ABSTRACT
Interleukin-10 (IL-10) is an immunomodulatory cytokine that is important for maintenance of epithelial cell (EC) survival and anti-inflammatory responses (AIR). The majority of HIV infections occur through the mucosal route despite mucosal epithelium acting as a barrier to human immunodeficiency virus (HIV). Therefore, understanding the role of IL-10 in maintenance of intestinal homeostasis during HIV infection is of interest for better characterization of the pathogenesis of HIV-mediated enteropathy. We demonstrated here changes in mucosal IL-10 signaling during simian immunodeficiency virus (SIV) infection in rhesus macaques. Disruption of the epithelial barrier was manifested by EC apoptosis and loss of the tight-junction protein ZO-1. Multiple cell types, including a limited number of ECs, produced IL-10. SIV infection resulted in increased levels of IL-10; however, this was associated with increased production of mucosal gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), suggesting that IL-10 was not able to regulate AIR. This observation was supported by the downregulation of STAT3, which is necessary to inhibit production of IFN-γ and TNF-α, and the upregulation of SOCS1 and SOCS3, which are important regulatory molecules in the IL-10-mediated AIR. We also observed internalization of the IL-10 receptor (IL-10R) in mucosal lymphocytes, which could limit cellular availability of IL-10 for signaling and contribute to the loss of a functional AIR. Collectively, these findings demonstrate that internalization of IL-10R with the resultant impact on IL-10 signaling and dysregulation of the IL-10-mediated AIR might play a crucial role in EC damage and subsequent SIV/HIV pathogenesis.
IMPORTANCE Interleukin-10 (IL-10), an important immunomodulatory cytokine plays a key role to control inflammatory function and homeostasis of the gastrointestinal mucosal immune system. Despite recent advancements in the study of IL-10 and its role in HIV infection, the role of mucosal IL-10 in SIV/HIV infection in inducing enteropathy is not well understood. We demonstrated changes in mucosal IL-10 signaling during SIV infection in rhesus macaques. Disruption of the intestinal epithelial barrier was evident along with the increased levels of mucosal IL-10 production. Increased production of mucosal IFN-γ and TNF-α during SIV infection suggested that the increased level of mucosal IL-10 was not able to regulate anti-inflammatory responses. Our findings demonstrate that internalization of IL-10R with the resultant impact on IL-10 signaling and dysregulation of the IL-10-mediated anti-inflammatory responses might play a crucial role in epithelial cell damage and subsequent SIV/HIV pathogenesis.
INTRODUCTION
The mucosal epithelium seems to be an efficient mechanical barrier against human immunodeficiency virus type 1 (HIV-1). However, mucosal transmission accounts for more than 90% of HIV infections (1–3). Intestinal epithelial cells (ECs) preferentially express viral coreceptors such as CCR5 and primary ECs have been shown to be able to transfer CCR5-tropic HIV more efficiently than CXCR4-tropic HIV through transcytosis to indicator cells (4, 5). These data suggest that ECs may be more actively involved in mucosal transmission of HIV than generally thought. Furthermore, studies have shown that mucosal ECs are impacted by HIV/simian immunodeficiency virus (SIV) infection and respond directly to HIV envelope glycoproteins by upregulating inflammatory cytokines that lead to impairment of barrier functions (6–8). We have recently shown the presence of early EC apoptosis and upregulation of ICAM-1 and HLA-DR by intestinal ECs, which may be key features in SIV-mediated enteropathy (9). Intestinal permeability allows nutrients to pass through the gut, while maintaining a barrier against gut microbiota from leaving the intestine and migrating to the body. Increased permeability due to compromised barrier function could facilitate gut microbiota crossing the mucosal epithelium and entering circulation (microbial translocation) (10). Epithelial injury and impaired epithelial regeneration are considered key factors in the pathogenesis of AIDS contributing to generalized HIV-induced immune cell activation (9, 11, 12).
Interleukin-10 (IL-10), is an important immunomodulatory cytokine and was described as an inhibitory factor for the production of T-helper 1 (Th1) cytokines (13). We have recently demonstrated the role of IL-10 in maintaining the survival of ECs and regulating crypt breadth using colon explant cultures (14). Our study suggested that IL-10 played an obligate role in maintaining mucosal homeostasis by regulating the production of mucosal gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) cytokines. Studies in IL-10-deficient mice and the murine colitis model had shown that maintenance and generation of mucosal IL-10 was crucial to regulate intestinal immune inflammation and to prevent colitis (15). IL-10 signaling is mediated by the interaction of IL-10 and IL-10 receptors (IL-10R) that activate Janus kinase 1 (Jak1) and tyrosine kinase 2 (Tyk2) and eventually upregulate signal transducer and activator of transcription 3 (STAT3), a transcription factor that is essential for anti-inflammatory responses (AIR) (16). A recent report suggests that both STAT3 and IL-10 are essential parts of AIR and cannot be replaced by any other transcription factors or cytokines (17). Suppressors of cytokine signaling (SOCS1 and SOCS3) proteins also bind to Jak or certain cytokine receptors and suppress further signaling events and regulate adaptive immunity (18).
The role of IL-10 in HIV/SIV infection is complex. Upregulation of IL-10 expression in peripheral blood impaired T-cell activation and effector functions, which lead to prolonged HIV persistence in the host (19, 20). Conversely, IL-10 polymorphisms associated with increased IL-10 production might have a protective role against rapid disease progression by suppressing chronic immune activation and reducing CD4 T-cell loss (21). Despite the role of IL-10 as an immunomodulatory cytokine in maintaining the EC barrier in colon explants (14), the main source of IL-10 in intestinal tissues has not been well defined. The role of IL-10 in maintaining intestinal homeostasis during SIV infection, as well as its effect on mucosal IFN-γ and TNF-α cytokines, is also not well understood. Lastly, the role of IL-10 in regulating STAT3- and SOCS3-mediated AIR in SIV-infected intestinal tissues is also not well described.
We have identified the major IL-10-producing cells in the intestine of Indian origin rhesus macaques (InRMs). We also quantified EC apoptosis and identified the expression of IL-10R in mucosal cells. The data suggested that internalization of IL-10R was a key feature in SIV pathogenesis that might be preventing the interaction between IL-10R and IL-10, leading to decreased ability to modulate Th1 responses in the intestine. Furthermore, downregulation of STAT3 and upregulation of SOCS1 and SOCS3 RNA suggested the dysfunction of IL-10-mediated AIR where increased expression of IFN-γ and/or TNF-α produced by mucosal lymphoid cells might be important in inducing EC damage in SIV-infected macaques.
MATERIALS AND METHODS
Animal sampling.
A total of 41 InRMs (Macaca mulatta) of both sexes between 2.5 and 14.1 years of age were grouped into uninfected controls (n = 15), acute infection (n = 14), and chronic infection (n = 12) based on days after SIVMAC251 infection (Table 1). All animals were negative for HIV-2, SIV, type D retrovirus, and STLV-1 infection at the beginning of the study. Animals were housed at the Tulane National Primate Research Center (TNPRC) and were under the care of TNPRC veterinarians in accordance with the standards incorporated in the Guide for the Care and Use of Laboratory Animals. All experiments using InRMs were approved by the Tulane Institutional Animal Care and Use Committee (IACUC). Macaques were socially housed indoors in climate-controlled conditions, were monitored continuously by veterinarians to ensure their welfare, and were fed commercially prepared monkey chow twice daily. The TNPRC environmental enrichment program is reviewed and approved by the IACUC semiannually. Veterinarians at the TNPRC Division of Veterinary Medicine have established procedures to minimize pain and distress through several means. All clinical procedures, including administration of anesthesia and analgesics, were carried out under the direction of a laboratory animal veterinarian. Animals were anesthetized with ketamine hydrochloride for blood collection procedures. Laboratory animal veterinarians performed all surgeries and tissue collections. Animals were preanesthetized with ketamine hydrochloride, acepromazine, and glycopyrolate, intubated, and maintained on a mixture of isoflurane and oxygen. Buprenorphine was given intraoperatively and postoperatively for analgesia.
TABLE 1.
