strains cannot efficiently coutilize hexoses and pentoses, due to Saccharomyces cerevisiae's strong preference for glucose (5). In northern regions of the world, a lot of attention is focused on ethanol production from spruce, since it is abundantly available. Spruce is often steam pretreated with addition of SO2 or H2SO4, which can result in degradation products that will inhibit the fermentation (6). The inhibitors are usually divided into the categories carboxylic acids, furan aldehydes, and phenolic compounds, and the respective amounts of these depend on both the source of the raw material and its pretreatment and hydrolysis (3, 7, 8). The effects of the inhibitors on the fermenting cells include, e.g., direct inhibition of catabolic enzymes, generation of reactive oxygen species, decreased intracellular pH, ATP depletion, toxic anion accumulation, and disturbance of membrane integrity (8). This can lead to increased lag time, slower fermentation rate, decreased viability and, ultimately, stuck fermentation. Inhibitory compounds can be further categorized into those that can be rapidly converted to less inhibitory compounds, such as the furan aldehydes (9, 10), and those that are not converted, such as carboxylic acids under anaerobic conditions (11). In the wide array of phenolic compounds there are both convertible and nonconvertible inhibitors (12). For in situ detoxification, it is necessary that the inhibitors are kept at a low level, at least relative to the amount of metabolically active cells: yeast at a high cell density using a membrane bioreactor could tolerate high concentrations of furfural (13). Other strategies to handle the toxicity of lignocellulosic hydrolysates involve, e.g., fed-batch processes, where the inhibitors can be kept at a low concentration inside the reactor (14). Evolutionary engineering, as well as the overexpression of specific genes, has also been shown to increase the tolerance of yeast to toxic hydrolysates. The evolutionary engineering strategies likely lead to beneficial mutations in genes important for the performance of the cells in the hydrolysates (15). As a recent example of metabolic engineering, the overexpression of genes leading to increased glutathione biosynthesis led to better performance of the cells in simultaneous saccharification and fermentation of pretreated spruce (16).
It has recently been shown that the tolerance can be improved also at a low average cell concentration without changes to the yeast cells. This happens if the cells are encapsulated inside a semipermeable membrane, giving a high local cell density (11). In this case, the increased tolerance to inhibitors has two reasons. First, the cells experience a certain stress level due to the encapsulated state where inner lying cells become nutrient limited (11, 17). The slight stress response increases the ability of the cells to cope with the stress deriving from inhibitory compounds (11) and even increases the thermotolerance of the cells (18). Second, it was shown that conversion of the inhibiting compounds is necessary for the increased robustness, and a model was proposed in which the cells close to the capsule membrane convert inhibitors, leaving subinhibitory levels for inner lying cells (11). As an extension of this, it has also been shown that the simultaneous glucose and xylose utilization by a recombinant S. cerevisiae was improved by encapsulation (19). Glucose is presumably consumed by cells close to the membrane, while xylose is consumed by cells closer to the core (19). With this reasoning, the protective effect does not mainly come from the membrane of the capsule, and hypothetically it would be observed also if the cells were kept tightly together by other means.
Such tight agglomeration of cells can be achieved by flocculation, where cells are kept together by lectin-like cell wall proteins, so-called flocculins, that attach to carbohydrates in the cell wall of neighboring cells (20). Flocculation has since long been used in breweries and other ethanol industries as a means of easy separation of the yeast at the end of the fermentation. In this case the yeast cells do not flocculate during the batch, mainly due to the presence of sugars which competitively inhibit the flocculation by binding to the flocculins (21). For microbiologists, flocculation has often been considered a nuisance due to the difficulties of working with heterogeneous cell suspensions rather than the homogenous ones given by nonflocculating cells. Common lab strains, such as the sequenced S. cerevisiae S288c, do not flocculate. This can be due to different reasons, such as a defective transcription factor controlling the flocculation genes (22), or that the flocculation genes are simply missing. The latter is the case for the recently sequenced S. cerevisiae CEN.PK 113-7D, where the major flocculation genes FLO1, FLO5, and FLO9 are missing (23). However, naturally flocculating strains have been shown to be good at fermenting inhibitory hydrolysates (24–26), and flocculation has also been shown to increase the ethanol tolerance of S. cerevisiae (27, 28). Therefore, it is plausible that flocculation can indeed be a beneficial trait in second-generation bioethanol production.
In this study, we tested the hypothesis that strong flocculation would lead to improved fermentation performance in lignocellulose-derived inhibitory media, by generation of clustered cells. To this end, we constructed strains constitutively expressing variants of FLO1 in CEN.PK 113-7D, resulting in different flocculation strengths, and investigated the effects on fermentation of inhibitory media.
MATERIALS AND METHODS
Saccharomyces cerevisiae strains.
The three recombinant mutants used in the present study all originated from CEN.PK 113-7D (MATa MAL2-8C SUC2) (29) (kindly provided by Peter Kötter, Biozentrum, Frankfurt, Germany), which was used as reference strain. Inoculations of precultures were done by picking a colony from fresh yeast extract-peptone-dextrose (YPD) agar plates (i.e., 10 g/liter of yeast extract [Scharlau], 20 g/liter of soy peptone [Fluka], and 20 g/liter of glucose [Fisher Scientific]), prepared using cells stored in glycerol at −80°C.
Construction of the flocculating yeast strains.
The TDH3 promoter (TDH3p) was amplified from genomic DNA of S. cerevisiae CEN.PK 113-7D by PCR using the primers EcoRV_TDH3p_FW and SpeI_TDH3p-RV (Table 1) and cloned in the pUG6 vector (30). The resulting vector was used as the template for amplification of the kanMX-TDH3p cassette using the primers SphI_KAN_FW and SalI_TDH3p-RV (Table 1). The cassette was cloned in Yiplac211 (31) and thereafter amplified by PCR using the primers HO_KAN-FW and TDH3p_FLO1-RV (Table 1), giving flanking ends homologous to the HO locus in S. cerevisiae CEN.PK 113-7D and to FLO1 from S. cerevisiae S288c (GenBank accession number NM_001178230.1), respectively. FLO1 was amplified from S288c chromosomal DNA using the primers FLO1-FW and FLO1_HO-RV (Table 1), giving a region homologous to the HO-locus in S. cerevisiae CEN.PK 113-7D. By fusion PCR the kanMX-TDH3p cassette and the FLO1 gene were merged together and amplified using the primers HO-FW and HO-RV (Table 1) in two PCRs. Specifically, in the first step the FLO1 gene and the kanMX-TDH3p cassette were mixed in a single PCR for PCR-based fusion, using the Phusion polymerase (Thermo Scientific). The DNA fragment resulting from the fusion was used as the template for the second PCR using the primers HO-FW and HO-RV. This yielded a PCR product with flanking regions homologous to the HO locus that was used for homologous recombination in CEN.PK 113-7D using the lithium acetate-based transformation method (32). Transformants were selected on YPD plates containing G418 (Sigma-Aldrich) at 200 μg/ml, after replica plating once, and were tested for flocculation ability in 4 ml of YPD medium in 12-ml tubes, with shaking overnight. The correct integration into the HO locus was confirmed by PCR using the primers SapI_KAN-RV and Ctrl_HO-FW (Table 1). The nucleotide sequences of the recombinant genes were determined by cycle sequencing (Eurofins MWG Operon, Ebersberg, Germany).
TABLE 1.
