Abstract
In microaerophilic or anaerobic environments, Pseudomonas aeruginosa utilizes nitrate reduction for energy production, a process dependent on the availability of the oxyanionic form of molybdenum, molybdate (MoO42−). Here, we show that molybdate acquisition in P. aeruginosa occurs via a high-affinity ATP-binding cassette permease (ModABC). ModA is a cluster D-III solute binding protein capable of interacting with molybdate or tungstate oxyanions. Deletion of the modA gene reduces cellular molybdate concentrations and results in inhibition of anaerobic growth and nitrate reduction. Further, we show that conditions that permit nitrate reduction also cause inhibition of biofilm formation and an alteration in fatty acid composition of P. aeruginosa. Collectively, these data highlight the importance of molybdate for anaerobic growth of P. aeruginosa and reveal novel consequences of nitrate reduction on biofilm formation and cell membrane composition.
INTRODUCTION
Pseudomonas aeruginosa is a ubiquitous environmental organism, capable of survival and proliferation under diverse conditions. In anaerobic environments, P. aeruginosa is capable of dissimilatory reduction of nitrate for energy production (1, 2). The major dissimilatory nitrate reduction pathway employs four enzymatic complexes to reduce nitrate to nitrite (NarGHI), nitrite to nitric oxide (NirS), nitric oxide to nitrous oxide (NorCB), and, finally, nitrous oxide to dinitrogen (NosZ) (3). P. aeruginosa also possesses a periplasmic nitrate reductase complex (NapAB), although this is not believed to play a major role in anaerobic growth (4, 5). The anaerobic nitrate regulator, Anr, of the fumarate and nitrate reductase (Fnr) family of transcriptional regulators, controls transcription of the dissimilatory nitrate reduction pathway (6, 7). Anr senses oxygen tension via its [4Fe-4S]2+ cluster (8). Under low oxygen tension, Anr upregulates expression of the nar operon and the dissimilatory nitrate reductase pathway regulator, dnr, which further modulates expression of the nar operon, the nirQ regulatory gene, and norCB (3, 7, 9, 10). As a consequence, in response to low oxygen tension, the cell is able to reduce nitrate to dinitrogen and generate energy for growth.
Each enzyme of the dissimilatory nitrate reductase pathway utilizes a transition metal cofactor for its activity, namely, iron, copper, or molybdenum (11). The initial enzymatic complex NarGHI, which reduces nitrate to nitrite, requires molybdenum incorporated in a modified molybdenum cofactor, Mo-bis molybdopterin guanine dinucleotide (MGD) (12). Cellular molybdenum uptake occurs in the form of its oxyanion, molybdate (MoO42−), referred to here as Mo. Upon uptake, Mo is introduced into a complex molybdopterin molecule to generate the molybdenum cofactor (MoCo), which may then be further modified prior to insertion into MoCo-dependent proteins (13). Such proteins include dimethyl sulfoxide (DMSO) reductase, xanthine oxidase, and sulfite oxidase, all of which have broad roles in nitrogen, carbon, and sulfur metabolism (13, 14). Acquisition of Mo by prokaryotes occurs primarily via a high-affinity ATP-binding cassette (ABC) permease, ModABC. Secondary transport has been reported via sulfate/thiosulfate ABC permeases and a nonspecific anion importer in Escherichia coli (15), as well as a low-affinity Mo ABC permease, MolABC, in Haemophilus influenzae (16). However, no such MolABC ortholog has been identified in P. aeruginosa (17). The high-affinity Mo ABC importer, ModABC, comprises a solute binding protein (SBP) (ModA), responsible for binding Mo; a dimer of nucleotide binding domains (ModC) which hydrolyze ATP in the cytoplasm to energize the transporter; and two transmembrane domains (ModB) which traverse the membrane and to which ModA delivers its cargo for transport (18). In Gram-negative organisms, ModA is a soluble, freely diffusible, periplasmic SBP that delivers Mo to the assembled ModB2C2 permease (19), whereas in Gram-positive bacteria, ModA is lipid anchored to the cell membrane (20). The ModABC system has been extensively characterized in E. coli, where its expression is under the control of the Mo-binding LysR transcriptional regulator, ModE (21–23). In addition to binding Mo, E. coli ModA has been shown to bind its structurally similar oxyanion, tungstate (WO42−) (24), referred to here as W. Furthermore, substitution for Mo has also been observed in ModE (22), likely exerting deleterious effects on gene regulation. Despite the ability of W to be bound by ModA, potentially facilitating transport, there are currently no physiological roles for W in E. coli. Organisms that specifically utilize W, such as the archaea Pyrococcus furiosus and Archaeoglobus fulgidus and the bacterium Campylobacter jejuni, encode W-specific (TupABC) or Mo/W (WtpABC) ABC importers in their genomes (25–27). No orthologs of these systems have been identified in P. aeruginosa (17). Whether, similarly to E. coli, P. aeruginosa ModABC is capable of W binding and import remains to be determined.
In this study, we have characterized the P. aeruginosa PAO1 ModA component of the Mod permease and assessed the contribution of molybdate uptake to nitrate reduction, biofilm formation, and cellular physiology under oxygen-limiting or anaerobic conditions. This work also provides new insight into the ability of W to substitute for Mo in transport, regulation, and functional roles.
MATERIALS AND METHODS
Bacterial strains, media, and growth.
The wild-type P. aeruginosa strain used in this study was PAO1, with the ΔmodA deletion mutant made using PAO1 according to the method of Choi and Schweizer (28) with primers listed in Table 1. P. aeruginosa was grown in a semisynthetic cation-defined medium (CDM) containing 8.45 mM Na2HPO4, 4.41 mM KH2PO4, 1.71 mM NaCl, and 3.74 mM NH4Cl, supplemented with 0.5% yeast extract (Difco, Becton Dickinson, USA) and vitamins (0.2 μM biotin, 0.4 μM nicotinic acid, 0.24 μM pyridoxine-HCl, 0.15 μM thiamine-HCl, 66.4 μM riboflavin-HCl, and 0.63 μM calcium pantothenate) and Chelex-100 treated. CaCl2 and MgSO4 were subsequently added to 0.1 mM and 2 mM, respectively. Metal concentrations of the CDM were ascertained by inductively coupled plasma mass spectroscopy (ICP-MS), with molybdenum and tungsten both present at ≤10 nM, respectively. When required, cultures were supplemented with 100 mM KNO3 and with 10 mM Na2MoO4 and/or Na2WO4.
TABLE 1.