Adult Indian rhesus macaques examined
| Category | Animal no. | Age (yr) | Sexa | Virus | Days of infection | Dose (TCID50 or AID)b | Routec | Terminal plasma viral load (RNA copies/ml) |
|---|---|---|---|---|---|---|---|---|
| Normal | AG71 | 11.1 | F | Nil | ||||
| EI56 | 7.5 | F | Nil | |||||
| EV39 | 6.2 | M | Nil | |||||
| FF15 | 6.9 | F | Nil | |||||
| FK25 | 5.8 | M | Nil | |||||
| FT23 | 5.9 | F | Nil | |||||
| GB61 | 5.6 | M | Nil | |||||
| GI92 | 4.2 | M | Nil | |||||
| GJ06 | 4.9 | F | Nil | |||||
| GN70 | 10.1 | F | Nil | |||||
| GN74 | 13.3 | F | Nil | |||||
| GT20 | 4.6 | M | Nil | |||||
| HN64 | 3.0 | M | Nil | |||||
| HP24 | 3.0 | M | Nil | |||||
| IT16 | 4.6 | M | Nil | |||||
| Acute infection | AV63 | 4.4 | F | SIVMAC251 | 8 | 10* | i.v. | 1,900,000 |
| AV85 | 8.1 | F | SIVMAC251 | 21 | 10* | i.v. | 230,000 | |
| AV91 | 14.1 | M | SIVMAC251 | 10 | 500 | i.v | 157,190,000 | |
| BA17 | 8.3 | M | SIVMAC251 | 13 | 10* | i.v. | 11,000,000 | |
| BA57 | 14 | F | SIVMAC251 | 8 | 500 | i.v. | 14,288,200 | |
| BN37 | 2.5 | M | SIVMAC251 | 21 | 100 | i.v. | 340,000 | |
| CB74 | 3.2 | M | SIVMAC251 | 21 | 10* | i.v. | 12,000,000 | |
| CF65 | 12.3 | F | SIVMAC251 | 21 | 500 | i.vag. | 10,100,000 | |
| EK98 | 8.7 | F | SIVMAC251 | 21 | 500 | i.vag. | 26,800,000 | |
| FT35 | 6.7 | F | SIVMAC251 | 21 | 500 | i.vag. | 3,540,000 | |
| GI28 | 5.9 | F | SIVMAC251 | 21 | 500 | i.vag. | 5,830,000 | |
| HI53 | 6.6 | F | SIVMAC251 | 8 | 100 | i.v. | 3,555,700 | |
| HN29 | 12.6 | F | SIVMAC251 | 10 | 100 | i.v. | 110,000,000 | |
| M992 | 16 | F | SIVMAC251 | 13 | 500 | i.v. | 34,949,800 | |
| Chronic infection | AP53 | 6.4 | F | SIVMAC251 | 63 | 10* | i.v. | 4,100,000 |
| BC35 | 11.9 | F | SIVMAC251 | 422 | 300 | i.vag. | 428,298 | |
| BD03 | 12.8 | F | SIVMAC251 | 167 | 500 | i.vag. | 14,788,890 | |
| CL86 | 11.1 | F | SIVMAC251 | 281 | 500 | i.vag. | 972,889 | |
| DE50 | 9.7 | F | SIVMAC251 | 150 | 500 | i.vag. | 304,000 | |
| DR59 | 6.3 | F | SIVMAC251 | 250 | 1,000 | i.vag. | 288,441 | |
| EB09 | 6.3 | F | SIVMAC251 | 250 | 100 | i.vag. | 750,720 | |
| EJ26 | 6.2 | F | SIVMAC251 | 309 | 100 | i.v. | 397,806 | |
| FK88 | 6.5 | F | SIVMAC251 | 226 | 500 | i.vag. | 2,314,583 | |
| GN91 | 4.9 | F | SIVMAC251 | 401 | 500 | i.vag. | 4,190 | |
| HG58 | 9.1 | F | SIVMAC251 | 288 | 300 | i.vag. | 7,074 | |
| R544 | 7.9 | F | SIVMAC251 | 389 | 500 | i.vag. | 1,700,000 |
F and M represent female and male, respectively.
All values are expressed as the TCID50, except values denoted by an asterisk (*), which are AID values. AID and TCID50 represent the animal infectious dose and tissue culture infectivity dose at 50%, respectively.
i.v. and i.vag. denote intravenous and intravaginal routes, respectively.
Isolation of epithelial cells and IE and LP lymphocytes from the jejunum and colon.
Epithelial cells and lymphocytes from jejunum and colon were isolated as described earlier (9, 22–24). Both intraepithelial (IE) and lamina propria (LP) lymphocytes were isolated from intestinal biopsy specimens, resection surgery, or necropsy tissues. Intestinal lymphocytes were >90% viable as assessed by trypan blue dye exclusion.
Colon explant experiments.
Colon specimens ∼8 cm in length were collected in ice-cold Hanks balanced salt solution and were processed as described previously (14, 25). Mucosal explants were treated with either anti-IL-10 monoclonal antibodies (MAbs; 5 μg/ml; Biolegend), recombinant IL-10 (rIL-10) protein (50 ng/ml; Biolegend), or isotype control (5 μg/ml; Biolegend) for 6 h as reported earlier (14). After incubation, explant cultures were fixed in 2% paraformaldehyde and cryopreserved in 30% sucrose (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS), and frozen in cryomolds containing optimal cutting temperature (OCT) compound (Sakura Fintek, Inc., Torrance, CA) as previously described (9).
Immunodetection and quantitation of tissues.
Tissue sections were processed for immunofluorescent and immunohistochemistry staining with one or a combination of primary antibodies (see Table S1 in the supplemental material) as reported earlier (9). Nuclear staining was performed with ToPro3 (1 μM; Life Technologies). Labeled tissue sections were mounted using Prolong Gold antifade medium (Invitrogen) and imaged using a TCS SP2 confocal laser scanning microscope (Leica, Germany) equipped with an argon-krypton laser at 488 nm (green), a krypton laser at 568 nm (red), and a helium-neon laser at 633 nm (blue). Negative-control slides were incorporated in each experiment either by omitting the primary antibody or using isotype IgG1 and IgG(H+L) controls (9, 14). ImageJ (version 1.49d; National Institutes of Health [NIH], Bethesda, MD) and Adobe Photoshop CS5 Extended (USA) were used to assign colors to the channels collected. For quantification of apoptotic intestinal ECs, active caspase-3 (AC-3+) was detected in a minimum of 20 fields using a Nuance FX multispectral imaging system at a 500- to 720-nm spectral range with colors assigned using Nuance Version 2.10 software (CRi, USA). The data from enterocytes positive for AC-3 were expressed as percentages of the total number of enterocytes (ToPro3+ cytokeratin+). We performed quantitative fluorescence densitometry using ImageJ software to quantify zonula occludens (ZO-1) expression in the colon and jejunum. An average of five high-power fields chosen at random (0.14 mm2/field; five to six optical slices [0.2 μm]/field) were quantified. The intensity of ZO-1 expression was represented as fluorescence pixel values.
To quantify phospho-STAT3 (pSTAT3)-positive cells, slides were stained with pSTAT3 MAbs using a Mach 3 Rabbit AP-Polymer detection kit (Biocare Medical, USA). The negative control consisted of control rabbit Ig fractions (Dako, USA) used at the same concentration as the pSTAT3 MAbs to determine the background staining. Labeling was detected with BCIP/NBT (Dako), followed by nuclear Fast Red counterstaining. The slides were then mounted with Vecta Mount AQ (Vector Laboratories, USA). An average of 20 fields (40× magnification) were manually counted in each of the slides to quantify pSTAT3-positive cells. The sites for all tissue evaluations were selected randomly from each tissue and counted by two different individuals blinded to the samples to avoid bias.
Morphometric analysis.
Paraffin-embedded colon and jejunum specimens from uninfected control, acute, and chronically SIV-infected InRMs were processed for hematoxylin and eosin staining and used for morphometric analysis. Crypt length and breadth in the colon and villus height, crypt length, and villus/crypt ratios in the jejunum were measured by using Image-Pro Plus (v4.5) software as reported earlier (14).
Viral load quantification.
Plasma viral RNA was quantified by either a bDNA signal amplification assay (Siemens Diagnostics, USA) or quantitative reverse transcription-PCR (RT-PCR) at the Wisconsin National Primate Research Center with a lower detection limit of 125 and 60 SIV RNA copies/ml of plasma, respectively (26, 27).
Immunofluorescent staining and flow cytometry analysis.
To examine cell phenotypes and cytokine production, a cytokine flow cytometry assay was used on freshly isolated samples to detect either CD3+ or CD3− lymphocytes spontaneously producing cytokines (IFN-γ, TNF-α, and IL-10) according to the methods previously described (23, 28–30). Briefly, the intestinal IE and LP lymphocytes were resuspended at 106 cells/100 μl, stained with aqua fluorescent reactive Live/Dead stain kit (Life Technologies), and surface stained with directly conjugated MAbs to CD3, CD4, and/or CD45 (see Table S1 in the supplemental material). Cells were washed, fixed, and permeabilized using Cytofix/Cytoperm (BD Biosciences), washed twice in perm buffer (BD Biosciences), and stained with MAbs to IFN-γ, TNF-α, and IL-10 at room temperature for 25 min (see Table S1 in the supplemental material). After being washed in wash buffer, cells were resuspended in 1× BD stabilizing and fixative buffer (BD Biosciences). Whole-blood samples from normal InRMs were used for fluorochrome compensations. For detecting intracellular expression of IL-10R, the cells were fixed and permeabilized using Cytofix/Cytoperm and then stained with IL-10R MAbs (see Table S1 in the supplemental material). Polychromatic (6-10 parameter) flow cytometric acquisition was performed on a BD LSRII instrument. At least 100,000 events were collected from each sample by gating on live cells, and data were analyzed by using FlowJo software (Tree Star, Inc.). For quantifying cytokine (IFN-γ, TNF-α, and IL-10) responses and IL-10R expression, cells were gated on singlets, lymphocytes, followed by live cells, and then on CD3+ T cells and CD3− lymphocytes. CD3+ T cells and CD3− lymphocytes were further analyzed for the presence of IFN-γ-, TNF-α-, IL-10-, and IL-10R-positive cells. The positive staining for each cytokine was determined based on the fluorescent minus one control tubes (see Fig. S1 in the supplemental material). We have performed surface and intracellular IL-10R staining in parallel on different aliquots of the same sample. The percentages of IL-10R expression in cell cytoplasm were determined by subtracting surface expression values from values obtained after intracellular staining. To detect ECs, intracellular staining was performed by using antiepithelial antigen as reported earlier (see Table S1 in the supplemental material) (9).
Quantification of SOCS1, SOCS3, and STAT3 gene expression in intestinal tissues.
