ABSTRACT
Delineating the key early events that lead to the development of broadly neutralizing anti-HIV-1 antibodies during natural infection may help guide the development of immunogens and vaccine regimens to prevent HIV-1 infection. In this study, we monitored two HIV-1-positive subjects, VC20013 and VC10014, over the course of infection from before they developed broadly neutralizing antibody (bNAb) activity until several years after neutralizing breadth was detected in plasma. Both subjects developed bNAb activity after approximately 1 year postinfection, which ultimately mapped to the membrane-proximal external region (MPER) in VC20013 and an epitope that overlaps the CD4 receptor binding site in VC10014. In subject VC20013, we were able to identify anti-MPER activity in the earliest plasma sample that exhibited no bNAb activity, indicating that this epitope specificity was acquired very early on, but that it was initially not able to mediate neutralization. Escape mutations within the bNAb epitopes did not arise in the circulating envelopes until bNAb activity was detectable in plasma, indicating that this early response was not sufficient to drive viral escape. As bNAb activity began to emerge in both subjects, we observed a simultaneous increase in autologous antienvelope antibody binding affinity, indicating that antibody maturation was occurring as breadth was developing. Our findings illustrate one potential mechanism by which bNAbs develop during natural infection in which an epitope target is acquired very early on during the course of infection but require time and maturation to develop into broadly neutralizing activity.
IMPORTANCE One major goal of HIV-1 vaccine research is the development of a vaccine that can elicit broadly neutralizing antibodies (bNAbs). Although no such vaccine exists, bNAbs develop in approximately 20% of HIV-1-infected subjects, providing a prototype of the bNAbs that must be reelicited by vaccine. Thus, there is significant interest in understanding the mechanisms by which bNAbs develop during the course of infection. We studied the timing, epitope specificity, and evolution of the bNAb responses in two HIV-1-positive patients who developed bNAb activity within the first several years after infection. In one subject, antibodies to a broadly neutralizing epitope developed very early but were nonneutralizing. After several months, neutralizing activity developed, and the virus mutated to escape their activity. Our study highlights one mechanism for the development of bNAbs where early epitope acquisition followed by sufficient time for antibody maturation drives the epitope-specific antibody response toward broadly neutralizing activity.
INTRODUCTION
The HIV-1 pandemic continues to exact a massive human toll as the pandemic nears the end of its third decade. At present, more than 35 million people are infected with HIV-1 worldwide, causing more than 1.5 million deaths per year (1). Although significant progress has been made in expanding universal treatment options in areas where HIV-1 is endemic and despite successful trials involving prophylactic drug use and microbicides, a universal vaccine remains the best option to stop the spread of HIV-1 (2). In 2009, the RV144 efficacy trial provided the first direct evidence that preventing HIV-1 acquisition by vaccination was possible (3–6). This trial achieved a modest reduction in HIV-1 acquisition, which was associated with the presence of vaccine-elicited antibody responses to the V1V2 region of the HIV-1 envelope (Env) (3, 6).
Eliciting protective antibodies against HIV-1 remains a difficult prospect. Neutralizing antibodies elicited by a successful anti-HIV-1 vaccine must be able to cope with an array of immune evasion techniques employed by the virus. Foremost is the massive genetic diversity of Env, the sole target of anti-HIV-1 neutralizing antibodies, which is driven by the ability of the virus to mutate rapidly to escape the host immune response (7). To cope with this genetic diversity, a vaccine must elicit antibodies that are able to bind to and neutralize a broad diversity of circulating isolates. Such broadly neutralizing antibodies (bNAbs) have not yet been elicited by vaccination with Env, but they are known to develop during the course of natural infection (8–13). Over the last several years, tremendous strides have been made in understanding the genesis of bNAbs, which develop in 20 to 30% of HIV-1-infected subjects (8, 10, 11, 14). Their development typically occurs within the first 3 years of infection (11, 13) and is associated with a moderate, sustained viral load (8, 15, 16). In addition, the frequency of circulating CD4+ T follicular helper cells in peripheral blood has been reported to correlate with the presence of bNAbs (17), implying that CD4+ T cell helper function may be important for the development of neutralizing breadth.
The Env epitope targets and mechanisms of neutralization of anti-HIV-1 bNAbs have been thoroughly characterized through the study of monoclonal antibodies (MAbs) isolated from chronically infected subjects (18–29). They target a small number of well-conserved epitopes on Env, including the CD4 binding site (CD4-BS) (24–27, 29, 30), glycopeptide epitopes on the trimer surface (21, 22, 31), high-mannose glycan residues, the coreceptor binding site, and the membrane-proximal external region (MPER) of gp41 (19, 23, 32). In addition, these antibodies have common features that help inform vaccine design, and hint as to how they developed. Many bNAbs have undergone extensive somatic hypermutation and can diverge from germ line sequences by as much as 46% (21, 22, 24, 27, 30). For potent CD4-BS bNAbs, the VH gene usage is highly restricted to use mainly the IGHV1–2*02 human VH allele, hinting that host genomics likely are an important factor (24, 30). Thus, vaccine strategies and immunogens that will stimulate the correct bNAb precursor genes and drive somatic hypermutation to a degree that will confer broadly neutralizing activity are needed.
Despite this wealth of understanding, it is not fully understood how bNAb responses develop during the first few years of infection or how the temporal interplay between host antibody recognition and subsequent viral escape may drive the development of bNAb responses. In a few well-characterized cases, early neutralizing breadth was reported to be developed in “waves” in which the subject sequentially developed multiple epitope specificities over time (33, 34). Initially, these subjects developed bNAb responses against a single neutralizing epitope against their autologous virus, but the virus was able to escape those bNAbs. As escape variants arose, they stimulated the development of new bNAb specificities, further broadening the broadly neutralizing activity present in plasma. Thus, one mechanism by which early breadth may develop is as a result of the cycles of antibody acquisition and escape driving an ever-broadening neutralizing response. In such cases, the bNAb response is composed of multiple bNAb specificities that all contribute to neutralizing breadth.
Other subjects that developed broadly neutralizing activity within the first 3 years of infection have been reported, but they do not have multiple bNAb specificities to a significant degree (11, 13, 35). Thus, it is possible that early neutralizing breadth also develops with the acquisition of a single bNAb specificity, where antibody-driven escape drives maturation against that single epitope, thus resulting in a potent bNAb response without the necessity of multiple epitope targets (36, 37). Indeed, this process has been investigated in great detail in two recent landmark studies for the development of bNAbs against epitopes within the CD4-BS (37) and the V1V2 regions of Env (36). In both cases, the authors concluded that a diversifying viral envelope population was a key driver of the broadening bNAb response.
In this study, we monitored two HIV-1-infected individuals, VC10014 and VC20013, from the early stages of infection until well after they had developed broadly neutralizing activity. We thoroughly characterized the stepwise development of bNAb activity in both individuals throughout the early stages of infection. In addition, we assessed the neutralization properties of longitudinally spaced autologous Env proteins (Envs) and mapped the epitope specificities of the autologous neutralizing responses. In both cases, the bNAb activity developed about 1 year after infection and mapped to a single epitope. Neither subject appeared to develop multiple bNAb specificities that contributed to neutralizing breadth during this time frame, indicating that in both cases the early development of neutralizing breadth did not follow a pattern of multiple specificities developing in waves. Interestingly, the development of neutralization breadth was coincidental with a stepwise increase in plasma anti-envelope antibody affinity to the circulating autologous Env, indicating that antibody maturation may be a critical step in the early development of breadth. Thus, bNAb activity in these two individuals appears to have developed with the acquisition of a single epitope very early during the course of infection. Finally, after the detection of bNAbs in plasma, we noted a rapid increase in viral diversity, highlighting the role of envelope diversification in driving the development of bNAbs.
MATERIALS AND METHODS
HIV-1-infected subjects.
Subjects VC10014 and VC20013 were initially described as part of a systematic survey of HIV-1-positive cohorts to establish the relative frequencies with which broadly neutralizing antibodies (bNAbs) are developed (8) and are known to be infected with clade B HIV-1 variants. Both subjects entered observation within the first year of infection with HIV-1 and developed broadly neutralizing antibody responses within the first 3 years of infection. Subject VC10014 was under observation for approximately 6 years, while subject VC20013 was under observation for approximately 4 years. Plasma and peripheral blood mononuclear cell (PBMC) samples were taken at multiple time points over the period of observation. During the period of observation, both subjects had steady CD4+ T cell counts, were without antiretroviral therapy, and had no clinical signs of AIDS. Before use in any in vitro assays, plasma samples were heat inactivated for 1 h at 54°C and centrifuged at 17,000 × g for 10 min.