Oligonucleotide primers used in this study
| Name | Target | Sequence (5′→3′) | Reference |
|---|---|---|---|
| Gm-F | Gmr | CGAATTAGCTTCAAAAGCGCTCTGA | 28 |
| Gm-R | Gmr | CGAATTGGGGATCTTGAAGTTCCT | 28 |
| GW-attB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCT | 28 | |
| GW-attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGT | 28 | |
| PA1863-UpF-GWL | modA | TACAAAAAAGCAGGCTCCTGCCCCAGCTATTGC | This study |
| PA1863-UpR-GWL | modA | TCAGAGCGCTTTTGAAGCTAATTCGACATAGCCGGCCTTGG | This study |
| PA1863-DnF-GWL | modA | AGGAACTTCAAGATCCCCAATTCGCCGAAGCTGGTGGAAGG | This study |
| PA1863-DnR-GWL | modA | TACAAGAAAGCTGGGTGGACTTGATCAGCGCG | This study |
| PA1863LIC1F | modA | TGGGTGGTGGATTTCCTGACGAG GTGCAGGTCG | This study |
| PA1863LIC1R | modA | TTGGAAGTATAAATTTCCGAGTTCGTAGCCGTAGGA | This study |
| qPA16S_F | 16S | GGAGAAAGTGGGGGATCTTC | This study |
| qPA16S_R | 16S | CGTAGGAGTCTGGACCGTGT | This study |
| qPA0576_F | rpoD | CGATATAGCCGCTGAGGA | This study |
| qPA0576_R | rpoD | GAGATCGAAATCGCCAAG | This study |
| qPA0487_F | modE | TGTTCAGTTCGTCGATGG | This study |
| qPA0487_R | modE | ATGTCCGCACTCTCCACT | This study |
| qPA1846_F | cti | GTCCAGCTACCCGAACTTCA | This study |
| qPA1846_R | cti | TAGCTCCAGAACTGCGGATT | This study |
| qPA1861_F | modC | GTTGGCATCCTGGAACAC | This study |
| qPA1861_R | modC | ACTTGCCTGCGCTGTATC | This study |
| qPA1863_F | modA | CTTCCTGCTCCAGTTTCG | This study |
| qPA1863_R | modA | GCCAAGGAATTCGAGAAA | This study |
| qPA3393_F | nosD | AGGCGCTGTTCATCTACAA | This study |
| qPA3393_R | nosD | TGGCGACGTACTTGACCT | This study |
| qPA3877_F | narK1 | CCATGTTGCTCGGGTAGT | This study |
| qPA3877_R | narK1 | ACCACCATGACCATCCAC | This study |
| qPA3918_F | moaC | GAGCTTGACGCTGGTGA | This study |
| qPA3918_R | moaC | AACCCTGCAACTGATCCA | This study |
For bacterial growth, medium with nitrate and metal supplementation where appropriate was inoculated to an optical density at 600 nm (OD600) of 0.05 using overnight culture. Cells were grown to an OD600 of 0.6 on an Innova 40R shaking incubator (Eppendorf, Germany) at 240 rpm, 37°C. If required for anaerobic shift, the culture was then transferred to a smaller sterile tube, completely filling it to minimize the amount of oxygen available. Cultures were then statically incubated with tightened lids at 37°C for 2 h, allowing oxygen depletion and a switch to anaerobic respiration. To confirm cell viability, CFU · ml−1 and OD600 were determined before and after the anaerobic shift experiment, with no change observed. Twenty-four-hour cultures were inoculated similarly; however, lids of tubes were kept loose followed by static incubation at 37°C for 24 h. Those for anaerobic growth were placed in a GasPak EZ anaerobic chamber with a single GasPak EZ anaerobic chamber sachet and oxygen indicator (Becton Dickinson, USA). The chamber was closed securely and placed at 37°C for 24 h.
Whole-cell metal accumulation.
P. aeruginosa was grown to an OD600 of 0.6 at 37°C, on an Innova 40R shaking incubator at 240 rpm. The bacteria were then washed, by resuspension and centrifugation at 7,000 × g for 8 min, 3 times with phosphate-buffered saline (PBS) containing 5 mM EDTA and then 6 times with PBS. Bacterial pellets were desiccated at 95°C overnight. The dry cell weight was measured, and the pellets were resuspended in 35% HNO3 and boiled at 95°C for 1 h prior to removal of debris by centrifugation. Samples were diluted to a final concentration of 3.5% HNO3 and analyzed by ICP-MS on an Agilent 7500cx ICP-MS (Adelaide Microscopy, University of Adelaide) (29).
Expression and purification of ModA.
Recombinant ModA was generated by PCR amplification of P. aeruginosa PAO1 modA using ligation-independent cloning and primers listed in Table 1 to insert the gene into a C-terminal dodecahistidine tag-containing vector, pCAMcLIC01, to generate pCAMcLIC01-ModA. Protein expression was performed in E. coli LEMO21(DE3), by growing the cells in an autoinducing TB medium (Overnight Express; Merck, USA) using UltraYield flasks (Thomson Instrument Company, USA) for 18 h at 27°C, on an Innova 44R shaking incubator at 215 rpm. Cells were harvested; resuspended in 20 mM morpholinepropanesulfonic acid (MOPS) (pH 7.2), 200 mM NaCl, 15 mM imidazole, 10% glycerol buffer with 80 mM (either) Mo or W; and disrupted at 35,000 lb/in2 by a Constant Systems cell disruptor, and the soluble supernatant was isolated by centrifugation at 4°C for 60 min at 120,000 × g. Purification of ModA was achieved by a Histrap HP column (GE Healthcare, United Kingdom) on an Äkta purifier (GE Healthcare) using a low-salt buffer containing 50 mM MOPS (pH 7.8), 200 mM NaCl, 10% glycerol, and 15 mM imidazole and a high-salt buffer containing 50 mM MOPS (pH 6.6), 200 mM NaCl, 10% glycerol, and 1 M imidazole. ModA was then desalted on a HiPrep 26/10 desalting column (GE Healthcare), and homogeneity was confirmed by gel permeation chromatography on a 16/60 HiPrep Sephacryl S-200 HR column (GE Healthcare) using an Äkta purifier (GE Healthcare) prior to characterization.
ModA metal-binding assays.
Metal loading assays were performed on Mo- or W-purified ModA (30 μM) by mixing with a 10-fold molar excess of Mo or W (300 μM Na2MoO4 or Na2WO4) in a total volume of 2 ml into the binding assay buffer (20 mM MOPS, pH 7.2, 100 mM NaCl) for 60 min at 4°C. Unbound metal was removed by desalting on a PD10 column (GE Healthcare) into the binding assay buffer, and the protein concentration was determined. An aliquot of protein was then kept for ICP-MS analysis, and the remainder was mixed with a 10-fold molar excess of the other metal (Mo or W) in a total volume of 2 ml for 60 min at 4°C. Samples were then desalted on a PD10 column. Solutions (2 to 3 μM) of metal-loaded protein were prepared in 3.5% HNO3 and boiled for 15 min at 95°C. Samples were then cooled and centrifuged for 20 min at 14,000 × g. The supernatant was then analyzed by ICP-MS, and the metal-to-protein ratio was determined. The thermodynamic stability assay (TSA) was performed as previously described (29, 30).
RNA isolation.
Cells were grown aerobically or statically for 24 h as detailed above and then harvested at 7,000 × g for 8 min at 4°C and lysed in TRIzol reagent (Life Technologies, USA) and chloroform. Following phase separation by centrifugation, RNA was isolated from the aqueous phase using a PureLink RNA minikit (Life Technologies). DNase I treatment was performed on 1 μg total RNA using 100 units of recombinant RNase-free DNase I (Roche, Germany) at 37°C for 15 min, prior to inactivation of the enzyme by the addition of EGTA (pH 8.0) to a final concentration of 2 mM and incubation at 65°C for 10 min. Samples were analyzed by quantitative reverse transcription-PCR (qRT-PCR) or stored at −80°C until required.
qRT-PCR.