Changes in gene expression were determined using relative real-time RT-PCR. Total RNA was extracted from sections of cryopreserved colon explants or jejunum previously embedded in OCT compound using Purelink FFPE RNA Isolation kit (Life Technologies). Briefly, sections were washed twice with PBS to remove OCT. Samples were then incubated at 60°C in melting buffer containing proteinase K and processed according to the manufacturer's protocol. RNA was purified using the RNA Clean & Concentrator kit (Zymo Research, USA) with on-column DNase I treatment (Life Technologies) and finally quantified. Total RNA was converted to cDNA using Invitrogen Superscript III first-strand synthesis system (Life Technologies) with random hexamer primers. Quantification of SOCS1, SOCS3, and STAT3 transcripts was performed using the TaqMan gene expression assays Hs00705164_s1, Hs02330328_s1, and Rh01047585_m1, respectively (Life Technologies). Gene expression was normalized against 18S rRNA expression for each sample using TaqMan 18S rRNA endogenous control assay (Life Technologies) and validation experiments were run to determine target dynamic range and ensure equal amplification efficiencies between all assays. cDNA was amplified in accordance with the manufacturer's protocols on an ABI 7900HT Fast PCR system (Applied Biosystems). The relative gene expression was determined across treatments using the comparative threshold cycle (CT) method. Acutely and chronically SIV-infected jejunum samples were calibrated to the mean of the uninfected controls. Colon explant samples were calibrated to the mean of the media control. Fold changes in the expression were calculated using 2−ΔΔCT (31).
Statistics.
Graphical presentation and statistical analysis of the data were performed using GraphPad Prism (v5.0f; GraphPad Software, CA). The results between experimental groups were compared using a one-way analysis of variance and the nonparametric Mann-Whitney t test. P values of <0.05 were considered statistically significant.
RESULTS
Early intestinal epithelial cell apoptosis and loss of tight junction protein are characteristics of SIV infection.
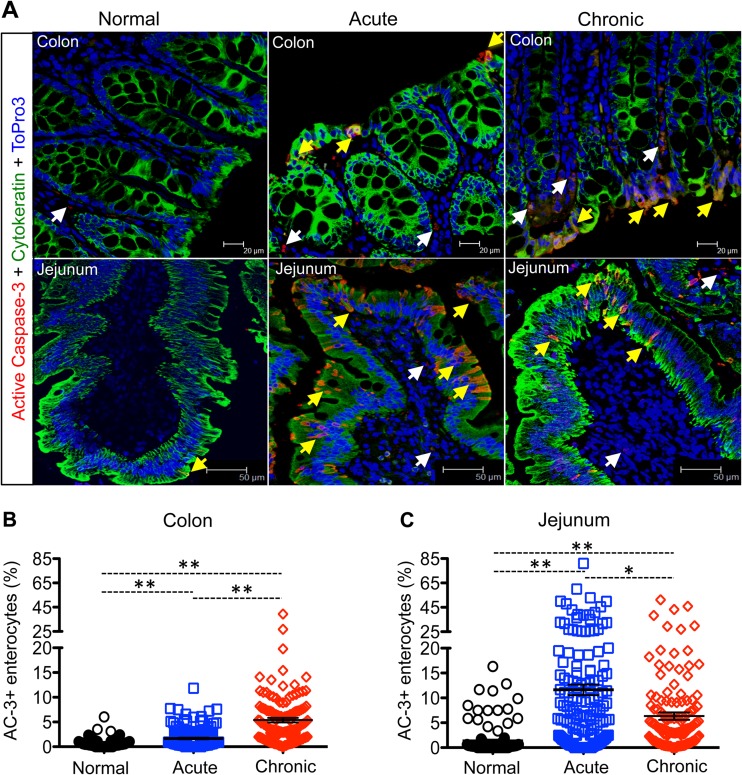
We quantified the percentages of apoptotic ECs (AC-3+ cytokeratin+) in uninfected controls and acutely and chronically SIV-infected InRMs to evaluate the integrity of the intestinal epithelial barrier (Fig. 1). In colon, a significant increase in EC apoptosis compared to controls was detected at acute and chronic stage of infection (P < 0.001, Fig. 1A and B). We also observed a significant increase in apoptosis of jejunal ECs in both acutely and chronically SIV-infected macaques compared to uninfected controls (P < 0.001; Fig. 1A and C). Although apoptosis of jejunal ECs in chronically infected animals was decreased significantly compared to the acute phase (P < 0.005), it was still significantly elevated compared to uninfected control macaques (Fig. 1A and C). To determine how early EC apoptosis occurs during SIV infection, we compared percentages of EC apoptosis of colon and jejunum in animals from 8 to 21 days postinfection (dpi) time points. In colon, increased EC apoptosis was detected from 10 dpi onward compared to uninfected controls, whereas in one animal (BA57), the EC apoptosis was detected as early as 8 dpi (see Table S2 in the supplemental material). However, increased EC apoptosis was observed in jejunum as early as 8 dpi, and the number of apoptotic enterocytes increased further at 21 dpi compared to uninfected controls (see Table S2 in the supplemental material). No correlation between plasma viral load and mean percentages of apoptotic ECs in either colon or jejunum from SIV-infected macaques was found by linear regression analysis (see Fig. S2 in the supplemental material).
FIG 1.
Intestinal epithelial cell (EC) apoptosis increases during SIV infection. (A) Representative immunofluorescence analysis of the colon and jejunum showing increased apoptotic ECs (active caspase-3 [AC-3]+ cytokeratin+) in colon at the chronic phase (colon, R544 at 389 dpi; jejunum, FK88 at 226 dpi) and acute phase (AV85 at 21 dpi) compared to uninfected control macaques (colon, AG71; jejunum, GN74). Apoptotic ECs and lamina propria cells are indicated by yellow and white arrows, respectively. Scatter plots (indicating means ± the standard errors) of apoptotic enterocytes are shown for both the colon (B) and jejunum (C) in normal (n = 6) and in acutely (n = 8) and chronically (n = 7) SIV-infected macaques. Asterisks indicate statistically significant differences between the respective animal groups (*, P < 0.005; **, P < 0.001).
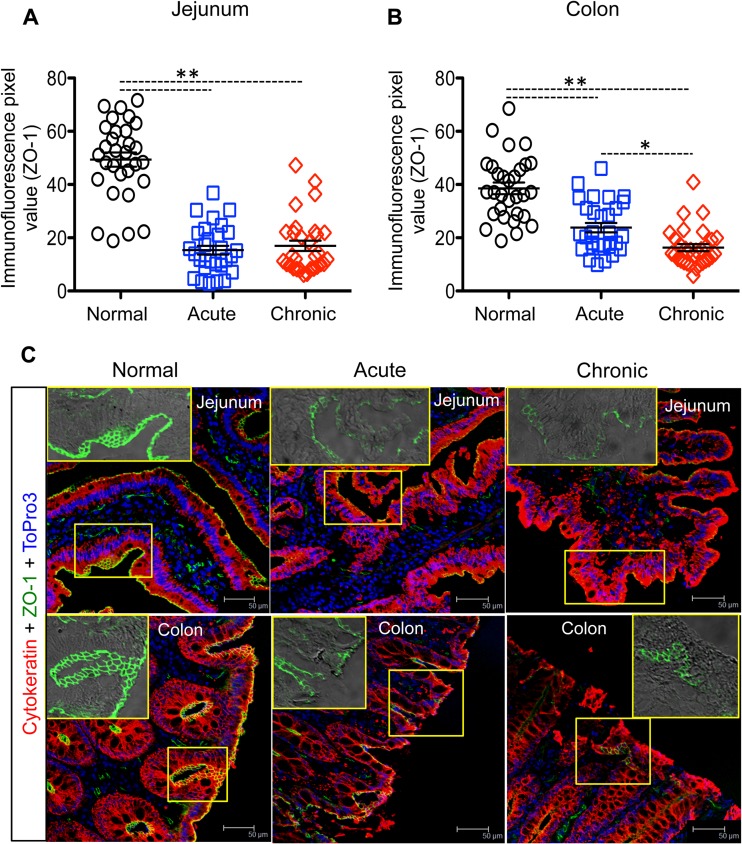
To evaluate the integrity of the intestinal epithelial barrier, we analyzed ZO-1 expression in intestinal tissues. Expression of ZO-1 protein was significantly reduced in both jejunum and colon in both acutely and chronically SIV-infected macaques compared to uninfected controls (P < 0.0001; Fig. 2A and B). In addition to an overall loss of ZO-1 expression the pattern of staining also changed from typical honeycomb pattern to a more discontinuous staining pattern (Fig. 2C). In the colon but not in the jejunum there was also a significant difference in ZO-1 expression between the acute and chronic stages of SIV infection (P < 0.001; Fig. 2B), suggesting different dynamics in ZO-1 expression between jejunum and colon. Together, the data clearly demonstrated the loss of epithelial barrier integrity in jejunum and colon beginning during SIV infection. Despite the observation that ZO-1 protein was simultaneously downregulated both in the jejunum and the colon, EC apoptosis was more evident in the small intestine than in the large intestine during acute SIV infection.
FIG 2.
Decreased expression of ZO-1 in SIV-infected macaques. Immunofluorescence pixel values of ZO-1 expression (indicating means ± the standard errors) in jejunum (A) and colon (B) from normal and acutely and chronically infected animals are shown (n = 6). Asterisks indicate statistically significant differences between the respective animal groups (*, P < 0.001; **, P < 0.0001). (C) Representative immunofluorescent confocal images in the jejunum and colon revealed a loss of ZO-1 expression in acutely (BA57 at 8 dpi) and chronically (AP53 at 63 dpi) SIV-infected macaques (scale bars, 50 μm) compared to normal uninfected macaques (jejunum, GN74; colon, GN70). Insets for each panel show patterns of ZO-1 expression alone. The apical mucosal surfaces of jejunum and colon exhibit ZO-1 with a typical honeycomb structure in normal uninfected animals.
