ABSTRACT
The majority of plant viruses are vectored by arthropods via persistent-circulative or noncirculative transmission. Previous studies have shown that specific binding sites for noncirculative viruses reside within the stylet or foregut of insect vectors, whereas the transmission mechanisms of circulative viruses remain ambiguous. Here we report the critical roles of whitefly primary salivary glands (PSGs) in the circulative transmission of two begomoviruses. The Middle East Asia Minor 1 (MEAM1) species of the whitefly Bemisia tabaci complex efficiently transmits both Tomato yellow leaf curl China virus (TYLCCNV) and Tomato yellow leaf curl virus (TYLCV), whereas the Mediterranean (MED) species transmits TYLCV but not TYLCCNV. PCR and fluorescence in situ hybridization experiments showed that TYLCCNV efficiently penetrates the PSGs of MEAM1 but not MED whiteflies. When a fragment of the coat protein of TYLCCNV was exchanged with that of TYLCV, mutated TYLCCNV accumulated in the PSGs of MED whiteflies, while mutant TYLCV was nearly undetectable. Confocal microscopy revealed that virion transport in PSGs follows specific paths to reach secretory cells in the central region, and the accumulation of virions in the secretory region of PSGs was correlated with successful virus transmission. Our findings demonstrate that whitefly PSGs, in particular the cells around the secretory region, control the specificity of begomovirus transmission.
IMPORTANCE Over 75% of plant viruses are transmitted by insects. However, the mechanisms of virus transmission by insect vectors remain largely unknown. Begomoviruses and whiteflies are a complex of viruses and vectors which threaten many crops worldwide. We investigated the transmission of two begomoviruses by two whitefly species. We show that specific cells of the whitefly primary salivary glands control viral transmission specificity and that virion transport in the glands follows specific paths to reach secretory cells in the central region and then to reach the salivary duct. Our results indicate that the secretory cells in the central region of primary salivary glands determine the recognition and transmission of begomoviruses. These findings set a foundation for future research not only on circulative plant virus transmission but also on other human and animal viruses transmitted by arthropod vectors.
INTRODUCTION
The majority of plant viruses depend on insect vectors for transmission. Depending on the mode of transmission, plant viruses are classified into three categories, i.e., nonpersistent, semipersistent, and persistent, and the viruses in the latter category can be classified further, as circulative-propagative and circulative-nonpropagative viruses. Nonpersistent and semipersistent viruses have transient relationships with their vectors and are associated only with the mouthparts or foregut. In contrast, persistent viruses circulate in the vector body and have developed intimate interactions with internal organs and components of vectors (1–5). Recent studies showed that the noncirculative Cauliflower mosaic virus (CaMV) and Lettuce infectious yellows virus (LIYV) were specifically retained in the stylet of an aphid and the foregut of a whitefly vector, respectively, and specific viral proteins bound to these retention sites correlated with virus transmission (6, 7). These studies provided a better understanding of the transmission of noncirculative viruses by insect vectors. In addition, the transmission mechanisms of Barley yellow dwarf virus, which is transmitted by aphids in a circulative, nonpropagative manner, have been documented in detail. During feeding, viral particles are acquired through gut tissue into the aphid hemocoel and then exit the transmission pathway through salivary tissues. In this process, the accessory salivary gland (ASG) basal lamina functions as the transmission barrier (8–10).
Begomoviruses (genus Begomovirus, family Geminiviridae) are a very-well-studied group of single-stranded DNA (ssDNA) viruses and are exclusively transmitted by the whitefly Bemisia tabaci in a circulative, probably nonpropagative manner (11, 12). During their journey in the whitefly body, begomoviruses breach multiple membrane-based barriers, including midgut (MG) epithelial cells, the apical plasmalemma of these cells, and the basal lamina of primary salivary glands (PSGs) (13, 14). Whitefly PSGs, not the accessory salivary glands, are the key organs in regulating virus circulation. Previous studies on the ultrastructure of PSGs showed that the PSGs are compact and kidney shaped. Each is composed of three regions: the central region and two darker-staining endcaps, one on either side. The central region is very complex and consists of two distinct sections: the ductal and secretory sections. These results have contributed valuable information on the physiological complexity of PSGs in virus transmission (15–17). Circulation of begomoviruses in the insect body is likely to involve complex interactions between virus-encoded proteins and vector organs and proteins (13, 18–21). Recent studies demonstrated the involvement of a GroEL homolog protein encoded by whitefly-endosymbiotic bacteria and the whitefly-encoded proteins BtHSP16 and BtHSP70 in virus transmission through interactions with the viral coat protein (CP) (22–24).
Tomato yellow leaf curl virus (TYLCV) is one of the most devastating begomoviruses and has caused extensive damage to tomato crops worldwide (11, 25, 26). However, Tomato yellow leaf curl China virus (TYLCCNV), which is closely related to TYLCV, is restricted to southern China and infects tomato and tobacco plants (27, 28). Both TYLCV and TYLCCNV are exclusively transmitted by the whitefly B. tabaci, which is now recognized as a complex containing at least 35 cryptic species (29, 30). During the past 20 years, two species of the B. tabaci complex, Middle East Asia Minor 1 (MEAM1) and Mediterranean (MED), which have commonly been referred to as biotype B and biotype Q, respectively, have invaded many countries worldwide and have coexisted in sympatry at various localities (31). Previous studies have shown that the transmission specificity and efficiency of different begomoviruses vary among different whitefly species (32, 33). However, the mechanisms underlying the varied transmission specificities and competences of begomoviruses in different whiteflies remain largely unknown.
Here we investigated TYLCV and TYLCCNV transmission specificity by the whitefly cryptic species MEAM1 and MED. We report that while MEAM1 whiteflies efficiently transmit TYLCV and TYLCCNV, MED whiteflies can ingest and retain both viruses but fail to transmit TYLCCNV. Virus localization experiments indicated that the transmission specificity of MED whiteflies resides in PSGs. Construction of viral CP mutants revealed specific interactions between the CP central domain and vector salivary components. Finally, exploring the virus route in PSGs revealed that virions were retained only in specific cells in the central region of PSGs that seem to be crucial for successful virus transmission. Our results propose that PSGs are a key vector component mediating the specificity of virus transmission and provide novel insights into the transmission mechanisms of circulative viruses.
MATERIALS AND METHODS
Insects, viruses, and plants.
Whiteflies were reared on cotton plants (Gossypium hirsutum L. cv. Zhemian 1793) in insect-proof cages at 26 ± 1°C with a 16-h light–8-h dark cycle in a temperature-controlled room. All experiments were conducted with this temperature and light regimen. Clones of TYLCCNV isolate Y10 (GenBank accession number AJ319675.1) with a beta-satellite (GenBank accession number AJ421621.1) and TYLCV isolate SH2 (GenBank accession number AM282874.1) were agroinoculated into tobacco (Nicotiana tabacum L. cv. NC89) and tomato (Solanum lycopersicom L. cv. Hezuo903) plants, respectively (27, 34). Uninfected tomato and tobacco plants were grown in insect-proof cages under natural lighting and at ambient temperature.