PCR primers
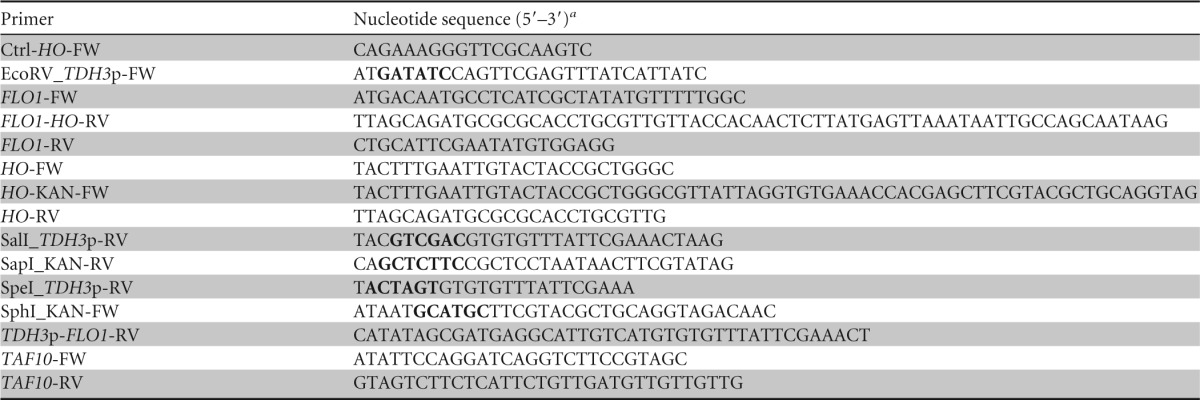
Boldface type indicates restriction sites.
Media.
Aerobic cultures for cell propagation were grown in 250-ml cotton-plugged conical flasks in a shaker bath (125 rpm) at 30°C. The growth medium used for cell propagation for the flocculation trials and the “microbial adhesion to hydrocarbons” (MATH) test was YPD medium containing 20 g/liter glucose. The growth medium used for the batch cultivations was a defined glucose medium (DGM) as previously reported (33), with the additional supplementation of CaCl2 at 1 g/liter to ensure that the degree of flocculation was not limited by possible Ca2+ shortage.
The media for the anaerobic batch cultivations were designed to include both readily convertible and nonconvertible inhibitors found in lignocellulosic hydrolysates, namely, furan aldehydes and carboxylic acids. The defined inhibitory media contained the same composition as the DGM described above, with the addition of either 5 g/liter furfural (Sigma-Aldrich) or 200 mM acetic (Sigma-Aldrich), formic (Sigma-Aldrich), and levulinic acid (Aldrich) at 12.0, 9.2, and 23.2 g/liter, respectively. DGM was used for comparison, as well as a hydrolysate medium, diluted to ca. 60% due to the addition of salts, vitamins, trace metals, and ergosterol (Sigma) as in DGM, and the cell suspension from the precultivation. A more inhibitory hydrolysate medium was achieved by adding 1.5 g/liter furfural to the spruce hydrolysate medium.
All of the media, with the exception of the hydrolysate medium, had a final glucose concentration of ∼20 to 21 g/liter. The pH of all media was adjusted to 5.5 with concentrated NaOH.
The hydrolysate was produced from spruce chips treated at pH 2.0 (by SO2 addition) under 18-bar pressure for 5 to 7 min. The hydrolysate was stored refrigerated at low pH (<pH 2) until use. Before use, the pH was adjusted to 5.5 with 10 M NaOH, and the hydrolysate was autoclaved, followed by centrifugation to remove solid particles. The hydrolysate medium used for anaerobic fermentations contained the following: glucose, 9.0 ± 0.2 g/liter; mannose, 11.9 ± 0.1 g/liter; galactose, 2.5 ± 0.2 g/liter; xylose, 5.3 ± 0.1 g/liter; arabinose, 1.9 ± 0.1 g/liter; acetic acid, 2.4 ± 0.1 g/liter; furfural, 1.78 ± 0.05 g/liter; and 5-hydroxymethyl furfural (HMF), 0.69 ± 0.03 g/liter (n = 16).
Flocculation trials.
The flocculation trials were performed with stationary-phase yeast cells harvested 48 h after inoculation in YPD medium according to a previously reported protocol (26), with slight modifications. In short, EDTA-deflocculated cells were heat killed and mixed at a concentration of ∼108 cells/ml in citrate buffer (50 mM, pH 4.5) containing 4 mM CaCl2 and various concentrations of the sugars tested in a total volume of 2 ml placed in a 12-ml round-bottom tube. The tubes were placed on an orbital shaker and agitated at 160 rpm at 25°C at an angle of ∼30° for 4 h to ensure equilibrium. A sample of 150 μl was taken from just below the meniscus after the tubes had been left stationary in a vertical position for 30 s. The sample was dispersed in 850 μl of 100 mM EDTA solution, and the cell concentration was measured as the optical density at 600 nm (OD600). The flocculation was expressed as the percentage of free cells and was calculated as follows: % free cells = [(OD600 of sample)/(OD600 of reference without CaCl2)] × 100.
Hydrophobicity test.
The hydrophobicity of cells was tested by the MATH assay according to the method of Westman et al. (26). The hydrophobicity is reported as the relative difference between the absorbance of the aqueous phase before and after vortexing with octane and was calculated as follows: hydrophobicity = [1 − (OD600 after vortexing/OD600 before vortexing)] × 100.
Quantitative PCR.
Yeast cells grown aerobically in YPD medium were harvested in the exponential phase, and the total RNA was immediately isolated using an RNeasy kit (Qiagen) with DNase treatment according to the manufacturer's protocol. The RNA was subjected to reverse transcription to cDNA with a RevertAid H Minus First Strand cDNA synthesis kit (Thermo Scientific), with 500 ng of RNA in 20-μl reactions. The expressions of the FLO1 variants and the reference gene TAF10, which showed a stable expression in all samples, were analyzed by quantitative PCR using a Brilliant II SYBR green QPCR master mix (Stratagene), 0.5 μM concentrations of forward and reverse primers, and 1 μl of cDNA. The experiments were performed on a Stratagene Mx3005P instrument, with an initial denaturation for 10 min at 95°C and amplification using 40 cycles of 30 s at 95°C and 1 min at 60°C. A denaturation curve analysis was included at the end of the program to verify the specificity of the primers. The primers used were FLO1-FW and FLO1-RV for the FLO gene variants and TAF10-FW and TAF10-RV for the TAF10 gene (34) (Table 1). The relative expression was calculated from the threshold cycle (CT) according to the following formula: relative gene expression = 2CT(TAF10) − CT(FLO1).
Batch cultivations with inhibitors.
Due to the inherent difficulties of cultivating a flocculating yeast strain reproducibly (growth cannot be monitored by OD600 measurements or withdrawal of samples for dry weight determination), a previously developed cultivation method was used (26) in order to avoid deflocculation of the cells, e.g., with EDTA. Hence, the entire precultivations were used as inocula for the batch cultivations in order to get reproducible data. Separate precultivations were performed for analysis of the initial biomass amounts. Yields were calculated from data at the end of the cultivations, where all biomass could be subjected to dry weight determination.
The batch cultivations were carried out in 250-ml conical flasks, cotton plugged for aerobic cultivation, and equipped with rubber stoppers fitted with stainless steel capillaries and a glass tube with a loop trap for anaerobic cultivations as previously described (35). Sterile water was used in the loop traps to permit produced CO2 to leave the flasks. The cultivations were started with a 36-h aerobic cultivation in 40 ml of DGM containing glucose at 25 g/liter in a shaker bath (125 rpm) at 30°C. The anaerobic cultivations were subsequently started by addition of 80 ml of fresh medium of different compositions, giving a total volume of ∼120 ml and the desired concentrations. Samples of the medium for analysis of extracellular medium by high-pressure liquid chromatography (HPLC) were taken through the steel capillaries.
Analytical methods.
The concentrations of metabolites and inhibitors were quantified by HPLC using an Aminex HPX-87H column (Bio-Rad) at 60°C with 5 mM H2SO4 as the eluent at a flow rate of 0.6 ml/min. A refractive index detector was used for glucose, formic acid, acetic acid, levulinic acid, glycerol, ethanol, furfural, and HMF. For the hydrolysate samples, an Aminex HPX-87P (Bio-Rad) column at 85°C with ultrapure water as eluent at a flow rate of 0.6 ml/min was also used to analyze the glucose, xylose, arabinose, galactose and mannose concentrations, using a refractive index detector. The cell dry weight was measured in predried and preweighed glass tubes. The cells were washed with ultrapure water before drying for 24 h at 105°C.
Statistics, yields, rates, and elemental balance calculations.
The biomass and metabolite yields were calculated from the concentrations determined at the beginning and the end of the anaerobic fermentations. Produced carbon dioxide was considered to be at the same molar ratio as ethanol and acetate. The biomass composition CH1.76O0.56N0.17 (36) was used in the carbon balance calculations. The consumption and production rates, qS, were calculated according to the formula:
where is the concentration of substrate or product consumed or produced at the time point tn and is the estimated biomass concentration in the middle of the time interval, assuming a linear relationship between ethanol and biomass production.
