ABSTRACT
Simian foamy viruses (SFV) are retroviruses that are widespread among nonhuman primates. SFV can be transmitted to humans, giving rise to a persistent infection. Only a few data are available concerning the distribution of SFV in human blood cells. Here we purified blood mononuclear cell subsets from 11 individuals infected with a Gorilla gorilla SFV strain and quantified SFV DNA levels by quantitative PCR. SFV DNA was detected in the majority of the CD8+, CD4+, and CD19+ lymphocyte samples and rarely in CD14+ monocyte and CD56+ NK lymphocyte samples. The median (interquartile range [IQR]) SFV DNA counts were 16.0 (11.0 to 49.8), 11.3 (5.9 to 28.3), and 17.2 (2.0 to 25.2) copies/105 cells in CD8+ T lymphocytes, CD4+ T lymphocytes, and CD19+ B lymphocytes, respectively. In the CD4 compartment, SFV DNA was detected in both memory and naive CD4+ T lymphocytes. SFV DNA levels in CD4+ T cells were positively correlated with the duration of the infection. Our study shows with a quantitative method that CD8+, CD4+, and B lymphocytes are major cellular targets of SFV in the blood of infected humans.
IMPORTANCE Investigation of SFV infections in humans is important due to the origin of human immunodeficiency viruses (HIV) and human T cell lymphotropic viruses (HTLV) from cross-species transmission of their simian counterparts to humans. Surprisingly little is known about many aspects of the biology of SFV in infected humans, including quantitative data concerning the cellular targets of SFV in vivo. Here we show that the distribution of SFV DNA among the different leukocyte populations is not homogeneous and that viral load in CD4+ T lymphocytes is correlated with the duration of infection. These new data will help in understanding the biology of retroviral infections in humans and can be useful in the growing field of SFV-based gene therapy.
INTRODUCTION
Like simian immunodeficiency viruses (SIVs) and simian T cell lymphotropic viruses (STLVs), simian foamy viruses (SFVs) are complex retroviruses that can infect humans after cross-species transmission. No pathology has been reported so far in the natural hosts or in humans, although large-scale dedicated studies have not yet been performed (1, 2). Humans are not the natural hosts of foamy viruses, but more than 100 cases of SFV infection have been reported so far within individuals in close contact with nonhuman primates (NHP). In such persons, SFV infection is persistent, and the SFV viral load ranges from 1 to >1,000 SFV DNA copies/105 blood cells (3–6). While it is known that both retroviruses, human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic virus (HTLV-1), can infect T cells (7, 8), little is known about the distribution of SFV in human blood cells (9). One pioneer study with chimpanzees and African green monkeys revealed a preferential infection of lymphocytes, especially CD8+ T cells, in 13 SFV-infected animals (10). In humans, using semiquantitative PCR assays, von Laer et al. found SFV DNA exclusively in CD8+ T cells in two tested individuals (10). Interestingly, SFV DNA was detected in monocytes and B lymphocytes, but not in CD4+ and CD8+ T cells, in one individual who was infected with an African green monkey viral strain containing deleterious mutations in the viral bet gene (11). However, partly due to the difficulty in obtaining, quickly processing, and accurately quantifying SFV DNA in samples from a series of SFV-infected individuals whose history of infection is known or presumed, no further data are available concerning SFV tropism in humans.
In this study, we aimed to quantify and analyze SFV viral load in the major populations of peripheral blood mononuclear cells (PBMCs) in a series of 11 hunters from Central Africa who were chronically infected with a gorilla strain of SFV (4). In contrast, the two previous studies were related to SFVs from African green monkeys and chimpanzees and comprise more isolated reports than this more systematic study (10, 11). Moreover, for the first time, quantitative assays were performed to better evaluate the levels of SFV DNA in the different cell populations.
MATERIALS AND METHODS
Participants.
We studied 11 participants; all of them live in villages or settlements in the rain forest of South Cameroon (Table 1). All participants received detailed information about the study and gave their written consent. The study received administrative and ethical clearance in Cameroon from the National Committee of Ethics, and in France, from the Comité de Protection des Personnes and the Commission Nationale de l'Informatique et des Libertés.
TABLE 1.