To further assess alteration in intestinal structure during SIV infection, we measured jejunum villus height and crypt length, as well as the colonic crypt (length and breadth), in normal and SIV-infected macaques. In the jejunum significant increases in villus height and crypt length were detected during acute SIV infection (see Fig. S3 in the supplemental material). In contrast, a significant decrease in colon crypt length was observed during acute SIV infection (see Fig. S3 in the supplemental material). No significant differences in jejunum villus height or colonic measurements were observed in chronically SIV-infected animals compared to uninfected controls (see Fig. S3 in the supplemental material).
Multiple cell types in intestinal tissues produce IL-10.
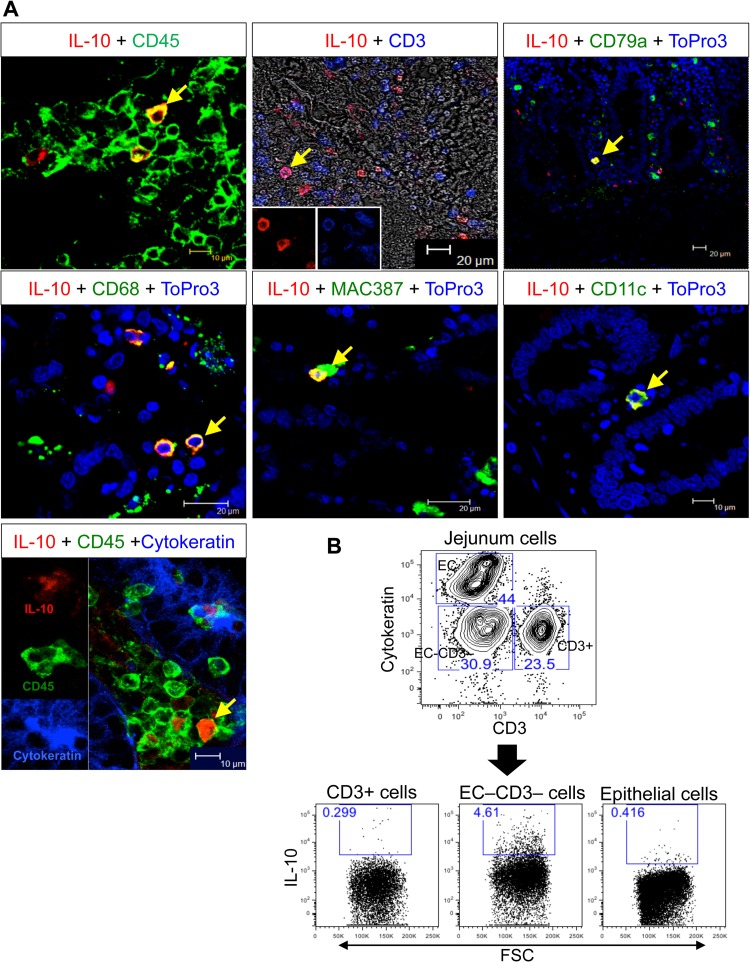
Since prior experiments demonstrated that IL-10 is important in maintaining normal EC function (14), we assessed the sources of IL-10 in the colon and jejunum from InRMs. Interestingly, by multilabel confocal microscopy, IL-10 was most commonly identified in cells expressing CD45 (leukocyte common antigen). This included monocytes/macrophages (CD68+, Mac387+), T cells (CD3), B cells/plasma cells (CD79a) (32), dendritic cells (CD11c) (Fig. 3A) with IL-10 expression by macrophages being the most common. Although we were not able to detect IL-10 expression in ECs using confocal microscopy, by flow cytometry we could detect IL-10 expression by a small percentage of ECs. Flow cytometry also showed that majority of the IL-10-positive cells were cytokeratin-negative, CD3-negative cells, in agreement with the confocal microscopy data (Fig. 3).
FIG 3.
Major IL-10-producing cells in intestinal tissues. (A) Expression of IL-10 by multilabel confocal microscopy in multiple cell types, including leukocytes (CD45+), T cells (CD3+), B cells/plasma cells (CD79a+), monocytes/macrophages (CD68+, Mac387+), dendritic cells (CD11c+), and epithelial cells (EC, cytokeratin+). The labels of each image are indicated at the top of each image. IL-10+ cells in lamina propria are indicated by yellow arrows. (B) Flow cytometry of cells isolated from the lower layer of a Percoll density gradient after EDTA treatment of the jejunum analyzed for the expression of cytokeratin, CD3, and IL-10. All cells were gated on singlets, followed by live cells, and then plotted based on CD3+ T cells and cytokeratin+ cells. Each gated cell population was further analyzed for IL-10 production as represented by dot plots. The data are representative of a normal uninfected InRM (i.e., macaque DE50, at the pre-SIV infection time point).
SIV infection induced increased levels of IFN-γ and IL-10 cytokines in intestinal tissues.
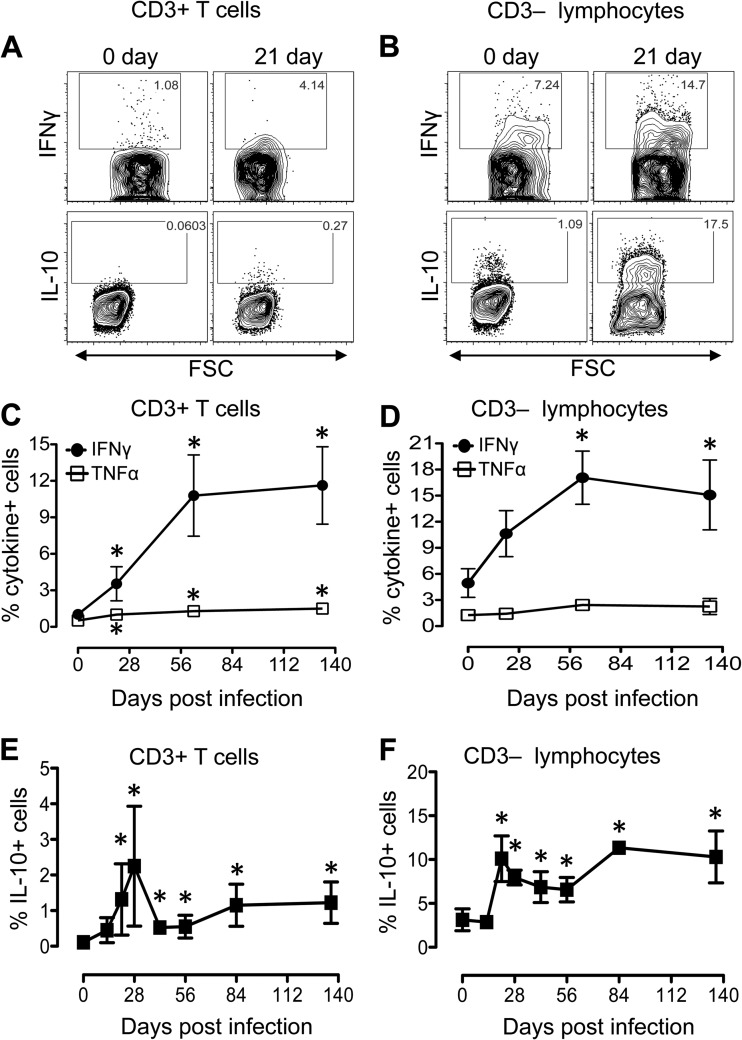
Earlier studies indicated that plasma IL-10 protein levels were elevated in acute HIV infection, and their levels positively correlated with plasma viral load and diminished after antiretroviral therapy (19, 33). However, the magnitude of IL-10 production in intestinal tissues has not been well documented. Here, we investigated constitutively produced IFN-γ, TNF-α, and IL-10 in CD3+ and CD3− lymphocytes isolated from jejunum LP of eight macaques (BD03, CL86, DE50, DR59, EB09, EJ26, FK88, and GN91; all listed in Table 1) at different time points after SIV infection. In all eight InRMs the plasma viral load peaked (mean vRNA load of log 107.2/ml) at 14 dpi and remained high (ranging between log10 106.0 and log10 107.1/ml of plasma) throughout the period of our study. Prior to SIV infection, a greater percentage of CD3− LP lymphocytes were found to be capable of producing IFN-γ, TNF-α, and IL-10 compared to CD3+ T lymphocytes (Fig. 4). After SIVMAC251 infection, the production of IFN-γ increased significantly in both CD3+ T cells and CD3− lymphocytes, whereas the level of TNF-α increased significantly only in CD3+ T cells (Fig. 4A to D).
FIG 4.
SIV infection induces increased levels of IFN-γ, TNF-α, and IL-10 cytokines in both CD3+ and CD3− lymphocytes in the jejunum lamina propria. (A) Representative contour plots showing differential expression of IFN-γ and IL-10 from one SIV-infected macaque (EB09) at days 0 and 21 dpi in CD3+ T cells (A) and CD3− lymphocytes (B). Mean percentages (± the standard errors) of CD3+ T cells (C) and CD3− lymphocytes (D) isolated from jejunum lamina propria producing IFN-γ and TNF-α cytokines are shown for SIV-infected macaques (n = 8). Increased production of IL-10 (mean ± the standard errors) was also detected in both CD3+ T cells (E) and CD3− lymphocytes (F) isolated from the jejunum lamina propria (n = 8). Asterisks indicate significant differences between day 0 and the specified time point (P < 0.05). All cells were gated on singlets, lymphocytes, followed by live cells, and then on CD3+ T cells and CD3− lymphocytes.