Engineering of infectious clones of CP mutant viruses.
Procedures for construction of infectious clones of mutated TYLCCNV (mTYLCCNV) and TYLCV (mTYLCV) were performed as previously described (27). Briefly, a 483-bp fragment (positions 212 to 694) of the TYLCCNV CP gene was replaced with a 486-bp fragment (positions 215 to 700) of the TYLCV CP gene by overlap extension PCR (see Fig. 3 for details of the replaced region). The fidelity of mTYLCCNV and mTYLCV was confirmed by Sanger sequencing.
FIG 3.

Comparison of amino acid sequences of TYLCV and TYLCCNV coat proteins. Alignments were done by GeneDoc. The exchanged region is indicated by underlining.
Transmission of TYLCCNV, TYLCV, and their mutants by MEAM1 and MED whiteflies.
Viruliferous MEAM1 and MED whiteflies were obtained by caging newly emerged adults on virus-infected tobacco plants for a 48-h acquisition access period (AAP). The viruliferous insects were then collected in groups of 10 and used to inoculate one uninfected tomato or tobacco plant. The inoculation was performed on the top of the second leaf at the plant's three- or four-true-leaf stage (∼3 weeks after sowing) for a 48-h inoculation access period (IAP), using leaf clip cages (33). Forty uninfected tomato or tobacco plants were used for each virus transmission test. The plants were then sprayed with imidacloprid at a concentration of 20 mg/liter to kill all the whiteflies and were kept until symptoms developed.
Quantification of TYLCCNV DNA in insects.
Whiteflies were given a 48-h AAP on TYLCCNV-infected tobacco plants. Half of the insects were then moved to cotton, a nonhost plant for TYLCCNV, for retention feeding, and the other half were allowed to feed continuously on infected tobacco (continuous feeding). Adult whiteflies from continuous feeding and retention feeding conditions were collected at different time points after the transfer (35). Total DNA was extracted from groups of 50 viruliferous whiteflies (cohorts with equally mixed sexes) by using previously described methods (36–38). For quantification of viral DNA in PSGs of viruliferous whiteflies fed on TYLCCNV-infected plants, 20 dissected PSGs were collected as one sample, and three replicates were examined. A partial sequence of the AV1 gene of TYLCCNV was chosen as the target, and the highly conserved β-actin gene was selected as the internal control. Primer sequences were as follows: for TYLCCNV, primer 1 (5′-TTAGAGATCGTCGTCCTAGTGG-3′) and primer 2 (5′-GCTCCTTACAAGCATATTGTCC-3′); for mTYLCCNV, primer 1 (5′-ATGTTACTCGTGGATCTGGAAT-3′) and primer 2 (5′-ATTGGGCTGTTTCCATAGGGCC-3′); for TYLCV and mTYLCV, primer 1 (5′-GAAGCGACCAGGCGATATAA-3′) and primer 2 (5′-GGAACATCAGGGCTTCGATA-3′); and for β-actin, primer 1 (5′-TCTTCCAGCCATCCTTCTTG-3′) and primer 2 (5′-CGGTGATTTCCTTCTGCATT-3′) (37). Quantitative PCR (qPCR) was performed on an ABI Prism 7500 Fast real-time PCR system (Applied Biosystems) with SYBR green detection (TaKaRa, China), and the relative abundance of viral DNA was calculated using the 2−ΔΔCT method.
Detection of viral DNA in individual whitefly tissues.
Viruliferous adult whiteflies were obtained after a 48-h AAP on infected plants and then transferred to healthy cotton plants. Female whiteflies were collected under a stereomicroscope. MGs and PSGs were dissected from single whiteflies in phosphate-buffered saline (PBS), flushed several times with sterile double-distilled water, and collected. The hemolymph (HL) droplet was collected from the abdomen by use of a 1- to 5-μl capillary pipette drawn to a fine point of ∼0.5 μm in diameter. Saliva samples were collected from 200 adult whiteflies fed on a whitefly feeding chamber with double-distilled water for a 12-h feeding period (39). In addition, nonviruliferous whiteflies were dissected as controls. For each organ and saliva sample of each species, 60 samples were collected in total, and three replicates were conducted. All the collected midgut, hemolymph, PSG, and saliva samples were then subjected to PCR analysis according to previous descriptions (18, 36, 40). Viral DNA was detected using the following primers: for TYLCCNV, primer 1 (5′-GAATTCATGACTATCAAATACAACAAC-3′) and primer 2 (5′-GGATCCTCATACATCTGAATTTGTAAATAG-3′); for mTYLCCNV, primer 1 (5′-CTACCCATGCAAGTAATCCTGTA-3′) and primer 2 (5′-ACTGGCAAAGCAACACAAAATA-3′); for TYLCV, primer 1 (5′-ATCGAAGCCCTGATATCCCCCGTGG-3′) and primer 2 (5′-CAGAGCAGTTGATCATG-3′); and for mTYLCV, primer 1 (5′-ATGCCTCTAATCCAGTGTATGCA-3′) and primer 2 (5′-TAAGGCGTAAGCGTGTAGACC-3′). An independent t test was used to compare levels of viruliferous organs and saliva between MEAM1 and MED whiteflies.
Localization of viral DNA.
Short oligonucleotide probes for TYLCCNV and mTYLCCNV (5′-Cy3-GGGACGTCAGGGCTTCTGTA-3′) and for TYLCV and mTYLCV (5′-Cy3-GGAACATCAGGGCTTCGATA-3′) were used to localize virions in dissected whitefly MGs and PSGs (41). Whiteflies were sampled using the method mentioned above. Twenty MGs and PSGs were dissected from viruliferous MEAM1 and MED adults. Dissected organs were used for fluorescence in situ hybridization (FISH) analyses as described by Ghanim et al. (41). In addition, no probe and nonviruliferous whiteflies were used as controls.
Entry of virus into whitefly PSGs.
Ten PSGs were collected from whiteflies fed on virus-infected plants for 3, 4, 6, 12, 24, 36, 48, or 72 h and then were fixed in 4% paraformaldehyde (MultiSciences Biotech, China) for 1 h at 37°C, permeabilized with 0.5% Triton X-100 for 30 min, and blocked with 5% nonfat milk dissolved in PBS-Tween 20 (PBST) for 2 h at 37°C. The tissues were further incubated with anti-CP monoclonal antibody (MAb) at a 1:500 dilution overnight at 4°C and finally incubated with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:5,000) (Jackson) for 1 h at 37°C. The localization signal was viewed with a Zeiss LSM710 confocal microscope (Zeiss, Germany).