Ethics statement.
All studies involving the enrollment, sample collection, and clinical follow-up of the subjects described here were approved by the Institutional Review Board at the Vanderbilt University School of Medicine (Vanderbilt University Medical Center, Nashville, TN, USA). The subjects described in this study provided written informed consent prior to participating in this study.
Kinetic analyses.
The description and production of the recombinant gp140 Envs used in these assays are given in the accompanying article by Malherbe et al. (38). The Env gp140s were constructed from plasma-derived envelope clones isolated from early infection. The env clones were modified to express as cleavage-defective gp140 trimers by the introduction of a stop codon at the terminus of the membrane-proximal external region and the removal of the primary and secondary cleavage sites between gp120 and gp41. Purified VC20013 (clone 013_9/23/04_C1 [see Fig. 3]) and VC10014 (014_4/15/04_F8 [see Fig. 4]) gp140 trimeric Env proteins were biotinylated (BT) at a 1:1 molar ratio using NHS-PEG4-Biotin system (Thermo Scientific, Rockford, IL) per the manufacturer's instructions. Zeba desalt spin columns (Thermo Scientific, Rockford, IL) were used to remove free biotin and exchange buffer to 1× phosphate-buffered saline (PBS).
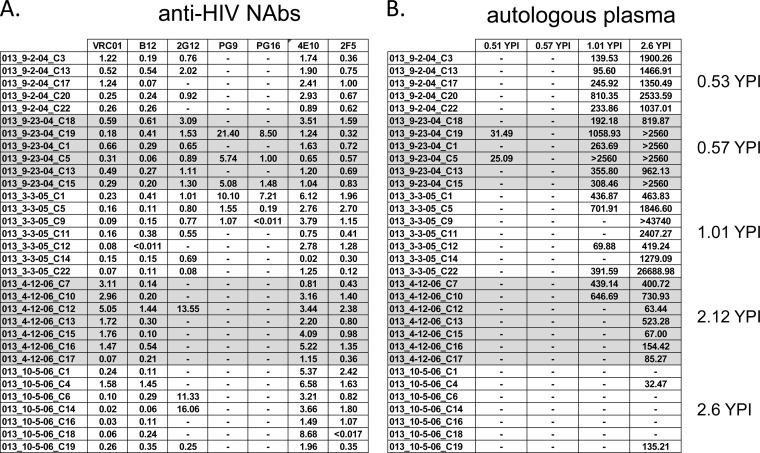
FIG 3.
Neutralization phenotype of the autologous viruses from subject VC20013. The neutralization properties of HIV-1 env clones isolated from temporally spaced plasma samples from subject VC20013 were determined. (A and B) Neutralization phenotypic profile of the autologous env clones against broadly neutralizing monoclonal antibodies (A) and autologous plasma (B). The values reported here are the IC50 values: antibody concentration (in micrograms per milliliter) (A) or reciprocal plasma dilution (B). Abbreviations and symbols are as defined in the legend to Fig. 1.
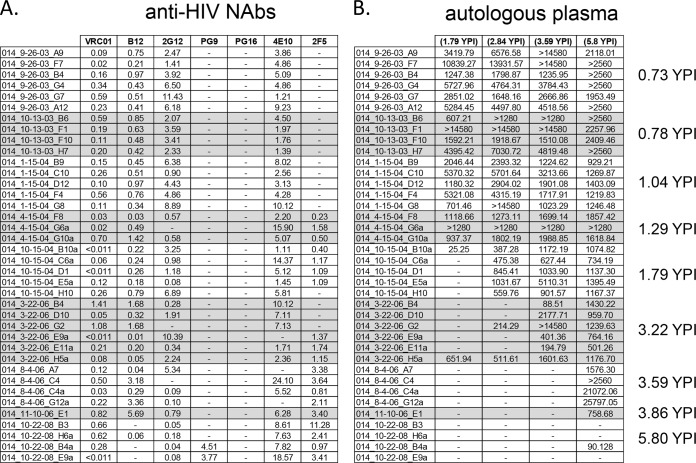
FIG 4.
Neutralization phenotype of the autologous viruses from subject VC20014. The neutralization properties of HIV-1 env clones isolated from temporally spaced plasma samples from subject VC20014 were determined. (A and B) Neutralization phenotypic profile of the autologous env clones against broadly neutralizing monoclonal antibodies (A) and autologous plasma (B). The values reported here are the IC50 values: antibody concentration (in μg/ml) (A) and reciprocal plasma dilution (B). Abbreviations and symbols are as defined in the legend to Fig. 1.
Binding affinities between BT-Env trimers and purified human polyclonal IgGs were measured by biolayer interferometry (BLI) using an Octet QKe instrument (ForteBio, Inc., Menlo Park, CA). BT-envelopes were immobilized directly on high-grade streptavidin biosensors (ForteBio) for 150 s at a single concentration of 2.5 μg/ml in kinetic buffer (KB) (1× PBS, 0.01% bovine serum albumin [BSA], 0.02% Tween 20, and 0.005% NaN3). After immobilization, baseline interference was read for 60 s in KB, then Env-bound sensors were immersed into wells containing six, 2-fold dilutions (from 1,000 nM down to 31.25 nM) of each purified human IgG sample for 900 s (association phase). The sensors were then moved into fresh KB for an additional 1,800 s to measure dissociation of the Env-bound IgG (dissociation phase). All kinetic interactions were measured with new sensors, and curve fitting was done using a 1:1 binding model using the data analysis software (ForteBio). A buffer-only reference was subtracted from all curves, and mean association constant (Kon), dissociation constant (Koff), and apparent equilibrium dissociation constant (KD) values were determined from at least three different concentrations of IgG that matched the theoretical fit with an R2 value of ≥0.95. The equilibrium dissociation constants were calculated as the kinetic dissociation rate constant divided by the kinetic association rate constant.
Envelope clones.
The amplification of HIV-1 envelope gene (env) sequences from subjects VC20013 and VC10014 was achieved by single-genome amplification (SGA) of the entire gp160 region according to the NIAID Center for HIV/AIDS Vaccine Immunology (CHAVI) standard operating procedures (39). Briefly, viral RNA was isolated from plasma samples and reverse transcribed using oligo(dT) primers. Viral envelope gp160 sequences were amplified by nested PCR, and the entire env-rev cassette was cloned into the pEMC* DNA plasmid vector, which places the gp160 sequences under transcriptional control of the cytomegalovirus (CMV) promoter. Finished clones were capable of expressing the entire gp160 portion of the HIV-1 env gene for production of pseudovirus (see below).
Pseudovirus production.
Pseudoviruses were produced by cotransfecting 293T cells plated at a density of 5 × 105 cells/ml, as previously described (8). Genejuice (EMD Millipore, Billerica, MA, USA) and Opti-MEM (Life Technologies, Carlsbad, CA, USA) were premixed for 5 min before adding 8 μg of the pNL4-3.Rev-.Env-.Luc+ (Luc stands for luciferase) HIV backbone and 4 μg of env plasmid DNA. The mixture was then allowed to precomplex for 15 min, and then it was added to HEK293T cells (Thermo Scientific, Waltham, MA, USA) for 4 h. After 4 h, the medium is removed, and fresh Dulbecco modified Eagle medium (DMEM) (Life Technologies, Carlsbad, CA, USA) supplemented with penicillin, streptomycin, l-glutamine, and 10% fetal bovine serum (FBS) was added to the culture. After incubation at 37°C for 3 days, the clarified cell supernatant was tested for p24 content (Zeptometrix, Franklin, MA, USA) and for functional entry and luciferase expression in TZM-bl cells. For neutralization assays to test glycan dependency, the pseudovirus production protocol was modified by supplementing the culture medium with kifunensine (25 μM) or swainsonine (20 μM) to alter the structures of the glycans on the surfaces of the virion as previously described (40).
Neutralization assays.