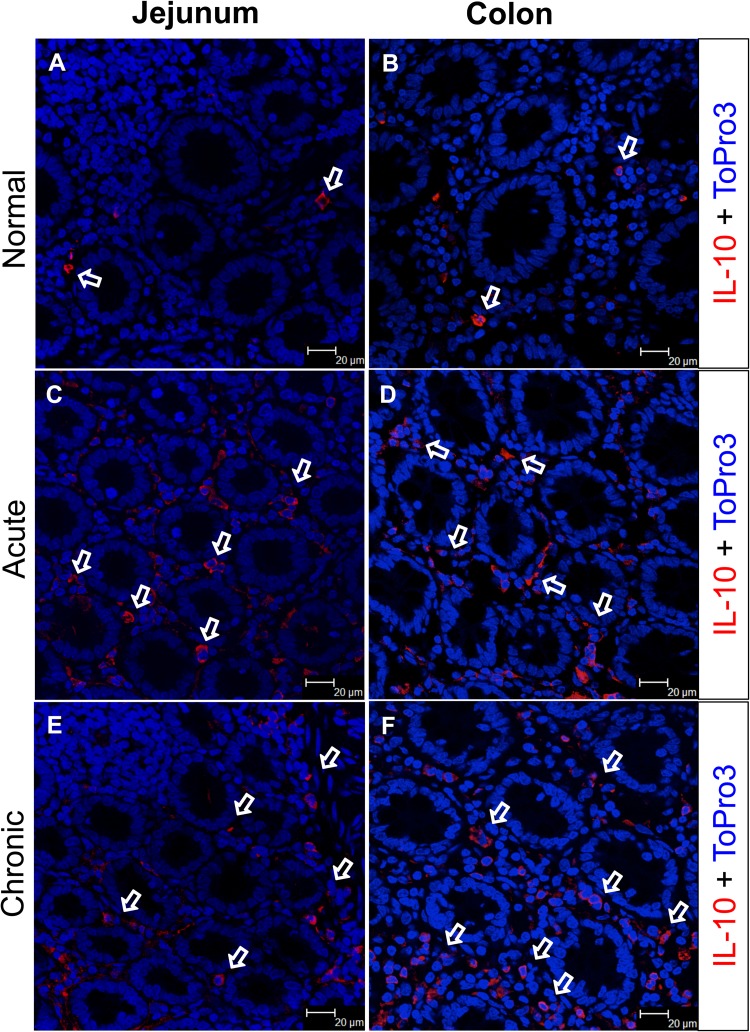
In both CD3+ T cells and CD3− lymphocytes, the percentages of IL-10+ cells increased significantly compared to preinfection in all SIV-infected InRMs (Fig. 4A, B, E, and F). Interestingly, higher percentages of IL-10-producing cells were observed in CD3− lymphocytes compared to CD3+ T cells. Immunofluorescent staining for IL-10 also confirmed increased production of IL-10 in the jejunum and colon during acute and chronic SIV infection (Fig. 5). The majority of IL-10-positive cells were present in the LP rather than within the intestinal epithelium in SIV-infected macaques. Despite significantly elevated levels of IL-10 expression by intestinal LP lymphocytes, there was also an increased proportion of IFN-γ- and TNF-α-expressing lymphocytes, suggesting a possible dysregulation of IL-10 signaling pathways in regulating IL-10-mediated immunosuppression.
FIG 5.
IL-10 expression increased in the intestinal lamina propria during SIV infection. IL-10 expression by lamina propria cells was visualized by immunofluorescence in the jejunum (A, C, and E) and colon (B, D, and F). Both jejunum and colon lamina propria cells (A and B) spontaneously express IL-10 in a normal uninfected animal (GT20). However, after SIV infection both the jejunum and colon demonstrated increased percentages of IL-10-producing cells in acute (C and D; HN29) and chronic (E and F; CL86) infections. White arrows denote the presence of IL-10-positive cells, with the majority distributed in the lamina propria region.
Evidence for IL-10 receptor (IL-10R) internalization during SIV infection.
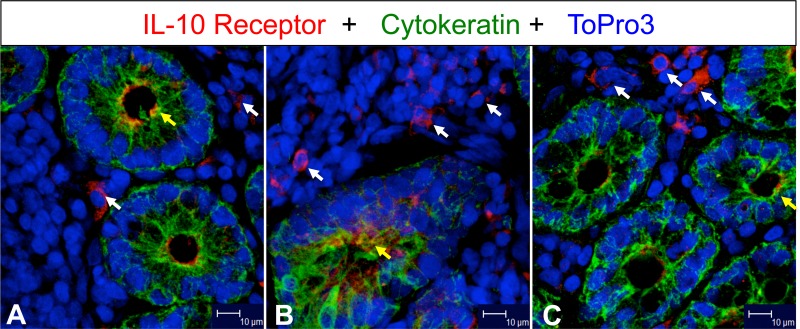
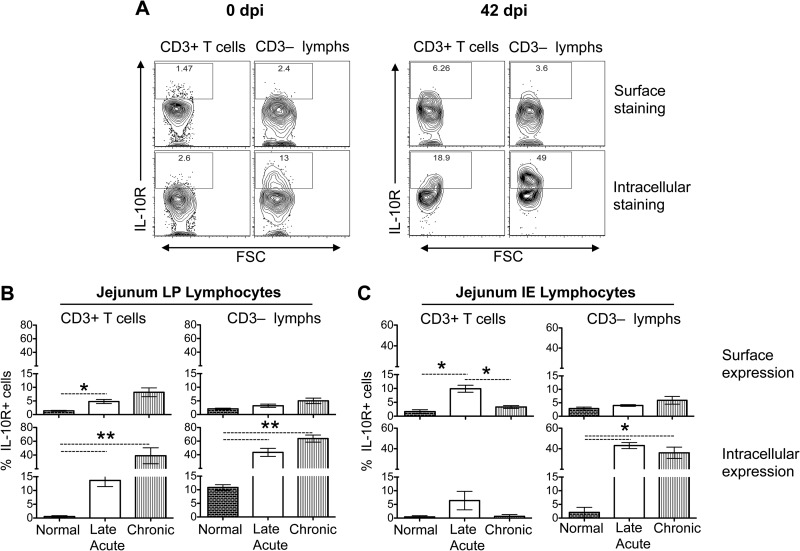
IL-10 has been shown to mediate anti-inflammatory activity in cells by interacting with IL-10R expressed on the cell surface (34). Increased IL-10R expression was detected by confocal microscopy in jejunum during SIV infection (Fig. 6). We also measured IL-10R expression on both cell surface and cell cytoplasm by flow cytometry on intestinal ECs, CD3+ T cells, and CD3− lymphocytes isolated from the LP and IE lymphocytes of uninfected controls (0 dpi) and SIV-infected animals in late acute (42 dpi), and chronic (150 to 281 dpi) time points (Fig. 7). No significant changes in surface (range, 0.6 to 3.3%) or intracellular IL-10R (range, 0.2 to 6.5%) expression in ECs between pre- and post-SIV infection time points were observed. A significant increase in surface IL-10R expression was observed in early infection in jejunum LP and IE CD3+ lymphocytes (P < 0.05). However, there were no significant differences in surface IL-10R expression during chronic SIV infection compared to uninfected controls (Fig. 7B and C). Increased intracellular IL-10R expression was detected in jejunum LP and IE CD3− lymphocytes during the early stage of SIV infection that was maintained through chronic infection (Fig. 7B and C). A significant difference in intracellular IL-10R expression was evident in both early and chronically SIV-infected macaques compared to macaques at their preinfection time points both in CD3+ T cells and CD3− LP lymphocytes (Fig. 7B). A significant increase in IL-10R surface expression was detected in jejunum LP CD3+ T cells at acute infection; however, the expression was much lower than the intracellular expression of IL-10R detected during acute and chronic infection in LP CD3+ T cells and IE CD3− lymphocytes (Fig. 7B and C). Our findings suggested that increased internalization of IL-10R in mucosal lymphocytes during SIV infection might be interfering with IL-10 responsiveness despite increased IL-10, which ultimately leads to uncontrolled mucosal immune activation (increased IFN-γ and TNF-α cytokines) and apoptosis of ECs.
FIG 6.
IL-10 receptor (IL-10R) expression increased in jejunum during SIV infection. White and yellow arrows indicate expression of IL-10R positive mononuclear cells in lamina propria and enterocytes, respectively, in jejunum from an uninfected control (A; GJ06) and from acutely (B; CF65 at 21 days postinfection) and chronically (C; AP53 at 63 days postinfection) SIV-infected macaques. Note that increased IL-10R expression was detected in both acutely and chronically SIV-infected macaques in lamina propria mononuclear cells.
FIG 7.
