Abstract
In Pseudomonas spp., the Gac-Rsm signal transduction system is required for the production of lipases. The current model assumes that the system induces lipase gene transcription mediated through the quorum-sensing (QS) system. However, there are no reports of a QS system based upon N-acyl homoserine lactones or the regulation of lipase gene expression in Pseudomonas protegens. In this study, we investigated the regulatory mechanism acting on lipA expression activated by the Gac-Rsm system in P. protegens Pf-5 through deletion and overexpression of gacA, overexpression of rsmA or rsmE, expression of various lacZ fusions, reverse transcription-PCR analysis, and determination of whole-cell lipase activity. The results demonstrated that the GacS-GacA (GacS/A) system activates lipA expression at both the transcriptional and the translational levels but that the translational level is the key regulatory pathway. Further results showed that the activation of lipA translation by the GacS/A system is mediated through RsmE, which inhibits lipA translation by binding to the ACAAGGAUGU sequence overlapping the Shine-Dalgarno (SD) sequence of lipA mRNA to hinder the access of the 30S ribosomal subunit to the SD sequence. Moreover, the GacS/A system promotes lipA transcription through the mediation of RsmA inhibiting lipA transcription via an unknown pathway. Besides the transcriptional repression, RsmA mainly activates lipA translation by negatively regulating rsmE translation. In summary, in P. protegens Pf-5, the Gac-RsmE system mainly and directly activates lipA translation and the Gac-RsmA system indirectly enhances lipA transcription.
INTRODUCTION
Lipases (triacylglycerol acylhydrolases; EC 3.1.1.3) are ubiquitous enzymes which are produced by various plants, animals, and microorganisms. They usually catalyze the hydrolysis of esters formed from glycerol and long-chain fatty acids in aqueous solutions. They also catalyze other reactions in micro- or nonaqueous environments, such as esterification, alcoholysis, aminolysis, or transesterification (1, 2). Owing to their versatility, lipases are widely applied in various processes, such as the manufacture of food (3, 4), detergents (5, 6), biodiesel (7, 8), and fine chemicals (9, 10) as well as waste treatment (11). Of these, the most widely used lipases are those originating from the genus Pseudomonas, due to their excellent properties (1, 12–15).
Although numerous bacterial lipases have been identified and characterized in past decades, rather limited reports on the molecular mechanisms regulating lipase gene expression are available. The transcription of the lipase gene is directly regulated by LipQ-LipR (LipQ/R) in Pseudomonas alcaligenes or CbrA-CbrB (CbrA/B) in P. aeruginosa. LipQ/R and CbrA/B are two-component systems of the NtrB-NtrC family that bind to a specific upstream activator sequence (UAS) with the help of RNA polymerase σ54 (or RpoN) (13, 16–18). In addition, two quorum-sensing (QS) systems, the las system and the rhl system, which are organized in a hierarchical manner, in which the las system transcriptionally activates the rhl system, enhance cbrAB transcription in P. aeruginosa (16, 19–22).
As we know, the global Gac-Rsm signal transduction system also plays an important role in the regulation of lipase gene expression. The two-component system GacS-GacA (GacS/A) has been reported to have a positive effect on lipase gene expression in Pseudomonas spp. (16, 19, 23). This highly conserved global regulatory system comprises the inner membrane-bound sensor kinase, GacS, which recognizes an as-yet-unidentified environmental signal and is activated through autophosphorylation, and the cognate response regulator, GacA, which is activated by the phosphorylated GacS via a phosphorelay mechanism. The current model assumes that the activated GacA specifically initializes the transcription of several small regulatory RNAs (sRNAs) of a common family, termed RsmY and RsmZ in P. aeruginosa or RsmX, RsmY, and RsmZ in P. protegens. Multiple single-stranded GGA motifs are a feature common to all of these sRNAs, and these motifs have a high affinity for RNA-binding protein(s) RsmA (and RsmE) of the RsmA-CsrA family. They can relieve translational repression by titrating out these RNA-binding proteins, which bind to specific motifs (usually ANGGA or GGA) overlapping or near the Shine-Dalgarno (SD) sequence of target mRNA to obstruct translation initiation (24–27). RsmA normally represses the two QS systems of P. aeruginosa and, consequently, inhibits expression of a variety of extracellular products, including lipase. However, RsmA also induces the production of lipase via an unknown mechanism in P. aeruginosa (20, 28).
Given the less well-documented scenario of the regulation of lipase gene expression, the aim of this study was to investigate in more detail the role of the Gac-Rsm signal transduction system in lipase gene expression, using the well-characterized soil bacterium P. protegens Pf-5 (previously called P. fluorescens Pf-5) (29, 30) as the model organism and lipase production as the readout. The lipA product, LipA, an intracellular lipase, exhibits excellent stability and high activity at moderate temperatures, under alkaline conditions, and in the presence of heavy metal ions, surfactants, and organic solvents (31). In terms of its excellent properties, the regulatory mechanism of lipA expression is worthy of study and is of theoretical and practical significance. By deleting and/or overexpressing specific components of the Gac-Rsm regulatory cascade, our results demonstrated that the GacS/A system mainly activates lipA translation through RsmE binding to the ACAAGGAUGU sequence, which overlaps the SD sequence of lipA mRNA, to obstruct the interaction of the SD sequence with the 30S ribosomal subunit and, hence, translation initiation. Furthermore, the GacS/A system also promotes lipA transcription via a mechanism in which RsmA indirectly inhibits lipA transcription by an unknown pathway. In addition to the transcriptional repression, RsmA stimulates lipA translation by repressing the translation of rsmE, and we found that this translational control is the key regulatory pathway.
MATERIALS AND METHODS
Strains, plasmids, culture conditions, and general methods.
The bacterial strains and plasmids used in this study are listed in Table 1. P. protegens (28°C) and Escherichia coli (37°C) strains were propagated in liquid or solid (1.5%, wt/vol, agar) LB medium. The following antibiotics were used: for P. protegens, ampicillin (Ap; 100 μg/ml), gentamicin (Gm; 50 μg/ml), and kanamycin (Km; 40 μg/ml); for E. coli, spectinomycin (Sp; 100 μg/ml), gentamicin (50 μg/ml), and kanamycin (40 μg/ml). PrimeSTAR HS DNA polymerase, restriction enzymes, and a DNA ligation kit were purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. (Dalian, China). Genomic DNA extraction, DNA gel extraction, and plasmid preparation were carried out with commercial kits (Omega Bio-Tek, Doraville, GA) according to the manufacturer's protocols. Oligonucleotide primers were synthesized by Wuhan Anygene Biological Technology Co., Ltd. (Wuhan, China). DNA sequencing was performed by Shanghai Sunny Biotechnology Co., Ltd. (Shanghai, China). All other routine manipulations were performed by standard methods (32).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid purpose and plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| Top10 | mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| BL21(DE3) | F− ompT hsdSB(rB− mB−) dcm gal(DE3) | Novagen |
| BL/pET-28a | BL21(DE3) with pET-28a; Kmr | This study |
| BL/pET-rsmE | BL21(DE3) with pET-rsmE; Kmr | This study |
| P. protegens | ||
| Pf-5 | Rhizosphere isolate; Apr | 29 |
| Pf3563 | ΔgacA derivative of Pf-5; Apr | This study |
| Pf-5F3 | pJQ003 conjugated into Pf-5; Gmr | This study |
| Pf-5F4 | pJQ004 conjugated into Pf-5; Gmr | This study |
| Pf-5F5 | pJQ005 conjugated into Pf-5; Gmr | This study |
| Pf-5F6 | pJQ006 conjugated into Pf-5; Gmr | This study |
| Pf3563F3 | pJQ003 conjugated into Pf3563; Gmr | This study |
| Pf3563F4 | pJQ004 conjugated into Pf3563; Gmr | This study |
| Plasmids | ||
| Triparental mating, pRK2073 | Helper plasmid for triparental mating; Spr | 37 |
| Markerless deletion mutation | ||
| pJQ200SK | Suicide vector with sacB counterselectable marker used for homologous recombination; Gmr | 33 |
| pJQΔgacA | pJQ200SK carrying a 1.772-kb XbaI insert with a deletion in the coding region of gacA; Gmr | This study |
| Overexpression | ||
| pBBR1MCS-5 | Broad-host-range vector; Gmr | 34 |
| pBBR1Km | NcoI-BglII-digested kanamycin resistance cassette subcloned in pBBR1MCS-5 digested with the same endonucleases | This study |
| pBBRKm | pBBR1Km with a 1,280-bp BamHI-XbaI fragment harboring lacIq-Plac; Kmr | This study |
| pBBRKgacA | pBBRKm with a 655-bp BamHI-HindIII fragment harboring the coding region of gacA; Kmr | This study |
| pBBRKrsmA | pBBRKm carrying a 260-bp BamHI-HindIII fragment harboring the coding region of rsmA; Kmr | This study |
| pBBRKrsmE | pBBRKm carrying a 263-bp BamHI-HindIII fragment harboring the coding region of rsmE; Kmr | This study |
| pET-28a | Expression vector carrying an N-terminal His tag-thrombin-T7 tag configuration plus an optional C-terminal His tag sequence; Kmr | Novagen |
| pET-rsmE | pET-28a carrying a 262-bp NdeI-HindIII fragment harboring the coding region of rsmE; Kmr | This study |
| Plasmid-borne lacZ fusion | ||
| pBBR001 | pBBR1MCS-5 derivative with a translational ′lacZ fusion; Gmr | This study |
| pBBR002 | pBBR1MCS-5 derivative with a transcriptional lacZ fusion; Gmr | This study |
| pBBR003 | pBBR001 derivative with a translational lipA′-′lacZ fusion; Gmr | This study |
| pBBR004 | pBBR002 derivative with a transcriptional lipA-lacZ fusion; Gmr | This study |
| pBBR005 | pBBR001 derivative with a translational rsmA′-′lacZ fusion; Gmr | This study |
| pBBR006 | pBBR001 derivative with a translational rsmE′-′lacZ fusion; Gmr | This study |
| pBBR00A10U | pBBR001 derivative with a translational lipA′-A10U-′lacZ fusion; Gmr | This study |
| pBBR00G11C | pBBR001 derivative with a translational lipA′-G11C-′lacZ fusion; Gmr | This study |
| pBBR00G12A | pBBR001 derivative with a translational lipA′-G12A-′lacZ fusion; Gmr | This study |
| pBBR00A14U | pBBR001 derivative with a translational lipA′-A14U-′lacZ fusion; Gmr | This study |
| Chromosome-borne lacZ fusion | ||
| pJQ003 | pJQ200SK derivative with a translational lipA′-′lacZ fusion; Gmr | This study |
| pJQ004 | pJQ200SK derivative with a transcriptional lipA-lacZ fusion; Gmr | This study |
| pJQ005 | pJQ200SK derivative with a translational rsmA′-′lacZ fusion; Gmr | This study |
| pJQ006 | pJQ200SK derivative with a translational rsmE′-′lacZ fusion; Gmr | This study |
Construction of suicide plasmid pJQΔgacA.
