Abstract
Lactobacillus rhamnosus GG is one of the best-characterized lactic acid bacteria and can be considered a probiotic paradigm. Comparative and functional genome analysis showed that L. rhamnosus GG harbors a genomic island including the spaCBA-srtC1 gene cluster, encoding the cell surface-decorating host-interacting pili. Here, induced mutagenesis was used to study pilus biogenesis in L. rhamnosus GG. A combination of two powerful approaches, mutation selection and next-generation sequencing, was applied to L. rhamnosus GG for the selection of pilus-deficient mutants from an enriched population. The isolated mutants were first screened by immuno-dot blot analysis using antiserum against pilin proteins. Relevant mutants were selected, and the lack of pili was confirmed by immunoelectron microscopy. The pilosotype of 10 mutant strains was further characterized by analyzing pilin expression using Western blot, dot blot, and immunofluorescence methods. A mucus binding assay showed that the mutants did not adhere to porcine intestinal mucus. Comparative genome sequence analysis using the Illumina MiSeq platform allowed us to determine the nature of the mutations in the obtained pilus-deficient derivatives. Three major classes of mutants with unique genotypes were observed: class I, with mutations in the srtC1 gene; class II, with a deletion containing the spaCBA-srtC1 gene cluster; and class III, with mutations in the spaA gene. Only a limited number of collateral mutations were observed, and one of the pilus-deficient derivatives with a deficient srtC1 gene contained 24 other mutations. This strain, PB12, can be considered a candidate for human trials addressing the impact of the absence of pili.
INTRODUCTION
Lactobacillus rhamnosus GG is one of the best-studied lactic acid bacteria and is marketed worldwide in probiotic products (1, 2). Initially, it was isolated from the intestine of a healthy female subject (3), and ever since, it has been used in various large and well-controlled probiotic trials that showed positive effects, i.e., symptom alleviation or inhibition of disease onset, on respiratory tract infections (4), atopic disease in children (5, 6), and also various types of diarrhea (7).
The genome of L. rhamnosus GG has recently been sequenced and was found to contain a genomic island harboring a functional gene cluster coding for proteinaceous pili that are decorated by the mucus binding SpaC protein (8). The pili, located at the cell surface, were further studied and found to consist of polymers containing the major subunit SpaA and the minor pilins SpaB and SpaC (9). The major pilin SpaA forms the pilus shaft, while the minor pilin SpaB decorates the shaft and is supposedly also located at the base of the pilus structure, where it is involved in arresting pilus biosynthesis and the docking of the pilus into the peptidoglycan. The latter reaction involves the activity of the housekeeping sortase SrtA (10), and we have recently provided evidence for the involvement of the pilus-specific srtC1 gene product in the specific recognition and polymerization of the SpaA and SpaC pilin subunits (11). The SpaC pilin, situated at the tip and the sides of the pilus structure, is involved in mediating host interactions (8, 12). Among the Gram-positive bacteria, pili have been previously found on the surface of pathogens such as Corynebacterium diphtheriae (13) and Enterococcus faecalis (14), where they are considered virulence factors and mediate interactions with the host. Hence, it is likely that L. rhamnosus GG pili are also involved in host signaling; their immunomodulatory properties have been established in a recent model system (15), and as demonstrated by a recent study, SpaC pilin is required for L. rhamnosus GG-induced cellular responses (16).
Comparative genome analysis of bacterial strains has been instrumental in determining their stability and genetic variation. We recently showed by genomic resequencing analysis that the pilus production capacity of L. rhamnosus GG is a stable trait found in all tested commercialized probiotic products and that its expression is under the control of a promoter generated by an iso-IS30 element just upstream of the spaCBA-srtC1 gene cluster (17). However, in some cases, strains incapable of producing pili have been isolated from products claimed to contain L. rhamnosus GG, but it is not known whether these strains are truly derived from L. rhamnosus GG, nor is it clear whether the pilus production deficiency was due to treatments that induce instability or select for the absence of pili (18). This is of considerable importance, as the absence of pili is likely to affect the probiotic properties of L. rhamnosus GG products.
We studied L. rhamnosus GG pilus biogenesis using a random chemical mutagenesis approach, by which a range of different pilus-deficient mutants were readily obtained. Detailed biochemical and genomic characterization of these pilus-deficient derivatives showed the presence of three classes of mutations: those affecting the srtC1 gene, those affecting the spaA gene, or those affecting the entire genomic island containing the spaCBA-srtC1 gene cluster. The present study not only provided new insight into pilus biogenesis but also generated pilus-deficient L. rhamnosus GG strains that have a limited number of collateral mutations. These strains may be instrumental in human trials addressing the impact of the absence of pili on the adhesion of L. rhamnosus GG. Since European Union Directive 2001/18/EC on the deliberate release of genetically modified organisms (GMOs) into the environment indicated that mutagenesis is excluded from methods yielding GMOs, the obtained pilus-deficient strains are not considered as such (19).
MATERIALS AND METHODS
Bacterial strains, growth conditions, and mutagenesis.
L. rhamnosus GG (ATCC 53103) and L. rhamnosus Lc705 (DSM 7061) were obtained from Valio Ltd., Helsinki, Finland. All L. rhamnosus strains were grown in De Man-Rogosa-Sharpe (MRS) medium and agar (Difco) at 37°C. Stationary-phase L. rhamnosus GG cells were treated with 2% (vol/vol) ethyl methanesulfonate (EMS; Sigma-Aldrich). Samples were incubated at 37°C for 2 h, after which the cells were washed several times with phosphate-buffered saline (PBS) (pH 7.3; Oxoid) and resuspended in MRS broth. Survival of the bacteria after the mutagenesis was estimated by plating the samples as a dilution series. At various stages in the mutation selection process, cultures were supplemented with 20% glycerol and stored at −80°C.
Enrichment of pilus-deficient cells and high-throughput whole-cell dot blot screening of colonies.