Error intervals are expressed as ±1 standard deviation unless otherwise noted. Statistical tests were performed using a two-tailed t test assuming unequal variance, which is a stricter criterion than assuming equal variance.
Nucleotide sequence accession numbers.
The nucleotide sequences for the weakly, intermediately, and strongly flocculating FLO1 variants were deposited in the GenBank database under accession numbers KM366093, KM366094, and KM366095, respectively.
RESULTS AND DISCUSSION
Flocculating mutants constitutively expressing FLO1 variants.
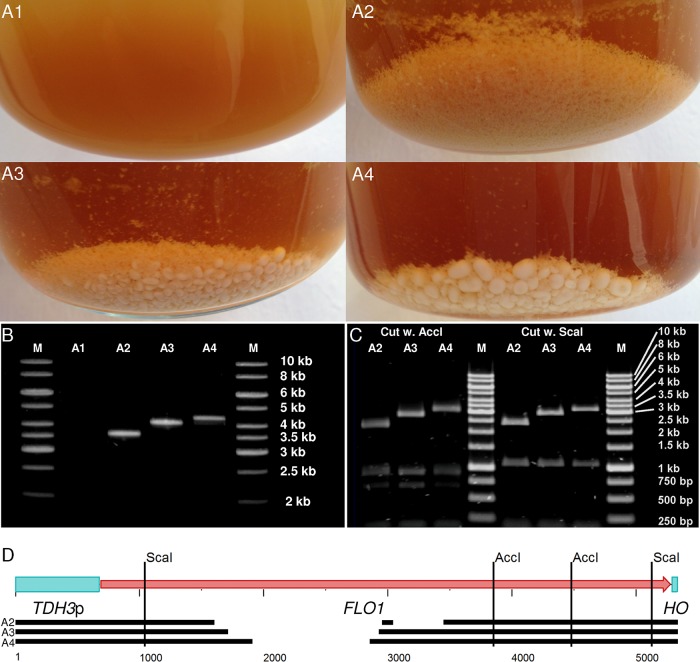
The major flocculation gene, FLO1 (20), and the TDH3 promoter region were isolated from genomic DNA of S. cerevisiae S288c and CEN.PK 113-7D, respectively, and merged together with the kanMX selectable marker (37) cloned from pUG6 (30) to yield an integration cassette flanked by regions homologous to the HO locus, which would lead to integration of the recombinant construct into the HO locus. Despite numerous attempts, a PCR protocol yielding a single PCR product of the kanMX-TDH3p cassette with the native, full-length FLO1 could not be developed. Subcloning of the FLO1 gene in Escherichia coli was also unsuccessful, as has been observed before (38). Nevertheless, the obtained partial gene PCR products were used for transformation and integration into the S. cerevisiae CEN.PK 113-7D genome. This resulted in several transformants that were selected and grown in liquid YPD medium, whereby mutants with different degrees of flocculation could be isolated (Fig. 1A). Three mutants were chosen for further analysis. Below, these are referred to as weakly, intermediately, and strongly flocculating, depending on the size of the flocs formed. The strongly flocculating mutant formed the largest and most compact flocs.
FIG 1.
Different flocculation characteristics depending on the size of the FLO gene. (A) The three chosen flocculating transformants showed distinct differences in their flocculation strength, with the weakly flocculating mutant (A2) not forming flocs as dense as the ones of the intermediate (A3) and strongly (A4) flocculating mutants. A1 shows the nonflocculating untransformed CEN.PK 113-7D. (B) The PCR product, when amplified using the EcoRV_TDH3p-FW and HO-RV primers from the genomic DNA of the different strains, showed that the inserts had different lengths, with longer inserts corresponding to stronger flocculation. (C) Restriction with AccI and ScaI, respectively, showed that the difference in the inserts localized to the middle region of the FLO1 gene. None of the inserts contained the entire FLO1 gene and the difference in length of the FLO1 variants was in a 2809-bp region in the middle of the wild-type FLO1. (D) Schematic representation of the TDH3p-FLO1-HO recombinant construct. The ScaI and AccI restriction sites are indicated. The full-length FLO1 is shown with the approximate regions present in the variants (A2-A4), as identified through sequencing, represented by black lines below. An alignment of the full sequences is available in Fig. S1 in the supplemental material.
Longer FLO genes lead to stronger flocculation.
Amplification of the integrated TDH3p-FLO1 by PCR using the primers EcoRV_TDH3p-FW and HO-RV (Table 1) confirmed that the gene products in the three selected transformants were of different lengths (Fig. 1B). The gene region(s) missing in the recombinant versions of FLO1 was narrowed down by restriction analysis of the TDH3p-FLO1 products using the enzymes AccI and ScaI (Fig. 1C and D). Sequencing of the TDH3p-FLO1 products showed that the missing regions were different portions of the previously described internal repeats (39) and that a few point mutations occurred with only minor differences in the amino acid sequences (Table 2; see also Fig. S1 in the supplemental material). There were no changes in the TDH3 promoter sequence between the three mutants. Quantitative PCR analysis showed that there were no significant differences in expression of the different flocculation genes (Fig. 2).The only difference between the FLO genes in the N-terminal part of the sequence, which is responsible for the carbohydrate binding (40), was a single silent mutation in the intermediately flocculating mutant (see Fig. S1 in the supplemental material). The deleted regions were multiples of 135 bp, translating to 45 amino acids (Table 2), which has been reported to be the length of the repeats (41). These are strong indications that the differences in flocculation strength between the mutants depended solely on the number of repeats in the middle region of the proteins, which has also been suggested previously (39). Differences in protein concentration and localization are also potential reasons for the differences in flocculation strength, but analysis of this lay beyond the scope of the present study. Treating the mutant flocculating strains with the chelating agent EDTA effectively and reversibly deflocculated the cells by removal of Ca2+, showing the expected Ca2+ dependence of the flocculation (42).
TABLE 2.
Differences in the flocculation genes/proteins from the characterized mutants compared to FLO1a
| Mutant type | Deleted region(s) (nt) | No. of deleted 45-aa repeats | Point mutation(s) |
|---|---|---|---|
| Weakly flocculating | 948–2297, 2418–2822 | 10 + 3 | |
| Intermediately flocculating | 1029–2243 | 9 | H926T |
| Strongly flocculating | 1275–2219 | 7 | I882V, I906V, S907T, H926T, V1361A |
nt, nucleotides; aa, amino acids.
FIG 2.
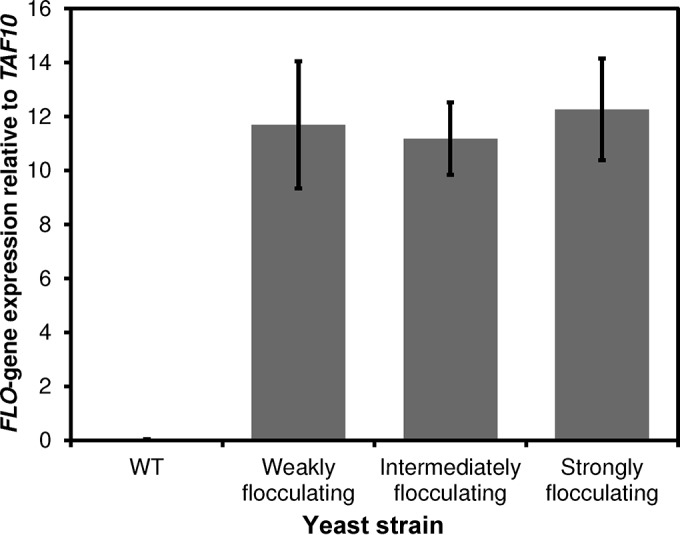
Equal expression of flocculation genes. The expression of the FLO1 gene variants was analyzed by quantitative PCR and was shown to be equal for the different strains relative to the reference gene, TAF10. Averages of duplicate biological replicates with duplicate technical replicates are shown with ±1 standard deviation (n = 2).