PCR amplicons, including the homology arms 906 bp upstream (bp −921 to −16 relative to the translational start site) and 866 bp downstream (bp +407 to +1272 relative to the translational start site) of gacA, were used to produce the markerless deletion mutation cassette of the coding region by overlap PCR. An XbaI-digested mutant cassette was ligated into the suicide plasmid pJQ200SK (33) for markerless deletion of the gacA coding region; this was followed by E. coli Top10 transformation. Transformants were screened on LB plates containing 50 μg/ml gentamicin and further verified by colony PCR. Then, plasmid DNA was isolated and sequenced. The recombinant plasmid was named pJQΔgacA (Table 1).
Construction of expression plasmids.
An NcoI- and BglII-digested kanamycin resistance cassette, amplified from plasmid pET-28a, was ligated into plasmid pBBR1MCS-5 (34) to create plasmid pBBR1Km (Table 1). Then, a PCR product carrying lacIq-Plac, obtained by using E. coli BL21(DE3) genomic DNA as the template, was digested with BamHI and XbaI and cloned into plasmid pBBR1Km to create plasmid pBBRKm (Table 1). Subsequently, PCR amplicons encoding the coding regions of the gacA (655 bp), rsmA (260 bp), and rsmE (263 bp) genes were digested with BamHI and HindIII and cloned into expression plasmid pBBRKm under the control of the lacZ promoter, creating recombinant plasmids pBBRKgacA, pBBRKrsmA, and pBBRKrsmE, respectively (Table 1). Similarly, the PCR amplicon encoding the coding region of the rsmE gene (262 bp) was digested with NdeI and HindIII and cloned into the expression plasmid pET-28a (Table 1), creating recombinant plasmid pET-rsmE (Table 1).
Construction of lacZ fusion plasmids.
To construct lacZ translational and transcriptional fusions (Fig. 1), PCR amplicons of ′lacZ (bp +22 to +3110 relative to the start site of translation), which lacked its own SD sequence and the first seven codons, and wild-type lacZ (bp −18 to +3110 relative to the start site of translation), which contained its own SD sequence, were amplified from E. coli BL21(DE3) genomic DNA. Then, BamHI- and HindIII-digested ′lacZ and lacZ were cloned into plasmid pBBR1MCS-5 to create translational fusion plasmid pBBR001 and transcriptional fusion plasmid pBBR002, respectively (Table 1). Next, PCR amplicons of lipA (bp −613 to +18 relative to the start site of translation), rsmA (bp −517 to +7 relative to the start site of translation), and rsmE (bp −499 to +7 relative to the start site of translation) were digested with KpnI and HindIII and cloned into plasmid pBBR001, creating plasmids pBBR003, pBBR005, and pBBR006, respectively (Table 1). Similarly, PCR amplicons containing lipA (bp −613 to −12 relative to the start site of translation) were digested with KpnI and HindIII and cloned into plasmid pBBR002, and the resulting plasmid was named pBBR004 (Table 1). To clone lipA′-′lacZ, lipA-lacZ, rsmA′-′lacZ, and rsmE′-′lacZ fusions into the suicide plasmid for introduction into the chromosome, pBBR003, pBBR004, pBBR005, and pBBR006 were digested with SphI and BamHI and cloned into plasmid pJQ200SK, creating plasmids pJQ003, pJQ004, pJQ005, and pJQ006, respectively (Table 1). For the single-base substitution mutations of the ANGGAN motif in the SD sequence of lipA mRNA, a common forward primer and specific reverse primers carrying the desired substitutions (Table 2) were used to introduce the mutations A10U, G11C, G12A, and A14U (relative to the start site of translation) (35). Then, these amplicons were digested with KpnI and HindIII and cloned into plasmid pBBR001, creating recombinant plasmids pBBR00A10U, pBBR00G11C, pBBR00G12A, and pBBR00A14U, respectively (Table 1).
FIG 1.
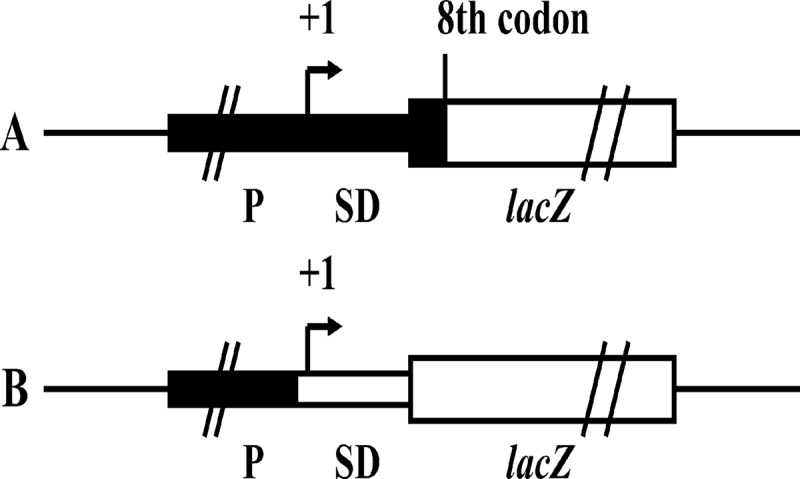
Schematic representation of lacZ translational fusion (A) and lacZ transcriptional fusion (B). (A) In the construct shown, the transcription of ′lacZ is driven by the x promoter (P) and the mRNA produced is translated from the SD sequence of x mRNA to produce a hybrid protein with several N-terminal amino acids of X and a large functional C-terminal fragment of β-galactosidase. The translational fusion data can reflect the strength of the promoter and the efficiency of initiation of translation of the target gene. (B) In the construct shown, the transcription of lacZ is driven by the x promoter and the mRNA produced is translated by the SD sequence of lacZ mRNA to produce a wild-type β-galactosidase. The transcriptional fusion data can reflect the strength of the promoter of the target gene.
TABLE 2.