SIV infection induces increased internalization of IL-10 receptors (IL-10R) in intestinal lymphoid cells compared to the expression of IL-10R on the cell surface. (A) Representative contour plots showing IL-10R expression after surface or intracellular staining in CD3+ T cells and CD3− lymphocytes in jejunum lamina propria (LP) lymphocytes from one SIV-infected macaque (EB09) at days 0 and 42 dpi. Bar charts showing the mean (± the standard errors) IL-10R surface and intracellular expression in CD3+ T cells and CD3− lymphocytes from both LP (B) and IE lymphocytes (C) derived from the jejunum tissues of normal, uninfected macaques (0 dpi) and late acutely (42 dpi) and chronically (150 to 281 dpi) SIV-infected macaques (n = 8). All cells were gated on singlets, lymphocytes, followed by live cells, and then on CD3+ T cells and CD3− lymphocytes. The percentages of IL-10R expression in cell cytoplasm were calculated by subtracting values of surface staining from values obtained from intracellular staining. Asterisks indicate statistically significant differences between the respective animal groups (*, P < 0.05; **, P < 0.005).
Upregulation of SOCS1 and SOCS3 and downregulation of STAT3 and pSTAT3 expressions occur in the jejunum during SIV infection.
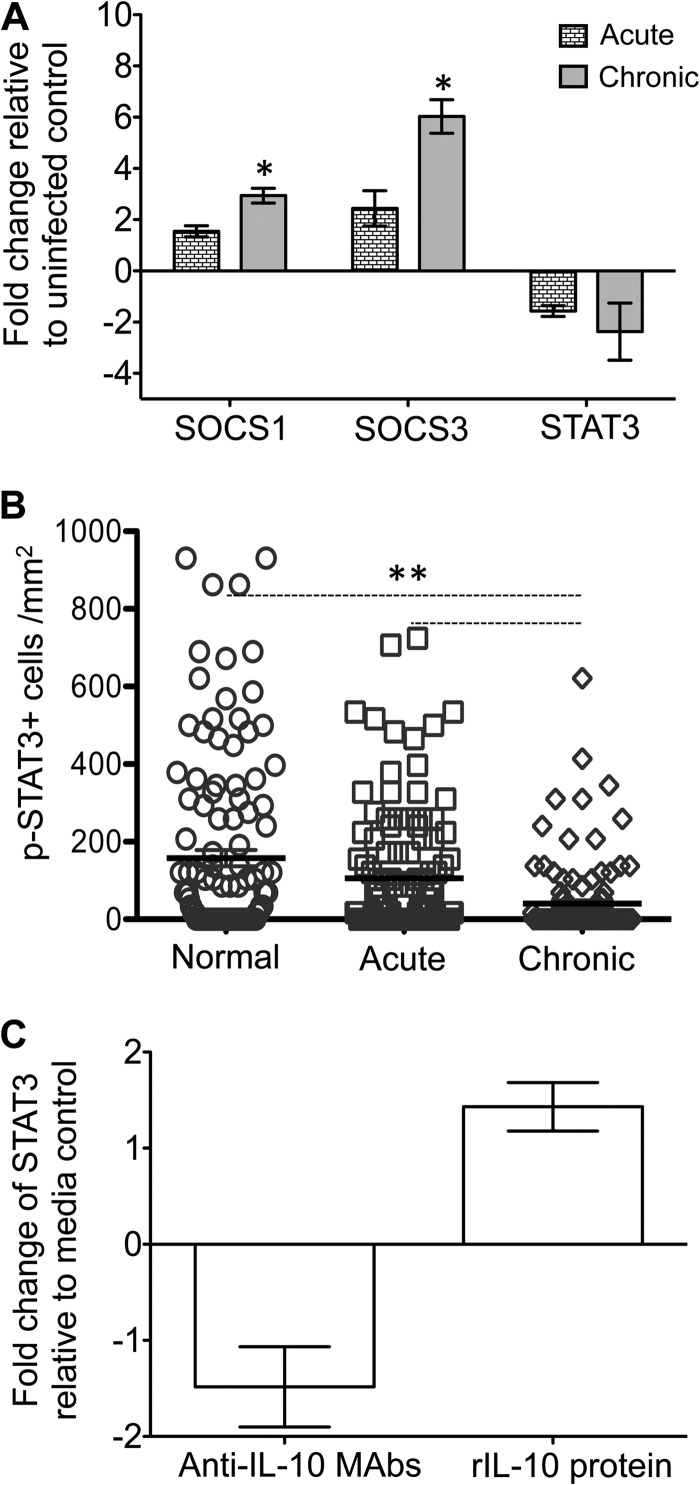
IL-10 initiates the AIR by the upregulation of STAT3 expression and its phosphorylation. SOCS1 and SOCS3 play a negative regulatory role in STAT3 activation and thereby suppressing further signaling events. We are interested to understand the expression of these key regulators in regulating IL-10-mediated AIR in normal and SIV-infected InRMs. During acute infection, SOCS1 and SOCS3 were upregulated 1.5- and 2.4-fold (mean values), respectively, compared to the normal uninfected groups; however, neither of these changes was statistically significant (Fig. 8A). At chronic time points, significant upregulation of both SOCS1 (P = 0.005) and SOCS3 (P < 0.001) was observed compared to normal uninfected InRMs. Conversely, STAT3 was downregulated in both the acutely (mean, 1.6-fold) and the chronically (mean, 2.4-fold) SIV-infected groups; however, neither of these changes was statistically significant (P > 0.05). We also measured pSTAT3 protein expression in the same group of normal uninfected and acutely and chronically SIV-infected InRMs, and a significant decrease in pSTAT3 expression detected in chronically SIV-infected InRMs (Fig. 8B; see Fig. S4 in the supplemental material), suggesting that IL-10-mediated STAT3 upregulation and immunoregulation were defective in SIV-infected macaques.
FIG 8.
Expression patterns of SOCS1, SOCS3, STAT3, and pSTAT3 in SIV-infected macaques and colon explant cultures. (A) Increased fold change of SOCS1 and SOCS3 and decreased fold change of STAT3 gene expression were observed in jejunum from acute and chronically SIV-infected rhesus macaques compared to uninfected normal macaques using relative RT-PCR (mean ± the standard error; n = 6). Samples were normalized against 18S rRNA expression. (B) Scatter plots (with means ± the standard errors) of pSTAT3+ cells in uninfected normal macaques and acutely and chronically SIV-infected macaques quantified by immunohistochemistry staining are shown (n = 6; a minimum 20 fields were measured for each sample). (C) The relative fold changes (means ± the standard errors) of STAT3 gene expression are indicated for colon explants in the presence of either anti-IL-10 MAbs or recombinant IL-10 (rIL-10) protein as determined using relative RT-PCR (n = 3). Asterisks indicate significant differences between groups for the specified genes and proteins (*, P ≤ 0.005; **, P < 0.0001).
Anti-IL-10 MAbs treatment induces STAT3 downregulation in colon explants.
To understand the role of IL-10 in regulating STAT3 signaling in colon explants, we have treated normal colon explants with either anti-IL-10 MAbs or rIL-10 protein and measured STAT3 expression in explant cultures. Endogenous IL-10 blocking with anti-IL-10 MAbs and rIL-10 protein treatment downregulated (1.5-fold) and upregulated (1.4-fold) STAT3 expression, respectively, compared to medium controls (Fig. 8C). However, these changes were not statistically significant.
DISCUSSION
IL-10 is an important immunoregulatory cytokine that plays a key role in the control of inflammation, function, and homeostasis of the gastrointestinal immune system (35, 36). Despite the report of increased IL-10 mRNA expression in intestinal tissues from HIV-infected patients with or without antiretroviral therapy (37, 38), the role of IL-10 in regulating mucosal cytokine production and its effect on maintaining the EC barrier and integrity in HIV/SIV infection are not well described. In our recent study, we demonstrated the role of IL-10 in maintaining the survival of ECs and regulating crypts breadth using rhesus colon explant cultures (14). The present studies used a multiplatform experimental approach using both in vivo SIV-macaque studies and ex vivo colon explant cultures to delineate the mechanism by which IL-10-mediated SIV enteropathy develops. The data indicated that increased internalization of IL-10R, upregulation of SOCS1 and SOCS3 and downregulation of STAT3 expression in the intestine of SIV-infected macaques might play a key role in IL-10 unresponsiveness in regulating cytokines, including IFN-γ and TNF-α, that are thought to be involved in compromising epithelial barrier function and EC apoptosis.
Early EC apoptosis was detected during acute SIV infection in both small and large intestines, a finding which is in agreement with those of others (39); however, the EC apoptosis was more pronounced in the jejunum compared to colon tissues. Increased EC apoptosis during acute SIV infection might be due to the imbalance between crypt repair and EC regeneration that was maintained during chronic SIV infection. The majority of EC apoptosis in jejunum was detected in villus tips, where the cells are more differentiated mature cells compared to the cells in crypts, where the cells were either new or stem cells (40). However, we did not observe any significant differences in villus height and crypt length ratio between normal and acutely SIV-infected InRMs until the animals were in there chronic phase of infection, which was in agreement with other studies (41). We also did not find any significant morphological changes in jejunum and colon due to opportunistic infection in either acute or chronic infection as reported elsewhere (data not shown) (42).
Considering the marked apoptosis of jejunum ECs during acute SIV infection, it was presumed that epithelial integrity was compromised. In our study, we observed not only a loss of tight-junction protein ZO-1 in the jejunum but also in the colon during acute infection. Loss of ZO-1 expression appeared along with the loss of ECs in intestinal tissues during acute infection, suggesting a possible direct correlation between the loss of tight junction protein and with the upregulation of IFN-γ and TNF-α cytokine responses, as reported earlier (43). Loss of epithelial integrity has also been demonstrated by the reduction of claudin-3 tight junction protein and epithelial resistance and/or increased mucosal mannitol permeability in colon and jejunum tissues, respectively, during acute and chronic SIV/HIV infection (6, 44). Early disruption of the epithelial barrier in both jejunum and colon was in agreement with an earlier report which suggests that microbial translocation had started both in small and large intestines during the acute phase and was more pronounced during chronic SIV infection (44).