Pilus-deficient strains were enriched from the EMS-treated bacterial population by using an immunological method based on the separation of cell types using immobilized IgG purified from anti-SpaC rabbit antiserum, which was previously described (8). For this purpose, wells of a Maxisorp microtiter plate (Nunc) were coated with a 10-μg/ml solution of purified SpaC IgG overnight at 4°C, washed with PBS, blocked for at least 1 h at room temperature (RT) with 1% bovine serum albumin (BSA; Sigma-Aldrich) in PBS, and then washed again. Mutated bacterial cells from cultures grown overnight were washed with PBS, the optical densities were adjusted to an A600 of 0.25, and aliquots (150 μl) of the resulting cultures were added into wells and incubated for 3 to 4 h at 37°C to enable the cells to sink to the bottom of the wells. Subsequently, the wells were emptied and washed once with PBS. The PBS wash was used to inoculate new cultures grown overnight. The enrichment procedure was repeated three times, and after the last enrichment, a dilution series of the sample was plated. Colonies were picked and grown overnight in 1 ml MRS medium in 96-well storage plates, amended with 20% glycerol, and subsequently frozen at −80°C until further analysis. The generated mutant library was grown overnight and screened with anti-SpaC antiserum by using dot blot analysis, along with controls L. rhamnosus strain GG and pilusless L. rhamnosus strain Lc705 (8). Dot blots were prepared by using a Bio-Rad 96-well Bio-Dot apparatus and nitrocellulose membranes (0.45 μm; Bio-Rad). Membranes were blocked, and the proteins were detected by using chemiluminescence according to the instructions of the ECL Advance Western blotting detection kit (GE Healthcare). Unpurified SpaC antiserum was used here and diluted 1:10,000 in blocking buffer, and horseradish peroxidase (HRP)-labeled goat anti-rabbit blotting-grade secondary antibody was used at a dilution of 1:100,000 (Bio-Rad).
Immunoelectron microscopy (immuno-EM).
L. rhamnosus cells were labeled by using rabbit antibodies generated against SpaA and SpaC, as previously described (8, 9, 12). Cells were grown overnight to stationary phase and washed gently with PBS, after which Formvar-carbon-coated grids were incubated on top of cell suspension drops for 1 h at RT. Unlike L. rhamnosus GG, pilus-deficient strains did not readily adhere to the sample grids. Hence, recently prepared grids had to be used. Grids were washed several times with 0.02 M glycine in PBS and blocked by using 1% BSA in PBS for 15 min. After the grids were blocked, they were incubated for 1 h with antiserum diluted 1:100 in blocking solution. Before protein A-gold labeling, grids were washed several times with 0.1% BSA in PBS. The protein A-gold suspension containing either 5- or 10-nm gold particles was diluted 1:55, and the grids were labeled by incubating them on top of the dilution drops for 20 min. Grids were washed several times with PBS, fixed with 1% (vol/vol) glutaraldehyde (Sigma-Aldrich), and washed with water. After several washings with water, either the protocol was continued with a second labeling or the grids were stained with a 1.8% methylcellulose–0.4% uranyl acetate mixture on ice. For double-labeling experiments, SpaC was first labeled with protein A conjugated to 5-nm gold particles, and for the second labeling, SpaA was labeled with protein A conjugated to 10-nm gold particles. The samples were examined by using a JEM-1400 transmission electron microscope (JEOL).
Genome sequencing.
Genomic DNA of the L. rhamnosus GG strains was isolated by using the Wizard Genomic DNA purification kit (Promega). Genomes of the strains were sequenced in paired-end mode by using the MiSeq platform (Illumina). The obtained reads for each strain were mapped against the L. rhamnosus GG genome (8) by using the SHRiMP2 v. 2.2.2 (20) command gmapper-ls with the option --qv-offset 33. The Samtools v. 0.1.18 (21) command mpileup, with the option -C50, and the vcfutils vcf-to-fastq conversion script (22) were used to build consensus sequences of the mappings. To resolve the differences between the parental and the mutant strains, the consensus sequences were aligned with the L. rhamnosus GG genome by using the nucmer algorithm with the options --maxmatch and -c 100 and by using the show-snps command with the option -C from the MUMmer 3.0 software package (23). The mutations were annotated by using the Artemis software (24). The mutations in the spaCBA-srtC1 gene cluster were verified by Sanger sequencing.
Expression analysis of pilus-associated proteins.
Cell wall extract, cell extract containing cytosol-associated proteins, and proteins secreted into the culture medium were analyzed by using Western blotting. Extraction of proteins was performed as described previously (25). Cells grown overnight were centrifuged and washed with PBS. The growth medium containing the secreted proteins was saved for Western analysis. The optical density of the washed cells was adjusted to an A600 of 8.0, and the cells were disrupted by bead beating in the presence of 0.1-mm zirconium beads (FastPrep). Subsequently, PBS was added, and the samples were centrifuged at 1,000 × g for 1 min to remove the beads. Supernatants were collected and centrifuged at 16,000 × g for 30 min at 4°C. The supernatant containing the cell extract of the proteins was saved for Western analysis. The pellet containing the cell wall extract was resuspended in digestion buffer (50 mM Tris-HCl [pH 8.0], 5 mM MgCl2, 5 mM CaCl2, 10 mg/ml lysozyme, 150 U/ml mutanolysin) and incubated at 37°C for 3 h. The cell wall extract, cell extract, and growth medium proteins were separated by using SDS-PAGE (NuPAGE Novex 4 to 12% Bis-Tris gels; Life Technologies). The gels were run first at 150 V for 15 min and then at 180 V for 1 h. Proteins were transferred onto an Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore) at 100 V for 1 h, and the membranes were blocked and labeled according to the instructions for the ECL Prime blocking agent (Life Technologies). To detect SpaA and SpaC in the blots, 1:30,000-diluted antiserum and a 1:100,000-diluted HRP-labeled goat anti-rabbit blotting-grade secondary antibody (Bio-Rad) were used, while for detection of SpaB, 1:10,000-diluted antiserum was used (8, 9, 12). The pilins were visualized on the blots with ECL Select Western blotting detection reagents (Life Technologies).
Quantification of surface-located SpaA and SpaC.