Primers used in this study
| Purpose and gene | Primer name | Sequencea (5′–3′) |
|---|---|---|
| Markerless deletion mutation, gacA | gacAU-U-XbaI | GGTCTAGAGGTGGGAGAGGATGATT |
| gacAU-L | GGAAGGCTGGAATGACTTATGGTCTTTACAGGTTGC | |
| gacAD-U | AGTCATTCCAGCCTTCC | |
| gacAD-L-XbaI | GGTCTAGAGAGTTCGTCGGTCAG | |
| gacAF | CAAGGAGGATCAGGATG | |
| gacAR | AGCGGTAGGTATTCACG | |
| Overexpression | ||
| Km resistance | KmF-NcoI | CACCATGGATACCTGTCCGCCTTT |
| KanR-BglII | CCAAGATCTACAACACTCAACCCTATCTCG | |
| lacIq-Plac | lac_PF-BamHI | TTGGATCCAGCTGTTTCCTGTGTGAAATTGT |
| lac_PR-XbaI | CCTCTAGACCATCGAATGGCGCAAAACC | |
| gacA | gacAF-BamHI | TTGGATCCTTGATAAGGGTGCTAGTAG |
| gacAR- HindIII | GGTAAGCTTGTTCGGTCATTTCAGAG | |
| rsmA | rsmAF-BamHI | AGGGATCCATGCTGATTCTGACTCG |
| rsmAR-HindIII | CCTAAGCTTCAGCTCTCCGCAACAC | |
| rsmE | rsmEF-BamHI | TCGGATCCATGCTGATACTCACCC |
| rsmEF-NdeI | CCCTCATATGCTGATACTCACCCG | |
| rsmER-HindIII | CCAAAGCTTCAAGGCAAACAAGACAG | |
| lacZ fusion | ||
| lacZ | lacZF-BamHI | GAAGGATCCGAAATACGGGCAGACAT |
| ′lacZR-HindIII | GGAAAGCTTCTGGCCGTCGTTTTACAA | |
| lacZR-HindIII | CGAAAGCTTCACACAGGAAACAGC | |
| lipA | lipA-PF-KpnI | AAGGTACCGAGCATGAAGCGATGAAC |
| lipA′-PR-HindIII | GGAAAGCTTGGCAAGCTCTTGGGACAT | |
| lipA-PR- HindIII | GTAAAGCTTGTGCGAAGGCAGCG | |
| lipA′-A10U-PR-HindIII | GGAAAGCTTCATGCGGCGACAaCCTTGTG | |
| lipA′-G11C-PR-HindIII | GGAAAGCTTCATGCGGCGACATgCTTGTG | |
| lipA′-G12A-PR-HindIII | GGAAAGCTTCATGCGGCGACATCtTTGTG | |
| lipA′-A14U-PR-HindIII | GGAAAGCTTCATGCGGCGACATCCTaGTG | |
| rsmA | rsmA′-PF-KpnI | AGGGTACCACCGACTTCACC |
| rsmA′-PR-HindIII | CCAAAGCTTCAGCATACCTTTCTCC | |
| rsmE | rsmE′-PF-KpnI | TGGGTACCGACCACGACCAG |
| rsmE′-PR-HindIII | CCTAAGCTTCAGCATGATCTTCTCCTT | |
| rt-rpoDR | TCACTTCACGGATACCCTC | |
| RT-PCR | ||
| lipA | rt-lipAF | CAATTCCAGCGAGGTG |
| rt-lipAR | GCCGATCAGGTTGACTC | |
| rsmX | rt-rsmXF | AGGATCAGGGAAGGTCG |
| rt-rsmXR | TCCAAGACCATTATGACTTC | |
| rsmY | rt-rsmYF | TGGACGTCGCGCAGGAAG |
| rt-rsmYR | GCGGGGCTTTGCAGACTG | |
| rsmZ | rt-rsmZF | TGTCGACGGATAGACAC |
| rt-rsmZR | CCCGCCCACATTTTTCC | |
| rsmE | rt-rsmEF | ATGCTGATACTCACCCG |
| rt-rsmER | CTGCCTGAATGCGTTG | |
| rpoD | rt-rpoDF | ATACCGACGAAGCAGCAG |
| rt-rpoDR | TCACTTCACGGATACCCTC |
Underlining indicates restriction enzyme cutting sites, and lowercase nucleotides represent substitution mutations.
Construction of strains.
A mutant with a markerless deletion of the gacA coding region was constructed according to a previously described method (31) through a double-crossover recombination event with a gene replacement system with plasmid pJQΔgacA (33, 36), and double-crossover colonies were confirmed by colony PCR and sequenced. All plasmids used in this study were introduced into P. protegens by triparental mating with pRK2073 as a helper plasmid (37).
β-Galactosidase activity assay.
The β-galactosidase activity of various P. protegens and E. coli strains carrying a lacZ fusion on o-nitrophenyl-β-d-galactopyranoside (ONPG) was determined by the method of Miller (38), normalized to the optical density at 600 nm (OD600) of the bacterial culture, and expressed in Miller units. To induce expression, 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to cultures of strains containing pBBRKm or pET-28a derivatives.
RT-PCR analysis.
Total RNA was isolated from various P. protegens strains without a lacZ fusion on the chromosome (OD600 ≈ 6.0) grown in LB medium at 28°C overnight using an RNApure Bacteria kit (DNase I) (CWBIO, Beijing, China) according to the manufacturer's instructions. By following the manufacturer's protocols, 2 μg of total RNA was reverse transcribed with random hexamer primers using a Thermo Scientific RevertAid first-strand cDNA synthesis kit. PCR amplifications were performed with Taq DNA polymerase (CWBIO) and the primers presented in Table 2. Gene expression was normalized by using rpoD as an internal control. Reverse transcription-PCR (RT-PCR) products were loaded on a 1.0% (wt/vol) agarose gel and visualized by ethidium bromide fluorescence. Experiments were carried out at least twice.
Lipase activity assay.
As LipA has been identified to be an intracellular lipase (31), the lipase activity of LipA was expressed as whole-cell lipase activity. Cultures (OD600 ≈ 6.0) of various P. protegens strains without a lacZ fusion on the chromosome were grown in LB medium at 28°C overnight. After that, whole-cell samples were prepared according to a previously described method (39). Lipase activity on p-nitrophenyl caprylate (pNPC) was determined by a previously described spectrophotometric method (40), with some modifications. The reaction mixture for the system consisted of 2.9 ml Tris-HCl buffer (50 mM, pH 9.0) and 30 μl pNPC (10 mM pNPC in acetonitrile). The mixtures were preincubated at 55°C for 5 min, and 70 μl whole-cell samples was subsequently added. After 5 min of incubation at 55°C, the reaction mixtures were centrifuged (12,000 rpm, 2 min) at 4°C and the OD410 was measured. One unit of lipase activity was defined as the amount of enzyme needed to release 1 μmol of p-nitrophenol per minute by 1.0 ml sample with an OD600 of 1.0. The activity of the whole-cell lipase was expressed as U/ml · OD600.
Statistical analysis.
Statistical significance was determined by calculating the P values using a two-tailed, unpaired Student's t test, and differences with a P value of <0.05 were considered statistically significant.
RESULTS
GacA activates the transcription and translation of lipA.
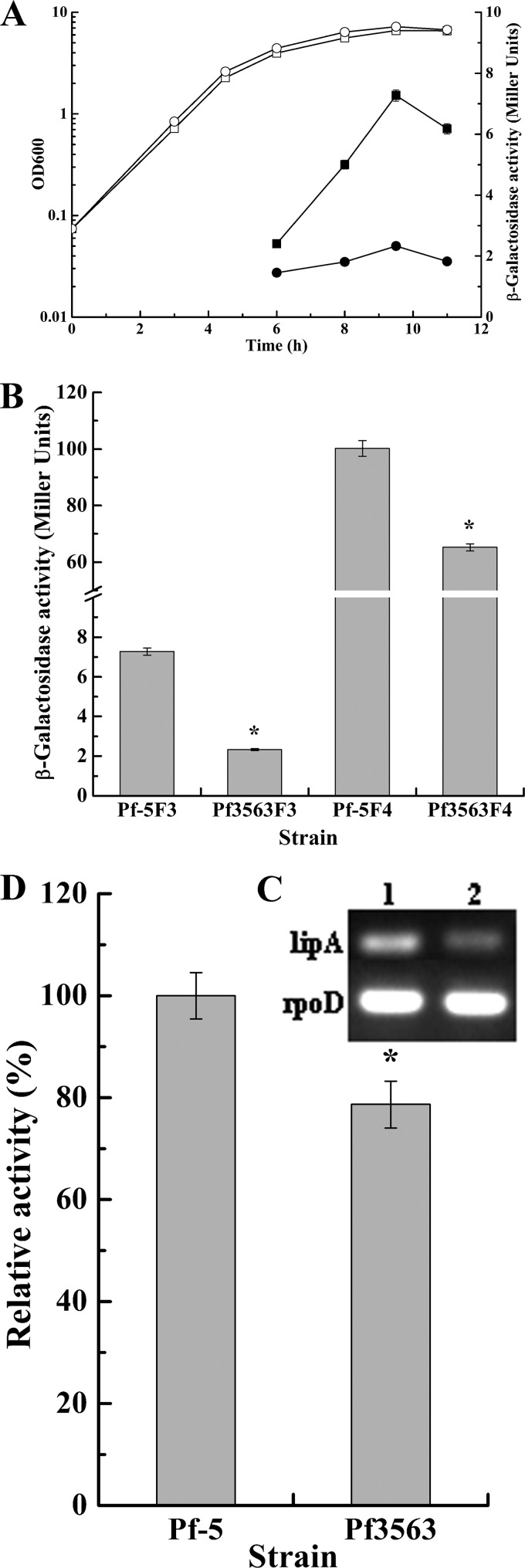
In P. aeruginosa, the response regulator GacA generally activates the transcription of lipase genes through QS systems. However, no QS system based upon N-acyl homoserine lactones has been found in P. protegens Pf-5 (41), and so far, no report on the regulation of lipase gene expression in P. protegens is available. To study the role of GacA in the regulation of lipA expression in Pf-5, a gacA deletion mutant, Pf3563, was constructed using a gene replacement system with plasmid pJQΔgacA through a double-crossover recombination event. The effect of GacA on the expression of lipA was studied by determining the β-galactosidase activities of chromosome-borne lipA′-′lacZ and lipA-lacZ fusions and the relative whole-cell lipase activities of cells lacking these fusions and by performing RT-PCR analysis of lipA transcription in wild-type strain Pf-5 and the gacA mutant Pf3563. As shown in Fig. 2A, gacA deletion significantly repressed lipA expression at all stages of growth, while it slightly increased the biomass of the bacteria. However, lipA expression displayed similar kinetics in Pf-5 and Pf3563, with expression sharply increasing in the late exponential phase and peaking in the stationary phase. Subsequently, to investigate at which regulatory level GacA activates lipA expression, the expression of chromosome-borne lipA′-′lacZ and lipA-lacZ fusions was examined in the Pf-5 and Pf3563 backgrounds. As shown in Fig. 2B, GacA positively controlled lipA expression at both the transcriptional and the translational levels, with the translational level being the key regulatory pathway. RT-PCR analysis of lipA transcription as well as relative whole-cell lipase activities in Pf-5 and Pf3563 further confirmed the results described above (Fig. 2C and D).