Epidemiological features of the 11 simian foamy virus-infected humans
| Individuala | Ethnicity | Age at contactb (yr) | Age at sampling (yr) | Estimated duration of infection (yr) | Wound location | Severityc | Sampling date (mo/day/yr) (study)d | Complete SFV genome availablee (GenBank accession no.) |
|---|---|---|---|---|---|---|---|---|
| AKO394 | Bantu | 53 | 58 | 5 | Thigh | 1 | 9/18/2012 (P) | No |
| BAD348 | Bantu | 19 | 32 | 13 | Leg | 3 | 3/14/2013 (P); 11/19/2013 (P + M) | No |
| BAD447 | Bantu | 40 | 60 | 20 | Hand | 2 | 3/10/2013 (P) | No |
| BAD456 | Bantu | 24 | 34 | 10 | Leg | 2 | 3/10/2013 (P) | No |
| BAD463 | Bantu | 37 | 47 | 10 | Leg | 3 | 11/19/2013 (P + M) | No |
| BAD468 | Bantu | 25 | 38 | 13 | Several | 3 | 9/04/2012 (P); 3/10/2012 (M); 11/15/2013 (P) | Yes (JQ867465.1) |
| BAK177 | Pygmy | 26 | 40 | 14 | Leg | 3 | 11/15/2013 (P) | No |
| BAK232 | Pygmy | 40 | 63 | 23 | Hand | 2 | 11/19/2013 (P + M) | No |
| BAK55 | Pygmy | 30 | 70 | 40 | Arm | 2 | 11/15/2013 (P) | No |
| BAK74 | Pygmy | 26 | 51 | 25 | Foot | 2 | 9/04/2012 (P); 03/14/2012 (M); 11/15/2013 (P) | Yes (JQ867464.1) |
| LOBAK2 | Pygmy | 37 | 64 | 27 | Thigh | 3 | 03/10/2013 (P) | No |
All individuals lived in South Cameroon rainforest villages/settlements.
All individuals had been bitten by a gorilla during hunting activities and are infected with a gorilla SFV (3). Age at contact was assumed, based on interview.
Wound bite severity (based on interview and physical injury): 1 = low, 2 = medium, 3 = high.
P corresponds to sampling for study of the general tropism of SFV in PBMCs, and M corresponds to sampling for study of the tropism of SFV in naive and memory cells.
Complete sequences of isolated viral strains from the indicated individuals are available in our previous study (14), and their GenBank accession numbers are provided.
SFV status of enrolled individuals.
We enrolled in the present study 11 participants who had been shown to be infected with a gorilla SFV strain in our previous work (4) (Table 1). Briefly, the 11 participants were determined to be infected with SFV based on both serological results (Western blot positivity) and molecular studies on PBMCs (SFV detection by nested PCR on the pol-in fragment [integrase-coding sequence of the polymerase gene] and/or the long terminal repeat [LTR] fragment). All of the participants have been bitten by a gorilla, which is very probably the cause of SFV infection, as suggested previously (4). Of note, the strain of SFV infecting each individual always matched the NHP species they were bitten by (herein, gorillas), as declared in the interview.
Purification of PBMC subpopulations.
Fresh blood samples were collected in EDTA tubes and shipped at room temperature from the Pasteur Institute of Yaoundé to the Pasteur Institute of Paris for processing. Within 20 h following sampling, fresh PBMCs were isolated by a Ficoll-Hypaque gradient. PBMC subpopulations were purified by sequential separation using magnetic beads; CD19+ B lymphocytes, CD14+ monocytes, CD4+ T cells, CD56+ NK cells, and CD8+ T cells were isolated using the corresponding positive-selection kits (Miltenyi Biotec, Paris, France). For the “memory study,” CD4+ T cells were first selected by T cell isolation kits followed by positive selection of CD45RO+ memory cells (Miltenyi Biotec, Paris). The flow-through contained the naive CD4+ T cell-enriched fraction. The purity of the separated cells was checked by flow cytometry using a BD FacsCanto system for data acquisition and FlowJo V8.7.1 software for data analysis after labeling with a composition of the following antibodies: anti-CD3-fluorescein isothiocyanate (FITC), anti-CD45RA-V450 (BD Biosciences, Rungis, France), anti-CD8-allophycocyanin (APC), anti-CD14-phycoerythrin (PE) (Beckman Coulter, Villepinte, France), anti-CD16-V450, anti-CD56-PC7, anti-CD20-PC5 (BD Pharmingen, Rungis, France), and anti-CD4-APC-H7 (eBioscience, Paris, France). Live/Dead Fixable Dead Cell Stain kits (Life Technologies, Saint Aubin, France) were used to identify viable cells. A gating strategy among live cells identified CD3+ CD4+ cells as CD4+ T lymphocytes, CD3+ CD8+ cells as CD8+ T lymphocytes, CD3− CD19+ cells as B lymphocytes, CD3− CD14+ as monocytes, and both CD3− CD56+ and CD3− CD16+ cells as NK lymphocytes. The purities of CD4+, CD8+, and CD19+ lymphocytes, CD14+ monocytes, and CD56+ NK cells were 94.0% ± 3.1%, 87.9% ± 8.1%, 79.9% ± 7.5%, 73.2% ± 15.2%, and 61.4% ± 16.4%, respectively (see Table S1 in the supplemental material). The purity of the CD4+ naive cells was 77.1% ± 18.5%, and the purity of the CD4+ memory cells was 86.5% ± 4.1% (data not shown).
Quantification of SFV DNA viral loads.