For the quantification of the pilus monomers using immunofluorescence labeling, cells grown overnight were washed with PBS, and their optical densities were adjusted (A600 = 1.0). Primary antibody for either SpaA or SpaC was added at a 1:100 ratio, and the samples were mixed and incubated for at least 30 min at room temperature. Cells were washed with PBS and resuspended in Alexa Fluor 488-labeled goat anti-rabbit antibody (Invitrogen) diluted 1:1,000 in PBS. The cells were incubated for at least 30 min at room temperature in the dark. After being washed once with PBS, the intensity of the fluorescence in the samples was measured with a Victor3 1420 multilabel counter (PerkinElmer). In addition, microscope slides were prepared, and images of the cells were obtained by using an epifluorescence microscope (Leica DM 4000B) and Cell^P imaging software (Olympus). Identical exposure times were used for all samples.
Adherence to porcine type II mucus.
A mucus binding assay was performed with porcine type II mucus (Sigma), essentially as described previously (26). Wells of a Maxisorp microtiter plate (Nunc) were coated with mucus by incubating a solution containing 0.5 mg/ml mucus–PBS in the wells overnight at +4°C. Control and mutant strains were grown overnight in MRS broth supplemented with 10 μl/ml [5′-3H]thymidine (17.0 Ci/mmol; PerkinElmer). L. rhamnosus Lc705 was used as a pilusless control. The next day, the cells were washed with PBS. After the optical density of the cultures was adjusted (A600 = 0.25), the wells were washed several times with PBS, and cells were added to the wells. Six technical replicates were prepared for each sample. Plates were centrifuged at 2,600 × g for 10 min to maximize the duration of the bacterium-mucus interaction and incubated at 37°C for 1 h. After incubation, the wells were washed several times with PBS to remove loosely bound cells. Lysis solution (1% SDS, 1 M NaOH) was added to the wells, and the plate was incubated at 60°C for 1 h. Radioactivity of the samples was measured by using a Wallac 1480 Wizard 3 automatic gamma counter.
Nucleotide sequence accession numbers.
The genome sequences can be found in NCBI's Sequence Read Archive (SRA) under study accession number SRP045628. The accession numbers for sample, experiment, and run, respectively, for each strain are as follows: SRS686857, SRX683693, and SRR1554404 for LrGG-PB11; SRS686858, SRX683685, and SRR1554393 for LrGG-PB12; SRS686867, SRX683696, and SRR1554407 for LrGG-PB13; SRS686869, SRX683698, and SRR1554410 for LrGG-PB21; SRS686872, SRX683701, and SRR1554411 for LrGG-PB22; SRS686873, SRX683705, and SRR1554430 for LrGG-PB23; SRS686888, SRX683720, and SRR1554448 for LrGG-PB24; SRS686901, SRX683733, and SRR1554450 for LrGG-PB25; SRS686903, SRX683735, and SRR1554460 for LrGG-PB31; and SRS686912, SRX683744, and SRR1554461 for LrGG-PB32.
RESULTS AND DISCUSSION
In the present study, we investigated the pilus production machinery of L. rhamnosus GG and addressed the possibility of producing pilusless isogenic mutants of L. rhamnosus GG without the use of GMO-generating methods. We selected random chemical mutagenesis with ethyl methanesulfonate (EMS), as it is an alkylating agent known to cause mainly transition mutations (27, 28). EMS and other mutagens are used routinely in industrial settings to enhance the qualities of industrial microbial strains (28–30). An enrichment method was established in which an excess of immobilized anti-SpaC IgG was used to trap piliated L. rhamnosus GG cells while nonpiliated mutants remain unbound. We screened 384 colonies from the enriched, EMS-treated sample and isolated 10 pilus-deficient strains, which represent 2.6% of the population. When a library of 192 mutagen-treated but nonenriched strains was screened for pilus-deficient strains, none were found. Hence, the enrichment method proved to be effective. Moreover, enrichment with nonmutagenized cells did not generate any pilus-deficient mutants with this sensitive system. This emphasizes the stability of pilus production in L. rhamnosus GG grown under these conditions, in line with our previous studies of pilus production of strains isolated from commercial products made with L. rhamnosus GG (17).
Immunoelectron microscopy analysis was performed and confirmed that all 10 mutants were devoid of the protruding pili found in L. rhamnosus GG (Fig. 1). Moreover, the genomes of the strains were sequenced by using the MiSeq platform, resulting in 162- to 343-fold coverage. Subsequent comparative analysis with the genome sequence of L. rhamnosus GG (8) was used to identify the mutations that resulted from mutagenesis (Table 1; see also Tables S1 and S2 in the supplemental material). The phenotypic and functional traits of the strains were also analyzed. Expression and localization of the pilin monomers in the cells were determined by Western blotting (Fig. 2; see also Fig. S3 in the supplemental material). SpaA and SpaC were detected in cell wall-associated, cytosolic (cell extract), and secreted protein fractions. SpaB was also analyzed, but it was detected only in the cell wall fraction. It was previously reported that the total amount of SpaB in the cells is very small (9). To obtain quantitative data on the amounts of SpaA and SpaC pilins on the cell surface, the strains were analyzed by using an immunofluorescence assay (Fig. 3). In addition, the adhesion of the strains to porcine intestinal mucus was measured to demonstrate the functional effect of the pilus deficiency (Fig. 4).
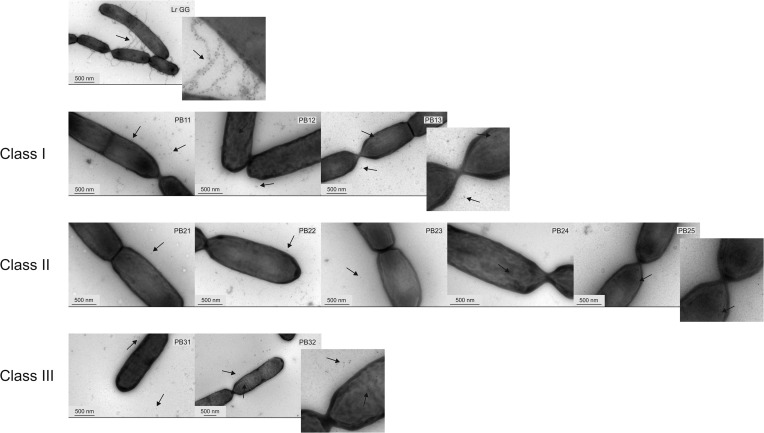
FIG 1.