FIG 2.
Effect of gacA mutation on lipA expression. (A) Influence of a gacA mutation on expression of a chromosome-borne lipA′-′lacZ translational fusion and growth of the bacteria. β-Galactosidase activity (solid symbols) and growth (open symbols) were measured in the wild-type strain (Pf-5F3; squares) and the gacA mutant (Pf3563F3; circles) at various time points after inoculation into 50 ml LB medium. (B) Influence of the gacA mutation on expression of a chromosome-borne lipA′-′lacZ translational fusion (wild-type strain, Pf-5F3; gacA mutant, Pf3563F3) and a chromosome-borne lipA-lacZ transcriptional fusion (wild-type strain, Pf-5F4; gacA mutant, Pf3563F4). The β-galactosidase activity of each strain was measured in the stationary phase after inoculation into 50 ml LB medium. (C) RT-PCR analysis of lipA expression in the wild-type strain (Pf-5; lane 1) and the gacA mutant (Pf3563; lane 2). (D) Relative whole-cell lipase activity in the wild-type strain (Pf-5) and the gacA mutant (Pf3563). All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05 compared with the control.
In addition, the overexpression of gacA in both Pf-5 and Pf3563 via the gacA expression plasmid pBBBRKgacA was investigated along with that in Pf-5/pBBRKm and Pf3563/pBBRKm as controls to examine the effect of gacA overexpression on lipA expression in the Pf-5 and Pf3563 backgrounds. As shown in Fig. 3, lipA expression was higher in Pf3563/pBBRKgacA than in Pf3563/pBBRKm or Pf-5/pBBRKm. Moreover, Pf-5/pBBRKgacA and Pf3563/pBBRKgacA exhibited similar levels of lipA expression, but the intensity of lipA expression in Pf-5/pBBRKgacA was higher than that in Pf3563/pBBRKgacA. Taken together, the intensity of gacA expression determines the intensity of lipA expression.
FIG 3.
Effect of gacA overexpression on lipA expression. (A) Influence of gacA overexpression on expression of a chromosome-borne lipA′-′lacZ translational fusion in different strains. The β-galactosidase activity of each strain was measured in the stationary phase after inoculation into 50 ml LB medium. (B) Influence of gacA overexpression on relative whole-cell lipase activity in different strains. All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05 compared with the control; **P < 0.01 compared with the control.
gacA deletion partly inhibits the transcription of rsmX, rsmY, and rsmZ.
Previous studies have shown that the transcription of rsmX, rsmY, and rsmZ is completely abolished by the deletion of gacA in the closely related strain P. protegens CHA0 (42, 43). However, RT-PCR analysis of rsmX, rsmY, and rsmZ transcription in Pf-5 and Pf3563, which was performed to investigate the effect of gacA deletion on their transcription, indicated that Pf3563 did initiate the transcription of rsmX, rsmY, and rsmZ, although at a level lower than that in Pf-5 (Fig. 4), being consistent with the partial expression of lipA in Pf3563 (Fig. 2).
FIG 4.
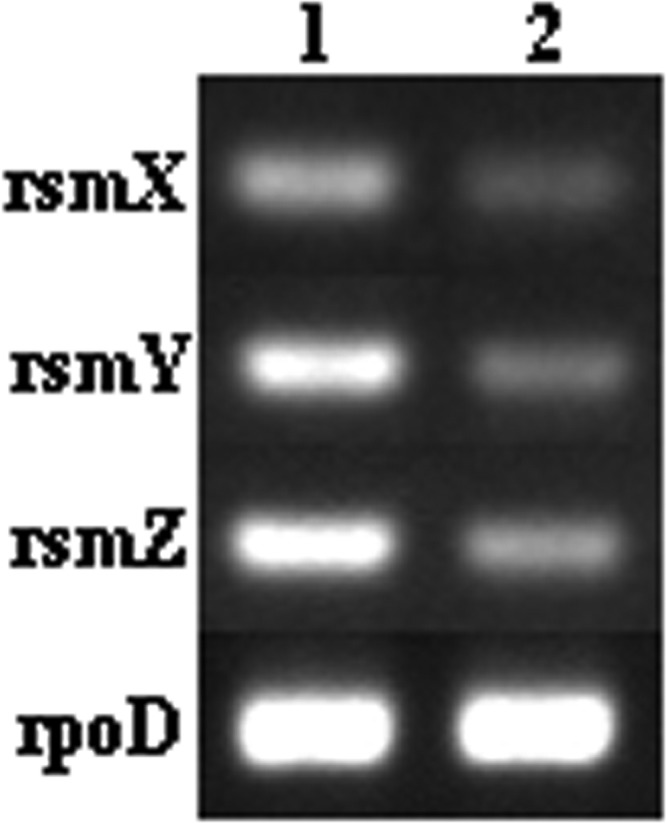
RT-PCR analysis of rsmX, rsmY, and rsmZ expression in the wild-type strain (Pf-5; lane 1) and the gacA mutant (Pf3563; lane 2).
RsmA and RsmE differentially regulate lipA expression.
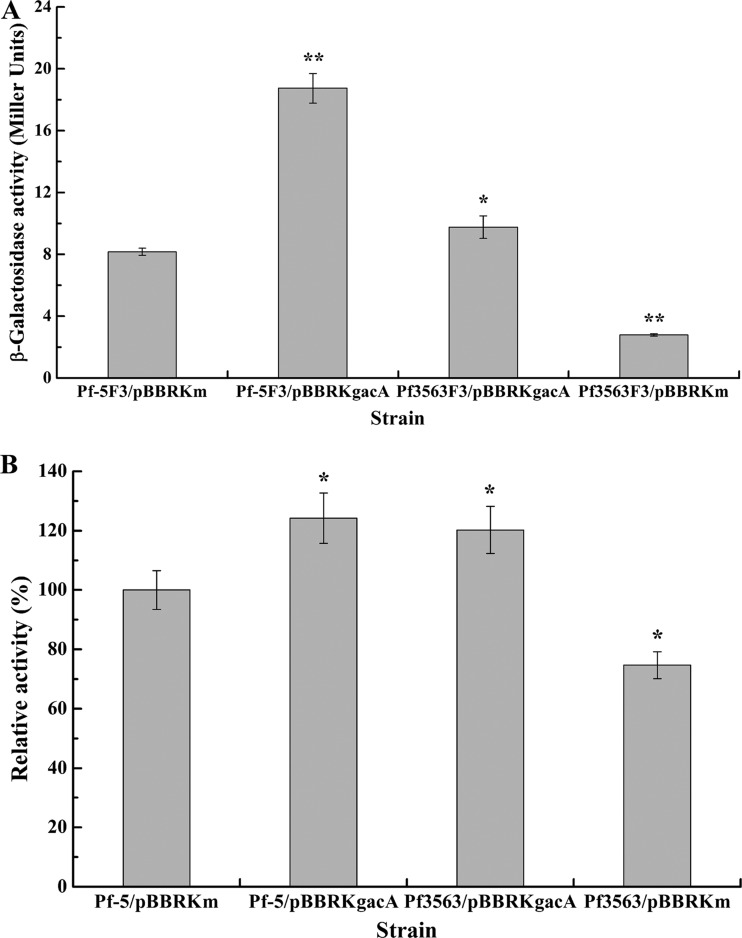
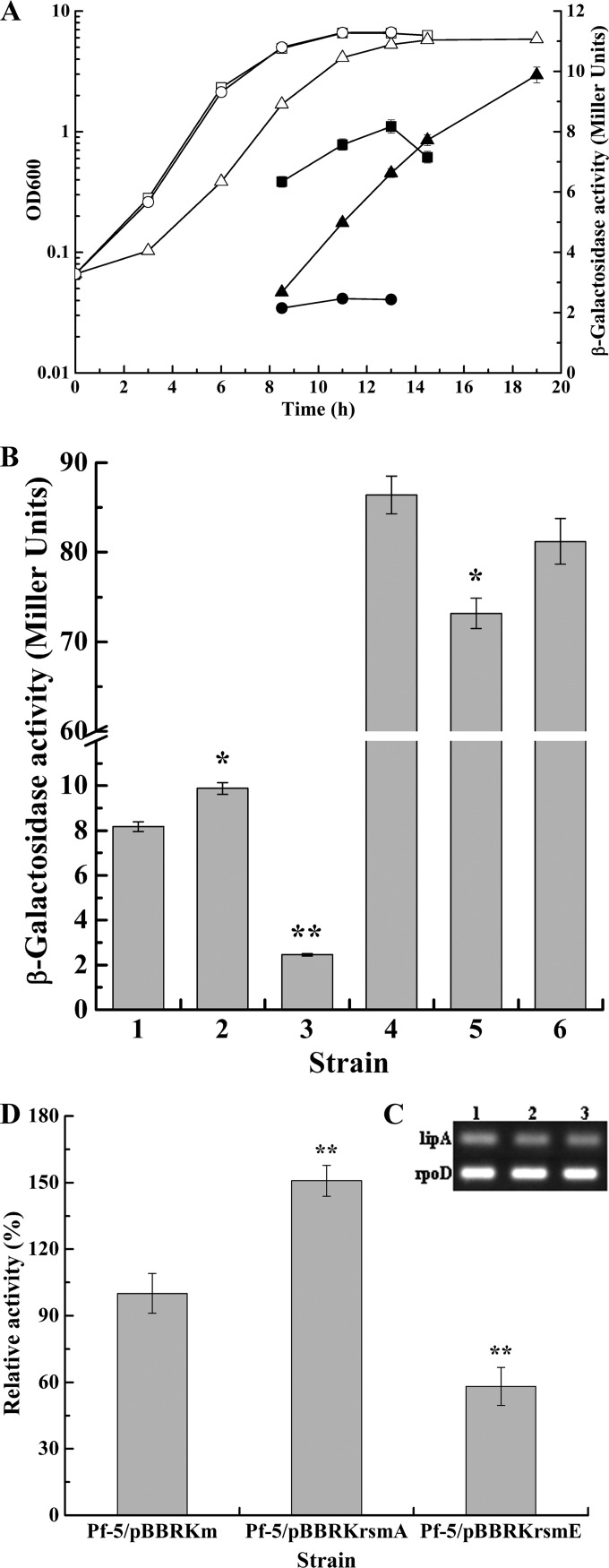
In many gammaproteobacteria, the RNA-binding proteins of the RsmA-CsrA family account for the translational control of the Gac-Rsm signal transduction system (24). Moreover, the Gac-Rsm system contains two members of the RsmA-CsrA family, RsmA and RsmE, in P. protegens Pf-5 (42). In practice, it is often more convenient and reliable to assess the function of the RsmA-CsrA family proteins by their overexpression than to use a comparison of the wild-type strain and the mutants (24, 35). Therefore, to examine how Rsm proteins act on the regulation of lipA expression, strains overexpressing rsmA and rsmE were constructed by triparental mating, and the roles of the Rsm