For transcriptional analysis of aerobic or anaerobic shift cultures with an OD600 of 0.6, qRT-PCR was performed using a two-step method as previously described (31, 32). Briefly, cDNA was synthesized using random hexamers (Sigma-Aldrich, USA) and Moloney murine leukemia virus RNase H-minus point mutant (M-MLV, RNase H minus) reverse transcriptase (Promega, USA), per the manufacturer's protocol. Quantitative PCR (qPCR) was performed on a LightCycler 480 (Roche) using DyNAmo ColorFlash SYBR green qPCR mix (ThermoFisher Scientific, USA). For analysis of RNA from 24-h cultures, qRT-PCR was performed on a LightCycler 480 (Roche) using the SuperScript III Platinum SYBR One-Step qRT-PCR kit (Life Technologies). Oligonucleotides used in this study were designed using Primer3 integrated within UGENE v1.11.4 (Unipro) (33) and are listed in Table 1. 16S rRNA and the constitutively expressed sigma factor gene rpoD (PA0576) were used as controls to normalize gene expression, with the data representing biological triplicates.
Nitrate reductase assay.
Cultures were grown with 100 mM KNO3, either to an OD600 of 0.6 prior to a 2-h anaerobic shift or statically for 24 h, as described above. The assay was performed on whole cells, principally as described by Filiatrault and coworkers (34). Following growth, chloramphenicol was added to 25 μg · ml−1 and incubated for 5 min prior to harvest by centrifugation at 2,250 × g, resuspension, and washing of cell pellets twice in 50 mM phosphate buffer (pH 7.2). Washed cell pellets were resuspended in 1 ml of 50 mM phosphate buffer (pH 7.2), and OD600 was determined using 100 μl culture. One hundred microliters of freshly prepared 0.5 mg · ml−1 methyl viologen was added to 800 μl of cell suspension, plus 100 μl of a solution containing 4 mg · ml−1 Na2S2O4, 4 mg · ml−1 NaHCO3, and 100 mM KNO3. Control reaction mixtures received an equivalent solution that lacked Na2S2O4. Samples were incubated without agitation for 5 min and appeared blue. The reaction was stopped by vortexing until clear. Immediately, 1 ml of 1% sulfanilic acid prepared in 20% HCl was added to stop the reaction, followed by vortexing for 15 s. One milliliter of 1.3 mg · ml−1 N-(1-naphthyl)ethylenediamine dihydrochloride was then added to permit formation of a pink azo dye. Samples were incubated for 13 min, prior to centrifugation to remove cell debris. One hundred fifty microliters of clarified solution was transferred to a microtiter plate (Greiner Bio-One, Germany) to permit analysis at OD540 and OD460 using a PHERAstar FS spectrophotometer (BMG Labtech, Germany). Nitrate reductase activity was expressed in arbitrary units, as calculated using the following formula:
| (1) |
where T = 5 min and V = 3 ml.
Biofilm analyses.
Planktonic cells and media were discarded from 5-ml 24-h static cultures, which were prepared as described above. Biofilm cells were washed twice in tap water and stained with 0.1% crystal violet for 10 min. Crystal violet was then discarded, followed by 3 washes of the stained cells in tap water, and then left to dry. To determine relative biofilm formation, with the exclusion of the pellicle or air-liquid interface biofilm, 3 ml 30% acetic acid was carefully added and allowed to solubilize the biofilm for 10 min, after which 200 μl was transferred to a 96-well microtiter plate (Costar, Corning, USA) for analysis by well scanning at OD595 using a PHERAstar FS spectrophotometer.
FA analyses.
Strains for fatty acid (FA) analysis were grown statically for 24 h as described above. Total cellular lipids were extracted using chloroform-isopropanol and were subsequently methylated using a 1% sulfuric acid solution in methanol. The fatty acid methyl esters were analyzed by gas chromatography at the School of Agriculture, Food and Wine, University of Adelaide, as previously described (35).
BATH.
Cultures for the bacterial-adherence-to-hydrocarbons (BATH) assay were prepared from overnight seed cultures diluted 1 in 25 into fresh medium prior to 24-h static growth at 37°C. Cells were harvested by centrifugation at 2,250 × g and 25°C for 20 min. Cell pellets were resuspended in potassium-urea-magnesium (PUM) buffer (127 mM K2HPO4, 53 mM KH2PO4, 29.97 mM urea, 0.8 mM MgSO4 · 7H2O) for two 5-ml washes. Cells were resuspended in 5 ml PUM buffer prior to dilution to 6 ml of an OD600 of 0.25. Three milliliters xylene was added to each sample, vortexed for 30 s, and allowed to separate over the course of 5 min. Glass Pasteur pipettes were used to extract 1 ml of the hydrophilic layer to determine OD600. Hydrophobicity (association with xylene) was expressed as a percentage, as calculated using the following formula:
| (2) |
RESULTS
ModA is required for Mo acquisition.
Molybdate acquisition in prokaryotes, such as E. coli, occurs primarily through a high-affinity ModABC ABC permease. In P. aeruginosa, PA1863 encodes a 251-amino-acid protein that shares ∼30% identity with the cluster D-III SBP ModA from E. coli (36) (see Fig. S1 in the supplemental material). The proposed P. aeruginosa modA is clustered with two other genes in a putative operon that includes PA1861 (modC) and PA1862 (modB). Overall, the genetic organization is indicative of a canonical ABC import pathway (37), and orthologous operons can be found in many other Pseudomonas species, including P. fluorescens, P. putida, and P. syringae. Consequently, we sought to ascertain whether the putative modA (PA1863) was responsible for the high-affinity acquisition of Mo by P. aeruginosa. We constructed a modA deletion strain (ΔmodA) in P. aeruginosa PAO1, and analysis by ICP-MS showed that loss of the SBP abrogated Mo accumulation (Fig. 1A). Molybdate uptake could be restored by supplementation of the ΔmodA strain with 10 mM Na2MoO4. Collectively, these data indicate that ModA has a crucial role in Mo uptake at nanomolar concentrations, but at elevated, nonphysiological Mo concentrations, another unidentified low-affinity or nonspecific uptake pathway(s) could acquire Mo.
FIG 1.

Deletion of modA alters P. aeruginosa Mo accumulation and expression of modC. (A and B) In vitro accumulation of Mo by wild-type PAO1 and ΔmodA cultures was assessed via growth in CDM with and without 10 mM Mo and/or W supplementation. Metal content was expressed as μg of Mo per gram of dry cells, as determined by ICP-MS. Data correspond to means ± standard errors of the means, with duplicate readings taken from each biological replicate grown on 3 individual days. Statistical significance was determined using a two-tailed unpaired Student t test, where * represents a P value of <0.05 and ** represents a P value of <0.01. “b.d.” represents values below the limit of detection. (C) Relative expression of modC, corrected for rpoD, was assessed for PAO1 and ΔmodA strains grown in CDM. Where indicated, Mo (Na2MoO4) and W (Na2WO4) were each supplemented at 10 mM. Data represent the means ± standard errors of the means, with n ≥ 3 biological replicates. Statistical significance was determined using a two-tailed unpaired Student t test, where * represents a P value of <0.05.