Quantitative PCRs (qPCRs) targeting the SFV polymerase gene (pol) were performed mainly as previously described (5). Briefly, the primers used (GF5qpcr-TAGACCTGAAGGAACCAAAATAATTCC, and GR5qpcr-TCCTTCCTCATATTAGGCCACC) were designed to detect a 144-bp nucleic acid region of the gorilla SFV polymerase gene. We used the Eppendorf Mastercycler realplex gradient detection system and Quantitect SYBR green (Qiagen) in a 20-μl-volume reaction mixture containing 10 μl of SYBR green buffer, 150 nM each primer, and 500 ng of a DNA sample (an approximately 75,000-cell equivalent) quantified by a NanoDrop 8000 spectrophotometer (Nanodrop Technologies). The optimized qPCR conditions used were as follows: 95°C for 15 min and 40 cycles at 95°C for 15s, 60°C for 30s, and 72°C for 30s. Samples were tested in triplicate in two independent assays, and the mean and standard deviation (SD) were calculated.
To standardize the qPCR, we cloned a 465-bp region that included the PCR target sequence from one primary isolate (BAK74, a gorilla strain of SFV) into a PCR cloning plasmid using a TOPO TA cloning kit (Invitrogen) and diluted it in 500 ng of human genomic DNA (Promega). All SFV qPCRs included a standard curve (from 3 to 37 = 2,187 SFV DNA copies; 3-fold serial dilutions) and were performed in triplicate. In each assay, SFV DNA copies from 3 to 37 were in the range of linear detection, and the correlation coefficient of SFV standard curves was >0.95. The limit of detection (LOD) was determined as the number of SFV DNA copies corresponding to the threshold cycle at which at most 5% of true positive samples scored arbitrarily negative. Using this definition, we determined the LOD to be equivalent to 3 SFV DNA copies/75,000 cells (equivalent to 4 SFV DNA copies/105 cells). qPCRs below the LOD were arbitrarily set up at half the LOD (equivalent to 2 SFV DNA copies/105 cells).
A 141-bp cellular albumin qPCR was performed on a 250× dilution of the samples (2 ng) to normalize the result with that for cellular DNA content (albF-AAACTCATGGGAGCTGCTGGTT, albR-GCTGTCATCTCTTGTGGGCTGT) using the same PCR conditions as for SFV quantification. All albumin qPCR assays included an albumin standard curve generated by adding 5-fold dilutions of human genomic DNA (Promega), ranging from 0.08 ng of DNA to 50 ng of DNA, to the PCR reagents. The correlation coefficient of albumin standard curves was >0.95.
We checked in every individual assay the specificity of the primers by using a melting curve.
SFV bet DNA coding sequences.
We performed strain-specific nested PCR to sequence the entire SFV bet coding DNA sequence (CDS) from genomic DNA of PBMCs of SFV-infected individuals. We used nested PCR to generate three PCR products, A (572-bp bet open reading frame 1 [orf-1]), B (761-bp 5′ fragment of bet orf-2), and C (861-bp 3′ fragment of bet orf-2, overlapping with B).
The primers used to generate PCR product A were primers A1 (5′-AACTGGAGAGAGCTAAAGCAG-3′) and A2 (5′-CCAGACACCAACATCAGCA-3′) for the outer PCR and primers A3 (5′-ATTGGTAACTTCTTAACTGG-3′) and A4 (5′-GTAACAGGCATAGGTTCATG-3′) for the inner PCR. The reaction conditions were 95°C for 15 min and 40 cycles at 95°C for 15 s, 50°C for 1 min, and 72°C for 1 min, followed by 72° for 7 min.
The primers used to generate PCR product B were primers B1 (5′-CATGAACCTATGCCTGTTAC-3′) and B2 (5′-CTGATCTGAAAGATTTGCAGC-3′) for the outer PCR and primers B3 (5′-TGCTGATGTTGGTGTCTGG-3′) and B4 (5′-CTTGATAAGCATATTGGAGCC-3′) for the inner PCR. The reaction conditions were 95°C for 15 min and 40 cycles at 95°C for 15s, 65°C for 1 min, and 72°C for 1 min, followed by 72° for 7 min.
The primers used to generate PCR product C were primers C1 (5′-CAGAACCTGATGTATGGTG-3′) and C2 (5′-CCTCTCTAGGGATTATACAG-3′) for the outer PCR and primers C3 (5′-GGTGTAGTGCAGCACTTTG-3′) and C4 (5′-CTCTTCTTACACTACTTCC-3′) for the inner PCR. The reaction conditions were 95°C for 15 min and 40 cycles at 95°C for 15s, 50°C for 1 min, and 72°C for 1 min, followed by 72° for 7 min.
Inner primers A3 and A4, B3 and B4, or C3 and C4 were used to sequence the PCR products A, B, and C, respectively. For each donor, one of each PCR product A, B, and C was sequenced by direct sequencing of the PCR product (this implies sequencing of the major variants). Each base was sequenced twice, using forward and reverse primers. Sequences were aligned using CLC Genomics Workbench software (Gap open cost, 10.0; Gap extension cost, 1.0).