proteins in regulating lipA expression were explored by measuring the β-galactosidase activities of chromosome-borne lipA′-′lacZ and lipA-lacZ fusions and the relative whole-cell lipase activities of cells lacking these fusions and by carrying out RT-PCR analysis of lipA transcription in Pf-5/pBBRKm, Pf-5/pBBRKrsmA, and Pf-5/pBBRKrsmE. As shown in Fig. 5A, overexpressed RsmA significantly enhanced lipA expression when bacteria entered the late stationary phase, whereas overexpressed RsmE notably inhibited lipA expression throughout the growth cycle. In addition, rsmA overexpression resulted in bacteria exhibiting a delayed growth cycle and slightly decreased the biomass. To further determine the regulatory mechanisms of RsmA and RsmE in lipA expression, the expression of chromosome-borne lipA′-′lacZ and lipA-lacZ fusions was investigated in the Pf-5/pBBRKm, Pf-5/pBBRKrsmA and Pf-5/pBBRKrsmE backgrounds. RsmA transcriptionally repressed but also translationally stimulated lipA expression, and the translational control was found to be the key regulatory pathway. In contrast, RsmE almost repressed lipA translation and also weakly inhibited lipA transcription (Fig. 5B and C). The relative whole-cell lipase activities in Pf-5/pBBRKm, Pf-5/pBBRKrsmA, and Pf-5/pBBRKrsmE further demonstrated that RsmA activated lipA expression, while RsmE inhibited lipA expression (Fig. 5D). As stated above, in P. protegens Pf-5, RsmA and RsmE differentially regulated lipA expression and exhibited different regulatory modes in some aspects.
FIG 5.
Effects of rsmA and rsmE overexpression on lipA expression. (A) Influences of rsmA and rsmE overexpression on expression of a chromosome-borne lipA′-′lacZ translational fusion and growth of bacteria. β-Galactosidase activity (solid symbols) and growth (open symbols) were measured in the wild-type strain (Pf-5F3/pBBRKm; squares), an rsmA-overexpressing strain (Pf-5F3/pBBRKrsmA; triangles), and an rsmE-overexpressing strain (Pf-5F3/pBBRKrsmE; circles) at various time points after inoculation into 50 ml LB medium. (B) Influences of rsmA and rsmE overexpression on expression of a chromosome-borne lipA′-′lacZ translational fusion (bar 1, wild-type strain Pf-5F3/pBBRKm; bar 2, rsmA-overexpressing strain Pf-5F3/pBBRKrsmA; bar 3, rsmE-overexpressing strain Pf-5F3/pBBRKrsmE) and a chromosome-borne lipA-lacZ transcriptional fusion (bar 4, wild-type strain Pf-5F4/pBBRKm; bar 5, rsmA-overexpressing strain Pf-5F4/pBBRKrsmA; bar 6, rsmE-overexpressing strain Pf-5F4/pBBRKrsmE). The β-galactosidase activity of each strain was measured in the stationary phase after inoculation into 50 ml LB medium. (C) RT-PCR analysis of lipA expression in wild-type strain Pf-5/pBBRKm (lane 1), rsmA-overexpressing strain Pf-5/pBBRKrsmA (lane 2), and rsmE-overexpressing strain Pf-5/pBBRKrsmE (lane 3). (D) Relative whole-cell lipase activity in wild-type strain Pf-5/pBBRKm, rsmA-overexpressing strain Pf-5/pBBRKrsmA, and rsmE-overexpressing strain Pf-5/pBBRKrsmE. All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05 compared with the control; **P < 0.01 compared with the control.
RsmA activates lipA translation by inhibiting rsmE translation.
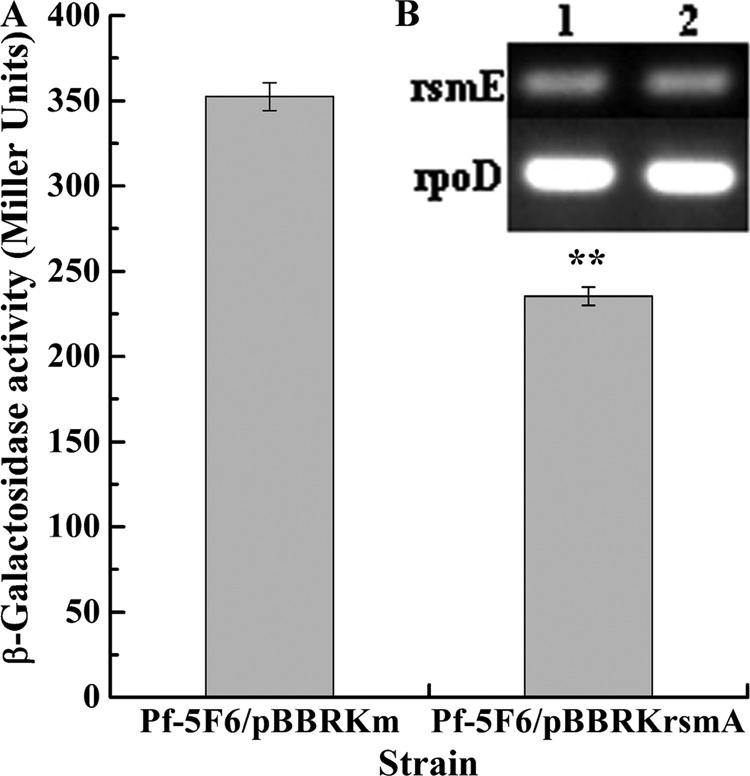
RsmA has been proven to repress the expression of rsmE in P. protegens CHA0 (42), suggesting that the activation of lipA translation by RsmA may be mediated by RsmE. Consequently, we next investigated the effect of RsmA on rsmE expression by analyzing the expression of the chromosome-borne rsmE′-′lacZ fusion and by implementing RT-PCR analysis of rsmE transcription in Pf-5/pBBRKm and Pf-5/pBBRKrsmA. As shown in Fig. 6A, overexpressed RsmA notably repressed rsmE expression. The RT-PCR analysis further showed that RsmA did not regulate rsmE transcription (Fig. 6B). Taken together, these results indicate that RsmA represses rsmE translation, confirming the suggestion made above.