RESULTS
Specificity of TYLCCNV and TYLCV transmission in whiteflies.
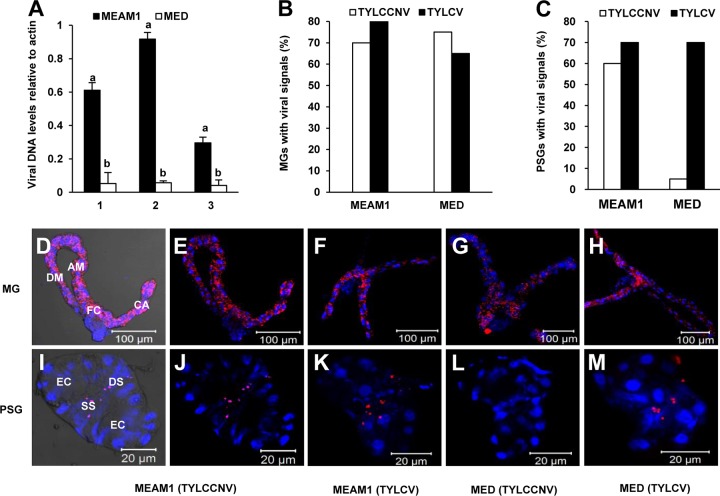
We examined the ability of MEAM1 and MED whiteflies to transmit TYLCCNV and TYLCV. MEAM1 whiteflies transmitted both viruses, while MED whiteflies transmitted TYLCV but not TYLCCNV (Fig. 1A). To identify whether this difference was due to the inability of MED whiteflies to ingest and accumulate TYLCCNV, two experiments were conducted. In the first experiment, the amount of virus ingested by whiteflies continuously feeding on virus-infected tomato plants for 14 days was examined. The results showed that during continuous feeding on virus-infected plants, MED whiteflies accumulated more viruses than did MEAM1 whiteflies (Fig. 1B). In the second experiment, after a 48-h AAP of feeding on virus-infected tomato plants, virus retention by whiteflies on cotton, a virus nonhost plant, was measured. This experiment showed that in the first day on cotton plants, the relative abundance of TYLCCNV was slightly higher in MEAM1 whiteflies, while the amount of virus in MED whiteflies remained higher from 3 to 12 days (Fig. 1C). Altogether, these results suggest that the failure of MED whiteflies to transmit TYLCCNV was not due to an inability to ingest and retain the virus.
FIG 1.
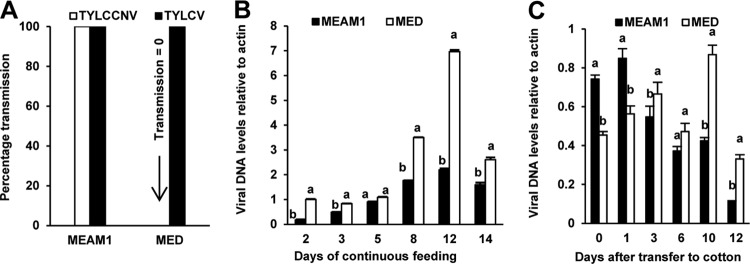
TYLCCNV and TYLCV transmissibility and virus dynamics in MEAM1 and MED whiteflies. (A) TYLCCNV and TYLCV transmissibility in MEAM1 and MED whiteflies. Whiteflies were fed on virus-infected plants for a 48-h AAP and then were used for inoculation tests in groups of 10 insects per healthy tomato plant for a 48-h IAP. Plant infection was determined by monitoring disease symptoms and by PCR amplification of the viral DNA 30 days after inoculation. Data on virus transmission were obtained from both 40 tomato plants and 40 tobacco plants for each combination of a whitefly species and a virus. (B) Quantitative PCR analysis of TYLCCNV DNA in groups of 50 MEAM1 or MED whiteflies that were maintained on TYLCCNV-infected tobacco plants (continuous feeding) for the time intervals indicated. (C) Quantitative PCR analysis of TYLCCNV DNA in groups of 50 MEAM1 or MED whiteflies that were transferred to nonhost cotton plants after a 48-h AAP on TYLCCNV-infected tobacco plants (retention feeding). Whiteflies were sampled at the time intervals indicated after transfer onto cotton plants.
Retention of virions in PSGs is a prerequisite for virus transmission.
We next examined whether potential barriers inside the whitefly body blocked TYLCCNV passage by tracing viral DNA in different whitefly tissues along the virus transmission pathway, including the MG, HL, PSGs, and saliva (21). PCR results showed that almost all collected MGs and HL of the two viruliferous species were TYLCCNV positive (Table 1), indicating that TYLCCNV can penetrate the MG barriers of both MEAM1 and MED whiteflies. However, while 81.4% of PSGs of MEAM1 whiteflies were positive, only 30.7% of PSGs of MED whiteflies were positive. Furthermore, TYLCCNV could be detected in 53.3% of the collected MEAM1 saliva samples, while all the MED saliva samples were TYLCCNV negative (Table 1). These results demonstrate that (i) the efficiency of TYLCCNV penetration into PSGs of MED whiteflies is low; and (ii) even if TYLCCNV virions can penetrate the PSGs of MED whiteflies, they fail to move outside the glands to the salivary duct and thus cannot be secreted together with the saliva.
TABLE 1.
Detection of TYLCCNV in MG, HL, PSGs, and saliva of viruliferous whiteflies
| Whitefly species | % Organs and saliva with TYLCCNV DNA (mean± SE)a |
|||
|---|---|---|---|---|
| MG | HL | PSGs | Saliva | |
| MEAM1 | 98.3 ± 0.02a | 94.9 ± 0.03a | 81.4 ± 0.05a | 53.3 ± 0.07a |
| MED | 94.7 ± 0.04a | 88.7 ± 0.08a | 30.7 ± 0.08b | 0b |
For each of the three organs and saliva for each of the two species, 60 samples were collected in total, and three replicates were conducted. Data in the same column that are followed by different letters are significantly different (P < 0.05).
Because the titer of virus in PSGs may also determine virus transmissibility to plants, the levels of viral DNA in PSGs of MEAM1 and MED whiteflies which had previously fed for the same interval on TYLCCNV-infected plants were compared. Results of qPCR analysis of the viral DNA indicated that while the abundances of β-actin DNA in the two PSG samples were at the same level, the virus titer in the PSGs of MEAM1 whiteflies was, on average, about 15 times higher than that in MED whiteflies (Fig. 2A). FISH analysis revealed that TYLCV DNA was traced in 65 to 80% of the tested MGs and PSGs of both MED and MEAM1 whiteflies. However, while TYLCCNV was detected in about 70% of MGs of both species and, furthermore, in 60% of the PSGs of MEAM1 whiteflies, it was detected in only 5% of MED PSGs (Fig. 2B and C). This result is consistent with the previous TYLCV transmission experiment (Fig. 1A). FISH experiments demonstrated that viruses were distributed in the entire MG, with noticeable enrichment in the filter chamber (Fig. 2D to H). Interestingly, in PSGs, TYLCCNV and TYLCV were specifically located in the central secretory region, along the ductules of the glands (Fig. 2I to M).