Neutralization assays were performed against pseudoviruses derived from the circulating envelope clones isolated from subjects VC20013 and VC10014 in the TZM-bl cell-based assay, as previously described (8). In addition, neutralization assays against heterologous HIV-1 pseudoviruses were performed. In those assays, the clade B and C isolates were taken from standardized virus panels (41, 42), and the clade A viruses were derived from early transmitted env genes, described previously (43) Briefly, plasma samples were titrated 2-fold from 1:20 to 1:2,560 and were incubated for 90 min at 37°C in the presence of single-round-competent virions. The virus/plasma mixture was added to TZM-bl cells that were plated at a density of 4 × 103 cells per well in a 96-well plate 24 h prior to inoculation. Seventy-two hours later, the cell supernatants were removed, and 100 μl of SteadyGlo luciferase reagent (Promega, Madison, WI, USA) was added to the cells of each well. The cell-associated luciferase activity (luminescence) for each well was determined on a Fluoroskan luminometer (Thermo-Fisher Scientific, Waltham, MA, USA). The data were imported into Prism 5 (GraphPad, La Jolla, CA, USA) and analyzed by nonlinear regression to fit a variable-slope sigmoidal dose-response curve y = [bottom + (top − bottom)]/[1 + 10(log IC50 − x) × hill slope], where the bottom and top are the plateaus and the hill slope is the slope factor. IC50 values were interpolated from each curve as part of the software analyses. The neutralization values reported here are the IC50, the plasma dilution at which viral entry was inhibited by 50% compared to the absence of plasma. When monoclonal antibodies (MAbs) are used instead of plasma, the IC50 values reported are the antibody concentrations (in micrograms per milliliter) that inhibit infection by 50%. MAb VRC01was kindly provided by J. Mascola (Vaccine Research Center [VRC], NIH), and MAbs 4E10, 2F5, 2G12, and b12 were purchased from Polymun Scientific (Vienna, Austria). MAbs PG9 and PG16 were obtained from Theraclone Sciences (Seattle, WA, USA).
HIV-1/HIV-2 MPER chimera neutralization assays.
Anti-membrane-proximal external region (anti-MPER) neutralization activity was measured against HIV-1/HIV-2 chimeric viruses, as previously described (9). The assay format is identical to that of the TZM-bl neutralization assay, which is described in detail above. We tested each plasma sample against wild-type (WT) HIV-2 and against six viral variants that contain a fragment of the HIV-1 MPER (C1YS, C3YS, C4YS, C6YS, C7YS, and C8YS). For a description of the portion of the HIV-1 MPER that corresponds to each variant, see Fig. 5B.
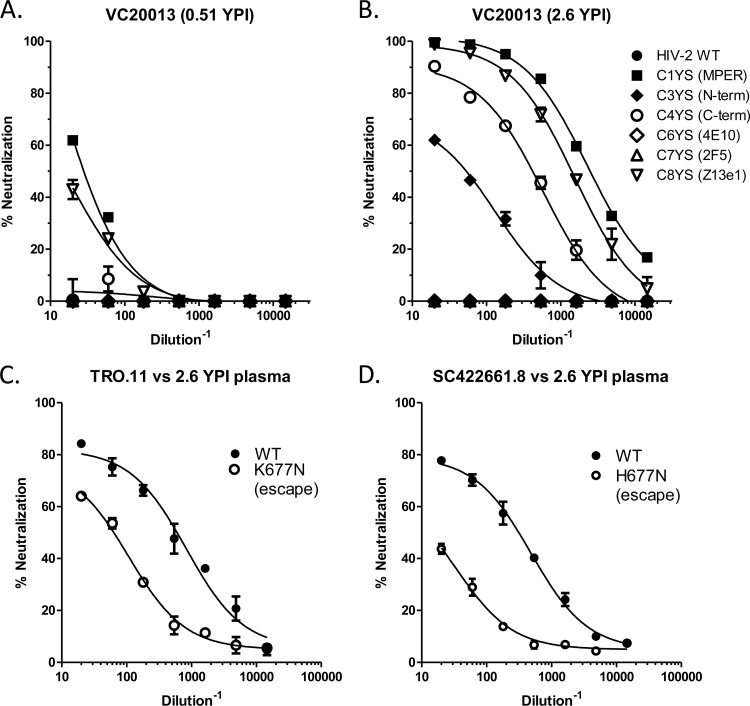
FIG 5.
Epitope specificity of the bNAb activity in subject VC20013. (A and B) Anti-MPER activity in subject VC20013 from 0.51 year postinfection (A) and 2.6 years postinfection (B) was measured against the HIV-2/HIV-1 MPER chimeras. The region of the MPER that has been inserted into the HIV-2 backbone is noted in parentheses in the legend in panel B (WT, wild type; N-term, N terminus). (C and D) Effects of the MPER escape mutations in modulating neutralization sensitivity to VC20013 plasma from 2.6 YPI. The escape mutations are K677N in TRO.11 (C) and H677N in SC422661.8 (D). WT, wild type (unmodified sequence).
Plasma IgG adsorptions on SF162 gp120- and SF162 gp120D368R-coated beads.
Plasma adsorptions were performed as previously described (8, 12), with some modifications. Plasma was diluted 1:10 in complete DMEM (Cellgro, Manassas, VA, USA) and serially adsorbed onto SF162 gp120-coated beads (MyOne Tosylactivated Dynabeads; Life Technologies, Carlsbad, CA, USA), and the flow through was collected. The antibodies bound to the gp120-coated beads were eluted by vortexing in increasingly acidic 0.1 M glycine solutions, followed by buffer exchange into PBS. Alternatively, diluted plasma was serially adsorbed onto SF162 gp120D368R-coupled beads to remove Abs that do not bind the CD4 binding site (CD4-BS). Each fraction described above was tested for residual neutralizing activity against heterologous and autologous isolates in the TZM-bl neutralization assay. The gp120-depleted plasma and the gp120D368R-depleted anti-gp120 fractions were tested for the presence of anti-CD4-BS antibodies, and the absence of non-CD4-BS gp120 Abs was tested by the Luminex assay (Luminex Corporation, Austin, TX, USA) against both wild-type SF162 gp120 and SF162 gp120D368R.
Generating mutations in gp160 env sequences.
Mutations into the coding sequence of certain env clones were introduced by site-directed mutagenesis. Mutagenesis reactions were carried out using the QuikChange II mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). High-performance liquid chromatography (HPLC)-purified complementary primer pairs coding for the desired point mutation were added to buffer, deoxynucleoside triphosphates (dNTPs), and DNA polymerase and denatured at 94°C for 10 min, followed by 19 cycles of the following conditions: 94°C for 30 s, 58°C for 30 s, and 72°C for 4 min. The reaction products were digested with DpnI for 1 h at 37°C to remove parental plasmids and were transformed into Escherichia coli DH5α cells (Life Technologies, Carlsbad, CA, USA). Single plasmid DNA clones were verified by DNA sequencing and then used to produce pseudovirus variants for use in cell culture assays as described above.
Nucleotide sequence accession numbers.
The sequences of all of the env clones used in this study have been submitted to GenBank, and the accession numbers are KJ698244 to KJ698348.
RESULTS
Incremental development of neutralization breadth within the first 3 years of infection.
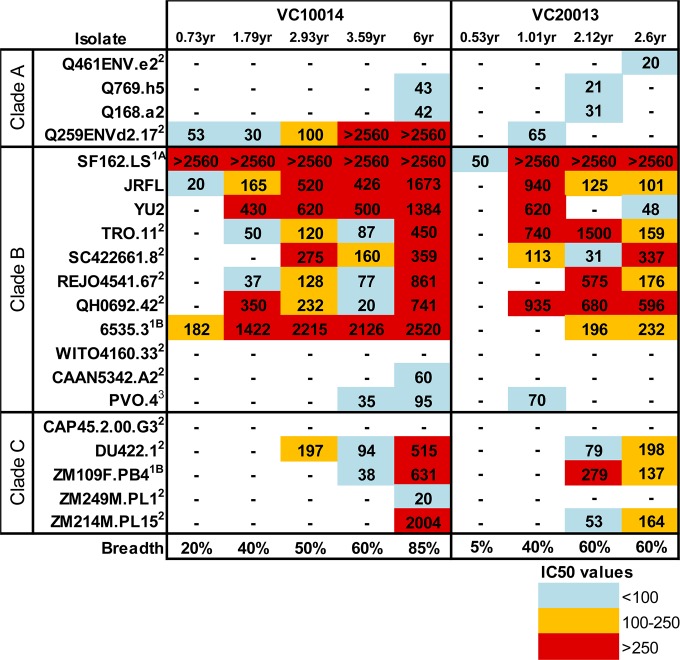
Both subject VC10014 and VC20013 entered observation within the first year of infection by HIV-1. Plasma samples from either subject during this first year showed little or no broadly neutralizing activity (Fig. 1). Approximately 1 year postinfection (YPI), both subjects' plasma antibodies began to exhibit broadly neutralizing activity and were able to neutralize several tier 2 heterologous viruses (i.e., isolates originating from different subjects). This initial activity was mainly restricted to viruses from clade B, but not viruses from clades A and C. By 2 years postinfection, both subjects developed cross-clade neutralizing activities, as their plasma antibodies were able to neutralize clade A and C viruses. By 6 years postinfection, plasma from subject VC10014 was able to neutralize 85% of viruses tested. By this time point, we also observed a marked increase in neutralization potency over previous time points, in particular against clade C viruses. The period of observation for subject VC20013 was shorter than that for subject VC10014, with samples being available only until approximately 3 years postinfection. Plasma from subject VC20013 eventually neutralized approximately 60% of viruses tested and generally displayed reduced ability to neutralize clade A isolates.