Immunoelectron microscopy double labeling of L. rhamnosus GG and pilus-deficient mutants. The cells were labeled with SpaA and SpaC antibodies, and detection was done by using 10-nm and 5-nm gold particles, respectively. The magnitude is depicted by a scale bar in each picture. Some of the gold particles are indicated by arrows to facilitate detection. The three mutant classes are grouped, and a representative of each class is shown in detail.
TABLE 1.
Mutations in pilus-deficient strainsa
| Strain | Class | Pilus deficiency-causing mutation |
Other mutation(s) |
||||
|---|---|---|---|---|---|---|---|
| Gene | Mutation | Position(s) (bp) | aa change | SNP | Deletion | ||
| PB11 | I | srtC1 | G→A | 443188 | Trp256STOP | 55 | |
| PB12 | G→A | 443187 | Trp256STOP | 23 | 1-bp deletion | ||
| PB13 | G→A | 443200 | Trp252STOP | 110 | 60-bp deletion in IS2, 80-bp deletion in IS10 | ||
| PB21 | II | Del | 383753–457279 (IS10–IS16) | 52 | |||
| PB22 | 383753–457279 (IS10–IS16) | 51 | |||||
| PB23 | 383753–526350 (IS10–IS29) | 21 | |||||
| PB24 | 348729–474511 (IS9–IS26) | 22 | 1-bp deletion | ||||
| PB25 | 348729–474511 (IS9–IS26) | 23 | |||||
| PB31 | III | spaA | G→A | 445030 | Met1Ile | 39 | |
| PB32 | C→T | 444293 | Ser247Phe | 20 | |||
aa, amino acid; SNP, single nucleotide polymorphism; Del, deletion.
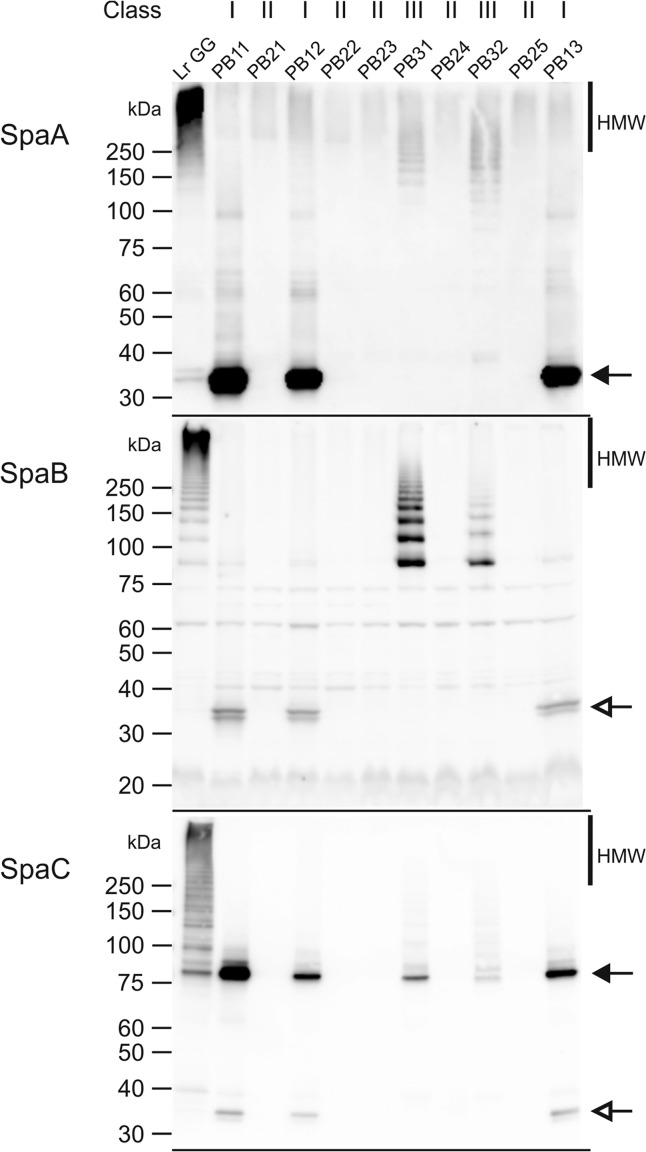
FIG 2.
Western blots of the cell wall protein extracts detected with either SpaA, SpaB, or SpaC antibodies. As the amounts of the pilin subunits vary (9) and the antibodies used have different sensitivities, we varied the amounts of loaded protein: the amount of protein extract in each lane is 84 times larger in the SpaB Western blot and 1.5 times larger in the SpaC blot than in the SpaA blot. In addition, in the SpaC blot, the amount of the L. rhamnosus GG sample is 10 times larger than the amount in other samples to better visualize the pilus ladder. The different mutant classes are depicted above the blots. Positions of monomeric pilins are indicated by black arrows. White arrows indicate the cross-reacting SpaA monomer in both SpaB and SpaC blots. Molecular masses (kDa) of the standard proteins are depicted on the left side of the blots. HMW, high-molecular-weight fraction.