Statistical analysis.
For qualitative analysis, SFV DNA detection rates across the 5 cell subsets were compared using Cochran's Q test and two-by-two comparisons were performed using McNemar's test. For quantitative analysis, the Friedman test was used to compare SFV DNA levels across T and B lymphocytes. Spearman's rank test was used to assess correlations between SFV DNA levels and variables related to SFV infection. GraphPad Prism software was used for statistical analysis.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences of bet orf-1 are KF982250 to KF982260, and for bet orf-2, KF982262 to KF982272.
RESULTS
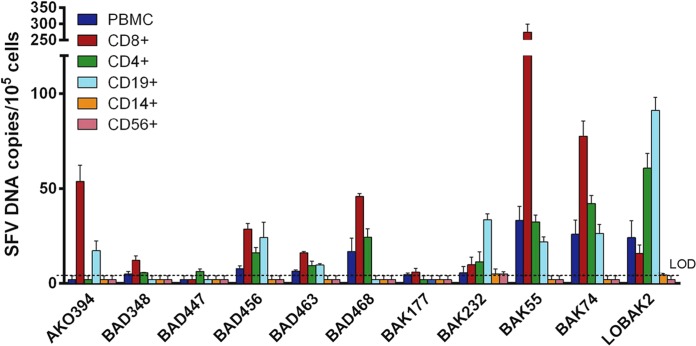
SFV DNA was quantified in selected subsets of peripheral blood cells from 11 individuals (Fig. 1). SFV DNA was detected in CD8+ T lymphocytes from 10 of 11 (91%) donors, in CD4+ T lymphocytes from 9 (82%) of them, and in CD19+ B lymphocytes from 7 (64%) of them. In contrast, SFV DNA levels were above the limit of detection in only two (18%) CD14+ monocyte samples and one (9%) CD56+ NK lymphocyte sample. The frequencies of SFV DNA detection across the 5 mononuclear cell subsets were significantly different (Cochran's Q test; P < 0.001). The frequency of SFV DNA detection in CD8+, CD4+, and CD19+ B lymphocytes was significantly higher than in CD56+ NK lymphocytes and in CD8+ and CD4+ T lymphocytes than in CD14+ monocytes (McNemar's test; P < 0.05 for each two-by-two comparison). There was a trend for a higher frequency of SFV DNA detection in B lymphocytes than in monocytes, although it did not reach statistical significance (McNemar's test; P = 0.07).
FIG 1.
SFV DNA loads in PBMC populations of 11 hunters infected with a Gorilla gorilla SFV strain. PBMC populations from 11 SFV-infected individuals were isolated, and the SFV DNA load was quantified in each PBMC population. Each quantification was performed twice in triplicate (e.g., n = 6). Means ± standard deviations are shown. Values below the limit of detection (LOD) were arbitrarily set as half the LOD, 2 SFV DNA copies/105 cells.
We then performed quantitative analysis on CD8+, CD4+, and CD19+ lymphocytes, as SFV DNA was generally above the limit of detection in these subsets. The mean (±SD) SFV DNA loads were 49.2 ± 78.1, 19.3 ± 18.9, and 21.1 ± 25.9 copies/105 cells in CD8+ T lymphocytes, CD4+ T lymphocytes, and CD19+ B lymphocytes, respectively. The median SFV DNA values were 16.0 (interquartile range [IQR], 11.0 to 49.8), 11.3 (IQR, 5.9 to 28.3), and 17.2 (IQR, 2.0 to 25.2) copies/105 cells in CD8+ T lymphocytes, CD4+ T lymphocytes, and B lymphocytes, respectively. SFV DNA levels in CD4+ T lymphocytes, CD8+ T lymphocytes, and B lymphocytes were not statistically different (Friedman's test; P = 0.12). SFV DNA loads in CD4+ T lymphocytes and B lymphocytes were correlated (Spearman's ρ = 0.646, P = 0.04) but did not correlate with the SFV DNA load in CD8+ T lymphocytes (for CD4+ T lymphocytes, Spearman's ρ = 0.469, P = 0.15; for B lymphocytes, Spearman's ρ = 0.312, P = 0.35).