The flocculation ability in the presence of different sugars was studied for the constructed flocculating mutants (Fig. 3). With no sugars present, the levels of free cells (means ± absolute standard deviations, n = 4) were 54% ± 6.0%, 5.7% ± 2.5%, and 2.5% ± 1.7% for the weakly, intermediately, and strongly flocculating mutants, respectively. The flocculation of the strongly flocculating mutant was inhibited only by mannose, which was expected since the inserted gene was based on FLO1 (43). For the intermediately flocculating mutant, a slight inhibition was also observed at the highest concentration of sucrose (Fig. 3). The flocculation of the weakly flocculating mutant was inhibited by all sugars tested. Mannose-dependent inhibition of the flocculation could potentially be a problem in high gravity spruce hydrolysates, which usually contain relatively large amounts of mannose (7). However, this is not a major issue, since at 0.8 M (144 g/liter) mannose, the fraction of free cells was still below 50% for the strongly flocculating mutant.
FIG 3.
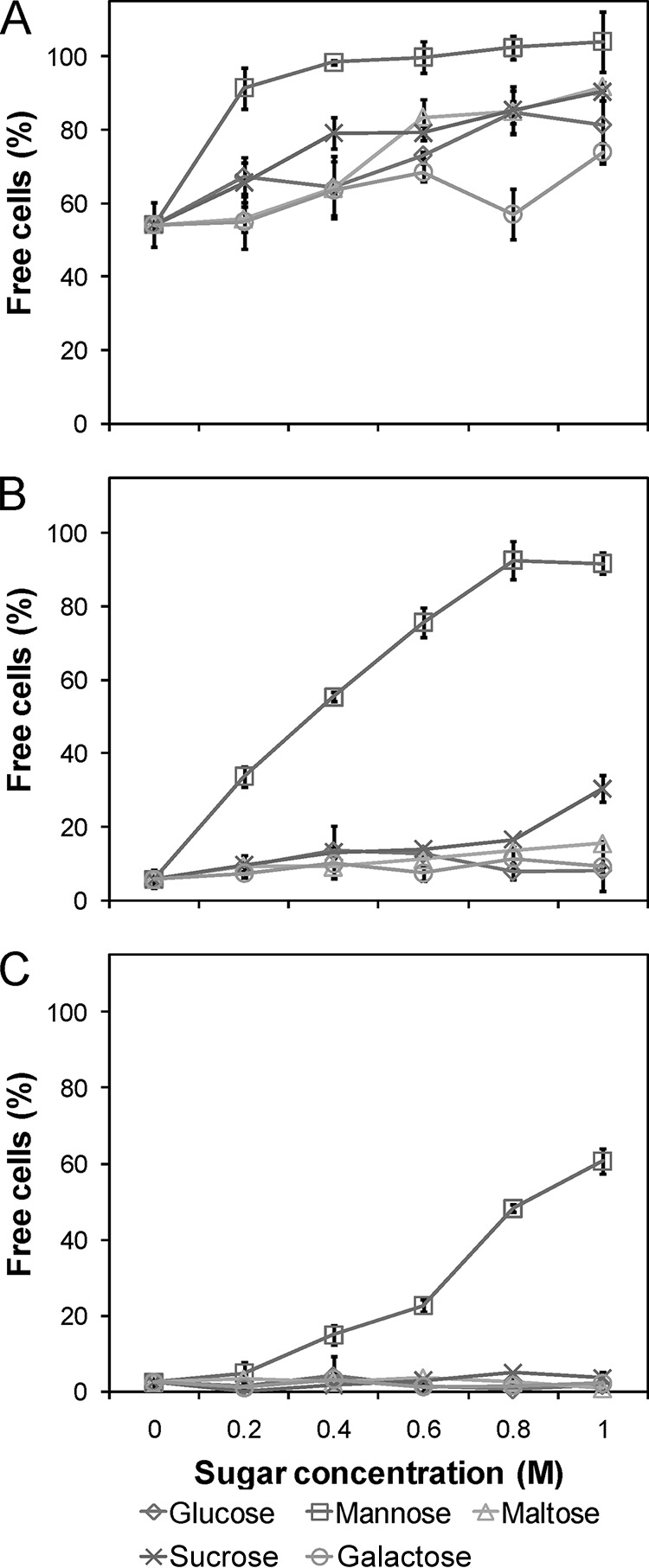
Sugar inhibition of flocculation. The amount of free cells in the presence of various sugars in different concentrations was measured by turbidimetry. (A) Weakly flocculating mutant. (B) Intermediately flocculating mutant. (C) Strongly flocculating mutant. The flocculation of all strains was inhibited by mannose and for the weakly flocculating strain to some degree also by various other sugars. Average values of duplicate experiments with duplicate technical replicates are shown with ±1 standard deviation (n = 2).
Longer flocculins increase the cell surface hydrophobicity.
The flocculating mutants all showed significantly higher cell wall hydrophobicity compared to the nonflocculating strain and the hydrophobicity also varied among each other (P < 0.063, two-tailed t test assuming unequal variance, n = 3) (Fig. 4). The higher hydrophobicity observed in the flocculating mutants is likely to be caused by the flocculins (44). In this way, the proteins would act in two ways to promote flocculation: by direct binding to carbohydrates and by hydrophobic interactions (45, 46). None of the mutants exhibited adhesive or invasive growth on YPD agar plates.
FIG 4.
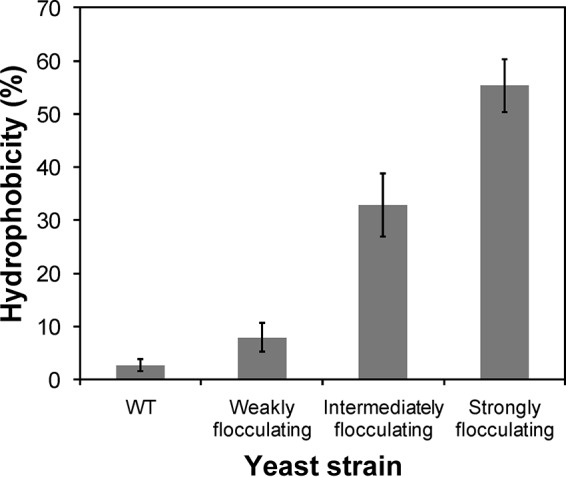
The cell wall hydrophobicity was greatly affected by the length of the flocculation gene. The stronger the flocculation and the larger the size of the inserted mutant variant of FLO1 and corresponding protein were, the higher the cell wall hydrophobicity. Average values of triplicate experiments with duplicate technical replicates are shown with ±1 standard deviation (n = 3).
Flocculation improves fermentation performance in inhibitory media.