FIG 6.
Effect of rsmA overexpression on rsmE expression. (A) Influence of rsmA overexpression on expression of a chromosome-borne rsmE′-′lacZ translational fusion (wild-type strain, Pf-5F6/pBBRKm; rsmA-overexpressing strain, Pf-5F6/pBBRKrsmA). The β-galactosidase activity of each strain was measured in the stationary phase after inoculation into 50 ml LB medium. (B) RT-PCR analysis of rsmE expression in wild-type strain Pf-5/pBBRKm (lane 1) and rsmA-overexpressing strain Pf-5/pBBRKrsmA (lane 2). All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. **P < 0.01 compared with the control.
RsmE represses lipA translation by binding to the SD sequence of lipA mRNA.
It has been reported that RsmE-mediated translational repression involves its binding to an (A/U)CANGGANG(U/A) consensus sequence, which overlaps the SD sequence of target mRNA, blocking the recruitment of the 30S ribosomal subunit and, hence, translation initiation (24, 35, 44). It is worth noting that the ACAAGGAUGU sequence overlapping the SD sequence of lipA mRNA fully matches the consensus sequence. In addition, RsmE from P. protegens Pf-5 is 100% identical to that from P. protegens CHA0, and the protein-RNA interaction between RsmE and the consensus sequence has been studied in P. protegens CHA0 (35, 42). To mimic the interaction between RsmE and the ACAAGGAUGU sequence, four single-base mutations at nucleotides A10, G11, G12, and A14 of lipA mRNA were constructed in the plasmid-borne lipA′-′lacZ fusions, and the fusions were expressed in E. coli BL21(DE3) with plasmid pET-rsmE or pET-28a. The effects of the A10U, G11C, G12A, and A14U mutations on translation regulation by RsmE are listed in Table 3. All these mutations influenced the regulatory control of RsmE, but the mutation of G11, G12, and A14 significantly eliminated the inhibitory effect of RsmE on the expression of the lipA′-′lacZ fusion. In short, these results demonstrate that RsmE inhibits lipA translation via binding to the ACAAGGAUGU sequence.
TABLE 3.
Effects of mutations in the SD sequence of lipA mRNA on translational repression by RsmE
| Plasmid | β-Galactosidase activity (Miller units) for strain: |
Repression factor | |
|---|---|---|---|
| BL/pET-28a | BL/pET-rsmE | ||
| pBBR003 | 1,312.5 ± 106.4 | 818 ± 75.3 | 1.60a |
| pBBR00A10U | 1,323.9 ± 110.8 | 1,018.6 ± 95.4 | 1.30a |
| pBBR00G11C | 611.5 ± 56.9 | 541.2 ± 42.8 | 1.13 |
| pBBR00G12A | 712 ± 69.5 | 608.5 ± 60.7 | 1.17 |
| pBBR00A14U | 1,025.6 ± 101.4 | 967.5 ± 98.3 | 1.06 |
P < 0.05 compared with the control.
Expression of rsmA and rsmE.
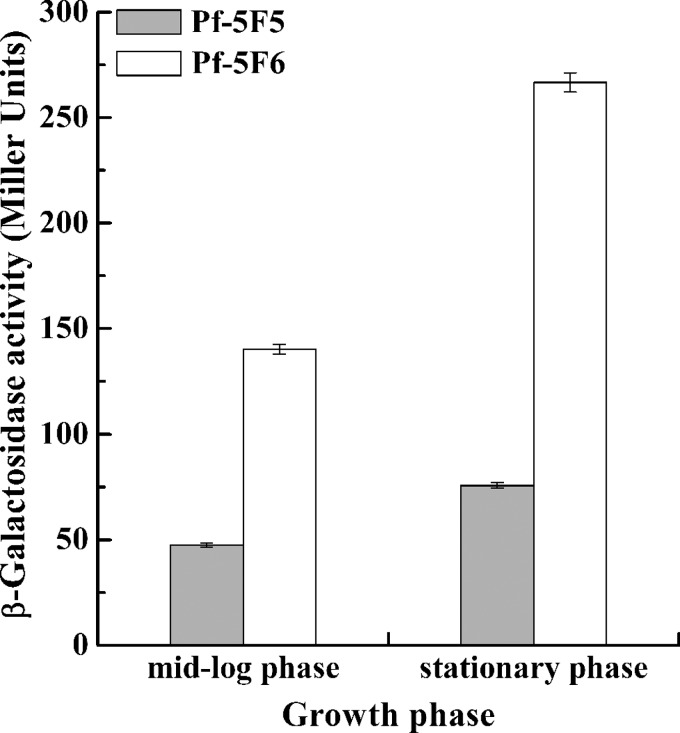
As mentioned above, overexpressed RsmA inhibited rsmE translation, thereby enhancing lipA translation. However, GacA activated lipA translation via the GacA-sRNAs-RsmE-LipA pathway rather than repress lipA translation through the GacA-sRNAs-RsmA-RsmE-LipA pathway, implying that the intensity of expression of rsmE is higher than that of rsmA. To assess the intensity of rsmA and rsmE expression, the β-galactosidase activities of chromosome-borne rsmA′-′lacZ and rsmE′-′lacZ fusions in the Pf-5 background were measured. As shown in Fig. 7, the intensity and variation of rsmA expression were less than those of rsmE expression. In addition, the expression of both rsmA and rsmE was enhanced with increasing cell density.
FIG 7.
Expression of a chromosome-borne rsmA′-′lacZ translational fusion (Pf-5F5) and a chromosome-borne rsmE′-′lacZ translational fusion (Pf-5F6) at different growth phases. The β-galactosidase activity of each strain was measured in the mid-log and stationary phases after inoculation into 50 ml LB medium.
DISCUSSION
In many gammaproteobacteria, the Gac-Rsm system regulates several unrelated pathways, such as primary and secondary metabolic pathways, biofilm formation, quorum sensing, oxidative stress, exoproduct formation, virulence, and pathogenesis (24, 45). Published studies on the regulation of lipase gene expression by the Gac-Rsm signal transduction system demonstrate that this system activates lipase gene transcription by mediation of the QS system and the CbrA/B two-component system in P. aeruginosa (16, 19). In the present study, a novel regulatory mechanism acting on lipase gene expression activated by the Gac-Rsm system was characterized in P. protegens Pf-5, which does not have a QS system based upon N-acyl homoserine lactones.
Although the GacS/A two-component system has previously been reported to activate the production of lipase (16, 19, 23), the effect of the GacS/A system on lipase gene expression in P. protegens is still unclear. To determine the status of the GacS/A system in P. protegens Pf-5, a gacA deletion mutant, Pf3563, and strains overexpressing gacA in the Pf-5 and Pf3563 background were constructed. On the basis of the available literature, GacA positively controls lipase gene transcription. However, the results in this study demonstrated that GacA mainly activates lipA translation (Fig. 2 and 3). It is worth noting that GacA also enhanced lipA transcription (Fig. 2B), which is consistent with the results of RT-PCR analysis of lipA transcription in Pf-5 and Pf3563 (Fig. 2C). Unlike the results obtained with the closely related strain P. protegens CHA0 (42, 43), the deletion of gacA in P. protegens Pf-5 partly abolished the transcription of rsmX, rsmY, and rsmZ (Fig. 4), which is in agreement with the regulatory effect of GacA on lipA expression. In addition, the deletion of gacA slightly increased the biomass of the bacteria (Fig. 2A), while the overexpression of gacA slightly decreased the biomass of the bacteria (data not shown), a result similar to that reported for other Pseudomonas species (24, 46). The role of GacA in regulating the biomass of bacteria may be due to the fact that the Gac-Rsm system is a part of the QS machinery (46–48).
Given that the Rsm system governs translational control in the Gac-Rsm regulatory cascade (27, 45), rsmA and rsmE overexpression strains Pf-5/pBBRKrsmA and Pf-5/pBBRKrsmE were constructed to investigate the regulatory mechanisms of Rsm proteins in lipA expression. The results showed that RsmA and RsmE differentially controlled the expression of lipA. RsmA regulated lipA expression in a biphasic manner, inhibiting its transcription but stimulating its translation, whereas RsmE repressed lipA translation (Fig. 5). To our knowledge, this is the first time that Rsm proteins have been reported to play such roles in the regulation of lipA expression.