Prior studies of the Mo ABC importer from E. coli (ModABC) have shown that although its physiological role was in Mo uptake, E. coli ModA was also able to bind W (24). However, the capability of E. coli ModA to interact with W has no physiological basis, as E. coli does not utilize W-cofactor-containing enzymes and, in many instances, W substitution for Mo results in enzyme inactivation (38). Only a single exception has been reported, with the in vivo substitution of W in the molybdoenzyme trimethylamine N-oxide (TMAO) reductase (39). The underlying molecular basis of W interaction with E. coli ModA is most likely a result of the amino acid side chains at the metal-binding site retaining the ligand-binding chemistry permissive for both Mo and W interaction. This limitation in ligand discrimination was recently exemplified in the manganese-recruiting cluster A SBP of Streptococcus pneumoniae, which was shown to have the capacity to interact with any first-row transition metal despite its sole physiological role in manganese acquisition (30). Thus, the inability of SBPs to discriminate between ligands with similar chemical properties may be a common feature of metal ion ABC importers. Consequently, we also sought to determine whether W supplementation impacted upon Mo accumulation in the wild-type and ΔmodA strains. We observed that upon supplementation with 10 mM Na2WO4, whole-cell accumulation of Mo by wild-type P. aeruginosa decreased by more than 90% (P = 0.006), consistent with the two ions competing for ModA-facilitated import (Fig. 1B). Supplementation with equimolar concentrations of Mo and W (10 mM) relieved the competition of W for ModA, allowing restoration of Mo accumulation (P = 0.0009, Fig. 1B). However, when the ΔmodA strain was supplemented with both Mo and W, ModA-independent Mo acquisition was also reduced (P = 0.0156; Fig. 1B). This suggests that the ModA-independent Mo acquisition pathway(s) had limited capacity to discriminate between Mo and W oxyanions. Collectively, these data indicate that ModA has a crucial role in Mo acquisition and that W can, at high extracellular concentrations, compete for binding to the SBP, reducing Mo uptake.
Molybdate acquisition in E. coli is under the control of the transcriptional regulator ModE (21–23). Accumulation of free Mo in the cytoplasm allows homodimeric ModE to bind two Mo ions and interact with its cognate DNA binding site, thereby repressing transcription of the E. coli modABCD genes (21, 40). The PAO1 genome contains PA0487 (designated modE), a gene that has 41% identity and 52% similarity to modE from E. coli. Unlike E. coli modE, which is divergently transcribed from modABCD, the putative PAO1 modE is located elsewhere in the genome. We examined the responsiveness of the modC gene, as a surrogate for the mod gene cluster, to Mo deficiency by use of not only the wild-type and ΔmodA strains but also the wild type supplemented with Mo and the structurally similar oxyanion, W (Fig. 1C). Intriguingly, modC was found to be upregulated in response to Mo deficiency in the ΔmodA strain only. In contrast, modC was repressed to wild-type levels when supplemented with Mo, Mo plus W, or W, with the latter resulting in a W-induced Mo deficiency. Taken together, these results show that the presence of either Mo or W can repress expression of modC, thus revealing the oxyanion dependency of modABC transcriptional regulation.
ModA has a single metal-binding site.
We then sought to ascertain the stoichiometry of metal binding by ModA. Recombinant ModA was expressed in E. coli, and the protein was purified after incubation with 80 mM Na2MoO4 or Na2WO4. Analysis of metal-bound ModA by gel permeation chromatography demonstrated the protein to be >95% homogeneous in size (see Fig. S2A in the supplemental material) and was confirmed by SDS-PAGE to be 28.6 kDa (Fig. S2B). ICP-MS was used to determine the metal content of the purified protein. Recombinant ModA purified with Na2MoO4 contained 0.99 ± 0.04 mol Mo · mol ModA−1 (Fig. 2A), while the protein purified with Na2WO4 contained 1.05 ± 0.04 mol W · mol ModA−1 (Fig. 2B). These data indicate that ModA contained a single metal-binding site, which was capable of accommodating either Mo or W. We then performed a competitive displacement assay by incubating Mo- or W-bound ModA with a 10-fold molar excess of the other metal (W or Mo, respectively; Fig. 2C and D). Incubation with the competing ligand resulted in complete displacement of Mo from Mo-ModA (0.04 ± 0.01 mol Mo · mol ModA−1) and partial loading with W (0.57 ± 0.03 mol W · mol ModA−1). Similarly, displacement of W from W-ModA resulted in decreased W occupancy (0.18 ± 0.05 mol W · mol ModA−1) and increased Mo loading (0.56 ± 0.01 mol Mo · mol ModA−1). Taken together, these data support the ICP-MS analysis regarding metal ion import (Fig. 1B), which indicates that W can compete with Mo for binding to ModA at the metal-binding site. We then examined the effect of metal binding on the thermostability of ModA. By subjecting ModA to an increasing temperature gradient, the stabilizing effect of the bound metal ion on the tertiary structure of the protein was assessed (Table 2). We observed that Mo induced a positive shift in the thermal stability of ModA (+17.6°C), slightly lower than that of W (+22.3°C). Collectively, these data indicate that ModA is able to bind either Mo or W oxyanions, consistent with the bioinformatic predictions and the phenotypic and metal ion accumulation studies.
FIG 2.
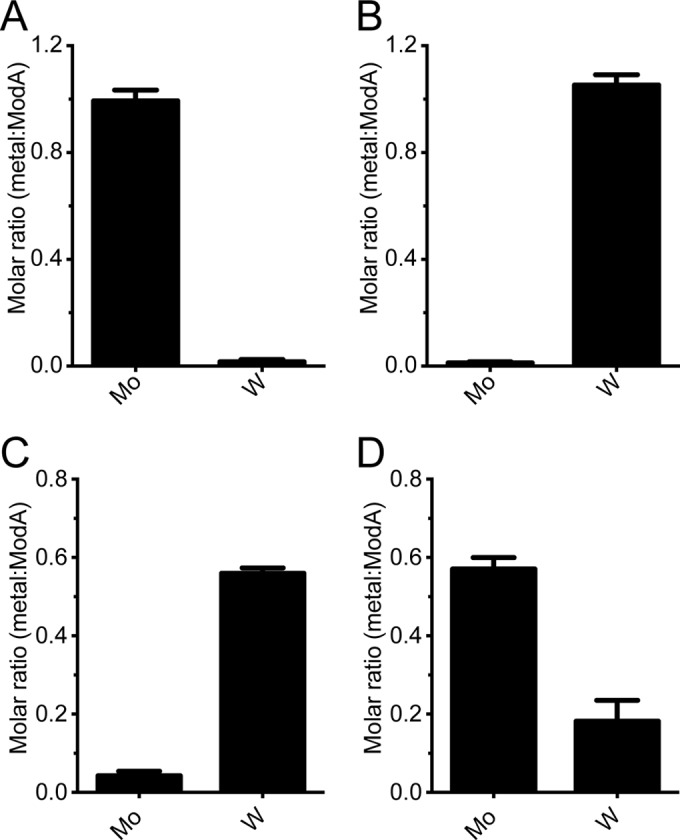
Metal loading of purified ModA. ICP-MS analysis was performed to determine metal loading of recombinant ModA. (A and B) Recombinant ModA purified in the presence of 80 mM Na2MoO4 (A) or 80 mM Na2WO4 (B). (C) Mo-purified ModA incubated with 10-fold molar excess of Na2WO4. (D) W-purified ModA incubated with 10-fold molar excess of Na2MoO4. Metal content is expressed as a molar ratio of metal to ModA. Data correspond to means ± standard errors of the means, with n ≥ 3.
TABLE 2.
Effect of Mo and W on apo-ModA thermal stabilitya
| Sample | Tm (°C) | ΔTm (°C) |
|---|---|---|
| Apo-ModA | 44.40 ± 0.85 | |
| Mo-ModA | 62.01 ± 0.17 | +17.6 |
| W-ModA | 66.72 ± 0.17 | +22.3 |
Values shown represent the mean (± standard error of the mean) from at least 3 independent measurements. Tm, melting temperature.
Anaerobic respiration and nitrate reduction are Mo dependent.