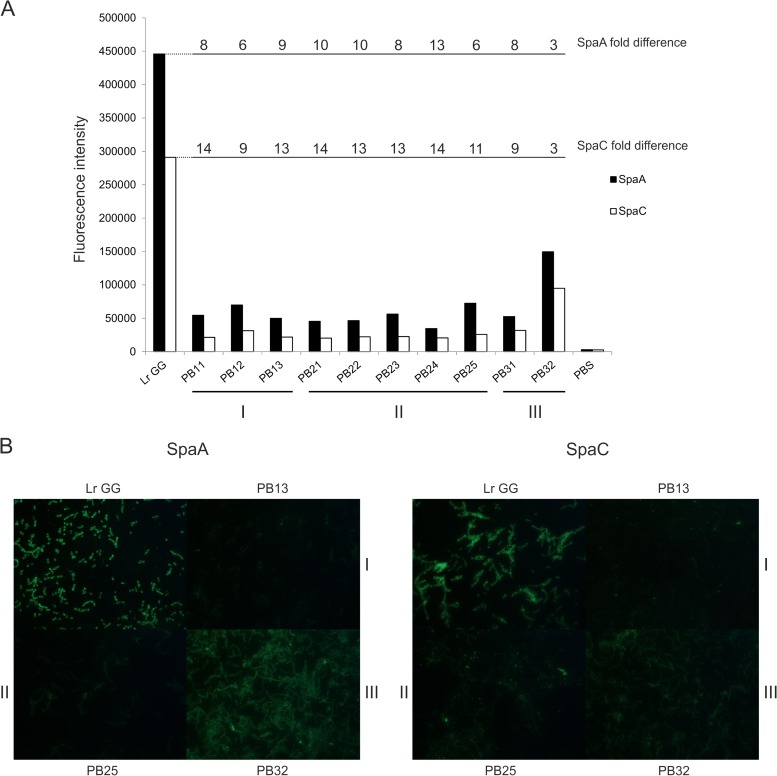
FIG 3.
Quantitation of SpaA and SpaC on the cell surface of L. rhamnosus GG and mutants strains. The cells were labeled with SpaA and SpaC antibodies, and the antibodies were detected with Alexa Fluor 488-labeled secondary antibody. (A) The absolute fluorescence intensity was measured by using a fluorescence plate reader. The fold differences in SpaA and SpaC intensities between L. rhamnosus GG and each mutant are depicted above the columns. PBS was used as a baseline sample. The strains are ordered according to class, and the classes are also depicted at the bottom. (B) The strains were visualized by using epifluorescence microscopy, and a representative strain from each class is shown.
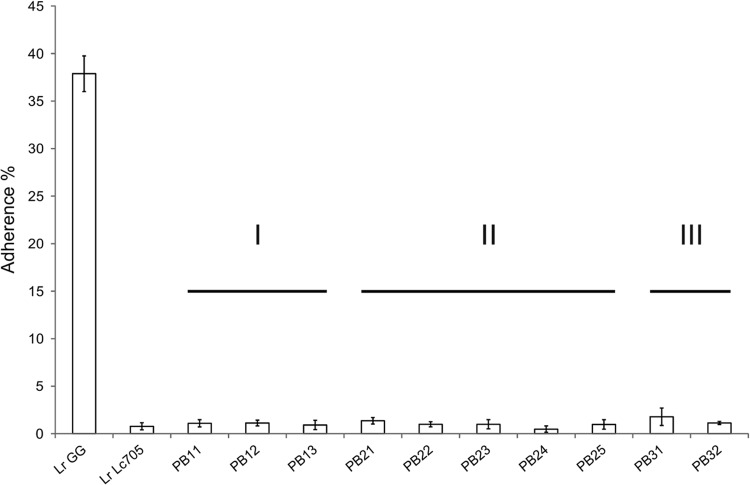
FIG 4.
Adherence of L. rhamnosus GG, the pilusless control L. rhamnosus Lc705, and pilus-deficient mutant strains to porcine mucus. The means ± standard deviations of data from 5 technical replicates (PB24 and PB31) or 6 technical replicates (other samples) from a representative experiment are shown. In the results for mutant strains PB24 and PB31, there was one technical replicate for each that clearly varied from other replicates (variation ≥2 times the average of the replicates) and hence was removed from the analysis. The strains are ordered according to class, and the classes are also depicted above the columns.
Three classes of mutants based on comparative genome analysis.
All 10 mutants were unique, as they contained different mutations (see Tables S1 and S2 in the supplemental material). Based on the nature of the mutations in the pilus biosynthesis pathway, three classes of mutants were identified (Table 1). In class I, the pilus-deficient phenotype was due to point mutations in the srtC1 gene for pilus-specific sortase C (strains PB11, PB12, and PB13, with the first number designating the class). Class II mutants had a large deletion of variable size in their genomes, which consistently included the spaCBA-srtC1 cluster of genes needed for pilus production (strains PB21, PB22, PB23, PB24, and PB25). Class III mutants had point mutations in the gene for the major pilus subunit SpaA (strains PB31 and PB32). In addition to the mutations affecting pilus production, the strains contained 20 to 112 collateral mutations. These mutations were evenly distributed across the genomes.
Class I mutants carry a defective srtC1 gene.
Class I mutants all shared a point mutation in the srtC1 gene, which codes for pilus-specific sortase C (Table 1). The mutations affected the same Trp256 residue (TGG) of the SrtC protein in strains PB11 and PB12 but with different substitutions: in PB11, it resided in the second base of the codon (TAG), and in PB12, it resided in the third base (TGA). In PB13, residue Trp252 was altered, as the second base of the codon was mutated (TGG→TAG). All mutations resulted in a stop codon and, hence, in a truncation of the C-terminal end of the enzyme and removal of about 100 amino acids. The substrate binding of the protein was probably not affected, since according to the secondary structure prediction for the mutated protein, the truncation occurred approximately 30 amino acids after the TLxTC catalytic site of the enzyme (31). It most likely affected the membrane-spanning domain of the enzyme (31, 32), preventing it from being retained on the cell membrane. Hence, it is likely that in the absence of the fully functional sortase SrtC, the polymerization reaction could not occur. In the class I mutants, the monomeric pilins accumulated on the cell surface and were also found in the cell extract, as shown by the Western blot results (Fig. 2; see also Fig. S3 in the supplemental material). SpaC was also observed among the secreted proteins, which might have resulted from its position at the tip of the pili and its supposed role as initiator of pilus biogenesis: the defective SrtC might have bound SpaC, but since it was probably unable to attach to the cell membrane, they both ended up in the culture medium. Although the class I strains could not produce mature pili, the immuno-EM pictures revealed small pilus oligomers containing ∼2 to 4 SpaA or SpaC pilins that are located in the milieu of the cells (Fig. 1), and these short pilin oligomers were also detected in very small amounts in the SpaA Western blot prepared with the cell wall extracts (Fig. 2). This finding suggests some residual activity of the sortase C enzyme despite its truncation. This finding also implies the presence of small pilin oligomers on the cell surface, but these were not observed in EM pictures, most likely because of the thick exopolysaccharide (EPS) layer, as it was shown previously to cover part of the pilus structure (33).