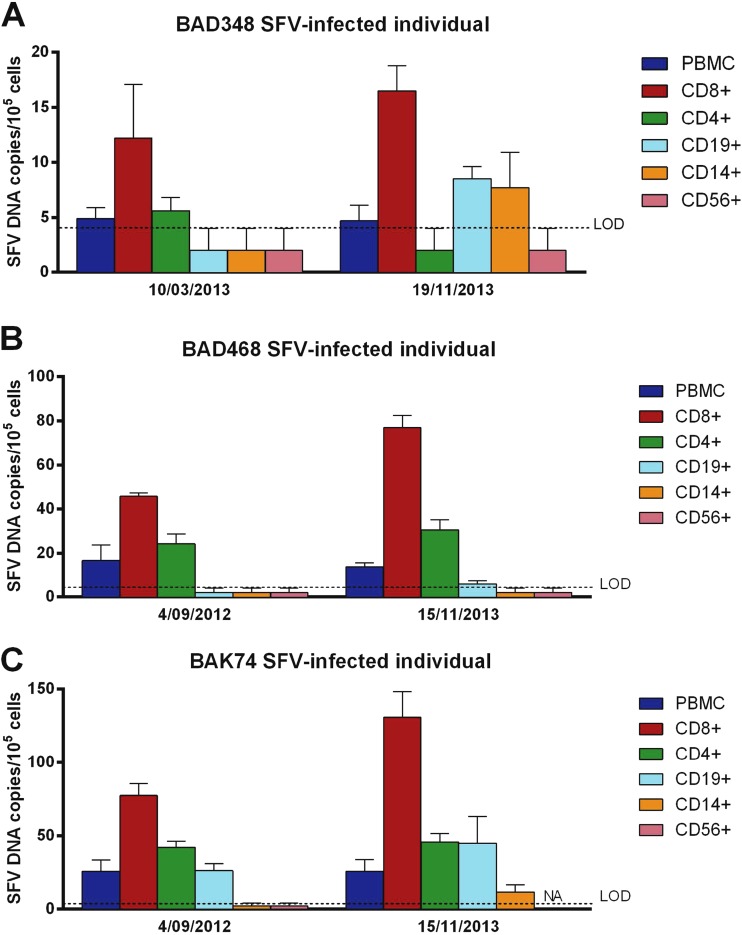
We repeated peripheral blood sampling, cell subset separation, and SFV DNA quantification 8 to 14 months later for three donors (BAD348, BAD468, and BAK74) (Fig. 2). For these three individuals, the CD8+ T lymphocytes contained the highest SFV DNA levels at both time points. In CD4+ T cells from BAD468 and in both CD4+ and CD19+ cells from BAK74, SFV DNA was detected at both time points. Finally, samples with an undetectable level of SFV DNA at one time point (CD14+ monocytes and CD56+ NK cells from the 3 donors, as well as CD4+ and CD19+ cells from BAD348 and CD19+ cells from BAD468) displayed undetectable or low levels (<10 copies/105 cells) of SFV DNA at the other time point. In conclusion, distribution of SFV DNA across blood cell subsets appeared to be mainly stable over an 8- to 14-month period.
FIG 2.
SFV DNA loads in PBMC populations of 3 hunters infected with a Gorilla gorilla SFV strain at two time points. PBMC populations from 3 SFV-infected individuals were isolated, and the SFV DNA load was quantified in each PBMC population at two different time points. Each quantification was performed twice in triplicate (e.g., n = 6). Means ± standard deviations are shown. NA, not available. Values below the limit of detection (LOD) were arbitrarily set as half the LOD, 2 SFV DNA copies/105 cells.
We quantified SFV DNA load in naive and memory CD4+ T cells from five individuals (Fig. 3). The SFV DNA load was above the limit of detection in memory CD4+ T cells from all donors and in naive CD4+ T cells from 3 donors. The mean (±SD) SFV DNA load was 21.8 ± 18.7 copies/105 cells in memory CD4+ T cells and 10.1 ± 11.3 copies/105 cells in naive CD4+ T cells; medians were 15.0 (IQR, 6.2 to 40.9) and 2.0 (IQR, 2.0 to 18.7) SFV DNA copies/105 cells in memory and naive CD4+ T cells, respectively. Thus, SFV targets both CD4+ naive and CD4+ memory cells. Due to limitations in the biological material available, analyses of SFV DNA loads in naive and memory CD8 T lymphocytes could not be performed.
FIG 3.
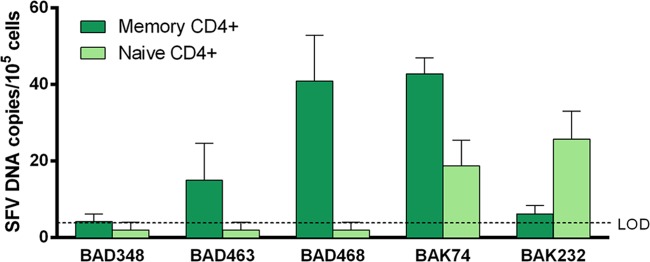
SFV DNA loads in memory and naive CD4+ T cells of 5 hunters infected with a Gorilla gorilla SFV strain. CD4+ cells were separated into naive and memory cells before quantification of the SFV DNA load in 5 SFV-infected individuals. Each quantification was performed twice in triplicate (e.g., n = 6). Means ± standard deviations are shown. Values below the limit of detection (LOD) were arbitrarily set as half the LOD, 2 SFV DNA copies/105 cells.