FIG 1.
Incremental development of broadly neutralizing antibody (bNAb) activity during early HIV-1 infection. Broadly neutralizing activity in subjects VC10014 and VC20013 against HIV-1 (4 clade A viruses, 12 clade B viruses, and 6 clade C viruses) in the TZM-bl neutralization assay was determined. The values reported here are the values for IC50, the reciprocal of the plasma dilution at which neutralizing activity is reduced to 50%. The IC50s are heat map color coded to indicate the relative potency of the response. When appropriate, the tier designation of each viral isolate is noted as a superscript (52). Neutralizing breadth is calculated as the percentage of viruses that each plasma sample is able to neutralize. −, no neutralization was observed.
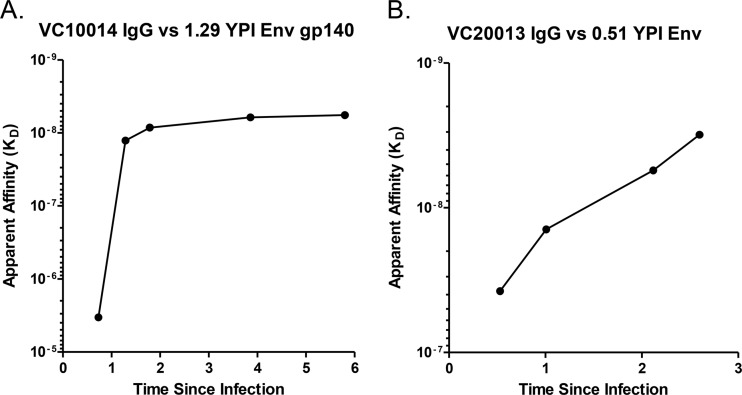
We also evaluated the binding affinity of plasma IgG to early autologous recombinant gp140 envelopes in subjects VC10014 and VC20013 by Octet biolayer interferometry (BLI). For both subjects, we evaluated binding of temporally spaced IgG samples against early serum-derived envelope clones to evaluate whether affinity was increasing against the circulating Envs over time. Interestingly, in both subjects, we observed a marked increase in plasma IgG anti-Env antibody binding affinity during the time of observation, which coincided with the broadening of the plasma neutralizing activities (Fig. 1 and 2). Importantly, we noted increases in the binding affinity in both subjects that were concomitant with the initial appearance of broadly neutralizing activity in plasma between the first and second years of infection. In subject VC10014, binding affinity to the autologous Env jumped from an equilibrium dissociation constant (KD) of 3.36 × 10−6 to 1.27 × 10−8 (where a decrease in KD equals an increase in binding affinity) when neutralizing breadth first appeared and ultimately reached nanomolar range by the end of observation (5.69 × 10−9) (Fig. 2A). The change in binding affinity was less pronounced for subject VC20013. For subject VC20013, the anti-Env affinity jumped from an initial prebreadth KD of 3.7 × 10−8 to 1.3 × 10−8, as breadth first developed at 1.01 YPI and ultimately reached 1.97 × 10−9 at 2.6 YPI (Fig. 2B). We previously reported on the general association between the development of broadly neutralizing antibodies (bNAbs) and plasma anti-HIV-1 envelope antibody avidity (8) and on the association between the frequency of peripheral CD4+ T follicular helper cells and the development of bNAb activity (11). Those previous findings, together with our observations that increases in binding affinity to autologous Env proteins occurred simultaneously with the appearance of bNAbs in subjects VC20013 and VC10014, suggest that antibody maturation during the early phases of infection is likely participating in the development of broadly neutralizing antibodies.
FIG 2.
Plasma IgG binding affinity to autologous Envs. The plasma IgG affinity to autologous Envs was determined by Octet biolayer interferometry (BLI). (A and B) The Env gp140 proteins used in this assay were derived from the early env sequences 014_04/15/04_F8 (A) and 013_9/23/04_C1 (B). The affinities of longitudinal IgG samples from subjects VC10014 (A) and VC20013 (B) were determined and plotted over time. The affinity measurements reported here are the equilibrium dissociation constants for each sample (KD), which were determined at multiple concentrations in replicate assays. YPI, year postinfection.
Neutralization phenotype of the circulating autologous viral isolates.
In order to assess the phenotype of the circulating autologous isolates, we made functional pseudoviruses bearing a number of autologous env sequences. Our analysis of the env sequences from both subjects confirmed that they were infected with clade B viruses. Further, our analyses did not reveal any evidence of superinfection (see Fig. S1 and S2 in the supplemental material). We tested 32 isolates from subject VC20013 from five time points spanning nearly 3 years of infection (Fig. 3 and Fig. S1). We tested 38 isolates from subject VC10014 from nine time points spanning nearly 6 years after infection with the virus (Fig. 4 and Fig. S2). We observed little or no contemporaneous autologous neutralization (that is, temporally matched plasma samples and viruses from the same time points), indicating that the circulating isolates escaped the contemporaneous antibody response (Fig. 3B and 4B). Plasma samples from both subjects were also unable to neutralize isolates from future time points, indicating that the contemporaneous escape became fixed in the circulating viral quasispecies. Only in the cases where plasma samples were tested against isolates from earlier time points did we observe robust and potent neutralizing activity. This pattern in which autologous plasma is able to neutralize isolates from previous time points, but not contemporaneous or future isolates, is in agreement with previous studies (44–46). The development of bNAb activity did not alter this pattern, as we observed escape in the autologous isolates in nearly all of the isolates from after heterologous neutralizing activity had appeared.
The isolates from each subject were also tested against well-known monoclonal bNAbs (Fig. 3A and 4A). We hypothesized that if bNAbs could neutralize the early isolates but not the late isolates, this could be an indication that the subject developed antibodies to those epitope targets and that the autologous Envs had escaped their activity. Interestingly, all of the isolates from subject VC20013 were very susceptible to bNAbs that target the CD4 binding site (CD4-BS), including MAbs b12 and VRC01 (18, 24). This potency did not change over the 3 years of observation. This lack of immune pressure to the CD4-BS implies that subject VC20013 did not develop anti-CD4-BS antibodies similar to MAbs VRC01 and b12. The isolates from subject VC10014 were also susceptible to anti-CD4-BS antibodies until the sixth year of infection, when the viruses became mostly resistant to neutralization by b12. It is possible that at this point during the course of infection that this subject developed neutralizing activity to the CD4-BS or to an epitope that overlaps with MAb b12. Interestingly, plasma from this time point showed a dramatic increase in the breadth and potency of cross-neutralizing responses, neutralizing 40% more isolates than the previous time point (Fig. 1).
We also tested PG9 and PG16, two clonally related bNAbs that target a glycopeptide epitope in the V2 region (21). A few isolates from approximately 1 year postinfection showed moderate sensitivity to neutralization in subject VC20013, but later isolates remained resistant. Thus, the earliest isolates from both subjects were resistant to neutralization by these bNAbs, indicating that the transmitter/founder virus very likely was already resistant to PG9/PG16. We tested the isolates for sensitivity to 2G12, a bNAb that targets a trio of high-mannose residues (20, 31). With only three exceptions, the isolates from subject VC10014 were sensitive to neutralization by 2G12. The earliest isolates from subject VC20013 were also sensitive to 2G12. Many of the isolates after the first year of infection were resistant to neutralization, although a few sensitive isolates remained present in those later samples, indicating that 2G12-senstive isolates were still circulating during these time points. Last, we surveyed two bNAbs that target the membrane-proximal external region (MPER) of the gp41 subunit of Env, 2F5 and 4E10. All of the isolates from VC20013 were sensitive to both bNAbs. All of the isolates from VC10014 were sensitive to neutralization by 4E10. However, the early isolates from VC10014 were resistant to 2F5, whereas isolates sampled from after 1 year postinfection were sensitive to neutralization by 2F5.