We observed increased expression of proinflammatory (TNF-α), inflammatory (IFN-γ), and anti-inflammatory (IL-10) cytokine in mucosal CD3+ T cells and CD3− lymphocytes. We also identified LP leukocytes, EC-negative CD3-negative cells, and macrophages/monocytes as the major source of IL-10 in intestinal tissues, in contrast to an earlier report wherein intestinal ECs were shown to be the major IL-10-producing cells in intestinal explants (45). A recent report also indicated that progressive SIV infection favors local expansion of mucosal IL-10+ regulatory T cells within the mucosal CD4+ T-cell population (46). An appropriate balance between inflammatory and anti-inflammatory cytokine responses are crucial for maintenance of a successful immune response against infectious diseases, specifically by limiting tissue damage and autoimmunity (47).
The present study showed that despite increased production of mucosal immunosuppressing IL-10-positive cells in CD3+ T and CD3− lymphocytes, IL-10 apparently lost its effectiveness in controlling inflammation, resulting in increased upregulation of proinflammatory (TNF-α) and Th1 (IFN-γ) cytokines in SIV-infected macaques, suggesting a failure of the cytokine balance in the mucosal environment which induced EC apoptosis. Despite an overall increased IL-10 expression in mucosal tissues, no significant changes in IL-10R surface expression was detected in CD3-negative lymphocytes between normal and SIV-infected macaques. Significant upregulation in surface IL-10R expression in jejunum LP and IE CD3+ T cells was detected during early SIV infection, but the fold increase in surface IL-10R expression was much lower than the fold increase in intracellular IL-10R expression. The increased expression of IL-10R in response to the higher levels of IL-10 associated with increased IL-10/IL-10R binding and increased ligand-dependent IL-10R internalization occurred during SIV infection but were apparently insufficient to regulate the production of TNF-α and IFN-γ cytokines. Upregulation of surface IL-10R expression is essential for IL-10-mediated cytokine modulation and was shown in both in vitro and in vivo models (48–51). Ligand-dependent IL-10R internalization and proteasome-mediated proteolysis were key factors in downregulating IL-10 signaling in tissues (52). Mutation in IL-10R genes resulting in impaired IL-10 signaling had also been associated with severe inflammation at mucosal site in inflammatory bowel disease (53, 54). Cytokines such as IFN-γ and TNF-α, based on their target cells, can induce antiviral immune responses or may damage the mucosal surfaces. Our recent ex vivo colon explant studies also suggested the role of increased IFN-γ and TNF-α cytokines in inducing intestinal LP lymphocytes and EC apoptosis (14). Therefore, increased mucosal IFN-γ and TNF-α cytokines detected in SIV-infected InRMs may lead to increased intestinal EC and LP lymphocytes apoptosis and may potentially compromise intestinal epithelial barrier and integrity. Increased inflammation and loss of ECs in inflammatory bowel disease was also mediated by increased effector T cells producing IFN-γ and TNF-α (55). It might be of interest to determine whether polymorphisms of IL-10R may enhance the internalization and degradation of IL-10R and whether this has an impact on IL-10-mediated cytokine regulation in HIV/SIV pathogenesis. A recent report showed that zymosan, a cell wall preparation from Saccharomyces cerevisiae, activated inflammatory pathways by internalization of surface IL-10R and increased the production of TNF-α (47). Conceivably, one or more of the HIV/SIV protein(s) may interfere with the increased expression of IL-10R on the cell surface or accelerate the internalization of IL-10R expression and dysregulate IL-10/IL-10R signaling.
STAT3 plays important roles not only in the IL-10-mediated AIR but is also involved in modulating several cellular functions. STAT3 can be proinflammatory or anti-inflammatory based on the cytokines responsible for the signaling. If the cells are stimulated by IL-6, STAT3 acts as a proinflammatory transcription factor and if the same cells are stimulated with IL-10, STAT3 works as anti-inflammatory transcription factor (17). Our in vitro colon explant culture blocking with anti-IL-10 MAbs supported the principal that decreased mucosal IL-10 signaling interferes with STAT3 expression. Reduced expression of STAT3 transcription factors and phosphorylated STAT3 proteins in acutely and chronically SIV-infected macaques suggested the lack of IL-10-mediated STAT3 activation, as well as lack a of AIR in mucosal tissues. SOCS proteins were identified as an important negative regulator of the cytokine-Jak-STAT pathway (18, 56). Upregulation of SOCS3 suppressed inflammatory conditions in inflammatory arthritis by inhibiting STAT3, with the idea that the IL-6- and IL-6-related cytokine-STAT3 pathway promotes inflammatory conditions (57). If STAT3 plays a protective anti-inflammatory role, the presence of increased SOCS1 and SOCS3 transcription factors determines its role as an inflammatory modulator. In the present study, we also observed increased expression of SOCS1 and SOCS3 (a significant increase in chronically SIV-infected animals) and decreased STAT3 and pSTAT3 (significant decrease in chronically SIV-infected animals) expression suggestive of the defect of IL-10-mediated STAT3 upregulation and the failure of AIR in mucosal tissues.
Several different potential mechanisms of intestinal EC apoptosis in SIV/HIV infection have been proposed, including virus-induced apoptosis mediated by the interaction of SIV and GPR15/Bob, an epithelial associated coreceptor for HIV-1/SIV (39), increased infiltration of perforin-positive mucosal cytotoxic T cells (6), upregulation of inflammatory cytokines by direct interaction of ECs with HIV-1 envelope protein (58), and viral protein- and cytokine-induced endoplasmic reticulum stress (12). In conclusion, this investigation clearly indicated that internalization of IL-10R was a key feature in SIV pathogenesis where the interaction between surface IL-10R and IL-10 might be important in regulating the proinflammatory and Th1 function in SIV-infected InRMs. Furthermore, downregulation of STAT3 and upregulation of SOCS1 and SOCS3 RNA suggested the dysfunction of IL-10-mediated AIR, where increased expression of IFN-γ and/or TNF-α produced by mucosal lymphoid cells might be responsible for inducing EC damage in SIV-infected macaques. Further studies are needed to explore the factor(s) that induce internalization of IL-10R that appear to play a leading role in regulating IL-10-mediated anti-inflammatory activity to maintain mucosal epithelial barrier integrity in SIV/HIV pathogenesis.
Our results provide a potential framework for further study to understand the virus factor(s) that play a key role in modulating IL-10 and IL-10R signaling in SIV/HIV pathogenesis and perhaps will lead to therapeutic strategies that can prevent early epithelial damage in acute and chronic HIV infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maury Duplantis and all animal care staff of the department of veterinary medicine for their technical assistance.
The study was supported by NIH grants P20 GM103458-09, R21 AI080395 (B.P.), and P51 RR00164-51 and the National Center for Research Resources and the Office of Research Infrastructure Programs of the National Institutes of Health through grant OD011104.
Footnotes
Published ahead of print 27 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01757-14.