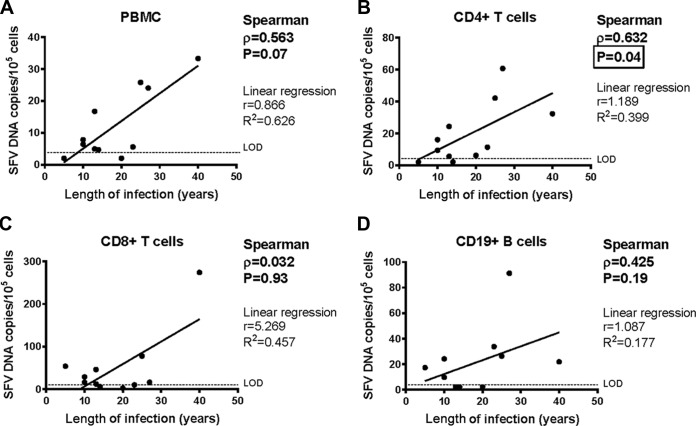
We then studied the association between SFV DNA load and the characteristics of infection. The SFV DNA load in PBMCs tended to be positively correlated with the duration of infection without reaching statistical significance (Spearman's ρ = 0.563, P = 0.07) (Fig. 4A). The SFV DNA load in CD4+ T cells was positively correlated with the duration of infection (Spearman's ρ = 0.632, P = 0.04) (Fig. 4B). In contrast, such a correlation was not found for CD8+ and CD19+ lymphocytes (Spearman's ρ = 0.032, P = 0.93 for CD8+ lymphocytes; Spearman's ρ = 0.425, P = 0.19 for CD19+ lymphocytes) (Fig. 4C and D). These data suggest a selective increase in viral load in CD4+ T lymphocytes over time when long periods of time (decades) are considered. The number of SFV DNA copies in the different cell subsets examined did not correlate with age at infection or age at sampling (data not shown).
FIG 4.
SFV DNA loads in PBMCs, CD4+ T cells, CD8+ T cells, and CD19+ B cells as a function of the duration of infection. PBMC populations from 11 individuals infected with a Gorilla gorilla SFV strain were isolated, and the SFV DNA load was quantified in each PBMC population. Each quantification was performed twice in triplicate (e.g., n = 6) and plotted as a function of the duration of infection. Time of infection was based on interviews, as described previously (4). Means are shown as well as Spearman's test results (bold characters). Of note, Spearman's test is nonparametric and thus appropriate for our set of data (there were no assumptions about the probability distributions of the variables, here viral loads). Linear regression (parametric) is provided for visualization but has no statistical relevance, as the correlation existing between viral load and the duration of infection is not linear. The values below the limit of detection (LOD) were arbitrarily set as half the LOD, 2 SFV DNA copies/105 cells.
Interestingly, premature stop codons in the viral accessory gene bet were reported for one SFV-infected individual (11). For this individual, SFV DNA was found in CD19+ B lymphocyte and CD14+ monocyte cells, but it was not detected in CD4+ T cells and CD8+ T cells, and the study's authors suggested that this may be linked to SFV bet mutations. Therefore, we sequenced the entire SFV bet CDS (1,443 bp) from the 11 individuals. We did not find deletion events or stop codons in the 11 bet gene sequences (see Fig. S1 and S2 in the supplemental material).
DISCUSSION
Here, we quantified SFV DNA load in the major PBMC subpopulations of 11 individuals infected with a Gorilla gorilla SFV strain. Previous reports on three infected persons showed that B and T lymphocytes as well as monocytes harbor SFV DNA (10, 11). Semiquantitative analysis of SFV DNA loads in the different PBMC populations of 2 individuals revealed that 104 to 105 CD8 T cells were sufficient to amplify SFV DNA, indicating a viral load of 1 to 10 SFV DNA copies/105 cells, which is slightly lower than our results and might be related to PCR sensitivity, the infecting strain of SFV, and/or the sample size. Our study is the first that accurately quantifies SFV DNA loads in PBMC populations in a series from SFV-infected individuals. We observed that the frequency of SFV detection in T and B lymphocytes was higher than in NK lymphocytes and monocytes. No difference in SFV DNA levels was observed between T and B lymphocytes. Among CD4+ T cells, SFV DNA was found in both naive and memory CD4+ T lymphocytes. Interestingly, HIV-1 (12) and HTLV-1 (8) preferentially infect memory CD4+ lymphocytes. More data would have been necessary to conclude whether there was a preferential infection of memory CD4+ T lymphocytes. Defining naive and memory CD8+ T lymphocytes and B lymphocytes relies on the coexpression of two molecules, and their purification requires a cell sorter in a biosafety level 2 laboratory, which was not available at the time of the study. Further experiments will be needed to determine if the observed cellular targets of SFV in humans are linked to biological properties of SFV, such as replication in the different cell subsets, as was previously suggested by a study in vitro (13). It would also be interesting to know whether infection of the T and B cell progenitor, clonal expansion of lymphocytes, and/or homeostatic CD8+ proliferation accounts for SFV DNA levels.