Molybdate plays an essential role in anaerobic respiration, where it serves as a component of the molybdenum cofactor in the first step of the nitrate reduction pathway, that is, the reduction of NO3− to NO2− by NarGHI (13, 41, 42). Consistent with our predictions, the ΔmodA strain, which lacks Mo, did not exhibit significant anaerobic growth over 24 h when supplemented with 100 mM KNO3 (Fig. 3A). While some growth was observed for the ΔmodA strain (OD600 of 0.25), this was likely to have occurred during the ∼2.5-h period required for the growth chamber to become anaerobic. Anaerobic growth in the presence of 100 mM KNO3 was also inhibited for the wild-type strain when supplemented with 10 mM W and for the ΔmodA strain under similar conditions (10 mM W; Fig. 3A), due to both strains being deficient in Mo accumulation (Fig. 1B). Anaerobic growth of the inhibited wild type (PAO1 plus W) could be restored by supplementation with Mo (PAO1 plus W plus Mo; Fig. 3A), indicating that it was a lack of Mo accumulation rather than an inhibitory effect of W that was preventing anaerobic growth. To confirm this, nitrate reductase assays, which measure nitrite production, were performed on 24-h static cultures of the ΔmodA strain, the ΔmodA strain plus W, or PAO1 plus W and demonstrated that when Mo accumulation was impaired, nitrate reductase activity was essentially abrogated (Fig. 3B). Furthermore, when sufficient intracellular Mo was present, as was the case for PAO1, the ΔmodA strain plus Mo, and PAO1 plus Mo plus W, nitrate reduction was facilitated. Taken together, these data indicate that ModA has an essential role in recruitment of Mo for nitrate reduction, a process prevented by W competing for import by ModA. We then examined the ability of P. aeruginosa to reduce nitrate in the presence of W by performing an anaerobic shift experiment. The wild-type and the ΔmodA strains were grown initially to an OD600 of 0.6 and then shifted to anaerobic conditions for 2 h. During this transition to anaerobiosis, the cells were forced to switch to nitrate reduction for energy production. Molybdenum limitation in the ΔmodA strain and ΔmodA strain plus W similarly resulted in impaired nitrate reductase activity (Fig. 3C). The inhibition of nitrate reduction activity in the ΔmodA strain supplemented with W also suggests that W was unable to functionally substitute for Mo within the Mo-bis MGD-dependent NarGHI nitrate reductase complex. Interestingly, the wild-type strain supplemented with W did not exhibit a significant impairment of nitrate reductase activity, with recorded levels similar to those of PAO1 and PAO1 plus Mo plus W (Fig. 3C). When considering the high concentration of W present in the cell (91.57 and 129.4 μg W · g cells−1 for PAO1 plus W and PAO1 plus Mo plus W, respectively), this result indicated that W was not inherently toxic to the nitrate reductase enzymes.
FIG 3.

Mo availability affects anaerobic growth and nitrate reductase activity. (A) Optical density (600 nm) of static cultures grown anaerobically in CDM supplemented with 100 mM KNO3 for 24 h. Means ± standard errors of the means shown for at least biological triplicates. (B and C) Nitrate reductase activity of 24-h static cultures (B) and 2-h anaerobic shift cultures (C) grown in CDM plus 100 mM KNO3. Nitrate reductase expressed in arbitrary units, as the mean ± standard error of the mean, with experiments performed in biological duplicates on 3 separate occasions. Where indicated, Mo and W were supplied at 10 mM as Na2MoO4 and Na2WO4, respectively. In each panel, statistical significance was determined using a two-tailed unpaired Student t test, where * represents a P value of <0.05, ** represents a P value of <0.01, *** represents a P value of <0.005, and **** represents a P value of <0.0001.
We then sought to confirm that the inability of the ΔmodA strain to reduce nitrate was due to Mo deficiency, rather than other effects, such as a lower abundance of nitrate reductase enzyme. Transcriptional analyses of the wild-type and ΔmodA P. aeruginosa strains revealed that the narK1K2GHJI operon was equally upregulated in the two strains during the 2-h anaerobic shift, as indicated by narK1 mRNA levels (Fig. 4A). Transcription of moaC and nosD (Fig. 4B and C), two other components of the nitrate reduction pathway, was also assessed, with upregulation observed in both strains during the nitrate-supplemented anaerobic shift (+ NO3 − O2). However, this upregulation was significantly lower in the ΔmodA strain than in the wild-type strain, most likely due to the lack of nitrate reduction, which is crucial for activating additional downstream transcription regulators via the production of nitric oxide (43). Taken together, these transcriptional analyses further support the crucial role of Mo homeostasis in the nitrate reduction pathway and the ability of P. aeruginosa to grow anaerobically.
FIG 4.

Transcriptional response to nitrate supplementation and anaerobic conditions. Relative expression levels of genes involved in nitrate reduction, corrected for rpoD, were determined for the PAO1 and ΔmodA strains grown to an OD600 of 0.6 in CDM. “+ NO3” indicates that cultures were supplemented with 100 mM KNO3 throughout growth, with − O2 denoting 2-h anaerobic shift cultures. Data represent means ± standard errors of the means, with n ≥ 3 biological replicates. Statistical significance was determined using a two-tailed unpaired Student t test, where * represents a P value of <0.05, ** represents a P value of <0.01, and **** represents a P value of <0.0001.
Nitrate reduction limits biofilm formation and alters cell membrane composition.
Biofilm formation by P. aeruginosa is of major significance for colonization of both biotic and abiotic surfaces (44, 45). We investigated in vitro biofilm formation on polycarbonate tubes by the wild-type and ΔmodA strains in the absence and presence of nitrate (Fig. 5). We observed that the inability to acquire Mo did not alter the production of biofilm by the ΔmodA strain in comparison with the wild type. However, upon supplementation with 100 mM KNO3, thereby facilitating nitrate reduction, the wild-type strain showed a significant 84% reduction in biofilm (P = 0.0139), whereas the ΔmodA strain showed no significant change. The reduction in biofilm was also achieved by the ΔmodA strain supplemented with 100 mM KNO3 and 10 mM Mo (P = 0.0012). Taken together, these data demonstrate that biofilm formation does not require ModA and hence, by extension, Mo. Furthermore, these data also show that nitrate reduction inhibits biofilm formation and may have a role in driving dispersal, although this requires further investigation.
FIG 5.

Anaerobic respiration reduces biofilm formation. Static biofilm formation after 24 h was assessed by 0.1% crystal violet staining and measuring absorbance at OD595. Means ± standard errors of the means shown for biological duplicates on 3 separate occasions. Statistical significance was determined using a two-tailed unpaired Student t test, where * represents a P value of <0.05 and ** represents a P value of <0.01.
Transition from the planktonic state to biofilm lifestyle has previously been shown to result in an alteration of the bacterial cell membrane lipid content (46). Consequently, we investigated the fatty acid (FA) composition of the wild-type and ΔmodA strains grown with and without KNO3 (Fig. 6). These analyses indicated that the P. aeruginosa cell membranes consist predominantly of 16- and 18-carbon FAs, with the saturated 16:0 (16-carbon acyl chain) and monounsaturated 18:1n-7 (18-carbon acyl chain with a double bond in the Δ7 position) FAs each comprising ∼30 to 40% of the total FA pool (Fig. 6A and E). The FA compositions of the wild-type and ΔmodA strains were essentially the same when grown in the absence of nitrate. Interestingly, the nitrate-reducing strain (PAO1 plus KNO3) exhibited a higher proportion of 16:0, 16:1n-7, and 18:0 than did the ΔmodA strain supplemented with nitrate (Fig. 6A, B, and D). Strikingly, major differences in the presence of trans monounsaturated FAs were seen. Culturing of PAO1 plus KNO3 resulted in significantly lower levels (>30-fold) of t16:1 (1 trans double bond) and t18:1n-7 (1 trans double bond in the Δ7 position) (Fig. 6C and F), an effect likely due to differences in cis-trans isomerization. Consequently, we assessed the transcription levels of the cis-trans isomerase (CTI; PA1846), and demonstrated CTI to be upregulated in strains that do not reduce nitrate (>3-fold) (Fig. 7A), corresponding with the higher levels of trans fatty acids (Fig. 6C and F). While the presence of unsaturated FAs leads to a more fluid cell membrane, the acyl chains of trans unsaturated FAs are straight, allowing tighter packing and providing increased rigidity. The benefits of a more rigid cell membrane under these conditions are currently unknown.