REFERENCES
- 1.Batman PA, Kapembwa MS, Miller AR, Sedgwick PM, Lucas S, Sewankambo NK, Serwadda D, Pudney J, Moody A, Harris JR, Griffin GE. 1998. HIV enteropathy: comparative morphometry of the jejunal mucosa of HIV-infected patients resident in the United Kingdom and Uganda. Gut 43:350–355. 10.1136/gut.43.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SG, Menzies IS, Lee CA, Johnson MA, Pounder RE. 1993. Intestinal permeability and function in patients infected with human immunodeficiency virus: a comparison with coeliac disease. Scand. J. Gastroenterol. 28:573–580. [DOI] [PubMed] [Google Scholar]
- 3.Ott M, Lembcke B, Staszewski S, Helm EB, Caspary WF. 1991. Intestinal permeability in patients with acquired immunodeficiency syndrome (AIDS). Klin. Wochenschr. 69:715–721 (In German.) 10.1007/BF01649441. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 5.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, Dandekar S. 2008. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 82:538–545. 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epple HJ, Allers K, Troger H, Kuhl A, Erben U, Fromm M, Zeitz M, Loddenkemper C, Schulzke JD, Schneider T. 2010. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology 139:1289–1300. 10.1053/j.gastro.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 7.Heise C, Miller CJ, Lackner A, Dandekar S. 1994. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169:1116–1120. 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 8.Cummins AG, LaBrooy JT, Stanley DP, Rowland R, Shearman DJ. 1990. Quantitative histological study of enteropathy associated with HIV infection. Gut 31:317–321. 10.1136/gut.31.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan D, Das A, Liu D, Veazey RS, Pahar B. 2012. Isolation and characterization of intestinal epithelial cells from normal and SIV-infected rhesus macaques. PLoS One 7:e30247. 10.1371/journal.pone.0030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tremaroli V, Backhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249. 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 11.Mohan M, Kaushal D, Aye PP, Alvarez X, Veazey RS, Lackner AA. 2013. Focused examination of the intestinal epithelium reveals transcriptional signatures consistent with disturbances in enterocyte maturation and differentiation during the course of SIV infection. PLoS One 8:e60122. 10.1371/journal.pone.0060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maingat F, Halloran B, Acharjee S, van Marle G, Church D, Gill MJ, Uwiera RR, Cohen EA, Meddings J, Madsen K, Power C. 2011. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB J. 25:2211–2220. 10.1096/fj.10-175992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentino DF, Bond MW, Mosmann TR. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan D, Das A, Lala W, Kenway-Lynch CS, Liu DX, Veazey RS, Pahar B. 2013. Interleukin-10 prevents epithelial cell apoptosis by regulating IFNγ and TNFα expression in rhesus macaque colon explants. Cytokine 64:30–34. 10.1016/j.cyto.2013.06.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosser DM, Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226:205–218. 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchins AP, Diez D, Miranda-Saavedra D. 2013. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief. Funct. Genomics 12:489–498. 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. 2012. SOCS, inflammation, and autoimmunity. Front. Immunol. 3:20. 10.3389/fimmu.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. 2009. IL-10 is upregulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356. 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP. 2010. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16:452–459. 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naicker DD, Wang B, Losina E, Zupkosky J, Bryan S, Reddy S, Jaggernath M, Mokgoro M, Goulder PJ, Kaufmann DE, Ndung'u T. 2012. Association of IL-10-promoter genetic variants with the rate of CD4 T-cell loss, IL-10 plasma levels, and breadth of cytotoxic T-cell lymphocyte response during chronic HIV-1 infection. Clin. Infect. Dis. 54:294–302. 10.1093/cid/cir811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahar B, Lackner AA, Piatak M, Jr, Lifson JD, Wang X, Das A, Ling B, Montefiori DC, Veazey RS. 2009. Control of viremia and maintenance of intestinal CD4+ memory T cells in SHIV(162P3)-infected macaques after pathogenic SIV(MAC251) challenge. Virology 387:273–284. 10.1016/j.virol.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Das A, Lackner AA, Veazey RS, Pahar B. 2008. Intestinal double-positive CD4+ CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood 112:4981–4990. 10.1182/blood-2008-05-160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenway-Lynch CS, Das A, Pan D, Lackner AA, Pahar B. 2013. Dynamics of cytokine/chemokine responses in intestinal CD4+ and CD8+ T cells during acute simian immunodeficiency virus infection. J. Virol. 87:11916–11923. 10.1128/JVI.01750-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dame MK, Veerapaneni I, Bhagavathula N, Naik M, Varani J. 2011. Human colon tissue in organ culture: calcium and multi-mineral-induced mucosal differentiation. In Vitro Cell. Dev. Biol. Animal 47:32–38. 10.1007/s11626-010-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valentine LE, Loffredo JT, Bean AT, Leon EJ, MacNair CE, Beal DR, Piaskowski SM, Klimentidis YC, Lank SM, Wiseman RW, Weinfurter JT, May GE, Rakasz EG, Wilson NA, Friedrich TC, O'Connor DH, Allison DB, Watkins DI. 2009. Infection with “escaped” virus variants impairs control of simian immunodeficiency virus SIVmac239 replication in Mamu-B*08-positive macaques. J. Virol. 83:11514–11527. 10.1128/JVI.01298-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303–312. 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 28.Pahar B, Gray WL, Phelps K, Didier ES, Deharo E, Marx PA, Traina-Dorge VL. 2012. Increased cellular immune responses and CD4+ T-cell proliferation correlate with reduced plasma viral load in SIV challenged recombinant simian varicella virus–simian immunodeficiency virus (rSVV-SIV) vaccinated rhesus macaques. Virol. J. 9:160. 10.1186/1743-422X-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pahar B, Amedee AM, Thomas J, Dufour JP, Zhang P, Nelson S, Veazey RS, Bagby GJ. 2013. Effects of alcohol consumption on antigen-specific cellular and humoral immune responses to SIV in rhesus macaques. J. Acquir. Immune Defic. Syndr. 64:332–341. 10.1097/QAI.0b013e31829f6dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenway-Lynch CS, Das A, Lackner AA, Pahar B. 2014. Cytokine/chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 88:9442–9457. 10.1128/JVI.00774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3:1101–1108. 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 32.Das A, Veazey RS, Wang X, Lackner AA, Xu H, Pahar B. 2011. Simian immunodeficiency virus infection in rhesus macaques induces selective tissue specific B cell defects in double positive CD21+ CD27+ memory B cells. Clin. Immunol. 140:223–228. 10.1016/j.clim.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris PJ, Pappalardo BL, Custer B, Spotts G, Hecht FM, Busch MP. 2006. Elevations in IL-10, TNF-α, and IFN-γ from the earliest point of HIV type 1 infection. AIDS Res. Hum. Retrovir. 22:757–762. 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Lu Y, Yuan L, Liu J. 2011. Regulation of interleukin-10 receptor ubiquitination and stability by β-TrCP-containing ubiquitin E3 ligase. PLoS One 6:e27464. 10.1371/journal.pone.0027464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 36.O'Shea JJ, Murray PJ. 2008. Cytokine signaling modules in inflammatory responses. Immunity 28:477–487. 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulbin H, Bode H, Stocker H, Schmidt W, Zippel T, Loddenkemper C, Engelmann E, Epple HJ, Arasteh K, Zeitz M, Ullrich R. 2008. Cytokine expression in the colonic mucosa of human immunodeficiency virus-infected individuals before and during 9 months of antiretroviral therapy. Antimicrob. Agents Chemother. 52:3377–3384. 10.1128/AAC.00250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGowan I, Elliott J, Fuerst M, Taing P, Boscardin J, Poles M, Anton P. 2004. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J. Acquir. Immune Defic. Syndr. 37:1228–1236. 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Estes JD, Duan L, Jessurun J, Pambuccian S, Forster C, Wietgrefe S, Zupancic M, Schacker T, Reilly C, Carlis JV, Haase AT. 2008. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J. Infect. Dis. 197:420–429. 10.1086/525046. [DOI] [PubMed] [Google Scholar]
- 40.Radtke F, Clevers H. 2005. Self-renewal and cancer of the gut: two sides of a coin. Science 307:1904–1909. 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 41.George MD, Reay E, Sankaran S, Dandekar S. 2005. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 79:2709–2719. 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heise C, Vogel P, Miller CJ, Halsted CH, Dandekar S. 1993. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques: functional, pathological, and morphological changes. Am. J. Pathol. 142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 43.Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. 2000. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 14:1749–1753. 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- 44.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, Hirsch VM, Silvestri G, Douek DC, Miller CJ, Haase AT, Lifson J, Brenchley JM. 2010. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6:e1001052. 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, Laboisse CL. 2008. Mucosal IL-10 and TGF-β play crucial roles in preventing LPS-driven, IFN-γ-mediated epithelial damage in human colon explants. J. Clin. Invest. 118:1132–1142. 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allers K, Loddenkemper C, Hofmann J, Unbehaun A, Kunkel D, Moos V, Kaup FJ, Stahl-Hennig C, Sauermann U, Epple HJ, Schneider T. 2010. Gut mucosal FOXP3+ regulatory CD4+ T cells and nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques. J. Virol. 84:3259–3269. 10.1128/JVI.01715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du Z, Kelly E, Mecklenbrauker I, Agle L, Herrero C, Paik P, Ivashkiv LB. 2006. Selective regulation of IL-10 signaling and function by zymosan. J. Immunol. 176:4785–4792. 10.4049/jimmunol.176.8.4785. [DOI] [PubMed] [Google Scholar]
- 48.Crepaldi L, Gasperini S, Lapinet JA, Calzetti F, Pinardi C, Liu Y, Zurawski S, de Waal Malefyt R, Moore KW, Cassatella MA. 2001. Upregulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. J. Immunol. 167:2312–2322. 10.4049/jimmunol.167.4.2312. [DOI] [PubMed] [Google Scholar]
- 49.Tamassia N, Calzetti F, Menestrina N, Rossato M, Bazzoni F, Gottin L, Cassatella MA. 2008. Circulating neutrophils of septic patients constitutively express IL-10R1 and are promptly responsive to IL-10. International immunology. 20:535–541. 10.1093/intimm/dxn015. [DOI] [PubMed] [Google Scholar]
- 50.Emmerich J, Mumm JB, Chan IH, Laface D, Truong H, McClanahan T, Gorman DM, Oft M. 2012. IL-10 directly activates and expands tumor-resident CD8+ T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 72:3570–3581. 10.1158/0008-5472.CAN-12-0721. [DOI] [PubMed] [Google Scholar]
- 51.Ding Y, Qin L, Zamarin D, Kotenko SV, Pestka S, Moore KW, Bromberg JS. 2001. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J. Immunol. 167:6884–6892. 10.4049/jimmunol.167.12.6884. [DOI] [PubMed] [Google Scholar]
- 52.Wei SH, Ming-Lum A, Liu Y, Wallach D, Ong CJ, Chung SW, Moore KW, Mui AL. 2006. Proteasome-mediated proteolysis of the interleukin-10 receptor is important for signal downregulation. J. Interferon Cytokine Res. 26:281–290. 10.1089/jir.2006.26.281. [DOI] [PubMed] [Google Scholar]
- 53.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361:2033–2045. 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, Hugot JP, Daussy C, Verkarre V, Pigneur B, Fischer A, Klein C, Cerf-Bensussan N, Ruemmele FM. 2011. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am. J. Gastroenterol. 106:1544–1555. 10.1038/ajg.2011.112. [DOI] [PubMed] [Google Scholar]
- 55.Bouma G, Strober W. 2003. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3:521–533. 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. 2001. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 193:471–481. 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, Nagata K, Yoshimura A. 2001. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J. Clin. Invest. 108:1781–1788. 10.1172/JCI200113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. 2010. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 6:e1000852. 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.