We observed that the distribution of SFV DNA in peripheral blood cell subsets was stable over several months. However, our cross-sectional study suggests that SFV cellular targets may evolve over several decades. We found that the SFV DNA load in CD4+ T cells was positively correlated with the duration of infection, whereas this correlation was not observed for CD19+ B cells or CD8+ T cells. Further longitudinal studies will be needed to better understand the evolution of SFV DNA load and cellular target in the course of infection (5). Finally, it would be interesting to have quantitative data on the natural host of SFV infections, gorillas in our case, but blood sampling of a large series of SFV-infected gorillas in this area is extremely challenging.
In humans, SFV DNA was found exclusively in CD8+ T cells from two tested individuals (10) and in monocytes and B lymphocytes from one individual who was infected with a viral strain containing deleterious mutations in the SFV bet accessory gene (11). Of note, we have also found a premature stop codon in the bet gene of the Pan troglodytes troglodytes strain of SFV (from 3 chimpanzees and 4 humans infected with SFV) resulting in a predicted Bet protein that is shortened by 2% (14). These observations led us to sequence the bet coding sequence from the 11 individuals. No sequences harbored stop codons or major deletions, suggesting that at least in our series of individuals infected with a gorilla foamy virus, deleterious mutations in bet are not required for infection of monocytes and B lymphocytes. Of note, 2 of 11 bet sequences were obtained after virus isolation in our previous study (14) and showed >99.5% similarity with the ones presented here. The number of cell fractions with detectable SFV DNA varied between one and five, and we did not observe a bimodal distribution of the virus across the mononuclear cell subsets. The variations across the two previous studies and our results may be related to the small size of the study populations, the differences in assay sensitivity, the interindividual variations, or distinct specificities of SFV from different clades. Indeed, we have studied individuals infected with SFV from gorillas, whereas studies reported by Callahan et al. and van Laer et al. focused on individuals infected with SFV from African green monkeys or chimpanzees (10, 11).
The sample size is small, but this comes as a result of the relative rarity of SFV-infected individuals and the difficulty in obtaining and preserving high-quality samples in the field. For most donors, SFV DNA loads in purified CD8, CD4, and CD19 subsets were similar or higher than in whole PBMCs, in line with the preferential infection of these lymphocyte subsets. For some samples, a fraction of PBMCs partially lost the expression of CD markers after overnight shipment or was undergoing apoptosis. As a consequence, the SFV DNA load could be higher in enriched viable subsets than in whole PBMCs (as for donor AKO394). Importantly, SFV DNA loads in different PBMCs and/or buffy coat samples from these same individuals were reported by our team in previous studies, and they were in the same range (4, 5). Moreover, the lower level of purity of certain cell subsets (especially NK cells) is another limitation of our study, although SFV DNA in NK cell subsets is generally undetectable. These technical issues do not change our major conclusion that CD8+ and CD4+ T lymphocytes and B lymphocytes are preferentally infected in individuals infected with a gorilla SFV strain.
Our findings raise concerns about the consequences of in vivo retroviral coinfections, because we found that SFV, like HIV-1 and HTLV-1, shows long-term persistence in leukocytes, including T lymphocytes. Furthermore, SFV can increase HIV binding at the cell surface via heparan sulfate glycosaminoglycans and can upregulate HIV-1 LTR transactivation (15, 16). Cases of HIV-1/SFV (17) or HTLV-1/SFV (unpublished data) coinfections have been found in humans. In macaques, experimental SIV infection in either non-SFV-infected or naturally SFV-infected animals revealed that SFV infection increases the pathology of SIV infection (18). Given the ongoing risk of exposure to these three primate retroviruses, especially in Central Africa, microbiological surveillance is required to better evaluate the potential clinical and physiopathological impact of such retroviral interactions. Furthermore, our findings indicate the cellular distribution of SFV in vivo. This could have important implications for the development of SFV-based viral vectors for use in human gene therapy. For instance, recent approaches for treating blood-related diseases consist of direct intravenous administration of SFV viral vectors. Identifying the cellular targets of SFV is critical to better estimate the efficiency and safety of such vectors (19, 20).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Institut de Recherche pour le Développement (IRD) and the Centre Pasteur du Cameroun for their collaborations concerning the field work in Cameroon. We thank Philippe Afonso for critical reading of the manuscript. This text has been verified by a native English speaker.
This work was supported by the Institut Pasteur in Paris, France, by Programme Transversal de Recherche 437 from the Institut Pasteur, and by the French government program Investissement d'Avenir (grant ANR-10-LABX-62-IBEID). R. Rua was supported by the Bourse de l'Ecole Normale Supérieure from the Faculté Paris Diderot. E. Betsem was supported by the association Virus Cancer Prevention and by the Institut National pour le Cancer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no competing financial interests.
R.R., E.B., T.M., and A.G. performed experiments; R.R. and F.B. analyzed data; and R.R., F.B., and A.G. wrote the manuscript. A.G. supervised the research project.
Footnotes
Published ahead of print 10 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01801-14.