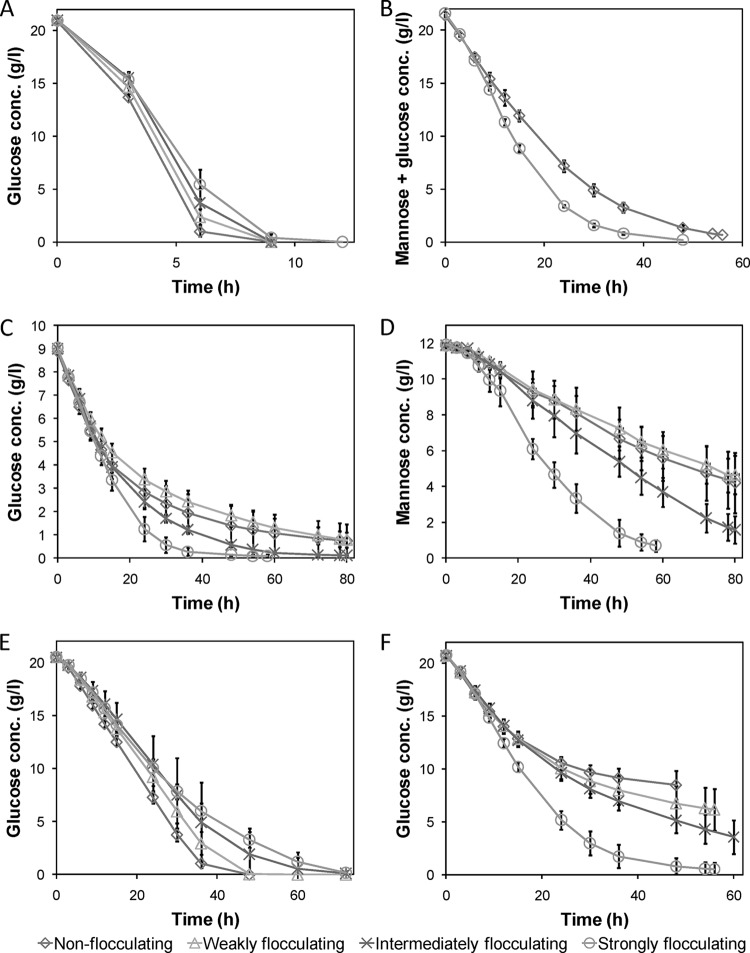
In order to further investigate our hypothesis that high local cell density increases the tolerance toward convertible inhibitory compounds such as furfural (11), the new flocculating mutants and the nonflocculating parental strain were tested for their ability to ferment different media. The batch cultivations were started with a 36-h aerobic precultivation step in 40 ml of defined medium containing 25 g/liter glucose in 250-ml E-flasks. In this setup, none of the mutants created large dense cell flocs during the precultivation, and all mutants hence exhibited similar growth, as seen by the measured biomass and metabolites at the start of the anaerobic cultivations. Separate experiments using different volume of medium showed that more than 40 ml of medium was required for formation of larger flocs. The anaerobic batch fermentations were initiated by addition of 80 ml of medium, giving the final concentrations in the media as mentioned in Materials and Methods. The starting cell concentrations in the batches were 886 ± 3, 867 ± 22, 833 ± 2, and 831 ± 19 mg (dry weight)/liter (n = 3) for the nonflocculating, weakly, intermediately, and strongly flocculating mutants, respectively. The starting cell concentration was thus slightly lower for the two most strongly flocculating mutants than for the wild-type strain due to a lower biomass yield during the aerobic precultivation. After the start of the fermentations, it took a few hours before the dense cell flocs were formed for the strongly flocculating mutant and longer still for the intermediately flocculating mutant. However, it was clear that constitutive flocculation occurred for all flocculating strains.
In noninhibitory defined glucose medium, the flocculating mutants had slower glucose consumption rate the stronger the flocculation (Fig. 5A and Table 3). This was most likely due to mass transfer limitations of nutrients to cells inside the flocs and the slightly lower initial biomass amount. Another contributing factor may have been the increased metabolic burden of the constitutive expression of the flocculin, leading to lower growth rates (27). However, the similar biomass yields indicate that the extra metabolic burden was very small (Table 3).
FIG 5.
The fermentation profiles of the flocculating strains in different media revealed distinct differences in inhibitor tolerance. The fermentation profiles of the different strains were distinctly different in the different media tested: defined noninhibitory medium (A), spruce hydrolysate (B), glucose (C), and mannose (D) in spruce hydrolysate with 1.5 g/liter extra furfural, defined medium with 200 mM (each) formic, acetic, and levulinic acid (E), and defined medium with 5 g/liter furfural (F). With strong flocculation creating dense cell flocs, the tolerance toward the readily convertible inhibitor furfural, as well as the spruce hydrolysate, was increased, leading to faster fermentations. For the not readily convertible acids, as well as the noninhibitory medium, mass transfer limitations through the flocs plausibly decreased the fermentation rates, leading to longer fermentation times the stronger the flocculation. Average values of three to four experimental replicates (duplicates for panel B) are shown with ±1 standard deviation.
TABLE 3.
Product yields and rates in anaerobic batch cultivationsa
| Medium and strain | Mean product yield ± SD |
Mean specific rate ± SD |
Mean CR (%) ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| YSE | YSAce | YSGly | YSX | qEtOH | qGlu | qMan | ||
| DGM | ||||||||
| WT | 414 ± 5 | 9 ± 2 | 53 ± 1 | 65 ± 3 | 0.89 ± 0.03 | 2.2 ± 0.1 | NA | 95 ± 2 |
| Weakly flocculating | 412 ± 5 | 13 ± 0* | 58 ± 1* | 67 ± 4 | 0.85 ± 0.04 | 2.1 ± 0.1 | NA | 96 ± 1 |
| Intermediately flocculating | 412 ± 4 | 13 ± 2* | 59 ± 3* | 69 ± 3 | 0.82 ± 0.04 | 2.0 ± 0.1 | NA | 96 ± 2 |
| Strongly flocculating | 413 ± 8 | 11 ± 1 | 59 ± 2* | 69 ± 2 | 0.77 ± 0.03* | 2.0 ± 0.1* | NA | 96 ± 2 |
| Furfural | ||||||||
| WT | 415 ± 13 | 22 ± 4 | 61 ± 8 | –1 ± 2 | 0.24 ± 0.03 | 0.61 ± 0.06 | NA | 90 ± 2 |
| Weakly flocculating | 435 ± 16 | 22 ± 2 | 59 ± 5 | –2 ± 1 | 0.24 ± 0.02 | 0.61 ± 0.06 | NA | 93 ± 3 |
| Intermediately flocculating | 440 ± 9* | 25 ± 2 | 52 ± 3 | 5 ± 3* | 0.25 ± 0.02 | 0.63 ± 0.05 | NA | 94 ± 2 |
| Strongly flocculating | 445 ± 5* | 26 ± 2 | 43 ± 2* | 8 ± 0* | 0.34 ± 0.02* | 0.81 ± 0.05* | NA | 94 ± 1 |
| Carboxylic acids | ||||||||
| WT | 421 ± 4 | 36 ± 4 | 71 ± 1 | 12 ± 1 | 0.23 ± 0.01 | 0.57 ± 0.02 | NA | 94 ± 0 |
| Weakly flocculating | 420 ± 1 | 47 ± 4* | 75 ± 2 | 19 ± 3* | 0.19 ± 0.01* | 0.49 ± 0.03* | NA | 96 ± 1 |
| Intermediately flocculating | 421 ± 2 | 45 ± 19 | 78 ± 1* | 25 ± 3* | 0.17 ± 0.04 | 0.42 ± 0.10 | NA | 98 ± 1 |
| Strongly flocculating | 430 ± 3* | 36 ± 5 | 69 ± 2 | 26 ± 4* | 0.20 ± 0.02* | 0.47 ± 0.04* | NA | 98 ± 1 |
| Hydrolysate + furfural (1.5 g/liter) | ||||||||
| WT | 431 ± 16 | 42 ± 8 | 20 ± 3 | –3 ± 4 | 0.22 ± 0.03 | 0.37 ± 0.05 | 0.10 ± 0.03 | 90 ± 3 |
| Weakly flocculating | 427 ± 18 | 40 ± 14 | 22 ± 3 | 3 ± 1* | 0.20 ± 0.03 | 0.35 ± 0.01 | 0.10 ± 0.02 | 90 ± 2 |
| Intermediately flocculating | 439 ± 15 | 35 ± 2 | 20 ± 2 | 9 ± 3* | 0.23 ± 0.02 | 0.39 ± 0.02 | 0.11 ± 0.03 | 94 ± 2 |
| Strongly flocculating | 468 ± 15* | 25 ± 2* | 23 ± 2 | 7 ± 3* | 0.30 ± 0.02* | 0.44 ± 0.04 | 0.20 ± 0.06* | 97 ± 3 |
Product yields are expressed as mg of product per g of consumed hexose. YSE, ethanol yield; YSAce, acetate yield; YSGly, glycerol yield; YSX, biomass yield. qEtOH, qGlu, and qMan, the average specific rates of ethanol production and glucose and mannose consumption during the first 15 h (6 h for DGM), are expressed as g (g biomass)−1 h−1. CR, carbon recovery; NA, not applicable; SD, standard deviation (n ≧ 3); *, significantly different from the wild type (WT; P < 0.05) as determined by a two-tailed t test assuming unequal variance.