Plasma neutralizing antibodies from subject VC20013 target a unique epitope within the MPER region of gp41.
Assessing the neutralization profiles of the autologous isolates to known bNAbs provided few clues as to the epitope specificities of the cross-neutralizing antibody activity. As such, we used well-developed mapping techniques to identify the epitope targets of both the autologous and heterologous neutralizing activity. We employed plasma adsorption techniques in which bead-coupled gp120 protein (and mutated variants) were used to identify the presence of anti-gp120 antibodies (8, 11, 12, 47). We incubated plasma with wild-type (WT) gp120 and variants containing mutations specific to the CD4-BS in order to fractionate the gp120 and CD4-BS antibody fractions (8, 12, 28). In addition, to assess whether specificities were present that were dependent on glycan residues, such as PG9, 2G12, and PGT128, we used a combination of viruses grown in glycosidase inhibitors (such as kifunensine) to alter the structure of the glycan shield and viruses in which N-linked glycosylation sites (NLGS) had been selectively removed (see Fig. S3 and S6 in the supplemental material) (11, 29, 33). Mutant and glycosidase inhibitor-treated viruses were prepared from both heterologous and autologous isolates. To assess any potential anti-MPER-directed activity, we employed HIV-2/HIV-1 MPER chimeras, in which the MPER from HIV-1 is inserted to replace the MPER in the HIV-2 envelope (9). The insertions range from the entire MPER region (C1YS) to only the N or C terminus (C3YS and C4YS, respectively) or have only the epitope regions of known MPER antibodies (C6YS, C7YS, and C8YS) (Fig. 5).
For subject VC20013, we were unable to map the neutralizing activity to the gp120 subunit using plasma adsorption techniques, in agreement with our previously published results (8). Further, our studies using NLGS mutant isolates and kifunensine-grown isolates failed to identify any potential glycan-dependent activity (see Fig. S3 in the supplemental material). Thus, it is likely that neither the autologous nor heterologous activity in subject VC20013 was due to CD4-BS activity or glycan-dependent specificities (such as PG9, PGT128, or 2G12) (Fig. S3). Plasma from VC20013 potently neutralized the chimeras containing the whole MPER (C1YS [Fig. 5A and B]), the C terminus of the MPER (C4YS), and a central portion of the MPER (C8YS). We detected low levels of this neutralizing activity at the earliest time point when cross-neutralizing activity was not evident (0.68 YPI) (Fig. 5A) but only at low potency and only against the whole MPER fragment. Thus, it is likely that this anti-MPER specificity was beginning to develop before cross-neutralizing activity became measurable in plasma but did not gain significant neutralization potency until more than 1 year postinfection (the time at which broadly neutralizing activity was readily detectable).
Analysis of the sequences isolated from subject VC20013 indicated that the 2F5 and 4E10 epitopes were strongly conserved throughout the course of infection, indicating a lack of immune pressure within those epitopes. However, at position 677 within the MPER (HXB2 numbering), we noted the appearance of an amino acid mutation that occurred at the time neutralization breadth developed that became fixed in isolates from later time points. During early infection, a lysine residue (K) was present in all isolates at this position (K677) (see Fig. S4 in the supplemental material). At the time neutralizing breadth initially appeared in plasma, the viral quasispecies contained K677, but there were also a small number of sequences that contained mutations to an asparagine (N677). In later samples, N677 became fixed in all of the circulating isolates, indicating that N677 was likely an escape mutation. Interestingly, position 677 has not been identified as a critical part of the epitopes of the anti-MPER antibodies 10E8, 2F5, 4E10, and Z13-e1 (19, 23, 48).
To determine whether the change from K to N at position 677 was responsible for the observed neutralization escape in subject VC20013, we mutated both autologous and heterologous isolates at this position and determined its effect on neutralizing activity. We introduced the K677N mutation into isolate TRO.11 and the H677N mutation into isolate SC422661.8, both clade B tier 2 isolates from standardized neutralization panels (41) that were neutralized by the VC20013 plasma. In the case of the TRO.11/K677N mutant virus, neutralization by plasma from 2.5 years postinfection was reduced by more than 1 log unit (Fig. 5C), and neutralizing susceptibility was nearly entirely abrogated for SC422661.8/H677N (Fig. 5D). The prevalence of N677 in the HIV-1 Env sequence compendium (http://www.hiv.lanl.gov) (in 2013), which contains 3,710 sequences from all clades and recombinant forms, is quite high and is present in more than 58% of sequences. These circulating isolates that already contain N677 are likely to be naturally resistant to neutralization by the specific anti-MPER bNAbs present in subject VC20013. The high incidence of circulating isolates that potentially are resistant to the VC20013 bNAbs is one likely explanation of why plasma from VC20013 did not exhibit more-extensive cross-reactive bNAb activity.
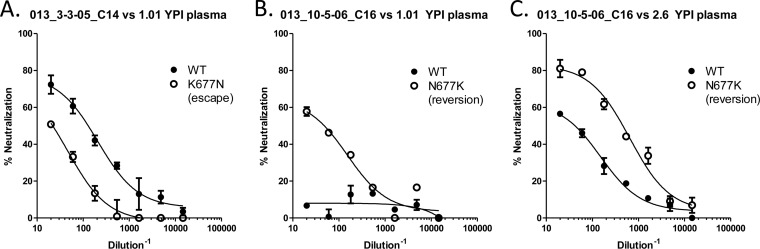
To investigate whether the K677N mutation contributed to escape of the autologous viruses, we introduced the K677N mutation into isolates that were sensitive to neutralization and the reverse mutation N677K into neutralization-resistant isolates. The introduction of K677N in the early, neutralization-sensitive isolates made them partially resistant to neutralization by the contemporaneously matched plasma (Fig. 6A), reducing the IC50 more than 6-fold from 1:132 to 1:20. K677N had no effect on the neutralizing activity of the later plasma samples against the early isolates (data not shown). This is not surprising, as the later plasma samples likely would have acquired new autologous neutralizing epitopes and would not be as affected by this mutation in much earlier isolates. Introduction of the reversion mutation N677K, which we anticipated would increase the neutralization sensitivity of resistant isolates, into the later isolates had a much more profound effect. IC50 titers for plasma from 1.19 YPI against an isolate from 2.6 YPI increased from undetectable against the WT virus to 1:47 in the N677K virus (Fig. 6B). Additionally, this reversion mutation rendered partially resistant isolates from later during infection more sensitive to neutralization by contemporaneously matched plasma samples, increasing the IC50 titers more than 1 log unit from 1:44 to 1:490 (Fig. 6C). Thus, the autologous neutralizing activity dependent on residue 677 appeared to have developed around 1 year postinfection (at the time cross-neutralizing breadth was also present) and was still present more than 2 years later.
FIG 6.
Anti-MPER NAbs mediate autologous neutralization in subject VC20013. The MPER mutations at residue 677 were inserted into autologous isolates from subject VC20013. The escape mutation K677N was inserted into a neutralization-sensitive isolate (A), which resulted in a reduction in neutralization. The reversion mutation N677K was inserted into two neutralization-resistant VC20013 isolates (B and C), which resulted in an increased ability to neutralize the virus. WT, wild type.
Together, these findings indicate that the anti-MPER activity is a major component of both the heterologous and autologous neutralizing activity in subject VC20013 and that it started to develop prior to cross-neutralizing activity became readily detectable in plasma. However, cross-neutralizing activity and the diagnostic escape mutations in the autologous isolates did not become apparent until more than a year after infection. Thus, in subject VC20013 it appears that initial early acquisition of a specific epitope in the MPER and sufficient time to mature the antibody response was necessary to achieve cross-neutralizing breadth.
Plasma neutralizing antibodies from subject VC10014 target an epitope overlapping the CD4-BS similar to MAb HJ16.