In general, Rsm proteins inhibit gene expression mainly through the translational pathway (24, 45). However, RsmA represses lipA transcription in P. protegens Pf-5, but the mechanism remains to be elucidated. We suggest that the regulatory network of lipA expression may include transcriptional factors controlled by RsmA. It has also been suggested that translational activation by Rsm proteins may involve their binding to mRNAs, which somehow stabilize them and/or facilitate their translation initiation, ultimately resulting in a positive effect on gene expression (20, 49). Similarly, it has been reported that binding of the ortholog of Rsm proteins, CsrA, to the highly structured untranslated leader of mRNA results in translational activation either by causing structural changes in the RNA (45) or by interfering with the 5′ end-dependent RNase E cleavage pathway of the transcript (50). However, the mechanism underlying the activation of lipA translation by RsmA differs from that proposal. It has been reported that RsmA inhibits rsmE expression in P. protegens CHA0 (42), suggesting that the mechanism by which RsmA realizes the activation of lipA translation involves translational repression of rsmE expression by RsmA, which is consistent with the effect of overexpressed RsmA on rsmE expression (Fig. 6). Although RsmA has been reported to positively control the ability to produce extracellular lipase in P. aeruginosa (20), the molecular mechanism remains unclear.
Translational repression by Rsm proteins generally involves their binding to multiple sites in the untranslated leader and/or the initially translated region of target mRNAs, one of which overlaps the cognate SD sequence (26, 27). It has been reported that RsmE from P. protegens CHA0, which is identical to that from P. protegens Pf-5, recognizes an (A/U)CANGGANG(U/A) consensus sequence overlapping the SD sequence of target mRNAs (35, 42). The ACAAGGAUGU sequence, which is located at the SD sequence of lipA mRNA, was found to fully match the consensus sequence, suggesting that RsmE represses lipA translation by binding to the sequence. Mutational analysis of the SD sequence of lipA mRNA verified that RsmE interacts with the SD sequence and, hence, inhibits the translation of lipA mRNA (Table 3), which demonstrates that the activation of lipA translation by GacA is mediated through RsmE binding to the SD sequence of lipA mRNA, and then the Gac-RsmE system in P. protegens Pf-5 directly activates lipA translation. However, in P. aeruginosa the Gac-Rsm system stimulates the expression of QS systems and the CbrA/B two-component system, thereby enhancing lipase gene transcription (16, 19). This may be due to the binding of RsmE to the SD sequence of lipA mRNA and the absence of a QS system based upon N-acyl homoserine lactones in P. protegens Pf-5 (41).
The question remains as to why RsmE rather than RsmA plays a major role in the regulatory network of lipA expression. Possible reasons are as follows. (i) The 5′ untranslated region of lipA mRNA contains only a GGA motif, which is in the ACAAGGAUGU sequence and which can be bound by RsmE but not RsmA. The interaction between RsmA and RNA is complex, as it depends on at least two RNA recognition sequences, as well as their spatial relationship and, potentially, their binding cooperativity (35). (ii) In our study, the intensity and variation of rsmE expression were higher than those of rsmA expression (Fig. 7), which may also be the reason why the Gac-Rsm system activates lipA translation via the Gac-RsmE-LipA pathway rather than represses lipA translation through the Gac-RsmA-RsmE-LipA pathway. In contrast, the level of rsmE expression is less than that of rsmA expression in P. protegens CHA0 (42). (iii) RsmE probably plays a role in the termination of GacA-controlled gene expression (42).
In conclusion, in P. protegens Pf-5 the Gac-Rsm signal transduction system mainly and directly activates lipA translation via the Gac-RsmE-LipA pathway and, in addition, indirectly enhances lipA transcription through the of Gac-RsmA-?-LipA pathway (Fig. 8).
FIG 8.
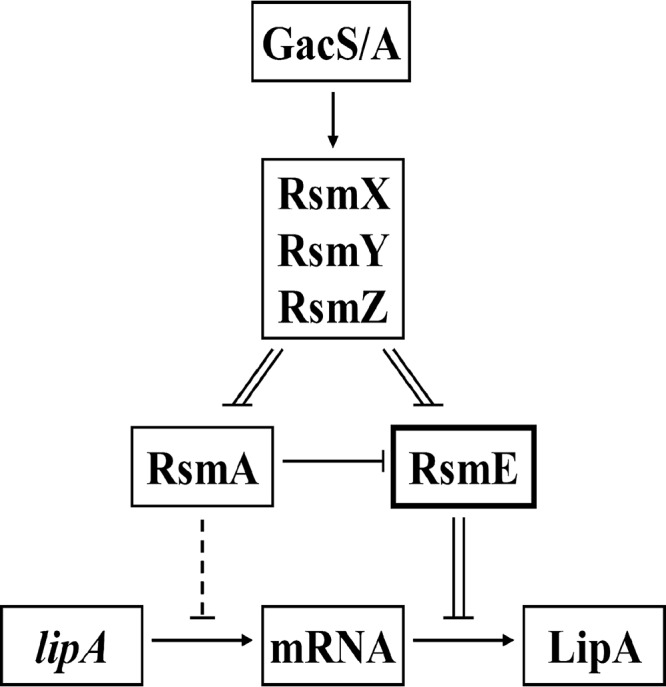
Schematic diagram depicting the activation of lipA expression by the Gac-Rsm system in P. protegens Pf-5. Transcription of three sRNAs (RsmX, RsmY, and RsmZ) depends on the presence of GacA. The function of sRNAs is to relieve translational repression of two RNA-binding proteins, RsmA and RsmE. RsmA mainly activates lipA translation by inhibiting rsmE translation, but it also represses lipA transcription via an unknown pathway. However, RsmE inhibits lipA translation by binding to the SD sequence of lipA mRNA. Among these regulatory pathways, the RsmE-mediated pathway plays a major role in the regulation of lipA expression. Solid line, direct regulation; dotted line, indirect regulation; double line, physical interaction; arrow, positive effect; bar, negative effect.
ACKNOWLEDGMENTS
We acknowledge the financial support of the National Natural Science Foundation of the People's Republic of China (NSFC; no. 31070089, 31170078, and J1103514), the National High Technology Research and Development Program of the People's Republic of China (863 Program; no. 2011AA02A204), and the Innovation Foundation of the Shenzhen Government (JCYJ20120831111657864).
Many thanks are due to Chen Jingyuan (Hubei Academy of Forestry, Wuhan, China) for kindly providing strain P. protegens Pf-5.
Footnotes
Published ahead of print 15 August 2014
REFERENCES
- 1.Gupta R, Gupta N, Rathi P. 2004. Bacterial lipases: an overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol. 64:763–781. 10.1007/s00253-004-1568-8. [DOI] [PubMed] [Google Scholar]
- 2.Angkawidjaja C, Kanaya S. 2006. Family I.3 lipase: bacterial lipases secreted by the type I secretion system. Cell. Mol. Life Sci. 63:2804–2817. 10.1007/s00018-006-6172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aravindan R, Anbumathi P, Viruthagiri T. 2007. Lipase applications in food industry. Indian J. Biotechnol. 6:141–158. [Google Scholar]
- 4.Pan XX, Xu L, Zhang Y, Xiao X, Wang XF, Liu Y, Zhang HJ, Yan YJ. 2012. Efficient display of active Geotrichum sp. lipase on Pichia pastoris cell wall and its application as a whole-cell biocatalyst to enrich EPA and DHA in fish oil. J. Agric. Food Chem. 60:9673–9679. 10.1021/jf301827y. [DOI] [PubMed] [Google Scholar]
- 5.Romdhane IB, Fendri A, Gargouri Y, Gargouri A, Belghith H. 2010. A novel thermoactive and alkaline lipase from Talaromyces thermophilus fungus for use in laundry detergents. Biochem. Eng. J. 53:112–120. 10.1016/j.bej.2010.10.002. [DOI] [Google Scholar]
- 6.Grbavcic S, Bezbradica D, Izrael-Zivkovic L, Avramovic N, Milosavic N, Karadzic I, Knezevic-Jugovic Z. 2011. Production of lipase and protease from an indigenous Pseudomonas aeruginosa strain and their evaluation as detergent additives: compatibility study with detergent ingredients and washing performance. Bioresour. Technol. 102:11226–11233. 10.1016/j.biortech.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 7.Yoo HY, Simkhada JR, Cho SS, Park DH, Kim SW, Seong CN, Yoo JC. 2011. A novel alkaline lipase from Ralstonia with potential application in biodiesel production. Bioresour. Technol. 102:6104–6111. 10.1016/j.biortech.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 8.Jin Z, Han SY, Zhang L, Zheng SP, Wang Y, Lin Y. 2013. Combined utilization of lipase-displaying Pichia pastoris whole-cell biocatalysts to improve biodiesel production in co-solvent media. Bioresour. Technol. 130:102–109. 10.1016/j.biortech.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Busto E, Gotor-Fernandez V, Gotor V. 2006. Kinetic resolution of 4-chloro-2-(1-hydroxyalkyl)pyridines using Pseudomonas cepacia lipase. Nat. Protoc. 1:2061–2067. 10.1038/nprot.2006.335. [DOI] [PubMed] [Google Scholar]
- 10.Chang IA, Kim IH, Kang SC, Hou CT, Kim HR. 2007. Production of 7,10-dihydroxy-8(E)-octadecenoic acid from triolein via lipase induction by Pseudomonas aeruginosa PR3. Appl. Microbiol. Biotechnol. 74:301–306. 10.1007/s00253-006-0662-5. [DOI] [PubMed] [Google Scholar]
- 11.Jeganathan J, Bassi A, Nakhla G. 2006. Pre-treatment of high oil and grease pet food industrial wastewaters using immobilized lipase hydrolyzation. J. Hazard. Mater. 137:121–128. 10.1016/j.jhazmat.2005.11.106. [DOI] [PubMed] [Google Scholar]