FIG 2.
Retention of TYLCCNV in the MGs and PSGs of MEAM1 and MED whiteflies. Adult whiteflies were allowed a 48-h AAP on TYLCCNV-infected plants and then maintained on a virus nonhost cotton plant. (A) Different abundances of TYLCCNV DNA within whitefly PSGs. Twenty PSGs dissected from viruliferous MEAM1 and MED whiteflies were collected separately for qPCR analysis. The numbers on the x axis represent three independent experiments. The numbers on the y axis represent abundances of TYLCCNV DNA relative to that of whitefly β-actin DNA. Vertical bars on columns indicate standard errors. (B and C) Percentages of MGs (B) and PSGs (C) with fluorescence signals. The data were surveyed separately from 20 dissected MGs and PSGs. (D to M) MGs and PSGs were hybridized with a Cy3-labeled virus-specific probe (red), and nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (blue). Specimens were visualized under a confocal microscope. Panels D and I are bright-field images of panels E and J for better visualization. Annotations under the corresponding pictures represent whitefly species-virus combinations. CA, caeca; FC, filter chamber; DM, descending midgut; AM, ascending midgut; DS, ductal section of the central region; EC, endcap; SS, secretory section of the central region.
CP determines virus transmission specificity.
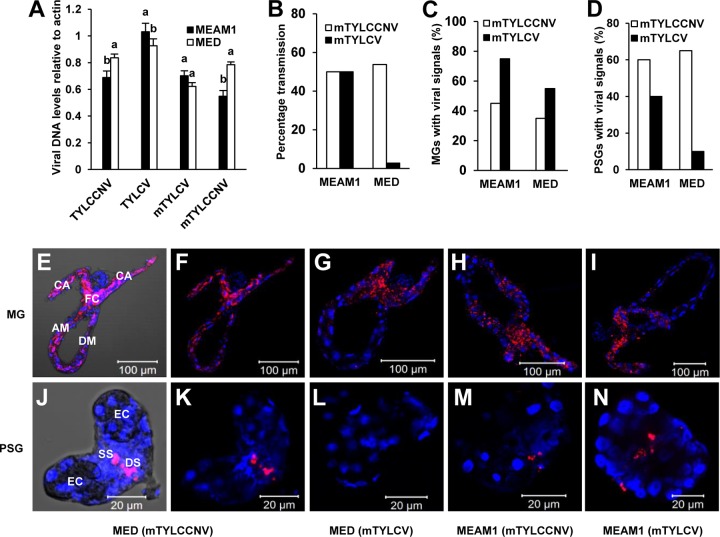
The results presented so far indicate that TYLCV and TYLCCNV transmissibility is correlated with efficient entry into whitefly PSGs. The CP of begomoviruses is the only virus-encoded protein that has been shown to determine vector competence (42–44). To investigate whether viral CP is implicated in virus transmission, we exchanged a partial CP sequence of TYLCV which has been shown to influence virus transmissibility (44, 45) with that of TYLCCNV (Fig. 3) and examined the transmissibility of the two mutant viruses by MEAM1 and MED whiteflies. First, we compared the abilities of wild-type virus accumulation and mutant virus accumulation by MEAM1 or MED whiteflies to exclude the possibility that the initial amounts of viruses in whiteflies might contribute to different efficiencies of virus transmission. The results indicated that after continuous feeding on virus-infected plants for 48 h, there was no significant difference in the abilities of both viruses to accumulate in each virus-whitefly combination (Fig. 4A). Thus, virus transmissibility is not dependent upon the initial amount of virus acquired from virus-infected plants. When tomato and tobacco plants were infested with 10 MEAM1 adults that had fed on mutant TYLCV (mTYLCV)- or mutant TYLCCNV (mTYLCCNV)-infected plants, about 50% became infected (Fig. 4B). However, similar to the case with TYLCV and TYLCCNV, the transmissibilities of the two mutant viruses by MED whiteflies differed significantly, with 54% of the plants infected with mTYLCCNV but only 3.75% of the plants infected by mTYLCV (Fig. 4B). These results confirmed the essential role of viral CP in vector-mediated virus transmission (42, 44).
FIG 4.
Transmission and localization of mutant viruses in MEAM1 and MED whiteflies. (A) Acquisition of wild-type and mutant viruses by MEAM1 and MED whiteflies. Adult whiteflies were allowed a 48-h AAP on virus-infected plants and then were collected for analysis of viral DNA abundance. Quantitative PCR analysis was used to evaluate viral DNA abundances in groups of 50 MEAM1 or MED whiteflies. Vertical bars on columns indicate standard errors. (B) mTYLCCNV and mTYLCV transmissibility by MEAM1 and MED whiteflies. For each combination of a whitefly species and a virus, data on transmission were obtained from 40 tomato and 40 tobacco plants. (C and D) Percentages of MGs (C) and PSGs (D) with viruses. The data were obtained separately from 20 dissected MGs and PSGs. (E to N) MGs and PSGs were hybridized with a virus-specific Cy3-labeled probe (red), stained with DAPI to detect nuclei (blue), and then visualized under a confocal microscope. Panels E and J are bright-field images of panels F and K for better visualization. Annotations under the corresponding pictures represent whitefly species-virus combinations. CA, caeca; FC, filter chamber; DM, descending midgut; AM, ascending midgut; DS, ductal section of the central region; EC, endcap; SS, secretory section of the central region.
To test whether PSGs were also involved in the transmission specificity of the two mutant viruses, we monitored the amounts of DNA of the two viruses in PSGs, HL, and saliva of MEAM1 and MED whiteflies. The results clearly indicated that for MEAM1 whiteflies, viral DNAs of the two mutants were detected in 40 to 75% of tested HL and PSG samples, as well as 55 to 70% of saliva samples (Table 2). In addition, mTYLCCNV and mTYLCV were detected in 70% and 55% of MED HL samples, respectively, indicating that both viruses can pass the MG barrier of MED whiteflies efficiently. However, while mTYLCCNV was detected in 45% of PSGs and 60% of saliva samples from MED whiteflies, mTYLCV DNA was present in only 22.5% of PSGs and 2.5% of saliva samples from this whitefly species (Table 2).
TABLE 2.