Class II mutants carry a large deletion containing the pilus gene cluster.
Five mutants, PB21, PB22, PB23, PB24, and PB25, belonged to class II. These mutants had a large deletion in their genomes in the region between bp 348700 and 526400, but the size of the deleted area varied among the strains (Table 1). The deletions resided between two insertion sequence (IS) elements, all belonging to the IS5 family, suggesting possible scenarios for these deletions. It is plausible that the stress caused by chemical mutagenesis resulted in the activation of transposable elements or that the mutations might have occurred by homologous or illegitimate recombination (34–37). Similar large areas of the genome were deleted in two L. rhamnosus strains from dairy products (18). However, in a survey of the genome sequences of 100 L. rhamnosus strains, we found this region to be highly variable (38). These results implicate the importance of this genomic region in adaptation to various environments. Careful analysis (see Table S2 in the supplemental material) showed that in addition to the spaCBA-srtC1 gene cluster, the region contained various genes for the metabolism of sugars. As expected from the deletion of the spaCBA-srtC1 genes, there were no SpaC or SpaA pilins observed in the Western blots (Fig. 2) or in the immuno-EM pictures (Fig. 1).
Class III mutants carry mutations in the spaA gene.
The class III mutants, PB31 and PB32, were pilus deficient due to a single nucleotide change in the spaA gene (Table 1). SpaA is the major subunit in heterotrimeric L. rhamnosus GG pili, and it forms the backbone of the pilus structure (9). PB31 showed a mutation in the start codon (ATG) of the gene, resulting in an ATA codon. This mutation did not completely abolish SpaA production, but the translation initiation efficiency appeared to be reduced, as the amount of SpaA on the Western blots was significantly smaller than that in the wild-type strain (Fig. 2; see also Fig. S3 in the supplemental material). In addition, pili were produced by the cells in small amounts, as seen in the Western blots, although they were not of mature length. Again, these pili were probably mostly covered by the EPS layer on the cell surface in the immuno-EM pictures (33), but pilin oligomers were seen in the milieu of the cells (Fig. 1). It is possible that ATA still acted as a start codon or that translation started from the next possible ATG start codon, which is situated 48 bp downstream from the original start site. The latter hypothesis would most likely lead to accumulation of the protein in the cytoplasm, because the N-terminal secretion signal peptide would be missing, but accumulation was not detected by Western blot analysis. Hence, decreased expression resulting from the mutated start codon (ATA) is a more probable explanation.
In PB32, the mutation was in codon 247 of the spaA pilin gene, resulting in an amino acid substitution from serine to phenylalanine. Based on the phenotype of PB32, this mutation most likely disrupted the structure of the protein, and hence, the pili were polymerized less efficiently, resulting in fewer pili, as demonstrated by Western blotting. In the cell wall and cell extract blots, the electrophoretic mobility of the SpaA monomer was also slightly lower than in L. rhamnosus GG and class I mutants, suggesting the presence of an uncleaved signal peptide (Fig. 2; see also Fig. S3 in the supplemental material). In the EM pictures, few gold particles, supposedly attached to the tips of the pilus structures, were seen on the surface of the cells (Fig. 1). Detached pilins and pilin oligomers were also found in cell surroundings, and according to the Western blot results, a rather large amount of immature pili was observed in the growth medium. This finding suggested that the mutation also affected SrtA coupling and that the pili were not attached to the cell wall efficiently. The short length of the pili in the SpaA mutants might be explained by the scarce supply of SpaA: when there is less SpaA available, the terminating SpaB could be incorporated more often into the pilus backbone. The housekeeping sortase SrtA, which attaches pili to the cell wall and terminates pilus elongation, has been suggested to recognize the base pilin SpaB more efficiently than the other pilins (11). SpaB was assumingly more easily available for SrtA than SpaA in the class III mutants, especially in PB31, consequently leading to activation of SrtA, early termination of pilus synthesis, and formation of shorter pili. When the SpaA mutants were compared, there seemed to be less SpaB in the pili of PB32 than in the pili of PB31, but on the other hand, PB32 produced more pili than did PB31. These observations also support the concept that the amount of available SpaA is the limiting factor in pilus biogenesis.
Collateral mutations in PB12.
One of the class I mutants, PB12, is the most suitable derivative to be used as a pilusless L. rhamnosus GG mutant in in vivo trials, since among all the mutants, it has the fewest collateral mutations with a class III mutant, PB32 (Table 1), and it has a lower level of production of pilin mono- and oligomers than PB32 (Fig. 2; see also Fig. S3 in the supplemental material). The 24 collateral mutations of PB12 consist of 9 silent, 6 intergenic, and 9 potentially effective mutations. The latter mutations are described in Table S4 in the supplemental material. None of the gene products related to these mutations were previously characterized in L. rhamnosus GG, but based on the current genome annotation of L. rhamnosus GG, the possible gene products are associated with membrane transport, nucleic acid synthesis and function, translation, and fatty acid synthesis. However, the growth rate of the strain and the results of API 50 CH tests (bioMérieux) are similar to those of the parental strain (results not shown), indicating that it is unlikely that the mutations have an extensive impact on the metabolism of the strain.
In conclusion, half of the mutants were pilus deficient due to a deletion of the SpaCBA-srtC1 gene cluster, and half of them had a single mutation inside the cluster, which destroyed their ability to produce pili. In addition to these mutations, the mutants showed quite similar quantities of collateral mutations.
Quantification of pilins and functional effect of pilus deficiency.