FIG 6.
Fatty acid (FA) compositions of PAO1 and ΔmodA strains. The major constituents of cellular fatty acids are depicted, indicating differences in relative abundance between PAO1 and the ΔmodA strain grown in CDM with (+ NO3) and without 100 mM KNO3. (A) 16:0, saturated FA of 16 carbon chains. (B) 16:1n-7, a monounsaturated FA of 16 carbons, with a cis double bond at the 7th carbon. (C) t16:1, a trans monounsaturated fatty acid with 16 carbons. The position of the double bond was not determined. (D) 18:0, saturated FA of 18 carbon chains. (E) 18:1n-7, a monounsaturated FA of 18 carbons with a cis double bond at the 7th carbon. (F) t18:1n-7, a trans monounsaturated fatty acid of 18 carbons, with the double bond at the 7th carbon. The data represent the means ± standard errors of the means, with n ≥ 3 biological replicates. Statistical significance was determined using a two-tailed unpaired Student t test, where * represents a P value of <0.05, ** represents a P value of <0.01, *** represents a P value of <0.005, and **** represents a P value of <0.0001.
FIG 7.

cis-trans isomerase (CTI) expression affects Pseudomonas membrane hydrophobicity. (A) Relative mRNA expression levels of CTI (PA1846) were assessed for 24-h static cultures of PAO1 and ΔmodA strains, corrected for 16S rRNA abundance. (B) The bacterial-adherence-to-hydrocarbons (BATH) assay was performed using xylene. Changes in membrane lipid hydrophobicity are reflected in altered adherence to xylene. The “+ NO3” notation indicates that cultures were grown with 100 mM KNO3. Data represent the means ± standard errors of the means, where n is ≥3 independent biological replicates. Statistical significance was determined using a two-tailed unpaired Student t test, where * represents a P value of <0.05 and *** represents a P value of <0.005.
In contrast to our data, most studies to date have suggested that pseudomonal CTIs are constitutively expressed (47). However, resistance to hydrocarbons, in particular toluene, has previously been linked with the isomerization of cis to trans FAs through an upregulation of CTI (up to 1.4-fold) in P. putida (48). Consistent with the >3-fold upregulation of CTI that we observed in cells unable to reduce nitrate, we sought to determine whether a lack of nitrate reduction, and hence upregulation of CTI and trans FA abundance, provided P. aeruginosa with the ability to associate with hydrocarbons via increased hydrophobicity. To do so, we incubated P. aeruginosa with xylene in a bacterial-adhesion-to-hydrocarbons (BATH) assay. Our results corroborate previous observations, in that a greater abundance of trans FAs correlates with the ability of cells to associate with the hydrophobic xylene phase (48), in contrast to cells containing very low levels of trans FAs (Fig. 7B), which associate with the aqueous/hydrophilic buffer. Collectively, these data show that cells unable to reduce nitrate have a distinct FA composition compared to those capable of nitrate reduction, with this difference attributable to the differential expression of CTI. Consequently, this may facilitate an enhanced tolerance to hydrocarbons and assist with the persistence of the bacterium in diverse environments.
DISCUSSION
Molybdate is an essential transition element. Here, we have reported on the PA1861-PA1863 operon and provided direct evidence for its role as the Mo ABC import system, modABC, of P. aeruginosa. Deletion of the SBP component of the ABC permease, ModA, abrogated Mo accumulation. This impairment was consistent with characterization of other ABC permeases, in which transport is dependent on the SBP to deliver the cargo for cellular uptake. A detailed biochemical characterization of ModA further confirmed its role as a Mo-recruiting SBP, and primary sequence analysis supported its classification within the cluster D-III subgroup of ABC transporter-associated SBPs, along with other Mo-recruiting SBPs. The limited bioavailability of Mo in some environmental niches has led to certain organisms also utilizing W due to its capacity for similar redox chemistry. Biochemical characterization of P. aeruginosa ModA also revealed that, similarly to ModA from E. coli (24), the SBP was also competent for interaction with W. As W has no known role in Pseudomonas, and the relative environmental abundance of W is on the order of 100-fold lower in most environments, this interaction is most likely nonphysiological (49). However, as P. aeruginosa is unlikely to encounter significant concentrations of W in its native environments, the capacity of ModA to interact with W does not appear to exert a fitness cost on the bacterium. Thus, our findings also highlight an apparent limitation in how ModA and related cluster D SBPs discriminate between structurally similar oxyanions. Overall, this observation is consistent with a growing body of evidence which indicates that the selectivity of the SBPs is ultimately constrained by the composition of the ligand-binding site. That is, the chemical properties of the side chains required to coordinate the cognate ligand, e.g., Mo, also permit other ligands with similar structural or chemical characteristics, e.g., W, to interact with the SBP. In this work, we have shown that W is capable of competing with Mo for ModA in P. aeruginosa and results in cellular Mo depletion.
Although W is capable of similar redox chemistry, it does not appear to be capable of functionally replacing Mo in the NarGHI nitrate reductase complex. Whether this arises from issues in the formation of the oxyanion-derived cofactor or is due to the inability of NarG to utilize a W cofactor remains to be determined. Rather, the toxicity of W is achieved via its competition with Mo binding to ModA and potential repression of modABC transcription via substitution in ModE. Together, these behaviors lead to depletion of intracellular Mo and impairment of the organism's capacity to further acquire the oxyanion.
Here, we have shown that Mo acquisition is directly linked to anaerobic nitrate reduction for in vitro growth. Interestingly, we observed that biofilm formation was inhibited when P. aeruginosa employed anaerobic nitrate respiration. Furthermore, anaerobic cultures grown in vitro in the presence of nitrate did not form biofilms after 24 h (data not shown). Nitric oxide, a product of nitrate reduction, has been shown to act as a signaling molecule when present at sublethal concentrations, driving dispersal of biofilms (50). Consequently, this by-product of nitrate respiration could be responsible for the inhibition of biofilm formation observed in our experiments.
The effect of Mo accumulation and nitrate reduction upon the fatty acid composition of Pseudomonas cell membranes has not, to our knowledge, been previously investigated. Consequently, we sought to ascertain the impact of these environmental effects on the trans fatty acid content. cis-trans isomerization has previously been reported in association with environmental stresses such as changes in elevated temperatures or the presence of toxic substances (51). Similar to saturated FAs, trans FAs are capable of more dense packing within the membrane, making it more rigid and less permeable. We observed that the inability to reduce nitrate was associated with a higher trans FA content. The isomerization of preexisting cis to trans FAs may be a stress response of cells unable to reduce nitrate due to a lack of Mo. Accordingly, our transcriptional analysis demonstrated the gene encoding CTI, the enzyme responsible for converting cis FAs to trans FAs, to be upregulated in strains unable to reduce nitrate, providing an explanation for the higher trans FA content. Although this finding contradicts a previous report, which indicated that CTI was constitutively expressed (52), our results indicate that cis-trans isomerization of the P. aeruginosa FAs may be induced as a component of cellular stress response mechanisms. However, the consequences of such changes in the fatty acid composition, and consequently membrane fluidity and hydrophobicity, require further investigation.