REFERENCES
- 1.Khan AS. 2009. Simian foamy virus infection in humans: prevalence and management. Expert Rev. Anti Infect. Ther. 7:569–580. 10.1586/eri.09.39. [DOI] [PubMed] [Google Scholar]
- 2.Gessain A, Rua R, Betsem E, Turpin J, Mahieux R. 2013. HTLV-3/4 and simian foamy retroviruses in humans: discovery, epidemiology, cross-species transmission and molecular virology. Virology 435:187–199. 10.1016/j.virol.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calattini S, Betsem EB, Froment A, Mauclère P, Tortevoye P, Schmitt C, Njouom R, Saib A, Gessain A. 2007. Simian foamy virus transmission from apes to humans, rural Cameroon. Emerg. Infect. Dis. 13:1314–1320. 10.3201/eid1309.061162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betsem E, Rua R, Tortevoye P, Froment A, Gessain A. 2011. Frequent and recent human acquisition of simian foamy viruses through apes' bites in central Africa. PLoS Pathog. 7:e1002306. 10.1371/journal.ppat.1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rua R, Betsem E, Gessain A. 2013. Viral latency in blood and saliva of simian foamy virus-infected humans. PLoS One 8:e77072. 10.1371/journal.pone.0077072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Switzer WM, Tang S, Ahuka-Mundeke S, Shankar A, Hanson DL, Zheng H, Ayouba A, Wolfe ND, LeBreton M, Djoko CF, Tamoufe U, Esteban A, Heneine W, Peeters M, Wright LL, Muyembe-Tamfum JJ, Wemakoy EO, Mulembakani P, Hoff NA, Rimoin AW. 2012. Novel simian foamy virus infections from multiple monkey species in women from the Democratic Republic of Congo. Retrovirology 9:100. 10.1186/1742-4690-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clapham PR, McKnight A. 2001. HIV-1 receptors and cell tropism. Br. Med. Bull. 58:43–59. 10.1093/bmb/58.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Richardson JH, Edwards AJ, Cruickshank JK, Rudge P, Dalgleish AG. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meiering CD, Linial ML. 2001. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 14:165–176. 10.1128/CMR.14.1.165-176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Laer D, Neumann-Haefelin D, Heeney JL, Schweizer M. 1996. Lymphocytes are the major reservoir for foamy viruses in peripheral blood. Virology 221:240–244. 10.1006/viro.1996.0371. [DOI] [PubMed] [Google Scholar]
- 11.Callahan ME, Switzer WM, Matthews AL, Roberts BD, Heneine W, Folks TM, Sandstrom PA. 1999. Persistent zoonotic infection of a human with simian foamy virus in the absence of an intact orf-2 accessory gene. J. Virol. 73:9619–9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. 1990. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl. Acad. Sci. U. S. A. 87:6058–6062. 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikovits JA, Hoffman PM, Rethwilm A, Ruscetti FW. 1996. In vitro infection of primary and retrovirus-infected human leukocytes by human foamy virus. J. Virol. 70:2774–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rua R, Betsem E, Calattini S, Saib A, Gessain A. 2012. Genetic characterization of simian foamy viruses infecting humans. J. Virol. 86:13350–13359. 10.1128/JVI.01715-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller A, Garrett ED, Cullen BR. 1992. The Bel-1 protein of human foamy virus activates human immunodeficiency virus type 1 gene expression via a novel DNA target site. J. Virol. 66:3946–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffer C, Lecellier CH, Mannioui A, Felix N, Nelson E, Lehmann-Che J, Giron ML, Gluckman JC, Saib A, Canque B. 2004. Persistent infection with primate foamy virus type 1 increases human immunodeficiency virus type 1 cell binding via a Bet-independent mechanism. J. Virol. 78:11405–11410. 10.1128/JVI.78.20.11405-11410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Switzer WM, Garcia AD, Yang C, Wright A, Kalish ML, Folks TM, Heneine W. 2008. Coinfection with HIV-1 and simian foamy virus in West Central Africans. J. Infect. Dis. 197:1389–1393. 10.1086/587493. [DOI] [PubMed] [Google Scholar]
- 18.Choudhary A, Galvin TA, Williams DK, Beren J, Bryant MA, Khan AS. 2013. Influence of naturally occurring simian foamy viruses (SFVs) on SIV disease progression in the rhesus macaque (Macaca mulatta) model. Viruses 5:1414–1430. 10.3390/v5061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindemann D, Rethwilm A. 2011. Foamy virus biology and its application for vector development. Viruses 3:561–585. 10.3390/v3050561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burtner CR, Beard BC, Kennedy DR, Wohlfahrt ME, Adair JE, Trobridge GD, Scharenberg AM, Torgerson TR, Rawlings DJ, Felsburg PJ, Kiem HP. 2014. Intravenous injection of a foamy virus vector to correct canine SCID-X1. Blood 123:3578–3584. 10.1182/blood-2013-11-538926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.