To obtain quantitative data on the amount of pilins produced, SpaA and SpaC were quantitated and visualized on the surface of the strains by using an immunofluorescence assay (Fig. 3). In the immunofluorescence assay, either the SpaA or the SpaC pilins were labeled with the fluorescent dye Alexa Fluor 488, and the amount of pilins on the cells was determined as the intensity of fluorescence that was measured with a multilabel counter and visualized by epifluorescence microscopy (Fig. 3). For the SpaA measurement, a 6- to 13-fold-lower fluorescence intensity was detected for the pilus-negative strains than for L. rhamnosus GG, and for the SpaC measurement, a 9- to 14-fold-lower intensity was observed. Strain PB32 differed from the other mutants since it had only a 3-fold difference compared to the parental strain. This finding can be explained by the expression of short pili, which were observed by Western blotting (Fig. 2; see also Fig. S3 in the supplemental material). The variation between the strains was also detected by fluorescence microscopy. To conclude, the quantification of SpaA and SpaC monomers on the surface of the cells verifies the results obtained by Western blotting (Fig. 2) and immuno-EM (Fig. 1). The mutants were also analyzed by using whole-cell dot blotting, which gave essentially similar results (results not shown).
Adhesion of the mutants to porcine type II intestinal mucus was measured to determine the functional impact of the mutations (Fig. 4). In the mucus binding assay, the adherence of the mutants ranged from 0.5 to 1.8%, while the adherence of L. rhamnosus GG was 37.9%. L. rhamnosus Lc705 was used as a pilus-negative control, as it was shown previously not to have SpaCBA pili (8). Its adherence to porcine mucus was 0.8%. Adherence was determined as the ratio of the amount of cells that remained bound to the mucus after washings to the total amount of cells added to the microtiter plate wells coated with mucus. The mucus binding assay was also performed with human mucus (8) for representatives of major mutant classes I and II (PB11, PB12, PB21, PB22, and PB23), and it was observed that this binding did not differ from the results obtained with porcine mucus (results not shown). The short pilin oligomers produced by a few of the strains, especially PB32, did not increase the adhesive properties, most likely because they are covered largely by the EPS layer on the surface of the cells and hence are not able to adhere to mucus (33). This result clearly indicates the role of mature pili in the adhesion of L. rhamnosus GG to mucus.
Conclusions.
We analyzed the genotype-phenotype relationships of a total of 10 pilus-deficient L. rhamnosus GG mutants and elucidated new aspects of the biogenesis of pili and the genomic region in which the spaCBA gene cluster resides. In addition, a new and efficient method for enrichment of strains according to the amount of SpaC pilin on the cell surface was developed, opening the possibility of implementing our mutagenesis method for other bacterial species of industrial relevance. No pilus-deficient mutants were found in the mutated population without the use of the enrichment. In line with our knowledge of pilus biology in Gram-positive bacteria, pilus biosynthesis critically relies on the functionality of pilin-specific sortase C, as revealed by the class I mutant strains, and the synthesis of the major pilin SpaA that forms the backbone of the pilus structure, as observed for the class III strains. The class II mutants, on the other hand, highlight the importance of the genomic area, in which the spaCBA-srtC1 gene cluster also resides, in the adaptation of L. rhamnosus GG to various environments.
The strains developed in this study can be used to further elucidate the effect of L. rhamnosus GG pili on the adhesion to and interaction with the host gastrointestinal tract more reliably than before. The cell morphology of the strains appeared similar to that of wild-type L. rhamnosus GG when analyzed by immuno-EM. Also, these strains grew at a rate similar to that of the parental strain, with comparable metabolic functions (results not shown). As may be expected from random mutagenesis, the mutant strains have a certain level of collateral mutations in their genomes. However, there are also some strains with only a few mutations, facilitating the prediction of their effect on the phenotype. As these pilus-deficient strains of L. rhamnosus GG are made without the use of GMO approaches, they can be used in human and other trials to define the effect of pili under in vivo conditions.
Supplementary Material
ACKNOWLEDGMENTS
The study was financially supported by the Finnish Funding Agency for Technology and Innovation (Tekes, grant 454/10), the European Research Council (grant 250172, MicrobesInside), and the Academy of Finland (grants 141130 and 252123).
We acknowledge Ross Crittenden, Soile Tynkkynen, and Tuomas Salusjärvi for their continuing interest in the project. We acknowledge the Palva group for providing some of the materials used in this study, and we are grateful to Outi Immonen for her excellent technical assistance. We thank the core units for electron microscopy and sequencing of the Institute of Biotechnology, University of Helsinki, for providing useful advice and facilities.
Footnotes
Published ahead of print 5 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02006-14.