Conclusions.
This study provides a detailed characterization of ModABC, the primary Mo uptake pathway of P. aeruginosa. Our study indicates that the toxicity of W is multifaceted and is mediated via the limitation of Mo import. Further, we have shown how anaerobic nitrate respiration modulates biofilm dispersal and the FA composition of the bacterial cell membrane. These properties, both of which facilitate the survival of P. aeruginosa in diverse and dynamic environments, have major implications for understanding how this bacterium adapts to environmental stimuli and may be applicable to other Gram-negative environmental organisms and opportunistic pathogens.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Australian Research Council (ARC) grant DP120103957 to C.A.M. and J.C.P., the National Health and Medical Research Council (NHMRC) project grant 1022240 to C.A.M. and program grant 565526 to J.C.P, and the Channel 7 Children's Research Foundation grant 13661 to V.G.P. J.C.P. is an NHMRC Senior Principal Research Fellow, and V.G.P. is supported by an Australian Cystic Fibrosis Research Trust scholarship.
We thank H. Schweizer for providing the pEX18ApGW and pFLP2 plasmids and C. Adolphe and A. G. McEwan for discussions.
Footnotes
Published ahead of print 29 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02465-14.
REFERENCES
- 1.Palmer KL, Brown SA, Whiteley M. 2007. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J. Bacteriol. 189:4449–4455. 10.1128/JB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, Allen HL, DeKievit TR, Gardner PR, Schwab U, Rowe JJ, Iglewski BH, McDermott TR, Mason RP, Wozniak DJ, Hancock REW, Parsek MR, Noah TL, Boucher RC, Hassett DJ. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593–603. 10.1016/S1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber K, Krieger R, Benkert B, Eschbach M, Arai H, Schobert M, Jahn D. 2007. The anaerobic regulatory network required for Pseudomonas aeruginosa nitrate respiration. J. Bacteriol. 189:4310–4314. 10.1128/JB.00240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noriega C, Hassett DJ, Rowe JJ. 2005. The mobA gene is required for assimilatory and respiratory nitrate reduction but not xanthine dehydrogenase activity in Pseudomonas aeruginosa. Curr. Microbiol. 51:419–424. 10.1007/s00284-005-0125-8. [DOI] [PubMed] [Google Scholar]
- 5.Van Alst NE, Sherrill LA, Iglewski BH, Haidaris CG. 2009. Compensatory periplasmic nitrate reductase activity supports anaerobic growth of Pseudomonas aeruginosa PAO1 in the absence of membrane nitrate reductase. Can. J. Microbiol. 55:1133–1144. 10.1139/W09-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawers RG. 1991. Identification and molecular characterization of a transcriptional regulator from Pseudomonas aeruginosa PAO1 exhibiting structural and functional similarity to the FNR protein of Escherichia coli. Mol. Microbiol. 5:1469–1481. 10.1111/j.1365-2958.1991.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 7.Trunk K, Benkert B, Quäck N, Münch R, Scheer M, Garbe J, Jänsch L, Trost M, Wehland J, Buer J, Jahn M, Schobert M, Jahn D. 2010. Anaerobic adaptation in Pseudomonas aeruginosa: definition of the Anr and Dnr regulons. Environ. Microbiol. 12:1719–1733. 10.1111/j.1462-2920.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SS, Karabulut AC, Lipscomb JD, Hennigan RF, Lymar SV, Groce SL, Herr AB, Howell ML, Kiley PJ, Schurr MJ, Gaston B, Choi K-H, Schweizer HP, Hassett DJ. 2007. Two-pronged survival strategy for the major cystic fibrosis pathogen, Pseudomonas aeruginosa, lacking the capacity to degrade nitric oxide during anaerobic respiration. EMBO J. 26:3662–3672. 10.1038/sj.emboj.7601787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giardina G, Rinaldo S, Johnson KA, Di Matteo A, Brunori M, Cutruzzolà F. 2008. NO sensing in Pseudomonas aeruginosa: structure of the transcriptional regulator DNR. J. Mol. Biol. 378:1002–1015. 10.1016/j.jmb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Giardina G, Castiglione N, Caruso M, Cutruzzola F, Rinaldo S. 2011. The Pseudomonas aeruginosa DNR transcription factor: light and shade of nitric oxide-sensing mechanisms. Biochem. Soc. Trans. 39:294–298. 10.1042/BST0390294. [DOI] [PubMed] [Google Scholar]
- 11.Kraft B, Strous M, Tegetmeyer HE. 2011. Microbial nitrate respiration—genes, enzymes and environmental distribution. J. Biotechnol. 155:104–117. 10.1016/j.jbiotec.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Bertero MG, Rothery RA, Palak M, Hou C, Lim D, Blasco F, Weiner JH, Strynadka NCJ. 2003. Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nat. Struct. Biol. 10:681–687. 10.1038/nsb969. [DOI] [PubMed] [Google Scholar]
- 13.Iobbi-Nivol C, Leimkühler S. 2013. Molybdenum enzymes, their maturation and molybdenum cofactor biosynthesis in Escherichia coli. Biochim. Biophys. Acta 1827:1086–1101. 10.1016/j.bbabio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez D, Dias FM, Rowe JJ. 1991. Nitrate transport and its regulation by O2 in Pseudomonas aeruginosa. Arch. Biochem. Biophys. 286:159–163. 10.1016/0003-9861(91)90022-B. [DOI] [PubMed] [Google Scholar]
- 15.Rosentel JK, Healy F, Maupin-Furlow JA, Lee JH, Shanmugam KT. 1995. Molybdate and regulation of mod (molybdate transport), fdhF, and hyc (formate hydrogenlyase) operons in Escherichia coli. J. Bacteriol. 177:4857–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tirado-Lee L, Lee A, Rees DC, Pinkett HW. 2011. Classification of a Haemophilus influenzae ABC transporter HI1470/71 through its cognate molybdate periplasmic binding protein, MolA. Structure 19:1701–1710. 10.1016/j.str.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FSL, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GKS, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock REW, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 18.Self WT, Grunden AM, Hasona A, Shanmugam KT. 2001. Molybdate transport. Res. Microbiol. 152:311–321. 10.1016/S0923-2508(01)01202-5. [DOI] [PubMed] [Google Scholar]
- 19.Rech S, Wolin C, Gunsalus RP. 1996. Properties of the periplasmic ModA molybdate-binding protein of Escherichia coli. J. Biol. Chem. 271:2557–2562. 10.1074/jbc.271.5.2557. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer H, Pantel I, Lindgren P-E, Götz F. 1999. Characterization of the molybdate transport system ModABC of Staphylococcus carnosus. Arch. Microbiol. 172:109–115. 10.1007/s002030050747. [DOI] [PubMed] [Google Scholar]
- 21.Anderson LA, Palmer T, Price NC, Bornemann S, Boxer DH, Pau RN. 1997. Characterisation of the molybdenum-responsive ModE regulatory protein and its binding to the promoter region of the modABCD (molybdenum transport) operon of Escherichia coli. Eur. J. Biochem. 246:119–126. 10.1111/j.1432-1033.1997.00119.x. [DOI] [PubMed] [Google Scholar]
- 22.Grunden AM, Self WT, Villain M, Blalock JE, Shanmugam KT. 1999. An analysis of the binding of repressor protein ModE to modABCD (molybdate transport) operator/promoter DNA of Escherichia coli. J. Biol. Chem. 274:24308–24315. 10.1074/jbc.274.34.24308. [DOI] [PubMed] [Google Scholar]