Viral DNAs in HL, PSGs, and saliva of viruliferous whiteflies
| Whitefly species | Virus | Whitefly tissue or salivaa | No. of samples with virus signal/no. of samples observed |
|---|---|---|---|
| MEAM1 | mTYLCCNV | HL | 30/40 |
| mTYLCCNV | PSGs | 21/40 | |
| mTYLCCNV | Saliva | 28/40 | |
| mTYLCV | HL | 25/40 | |
| mTYLCV | PSGs | 16/40 | |
| mTYLCV | Saliva | 22/40 | |
| MED | mTYLCCNV | HL | 28/40 |
| mTYLCCNV | PSGs | 18/40 | |
| mTYLCCNV | Saliva | 24/40 | |
| mTYLCV | HL | 22/40 | |
| mTYLCV | PSGs | 9/40 | |
| mTYLCV | Saliva | 1/40 |
Each saliva sample was collected from 200 whiteflies feeding on a membrane feeding chamber with double-distilled water.
Viral CP interaction with PSGs determines virus transmission specificity.
To further confirm the results presented above, the spatial localization of the two mutants in MGs and PSGs of MEAM1 and MED whiteflies was studied using FISH. Signals of both mutants were detected in 40 to 75% of collected organs from viruliferous MEAM1 whiteflies. However, in viruliferous MED whiteflies, while mTYLCCNV DNA was traced in 65% of PSGs, mTYLCV DNA was detected in only 10% of PSGs (Fig. 4C and D). These results are in line with the results of the virus transmission assays (Fig. 4B) and indicate that viral CP and whitefly PSGs collaboratively regulate virus transmission specificity. Similar to the case with the wild-type viruses, FISH analyses showed that mTYLCCNV and mTYLCV DNAs were enriched in the MG filter chamber (Fig. 4E to I) and specifically retained in the secretory region of PSGs (Fig. 4J to N).
Entry of TYLCV into whitefly PSGs follows a specific pathway.
The results so far demonstrated that all viruses were located in the central secretory region of PSGs, indicating that virion accumulation in the restricted PSG area might be required for successful virus transmission. To confirm this hypothesis, we investigated the accumulation dynamics of TYLCV and TYLCCNV in the PSGs of MED whiteflies by using immunofluorescence. Adult MED whiteflies were given various AAPs on virus-infected tomato plants, and then PSGs were dissected for immunofluorescence and confocal microscopy observations (Fig. 5 and 6). The results showed that with a 3-h AAP, no TYLCV was detected in the PSGs of MED whiteflies, and with 4-h to 6-h AAPs, TYLCV virions occupied the entire PSG. Interestingly, with 12-h to 24-h AAPs, virions gradually accumulated to the central region of PSGs. With 36-h to 72-h AAPs, the viral CP was observed only in the few specific cells along the salivary duct, and little signal was traced in other regions of PSGs (Fig. 5). Strikingly, with 4-h to 12-h AAPs, TYLCCNV virions were always distributed in circumference cells of MED PSGs. With 24-h to 72-h AAPs, virions were scarcely localized in PSGs, and few viral signals were detected on the periphery of PSGs (Fig. 6). To examine whether these phenomena were general, 10 PSGs were analyzed for each time point. The distribution of virions in nearly 90% of the samples was consistent with the patterns shown in Fig. 5 (data not shown). Next, we examined the kinetics of TYLCV and TYLCCNV in the PSGs of MEAM1 whiteflies. Interestingly, both viruses followed a pattern of kinetics similar to that of TYLCV in PSGs of MED whiteflies. An average of 80% of viral signals showed the same trend, where an increase of the AAP led to a gradual accumulation of virions in the gland secretory region (data not shown). The few PSGs with different patterns of virion distribution were probably due to the asynchrony of whitefly feeding and acquisition of the virus.
FIG 5.
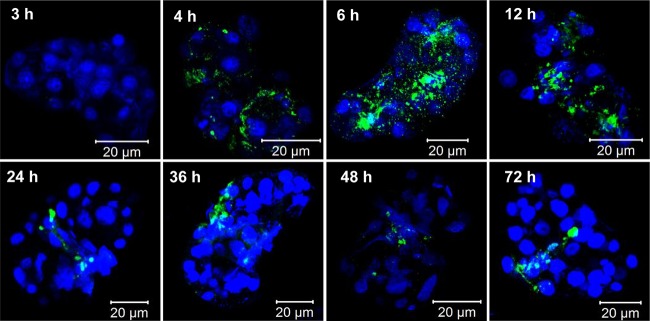
TYLCV accumulation and entry into PSGs. MED whiteflies were continuously fed on TYLCV-infected plants for 72 h. At each selected time point, 10 PSGs were dissected. TYLCV virions were detected by use of a mouse anti-TYLCV monoclonal antibody and a goat anti-mouse secondary antibody conjugated to FITC (green). Nuclei were stained with DAPI (blue) and then examined with a confocal microscope.
FIG 6.
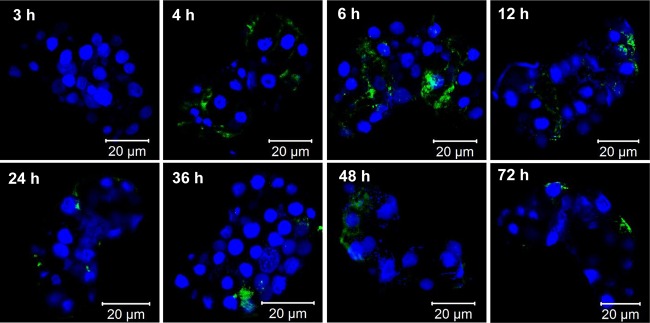
TYLCCNV accumulation and entry into PSGs. MED whiteflies were continuously fed on TYLCCNV-infected plants for 72 h. At each selected time point, 10 PSGs were dissected. TYLCCNV virions were detected by use of a mouse anti-TYLCCNV monoclonal antibody and a goat anti-mouse secondary antibody conjugated to FITC (green). Nuclei were stained with DAPI (blue) and then examined with a confocal microscope.