In an initial test of the ability to ferment a toxic medium, the nonflocculating strain and the strongest flocculating mutant were cultivated in a complete spruce hydrolysate containing 0.21 ± 0.00 g/liter furfural, 0.73 ± 0.00 g/liter HMF, and 2.4 ± 0.1 g/liter acetic acid (n = 4). The flocculation clearly increased the robustness of the strain, enabling it to utilize the glucose and mannose in the hydrolysate faster than the nonflocculating strain, despite the higher initial concentration of biomass for the nonflocculating strain (Fig. 5B). Since the spruce hydrolysate was not severely inhibiting even for the nonflocculating strain, 1.5 g/liter furfural was added to increase the toxicity of the medium. In this medium, the differences between the strongly flocculating mutant and the nonflocculating parental strain became even clearer. The intermediately flocculating mutant, which formed less dense cell flocs, had only a slightly increased robustness, and the weakly flocculating mutant did not show any significant difference in sugar consumption from the nonflocculating strain (Fig. 5C and D). The superior performance of the strongly flocculating cells was also reflected in a significantly higher ethanol production rate compared to the other strains (Table 3). This shows that dense cell flocs are required for the increased robustness of flocculating strains, since lower levels of flocculation were not effective in increasing yeast robustness under the tested conditions.
To further characterize the increased tolerance arising from strong flocculation, the strains were cultivated in defined media containing the readily convertible inhibitor furfural and a mix of the not readily convertible inhibitors formic, acetic, and levulinic acid. Flocculation did not increase the tolerance toward the acids, which cannot be converted anaerobically by the yeast (Fig. 5E). Instead, the differences between the strains were similar to those observed in the noninhibitory medium. On the other hand, in the furfural-supplemented medium, the strongly flocculating mutant was significantly faster at consuming the glucose (Table 3), while the other strains were unable to complete the fermentations (Fig. 5F). The intermediately flocculating mutant also showed an improved performance compared to the nonflocculating yeast, whereas the weakly flocculating mutant did not show a significant improvement. Despite the differences in sugar consumption, all strains were able to convert roughly all furfural within 48 h (Fig. 6C).
FIG 6.
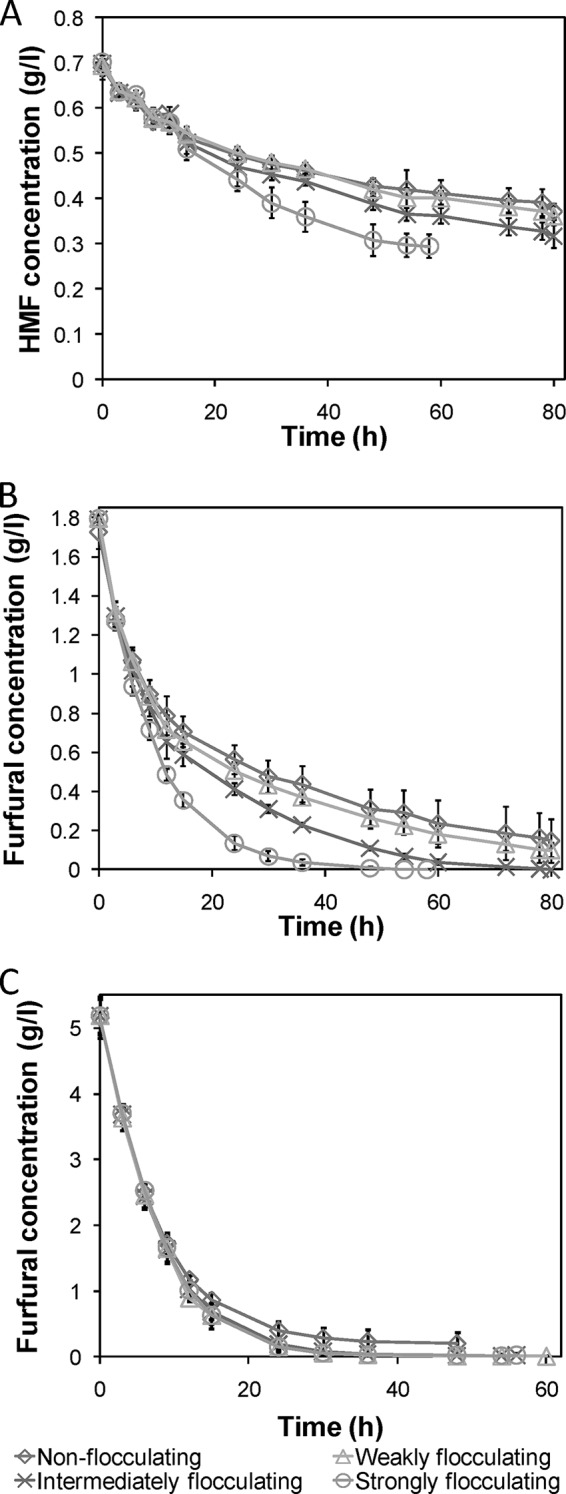
Furan aldehydes concentrations during batch cultivations. The furan aldehydes concentrations during batch cultivations of the different strains were distinctly different in the spruce hydrolysate medium, with added furfural, for HMF (A) and furfural (B), where the more tolerant strains converted the inhibitors faster. (C) In the medium with 5 g/liter furfural, the profiles were, however, rather similar, with only a slightly different conversion profile of the nonflocculating strain. Average values of four experimental replicates are shown with ±1 standard deviation.
Product yields.
In all inhibitory media, the ethanol yields were significantly higher (P < 0.05) for the strongly flocculating than for the nonflocculating strain (Table 3). This could be a consequence of a potentially altered energy requirement due to flocculation, requiring higher ATP production compared to the nonflocculating cells. Tentatively this was an effect of a higher requirement for maintenance energy by cells in the interior of the flocs, leading to more of the glucose being directed to ethanol production.
In the noninhibitory defined glucose medium, flocculation caused a slight increase in the glycerol yield. The reason for this is unknown and the effect was not observed in the other media. A decreased yield has been reported for encapsulated yeast, where the cells are also packed tightly together, why the reason for the increased yield in the current case remains elusive (17). The glycerol yields were significantly lower in the two furfural-containing media. Furfural is known to act as a redox sink and can replace glycerol for reoxidation of excess NADH formed in, e.g., biosynthetic reactions or due to acid formation (47).
The biomass yields were lower in all inhibitory media than in the noninhibitory medium (Table 3). In the inhibitory media, it could also be observed that the biomass yields were higher for the flocculating than for the nonflocculating strain. However, because the flocculating cells were not equally dispersed in the medium, sampling during the fermentation caused a decrease in the volume without decreasing the content of flocculating cells. Therefore, the cell yields of the strongly flocculating mutants were most likely overestimated.
High local cell density for increased robustness.
The results obtained for the strongly flocculating yeast are similar to what has been observed for a nonflocculating yeast strain which was encapsulated in a semipermeable gel membrane of alginate and chitosan (11). In that study, it was concluded that the tightly packed cell community created by the encapsulation of the cells increases the overall robustness of the yeast by letting cells close to the membrane convert inhibitors, allowing inner lying cells to ferment sugars because of lower inhibitor concentrations. By computer simulations, it has been shown that diffusion limitations in the encapsulated cell pellet lead to concentration gradients of both nutrients and convertible inhibitors (19). It has also been shown that encapsulation of yeast induces a starvation stress response which increases the robustness of the yeast community (11, 17). The fact that the cells grow rather slowly inside the capsules might also aid in increasing the robustness, since slow-growing cells have been shown to have increased stress tolerance (48).