- 23.Self WT, Grunden AM, Hasona A, Shanmugam KT. 1999. Transcriptional regulation of molybdoenzyme synthesis in Escherichia coli in response to molybdenum: ModE-molybdate, a repressor of the modABCD (molybdate transport) operon is a secondary transcriptional activator for the hyc and nar operons. Microbiology 145:41–55. 10.1099/13500872-145-1-41. [DOI] [PubMed] [Google Scholar]
- 24.Imperial J, Hadi M, Amy NK. 1998. Molybdate binding by ModA, the periplasmic component of the Escherichia coli mod molybdate transport system. Biochim. Biophys. Acta 1370:337–346. 10.1016/S0005-2736(98)00003-0. [DOI] [PubMed] [Google Scholar]
- 25.Bevers LE, Hagedoorn P-L, Krijger GC, Hagen WR. 2006. Tungsten transport protein A (WtpA) in Pyrococcus furiosus: the first member of a new class of tungstate and molybdate transporters. J. Bacteriol. 188:6498–6505. 10.1128/JB.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollenstein K, Frei DC, Locher KP. 2007. Structure of an ABC transporter in complex with its binding protein. Nature 446:213–216. 10.1038/nature05626. [DOI] [PubMed] [Google Scholar]
- 27.Smart JP, Cliff MJ, Kelly DJ. 2009. A role for tungsten in the biology of Campylobacter jejuni: tungstate stimulates formate dehydrogenase activity and is transported via an ultra-high affinity ABC system distinct from the molybdate transporter. Mol. Microbiol. 74:742–757. 10.1111/j.1365-2958.2009.06902.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi KH, Schweizer HP. 2005. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 5:30. 10.1186/1471-2180-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. 2011. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 7:e1002357. 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couñago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O'Mara ML, Cooper MA, McEwan AG, Paton JC, Kobe B, McDevitt CA. 2014. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat. Chem. Biol. 10:35–41. 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- 31.Eijkelkamp BA, Morey JR, Ween MP, Ong CL, McEwan AG, Paton JC, McDevitt CA. 2014. Extracellular zinc competitively inhibits manganese uptake and compromises oxidative stress management in Streptococcus pneumoniae. PLoS One 9:e89427. 10.1371/journal.pone.0089427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plumptre CD, Eijkelkamp BA, Morey JR, Behr F, Couñago RM, Ogunniyi AD, Kobe B, O'Mara ML, Paton JC, McDevitt CA. 2014. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol. Microbiol. 91:834–851. 10.1111/mmi.12504. [DOI] [PubMed] [Google Scholar]
- 33.Okonechnikov K, Golosova O, Fursov M, UGENE Team. 2012. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28:1166–1167. 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- 34.Filiatrault MJ, Tombline G, Wagner VE, Van Alst N, Rumbaugh K, Sokol P, Schwingel J, Iglewski BH. 2013. Pseudomonas aeruginosa PA1006, which plays a role in molybdenum homeostasis, is required for nitrate utilization, biofilm formation, and virulence. PLoS One 8:e55594. 10.1371/journal.pone.0055594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eijkelkamp BA, Stroeher UH, Hassan KA, Elbourne LDH, Paulsen IT, Brown MH. 2013. H-NS plays a role in expression of Acinetobacter baumannii virulence features. Infect. Immun. 81:2574–2583. 10.1128/IAI.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berntsson RPA, Smits SHJ, Schmitt L, Slotboom D-J, Poolman B. 2010. A structural classification of substrate-binding proteins. FEBS Lett. 584:2606–2617. 10.1016/j.febslet.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Davidson AL, Dassa E, Orelle C, Chen J. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72:317–364. 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kletzin A, Adams MWW. 1996. Tungsten in biological systems. FEMS Microbiol. Rev. 18:5–63. 10.1111/j.1574-6976.1996.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 39.Buc J, Santini C-L, Giordani R, Czjzek M, Wu L-F, Giordano G. 1999. Enzymatic and physiological properties of the tungsten-substituted molybdenum TMAO reductase from Escherichia coli. Mol. Microbiol. 32:159–168. 10.1046/j.1365-2958.1999.01340.x. [DOI] [PubMed] [Google Scholar]
- 40.Gourley DG, Schuttelkopf AW, Anderson LA, Price NC, Boxer DH, Hunter WN. 2001. Oxyanion binding alters conformation and quaternary structure of the C-terminal domain of the transcriptional regulator ModE. Implications for molybdate-dependent regulation, signaling, storage, and transport. J. Biol. Chem. 276:20641–20647. 10.1074/jbc.M100919200. [DOI] [PubMed] [Google Scholar]
- 41.Philippot L, Hojberg O. 1999. Dissimilatory nitrate reductases in bacteria. Biochim. Biophys. Acta 1446:1–23. 10.1016/S0167-4781(99)00072-X. [DOI] [PubMed] [Google Scholar]
- 42.Arai H. 2011. Regulation and function of versatile aerobic and anaerobic respiratory metabolism in Pseudomonas aeruginosa. Front. Microbiol. 2:103. 10.3389/fmicb.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arai H, Mizutani M, Igarashi Y. 2003. Transcriptional regulation of the nos genes for nitrous oxide reductase in Pseudomonas aeruginosa. Microbiology 149:29–36. 10.1099/mic.0.25936-0. [DOI] [PubMed] [Google Scholar]
- 44.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 45.Parsek MR, Singh PK. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677–701. 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 46.Benamara H, Rihouey C, Jouenne T, Alexandre S. 2011. Impact of the biofilm mode of growth on the inner membrane phospholipid composition and lipid domains in Pseudomonas aeruginosa. Biochim. Biophys. Acta 1808:98–105. 10.1016/j.bbamem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Kiran M, Annapoorni S, Suzuki I, Murata N, Shivaji S. 2005. Cis-trans isomerase gene in psychrophilic Pseudomonas syringae is constitutively expressed during growth and under conditions of temperature and solvent stress. Extremophiles 9:117–125. 10.1007/s00792-005-0435-6. [DOI] [PubMed] [Google Scholar]
- 48.Bernal P, Segura A, Ramos J-L. 2007. Compensatory role of the cis-trans-isomerase and cardiolipin synthase in the membrane fluidity of Pseudomonas putida DOT-T1E. Environ. Microbiol. 9:1658–1664. 10.1111/j.1462-2920.2007.01283.x. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz G, Mendel RR, Ribbe MW. 2009. Molybdenum cofactors, enzymes and pathways. Nature 460:839–847. 10.1038/nature08302. [DOI] [PubMed] [Google Scholar]
- 50.Barraud N, Hassett DJ, Hwang S-H, Rice SA, Kjelleberg S, Webb JS. 2006. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 188:7344–7353. 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heipieper HJ, Meinhardt F, Segura A. 2003. The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 229:1–7. 10.1016/S0378-1097(03)00792-4. [DOI] [PubMed] [Google Scholar]
- 52.von Wallbrunn A, Richnow HH, Neumann G, Meinhardt F, Heipieper HJ. 2003. Mechanism of cis-trans isomerization of unsaturated fatty acids in Pseudomonas putida. J. Bacteriol. 185:1730–1733. 10.1128/JB.185.5.1730-1733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