We performed similar epitope mapping analyses on temporally spaced plasma samples from subject VC10014. This subject exhibited no measurable activity against the HIV-2/HIV-1 MPER chimeras, indicating that no anti-MPER activity was present (data not shown). In addition, we did not detect any glycan-dependent antibody specificities, as neither the targeted removal of NLGS nor treatment of viruses with glycosidase inhibitors had any effect on neutralizing activity against either the heterologous (see Fig. S5 in the supplemental material) or autologous isolates (data not shown). We did observe a significant reduction in neutralization of both the heterologous (8) and autologous isolates after the removal of anti-gp120 antibodies by plasma adsorption studies, indicating that the neutralizing activity likely targets the gp120 subunit (Fig. S6). In this case, the neutralizing activity was removed by both wild-type gp120 and gp120 containing the D368R mutation in the CD4-BS, indicating that the antibodies do not target the CD4-BS (8).
We analyzed the sequences from subject VC10014 for signatures of antibody escape at the time when cross-neutralizing breadth first became measureable in plasma. We observed two regions in the autologous env sequences in which potential escape mutations arose at the time that broadly neutralizing activity became detectable in plasma and which became fixed in later env sequences. One of those regions was in the C2 region of Env, a region that constitutes one of the five discontinuous regions that fold together to form the CD4-BS. In the early samples up to nearly 2 years postinfection, F277 (HXB2 numbering) was universally present in the viral population, but as cross-neutralizing activity became detectable at 2 years postinfection, this position transitioned to I277 (F277I) and became fixed thereafter (see Fig. S7 in the supplemental material). In viral isolates from 2 years after breadth was detected (3.5 years postinfection), a mutation arose adjacent to I277 in the C2 region, N279D, and became fixed in the population. Interestingly, both I277 and N279 are known to make contact with the CD4 receptor (37). These two closely spaced mutations potentially reflect sequential escape mechanisms from continued antibody pressure at this site. Another potential escape mutation appeared in the variable region V4 of Env at the time cross neutralizing breadth was developed (Fig. S7). In this case, the mutation was a 4-amino-acid insertion that increased the size of the V4 loop from 31 to 35 amino acid residues. This insertion appears to have occurred in more than one Env lineage, as we detected at least two different insertion motifs (SSWN and DYTY), implying that this could be a general escape mechanism rather than a single virus that escaped and later proliferated.
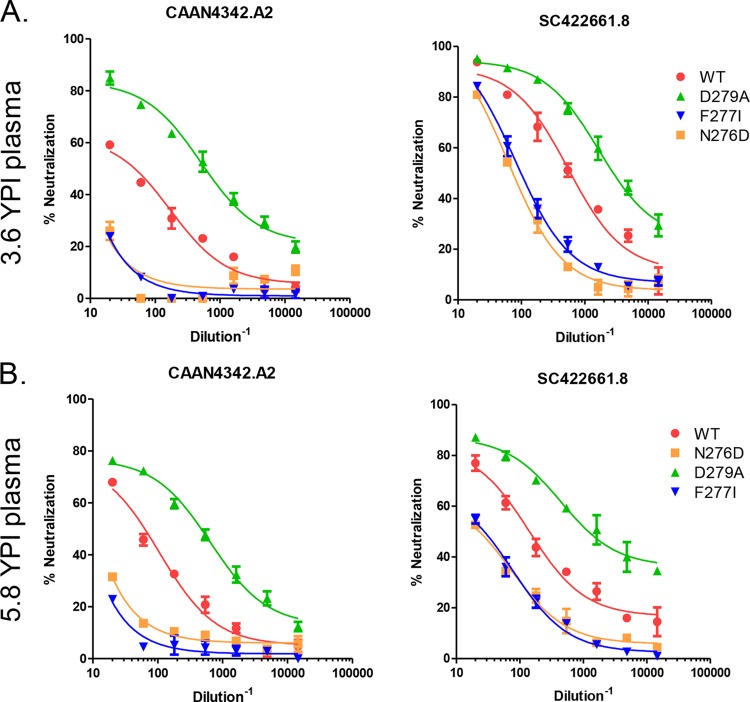
We introduced these potential escape mutations alone and in combination with one another into neutralization-sensitive autologous isolates and into heterologous clade B isolates to assess whether they would reduce neutralization. Introduction of F277I into the heterologous isolates CAAN4342.A2 and SC422661.8 resulted in a significant reduction in neutralization by plasma from both 3.6 and 5.8 years postinfection, indicating that the heterologous activity against these isolates is at least partially dependent on residue 277 (Fig. 7A and B). We did not introduce D279 in these isolates, as both of those isolates already had an aspartic acid (D) at those residues. However, the mutation D279A, which constitutes a reversion mutation from the escape residue, made both CAAN4342.A2 and SC422661.8 more sensitive to neutralization (Fig. 7A and B). We noted that position F277 is adjacent to an N-linked glycosylation site at position 276, creating the possibility that the escape mutations at position 277 may affect the exposure or orientation of the glycan moiety at position 276. Removal of the NLGS by mutation N276D in isolates CAAN4342.A2 and SC422661.8 yielded a reduction in neutralization activity that was nearly identical to that caused by the F277I mutation (Fig. 7A and B). These findings indicated that the heterologous activity in subject VC10014 was potentially similar to the MAb HJ16, which is known to target an epitope overlapping the CD4-BS but is not sensitive to the D368R mutation (49). Importantly, MAb HJ16 is also critically dependent on the glycan moiety at position 276 (50), similar to the activity in subject VC10014. However, the breadth and potency of the bNAb response in VC10014 far exceeded that of MAb HJ16, indicating that although they may target a similar region on the gp120 subunit, they are not identical.
FIG 7.
Epitope specificity of the bNAb activity in subject VC10014. We introduced mutations within the C2 region of Env to assess their effect on neutralizing activity. (A and B) Plasma samples from subject VC10014 from 3.6 YPI (A) and 5.8 YPI (B) were tested against the two tier 2 clade B isolates CAAN4342.A2 and SC422661.8, as well as variants in which the mutations D279A, F277I, and N276D had been introduced. The escape mutations F277I and N276D resulted in a reduction in neutralization, whereas the reversion mutation D279A increased neutralization sensitivity.
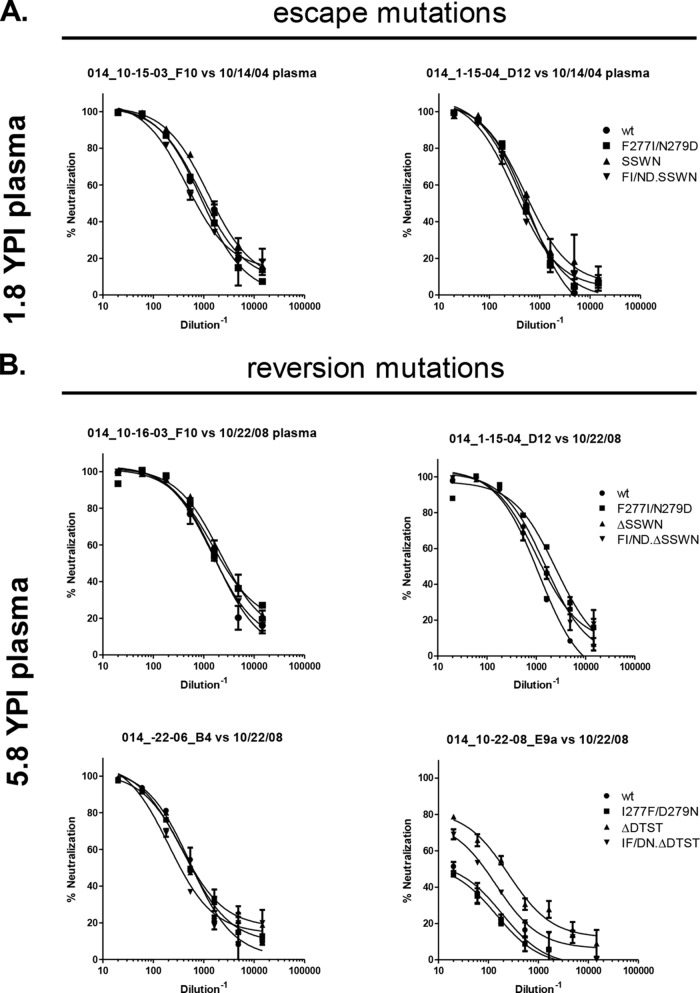
We also tested the C2 mutations and V4 insertions in the autologous isolates. In the neutralization-sensitive autologous isolates from 1 to 2 years postinfection, the introduction of F277I/N279D or the four amino acid insertion (SSWN) had no effect on neutralization sensitivity (Fig. 8A). Similarly, there was no effect when the mutations were introduced together as a triple mutant F277I/N279D.SSWN autologous virus (Fig. 8A). Reversion mutations were introduced in the neutralization-resistant isolates from later time points (I277F, D279N, or V4 amino acid deletion) to assess whether they increased neutralization sensitivity (Fig. 8B). The major effect that we observed was in a late, partially resistant isolate (014_10-22–08_E9a) from nearly 6 years postinfection. Removal of the 4-amino-acid motif (ΔDTST) in the V4 variable region increased neutralization sensitivity to the contemporaneously matched plasma more than 10-fold, increasing the IC50 from 1:20 to 1:214 (Fig. 8B). The reversion triple mutant version of the same late isolate, which contained the reversion mutations I227F/D279N/ΔDTST, had an intermediate phenotype, increasing the IC50 from 1:20 to 1:87. Thus, the insertion of four amino acids in the V4 region seems to have had the most profound effect on the neutralization sensitivity to the autologous plasma and the most significant contribution to the escape from the autologous neutralizing activity. Interestingly, so far, the V4 has not been identified as a major part of any epitope of broadly neutralizing MAbs. It is possible that in subject VC10014, the V4 is not a direct epitope target but rather is able to mediate escape by masking a distal neutralizing epitope.