REFERENCES
- 1.de Vos WM. 2011. Systems solutions by lactic acid bacteria: from paradigms to practice. Microb. Cell Fact. 10:S2. 10.1186/1475-2859-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM. 2005. Probiotic and other functional microbes: from markets to mechanisms. Curr. Opin. Biotechnol. 16:204–211. 10.1016/j.copbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. 1992. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig. Dis. Sci. 37:121–128. 10.1007/BF01308354. [DOI] [PubMed] [Google Scholar]
- 4.Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R. 2001. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 322:1327. 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357:1076–1079. 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 6.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361:1869–1871. 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 7.Szajewska H, Skorka A, Ruszczyński M, Gieruszczak-Białek D. 2013. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children—updated analysis of randomised controlled trials. Aliment. Pharmacol. Ther. 38:467–476. 10.1111/apt.12403. [DOI] [PubMed] [Google Scholar]
- 8.Kankainen M, Paulin L, Tynkkynen S, Von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx APA, Lebeer S, De Keersmaecker SCJ, Vanderleyden J, Hämäläinen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjärvi T, Auvinen P, De Vos WM. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. U. S. A. 106:17193–17198. 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reunanen J, von Ossowski I, Hendrickx APA, Palva A, de Vos WM. 2012. Characterization of the SpaCBA pilus fibers in the probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 78:2337–2344. 10.1128/AEM.07047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandlik A, Swierczynski A, Das A, Ton-That H. 2008. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol. 16:33–40. 10.1016/j.tim.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douillard FP, Rasinkangas P, von Ossowski I, Reunanen J, Palva A, de Vos WM. 2014. Functional identification of conserved residues involved in Lactobacillus rhamnosus GG sortase specificity and pilus biogenesis. J. Biol. Chem. 289:15764–15775. 10.1074/jbc.M113.542332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Ossowski I, Reunanen J, Satokari R, Vesterlund S, Kankainen M, Huhtinen H, Tynkkynen S, Salminen S, de Vos WM, Palva A. 2010. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 76:2049–2057. 10.1128/AEM.01958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ton-That H, Schneewind O. 2003. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 50:1429–1438. 10.1046/j.1365-2958.2003.03782.x. [DOI] [PubMed] [Google Scholar]
- 14.Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, Murray BE. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Invest. 116:2799–2807. 10.1172/JCI29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Ossowski I, Pietilä TE, Rintahaka J, Nummenmaa E, Mäkinen V-M, Reunanen J, Satokari R, de Vos WM, Palva I, Palva A. 2013. Using recombinant Lactococci as an approach to dissect the immunomodulating capacity of surface piliation in probiotic Lactobacillus rhamnosus GG. PLoS One 8:e64416. 10.1371/journal.pone.0064416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardita CS, Mercante JW, Kwon YM, Luo L, Crawford ME, Powell DN, Jones RM, Neish AS. 2014. Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses. Appl. Environ. Microbiol. 80:5068–5077. 10.1128/AEM.01039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douillard FP, Ribbera A, Järvinen HM, Kant R, Pietilä TE, Randazzo C, Paulin L, Laine PK, Caggia C, von Ossowski I, Reunanen J, Satokari R, Salminen S, Palva A, de Vos WM. 2013. Comparative genomic and functional analysis of Lactobacillus casei and Lactobacillus rhamnosus strains marketed as probiotics. Appl. Environ. Microbiol. 79:1923–1933. 10.1128/AEM.03467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sybesma W, Molenaar D, van IJcken W, Venema K, Kort R. 2013. Genome instability in Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 79:2233–2239. 10.1128/AEM.03566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Parliament and Council of the European Union. 2001. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. L106. Off. J. Eur. Commun. 44:1–38. [Google Scholar]
- 20.David M, Dzamba M, Lister D, Ilie L, Brudno M. 2011. SHRiMP2: sensitive yet practical SHort Read Mapping. Bioinformatics 27:1011–1012. 10.1093/bioinformatics/btr046. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R, 1000 Genomes Project Analysis Group 2011. The variant call format and VCFtools. Bioinformatics 27:2156–2158. 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12. 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 25.Åvall-Jaaskelainen S, Lindholm A, Palva A. 2003. Surface display of the receptor-binding region of the Lactobacillus brevis S-layer protein in Lactococcus lactis provides nonadhesive lactococci with the ability to adhere to intestinal epithelial cells. Appl. Environ. Microbiol. 69:2230–2236. 10.1128/AEM.69.4.2230-2236.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vesterlund S, Paltta J, Karp M, Ouwehand AC. 2005. Measurement of bacterial adhesion—in vitro evaluation of different methods. J. Microbiol. Methods 60:225–233. 10.1016/j.mimet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Sega GA. 1984. A review of the genetic effects of ethyl methanesulfonate. Mutat. Res. 134:113–142. 10.1016/0165-1110(84)90007-1. [DOI] [PubMed] [Google Scholar]
- 28.Parekh S, Vinci V, Strobel R. 2000. Improvement of microbial strains and fermentation processes. Appl. Microbiol. Biotechnol. 54:287–301. 10.1007/s002530000403. [DOI] [PubMed] [Google Scholar]
- 29.Tillich UM, Lehmann S, Schulze K, Dühring U, Frohme M. 2012. The optimal mutagen dosage to induce point-mutations in Synechocystis sp. PCC6803 and its application to promote temperature tolerance. PLoS One 7:e49467. 10.1371/journal.pone.0049467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margolles A, Sanchez B. 2012. Selection of a Bifidobacterium animalis subsp. lactis strain with a decreased ability to produce acetic acid. Appl. Environ. Microbiol. 78:3338–3342. 10.1128/AEM.00129-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrickx AP, Budzik JM, Oh S, Schneewind O. 2011. Architects at the bacterial surface—sortases and the assembly of pili with isopeptide bonds. Nat. Rev. Microbiol. 9:166–176. 10.1038/nrmicro2520. [DOI] [PubMed] [Google Scholar]
- 32.Marraffini LA, Dedent AC, Schneewind O. 2006. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 70:192–221. 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebeer S, Claes I, Tytgat HL, Verhoeven TL, Marien E, von Ossowski I, Reunanen J, Palva A, de Vos WM, Keersmaecker SC, Vanderleyden J. 2012. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 78:185–193. 10.1128/AEM.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aras RA, Kang J, Tschumi AI, Harasaki Y, Blaser MJ. 2003. Extensive repetitive DNA facilitates prokaryotic genome plasticity. Proc. Natl. Acad. Sci. U. S. A. 100:13579–13584. 10.1073/pnas.1735481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levinson G, Gutman GA. 1987. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol. Biol. Evol. 4:203–221. [DOI] [PubMed] [Google Scholar]
- 36.Bierne H, Ehrlich SD, Michel B. 1997. Deletions at stalled replication forks occur by two different pathways. EMBO J. 16:3332–3340. 10.1093/emboj/16.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tillier ER, Collins RA. 2000. Genome rearrangement by replication-directed translocation. Nat. Genet. 26:195–197. 10.1038/79918. [DOI] [PubMed] [Google Scholar]
- 38.Douillard FP, Ribbera A, Kant R, Pietilä TE, Järvinen HM, Messing M, Randazzo CL, Paulin L, Laine P, Ritari J, Caggia C, Lähteinen T, Brouns SJ, Satokari R, von Ossowski I, Reunanen J, Palva A, de Vos WM. 2013. Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet. 9:e1003683. 10.1371/journal.pgen.1003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.