- 12.Arpigny JL, Jaeger KE. 1999. Bacterial lipolytic enzymes: classification and properties. Biochem. J. 343:177–183. 10.1042/0264-6021:3430177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krzeslak J, Gerritse G, van Merkerk R, Cool RH, Quax WJ. 2008. Lipase expression in Pseudomonas alcaligenes is under the control of a two-component regulatory system. Appl. Environ. Microbiol. 74:1402–1411. 10.1128/AEM.01632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Ashby RD, Needleman DS, Lee KT, Solaiman DK. 2012. Cloning, sequencing, and characterization of lipase genes from a polyhydroxyalkanoate (PHA)-synthesizing Pseudomonas resinovorans. Appl. Microbiol. Biotechnol. 96:993–1005. 10.1007/s00253-012-4133-x. [DOI] [PubMed] [Google Scholar]
- 15.Panizza P, Syfantou N, Pastor FI, Rodriguez S, Diaz P. 2013. Acidic lipase Lip I.3 from a Pseudomonas fluorescens-like strain displays unusual properties and shows activity on secondary alcohols. J. Appl. Microbiol. 114:722–732. 10.1111/jam.12089. [DOI] [PubMed] [Google Scholar]
- 16.Rosenau F, Jaeger K. 2000. Bacterial lipases from Pseudomonas: regulation of gene expression and mechanisms of secretion. Biochimie 82:1023–1032. 10.1016/S0300-9084(00)01182-2. [DOI] [PubMed] [Google Scholar]
- 17.Abdou L, Chou HT, Haas D, Lu CD. 2011. Promoter recognition and activation by the global response regulator CbrB in Pseudomonas aeruginosa. J. Bacteriol. 193:2784–2792. 10.1128/JB.00164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krzeslak J, Papaioannou E, van Merkerk R, Paal KA, Bischoff R, Cool RH, Quax WJ. 2012. Lipase A gene transcription in Pseudomonas alcaligenes is under control of RNA polymerase σ54 and response regulator LipR. FEMS Microbiol. Lett. 329:146–153. 10.1111/j.1574-6968.2012.02516.x. [DOI] [PubMed] [Google Scholar]
- 19.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309–319. 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 20.Heurlier K, Williams F, Heeb S, Dormond C, Pessi G, Singer D, Camara M, Williams P, Haas D. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 186:2936–2945. 10.1128/JB.186.10.2936-2945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster M, Greenberg EP. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 296:73–81. 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Williams P, Camara M. 2009. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 12:182–191. 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Lalaouna D, Fochesato S, Sanchez L, Schmitt-Kopplin P, Haas D, Heulin T, Achouak W. 2012. Phenotypic switching in Pseudomonas brassicacearum involves GacS- and GacA-dependent Rsm small RNAs. Appl. Environ. Microbiol. 78:1658–1665. 10.1128/AEM.06769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapouge K, Schubert M, Allain FH, Haas D. 2008. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67:241–253. 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown D. 2010. A mathematical model of the Gac/Rsm quorum sensing network in Pseudomonas fluorescens. Biosystems 101:200–212. 10.1016/j.biosystems.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Sonnleitner E, Haas D. 2011. Small RNAs as regulators of primary and secondary metabolism in Pseudomonas species. Appl. Microbiol. Biotechnol. 91:63–79. 10.1007/s00253-011-3332-1. [DOI] [PubMed] [Google Scholar]
- 27.Marzi S, Romby P. 2012. RNA mimicry, a decoy for regulatory proteins. Mol. Microbiol. 83:1–6. 10.1111/j.1365-2958.2011.07911.x. [DOI] [PubMed] [Google Scholar]
- 28.Gooderham WJ, Hancock RE. 2009. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 33:279–294. 10.1111/j.1574-6976.2008.00135.x. [DOI] [PubMed] [Google Scholar]
- 29.Howell CR, Stipanovic RD. 1979. Control of Rhizoctonia solani on cotton seedlings with Pseudomonas fluorescens and with an antibiotic produced by the bacterium. Phytopathology 69:480–482. 10.1094/Phyto-69-480. [DOI] [Google Scholar]
- 30.Ramette A, Frapolli M, Fischer-Le Saux M, Gruffaz C, Meyer JM, Defago G, Sutra L, Moenne-Loccoz Y. 2011. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 34:180–188. 10.1016/j.syapm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Zha D, Xu L, Zhang H, Yan Y. 2014. Molecular identification of lipase LipA from Pseudomonas protegens Pf-5 and characterization of two whole-cell biocatalysts Pf-5 and Top10lipA. J. Microbiol. Biotechnol. 24:619–628. 10.4014/jmb.1312.12005. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 33.Quandt J, Hynes MF. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15–21. 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 34.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RN, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 35.Schubert M, Lapouge K, Duss O, Oberstrass FC, Jelesarov I, Haas D, Allain FH. 2007. Molecular basis of messenger RNA recognition by the specific bacterial repressing clamp RsmA/CsrA. Nat. Struct. Mol. Biol. 14:807–813. 10.1038/nsmb1285. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi DY, Reedy RM, Palumbo JD, Zhou JM, Yuen GY. 2005. A clp gene homologue belonging to the Crp gene family globally regulates lytic enzyme production, antimicrobial activity, and biological control activity expressed by Lysobacter enzymogenes strain C3. Appl. Environ. Microbiol. 71:261–269. 10.1128/AEM.71.1.261-269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leong SA, Ditta GS, Helinski DR. 1982. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J. Biol. Chem. 257:8724–8730. [PubMed] [Google Scholar]
- 38.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 39.Shu ZY, Wu JG, Cheng LX, Chen D, Jiang YM, Li X, Huang JZ. 2012. Production and characteristics of the whole-cell lipase from organic solvent tolerant Burkholderia sp. ZYB002. Appl. Biochem. Biotechnol. 166:536–548. 10.1007/s12010-011-9446-1. [DOI] [PubMed] [Google Scholar]
- 40.Liu W, Jia B, Zhao H, Xu L, Yan Y. 2010. Preparation of a whole-cell biocatalyst of Aspergillus niger lipase and its practical properties. J. Agric. Food Chem. 58:10426–10430. 10.1021/jf1008555. [DOI] [PubMed] [Google Scholar]
- 41.Mavrodi DV, Paulsen IT, Ren Q, Loper JE. 2007. Genomics of Pseudomonas fluorescens Pf-5, p 3–30 In Ramos J, Filloux A. (ed), Pseudomonas. Springer, Dordrecht, Netherlands. [Google Scholar]
- 42.Reimmann C, Valverde C, Kay E, Haas D. 2005. Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in the biocontrol strain Pseudomonas fluorescens CHA0. J. Bacteriol. 187:276–285. 10.1128/JB.187.1.276-285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kay E, Dubuis C, Haas D. 2005. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc. Natl. Acad. Sci. U. S. A. 102:17136–17141. 10.1073/pnas.0505673102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Lee SH, Seeve C, Yu JM, Pierson LR, Pierson EA. 2013. Roles of the Gac-Rsm pathway in the regulation of phenazine biosynthesis in Pseudomonas chlororaphis 30-84. Microbiologyopen 2:505–524. 10.1002/mbo3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romeo T, Vakulskas CA, Babitzke P. 2013. Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ. Microbiol. 15:313–324. 10.1111/j.1462-2920.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kay E, Humair B, Denervaud V, Riedel K, Spahr S, Eberl L, Valverde C, Haas D. 2006. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J. Bacteriol. 188:6026–6033. 10.1128/JB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez N, Heeb S, Valverde C, Kay E, Reimmann C, Junier T, Haas D. 2008. Genome-wide search reveals a novel GacA-regulated small RNA in Pseudomonas species. BMC Genomics 9:167. 10.1186/1471-2164-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humair B, Wackwitz B, Haas D. 2010. GacA-controlled activation of promoters for small RNA genes in Pseudomonas fluorescens. Appl. Environ. Microbiol. 76:1497–1506. 10.1128/AEM.02014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frangipani E, Visaggio D, Heeb S, Kaever V, Cámara M, Visca P, Imperi F. 2013. The Gac/Rsm and cyclic-di-GMP signaling networks coordinately regulate iron uptake in Pseudomonas aeruginosa. Environ. Microbiol. 6:676–688. 10.1111/1462-2920.12164. [DOI] [PubMed] [Google Scholar]
- 50.Yakhnin AV, Baker CS, Vakulskas CA, Yakhnin H, Berezin I, Romeo T, Babitzke P. 2013. CsrA activates flhDC expression by protecting flhDC mRNA from RNase E-mediated cleavage. Mol. Microbiol. 87:851–866. 10.1111/mmi.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]