FIG 8.
VC10014 epitope mapping in the autologous isolates. We tested the effects of mutations within the C2 region of Env and insertions and deletions in the V4 region of Env on the neutralization phenotype of autologous viruses isolated from subject VC10014. (A) Effects of potential escape mutations on neutralization sensitivity of early isolates to early or contemporaneously matched plasma. We tested variants in which we introduced F277I/N279D or the V4 insertion motif SSWN and variants with both the mutation and insertion (F277I/N279D/SSWN). (B) Effects of potential reversion mutations in the later, neutralization-resistant isolates against late or contemporaneously matched plasma. The reversion mutations include I277F/D279N, ΔSSWN, or ΔDTST and I277F/D279N/ΔSSWN or I277F/D279N/ΔDTST. YPI, years postinfection; wt, wild type; ΔSSWN or ΔDTST, deletion of the four amino acids; FI/ND/ΔDTST or FI/ND/ΔSSWN, I277F, D279N, and deletion of SSWN or DTST in the V4.
DISCUSSION
Deciphering the mechanisms behind the early development of broadly neutralizing antibodies to HIV-1 may lead to the development of better vaccine immunogens and vaccination protocols, and it remains a priority for the HIV-1 vaccine development field. It has been established that events within the first 3 to 4 years are critical in determining whether bNAbs will develop, as recent studies have shown (11, 13). Several critical factors are associated with the development of bNAbs during the early years of infection. First, sustained but moderate viral loads are associated with the development of bNAbs in multiple studies (8, 11, 15, 51). Second, circulating peripheral T follicular helper CD4+ T cells (TFH) cells are more frequent in subjects that develop bNAbs (11, 17). These findings hint at a potential interplay between the virus and the host immune system in which antigenic stimulation drives a maturing antibody response toward broadly neutralizing activity. However, the early immunological and virologic events preceding the development of neutralizing breadth are only now beginning to come into focus.
Different patterns have emerged of how neutralizing breadth gradually emerges during the early years of infection. In some subjects, breadth develops in several overlapping “waves” of bNAb specificities, where a single epitope specificity is acquired, the response peaks, and then it wanes (11, 34). In other cases, subjects may develop bNAb activity to a single epitope that can account for the majority of the heterologous neutralizing activity and do not appear to develop bNAbs to multiple epitopes (11, 13). The early viral and immunological events before the initial emergence of bNAb activity, no matter which pattern emerges, are of great interest to vaccine design efforts. Despite recent progress, the early stages of the development of neutralizing breadth against epitopes outside the CD4-BS or the V1V2 has yet to be defined, and it is unclear whether their development follows a similar pattern to what has been reported.
Our data indicate that subjects VC10014 and VC20013 both developed bNAb activity by the acquisition of a single bNAb specificity. In subject VC10014, the bNAb activity developed around 1 year postinfection and targets an epitope that overlaps the CD4-BS and is similar to (but distinct from) bNAb HJ16. In the case of VC20013, the bNAb activity targets a novel epitope in the MPER that is critically dependent on residue 677. Importantly, we were able to detect anti-MPER activity against the HIV-1/HIV-2 MPER chimeras well before plasma neutralizing activity was detectable against the heterologous isolates. Thus, the anti-MPER antibody specificity developed very early in the course of infection and was present in plasma long before it was able to mediate heterologous neutralization. As bNAb activity developed after 1 year postinfection in both subjects, we noted a simultaneous increase in the antibody binding affinity to the autologous Envs, indicating that antibody maturation could have been a key element in driving the antibody responses toward broadly neutralizing activity. These observations also may provide a mechanistic link between the observed increased frequency of circulating TFH cells (11, 17) and the initial development of bNAbs and highlight the potential role of antibody maturation during these early developmental processes.
In addition to maturation, it has been established that Env evolution is an important driver of bNAb development (36, 37). It is intriguing that the areas within or near the epitopes to which we mapped the broadly neutralizing activity (the MPER in subject VC20013 and the C2 and V4 regions in subject VC10014) were not evolving prior to the emergence of broadly neutralizing activity. Only after broadly neutralizing activity became detectable in plasma did escape mutations appear and become rapidly fixed in the viral populations. These findings imply that the initial development of neutralizing breadth in these two patients was not dependent on early evolution within the direct epitopes themselves, although we cannot rule out that the low level of mutation outside the epitopes was beneficial. However, the initial appearance of breadth, which was highly restricted to neutralizing clade B isolates, coincided with a rapid diversification of the circulating Envs. These observations suggest that increasing viral diversity was likely a key driver of the broadening response over subsequent time points, as has been recently reported (36, 37).
Importantly, our findings highlight that in the case of the anti-MPER responses in subject VC20013, the initial B cell receptor (BCR) acquisition of a broadly neutralizing epitope was not sufficient to achieve neutralizing breadth, but rather the response still required time in order to achieve broadly neutralizing activity. It is unclear to what extent such BCRs must undergo maturation in order to acquire broadly neutralizing activity, and it is likely that antibody responses to different epitopes have different minimum affinity maturation requirements. These differences may partially account for why the timing of bNAb development among HIV-1-positive subjects is somewhat variable. However, our findings here are in agreement with the findings of a recent report on the development of bNAb CH103, which was isolated approximately 2.5 years postinfection and targets the CD4-BS (37). In that study (37), Liao and colleagues were able to identify relevant BCR intermediates as early as 4 weeks postinfection and were able to track BCR maturation until the initial emergence of broadly neutralizing activity nearly a year later. As broadly neutralizing activity emerged in that subject, they noted the rapid emergence of escape mutations in the viral env genes, similar to what we observed here. Similar findings have also been recently reported for a subject that developed V1V2-targeted antibodies (36). It is unclear exactly how early the bNAb progenitors developed in subjects VC20013 and VC10014 or which exact stepwise mutations within the epitope-specific BCRs preceded the emergence of bNAb activity. A thorough longitudinal investigation of BCR maturation by deep sequencing or B cell isolation is necessary to definitively answer these questions. However, our observations that bNAbs to the MPER and HJ16 epitopes developed by a mechanism similar to what was reported for the CD4-BS and V1V2 suggests that these early developmental milestones likely are somewhat universal.
Overall, our findings illuminate several of the key early events that led to the development of neutralizing breadth in two HIV-1-positive subjects. Our findings, along with previous studies (36, 37), highlight a potential mechanism for the development of anti-HIV-1 bNAbs in which B cell receptors targeting neutralizing epitopes become stimulated very early on after infection. This initial BCR stimulation may not result in the production of a neutralizing antibody response immediately, but rather it is the first step in the process of driving epitope-specific antibody maturation toward broadly neutralizing activity. Given the proper immune environment in which the B cell receives sufficient antigenic stimulation and CD4+ T cell help over a period of time, these responses may mature to the point of acquiring broadly neutralizing activity (8, 11, 15, 17, 51). Our findings also reinforce the need for the development of vaccine regimens and delivery systems that provide sufficient antigenic stimulation and that are able simultaneously to drive B cell maturation.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Oliver, A. McGuire, M. Lange, and K. Cohen for critical readings of the manuscript and for their helpful comments.