DISCUSSION
The movement of begomoviruses through the whitefly body follows a specific pathway, starting in the stylet, passing through the esophagus, MG, HL, and PSGs, and finally ending with secretion with saliva via the salivary duct (21). Along this pathway, begomoviruses must overcome several membrane barriers that incur close relationships between vector components and virus-encoded proteins (13, 46). Previous research on begomovirus-whitefly interactions focused on parameters of virus acquisition, retention, and inoculation by whiteflies, and few attempts have been made to discover vector components and to unravel mechanisms that underlie these interactions (18, 23, 24, 32, 33, 47–49). Currently, only two heat shock proteins, BtHSP16 and BtHSP70, and a 63-kDa GroEL protein produced by secondary endosymbionts of B. tabaci have been implicated in direct interaction with the viral CP (22–24). Here we provided direct evidence that PSGs play a major role in virus transmission by examining factors that prevent TYLCCNV transmission in MED whiteflies. First, the results presented here suggest that the number of TYLCCNV virions in PSGs of MEAM1 whiteflies is 15 times larger than that in MED whiteflies, demonstrating that entry into PSGs is a key barrier in TYLCCNV transmission. Second, the results demonstrate that while TYLCCNV could be detected in the PSGs of a small percentage of MED whiteflies, viral DNA was never detected in whitefly saliva samples. Our data prove that even when TYLCCNV can occasionally penetrate the PSGs of MED whiteflies, it fails to move outside the gland. In a previous study, a nontransmissible Tomato yellow leaf curl Sardinia virus (TYLCSV) mutant was detected in PSGs of MEAM1 whiteflies but was never secreted with saliva (50). Similarly, some nontransmissible luteoviruses are able to traverse the basal membrane but not the basal plasmalemma of the ASGs of aphids (9, 10). Collectively, these findings provide strong evidence that PSGs are the major organs that determine the transmission efficiency and specificity of the begomoviruses examined in this study.
Previous studies demonstrated that B. tabaci secondary symbionts are involved in begomovirus transmission. A GroEL protein produced by Hamiltonella has been shown to interact with TYLCV CP and to protect virions from proteolysis by the whitefly immune system (48, 51). Recent studies on B. tabaci secondary symbiont Rickettsia-TYLCV interactions showed that a B. tabaci strain infected with Rickettsia acquired more TYLCV from infected plants, retained the virus longer, and transmitted the virus more efficiently than the noninfected strain (52). In the latter study, fluorescence in situ hybridization analysis showed that while high levels of Rickettsia in the midgut resulted in TYLCV concentration in the filter chamber, a favored site for virus translocation and transmission, low levels of Rickettsia in the midgut resulted in an even distribution of the virus, demonstrating that by infecting the midgut, Rickettsia increases TYLCV transmission efficacy (52). The MEAM1 species we used here harbored Hamiltonella and Rickettsia, while the MED species harbored only Hamiltonella (53). As shown in Fig. 1, the transmission efficiency of TYLCCNV by MEAM1 whiteflies is 100%, with no transmission by MED whiteflies. Rickettsia might contribute to the high transmission efficiency of TYLCCNV by MEAM1 whiteflies. However, since TYLCCNV titers were shown to be higher in MED whiteflies than in MEAM1 whiteflies, it is possible that some other factors are involved in enhancing the ability of the virus to accumulate and be retained in MED whiteflies yet to be transmitted poorly by this species. Given the fact that Rickettsia was shown only to contribute to transmission and was not a major factor that limited transmission, we do not expect that the absence of Rickettsia from MED species is an important factor responsible for the inability of this species to transmit the virus, and other factors, especially the PSGs, are far more important in determining this transmission specificity.
CP of geminiviruses is essential for virus transmission and determines the virus-vector specificity (42, 43, 54). Replacement of CP from the nontransmissible Abutilon mosaic virus (AbMV) with CP of the transmissible Sida golden mosaic virus (SiGMV) resulted in a transmissible AbMV chimera (44). The region of CP between amino acids 129 and 134 was shown to be involved in whitefly-specific transmission of TYLCV and correlated with virion formation (45). In the current study, we exchanged the central region of TYLCV CP with that of the MED-nontransmissible TYLCCNV. This exchange resulted in successful and efficient transmission of mTYLCCNV by MED whiteflies (Fig. 4). A possible reason is that the mTYLCCNV CP can be recognized by potential receptors or become compatible with the virus-binding site on the cell membranes of MED PSGs. Interestingly, our results showed that the two CP mutants could move through the PSGs of MEAM1 whiteflies but were less efficient than the wild-type viruses (Table 2). It is possible that the stability of the mutant viruses generated in this study was reduced or that their affinity for possible receptors in PSGs was lower than that of the wild-type viruses, which contributed to the lower efficiency of virus transmission.
A whitefly PSG is composed of 13 or more large cells that vary in density, size, and morphology. As elucidated, virions are secreted together with saliva that moves from the secretory region, through the internal and external ducts, to the salivary pump, and finally to the salivary canal in the stylet, to be ejected during whitefly feeding on plants (15–17). The kinetics of TYLCV and TYLCCNV in PSGs revealed several interesting observations. First, both TYLCV and TYLCCNV can enter the peripheral region of PSGs at early time points in both whitefly species (Fig. 5 and 6). This result suggests that entry into PSGs is not a specific barrier and cannot determine the specificity of virus transmission, but it may influence virus transmission efficiency. Second, transmissible viruses gradually accumulated in the cells along the internal ducts in the central region of PSGs (Fig. 5), while nontransmissible viruses (TYLCCNV in MED whiteflies) accumulated only in the peripheral area and never reached the central region of PSGs (Fig. 6). These results confirmed that secretory cells in the central region of whitefly PSGs are an important site that governs the transmission specificity of begomoviruses. It also suggests that possible receptors may reside in these cells and account for virus recognition and secretion through PSGs. The PSGs of MED whiteflies might lack some component in these specific cells that interact with TYLCCNV, resulting in no transmission. Third, for both viruses, the increase in AAP led to a gradual disappearance of viral signals in the peripheral region of PSGs, and virus entry appeared only in the central region (Fig. 5 and 6). We speculate that after a long AAP, the virus in the peripheral region might be degraded or some molecules used by the virus for entry might be consumed, and the presence of the virions in the PSGs might turn on the secretion process, creating some kind of functional funnel guiding virions to the central secretory duct. These findings substantially extend our understanding of the transmission mechanisms of begomoviruses by B. tabaci and provide invaluable clues to the interactions between PSGs and circulative viruses.
ACKNOWLEDGMENTS
We thank Jian-Xiang Wu for providing monoclonal antibodies for TYLCV and TYLCCNV, Jun-Bo Luan for helpful suggestions, Chang-Jun Huang for construction of infectious clones, and Yun-Qin Li for confocal microscopy.
This work was supported by the National Natural Science Foundation of China (grants 31390421, 31361140356, and 31101410) and The Special Fund for Agro-Scientific Research in the Public Interest China (grant 201003065).