Strong flocculation is similar to encapsulation in the sense that it also leads to high local cell concentrations. As in the case of encapsulated yeast, genes involved in stress resistance are upregulated in cells flocculating strongly because of FLO1 expression (27). Furthermore, diffusion limitations in yeast flocs have been observed previously. For example, Ge and Bai (49) showed that mass transfer limits the rates of growth and ethanol formation in flocs larger than 100 μm, i.e., significantly smaller than for the strongly flocculating mutant in the present study. Therefore, it is likely that the increased robustness of the flocculating yeast observed here can be attributed to similar phenomena as were concluded for the encapsulated yeast. When the cells flocculate strongly and form dense cell flocs up to several millimeters in diameter, the cells closest to the surface of the floc face the harshest conditions in a medium containing inhibitors. With convertible inhibitors, cells closer to the core experience lower levels, due to coupled diffusion and conversion reactions that cause radial concentration gradients in the floc. However, they also have access to sugars that the outer lying cells cannot utilize. As a whole, the cell community will thus be able to ferment media that are too inhibitory for nonflocculating strains, where all cells are exposed to the same inhibitor concentrations. Moreover, even after complete conversion of the inhibitors, the whole nonflocculating cell population may be severely affected by long-lasting effects of the inhibitors, e.g., a lack of energy reserves. In contrast, flocculating populations may contain cells that are still unaffected by the inhibitors, and are able to ferment the medium. This is illustrated by the significantly different fermentative performance of the different strains (Fig. 5F), despite very similar furfural conversion (Fig. 6C). In a more complex medium, such as the spruce hydrolysate used, additional inhibitory compounds likely play a role in increasing the difference between the strains, so that the nonflocculating and weakly flocculating cells cannot convert nearly as much of the furan aldehydes as the mutants forming dense cell flocs (Fig. 6A and B). However, flocculation does not protect against inhibitors that are not converted. For example, carboxylic acids can diffuse through the cell flocs without being converted and will therefore, eventually, be present at the same concentration around all cells. This also holds for encapsulated cells, unless the capsule membrane itself can stop diffusion of the inhibitor, as in the case of the hydrophobic inhibitor limonene (11, 50).
Another interesting observation made from the fermentation of the hydrolysate was that the rate of mannose consumption was 100% higher in the strongly flocculating mutant than in the nonflocculating strain (Fig. 5D and Table 3). Similar effects have recently been shown by encapsulation of a xylose-fermenting yeast, which led to improved simultaneous consumption of glucose, mannose, galactose, and xylose (19). This can be explained by the same reasoning as for the increased inhibitor tolerance. Mannose and glucose compete for the same transporter proteins, and because the cells in the periphery of the flocs utilize glucose, the competitive inhibition of mannose uptake by glucose is relieved for the cells closer to the core of the dense cell flocs (51).
In wild yeast strains, flocculation is an evolved trait that causes a large number of individual cells to form a community, mimicking multicellular organisms. As we have shown in our experiments, this enables fermentation of otherwise toxic media, likely through protection of part of the community of cells through sacrifice of the outer layer of cells while cells can divide inside the flocs. Furthermore, it has been shown that in a mixture of FLO1-expressing cells and cells that do not express FLO1, the latter cells will make up the outer layer of cells in the yeast flocs (27). This occurs since all cells have the mannose residues in their cell walls that are necessary for binding by flocculins, but the cells lacking FLO1 expression lack the ability to bind to an additional layer of nonflocculating cells. In a toxic medium, the strongest flocculating cells will thus be even better protected in a mixture of cells with different flocculation ability, driving evolution toward survival of the flocculin-expressing cells.
It has been shown previously that flocculation increases the ethanol tolerance of the yeast (27, 28). That strong flocculation, in itself, also increases fermentation rates of toxic lignocellulosic hydrolysates and, specifically, limits the inhibitory effect of high concentration of furfural on yeast fermentation performance is of great potential importance for second-generation ethanol production. Since the occurrence of dense cell flocs proved necessary for increased robustness, it is important to consider the reactor design and choice of operational parameters for good performance of a flocculating strain in such applications. Yeast flocs are sensitive to shear forces, and it is thus likely that too rapid stirring would lead to disruption of the flocs and abolished inhibitor tolerance. It is clear that further experiments are necessary for assessing the feasibility of using flocculation to improve the fermentation of toxic lignocellulose hydrolysates in large-scale bioreactors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tomas Brandberg at SEKAB AB, Sweden, for providing the spruce hydrolysate. We are grateful to Oskar Henriksson for performing initial trials.
This study was supported by the Swedish Research Council (grant 2009-4514) and the Chalmers Energy Initiative (http://www.chalmers.se/en/areas-of-advance/energy/cei/).
We declare that we have no competing interests.
Footnotes
Published ahead of print 29 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01906-14.
REFERENCES
- 1.Brown TR, Brown RC. 2013. A review of cellulosic biofuel commercial-scale projects in the United States. Biofuel Bioprod. Bioref. 7:235–245. 10.1002/bbb.1387. [DOI] [Google Scholar]
- 2.Larsen J, Haven MØ, Thirup L. 2013. Inbicon makes lignocellulosic ethanol a commercial reality. Biomass Bioenergy 46:36–45. 10.1016/j.biombioe.2012.03.033. [DOI] [Google Scholar]
- 3.Klinke HB, Thomsen AB, Ahring BK. 2004. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pretreatment of biomass. Appl. Microbiol. Biotechnol. 66:10–26. 10.1007/s00253-004-1642-2. [DOI] [PubMed] [Google Scholar]
- 4.Laluce C, Schenberg ACG, Gallardo JCM, Coradello LFC, Pombeiro-Sponchiado SR. 2012. Advances and developments in strategies to improve strains of Saccharomyces cerevisiae and processes to obtain the lignocellulosic ethanol: a review. Appl. Biochem. Biotechnol. 166:1908–1926. 10.1007/s12010-012-9619-6. [DOI] [PubMed] [Google Scholar]
- 5.Bertilsson M, Andersson J, Lidén G. 2008. Modeling simultaneous glucose and xylose uptake in Saccharomyces cerevisiae from kinetics and gene expression of sugar transporters. Bioprocess Biosyst. Eng. 31:369–377. 10.1007/s00449-007-0169-1. [DOI] [PubMed] [Google Scholar]
- 6.Stenberg K, Tengborg C, Galbe M, Zacchi G. 1998. Optimisation of steam pretreatment of SO2-impregnated mixed softwoods for ethanol production. J. Chem. Technol. Biotechnol. 71:299–308. . [DOI] [Google Scholar]
- 7.Taherzadeh MJ, Eklund R, Gustafsson L, Niklasson C, Liden G. 1997. Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind. Eng. Chem. Res. 36:4659–4665. 10.1021/ie9700831. [DOI] [Google Scholar]
- 8.Almeida JRM, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF. 2007. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 82:340–349. 10.1002/jctb.1676. [DOI] [Google Scholar]
- 9.Taherzadeh MJ, Gustafsson L, Niklasson C, Lidén G. 1999. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J. Biosci. Bioeng. 87:169–174. 10.1016/S1389-1723(99)89007-0. [DOI] [PubMed] [Google Scholar]
- 10.Taherzadeh MJ, Gustafsson L, Niklasson C, Lidén G. 2000. Physiological effects of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 53:701–708. 10.1007/s002530000328. [DOI] [PubMed] [Google Scholar]
- 11.Westman JO, Manikondu RB, Franzén CJ, Taherzadeh MJ. 2012. Encapsulation-induced stress helps Saccharomyces cerevisiae resist convertible lignocellulose derived inhibitors. Int. J. Mol. Sci. 13:11881–11894. 10.3390/ijms130911881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinke HB, Olsson L, Thomsen AB, Ahring BK. 2003. Potential inhibitors from wet oxidation of wheat straw and their effect on ethanol production of Saccharomyces cerevisiae: wet oxidation and fermentation by yeast. Biotechnol. Bioeng. 81:738–747. 10.1002/bit.10523. [DOI] [PubMed] [Google Scholar]
- 13.Ylitervo P, Franzén C, Taherzadeh M. 2013. Impact of furfural on rapid ethanol production using a membrane bioreactor. Energies 6:1604–1617. 10.3390/en6031604. [DOI] [Google Scholar]