This study was funded by HIVRAD P01 AI078064 to N.L.H. and L.S.
Footnotes
Published ahead of print 13 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01816-14.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). 2013. Global report. UNAIDS report on the global AID epidemic 2013. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 2.McElrath MJ, Haynes BF. 2010. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33:542–554. 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420. 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomaras GD, Haynes BF. 2010. Strategies for eliciting HIV-1 inhibitory antibodies. Curr. Opin. HIV AIDS 5:421–427. 10.1097/COH.0b013e32833d2d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 7.Buonaguro L, Tornesello ML, Buonaguro FM. 2007. Human immunodeficiency virus type 1 subtype distribution in the worldwide epidemic: pathogenetic and therapeutic implications. J. Virol. 81:10209–10219. 10.1128/JVI.00872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769. 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray ES, Taylor N, Wycuff D, Moore PL, Tomaras GD, Wibmer CK, Puren A, DeCamp A, Gilbert PB, Wood B, Montefiori DC, Binley JM, Shaw GM, Haynes BF, Mascola JR, Morris L. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925–8937. 10.1128/JVI.00758-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, Connors M, Hoxie J, Mascola JR, Wyatt R. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 83:1045–1059. 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7:e1001251. 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, Mascola JR. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 13:1032–1034. 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, CAPRISA002 Study Team 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85:4828–4840. 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, Wood B, Nathe C, Richman D, Tomaras GD, Bibollet-Ruche F, Robinson JE, Morris L, Shaw GM, Montefiori DC, Mascola JR. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651–11668. 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piantadosi A, Panteleeff D, Blish CA, Baeten JM, Jaoko W, McClelland RS, Overbaugh J. 2009. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J. Virol. 83:10269–10274. 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188–199. 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, International AIDS Vaccine Initiative Protocol C Principal Investigators. Poignard P, Crotty S. 2013. Human circulating PD-(+)1CXCR3(-)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39:758–769. 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, Lamacchia M, Garratty E, Stiehm ER, Bryson YJ, Cao Y, Moore JP, Ho DD, Barbas CF., III 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027. 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 19.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905. 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306–7321. 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators. Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators. Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, Scheid JF, Kraft Z, Liu Y, Pietzsch J, Hurley A, Poignard P, Feizi T, Morris L, Walker BD, Fatkenheuer G, Seaman MS, Stamatatos L, Nussenzweig MC. 2012. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J. Exp. Med. 209:1469–1479. 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diskin R, Scheid JF, Marcovecchio PM, West AP, Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293. 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietzsch J, Scheid JF, Mouquet H, Klein F, Seaman MS, Jankovic M, Corti D, Lanzavecchia A, Nussenzweig MC. 2010. Human anti-HIV-neutralizing antibodies frequently target a conserved epitope essential for viral fitness. J. Exp. Med. 207:1995–2002. 10.1084/jem.20101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640. 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O'Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang ZY, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, NISC Comparative Sequencing Program. Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602. 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scanlan CN, Pantophlet R, Wormald MR, Saphire EO, Calarese D, Stanfield R, Wilson IA, Katinger H, Dwek RA, Burton DR, Rudd PM. 2003. The carbohydrate epitope of the neutralizing anti-HIV-1 antibody 2G12. Adv. Exp. Med. Biol. 535:205–218. 10.1007/978-1-4615-0065-0_13. [DOI] [PubMed] [Google Scholar]
- 32.Nelson JD, Brunel FM, Jensen R, Crooks ET, Cardoso RM, Wang M, Hessell A, Wilson IA, Binley JM, Dawson PE, Burton DR, Zwick MB. 2007. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. J. Virol. 81:4033–4043. 10.1128/JVI.02588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikell I, Stamatatos L. 2012. Evolution of cross-neutralizing antibody specificities to the CD4-BS and the carbohydrate cloak of the HIV Env in an HIV-1-infected subject. PLoS One 7:e49610. 10.1371/journal.pone.0049610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wibmer CK, Bhiman JN, Gray ES, Tumba N, Abdool Karim SS, Williamson C, Morris L, Moore PL. 2013. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS Pathog. 9:e1003738. 10.1371/journal.ppat.1003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 6:e1001028. 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, Dekosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, Altae-Tran HR, Bailer RT, Crooks ET, Cupo A, Druz A, Garrett NJ, Hoi KH, Kong R, Louder MK, Longo NS, McKee K, Nonyane M, O'Dell S, Roark RS, Rudicell RS, Schmidt SD, Sheward DJ, Soto C, Wibmer CK, Yang Y, Zhang Z, NISC Comparative Sequencing Program. Mullikin JC, Binley JM, Sanders RW, Wilson IA, Moore JP, Ward AB, Georgiou G, Williamson C, Abdool Karim SS, Morris L, Kwong PD, Shapiro L, Mascola JR. 2014. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509:55–62. 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program. Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malherbe DC, Pissani F, Sather DN, Guo B, Pandey S, Sutton WF, Stuart AB, Robins H, Park B, Krebs SJ, Schuman JT, Kalams S, Hessell AJ, Haigwood NL. 2014. Envelope variants circulating as initial neutralization breadth developed in two HIV-infected subjects stimulate multiclade neutralizing antibodies in rabbits. J. Virol. 88:12949–12967. 10.1128/JVI.01812-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salazar-Gonzalez JF, Bailes E, Pham KT, Salazar MG, Guffey MB, Keele BF, Derdeyn CA, Farmer P, Hunter E, Allen S, Manigart O, Mulenga J, Anderson JA, Swanstrom R, Haynes BF, Athreya GS, Korber BT, Sharp PM, Shaw GM, Hahn BH. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952–3970. 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doores KJ, Burton DR. 2010. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J. Virol. 84:10510–10521. 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125. 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 80:11776–11790. 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long EM, Rainwater SM, Lavreys L, Mandaliya K, Overbaugh J. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retroviruses 18:567–576. 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- 44.Moore PL, Ranchobe N, Lambson BE, Gray ES, Cave E, Abrahams MR, Bandawe G, Mlisana K, Abdool Karim SS, Williamson C, Morris L, CAPRISA002 Study, NIAID Center for HIV/AIDS Vaccine Immunology (CHAVI) 2009. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog. 5:e1000598. 10.1371/journal.ppat.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, Allen SA, Pinter A, Shaw GM, Hunter E, Robinson JE, Gnanakaran S, Derdeyn CA. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 5:e1000594. 10.1371/journal.ppat.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaillon A, Braibant M, Hue S, Bencharif S, Enard D, Moreau A, Samri A, Agut H, Barin F. 2012. Human immunodeficiency virus type-1 (HIV-1) continues to evolve in presence of broadly neutralizing antibodies more than ten years after infection. PLoS One 7:e44163. 10.1371/journal.pone.0044163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sather DN, Carbonetti S, Kehayia J, Kraft Z, Mikell I, Scheid JF, Klein F, Stamatatos L. 2012. Broadly neutralizing antibodies developed by an HIV-positive elite neutralizer exact a replication fitness cost on the contemporaneous virus. J. Virol. 86:12676–12685. 10.1128/JVI.01893-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker CE, Deterding LJ, Hager-Braun C, Binley JM, Schulke N, Katinger H, Moore JP, Tomer KB. 2001. Fine definition of the epitope on the gp41 glycoprotein of human immunodeficiency virus type 1 for the neutralizing monoclonal antibody 2F5. J. Virol. 75:10906–10911. 10.1128/JVI.75.22.10906-10911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, Willems B, Zekveld MJ, Dreja H, O'Sullivan E, Pade C, Orkin C, Jeffs SA, Montefiori DC, Davis D, Weissenhorn W, McKnight A, Heeney JL, Sallusto F, Sattentau QJ, Weiss RA, Lanzavecchia A. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5:e8805. 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balla-Jhagjhoorsingh SS, Corti D, Heyndrickx L, Willems E, Vereecken K, Davis D, Vanham G. 2013. The N276 glycosylation site is required for HIV-1 neutralization by the CD4 binding site specific HJ16 monoclonal antibody. PLoS One 8:e68863. 10.1371/journal.pone.0068863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doria-Rose NA, Klein RM, Daniels MG, O'Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 84:1631–1636. 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452. 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.