Footnotes
Published ahead of print 10 September 2014
REFERENCES
- 1.Nault L. 1997. Arthropod transmission of plant viruses: a new synthesis. Ann. Entomol. Soc. Am. 90:521–541. [Google Scholar]
- 2.Ng JC, Perry KL. 2004. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 5:505–511. 10.1111/j.1364-3703.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 3.Ng JC, Falk BW. 2006. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 44:183–212. 10.1146/annurev.phyto.44.070505.143325. [DOI] [PubMed] [Google Scholar]
- 4.Bragard C, Caciagli P, Lemaire O, Lopez-Moya J, MacFarlane S, Peters D, Susi P, Torrance L. 2013. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 51:177–201. 10.1146/annurev-phyto-082712-102346. [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez S, Michalakis Y, Munster M, Blanc S. 2013. Plant feeding by insect vectors can affect life cycle, population genetics and evolution of plant viruses. Funct. Ecol. 27:610–622. 10.1111/1365-2435.12070. [DOI] [Google Scholar]
- 6.Uzest M, Gargani D, Drucker M, Hébrard E, Garzo E, Candresse T, Fereres A, Blanc S. 2007. A protein key to plant virus transmission at the tip of the insect vector stylet. Proc. Natl. Acad. Sci. U. S. A. 104:17959–17964. 10.1073/pnas.0706608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen AYS, Walker GP, Carter D, Ng JC. 2011. A virus capsid component mediates virion retention and transmission by its insect vector. Proc. Natl. Acad. Sci. U. S. A. 108:16777–16782. 10.1073/pnas.1109384108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gildow F, Gray S. 1993. The aphid salivary gland basal lamina as a selective barrier associated with vector-specific transmission of barley yellow dwarf luteoviruses. Phytopathology 83:1293–1302. 10.1094/Phyto-83-1293. [DOI] [Google Scholar]
- 9.Peiffer M, Gildow F, Gray S. 1997. Two distinct mechanisms regulate luteovirus transmission efficiency and specificity at the aphid salivary gland. J. Gen. Virol. 78:495–503. [DOI] [PubMed] [Google Scholar]
- 10.Gray S, Gildow F. 2003. Luteovirus-aphid interactions. Annu. Rev. Phytopathol. 41:539–566. 10.1146/annurev.phyto.41.012203.105815. [DOI] [PubMed] [Google Scholar]
- 11.Czosnek H, Laterrot H. 1997. A worldwide survey of Tomato yellow leaf curl viruses. Arch. Virol. 142:1391–1406. 10.1007/s007050050168. [DOI] [PubMed] [Google Scholar]
- 12.Czosnek H, Ghanim M, Morin S, Rubinstein G, Fridman V, Zeidan M. 2001. Whiteflies: vectors, and victims (?), of geminiviruses. Adv. Virus Res. 57:291–322. 10.1016/S0065-3527(01)57006-2. [DOI] [PubMed] [Google Scholar]
- 13.Hogenhout SA, Ammar ED, Whitfield AE, Redinbaugh MG. 2008. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46:327–359. 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- 14.Brown JK, Czosnek H. 2002. Whitefly transmission of plant viruses. Adv. Bot. Res. 36:65–100. 10.1016/S0065-2296(02)36059-2. [DOI] [Google Scholar]
- 15.Cicero JM, Brown JK. 2011. Functional anatomy of whitefly organs associated with Squash leaf curl virus (Geminiviridae: Begomovirus) transmission by the B biotype of Bemisia tabaci (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 104:261–279. 10.1603/AN10075. [DOI] [Google Scholar]
- 16.Cicero JM, Brown JK. 2012. Ultrastructural studies of the salivary duct system in the whitefly vector Bemisia tabaci (Aleyrodidae: Hemiptera). Ann. Entomol. Soc. Am. 105:701–717. 10.1603/AN12030. [DOI] [Google Scholar]
- 17.Ghanim M, Rosell RC, Campbell LR, Czosnek H, Brown JK, Ullman DE. 2001. Digestive, salivary, and reproductive organs of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) B type. J. Morphol. 248:22–40. 10.1002/jmor.1018. [DOI] [PubMed] [Google Scholar]
- 18.Ghanim M, Morin S, Czosnek H. 2001. Rate of Tomato yellow leaf curl virus translocation in the circulative transmission pathway of its vector, the whitefly Bemisia tabaci. Phytopathology 91:188–196. 10.1094/PHYTO.2001.91.2.188. [DOI] [PubMed] [Google Scholar]
- 19.Ammar E. 1994. Propagative transmission of plant and animal viruses by insects: factors affecting vector specificity and competence. Adv. Dis. Vector Res. 10:289–331. 10.1007/978-1-4612-2590-4_11. [DOI] [Google Scholar]
- 20.Rosell RC, Torres-Jerez I, Brown J. 1999. Tracing the geminivirus-whitefly transmission pathway by polymerase chain reaction in whitefly extracts, saliva, hemolymph, and honeydew. Phytopathology 89:239–246. 10.1094/PHYTO.1999.89.3.239. [DOI] [PubMed] [Google Scholar]
- 21.Czosnek H, Ghanim M, Ghanim M. 2002. The circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci—insights from studies with Tomato yellow leaf curl virus. Ann. Appl. Biol. 140:215–231. 10.1111/j.1744-7348.2002.tb00175.x. [DOI] [Google Scholar]
- 22.Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Kontsedalov S, Skaljac M, Brumin M, Sobol I, Czosnek H, Vavre F, Fleury F. 2010. The transmission efficiency of Tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J. Virol. 84:9310–9317. 10.1128/JVI.00423-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnesorge S, Bejarano E. 2009. Begomovirus coat protein interacts with a small heat-shock protein of its transmission vector (Bemisia tabaci). Insect Mol. Biol. 18:693–703. 10.1111/j.1365-2583.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 24.Götz M, Popovski S, Kollenberg M, Gorovits R, Brown JK, Cicero JM, Czosnek H, Winter S, Ghanim M. 2012. Implication of Bemisia tabaci heat shock protein 70 in begomovirus-whitefly interactions. J. Virol. 86:13241–13252. 10.1128/JVI.00880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriones E, Navas-Castillo J. 2000. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 71:123–134. 10.1016/S0168-1702(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 26.Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S. 2011. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 49:219–248. 10.1146/annurev-phyto-072910-095235. [DOI] [PubMed] [Google Scholar]
- 27.Cui X, Tao X, Xie Y, Fauquet CM, Zhou X. 2004. A DNAβ associated with Tomato yellow leaf curl China virus is required for symptom induction. J. Virol. 78:13966–13974. 10.1128/JVI.78.24.13966-13974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Zhou X, Zhang X, Xie Y. 2004. Molecular characterization of tomato-infecting begomoviruses in Yunnan, China. Arch. Virol. 149:1721–1732. 10.1007/s00705-004-0334-7. [DOI] [PubMed] [Google Scholar]
- 29.Mugiira R, Liu SS, Zhou X. 2008. Tomato yellow leaf curl virus and Tomato leaf curl Taiwan virus invade southeast coast of China. J. Phytopathol. 156:217–221. 10.1111/j.1439-0434.2007.01345.x. [DOI] [Google Scholar]