- 14.Taherzadeh MJ, Niklasson C, Liden G. 1999. Conversion of dilute-acid hydrolyzates of spruce and birch to ethanol by fed-batch fermentation. Bioresour. Technol. 69:59–66. 10.1016/S0960-8524(98)00169-2. [DOI] [Google Scholar]
- 15.Koppram R, Albers E, Olsson L. 2012. Evolutionary engineering strategies to enhance tolerance of xylose utilizing recombinant yeast to inhibitors derived from spruce biomass. Biotechnol. Biofuels 5:32. 10.1186/1754-6834-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ask M, Mapelli V, Hock H, Olsson L, Bettiga M. 2013. Engineering glutathione biosynthesis of Saccharomyces cerevisiae increases robustness to inhibitors in pretreated lignocellulosic materials. Microb. Cell Fact. 12:87. 10.1186/1475-2859-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westman JO, Taherzadeh MJ, Franzén CJ. 2012. Proteomic analysis of the increased stress tolerance of Saccharomyces cerevisiae encapsulated in liquid core alginate-chitosan capsules. PLoS One 7:e49335. 10.1371/journal.pone.0049335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ylitervo P, Franzén CJ, Taherzadeh MJ. 2011. Ethanol production at elevated temperatures using encapsulation of yeast. J. Biotechnol. 156:22–29. 10.1016/j.jbiotec.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Westman JO, Bonander N, Taherzadeh MJ, Franzén CJ. 2014. Improved sugar co-utilization by encapsulation of a recombinant Saccharomyces cerevisiae strain in alginate-chitosan capsules. Biotechnol. Biofuels 7:102. 10.1186/1754-6834-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki BLA, Poon NH, James AP, Seligy VL. 1982. Possible mechanism for flocculation interactions governed by gene FLO1 in Saccharomyces cerevisiae. J. Bacteriol. 150:878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstrepen KJ, Derdelinckx G, Verachtert H, Delvaux FR. 2003. Yeast flocculation: what brewers should know. Appl. Microbiol. Biotechnol. 61:197–205. 10.1007/s00253-002-1200-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Styles CA, Fink GR. 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nijkamp J, van den Broek M, Datema E, de Kok S, Bosman L, Luttik M, Daran-Lapujade P, Vongsangnak W, Nielsen J, Heijne W, Klaassen P, Paddon C, Platt D, Kotter P, van Ham R, Reinders M, Pronk J, de Ridder D, Daran J-M. 2012. De novo sequencing, assembly and analysis of the genome of the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microb. Cell Fact. 11:36. 10.1186/1475-2859-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez i Nogue V, Bettiga M, Gorwa-Grauslund M. 2012. Isolation and characterization of a resident tolerant Saccharomyces cerevisiae strain from a spent sulfite liquor fermentation plant. AMB Express 2:68. 10.1186/2191-0855-2-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushika A, Inoue H, Murakami K, Takimura O, Sawayama S. 2009. Bioethanol production performance of five recombinant strains of laboratory and industrial xylose-fermenting Saccharomyces cerevisiae. Bioresour. Technol. 100:2392–2398. 10.1016/j.biortech.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 26.Westman JO, Taherzadeh MJ, Franzén CJ. 2012. Inhibitor tolerance and flocculation of a yeast strain suitable for second-generation bioethanol production. Electron. J. Biotechnol. 15 10.2225/vol15-issue3-fulltext-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smukalla S, Caldara M, Pochet N, Beauvais A, Guadagnini S, Yan C, Vinces MD, Jansen A, Prevost MC, Latgé J-P, Fink GR, Foster KR, Verstrepen KJ. 2008. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell 135:726–737. 10.1016/j.cell.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue C, Zhao XQ, Bai FW. 2010. Effect of the size of yeast flocs and zinc supplementation on continuous ethanol fermentation performance and metabolic flux distribution under very high concentration conditions. Biotechnol. Bioeng. 105:935–944. 10.1002/bit.22610. [DOI] [PubMed] [Google Scholar]
- 29.van Dijken JP, Bauer J, Brambilla L, Duboc P, Francois JM, Gancedo C, Giuseppin MLF, Heijnen JJ, Hoare M, Lange HC, Madden EA, Niederberger P, Nielsen J, Parrou JL, Petit T, Porro D, Reuss M, van Riel N, Rizzi M, Steensma HY, Verrips CT, Vindeløv J, Pronk JT. 2000. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb. Technol. 26:706–714. 10.1016/S0141-0229(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 30.Güldener U, Heck S, Fiedler T, Beinhauer J, Hegemann JH. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519–2524. 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gietz RD, Akio S. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534. 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 32.Gietz RD, Woods RA. 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350:87–96. 10.1016/S0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 33.Taherzadeh MJ, Lidén G, Gustafsson L, Niklasson C. 1996. The effects of pantothenate deficiency and acetate addition on anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 46:176–182. 10.1007/s002530050801. [DOI] [PubMed] [Google Scholar]
- 34.Teste M-A, Duquenne M, Francois JM, Parrou J-L. 2009. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol. Biol. 10:99. 10.1186/1471-2199-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taherzadeh MJ, Niklasson C, Lidén G. 1997. Acetic acid-friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem. Eng. Sci. 52:2653–2659. 10.1016/S0009-2509(97)00080-8. [DOI] [Google Scholar]
- 36.Verduyn C, Postma E, Scheffers WA, van Dijken JP. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395–403. 10.1099/00221287-136-3-395. [DOI] [PubMed] [Google Scholar]
- 37.Wach A, Brachat A, Pohlmann R, Philippsen P. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793–1808. 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 38.Teunissen AW, van den Berg JA, Steensma HY. 1993. Physical localization of the flocculation gene FLO1 on chromosome I of Saccharomyces cerevisiae. Yeast 9:1–10. 10.1002/yea.320090102. [DOI] [PubMed] [Google Scholar]
- 39.Verstrepen KJ, Jansen A, Lewitter F, Fink GR. 2005. Intragenic tandem repeats generate functional variability. Nat. Genet. 37:986–990. 10.1038/ng1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi O, Hayashi N, Kuroki R, Sone H. 1998. Region of Flo1 proteins responsible for sugar recognition. J. Bacteriol. 180:6503–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goossens K, Willaert R. 2010. Flocculation protein structure and cell-cell adhesion mechanism in Saccharomyces cerevisiae. Biotechnol. Lett. 32:1571–1585. 10.1007/s10529-010-0352-3. [DOI] [PubMed] [Google Scholar]
- 42.Stratford M. 1989. Yeast flocculation: calcium specificity. Yeast 5:487–496. 10.1002/yea.320050608. [DOI] [Google Scholar]
- 43.Stratford M, Assinder S. 1991. Yeast flocculation: Flo1 and NewFlo phenotypes and receptor structure. Yeast 7:559–574. 10.1002/yea.320070604. [DOI] [PubMed] [Google Scholar]
- 44.Straver MH, Smit G, Kijne JW. 1994. Purification and partial characterization of a flocculin from brewer's yeast. Appl. Environ. Microbiol. 60:2754–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Mulders SE, Christianen E, Saerens SMG, Daenen L, Verbelen PJ, Willaert R, Verstrepen KJ, Delvaux FR. 2009. Phenotypic diversity of Flo protein family-mediated adhesion in Saccharomyces cerevisiae. FEMS Yeast Res. 9:178–190. 10.1111/j.1567-1364.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 46.Smit G, Straver MH, Lugtenberg BJJ, Kijne JW. 1992. Flocculence of Saccharomyces cerevisiae cells is induced by nutrient limitation, with cell surface hydrophobicity as a major determinant. Appl. Environ. Microbiol. 58:3709–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sárvári Horváth I, Franzen CJ, Taherzadeh MJ, Niklasson C, Liden G. 2003. Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl. Environ. Microbiol. 69:4076–4086. 10.1128/aem.69.7.4076-4086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zakrzewska A, van Eikenhorst G, Burggraaff JEC, Vis DJ, Hoefsloot H, Delneri D, Oliver SG, Brul S, Smits GJ. 2011. Genome-wide analysis of yeast stress survival and tolerance acquisition to analyze the central trade-off between growth rate and cellular robustness. Mol. Biol. Cell 22:4435–4446. 10.1091/mbc.E10-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge XM, Bai FW. 2006. Intrinsic kinetics of continuous growth and ethanol production of a flocculating fusant yeast strain SPSC01. J. Biotechnol. 124:363–372. 10.1016/j.jbiotec.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 50.Pourbafrani M, Talebnia F, Niklasson C, Taherzadeh MJ. 2007. Protective effect of encapsulation in fermentation of limonene-contained media and orange peel hydrolyzate. Int. J. Mol. Sci. 8:777–787. 10.3390/i8080777. [DOI] [Google Scholar]
- 51.Reifenberger E, Freidel K, Ciriacy M. 1995. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol. Microbiol. 16:157–167. 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.