- 30.Firdaus S, Vosman B, Hidayati N, Supena J, Darmo E, Visser GFR, van Heusden AW. 2013. The Bemisia tabaci species complex: additions from different parts of the world. Insect Sci. 20:723–733. 10.1111/1744-7917.12001. [DOI] [PubMed] [Google Scholar]
- 31.Hu J, De Barro P, Zhao H, Wang J, Nardi F, Liu SS. 2011. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS One 6:e16061. 10.1371/journal.pone.0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Hu J, Xu FC, Liu SS. 2010. Transmission of Tomato yellow leaf curl virus by two invasive biotypes and a Chinese indigenous biotype of the whitefly Bemisia tabaci. Int. J. Pest Manag. 56:275–280. 10.1080/09670871003743428. [DOI] [Google Scholar]
- 33.Jiu M, Zhou XP, Liu SS. 2006. Acquisition and transmission of two begomoviruses by the B and a non-B biotype of Bemisia tabaci from Zhejiang, China. J. Phytopathol. 154:587–591. 10.1111/j.1439-0434.2006.01151.x. [DOI] [Google Scholar]
- 34.Zhou X, Xie Y, Tao X, Zhang Z, Li Z, Fauquet CM. 2003. Characterization of DNAβ associated with begomoviruses in China and evidence for co-evolution with their cognate viral DNA-A. J. Gen. Virol. 84:237–247. 10.1099/vir.0.18608-0. [DOI] [PubMed] [Google Scholar]
- 35.Ohnishi JK, Terami T, Honda FK. 2009. A selective barrier in the midgut epithelial cell membrane of the nonvector whitefly Trialeurodes vaporariorum to Tomato yellow leaf curl virus uptake. J. Gen. Plant Pathol. 75:131–139. 10.1007/s10327-009-0147-3. [DOI] [Google Scholar]
- 36.Luo C, Yao Y, Wang RJ, Yan FM, Hu DX, Zhang ZL. 2002. The use of mitochondrial cytochrome oxidase I (mt CO I.) gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomol. Sin. 45:759–763. [Google Scholar]
- 37.Sinisterra XH, McKenzie C, Hunter WB, Powell CA, Shatters RG. 2005. Differential transcriptional activity of plant-pathogenic begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyrodidae). J. Gen. Virol. 86:1525–1532. 10.1099/vir.0.80665-0. [DOI] [PubMed] [Google Scholar]
- 38.Mason G, Caciagli P, Accotto GP, Noris E. 2008. Real-time PCR for the quantitation of Tomato yellow leaf curl Sardinia virus in tomato plants and in Bemisia tabaci. J. Virol. Methods 147:282–289. 10.1016/j.jviromet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Salvucci ME, Crafts-Brandner SJ. 2000. Effects of temperature and dietary sucrose concentration on respiration in the silverleaf whitefly, Bemisia argentifolii. J. Insect Physiol. 46:1461–1467. 10.1016/S0022-1910(00)00070-6. [DOI] [PubMed] [Google Scholar]
- 40.Qian Y, Zhou X. 2005. Pathogenicity and stability of a truncated DNA [beta] associated with Tomato yellow leaf curl China virus. Virus Res. 109:159–163. 10.1016/j.virusres.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Ghanim M, Brumin M, Popovski S. 2009. A simple, rapid and inexpensive method for localization of Tomato yellow leaf curl virus and Potato leafroll virus in plant and insect vectors. J. Virol. Methods 159:311–314. 10.1016/j.jviromet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Briddon R, Pinner M, Stanley J, Markham P. 1990. Geminivirus coat protein replacement alters insect specificity. Virology 177:85–94. 10.1016/0042-6822(90)90462-Z. [DOI] [PubMed] [Google Scholar]
- 43.Azzam O, Frazer J, De La Rosa D, Beaver J, Ahlquist P, Maxwell D. 1994. Whitefly transmission and efficient ssDNA accumulation of bean golden mosaic geminivirus require functional coat protein. Virology 204:289–296. 10.1006/viro.1994.1533. [DOI] [PubMed] [Google Scholar]
- 44.Hofer P, Bedford ID, Markham PG, Jeske H, Frischmuth T. 1997. Coat protein gene replacement results in whitefly transmission of an insect nontransmissible geminivirus isolate. Virology 236:288–295. 10.1006/viro.1997.8751. [DOI] [PubMed] [Google Scholar]
- 45.Noris E, Vaira A, Caciagli P, Masenga V, Gronenborn B, Accotto G. 1998. Amino acids in the capsid protein of Tomato yellow leaf curl virus that are crucial for systemic infection, particle formation, and insect transmission. J. Virol. 72:10050–10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanc S, Uzest M, Drucker M. 2011. New research horizons in vector-transmission of plant viruses. Curr. Opin. Microbiol. 14:483–491. 10.1016/j.mib.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Ghanim M, Sobol I, Czosnek H. 2007. Horizontal transmission of begomoviruses between Bemisia tabaci biotypes. Arthropod-Plant Interact. 1:195–204. 10.1007/s11829-007-9018-z. [DOI] [Google Scholar]
- 48.Morin S, Ghanim M, Sobol I, Czosnek H. 2000. The GroEL protein of the whitefly Bemisia tabaci interacts with the coat protein of transmissible and nontransmissible begomoviruses in the yeast two-hybrid system. Virology 276:404–416. 10.1006/viro.2000.0549. [DOI] [PubMed] [Google Scholar]
- 49.Luan JB, Li JM, Varela N, Wang YL, Li FF, Bao YY, Zhang CX, Liu SS, Wang XW. 2011. Global analysis of the transcriptional response of whitefly to Tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J. Virol. 85:3330–3340. 10.1128/JVI.02507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caciagli P, Piles VM, Marian D, Vecchiati M, Masenga V, Mason G, Falcioni T, Noris E. 2009. Virion stability is important for the circulative transmission of Tomato yellow leaf curl Sardinia virus by Bemisia tabaci, but virion access to salivary glands does not guarantee transmissibility. J. Virol. 83:5784–5795. 10.1128/JVI.02267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghanim M. 2014. A review of the mechanisms and components that determine the transmission efficiency of Tomato yellow leaf curl virus (Geminiviridae; Begomovirus) by its whitefly vector. Virus Res. 186:47–54. 10.1016/j.virusres.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Kliot A, Cilia M, Czosnek H, Ghanim M. 2014. Implication of the bacterial endosymbiont Rickettsia spp. in interactions of the whitefly Bemisia tabaci with Tomato yellow leaf curl virus. J. Virol. 88:5652–5660. 10.1128/JVI.00071-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bing XL, Ruan YM, Rao Q, Wang XW, Liu SS. 2013. Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci. 20:194–206. 10.1111/j.1744-7917.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Bedford ID, Briddon RW, Markham PG. 1997. Efficient whitefly transmission of African cassava mosaic geminivirus requires sequences from both genomic components. J. Gen. Virol. 78:1791–1794. [DOI] [PubMed] [Google Scholar]